- 1Department of Neurosurgery, Lanzhou University Second Hospital, Lanzhou, China
- 2Academician Workstation of The Second Hospital & Clinical Medical School, Lanzhou University, Lanzhou, China
- 3The Second Medical College of Lanzhou University, Lanzhou University, Lanzhou, China
- 4The First School of Clinical Medicine, Lanzhou University, Lanzhou, China
- 5Université Paris Cité, NeuroDiderot, INSERM, Paris, France
- 6Department of Neurology, West China Hospital, Sichuan University, Chengdu, China
Individuals with brain tumors are more susceptible to comorbid sleep disturbances, which significantly impair daytime functioning, quality of life, and long-term prognosis. A bidirectional relationship between sleep and brain tumors has been suggested, with sleep disturbances in this population being diverse and multifactorial, stemming from neurotransmitter imbalances, treatment interventions, and comorbidities conditions throughout the disease course. While sleep assessment and intervention guidelines exist for cancer more broadly, specific recommendations for neuro-oncological etiology populations remain limited. As awareness grows regarding the negative impact of poor sleep in patients with brain tumors, there is an urgent need for more targeted research to systematically characterize sleep disturbances and explore therapeutic implications. In this context, we conducted a narrative review current on sleep research in patients with brain tumors.
1 Introduction
Brain tumors include primary neoplasms arising from cerebral cells or metastatic lesions in the central nervous system (CNS). The average age-adjusted annual incidence rate of CNS tumors is 24.7 per 100,000 population (1), accounting for approximately 1.4% of all cancers. Among these, benign brain tumors, such as meningiomas and craniopharyngiomas have an incidence of 17.69 per 100,000, while malignant tumors, including gliomas and metastases, occur at a rate of 7.02 per 100,000 (1). Despite advances in treatment, the five-year overall survival rate following surgery for brain tumor patients remains below 30% (2).
The development of brain tumors is influenced by various factors, including genetic predisposition, radiation exposure, and viral infections. Symptoms including cognitive decline (36%), seizures (35%), headaches (30%), and neurological deficits like aphasia (20%) and motor deficits (20%) are relative common in patients with brain tumors (3, 4). Importantly, these symptoms often occur in clusters and may persist as long-term comorbidities even after surgical intervention (5–7), contributing to higher recurrence rates and poorer prognosis. Among these comorbidities, sleep disturbances are of growing concern due to their significant impact on caner progression and management. Studies reported higher prevalence of sleep disturbances in individuals with cancer (30% - 93.1%) compared to the general population (9% - 20.9%) (8–11), with brain tumor patients being particularly vulnerable (12, 13). Previous surveys indicated that 61.5% of brain tumor patients reported poor sleep quality 21.5% and insomnia symptoms (14), and 5% with excessive daytime sleepiness (EDS) (15). Structural factors such as tumor-induced compression or postoperative changes may damage key sleep–wake regulatory nuclei in the brain (16–18), leading to sleep disruption and a broad range of symptoms (19, 20). For instance, craniopharyngiomas in the saddle area can compress or infiltrate the hypothalamus, impairing hypothalamic secretions and inducing secondary narcolepsy. Given the critical role of sleep in physical and mental health, sleep disturbances can cause daytime dysfunction (19, 21, 22), accelerate tumor progression (13), reduce treatment efficacy, and negatively affect long-term prognosis (23).
Despite increasing recognition of sleep disturbances in patients with brain tumors, existing research remains limited to a few single-center, small-sample observational studies. Current knowledge of the relationship between brain tumors and sleep is fragmented, and the associated clinical characteristics and risk factors are not yet well-defined. To enhance the understanding and raise awareness of these co-occurring disturbances, we conducted a narrative review of recent evidence, offering a comprehensive synthesis of clinical findings and their connections.
2 Clinical characteristics of sleep disturbances in brain tumor patients
Sleep surveys have found that 57% to 81.8% of patients with brain tumor experience poor sleep quality (13, 15, 24–27) primarily due to various forms of sleep disturbances.
2.1 Insomnia
Current research identifies insomnia as the most prevalent sleep disturbance among patients with brain tumors, typically characterized by difficulties in initiating and maintaining sleep (28). In a Korean national cohort of 4,851 patients with malignant brain tumors (29), the preoperative prevalence of insomnia was 18.8%, with an additional 9.2% developing new-onset insomnia after surgery (29). A longitudinal study further indicated that insomnia was associated with increased post-operative mortality within two years, despite the duction of symptom severity following surgery (29). Persistent insomnia is reported in approximately 50% of patients with low-grade gliomas, pituitary adenomas, and recurrent gliomas (30–32). Its severity may worsen when accompanied by postoperative complications such as headaches and epilepsy (31) or as a side effect of treatments including corticosteroids and radiotherapy (33, 34). However, the specific pathophysiological links between insomnia and distinct brain tumor types remain insufficiently understood. Using the Athens Insomnia Scale, one study found a higher prevalence of insomnia in patients with malignant brain tumors (61.8%, n = 35) compared to those with benign brain tumors (54.3%, n = 68) (15). Tumor location, such as suprasellar versus non-suprasellar regions, did not significantly influence insomnia prevalence (64.5%, n = 31 vs. 61.1%, n = 67, respectively) (15). Cross-sectional studies also reported high rates of insomnia (46.8% - 59.2%) in untreated pituitary adenoma and meningioma patients (15, 27). In a prospective study of 70 patients awaiting tumor resection, those with bilateral tumors exhibited more severe insomnia (35). Objective assessments using polysomnography (PSG) remain rare. Small sample PSG studies in glioma patients have yielded inconsistent results regarding sleep onset latency (SOL) (36–38), a key marker of insomnia severity. Importantly, patients with malignant brain tumors are particularly vulnerable to pre- and post-operative anxiety and depression (15, 39, 40), factors strongly correlated with insomnia (24, 26). For example, in a cohort of 358 patients with glioma, while sleep quality briefly improved post-surgery, long-term anxiety about disease progression was linked to sleep disruption (41). Similarly, a pediatric study in post-operative medulloblastoma patients (n = 37), found that emotional distress was associated with longer SOL, more nocturnal awakenings, and reduced sleep efficiency (42). In contrast, such associations were not consistently observed in patients with benign brain tumors (28) warranting caution when generalizing findings across tumor types. Collectively, evidence suggests that insomnia is highly prevalent in brain tumor populations and may serve as a prognostic indicator, particularly in malignant cases. Its impact appears closely tied to severity and the presence of psychological comorbidities (28, 30, 43).
2.2 Excessive daytime sleepiness
EDS, also known as hypersomnolence disorder, is a significant sleep disturbance in patients with brain tumors, characterized by reduced alertness and episodes of unintended daytime sleep. It can critically impair cognitive functions, particularly attention and memory (44). Based on the Epworth Sleepiness Scale (ESS), one study reported that 4.9% (n = 103) of brain tumor patients experienced EDS, defined as an ESS score > 10 (15). EDS is most prevalent in patients with tumors affecting the basal ganglia, hypothalamus and brainstem, such as craniopharyngiomas and pituitary adenomas (34, 45). PSG and multiple sleep latency test (MSLT) studies have reported EDS prevalence rates ranging from 50% to 83% in this population (45–47). In pediatric cohorts, the incidence of secondary narcolepsy following childhood brain tumors is approximately 1.67% (n = 2,336) (34) with EDS as a primary symptom. Secondary narcolepsy is more common in tumors of the sellar region (48), with incidence rates between 14.3% and 35% among craniopharyngioma patients (46, 49). These tumors typically lead to reduced cerebrospinal fluid orexin concentrations, though levels often normalize following tumor resection (50–52). EDS may be exacerbated by hypothalamic involvement and associated weight gain, which can increase the risk of sleep apnea. Sleep apnea disrupts sleep macrostructure via hypoxemia, thereby intensifying EDS (49, 53). A notable case report documented marked improvement in EDS after surgical resection of a Grade II temporal lobe hippocampal glioma causing secondary narcolepsy, with ESS scores decreasing from 16 to 3 (54). In pediatric craniopharyngioma patients, postoperative hypothalamic damage may also impair nighttime melatonin secretion further contributing to EDS (53, 55). Current research on EDS in brain tumor patients largely focuses on the craniopharyngiomas and pituitary adenomas, underscoring the need for broader investigations across diverse tumor types (31).
2.3 Sleep-related breathing disorders
A meta-analysis of post-operative PSG in survivors of pediatric brain tumors revealed that approximately 64% had comorbid sleep-related breathing disorders (SBDs) (56). SBD is frequently associated with EDS in clinical settings, a pattern consistently observed in brain tumor patients. In one study of 31 pediatric brain tumor patients who developed postoperative EDS 58.1% met diagnostic criteria for SBDs (45). Notably, younger patients (0–12 years) were more affected, with 83.3% exhibiting an apnea-hypopnea index (AHI) > 1 event/hour compared to 40% of older patients (aged 12–18 years) with AHI > 5 events/hour. Among these 31 children, central sleep apnea was identified in 22.2% of cases, highlighting the importance of comprehensive SBD screening, particularly when tumors involve regions regulating respiratory control, such as the sellar area, hypothalamus, basal ganglia, and brainstem (57). In adult brain tumor patients, findings from the STOP-BANG questionnaire indicate that the severity of obstructive sleep apnea (OSA) is significantly correlated with poorer prognosis and higher readmission rates within 30- and 90-days post-surgery (58, 59).
2.4 Circadian arrhythmia and parasomnias
Circadian rhythm disturbances and rapid eye movement (REM) sleep behavior disorder (RBD) have also been reported in brain tumor patients and should be carefully distinguished from EDS. Actigraphy monitoring revealed circadian rhythm disruption in 70% (n = 35) of patients with malignant brain tumors and 57.7% (n = 68) benign brain tumors (19). Specifically 59.3% of untreated patients with pituitary tumors and meningiomas(n = 77), exhibited disrupted circadian patterns (25). These alterations are likely associated with lesions in the sellar region, which can impair melatonin secretion (19, 60). RBD, a parasomnia occurring during REM sleep, is characterized by dream-enacting behaviors and REM sleep without atonia on polysomnographic recordings (61). To date, brain tumor-associated has been reported primarily in case studies. For instance, one patient with brainstem lymphoma exhibited vivid dreams and violent behavior consistent with RBD (62), which markedly improved following surgical resection (62). A retrospective analysis of eight patients with brain tumors and RBD suggested that the disorder may be linked to tumor-related damage in the brainstem and limbic system (63). Since RBD is often a prodromal feature of α-synucleinopathy-related neurodegenerative diseases, the long-term risk of neurodegenerative risk in brain tumor patients with RBD remains unknown (Figure 1).
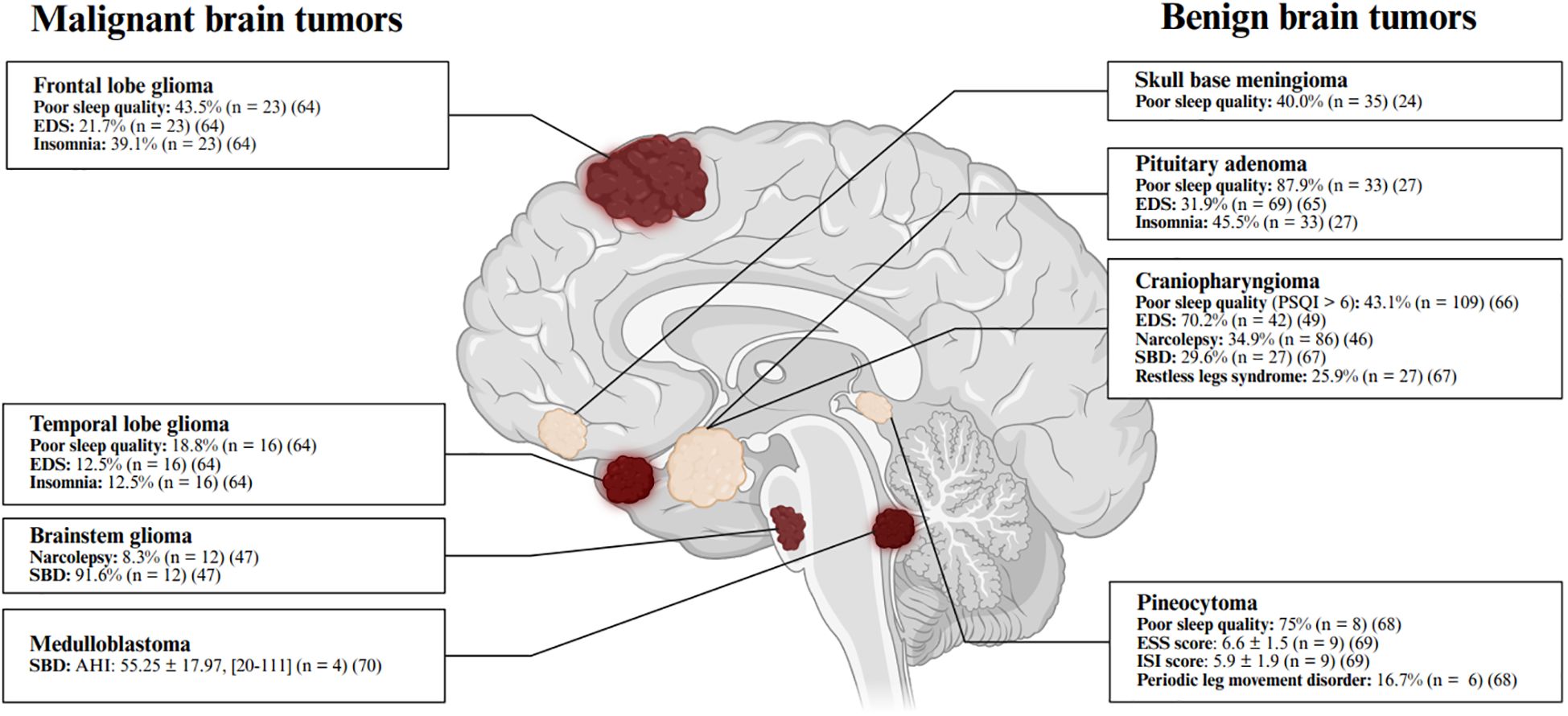
Figure 1. Commonly reported sleep disturbances in patients with brain tumors. The prevalence of sleep disturbances varies by tumor type (24, 27, 47, 49, 64–70), with specific rates and sample sizes detailed in the corresponding sections and summarized in this figure (64–70). Continuous variables are presented as mean ± standard error of the mean (SEM) and [range]. Poor sleep quality is defined as a Pittsburgh Sleep Quality Index (PSQI) score > 5. Excessive daytime sleepiness (EDS) is defined as an Epworth Sleepiness Scale (ESS) score > 10. Insomnia is indicated by an Insomnia Severity Index (ISI) score > 7. Narcolepsy is diagnosed based on polysomnography (PSG) and multiple sleep latency test (MSLT) results. Sleep-related breathing disorder (SBD) is diagnosed either by self-report or by an apnea-hypopnea index (AHI) > 5 events/hour. Periodic limb movement disorder (PLMD) is considered clinically significant when periodic limb movements exceed 10 per hour. Figure created with BioRender.com.
3 Risk factors and mechanism underlying sleep disturbances in brain tumor patients
3.1 Pre-existing sleep disorders
While brain tumors can lead to newly onset sleep disturbances - often linked to tumor location, type, or treatment modalities (13, 30–32, 42, 65–69) — patients may also present with pre-existing sleep disorders such as insomnia prior to diagnosis. One study reported that up to 62% of patients experienced sleep disturbances before their tumor was identified (13). As the tumor progresses, these pre-existing conditions may worsen, typically presenting as reduced total sleep time and increased nocturnal awakenings (71, 72).
3.2 Brain tumors and the intervention
Previous studies have shown that brain tumors can induce new-onset sleep disturbances, which may be associated with tumor characteristics (73) or therapeutic interventions (13, 30–32, 41, 74–77) such as surgery, radiotherapy, and medication. Brain tumor-related treatments, including surgical resection (30, 31, 41, 74), radiotherapy (75–77), and specific medications such as corticosteroid use (13, 32), can collectively disrupt sleep-wake regulation. Damage to the sleep-regulatory nuclei or neural pathways, or the neurotransmitters or hormone systems involved in sleep may result from both the tumor and its treatment, thereby increasing the risk of sleep disturbances (45). Neuroendocrine dysfunction is a key contributor to sleep disruption in brain tumor patients. For example, impairment of the suprachiasmatic nucleus can reduce melatonin secretion, altering circadian rhythms and degrading sleep quality (23). Hypothalamic involvement may lead to decreased orexin levels or dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis, resulting in EDS and fragmented nighttime sleep (23, 53). In a study of postoperative craniopharyngioma patients (n = 15), elevated evening and nighttime salivary cortisol levels were associated with increased nocturnal awakenings and reduced total sleep time (23). Pituitary tumors can also disrupt anterior pituitary hormone secretion, potentially altering sleep architecture (78). Although no direct cases have been reported, elevated prolactin or thyroid-stimulating hormone (TSH) levels may contribute to sleep disruption (79, 80), while excess growth hormone can lead to soft tissue proliferation in the upper airway, increasing the risk of OSA (81, 82). Postoperative increases in SBDs have been observed in pediatric brain tumor patients, rising from 4.6% to 64% (15, 56), possibly reflecting impaired respiratory control following surgery (47). Radiotherapy further compounds sleep issues. In glioma patients (n = 68), over 90% reported EDS after radiotherapy, along with fatigue and reduced daytime function (33). These symptoms peaked at two weeks into treatment and persisted for up to 10 weeks, potentially linked to high radiation doses ( > 30 Gy) (34) or radiation-induced HPA axis impairment (83). Radiotherapy may also lead to upper airway muscle fibrosis and neuromuscular injury, increasing OSA risk (84). Insomnia also tends to increase immediately after radiotherapy and decline thereafter. In a study of patients with low-grade glioma and meningioma (n = 23), insomnia prevalence rose to 23.3% during radiotherapy and declined to 8.7% one month after completing proton therapy (85). Similar trends were observed in glioblastoma patients (86). Potential mechanisms include hypothalamic and pineal gland dysfunction, as well as radiotherapy-induced mood changes, pain, and fatigue, which can contribute to insomnia (87, 88). Corticosteroids, commonly used perioperatively to manage vasogenic edema, nausea, and pain, are potent modulators of the HPA axis (89). They stimulate wake-promoting neural nuclei and may activate the ascending reticular activating system, which can reduce sleep drive and disrupt sleep-wake balance. A study of recurrent glioma patients (n = 340) found that corticosteroid users had a significantly higher prevalence (58.5% vs. 38.4%) and severity of insomnia compared to non-users (32).
3.3 Emotional problems
Studies have demonstrated that anxiety and depression are more prevalent in brain tumor patients than in the general population (24, 26, 78). Assessments using the Hospital Anxiety and Depression Scale indicate that depressive symptoms are more severe in patients with malignant brain tumors compared to those with benign tumors (15, 39). Notably, these emotional disturbances are closely associated with changes in sleep patterns both before and after surgical intervention (24, 26). Research has shown that the severity of pre-treatment anxiety and depression positively correlates with the intensity of sleep disturbances in patients with primary brain tumors (15). In a cohort of 358 glioma patients, sleep quality showed temporary improvement shortly after surgery; however, anxiety about tumor recurrence subsequently worsened sleep quality and contributed to long-term insomnia-like symptoms (41). This phenomenon appears to be less pronounced in patients with benign brain tumors (15). In pediatric populations, similar findings have been reported. For example, studies in children with medulloblastoma found that depressive symptoms were associated with prolonged sleep latency, increased nocturnal awakenings, and reduced sleep efficiency (42).
3.4 Other comorbidities
Comorbid conditions such as headache and epilepsy are known to exacerbate cognitive deficits, reduce quality of life, and contribute to various sleep disturbances in brain tumor patients. Headache has been identified as an independent predictor of insomnia in this population (n = 100) (24). Additionally, seizures have been associated with EDS in pediatric brain tumor patients (n = 70) (90). Fatigue is another prevalent symptom in brain tumor patients and is strongly linked to sleep disturbances (91). In patients with glioma, reduced physical activity due to fatigue may further exacerbate sleep problems (92). As a result, fatigue and sleep disturbances frequently co-occur, particularly in patients with recurrent gliomas. In one study (n = 340), over 80% of patients with comorbid insomnia also reported symptoms of fatigue (32). Although the bidirectional mechanisms linking fatigue and sleep disturbances remain poorly understood, several studies suggest that HPA axis dysregulation may play a central role in both conditions (32, 37, 93).
4 Assessments and interventions for sleep disturbances in brain tumor patients
4.1 Assessments
Sleep disturbances in individuals with brain tumors are typically assessed using both questionnaires and PSG, offering a comprehensive evaluation from subjective and objective perspectives. PSG is widely recognized as the gold standard for sleep assessment, enabling detailed evaluation of nocturnal sleep and daytime sleepiness (44, 94). As such, PSG is considered the primary diagnostic tool for identifying sleep disorders in brain tumor patients, particularly SBD, secondary narcolepsy, and RBD. Although no specific sleep questionnaire developed for brain tumor patients, the Pittsburgh Sleep Quality Index (PSQI) (n = 205, Cronbach’s alpha = 0.79) (95), Insomnia Severity Index (ISI) (n = 1,026, Cronbach’s alpha = 0.92) have demonstrated strong reliability and validity in cancer populations (96) and widely used in the sleep research in patient with cancers (15, 26). As well, the ESS showed great sensitivity and specificity in craniopharyngioma patients (97). Additionally, actigraphy is a non-invasive method for monitoring res and activity cycles, which could provide objective data on sleep duration and patterns. This is especially valuable for detecting circadian rhythm sleep–wake disorders in brain tumor patients.
4.2 Interventions
Multiple clinical interventions, both non-pharmacological and pharmacological, have shown effectiveness in managing sleep disturbances among brain tumor patients. The primary treatment strategy should involve early identification and, when possible, removal of the underlying cause, followed by targeted interventions.
4.3 Non-pharmacological approaches
Particularly, sleep hygiene education and cognitive behavioral therapy are recommended as first-line treatments in cancer populations to improve sleep-related cognition and behavior (98). Cognitive behavioral therapy for insomnia (CBT-I) has been validated in brain tumor patients, demonstrating efficacy in improving sleep and offering additional benefits such as reduced fatigue, depression, and anxiety, thereby enhancing quality of life (87, 88, 99). However, the long-term efficacy of CBT-I remains uncertain (100, 101). For patients with EDS, modified CBT-I protocols that emphasize sufficient nighttime sleep and structured daytime napping may be beneficial (48, 102). Given the critical role of sleep in cancer prognosis and recovery (103), CBT-I should be implemented thoughtfully and personalized to meet individual patient needs (104).
4.4 Pharmacological management of insomnia
Pharmacological treatments for insomnia in the general population include non-benzodiazepines, benzodiazepines, and melatonin receptor agonists (98, 105). Among cancer patients, non-benzodiazepine hypnotics, such as zopiclone, eszopiclone, and zolpidem, are often preferred due to their relatively mild side effect profiles (106). However, no randomized controlled trials (RCTs) have specifically investigated their efficacy in brain tumor patients. Still, studies in broader oncology populations suggest that sequential treatment involving cognitive behavioral therapy followed by zolpidem may be effective for long-term insomnia remission (107, 108). Zopiclone (3.75–7.5 mg) and eszopiclone (3 mg) have been shown to reduce SOL, increase total sleep time, and improve subjective sleep satisfaction in patients with other cancers (108–110). A small study reported that zolpidem (10 mg) improved insomnia symptoms in brain tumor patients (n = 7) (111); however, caution is warranted, as chronic use exceeding 300 mg/year has been associated with increased risks of oral cancer and mortality (112–114). Benzodiazepines are generally not recommended for brain tumor patients due to their potential to worsen OSA, EDS, and cognitive dysfunction (115–118). Melatonin supplementation has demonstrated efficacy in managing insomnia related to pineal tumors (119), while low-dose antidepressants such as mirtazapine and trazodone have proven beneficial in cancer patients more broadly, though data specific to brain tumors are lacking (120, 121). The potential role of selective orexin receptor antagonists remains unexplored in this population and presents a promising area for future research (122).
4.5 Pharmacological management of EDS
Managing EDS in brain tumor patients requires an etiology-focused approach, beginning with surgical resection to eliminate the underlying cause. Pharmacological options include stimulant medications such as modafinil, pitolisant, and methylphenidate. Pitolisant has shown efficacy in treating EDS, cataplexy, and hypnagogic hallucinations, particularly in craniopharyngioma patients with secondary narcolepsy (20, 49, 123). Modafinil has demonstrated modest benefits, particularly in cognitive functioning, although its overall utility in brain tumor patients remains limited (118, 124). Postoperative melatonin administration in craniopharyngioma patients has also shown promise in reducing EDS (55).
4.6 Management of SBD
SBD is frequently observed in patients with brainstem tumors or craniopharyngiomas, where surgical resection often leads to substantial symptom improvement (125). For persistent SBD after surgery, continuous positive airway pressure (CPAP) therapy is the first-line management (20, 36, 125, 126). Polysomnographic evaluations should be conducted both pre- and postoperatively to accurately diagnose and monitor SBD.
4.7 Exercise-based interventions
Moderate to high-intensity aerobic and resistance training may improve sleep quality, physical fitness, and mental well-being in brain tumor patients (127). However, findings are mixed. For example, one study of high-grade glioma patients undergoing postoperative radiotherapy found that regular aerobic exercise (yoga, 2–3 sessions/week, 60 minutes/session) significantly improved sleep quality (128). Conversely, a Danish RCT found no improvement in insomnia, EDS, or quality of life following a 6-week intensive physical therapy program (3 sessions/week, 90 minutes/session) (129). Longer-duration programs may offer greater benefit. A 6-month home-based aerobic exercise intervention (20–45 minutes/session, 3 times/week) monitored by physical therapists improved sleep quality (reduced PSQI scores), cardiopulmonary function, and cognitive performance in glioma patients (130). Despite inconsistent outcomes, structured exercise under professional guidance is generally recommended for this population.
5 Summary and future perspective
In the context of brain tumors, the symptomatology of sleep disturbances is complex, yet their underlying etiologies remain poorly understood. To date, a tumor feature–oriented sleep phenotype framework has not been established. However, current evidence indicates that insomnia is the most commonly reported sleep disorder in brain tumor patients, while EDS, particularly secondary narcolepsy, is frequently associated with tumors located in the sellar and thalamic regions. SBD also occurs in this population, and the use of PSG and CPAP therapy is strongly recommended during both pre- and postoperative care.
Given the substantial impact of poor sleep on quality of life and long-term prognosis, early identification and appropriate intervention are essential. Due to the limited accessibility and financial burden of PSG, consumer-grade portable devices (e.g., wearables, home electroencephalogram systems) offer promising alternatives for sleep monitoring, owing to their cost-effectiveness and ease of use (131, 132). Furthermore, validated digital questionnaires integrated with machine learning algorithms may provide efficient tools for initial sleep disturbance screening in routine clinical care (133). However, notable accuracy gaps persist between consumer devices and PSG in sleep staging (134).
Artificial intelligence (AI) represents a transformative area in sleep medicine. Emerging algorithms can analyze complex multimodal data from wearable sensors and electronic health records to improve the prediction and classification of sleep disorders. Previous studies have shown that AI models can accurately detect OSA using oximetry and photoplethysmography signals (135), or respiratory vibration signals from wearables (136). Nonetheless, the lack of standardized validation frameworks across populations and devices, along with ethical, safety, and legal concerns, remains a major barrier to clinical adoption (137, 138). Moreover, our current understanding of the mechanisms underlying sleep-wake dysregulation in brain tumor patients is fragmented, limiting the development of targeted treatments.
6 Limitations
This review has several limitations. Unlike previous systematic reviews on sleep in brain tumor patients (139–141), we did not formally assess the quality of evidence, as this study followed a narrative review format. Additionally, we did not stratify findings by age groups or tumor types due to the scarcity and heterogeneity of available data. Instead, we adopted an approach focused on specific sleep disorder types and their associated risk factors.
Author contributions
QLiang: Writing – review & editing, Writing – original draft. TH: Writing – original draft. TY: Writing – review & editing. YP: Writing – review & editing. KS: Writing – review & editing. XZ: Writing – review & editing, Supervision. QLi: Writing – review & editing, Project administration, Conceptualization.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This study was funded by the Natural Science Foundation of Gansu Province, China (Grant No. 25JRRA586).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Ostrom QT, Price M, Neff C, Cioffi G, Waite KA, Kruchko C, et al. Cbtrus statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2015-2019. Neuro Oncol. (2022) 24:v1–v95. doi: 10.1093/neuonc/noac202
2. Yuan Y, Shi Q, Li M, Nagamuthu C, Andres E, and Davis FG. Canadian brain cancer survival rates by tumour type and region: 1992-2008. Can J Public Health. (2016) 107:e37–42. doi: 10.17269/cjph.107.5209
3. Zelt S, Cooney T, Yu S, Daral S, Krebs B, Markan R, et al. Disease burden and healthcare utilization in pediatric low-grade glioma: A United States retrospective study of linked claims and electronic health records. Neurooncol Pract. (2024) 11:583–92. doi: 10.1093/nop/npae037
4. van den Bent MJ, Geurts M, French PJ, Smits M, Capper D, Bromberg JEC, et al. Primary brain tumours in adults. Lancet. (2023) 402:1564–79. doi: 10.1016/s0140-6736(23)01054-1
5. Slagboom TNA, Boertien TM, Bisschop PH, Fliers E, Baaijen JC, Hoogmoed J, et al. Controlled study of pre- and postoperative headache in patients with sellar masses (Heads-up study). Endocrinol Diabetes Metab. (2024) 7:e496. doi: 10.1002/edm2.496
6. Kahlenberg CA, Fadul CE, Roberts DW, Thadani VM, Bujarski KA, Scott RC, et al. Seizure prognosis of patients with low-grade tumors. Seizure. (2012) 21:540–5. doi: 10.1016/j.seizure.2012.05.014
7. Ouyang MW, McDonagh DL, Phillips-Bute B, James ML, Friedman AH, and Gan TJ. Comparison of postoperative nausea between benign and Malignant brain tumor patients undergoing awake craniotomy: A retrospective analysis. Curr Med Res Opin. (2013) 29:1039–44. doi: 10.1185/03007995.2013.811070
8. Miaskowski C, Lee K, Dunn L, Dodd M, Aouizerat BE, West C, et al. Sleep-wake circadian activity rhythm parameters and fatigue in oncology patients before the initiation of radiation therapy. Cancer Nurs. (2011) 34:255–68. doi: 10.1097/NCC.0b013e3181f65d9b
9. Lin S, Chen Y, Yang L, and Zhou J. Pain, fatigue, disturbed sleep and distress comprised a symptom cluster that related to quality of life and functional status of lung cancer surgery patients. J Clin Nurs. (2013) 22:1281–90. doi: 10.1111/jocn.12228
10. Bastien CH, Vallières A, and Morin CM. Validation of the insomnia severity index as an outcome measure for insomnia research. Sleep Med. (2001) 2:297–307. doi: 10.1016/s1389-9457(00)00065-4
11. Ohayon MM and Roth T. Place of chronic insomnia in the course of depressive and anxiety disorders. J Psychiatr Res. (2003) 37:9–15. doi: 10.1016/s0022-3956(02)00052-3
12. van Kooten J, Maurice-Stam H, Schouten AYN, van Vuurden DG, Granzen B, Gidding C, et al. High occurrence of sleep problems in survivors of a childhood brain tumor with neurocognitive complaints: the association with psychosocial and behavioral executive functioning. Pediatr Blood Cancer. (2019) 66:e27947. doi: 10.1002/pbc.27947
13. Willis KD, Ravyts SG, Lanoye A, and Loughan AR. Sleep disturbance in primary brain tumor: prevalence, risk factors, and patient preferences. Support Care Cancer. (2022) 30:741–8. doi: 10.1007/s00520-021-06476-3
14. Jacob L, Scholten PC, Kostev K, and Kalder M. Association between sleep disorders and the presence of breast cancer metastases in gynecological practices in Germany: A case-control study of 11,412 women. Breast Cancer Res Treat. (2018) 171:443–8. doi: 10.1007/s10549-018-4831-x
15. Lin PC, Chen PY, Wei KC, Lin JH, Lin MR, Wang HC, et al. Sleep disturbance in adults with untreated primary brain tumors: prevalence and impact on quality of life. Sleep Biol Rhythms. (2023) 21:201–9. doi: 10.1007/s41105-022-00436-y
16. Lin CL, Liu TC, Wang YN, Chung CH, and Chien WC. The association between sleep disorders and the risk of colorectal cancer in patients: A population-based nested case-control study. In Vivo. (2019) 33:573–9. doi: 10.21873/invivo.11513
17. Erren TC, Morfeld P, Foster RG, Reiter RJ, Groß JV, and Westermann IK. Sleep and cancer: synthesis of experimental data and meta-analyses of cancer incidence among some 1,500,000 study individuals in 13 countries. Chronobiol Int. (2016) 33:325–50. doi: 10.3109/07420528.2016.1149486
18. Büttner-Teleagă A, Kim YT, Osel T, and Richter K. Sleep disorders in cancer-a systematic review. Int J Environ Res Public Health. (2021) 18(21). doi: 10.3390/ijerph182111696
19. Armstrong TS, Shade MY, Breton G, Gilbert MR, Mahajan A, Scheurer ME, et al. Sleep-wake disturbance in patients with brain tumors. Neuro Oncol. (2017) 19:323–35. doi: 10.1093/neuonc/now119
20. Cordani R, Veneruso M, Napoli F, Milanaccio C, Verrico A, Consales A, et al. Sleep disturbances in craniopharyngioma: A challenging diagnosis. J Neurol. (2021) 268:4362–9. doi: 10.1007/s00415-021-10794-1
21. Shi T, Min M, Sun C, Zhang Y, Liang M, and Sun Y. Does insomnia predict a high risk of cancer? A systematic review and meta-analysis of cohort studies. J Sleep Res. (2020) 29:e12876. doi: 10.1111/jsr.12876
22. Tian S, Huangfu L, Bao Y, Ai S, Chang S, Wang Q, et al. Causal associations of sleep traits with cancer incidence and mortality. Front Genet. (2023) 14:1309069. doi: 10.3389/fgene.2023.1309069
23. Pickering L, Jennum P, Gammeltoft S, Poulsgaard L, Feldt-Rasmussen U, and Klose M. Sleep-wake and melatonin pattern in craniopharyngioma patients. Eur J Endocrinol. (2014) 170:873–84. doi: 10.1530/eje-13-1025
24. Zhang D, Wang J, Gu X, Gu Z, Li L, Dong C, et al. Prevalence, correlates, and impact of sleep disturbance in chinese meningioma patients. Support Care Cancer. (2022) 30:1231–41. doi: 10.1007/s00520-021-06504-2
25. Temiz NC, Kacar Y, Mehtiyev R, Ezgu MC, and Karakoc O. Comparison of sleep quality and sleepiness in patients operated for pituitary adenoma and in healthy individuals. Turk Neurosurg. (2018) 28:364–8. doi: 10.5137/1019-5149.Jtn.19510-16.0
26. Jeon MS, Dhillon HM, Koh ES, Nowak AK, Hovey E, Descallar J, et al. Exploring sleep disturbance among adults with primary or secondary Malignant brain tumors and their caregivers. Neurooncol Pract. (2021) 8:48–59. doi: 10.1093/nop/npaa057
27. Lin MR, Chen PY, Wang HC, Lin PC, Lee HC, and Chiu HY. Prevalence of sleep disturbances and their effects on quality of life in adults with untreated pituitary tumor and meningioma. J Neurooncol. (2021) 154:179–86. doi: 10.1007/s11060-021-03811-w
28. Riemann D, Espie CA, Altena E, Arnardottir ES, Baglioni C, Bassetti CLA, et al. The european insomnia guideline: an update on the diagnosis and treatment of insomnia 2023. J Sleep Res. (2023) 32:e14035. doi: 10.1111/jsr.14035
29. Choi HR, Song IA, Park HY, and Oh TK. Association between insomnia disorder and mortality among patients who underwent craniotomy for brain tumor resection: A South Korean nationwide cohort study. Sleep Breath. (2023) 27:329–36. doi: 10.1007/s11325-022-02586-2
30. Teng KX, Price B, Joshi S, Alukaidey L, Shehab A, Mansour K, et al. Life after surgical resection of a low-grade glioma: A prospective cross-sectional study evaluating health-related quality of life. J Clin Neurosci. (2021) 88:259–67. doi: 10.1016/j.jocn.2021.03.038
31. Waddle MR, Oudenhoven MD, Farin CV, Deal AM, Hoffman R, Yang H, et al. Impacts of surgery on symptom burden and quality of life in pituitary tumor patients in the subacute post-operative period. Front Oncol. (2019) 9:299. doi: 10.3389/fonc.2019.00299
32. Robertson ME, McSherry F, Herndon JE, and Peters KB. Insomnia and its associations in patients with recurrent glial neoplasms. Springerplus. (2016) 5:823. doi: 10.1186/s40064-016-2578-6
33. Powell C, Guerrero D, Sardell S, Cumins S, Wharram B, Traish D, et al. Somnolence syndrome in patients receiving radical radiotherapy for primary brain tumours: A prospective study. Radiother Oncol. (2011) 100:131–6. doi: 10.1016/j.radonc.2011.06.028
34. Khan RB, Merchant TE, Sadighi ZS, Bello MS, Lu Z, Sykes A, et al. Prevalence, risk factors, and response to treatment for hypersomnia of central origin in survivors of childhood brain tumors. J Neurooncol. (2018) 136:379–84. doi: 10.1007/s11060-017-2662-y
35. Mainio A, Hakko H, Niemelä A, Koivukangas J, and Räsänen P. Insomnia among brain tumor patients: A population-based prospective study of tumor patients in northern Finland. J Psychosoc Oncol. (2013) 31:507–16. doi: 10.1080/07347332.2013.822048
36. Pollak L, Shpirer I, Rabey JM, Klein C, and Schiffer J. Polysomnography in Patients with Intracranial Tumors before and after Operation. Acta Neurol Scand. (2004) 109:56–60. doi: 10.1034/j.1600-0404.2003.00176.x
37. Kitselaar WM, de Morree HM, Trompenaars MW, Sitskoorn MM, Rutten GJ, and Kop WJ. Fatigue after neurosurgery in patients with a brain tumor: the role of autonomic dysregulation and disturbed sleep. J Psychosom Res. (2022) 156:110766. doi: 10.1016/j.jpsychores.2022.110766
38. Nolan VG, Gapstur R, Gross CR, Desain LA, Neglia JP, Gajjar A, et al. Sleep disturbances in adult survivors of childhood brain tumors. Qual Life Res. (2013) 22:781–9. doi: 10.1007/s11136-012-0208-5
39. Sekely A, Bernstein LJ, Campbell KL, Mason WP, Laperriere N, Kalidindi N, et al. Neurocognitive impairment, neurobehavioral symptoms, fatigue, sleep disturbance, and depressive symptoms in patients with newly diagnosed glioblastoma. Neurooncol Pract. (2023) 10:89–96. doi: 10.1093/nop/npac068
40. Mugge L, Mansour TR, Crippen M, Alam Y, and Schroeder J. Depression and glioblastoma, complicated concomitant diseases: A systemic review of published literature. Neurosurg Rev. (2020) 43:497–511. doi: 10.1007/s10143-018-1017-2
41. Huang Y, Jiang ZJ, Deng J, and Qi YJ. Sleep quality of patients with postoperative glioma at home. World J Clin cases. (2020) 8:4735–42. doi: 10.12998/wjcc.v8.i20.4735
42. Graef DM, Crabtree VM, Srivastava DK, Li C, Pritchard M, Hinds PS, et al. Sleep and mood during hospitalization for high-dose chemotherapy and hematopoietic rescue in pediatric medulloblastoma. Psychooncology. (2018) 27:1847–53. doi: 10.1002/pon.4737
43. Umezaki S, Shinoda Y, Mukasa A, Tanaka S, Takayanagi S, Oka H, et al. Factors associated with health-related quality of life in patients with glioma: impact of symptoms and implications for rehabilitation. Jpn J Clin Oncol. (2020) 50:990–8. doi: 10.1093/jjco/hyaa068
44. Society CSR. Expert consensus on clinical diagnosis and treatment of excessive daytime sleepiness. Zhonghua Yi Xue Za Zhi. (2023) 103:1103–18. doi: 10.3760/cma.j.cn112137-20221228-02712
45. Mandrell BN, Wise M, Schoumacher RA, Pritchard M, West N, Ness KK, et al. Excessive daytime sleepiness and sleep-disordered breathing disturbances in survivors of childhood central nervous system tumors. Pediatr Blood Cancer. (2012) 58:746–51. doi: 10.1002/pbc.23311
46. Mandrell BN, LaRosa K, Hancock D, Caples M, Sykes A, Lu Z, et al. Predictors of narcolepsy and hypersomnia due to medical disorder in pediatric craniopharyngioma. J Neurooncol. (2020) 148:307–16. doi: 10.1007/s11060-020-03519-3
47. Pickering L, Main KM, Sehested A, Mathiasen R, Feldt-Rasmussen U, Klose M, et al. Brain tumours result in sleep disorders in children and adolescents. Sleep Med. (2021) 88:13–21. doi: 10.1016/j.sleep.2021.09.016
48. Bassetti CLA, Kallweit U, Vignatelli L, Plazzi G, Lecendreux M, Baldin E, et al. European guideline and expert statements on the management of narcolepsy in adults and children. J Sleep Res. (2021) 30:e13387. doi: 10.1111/jsr.13387
49. Dodet P, Noiray C, Leu-Semenescu S, Lefevre E, Nigam M, Faucher P, et al. Hypersomnia and narcolepsy in 42 adult patients with craniopharyngioma. Sleep. (2023) 46(5). doi: 10.1093/sleep/zsad032
50. Dauvilliers Y, Abril B, Charif M, Quittet P, Bauchet L, Carlander B, et al. Reversal of symptomatic tumoral narcolepsy, with normalization of csf hypocretin level. Neurology. (2007) 69:1300–1. doi: 10.1212/01.wnl.0000276948.46213.64
51. Nokura K, Kanbayashi T, Ozeki T, Koga H, Zettsu T, Yamamoto H, et al. Hypersomnia, asterixis and cataplexy in association with orexin a-reduced hypothalamic tumor. J Neurol. (2004) 251:1534–5. doi: 10.1007/s00415-004-0575-0
52. Tachibana N, Taniike M, Okinaga T, Ripley B, Mignot E, and Nishino S. Hypersomnolence and increased rem sleep with low cerebrospinal fluid hypocretin level in a patient after removal of craniopharyngioma. Sleep Med. (2005) 6:567–9. doi: 10.1016/j.sleep.2005.04.002
53. Müller HL, Handwerker G, Gebhardt U, Faldum A, Emser A, Kolb R, et al. Melatonin treatment in obese patients with childhood craniopharyngioma and increased daytime sleepiness. Cancer Causes Control. (2006) 17:583–9. doi: 10.1007/s10552-005-9012-7
54. Liao Y, He Y, Yang Y, Li X, and Huang F. Case report: narcolepsy type 2 due to temporal lobe glioma. Med (Baltimore). (2020) 99:e21002. doi: 10.1097/md.0000000000021002
55. Müller HL, Handwerker G, Wollny B, Faldum A, and Sörensen N. Melatonin secretion and increased daytime sleepiness in childhood craniopharyngioma patients. J Clin Endocrinol Metab. (2002) 87:3993–6. doi: 10.1210/jcem.87.8.8751
56. Helligsoe ASL, Weile KS, Kenborg L, Henriksen LT, Lassen-Ramshad Y, Amidi A, et al. Systematic review: sleep disorders based on objective data in children and adolescents treated for a brain tumor. Front Neurosci. (2022) 16:808398. doi: 10.3389/fnins.2022.808398
57. Verhulst SL, Schrauwen N, Haentjens D, Suys B, Rooman RP, Van Gaal L, et al. Sleep-disordered breathing in overweight and obese children and adolescents: prevalence, characteristics and the role of fat distribution. Arch Dis Child. (2007) 92:205–8. doi: 10.1136/adc.2006.101089
58. Caplan IF, Glauser G, Goodrich S, Chen HI, Lucas TH, Lee JYK, et al. Undiagnosed obstructive sleep apnea as predictor of 90-day readmission for brain tumor patients. World Neurosurg. (2020) 134:e979–e84. doi: 10.1016/j.wneu.2019.11.050
59. Caplan IF, Glauser G, Goodrich S, Chen HI, Lucas TH, Lee JYK, et al. Undiagnosed obstructive sleep apnea as a predictor of 30-day readmission for brain tumor patients. J Neurosurg. (2020) 133:624–9. doi: 10.3171/2019.4.Jns1968
60. Gapstur R, Gross CR, and Ness K. Factors associated with sleep-wake disturbances in child and adult survivors of pediatric brain tumors: A review. Oncol Nurs Forum. (2009) 36:723–31. doi: 10.1188/09.Onf.723-731
61. Sateia MJ. International classification of sleep disorders-third edition: highlights and modifications. Chest. (2014) 146:1387–94. doi: 10.1378/chest.14-0970
62. Jianhua C, Xiuqin L, Quancai C, Heyang S, and Yan H. Rapid eye movement sleep behavior disorder in a patient with brainstem lymphoma. Intern Med. (2013) 52:617–21. doi: 10.2169/internalmedicine.52.8786
63. McCarter SJ, Tippmann-Peikert M, Sandness DJ, Flanagan EP, Kantarci K, Boeve BF, et al. Neuroimaging-evident lesional pathology associated with rem sleep behavior disorder. Sleep Med. (2015) 16:1502–10. doi: 10.1016/j.sleep.2015.07.018
64. Mitterling T, Riffert V, Heimel S, Leibetseder A, Kaindlstorfer A, Heidbreder A, et al. Beyond sleep disturbance: structured analysis of sleep habits, chronotype and sleep disorders in adults with glioma. A cross-sectional exploratory study. Sleep Med. (2025) 125:146–54. doi: 10.1016/j.sleep.2024.11.033
65. Joustra SD, Kruijssen E, Verstegen MJ, Pereira AM, and Biermasz NR. Determinants of altered sleep-wake rhythmicity in patients treated for nonfunctioning pituitary macroadenomas. J Clin Endocrinol Metab. (2014) 99:4497–505. doi: 10.1210/jc.2014-2602
66. Lin B, Xiang S, Chen J, Jing Y, Ye Z, Zhang Y, et al. Assessment of quality of life in patients with craniopharyngioma and identification of risk factors for compromised overall wellness. Arch Endocrinol Metab. (2023) 68:e230001. doi: 10.20945/2359-4292-2023-0001
67. van der Klaauw AA, Biermasz NR, Pereira AM, van Kralingen KW, Dekkers OM, Rabe KF, et al. Patients cured from craniopharyngioma or nonfunctioning pituitary macroadenoma (Nfma) suffer similarly from increased daytime somnolence despite normal sleep patterns compared to healthy controls. Clin Endocrinol (Oxf). (2008) 69:769–74. doi: 10.1111/j.1365-2265.2008.03284.x
68. Slawik H, Stoffel M, Riedl L, Veselý Z, Behr M, Lehmberg J, et al. Prospective Study on Salivary Evening Melatonin and Sleep before and after Pinealectomy in Humans. J Biol Rhythms. (2016) 31:82–93. doi: 10.1177/0748730415616678
69. Krieg SM, Slawik H, Meyer B, Wiegand M, and Stoffel M. Sleep disturbance after pinealectomy in patients with pineocytoma who°I. Acta Neurochir (Wien). (2012) 154:1399–405; discussion 405. doi: 10.1007/s00701-012-1409-y
70. Lee A, Chen ML, Abeshaus S, Poliakov A, and Ojemann JG. Posterior fossa tumors and their impact on sleep and ventilatory control: A clinical perspective. Respir Physiol Neurobiol. (2013) 189:261–71. doi: 10.1016/j.resp.2013.05.027
71. Kalapurakal JA, Goldman S, Hsieh YC, Tomita T, and Marymont MH. Clinical outcome in children with craniopharyngioma treated with primary surgery and radiotherapy deferred until relapse. Med Pediatr Oncol. (2003) 40:214–8. doi: 10.1002/mpo.10247
72. van Litsenburg R, Kamara D, Irestorm E, Partanen M, de Vries R, McLaughlin Crabtree V, et al. Sleep Problems during and after Paediatric Brain Tumours. Lancet Child Adolesc Health. (2023) 7:280–7. doi: 10.1016/s2352-4642(22)00380-7
73. Zoli M, Sambati L, Milanese L, Foschi M, Faustini-Fustini M, Marucci G, et al. Postoperative outcome of body core temperature rhythm and sleep-wake cycle in third ventricle craniopharyngiomas. Neurosurg Focus. (2016) 41:E12. doi: 10.3171/2016.9.Focus16317
74. Fedorko S, Zweckberger K, and Unterberg AW. Quality of life following surgical treatment of lesions within the pineal region. J Neurosurg. (2019) 130:28–37. doi: 10.3171/2017.7.Jns17260
75. Ahn GS, Hwang K, Kim TM, Park CK, Chang JH, Jung TY, et al. Influence of concurrent and adjuvant temozolomide on health-related quality of life of patients with grade iii gliomas: A secondary analysis of a randomized clinical trial (Knog-1101 study). Cancer Res Treat. (2022) 54:396–405. doi: 10.4143/crt.2021.393
76. Panciroli C, Esteve A, Muñoz-Ferrer A, Abad J, Hernandez JM, Balaña C, et al. Prospective pilot study to explore the melatonin level in brain tumor patients undergoing radiotherapy. Sleep Breath. (2022) 26:469–75. doi: 10.1007/s11325-021-02365-5
77. Langegård U, Fransson P, Bjork-Eriksson T, Johansson B, Ohlsson-Nevo E, Sjövall K, et al. Health-Related Quality of Life in Patients with Primary Brain Tumors during and Three Months after Treatment with Proton Beam Therapy. Tech Innov Patient Support Radiat Oncol. (2021) 17:5–17. doi: 10.1016/j.tipsro.2021.01.004
78. Romijn JA. Pituitary diseases and sleep disorders. Curr Opin Endocrinol Diabetes Obes. (2016) 23:345–51. doi: 10.1097/med.0000000000000265
79. Erkoç A, Eroğlu İ, Erbas T, and Kutukcu EC. Muscle function, exercise capacity, physical activity level and cardiovascular disease risk factor knowledge in patients with prolactinoma. Endocrine. (2024) 85:1337–45. doi: 10.1007/s12020-024-03880-7
80. Shekhar S, Hall JE, and Klubo-Gwiezdzinska J. The hypothalamic pituitary thyroid axis and sleep. Curr Opin Endocr Metab Res. (2021) 17:8–14. doi: 10.1016/j.coemr.2020.10.002
81. Zhao X, Heng L, Qu Y, Jia D, Ren J, Sun S, et al. Densely granulated adenoma pattern is associated with an increased risk of obstructive sleep apnea in patients with acromegaly. Sleep Breath. (2022) 26:1381–7. doi: 10.1007/s11325-021-02468-z
82. Guo X, Gao L, Zhao Y, Wang M, Jiang B, Wang Q, et al. Characteristics of the upper respiratory tract in patients with acromegaly and correlations with obstructive sleep apnoea/hypopnea syndrome. Sleep Med. (2018) 48:27–34. doi: 10.1016/j.sleep.2018.04.011
83. Shankar RR, Jakacki RI, Haider A, Lee MW, and Pescovitz OH. Testing the hypothalamic-pituitary-adrenal axis in survivors of childhood brain and skull-based tumors. J Clin Endocrinol Metab. (1997) 82:1995–8. doi: 10.1210/jcem.82.6.4014
84. Alaoui AA, Alaoui S, Hajjar R, Urso D, and Gnoni V. Head and neck cancer radiotherapy and obstructive sleep apnea. Ann Palliat Med. (2022) 11:3592–5. doi: 10.21037/apm-22-972
85. Maquilan G, Grover S, Alonso-Basanta M, and Lustig RA. Acute toxicity profile of patients with low-grade gliomas and meningiomas receiving proton therapy. Am J Clin Oncol. (2014) 37:438–43. doi: 10.1097/COC.0b013e31827de86b
86. Reddy K, Gaspar LE, Kavanagh BD, Waziri A, Damek DM, Ney D, et al. Prospective evaluation of health-related quality of life in patients with glioblastoma multiforme treated on a phase ii trial of hypofractionated imrt with temozolomide. J Neurooncol. (2013) 114:111–6. doi: 10.1007/s11060-013-1159-6
87. Sathyapalan T and Dixit S. Radiotherapy-induced hypopituitarism: A review. Expert Rev Anticancer Ther. (2012) 12:669–83. doi: 10.1586/era.12.27
88. Kunni K, Langegård U, Ohlsson-Nevo E, Kristensen I, Sjövall K, Fessé P, et al. Symptom experience and symptom distress in patients with Malignant brain tumor treated with proton therapy: A five-year follow-up study. Tech Innov Patient Support Radiat Oncol. (2024) 31:100269. doi: 10.1016/j.tipsro.2024.100269
89. Schiff D, Lee EQ, Nayak L, Norden AD, Reardon DA, and Wen PY. Medical management of brain tumors and the sequelae of treatment. Neuro Oncol. (2015) 17:488–504. doi: 10.1093/neuonc/nou304
90. Rosen G and Brand SR. Sleep in children with cancer: case review of 70 children evaluated in a comprehensive pediatric sleep center. Support Care Cancer. (2011) 19:985–94. doi: 10.1007/s00520-010-0921-y
91. Crabtree VM, Rach AM, Schellinger KB, Russell KM, Hammarback T, and Mandrell BN. Changes in sleep and fatigue in newly treated pediatric oncology patients. Support Care Cancer. (2015) 23:393–401. doi: 10.1007/s00520-014-2356-3
92. Medysky ME, Temesi J, Culos-Reed SN, and Millet GY. Exercise, sleep and cancer-related fatigue: are they related? Neurophysiol Clin. (2017) 47:111–22. doi: 10.1016/j.neucli.2017.03.001
93. Cheng JX, Liu BL, Zhang X, Lin W, Zhang YQ, Liu WP, et al. Health-related quality of life in glioma patients in China. BMC Cancer. (2010) 10:305. doi: 10.1186/1471-2407-10-305
94. Kapur VK, Auckley DH, Chowdhuri S, Kuhlmann DC, Mehra R, Ramar K, et al. Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: an american academy of sleep medicine clinical practice guideline. J Clin Sleep Med. (2017) 13:479–504. doi: 10.5664/jcsm.6506
95. Tzeng JI, Fu YW, and Lin CC. Validity and reliability of the Taiwanese version of the pittsburgh sleep quality index in cancer patients. Int J Nurs Stud. (2012) 49:102–8. doi: 10.1016/j.ijnurstu.2011.08.004
96. Schulte T, Hofmeister D, Mehnert-Theuerkauf A, Hartung T, and Hinz A. Assessment of sleep problems with the insomnia severity index (Isi) and the sleep item of the patient health questionnaire (Phq-9) in cancer patients. Support Care Cancer. (2021) 29:7377–84. doi: 10.1007/s00520-021-06282-x
97. Crabtree VM, Klages KL, Sykes A, Wise MS, Lu Z, Indelicato D, et al. Sensitivity and specificity of the modified epworth sleepiness scale in children with craniopharyngioma. J Clin Sleep Med. (2019) 15:1487–93. doi: 10.5664/jcsm.7982
98. Chinese Society of Neurology SDS and Chinese Society of Neurology. Guideline for the evaluation and treatment of insomnia in chinese adults(2017). Chin J Neurol. (2018) 51:324–35. doi: 10.3760/cma.j.issn.1006-7876.2018.05.002
99. Loughan AR, Lanoye A, Willis KD, Fox A, Ravyts SG, Zukas A, et al. Telehealth group cognitive behavioral therapy for insomnia (Cbt-I) in primary brain tumor: primary outcomes from a single-arm phase 2 feasibility and proof-of-concept trial. Neuro Oncol. (2024) 26(3):516–27. doi: 10.1093/neuonc/noad193
100. Gao Y, Liu M, Yao L, Yang Z, Chen Y, Niu M, et al. Cognitive behavior therapy for insomnia in cancer patients: A systematic review and network meta-analysis. J Evid Based Med. (2022) 15:216–29. doi: 10.1111/jebm.12485
101. Ma Y, Hall DL, Ngo LH, Liu Q, Bain PA, and Yeh GY. Efficacy of cognitive behavioral therapy for insomnia in breast cancer: A meta-analysis. Sleep Med Rev. (2021) 55:101376. doi: 10.1016/j.smrv.2020.101376
102. Billiard M, Bassetti C, Dauvilliers Y, Dolenc-Groselj L, Lammers GJ, Mayer G, et al. Efns guidelines on management of narcolepsy. Eur J Neurol. (2006) 13:1035–48. doi: 10.1111/j.1468-1331.2006.01473.x
103. Zhou T, Wang Z, Qiao C, Wang S, Hu S, Wang X, et al. Sleep disturbances and the risk of lung cancer: A meta-epidemiological study. BMC Cancer. (2023) 23:884. doi: 10.1186/s12885-023-11392-2
104. Turner AD, Ong JC, Jones AL, Tu A, Salanitro M, and Crawford MR. Neurocognitive Functioning in Comorbid Insomnia and Sleep Apnea Patients Is Better after Positive Airway Pressure Therapy, but Worse after Cognitive Behavioral Therapy for Insomnia: Exploratory Analysis of Cognitive Outcomes from the Multidisciplinary Approach to the Treatment of Insomnia and Comorbid Sleep Apnea Study. Sleep. (2023) 46(8). doi: 10.1093/sleep/zsad128
105. Wilt TJ, MacDonald R, Brasure M, Olson CM, Carlyle M, Fuchs E, et al. Pharmacologic treatment of insomnia disorder: an evidence report for a clinical practice guideline by the american college of physicians. Ann Intern Med. (2016) 165:103–12. doi: 10.7326/m15-1781
106. Grassi L, Zachariae R, Caruso R, Palagini L, Campos-Ródenas R, Riba MB, et al. Insomnia in adult patients with cancer: esmo clinical practice guideline. ESMO Open. (2023) 8:102047. doi: 10.1016/j.esmoop.2023.102047
107. Morin CM, Edinger JD, Beaulieu-Bonneau S, Ivers H, Krystal AD, Guay B, et al. Effectiveness of sequential psychological and medication therapies for insomnia disorder: A randomized clinical trial. JAMA Psychiatry. (2020) 77:1107–15. doi: 10.1001/jamapsychiatry.2020.1767
108. Zhang Y, Su J, Wang J, Tang G, Hu W, Mao J, et al. Cognitive behavioral therapy for insomnia combined with eszopiclone for the treatment of sleep disorder patients transferred out of the intensive care unit: A single-centred retrospective observational study. Medicine. (2018) 97:e12383. doi: 10.1097/md.0000000000012383
109. Jakobsen G, Sjue K, Paulsen Ø, Kaasa S, Hjermstad MJ, and Klepstad P. Zopiclone versus placebo for short-term treatment of insomnia in patients with advanced cancer-a double-blind, randomized placebo-controlled clinical multicenter phase iv trial. Support Care Cancer. (2022) 31:60. doi: 10.1007/s00520-022-07537-x
110. Dimsdale JE, Ball ED, Carrier E, Wallace M, Holman P, Mulroney C, et al. Effect of eszopiclone on sleep, fatigue, and pain in patients with mucositis associated with hematologic Malignancies. Support Care Cancer. (2011) 19:2015–20. doi: 10.1007/s00520-010-1052-1
111. Chang MC and Chun MH. The effect of hypnotics on sleep quality and cognitive function in patients with brain tumors. J Korean Neurosurg Soc. (2020) 63:261–7. doi: 10.3340/jkns.2019.0057
112. Hwang S, Son H, Kim M, Lee SK, and Jung KY. Association of zolpidem with increased mortality in patients with brain cancer: A retrospective cohort study based on the national health insurance service database. J Clin Neurol. (2022) 18:65–70. doi: 10.3988/jcn.2022.18.1.65
113. Kripke DF, Langer RD, and Kline LE. Hypnotics’ Association with mortality or cancer: A matched cohort study. BMJ Open. (2012) 2:e000850. doi: 10.1136/bmjopen-2012-000850
114. Kao CH, Sun LM, Liang JA, Chang SN, Sung FC, and Muo CH. Relationship of zolpidem and cancer risk: A Taiwanese population-based cohort study. Mayo Clin Proc. (2012) 87:430–6. doi: 10.1016/j.mayocp.2012.02.012
115. Atkin T, Comai S, and Gobbi G. Drugs for insomnia beyond benzodiazepines: pharmacology, clinical applications, and discovery. Pharmacol Rev. (2018) 70:197–245. doi: 10.1124/pr.117.014381
116. Wang SH, Chen WS, Tang SE, Lin HC, Peng CK, Chu HT, et al. Benzodiazepines associated with acute respiratory failure in patients with obstructive sleep apnea. Front Pharmacol. (2018) 9:1513. doi: 10.3389/fphar.2018.01513
117. Jennum P, Baandrup L, Tønnesen P, Ibsen R, and Kjellberg J. Mortality and use of psychotropic medication in sleep apnoea patients: A population-wide register-based study. Sleep Med. (2018) 43:19–24. doi: 10.1016/j.sleep.2017.11.1142
118. Riss J, Cloyd J, Gates J, and Collins S. Benzodiazepines in epilepsy: pharmacology and pharmacokinetics. Acta Neurol Scand. (2008) 118:69–86. doi: 10.1111/j.1600-0404.2008.01004.x
119. Etzioni A, Luboshitzky R, Tiosano D, Ben-Harush M, Goldsher D, and Lavie P. Melatonin replacement corrects sleep disturbances in a child with pineal tumor. Neurology. (1996) 46:261–3. doi: 10.1212/wnl.46.1.261
120. Cankurtaran ES, Ozalp E, Soygur H, Akbiyik DI, Turhan L, and Alkis N. Mirtazapine improves sleep and lowers anxiety and depression in cancer patients: superiority over imipramine. Support Care Cancer. (2008) 16:1291–8. doi: 10.1007/s00520-008-0425-1
121. Kim SW, Shin IS, Kim JM, Kim YC, Kim KS, Kim KM, et al. Effectiveness of mirtazapine for nausea and insomnia in cancer patients with depression. Psychiatry Clin Neurosci. (2008) 62:75–83. doi: 10.1111/j.1440-1819.2007.01778.x
122. Wu X, Xue T, Chen Z, Wang Z, and Chen G. Orexin receptor antagonists and insomnia. Curr Psychiatry Rep. (2022) 24:509–21. doi: 10.1007/s11920-022-01357-w
123. Dauvilliers Y, Lecendreux M, Lammers GJ, Franco P, Poluektov M, Caussé C, et al. Safety and efficacy of pitolisant in children aged 6 years or older with narcolepsy with or without cataplexy: A double-blind, randomised, placebo-controlled trial. Lancet Neurol. (2023) 22:303–11. doi: 10.1016/s1474-4422(23)00036-4
124. Boele FW, Douw L, de Groot M, van Thuijl HF, Cleijne W, Heimans JJ, et al. The effect of modafinil on fatigue, cognitive functioning, and mood in primary brain tumor patients: A multicenter randomized controlled trial. Neuro Oncol. (2013) 15:1420–8. doi: 10.1093/neuonc/not102
125. Buller F, Kamal MA, Brown SK, Carruthers E, Montague ML, Ochieng D, et al. Obstructive sleep apnea syndrome as a rare presentation in a young girl with a central nervous system tumor. J Clin Sleep Med. (2022) 18:1211–4. doi: 10.5664/jcsm.9800
126. Fujimoto K, Kasai H, Kunii R, Terada J, and Tatsumi K. Obstructive sleep apnea in a severely obese child with combined central sleep apnea and sleep-related hypoventilation disorder caused by a medullary tumor. J Clin Sleep Med. (2018) 14:1071–4. doi: 10.5664/jcsm.7184
127. Paolucci EM, Loukov D, Bowdish DME, and Heisz JJ. Exercise reduces depression and inflammation but intensity matters. Biol Psychol. (2018) 133:79–84. doi: 10.1016/j.biopsycho.2018.01.015
128. Milbury K, Mallaiah S, Mahajan A, Armstrong T, Weathers SP, Moss KE, et al. Yoga program for high-grade glioma patients undergoing radiotherapy and their family caregivers. Integr Cancer Ther. (2018) 17:332–6. doi: 10.1177/1534735417689882
129. Hansen A, Pedersen CB, Jarden JO, Beier D, Minet LR, and Søgaard K. Effectiveness of physical therapy- and occupational therapy-based rehabilitation in people who have glioma and are undergoing active anticancer treatment: single-blind, randomized controlled trial. Phys Ther. (2020) 100:564–74. doi: 10.1093/ptj/pzz180
130. Gehring K, Stuiver MM, Visser E, Kloek C, van den Bent M, Hanse M, et al. A pilot randomized controlled trial of exercise to improve cognitive performance in patients with stable glioma: A proof of concept. Neuro Oncol. (2020) 22:103–15. doi: 10.1093/neuonc/noz178
131. Kwon S, Kim HS, Kwon K, Kim H, Kim YS, Lee SH, et al. At-home wireless sleep monitoring patches for the clinical assessment of sleep quality and sleep apnea. Sci Adv. (2023) 9:eadg9671. doi: 10.1126/sciadv.adg9671
132. Kwon S, Kim H, and Yeo WH. Recent advances in wearable sensors and portable electronics for sleep monitoring. iScience. (2021) 24:102461. doi: 10.1016/j.isci.2021.102461
133. Schwartz AR, Cohen-Zion M, Pham LV, Gal A, Sowho M, Sgambati FP, et al. Brief digital sleep questionnaire powered by machine learning prediction models identifies common sleep disorders. Sleep Med. (2020) 71:66–76. doi: 10.1016/j.sleep.2020.03.005
134. Birrer V, Elgendi M, Lambercy O, and Menon C. Evaluating reliability in wearable devices for sleep staging. NPJ Digit Med. (2024) 7:74. doi: 10.1038/s41746-024-01016-9
135. Attia S, Oksenberg A, Levy J, Ang A, Shani-Hershkovich R, Adler A, et al. Clinical validation of artificial intelligence algorithms for the diagnosis of adult obstructive sleep apnea and sleep staging from oximetry and photoplethysmography-sleepai. J Sleep Res. (2025):e70093. doi: 10.1111/jsr.70093
136. Chen Y, Ma G, Zhang M, Yang S, Yan J, Zhang Z, et al. Contactless screening for sleep apnea with breathing vibration signals based on modified U-net. Sleep Med. (2023) 107:187–95. doi: 10.1016/j.sleep.2023.04.030
137. Nguyen QNT, Le T, Huynh QBT, Setty A, Vo TV, and Le TQ. Validation framework for sleep stage scoring in wearable sleep trackers and monitors with polysomnography ground truth. Clocks Sleep. (2021) 3:274–88. doi: 10.3390/clockssleep3020017
138. Goldstein CA, Berry RB, Kent DT, Kristo DA, Seixas AA, Redline S, et al. Artificial intelligence in sleep medicine: an american academy of sleep medicine position statement. J Clin Sleep Med. (2020) 16:605–7. doi: 10.5664/jcsm.8288
139. Jeon MS, Dhillon HM, and Agar MR. Sleep disturbance of adults with a brain tumor and their family caregivers: A systematic review. Neuro Oncol. (2017) 19:1035–46. doi: 10.1093/neuonc/nox019
140. Martin JA, Hart NH, Bradford N, Naumann F, Pinkham MB, Pinkham EP, et al. Prevalence and management of sleep disturbance in adults with primary brain tumours and their caregivers: A systematic review. J Neurooncol. (2023) 162:25–44. doi: 10.1007/s11060-023-04270-1
Keywords: brain tumor, sleep disturbances, insomnia, narcolepsy, prognosis
Citation: Liang Q, Hu T, Yang T, Pan Y, Spruyt K, Zhang X and Li Q (2025) Sleep disturbances in brain tumors: a narrative review. Front. Oncol. 15:1594232. doi: 10.3389/fonc.2025.1594232
Received: 15 March 2025; Accepted: 15 August 2025;
Published: 25 September 2025.
Edited by:
Brian D Adams, Brain Institute of America, United StatesReviewed by:
Giorgia Abete Fornara, IRCCS Ca ‘Granda Foundation Maggiore Policlinico Hospital, Italyİmdat Eroğlu, Gazi University, Türkiye
Copyright © 2025 Liang, Hu, Yang, Pan, Spruyt, Zhang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qiang Li, c3Ryb25nOTA4QDE2My5jb20=; Xinyan Zhang, eGlueWFuLXpoYW5nQHNjdS5lZHUuY24=
†These authors contributed equally to this work and share first authorship
‡These authors contributed equally to this work and share last authorship
 Qiang Liang1,2†
Qiang Liang1,2† Tong Yang
Tong Yang Yawen Pan
Yawen Pan Qiang Li
Qiang Li