- 1Department of Endocrinology, The Second People’s Hospital of Liaocheng, Liaocheng, Shandong, China
- 2Department of Rehabilitation Medicine, The Affiliated Hospital of Qingdao University, Qingdao, Shandong, China
- 3Department of Joint Surgery, The Second People’s Hospital of, Liaocheng, Shandong, China
Objective: To evaluate the effects of exercise interventions on bone health and body composition in postmenopausal women with breast cancer.
Methods: A systematic search was conducted across PubMed, EMBASE, Web of Science, CENTRAL, and CNKI databases for randomized controlled trials (RCTs) published before October 2024. Data from eligible studies were extracted and analyzed using STATA software.
Results: Eight RCTs comprising 1099 participants were included. The results indicated no significant differences between exercise and control interventions in patients’ bone mineral density (BMD) at the lumbar spine (WMD = 0.116, 95% CI [-0.357, 0.589], p = 0.631), femoral neck (WMD = -0.214, 95% CI [-0.497, 0.068], p = 0.137), or total hip (WMD = 0.299, 95% CI [-0.283, 0.882], p = 0.314). For body composition parameters, exercise interventions led to significant improvements in lean mass (WMD = 0.192, 95% CI [0.023, 0.362], p = 0.026) and marked reductions in percent body fat (WMD = -1.327, 95% CI [-2.587, -0.066], p = 0.039) compared to the control. However, no significant differences were observed in body weight (WMD = -0.024, 95% CI [-0.193, 0.146], p = 0.784) or fat mass (WMD = -0.078, 95% CI [-0.703, 0.546], p = 0.806) between the two interventions.
Conclusion: The current evidence suggested that exercise interventions effectively improve lean mass and reduce percent body fat but have a limited impact on BMD in postmenopausal women with breast cancer. A multimodal, individualized exercise program is recommended to address the challenges of bone health and body composition in this population.
Systematic review registration: https://www.crd.york.ac.uk/prospero/, identifier CRD42024613744.
Introduction
Breast cancer is the most commonly diagnosed cancer among women globally, with postmenopausal women accounting for a substantial proportion of cases (1). Significant progress in treatment, including advancements in early detection and the development of more effective therapies, has led to a marked improvement in breast cancer survival rates (2). However, many breast cancer survivors continue to endure long-term side effects from treatments such as aromatase inhibitors and chemotherapy. These therapies are often linked to an increased risk of osteoporosis as well as adverse changes in body composition (3, 4). Such changes heighten the risks of fractures, metabolic disorders, and cardiovascular diseases, ultimately leading to a decline in overall life quality of affected individuals (5, 6).
In postmenopausal women, the natural decline in estrogen levels accelerates bone loss, a process further compounded by breast cancer treatments (7). Consequently, conditions such as osteopenia and osteoporosis are prevalent among these women, significantly increasing the risk of fractures and functional impairment (8). Besides concerns related to bone health, a concurrent loss of muscle mass and increase in fat mass are commonly observed, leading to diminished physical function and elevated risk of cardiovascular complications (9, 10). These adverse effects highlight the urgent need for effective interventions to mitigate the negative impact of breast cancer treatments on bone health and body composition, particularly in postmenopausal women.
Exercise is increasingly recognized as an effective non-pharmacological intervention to enhance bone health and counteract adverse changes in body composition among breast cancer survivors (11). Specifically, resistance and weight-bearing exercises have been shown to help maintain or improve bone mineral density (BMD) and preserve lean muscle mass, while aerobic exercises are associated with reductions in body fat (9, 12). Despite these promising effects, evidence regarding the efficacy of various types of exercise interventions for postmenopausal women with breast cancer remains inconsistent. While some studies report significant benefits, others indicate minimal or no effect (13, 14).
To address these inconsistencies, a systematic review and meta-analysis is necessary to synthesize the existing evidence on the effects of exercise on bone health and body composition in postmenopausal women with breast cancer. This review aims to provide robust, evidence-based support for the clinical application of exercise as an intervention for this population, thereby guiding healthcare providers and informing future treatment strategies.
Method
The present study was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guideline (15). The protocol was registered in PROSPERO with the registration number CRD42024613744.
Eligibility criteria
The PICOS framework were applied to determine study eligibility (16). Studies were included if they involved postmenopausal women diagnosed with breast cancer (Population), who participated in exercise interventions such as aerobic, resistance, or weight-bearing training (Intervention), with a comparison group that received either no exercise or usual care (Comparison). The outcomes of interest were changes in bone mineral density and/or body composition (Outcome). Only randomized controlled trials (Study design) were considered for inclusion, with no restrictions on language. Studies were excluded if they involved premenopausal women, patients with major comorbid conditions affecting bone health or body composition, or if they evaluated non-exercise interventions. Additionally, retrospective trials, case reports, and conference papers were excluded.
Search strategy
An electronic search of the PubMed, EMBASE, Web of science, CENTRAL and CNKI databases was performed to identify potentially relevant studies published before October 2024. Search strategies were customized for each database using a combination of medical subject headings (MeSH) and free-text keywords. For instance, in PubMed, the following search strategy was applied: ((“postmenopausal women”[MeSH] OR “postmenopausal”[tiab]) AND (“breast cancer survivors”[tiab] OR “breast neoplasms”[MeSH] OR “breast cancer”[tiab])) AND (“exercise”[MeSH] OR “physical activity”[tiab] OR “resistance training”[MeSH] OR “aerobic exercise”[MeSH] OR “weight-bearing exercise”[tiab]) AND (“bone mineral density”[MeSH] OR “BMD”[tiab] OR “bone health”[tiab] OR “body composition”[MeSH] OR “lean mass”[tiab] OR “fat mass”[tiab] OR “body weight”[tiab] OR “ percent body fat “[tiab]) AND (randomized controlled trial[pt] OR controlled clinical trial[pt] OR randomized[tiab] OR placebo[tiab] OR “randomly”[tiab] OR trial[tiab]) NOT (animals[mh] NOT humans[mh]). For the CNKI database, a combination of Chinese equivalents for the following terms was used: “postmenopausal,” “breast cancer,” “exercise,” “bone mineral density,” and “body composition.” Boolean operators such as “AND” and “OR” were applied to build the search strategy. Both subject terms and free-text keywords were utilized to ensure a comprehensive and sensitive search. Reference cited in all included studies were also manually examined for additional records.
Study screening and data extraction
Two reviewers independently screened and assessed whether the retrieved studies met the inclusion criteria. The following information was then extracted from each eligible study: first author, publication year, participant characteristics, exercise intervention details, control condition, outcome measures, and follow up duration. Any discrepancies in data extraction were resolved through consensus with a third reviewer.
Study quality assessment
The methodological quality of the included studies was assessed by two independent reviewers using the Cochrane Collaboration’s tool (17). The following aspects were evaluated as either low, unclear or high risk of bias: (1) random sequence generation, (2) allocation concealment, (3) blinding of participants and researchers, (4) blinding of outcome assessment, (5) incomplete outcome data, (6) selective reporting, and (7) other bias. Any disagreements between the reviewers were resolved through discussion consensus with a third reviewer.
Statistical analysis
Weighted mean difference (WMD) and 95% confidence interval (CI) were pooled to calculate effect sizes, as the included studies reported statistics and variances in consistent units. Between-study heterogeneity was assessed using the I2 statistic, where values of 50% serve as a cutoff for low and high heterogeneity levels (18). A fixed-effect model was applied in the absence of significant heterogeneity, whereas a random-effect model was used otherwise. A funnel plot and Egger’s test were employed to detect potential publication bias, provided that a sufficient number of studies was available (19). All statistical analyses were conducted using Stata software 16.0 (StataCorp LP, College Station, TX), with a p-value <0.05 considered statistically significant.
Results
Study selection
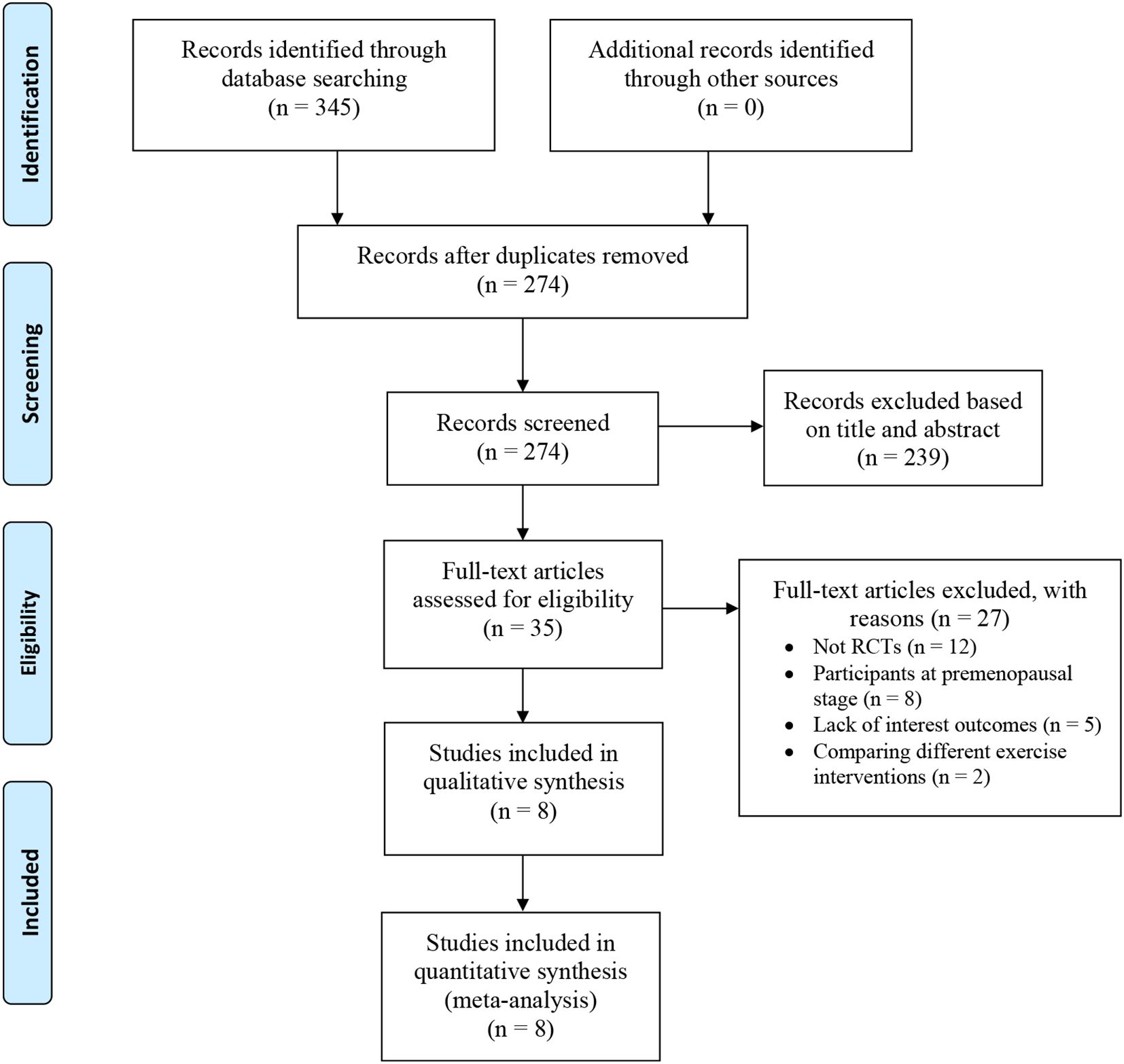
A total of 345 potentially relevant studies were identified in the preliminary search. After duplicated records were removed, 274 titles and abstracts were reviewed and screened for inclusion. Following the filtering process, 35 full-text articles were assessed for eligibility. Twenty-seven studies were excluded for the following reasons: 12 were non-randomized controlled studies, 8 included participants at the premenopausal stage, 5 lacked the outcomes of interest, and 2 compared different exercise interventions. Finally, 8 studies were included for data extraction and final meta-analysis (20–26). The study selection process, conducted based on the PRISMA statement was demonstrated in Figure 1.
Study characteristics
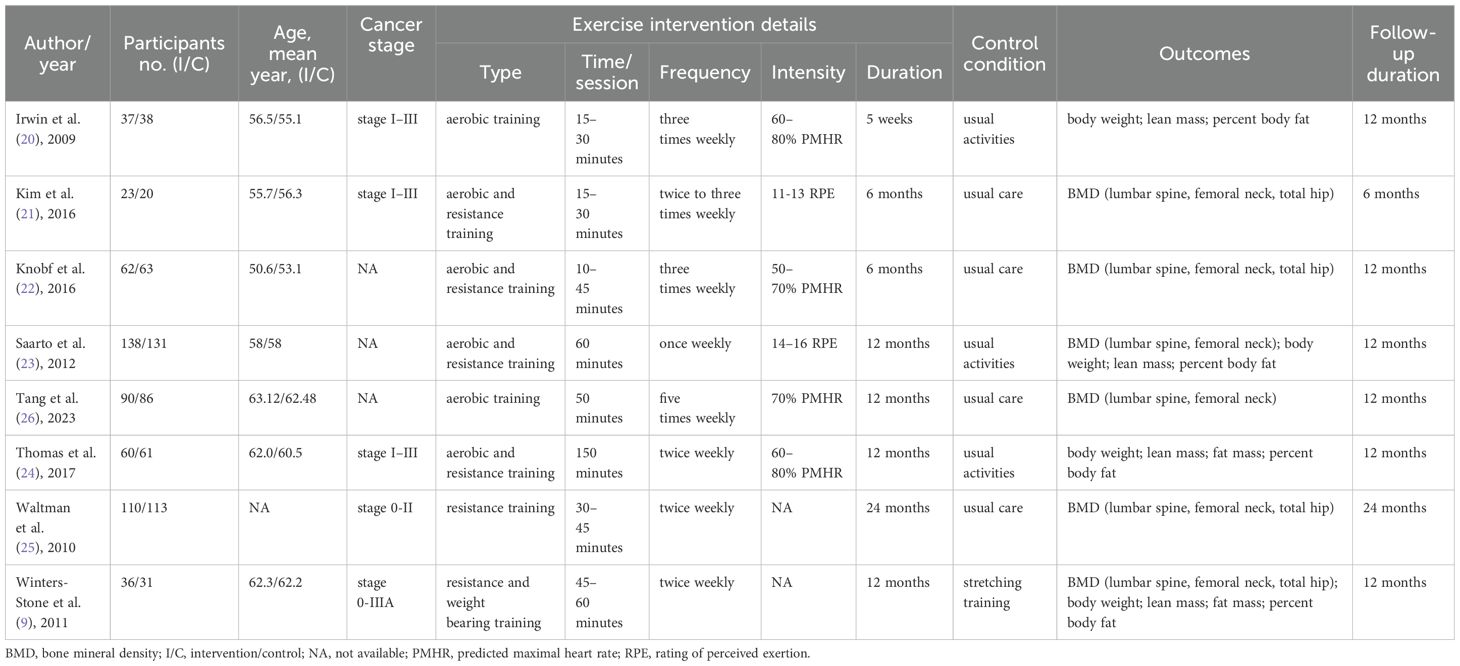
The included studies were published between 2009 and 2023, involving a total of 1099 participants. All participants were breast cancer survivors at various stages (0–III), with mean ages ranging from 50.6 to 63.12 years. There was notable heterogeneity in the exercise intervention programs across the studies. While most control group participants received usual care or engaged in usual activities (20–26), only one study employed stretching training as the control intervention (9). Bone mineral density was reported in the majority of studies, with measurements taken at the lumbar spine (21–23, 25, 26), femoral neck (9, 21–23, 25, 26), and total hip (9, 21, 22, 25). Body composition outcomes, including body weight (9, 20, 23, 24), lean mass (9, 20, 23, 24), fat mass (9, 23, 24), and percent body fat (9, 20, 24) were evaluated in five out of eight studies. Follow-up durations varied, ranging from 6 to 24 months, with most studies lasting 12 months. Table 1 illustrated the detailed characteristics of eligible studies.
Study quality assessment
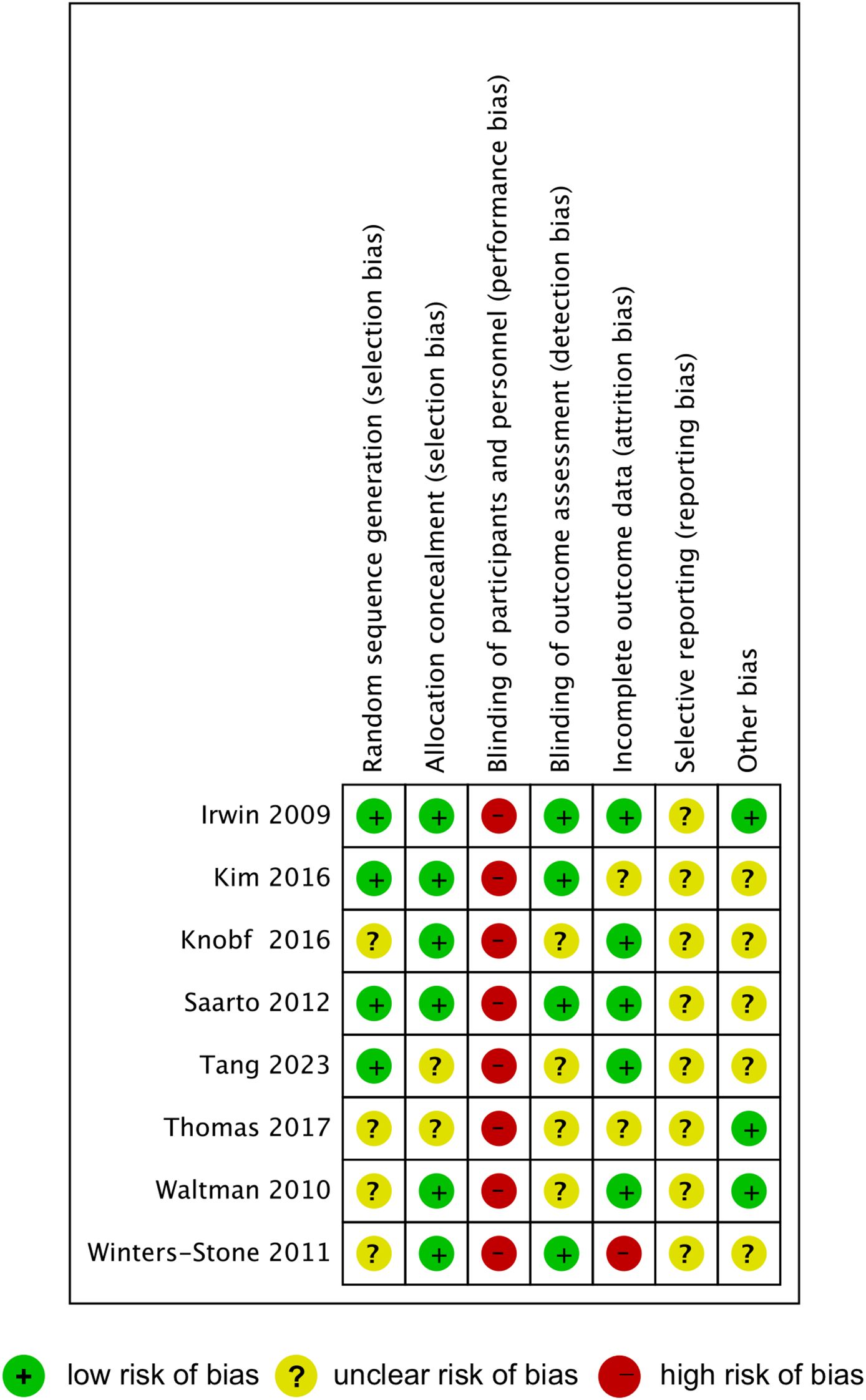
The results of quality assessment of the included studies were summarized in Figure 2. Four studies were rated as low risk for random sequence generation, while the other four studies were rated as unclear risk. Allocation concealment bias was generally low, with only two studies showing unclear risks. High risk of bias was observed across all studies in the blinding of participants and personnel, as exercise interventions inherently prevent effective blinding. Half of the included studies were classified as low risk in the blinding of outcome assessment, while the other half were categorized as unclear risk. Incomplete outcome data were assessed as low risk in five studies, unclear risk in two studies, and high risk in one study. Most studies demonstrated an unclear risk for selective reporting and other biases, except for two studies rated as low risks.
Meta-analysis
Effect of exercise on bone mineral density
Five studies (n= 423 participants) investigated the effect of exercise on lumbar spine BMD in postmenopausal women with breast cancer. A random-effect model was used due to the observed heterogeneity (p < 0.001, I2 = 90.90%), and the exercise intervention did not result in a significant improvement in lumbar spine BMD compared to the control group (WMD = 0.116; 95% CI: -0.357 to 0.589; p = 0.631; Figure 3A).
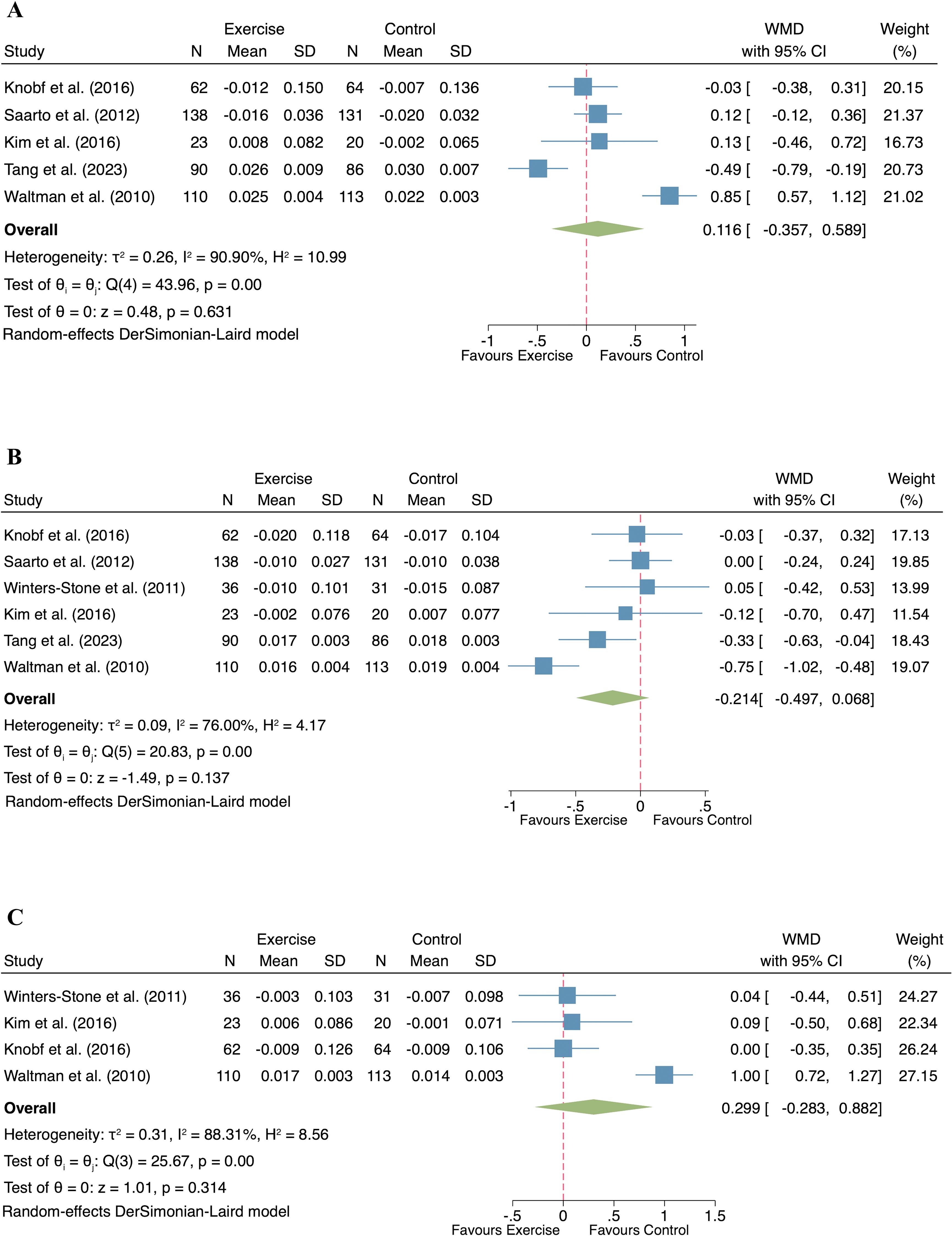
Figure 3. Forest plot of exercise compared to control on bone mineral density in postmenopausal women with breast cancer. ((A) Lumbar spine; (B) Femur neck; (C) Total hip).
The effect of exercise on femur neck BMD was evaluated in six studies comprising 459 participants. Given the observed heterogeneity (I² = 76.00%, p < 0.001), a random-effect model was applied. The pooled analysis showed no significant difference in femur neck BMD between the exercise and control groups (WMD = -0.214, 95% CI: -0.497 to 0.068, p = 0.137; Figure 3B).
Four studies, involving 231 breast cancer survivors, examined the influence of exercise on total hip BMD. With significant heterogeneity detected (I² = 88.31%, p < 0.001), a random-effect model was applied. The meta-analysis found no statistically significant effect of exercise on total hip BMD in postmenopausal women (WMD = 0.299, 95% CI: -0.283 to 0.882, p = 0.314; Figure 3C).
Effect of exercise on body composition
A total of four studies reported the effects of exercise on body composition in postmenopausal women with breast cancer (n= 271 participants) across four indicators: body weight, lean mass, fat mass and percent body fat. No significant heterogeneity was observed for any of the outcomes (body weight: I² = 0%, p = 0.54; lean mass: I² = 0%, p = 0.55; fat mass: I² = 0%, p = 0.65; percent body fat: I² = 0%, p = 0.45), justifying the application of fixed-effect models. The exercise intervention did not result in significant changes in body weight (WMD = -0.024, 95% CI: -0.193 to 0.146, p = 0.784; Figure 4A) and fat mass (WMD = -0.078, 95% CI: -0.703 to 0.546, p = 0.806; Figure 4C) compared to the control group. However, significant improvements in lean mass (WMD = 0.192, 95% CI: 0.023 to 0.362, p = 0.026; Figure 4B) and reductions in percent body fat (WMD = -1.327, 95% CI: -2.587 to -0.066, p = 0.039; Figure 4D) were evident in the exercise group compared to the control group.
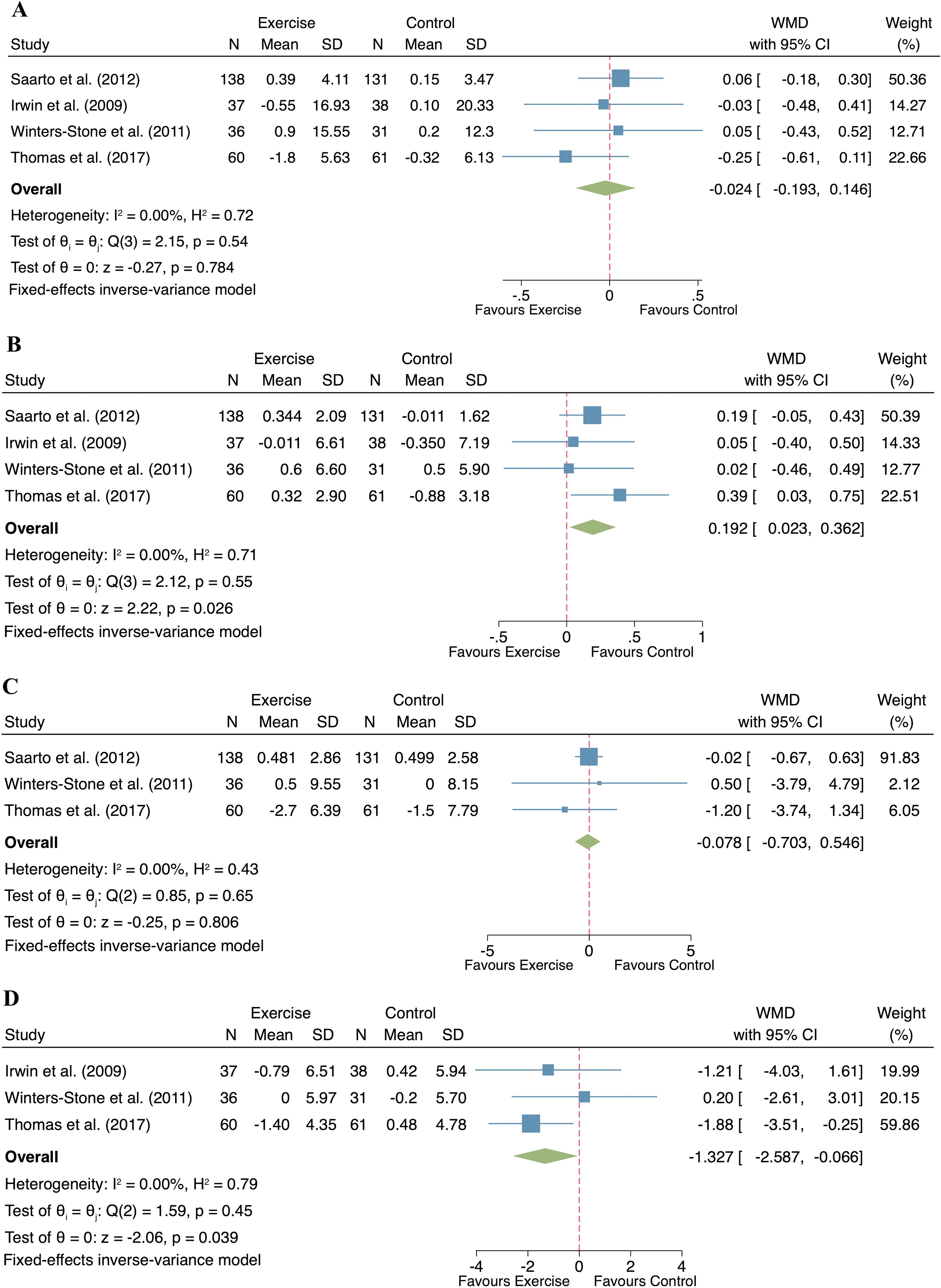
Figure 4. Forest plot of exercise compared to control on body composition in postmenopausal women with breast cancer. ((A). Body weight; (B). Lean mass; (C). Fat mass; (D). Percent body fat).
Publication bias
As shown in Figure 5, no significant publication bias was found according to the visualization of the funnel plot and the results from the Egger’s test. (p=0.881 for lumbar spine BMD; p=0.496 for femur neck BMD; p=0.279 for total hip BMD; p=0.613 for body weight; p=0.699 for fat mass; p=0.601 for lean mass; p=0.296 for percent body fat)
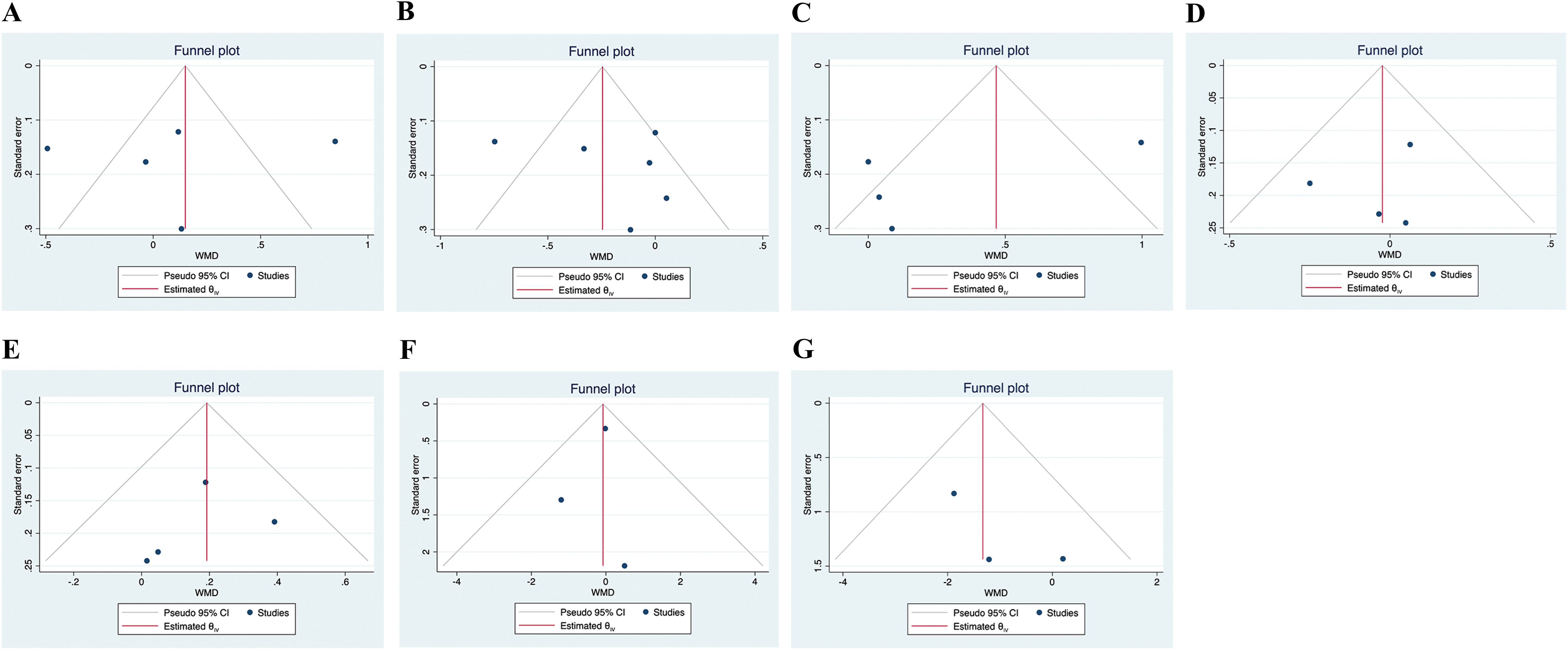
Figure 5. Funnel plots of the publication bias. ((A). Lumbar spine BMD; (B). Femur neck BMD; (C). Total hip BMD; (D). Body weight; (E). Lean mass; (F). Fat mass; (G). Percent body fat).
Discussion
The primary finding of this meta-analysis was that exercise interventions did not significantly improve BMD at key anatomical sites, including the lumbar spine, femoral neck, and total hip, in postmenopausal women with breast cancer. This lack of improvement in BMD with exercise aligns with previous research, such as the meta-analysis by Fornusek et al., which highlighted the limited osteogenic effect of exercise among postmenopausal breast cancer survivors (27). The postmenopausal status of participants, combined with the use of aromatase inhibitors (AIs), likely contributes to this limited response (28). AIs exacerbate estrogen deficiency, common in this population, which leads to increased bone resorption and diminishes the effectiveness of exercise as an osteogenic stimulus (29, 30). Although resistance or weight-bearing exercises generally provide mechanical loading that promotes bone formation (31), the hormonal imbalance resulting from reduced estrogen levels predisposes individuals to bone degradation, thereby limiting the efficacy of exercise in increasing BMD (32). Nevertheless, it is essential to note the substantial heterogeneity in our meta-analysis results, which may limit the strength of these conclusions. Possible explanations for this heterogeneity include variations in exercise types and intensities, participant characteristics such as baseline BMD and AI usage, diverse intervention durations affecting detectable bone changes, and methodological differences in BMD measurement. These considerations highlight the complexity involved in evaluating exercise effects on bone health and underscore the necessity for standardized exercise protocols and longer intervention periods in future studies. In contrast, evidence from real-world data by Abdel-Razeq et al. demonstrated that a comprehensive approach combining exercise with pharmacological interventions, such as bisphosphonates, is more effective in maintaining or improving BMD (13). Bisphosphonates suppress osteoclast activity, reduce bone resorption, and thus complement the osteogenic effects of exercise (33). This synergistic approach emphasizes the need for a multimodal strategy, where exercise serves as an adjunct to pharmacotherapy rather than as a standalone treatment to counteract bone loss in this population.
Beyond the effects on bone mineral density, this meta-analysis also assessed the impact of exercise interventions on body composition in postmenopausal women. The results indicated a reduction in percent body fat and an increase in lean mass, while total fat mass and body weight remained largely unchanged. These findings are consistent with previous studies, which reported that exercise interventions, particularly resistance exercise, enhances muscle mass and reduces fat percentage in breast cancer survivors, even though overall body weight often remains stable (34, 35). Although exercise significantly improved lean mass and reduced percent body fat, the absence of significant changes in total body weight and fat mass may reflect a recomposition effect, where muscle gain offsets fat loss, resulting in minimal net weight change (36). Furthermore, most included studies did not incorporate dietary control, which likely limited the potential for fat mass reduction, as exercise alone may not induce sufficient energy deficit (37). In contrast, the improvements in lean mass can be attributed to the anabolic effects of resistance or combined training, promoting muscle hypertrophy via increased protein synthesis (36). The reduction in percent body fat may also reflect enhanced metabolic efficiency, including improved insulin sensitivity and greater fat oxidation (12). This consistency underscores that exercise interventions primarily enhance physical fitness and body composition quality, as evidenced by increased lean mass and reduced body fat percentage, which are crucial for improving quality of life and reducing the risk of disease recurrence in breast cancer survivors (38, 39).
This study has several limitations. First, although substantial heterogeneity was observed in several pooled outcomes, subgroup or sensitivity analyses were not feasible due to the limited number of included studies and the inconsistent reporting of participant and intervention characteristics. This limitation should be considered when interpreting the meta-analytic results. Second, the relatively small sample sizes in several trials may have reduced the statistical power of the pooled estimates. Third, the duration of most interventions was relatively short (≤12 months), which may be insufficient to produce detectable changes in bone mineral density. Fourth, all included studies were rated as high risk for performance bias due to the inability to blind participants and personnel in exercise trials. This may have introduced subjective bias, particularly for self-reported outcomes such as body composition. However, BMD was objectively measured using standardized methods, which partially mitigates this concern. Finally, this review was based solely on quantitative data from randomized controlled trials. Future research incorporating qualitative approaches is needed to better understand patients’ individual needs and preferences, which may inform the design of more personalized and effective exercise interventions.
Conclusion
In conclusion, exercise interventions provide notable benefits for body composition but have limited impact on BMD in postmenopausal women with breast cancer. A comprehensive approach that integrates pharmacological treatments with individualized exercise programs may provide an optimal strategy for enhancing overall health outcomes in this population. These findings emphasize the necessity of multimodal and personalized interventions that address the diverse health challenges faced by breast cancer survivors, with a particular focus on preserving bone health while improving body composition.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
SY: Investigation, Writing – review & editing, Supervision, Writing – original draft. CG: Writing – review & editing, Formal Analysis, Software, Methodology, Conceptualization. CY: Methodology, Writing – review & editing, Visualization, Software, Formal Analysis. JH: Writing – original draft, Methodology, Data curation, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, and Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2018) 68:394–424. doi: 10.3322/caac.21492
2. Chen X, Hu G, and Yu Q. Impact of disulfidptosis-associated clusters on breast cancer survival rates and guiding personalized treatment. Front Endocrinol (Lausanne). (2023) 14:1256132. doi: 10.3389/fendo.2023.1256132
3. Pedersini R, Schivardi G, Laini L, Zamparini M, Bonalumi A, di Mauro P, et al. Changes in body composition in early breast cancer patients treated with aromatase inhibitors. J Endocrinol Invest. (2024) 47:3119–28. doi: 10.1016/j.breast.2024.103794
4. Nisha Y, Dubashi B, Bobby Z, Sahoo JP, Kayal S, Ananthakrishnan R, et al. Negative impact on bone homeostasis in postmenopausal women with non-metastatic breast cancer during cytotoxic chemotherapy. J Bone Miner Metab. (2023) 41:682–92. doi: 10.1007/s00774-023-01444-9
5. Pandey P, Sharma A, and Gogia A. Bone health in breast cancer. Curr Probl Cancer. (2023) 47:100959. doi: 10.1016/j.currproblcancer.2023.100959
6. Patnaik JL, Byers T, DiGuiseppi C, Dabelea D, and Denberg TD. Cardiovascular disease competes with breast cancer as the leading cause of death for older females diagnosed with breast cancer: a retrospective cohort study. Breast Cancer Res. (2011) 13:R64. doi: 10.1186/bcr2901
7. Cucciniello L, Garufi G, Di Rienzo R, Martinelli C, Pavone G, Giuliano M, et al. Estrogen deprivation effects of endocrine therapy in breast cancer patients: Incidence, management and outcome. Cancer Treat Rev. (2023) 120:102624. doi: 10.1016/j.ctrv.2023.102624
8. Rizzoli R, Bischoff-Ferrari H, Dawson-Hughes B, and Weaver C. Nutrition and bone health in women after the menopause. Womens Health (Lond). (2014) 10:599–608. doi: 10.2217/WHE.14.40
9. Winters-Stone KM, Dobek J, Nail L, Bennett JA, Leo MC, Naik A, et al. Strength training stops bone loss and builds muscle in postmenopausal breast cancer survivors: a randomized, controlled trial. Breast Cancer Res Treat. (2011) 127:447–56. doi: 10.1007/s10549-011-1444-z
10. Silverman SL, Delmas PD, Kulkarni PM, Stock JL, Wong M, and Plouffe L Jr. Comparison of fracture, cardiovascular event, and breast cancer rates at 3 years in postmenopausal women with osteoporosis. J Am Geriatr Soc. (2004) 52:1543–8. doi: 10.1111/j.1532-5415.2004.52420.x
11. Ficarra S, Thomas E, Bianco A, Gentile A, Thaller P, Grassadonio F, et al. Impact of exercise interventions on physical fitness in breast cancer patients and survivors: a systematic review. Breast Cancer. (2022) 29:402–18. doi: 10.1007/s12282-022-01347-z
12. Courneya KS, Segal RJ, Mackey JR, Gelmon K, Reid RD, Friedenreich CM, et al. Effects of aerobic and resistance exercise in breast cancer patients receiving adjuvant chemotherapy: a multicenter randomized controlled trial. J Clin Oncol. (2007) 25:4396–404. doi: 10.1200/JCO.2006.08.2024
13. Abdel-Razeq H, Al-Rasheed U, Mashhadani N, Al-Ibraheem A, Abdel-Razeq R, Jaradeh SA, et al. The efficacy of a comprehensive bone health program in maintaining bone mineral density in postmenopausal women with early-stage breast cancer treated with endocrine therapy: real-world data. Ir J Med Sci. (2022) 191:2511–5. doi: 10.1007/s11845-021-02897-5
14. Gonzalo-Encabo P, Valadés D, García-Honduvilla N, de Cos Blanco A, Friedenreich CM, and Pérez-López A. Exercise type and fat mass loss regulate breast cancer-related sex hormones in obese and overweight postmenopausal women. Eur J Appl Physiol. (2020) 120:1277–87. doi: 10.1007/s00421-020-04361-1
15. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Bmj. (2021) 372:n71. doi: 10.1136/bmj.n71
16. Amir-Behghadami M and Janati A. Population, Intervention, Comparison, Outcomes and Study (PICOS) design as a framework to formulate eligibility criteria in systematic reviews. Emerg Med J. (2020) 37:387. doi: 10.1136/emermed-2020-209567
17. Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. Bmj. (2011) 343:d5928. doi: 10.1136/bmj.d5928
18. Higgins JP, Thompson SG, Deeks JJ, and Altman DG. Measuring inconsistency in meta-analyses. Bmj. (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
19. Pustejovsky JE and Rodgers MA. Testing for funnel plot asymmetry of standardized mean differences. Res Synth Methods. (2019) 10:57–71. doi: 10.1002/jrsm.v10.1
20. Irwin ML, Alvarez-Reeves M, Cadmus L, Mierzejewski E, Mayne ST, Yu H, et al. Exercise improves body fat, lean mass, and bone mass in breast cancer survivors. Obes (Silver Spring). (2009) 17:1534–41. doi: 10.1038/oby.2009.18
21. Kim SH, Cho YU, Kim SJ, Hong S, Han MS, and Choi E. The effect on bone outcomes of adding exercise to supplements for osteopenic breast cancer survivors: A pilot randomized controlled trial. Cancer Nurs. (2016) 39:144–52. doi: 10.1097/NCC.0000000000000245
22. Knobf MT, Jeon S, Smith B, Harris L, Kerstetter J, Thompson AS, et al. Effect of a randomized controlled exercise trial on bone outcomes: influence of adjuvant endocrine therapy. Breast Cancer Res Treat. (2016) 155:491–500. doi: 10.1007/s10549-016-3693-3
23. Saarto T, Sievänen H, Kellokumpu-Lehtinen P, Nikander R, Vehmanen L, Huovinen R, et al. Effect of supervised and home exercise training on bone mineral density among breast cancer patients. A 12-month randomised controlled trial. Osteoporos Int. (2012) 23:1601–12. doi: 10.1007/s00198-011-1761-4
24. Thomas GA, Cartmel B, Harrigan M, Fiellin M, Capozza S, Zhou Y, et al. The effect of exercise on body composition and bone mineral density in breast cancer survivors taking aromatase inhibitors. Obes (Silver Spring). (2017) 25:346–51. doi: 10.1002/oby.21729
25. Waltman NL, Twiss JJ, Ott CD, Gross GJ, Lindsey AM, Moore TE, et al. The effect of weight training on bone mineral density and bone turnover in postmenopausal breast cancer survivors with bone loss: a 24-month randomized controlled trial. Osteoporos Int. (2010) 21:1361–9. doi: 10.1007/s00198-009-1083-y
26. Tang LC, Sun J, Jing YY, and Li L. Factors influencing the reduction of bone mineral density in postmenopausal breast cancer patients treated with endocrine therapy and analysis of the efficacy of aerobic exercise intervention. Chongqing Med J. (2023) 52:1384–9. doi: 10.3969/j.issn.1671-8348.2023.09.021
27. Fornusek CP and Kilbreath SL. Exercise for improving bone health in women treated for stages I-III breast cancer: a systematic review and meta-analyses. J Cancer Surviv. (2017) 11:525–41. doi: 10.1007/s11764-017-0622-3
28. Kharb R, Haider K, Neha K, and Yar MS. Aromatase inhibitors: Role in postmenopausal breast cancer. Arch Pharm (Weinheim). (2020) 353:e2000081. doi: 10.1002/ardp.202000081
29. Kim C, Na Y, Lee S, Park JY, Chung YJ, Song J, et al. A recent review of the management of postmenopausal symptoms in breast cancer survivors. J Menopausal Med. (2023) 29:85–91. doi: 10.6118/jmm.23016
30. Gallagher JC. Role of estrogens in the management of postmenopausal bone loss. Rheum Dis Clin North Am. (2001) 27:143–62. doi: 10.1016/S0889-857X(05)70191-5
31. Dieli-Conwright CM, Courneya KS, Demark-Wahnefried W, Sami N, Lee K, Sweeney FC, et al. Aerobic and resistance exercise improves physical fitness, bone health, and quality of life in overweight and obese breast cancer survivors: a randomized controlled trial. Breast Cancer Res. (2018) 20:124. doi: 10.1186/s13058-018-1051-6
32. Ramaswamy B and Shapiro CL. Osteopenia and osteoporosis in women with breast cancer. Semin Oncol. (2003) 30:763–75. doi: 10.1053/j.seminoncol.2003.08.028
33. Bonaiuti D, Shea B, Iovine R, Negrini S, Robinson V, Kemper HC, et al. Exercise for preventing and treating osteoporosis in postmenopausal women. Cochrane Database Syst Rev. (2002) 3:Cd000333. doi: 10.1002/14651858.CD000333
34. Al-Mhanna SB, Batrakoulis A, Norhayati MN, Mohamed M, Drenowatz C, Irekeola AA, et al. Combined aerobic and resistance training improves body composition, alters cardiometabolic risk, and ameliorates cancer-related indicators in breast cancer patients and survivors with overweight/obesity: A systematic review and meta-analysis of randomized controlled trials. J Sports Sci Med. (2024) 23:366–95. doi: 10.52082/jssm
35. Fraser SF, Gardner JR, Dalla Via J, and Daly RM. The effect of exercise training on lean body mass in breast cancer patients: A systematic review and meta-analysis. Med Sci Sports Exerc. (2022) 54:211–9. doi: 10.1249/MSS.0000000000002792
36. Yang H, Liu L, and Zhang X. Exercise interventions on body composition and quality of life of overweight/obese breast cancer survivors: a meta-analysis. BMC Womens Health. (2023) 23:484. doi: 10.1186/s12905-023-02627-2
37. Brown JC, Sarwer DB, Troxel AB, Sturgeon K, DeMichele AM, Denlinger CS, et al. A randomized trial of exercise and diet on body composition in survivors of breast cancer with overweight or obesity. Breast Cancer Res Treat. (2021) 189:145–54. doi: 10.1007/s10549-021-06284-7
38. Shaikh H, Bradhurst P, Ma LX, Tan SYC, Egger SJ, and Vardy JL. Body weight management in overweight and obese breast cancer survivors. Cochrane Database Syst Rev. (2020) 12:Cd012110. doi: 10.1002/14651858.CD012110
Keywords: exercise intervention, breast cancer, bone mineral density, body composition, meta - analysis
Citation: Yu S, Gao C, Yi C and Hei J (2025) Effects of exercise interventions on bone health and body composition in postmenopausal women with breast cancer: a systematic review and meta-analysis. Front. Oncol. 15:1593165. doi: 10.3389/fonc.2025.1593165
Received: 13 March 2025; Accepted: 04 June 2025;
Published: 18 June 2025.
Edited by:
Laikang Yu, Beijing Sport University, ChinaReviewed by:
Zhao Sun, First Affiliated Hospital of Zhengzhou University, ChinaLin Feng, Beijing Sport University, China
Copyright © 2025 Yu, Gao, Yi and Hei. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jingyou Hei, aGVpamluZ3lvdTE5ODZAMTI2LmNvbQ==
 Shan Yu
Shan Yu Chengfei Gao
Chengfei Gao Chuanjian Yi
Chuanjian Yi Jingyou Hei
Jingyou Hei