- 1Department of Neurosurgery, Chengdu Women’s and Children’s Central Hospital, School of Medicine, University of Electronic Science and Technology of China, Chengdu, China
- 2Department of Pediatric Surgery, Chengdu Women’s and Children’s Central Hospital, School of Medicine, University of Electronic Science and Technology of China, Chengdu, China
- 3Department of Neurosurgery, West China Hospital, Sichuan University, Chengdu, Sichuan, China
Background: Cavernous lymphangioma represents a common lesion of the lymphatic system, yet it is rarely encountered in the small intestine. The diagnosis of small intestinal cavernous lymphangioma poses significant clinical challenges. This case report presents the optimal diagnostic and therapeutic management of pediatric small intestinal cavernous lymphangioma.
Case description: A 27-month-old male presented with recurrent syncopal episodes, hematochezia, and anemia. Small intestinal abnormalities were detected via wireless capsule endoscopy. Single-port laparoscopic surgery was subsequently performed, intraoperatively revealing a 3×3×6 cm ileal lesion located 95 cm proximal to the ileocecal valve. Segmental bowel resection was completed, with postoperative histopathological confirmation of cavernous lymphangioma.
Conclusion: Pediatric cavernous lymphangioma of the small intestine is an exceedingly rare clinical disease with a multifaceted diagnostic process. Wireless capsule endoscopy is strongly recommended as a pivotal diagnostic modality for small intestine cavernous lymphangioma. Upon confirmation of the intestine lesion, immediate single-port laparoscopic resection should be implemented as the therapeutic imperative.
1 Introduction
Lymphangioma constitutes a congenital developmental anomaly or hamartomatous lesion of the lymphatic system, originating from lymphatic endothelial cells and vascular structures. Histopathologically, it is characterized by ectatic lymphatic channels lined by attenuated endothelial cells, surrounded by collagenous stroma or smooth muscle bundles (1). These lesions predominantly occur in cervical (75%) and axillary regions (20%), with rare involvement of orbits, mediastinum, adrenal glands, kidneys, bones, omentum, gastrointestinal tract (incidence <1%), retroperitoneum, liver, and pancreas (2). We present a 27-month-old male admitted with recurrent hematochezia and anemia. Diagnosis of small intestinal cavernous lymphangioma was confirmed via wireless capsule endoscopy, followed by successful single-port laparoscopic resection. The case report aims to enhance clinical recognition of small intestinal cavernous lymphangioma and emphasize the diagnostic significance of wireless capsule endoscopy in small intestinal lesion. This case report obtains Written informed consent from the patient’s legal guardians and adheres to the SCARE criteria (3).
2 Case report
2.1 General information
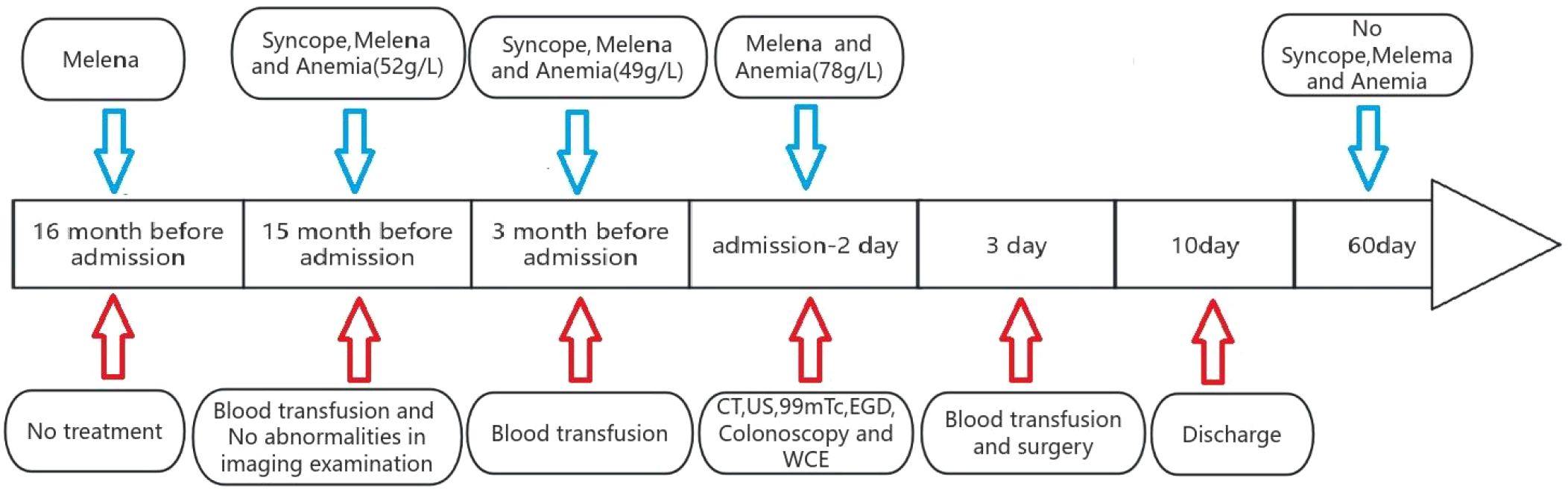
The pediatric patient was admitted with a 16-month history of recurrent melena and syncopal episodes. 16 months prior to admission(11-month-old), the child developed recurrent tarry stool. A syncopal event occurred 15 months before admission(12-month-old), prompting laboratory evaluation that revealed severe anemia (hemoglobin 52 g/L), requiring transfusion of leukocyte-depleted packed red blood cells. Subsequent abdominal ultrasonography and CT demonstrated no structural abnormalities. 3 months prior to admission(24-month-old), recurrent syncope necessitated hospitalization with critical anemia (hemoglobin 49 g/L) requiring repeat transfusion. 2 days prior to admission(27-month-old), patient’s legal guardians subsequently sought etiological investigation at our institution. Obstetric history indicated an uncomplicated pregnancy with no maternal medication exposure during the periconceptional period. The child was delivered via cesarean section at 38 weeks and 2 days gestation due to intrapartum asphyxia, with a birth weight of 3.35 kg and Apgar scores of 10 at 1 and 5 minutes.
2.2 Physical examination
The patient exhibited poor nutritional status with a conscious and alert mental state. Pallor was noted with mild cyanosis of the lips and pale nail beds. No petechiae or ecchymoses were observed on the skin. Oropharyngeal examination revealed non-erythematous mucosa and absence of tonsillar enlargement. Pulmonary auscultation demonstrated clear breath sounds bilaterally without adventitious sounds such as rhonchi or crackles. Cardiac evaluation showed regular rhythm with strong heart sounds and no detectable murmurs. Abdominal examination disclosed a soft, non-tender abdomen without guarding or rebound tenderness. Both hepatic and splenic margins remained non-palpable upon systematic palpation.
2.3 Laboratory examination
Hematological analysis revealed erythrocytopenia (RBC count 2.56×10¹²/L) with significant anemia (hemoglobin 78.00 g/L, hematocrit 25.3%). Biochemical profile demonstrated hypoproteinemia (total protein 50.0 g/L) characterized by hypoalbuminemia (albumin 34.9 g/L) and hypoglobulinemia (globulin 15.1 g/L). Fecal occult blood testing showed positivity. Serum tumor markers remained within normal detection limits.
2.4 Endoscopy
The patient underwent comprehensive colorectal endoscopy which demonstrated no significant abnormalities. Subsequent esophagogastroduodenoscopy (EGD) revealed unremarkable findings, with successful deployment of a wireless capsule endoscopy device in the descending duodenum. At the 4-hour post-insertion interval, capsule imaging identified a gray-brown irregular mass along the ileal wall (Figure 1). The device successfully traversed the ileocecal valve 5 hours after deployment.
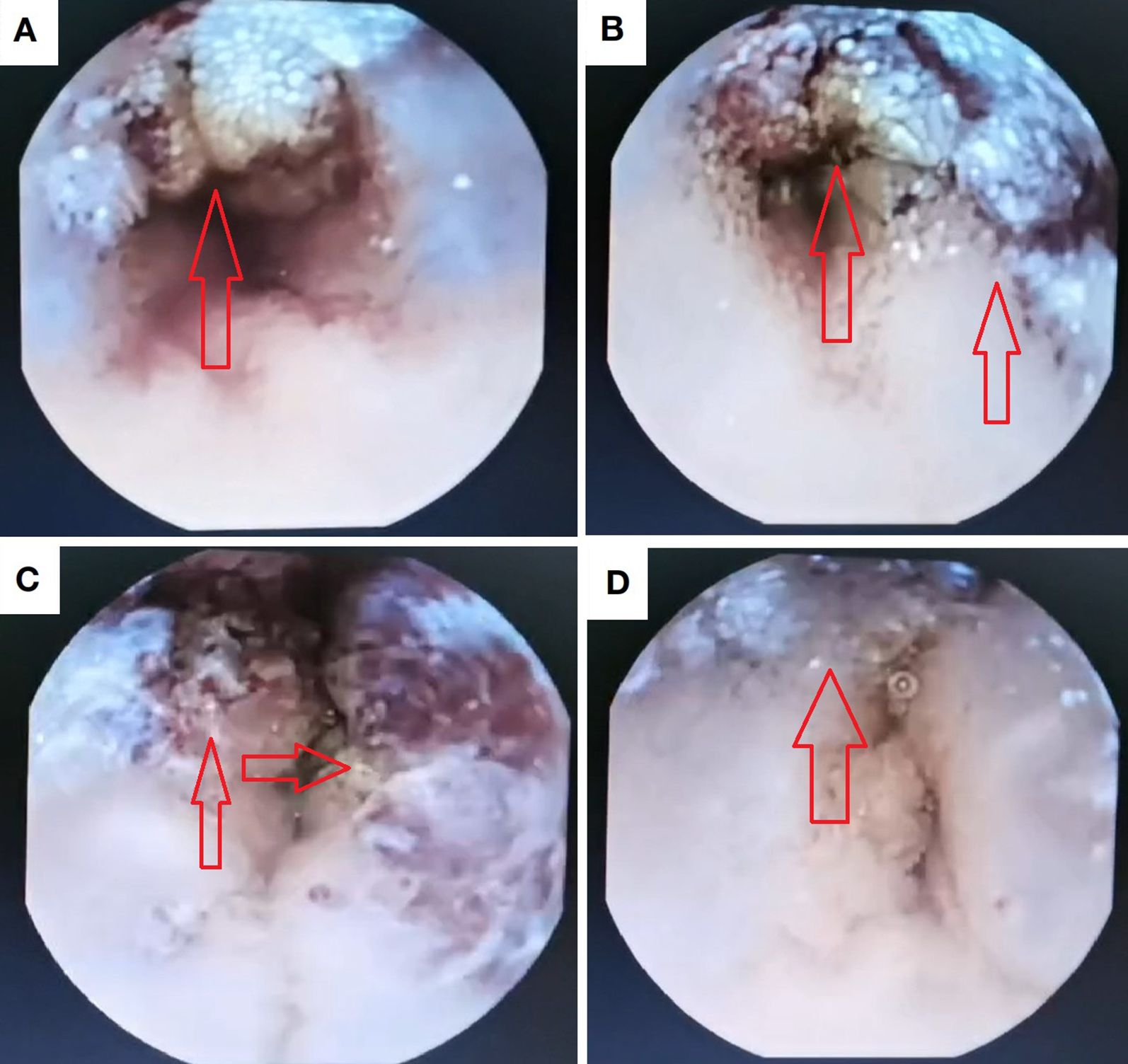
Figure 1. Intraluminal lesion identified via capsule endoscopy. (a, b) Proximal margins of the lesional bowel segment; (c) Intraluminal compartment within the pathological intestine; (d) Distal margins of the involved intestinal segment.
2.5 Imaging examination
Abdominal ultrasonography(US) revealed no significant abnormalities. Technetium-99m scintigraphy demonstrated absence of abnormal radiotracer accumulation in the abdominal region. Contrast-enhanced CT imaging identified a 65-mm segmental circumferential mural thickening in the distal ileum, located superior to the right aspect of the urinary bladder. The thickened bowel wall measured up to 10 mm in maximal cross-sectional diameter, demonstrating a precontrast attenuation value of 35 Hounsfield units (HU) with punctate calcification and mild enhancement post-contrast administration. Luminal narrowing was noted without proximal bowel dilatation. Perienteric fat planes remained preserved (Figure 2).
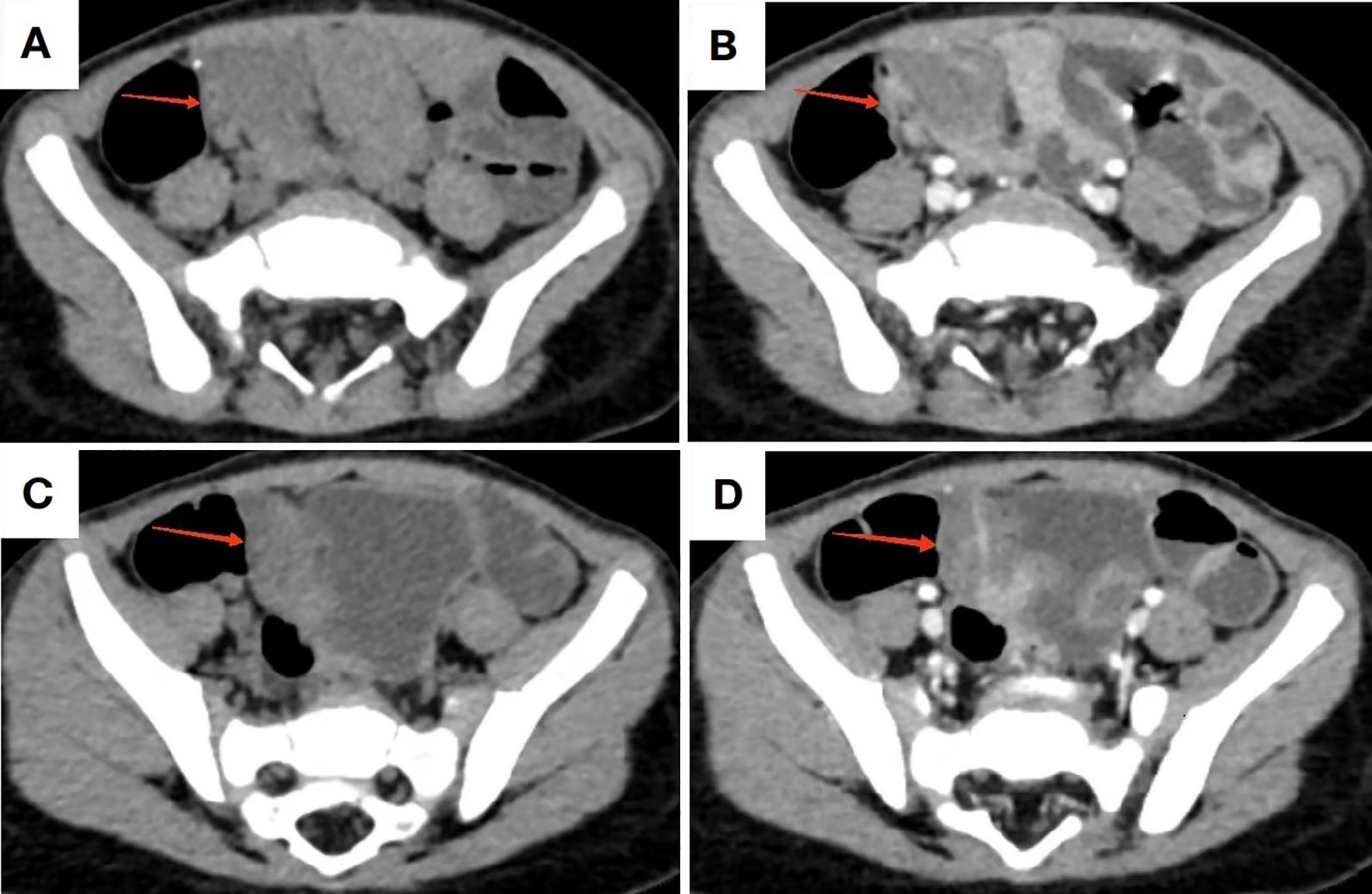
Figure 2. CT imaging of the lesion. (A, C) Non-contrast CT demonstrates bowel wall thickening in the distal ileum with minimal calcifications; (B, D) Contrast-enhanced CT reveals mild to moderate enhancement of the pathological segment.
2.6 Surgical procedure
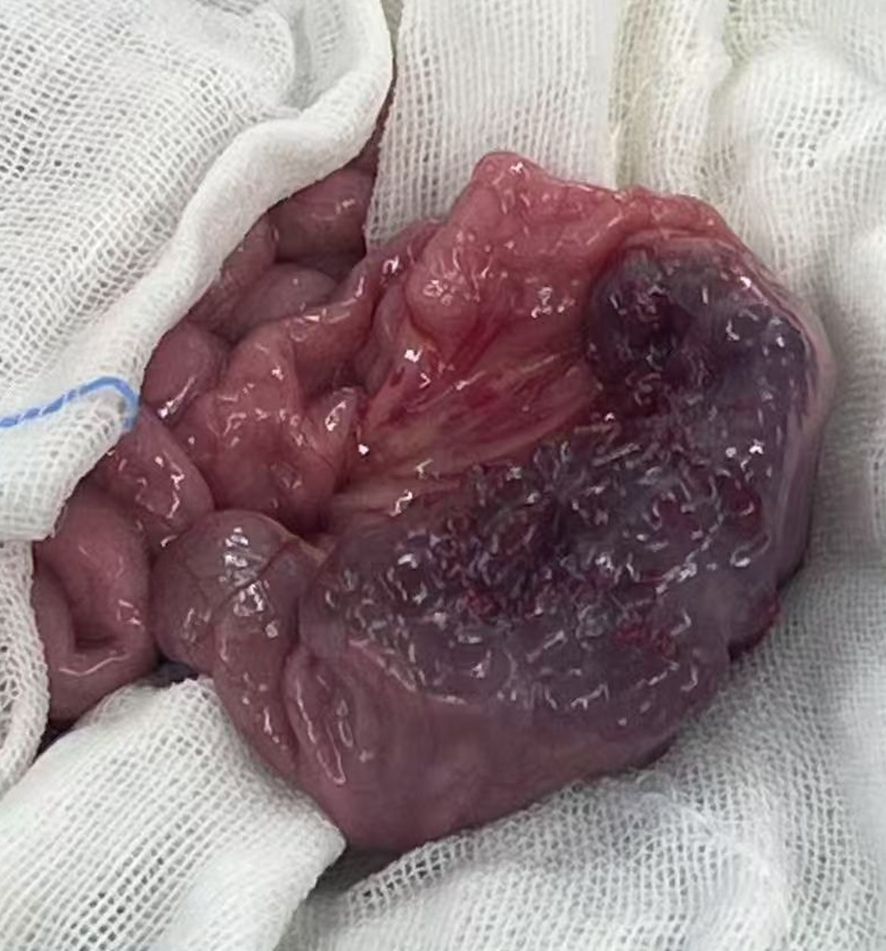
Based on the patient’s clinical history, physical examination, and ancillary investigations, a provisional diagnosis of small intestinal neoplasm was established, prompting single-port laparoscopic surgical intervention. Following successful anesthesia induction, the patient was positioned in the supine position with standard antiseptic preparation and draping. A 4-cm vertical transumbilical incision was created, through which a disposable wound retractor fixation system was deployed. The single-port laparoscopic platform and articulated laparoscopic instruments were prepared. Intraoperative exploration identified a gray-brown irregular mass (3×3×6 cm) involving the ileal wall 95 cm proximal to the ileocecal junction. We exteriorized it through the single-port incision (Figure 3). After meticulous dissection and ligation of the mesenteric vasculature supplying the affected bowel segment, the surgical team resected the affected bowel segment with 3 cm macroscopic margins proximal and distal to the lesion. End-to-end intestinal anastomosis was performed using interrupted sutures under tension-free conditions. Following confirmation of satisfactory anastomotic integrity, the bowel segment was carefully reintroduced into the abdominal cavity. Prior to closure, systematic reinspection confirmed absence of intestinal torsion or active hemorrhage. The wound retractor system was removed, and the fascial layers were closed with absorbable sutures.
2.7 Pathological findings
Gross examination revealed a gray-brown slightly elevated lesion (4.5×2.5 cm) on the mucosal surface. The serosal aspect demonstrated an irregular gray-brown mass measuring 3×3×6 cm, exhibiting a spongy cut surface with reddish-brown fluid accumulation. Histopathological examination showed dilated lymphatic channels within the intestinal mucosa and adjacent tissues, lined by attenuated endothelial cells (Figure 4). Immunohistochemical staining demonstrated positive immunoreactivity for D2-40. Pathological Diagnosis: Cavernous lymphangioma of the small intestine (WHO Classification of Soft Tissue Tumors, 2020).
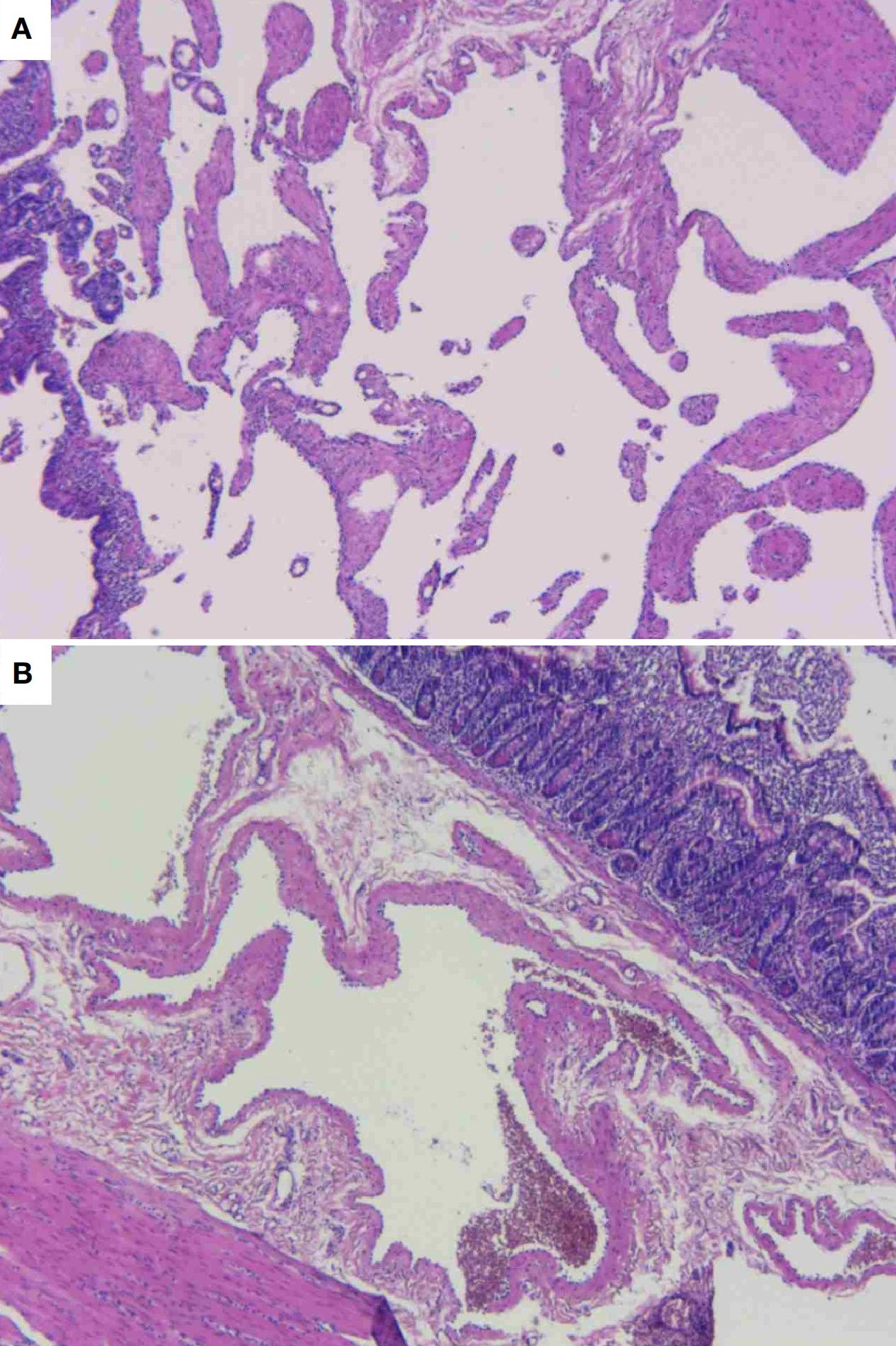
Figure 4. Histopathological photomicrograph of the lesion. (A) Dilated lymphatic channels exhibiting attenuated endothelial cells adherent to the vessel wall. (B) Dilated lymphatic channels in the mucosal and submucosal layers of the intestine, with associated muscular tissue in proximity.
2.8 Postoperative follow-up
The patient underwent scheduled follow-up assessments for two months postoperatively. Serial evaluations revealed normalization of dietary intake and bowel movements, with complete resolution of hematochezia. Serial hemoglobin measurements remained within normal physiological limits, demonstrating no significant declining trend (Figure 5).
3 Discussion
Lymphangioma is a malformation characterized by abnormal development of lymphatic system structures, which can be histopathologically classified into capillary lymphangioma, cavernous lymphangioma, and cystic lymphangioma (4). These lesions predominantly occur in the head and neck region (75%) and axilla (20%), with rare involvement of the gastrointestinal tract (<1%) (2) Typical clinical manifestations of small intestinal cavernous lymphangioma include hematochezia, intestinal obstruction, and abdominal pain (5). In this case report, the cavernous lymphangioma was localized to the ileum, 95 cm proximal to the ileocecal valve. Conventional diagnostic modalities, including esophagogastroduodenoscopy (EGD) and colonoscopy, failed to visualize the lesion. Additionally, cross-sectional imaging studies did not provide definitive guidance for clinical decision-making. However, wireless capsule endoscopy played a pivotal role in establishing the diagnosis and guiding subsequent management.
Prior to the development of wireless capsule endoscopy (WCE), evaluation of the small intestine was restricted to invasive procedures (e.g., intraoperative enteroscopy) or suboptimal diagnostic methods such as contrast radiography (6). Wireless capsule endoscopy revolutionized small bowel endoscopy by providing mucosal visualization with noninvasive method (7). Wireless capsule endoscopy is an advanced medical technology that employs a wireless, capsule-sized miniature camera to perform noninvasive visualization and diagnostic assessment of the gastrointestinal tract (8). Originally designed for evaluating small bowel pathologies, this innovation addresses the inherent limitations of conventional endoscopy, which cannot reliably access the entirety of the small intestine (9). The capsule progresses passively through the digestive tract via physiological peristalsis, capturing images at a rate of several frames per second via an integrated miniature camera (10). These images are transmitted wirelessly to an external recording device for subsequent clinician interpretation. Wireless capsule endoscopy is strongly recommended as a effective diagnostic method for patients of recurrent gastrointestinal hemorrhage that remain undiagnosed following conventional ancillary investigations (11).
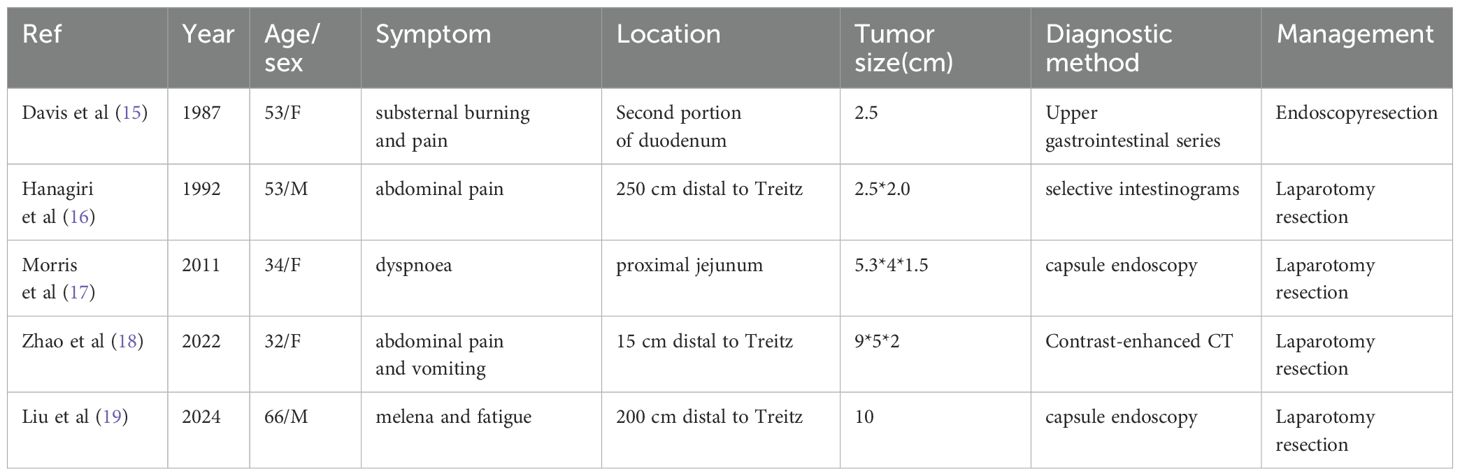
The management of lymphangioma is determined by factors including anatomic location, tumor dimensions, and invasiveness (12). Surgical resection of the affected bowel segment remains the first-line therapeutic approach for intestinal lymphangioma (13). Given the benign nature of lymphangiomas and their absence of distant metastasis to tissues or organs, localized excision of the diseased bowel typically achieves favorable long-term prognosis (13). Contemporary surgical practice has shifted toward minimally invasive and precision-oriented techniques, with both surgeons and patients sharing a priority of minimizing surgical scarring. The umbilicus has gained attention as an optimal site for scar-concealed incisions (14). While not the first reported case globally, cavernous lymphangioma predominantly occurs in the head and neck region, with ileal wall involvement representing an exceptionally rare presentation (Table 1). This case exemplifies an exemplary management paradigm by utilizing capsule endoscopy for diagnosis and single-port laparoscopy as the minimally invasive surgical modality, constituting a reference-worthy diagnostic and therapeutic framework for such rare entities. In the present case, we employed a single-incision laparoscopic exploration to localize the lesion within the small intestine, followed by ex vivo resection of the lymphangioma via a transumbilical approach. This method represents a synthesis of conventional surgical principles and laparoscopic advantages, optimizing both surgery accuracy and cosmetic outcomes.
4 Conclusions
In summary, small intestinal lymphangioma represents a rare etiology of gastrointestinal hemorrhage, frequently underdiagnosed due to limitations of conventional diagnostic modalities. Wireless capsule endoscopy is recommended as the first-line diagnostic method for small intestinal lymphangioma, though its cost-prohibitive nature often delays implementation.
Given the propensity of intestinal lymphangiomas to cause persistent hemorrhage, complete surgical excision provides the only pathway to definitive resolution. Therefore, expedited surgical intervention should be pursued upon clinical suspicion, in accordance with emergency surgery protocols.
Patient's perspective
The child’s parents stated that they had sought medical attention at six different hospitals prior to this consultation, undergoing both colonoscopy and gastroscopy without lesion detection. Their child received three blood transfusions totaling five therapeutic units. The parents expressed profound gratitude for the capsule endoscopy that successfully identified the lesion, and the subsequent single-port laparoscopic resection which they described as a “therapeutic milestone”.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Research Ethics Committee of Chengdu Women and Children’s Central Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin. Written informed consent was obtained from the minor(s)’ legal guardian/next of kin for the publication of any potentially identifiable images or data included in this article.
Author contributions
XY: Writing – original draft, Writing – review & editing. JG: Writing – review & editing. YZ: Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2025.1594332/full#supplementary-material
Abbreviations
EGD, Esophagogastroduodenoscopy; WCE, Wireless Wapsule Endoscopy; US Ultrasonography; CT, Computed Tomography; RBC, Red Blood Cells.
References
1. Roisman I, Manny J, Fields S, and Shiloni E. Intra-abdominal lymphangioma. Br J Surg. (1989) 76:485–9. doi: 10.1002/bjs.1800760519
2. Qi W, Yin CG, Wang S, Pan D, Chen XL, and Hu GD. Sclerotherapy combined with sirolimus for the treatment of complex cervicofacial lymphatic malformations in infants: avoiding the need for tracheostomy. J neurointerv Surg. (2025), 022908. doi: 10.1136/jnis-2024-022908
3. Sohrabi C, Mathew G, Maria N, Kerwan A, Franchi T, Agha RA, et al. The SCARE 2023 guideline: updating consensus Surgical CAse REport (SCARE) guidelines. Int J Surg. (2023) 109:1136–40. doi: 10.1097/JS9.0000000000000373
4. Tas B, Andac S, and Caglar A. Silhouette lymphangioma: an unknown macular form of cutaneous lymphangioma. Arch Iran Med. (2022) 25:456–9. doi: 10.34172/aim.2022.75
5. Huang MY, Chang HM, Gao HW, and Huang TY. Small intestinal lymphangioma with lymphangioectasia causing obscure gastrointestinal bleeding. Am J Gastroenterol. (2017) 112:413. doi: 10.1038/ajg.2016.505
6. ASGE Technology Committee, Wang A, Banerjee S, Barth BA, Bhat YM, Chauhan S, et al. Wireless capsule endoscopy. Gastrointest Endosc. (2013) 78:805–15. doi: 10.1016/j.gie.2013.06.026
7. Soffer S, Klang E, Shimon O, Nachmias N, Eliakim R, Ben-Horin S, et al. Deep learning for wireless capsule endoscopy: a systematic review and meta-analysis. Gastrointest Endosc. (2020) 92:831–839.e8. doi: 10.1016/j.gie.2020.04.039
8. Dey N, Ashour AS, Shi F, and Sherratt RS. Wireless capsule gastrointestinal endoscopy: direction-of-arrival estimation based localization survey. IEEE Rev BioMed Eng. (2017) 10:2–11. doi: 10.1109/RBME.2017.2697950
9. B S and P A. Recent developments in wireless capsule endoscopy imaging: Compression and summarization techniques. Comput Biol Med. (2022) 149:106087. doi: 10.1016/j.compbiomed.2022.106087
10. Cao Q, Deng R, Pan Y, Liu R, Chen Y, Gong G, et al. Robotic wireless capsule endoscopy: recent advances and upcoming technologies. Nat Commun. (2024) 15:4597. doi: 10.1038/s41467-024-49019-0
11. Bang CS, Lee JJ, and Baik GH. Computer-aided diagnosis of gastrointestinal ulcer and hemorrhage using wireless capsule endoscopy: systematic review and diagnostic test accuracy meta-analysis. J Med Internet Res. (2021) 23:e33267. doi: 10.2196/33267
12. Dalmády S, Csoma Z, Besenyi Z, Bottyán K, Oláh J, Kemény L, et al. New treatment option for capillary lymphangioma: bleomycin-based electrochemotherapy of an infant. Pediatrics. (2020) 146:e20200566. doi: 10.1542/peds.2020-0566
13. Yang J, Zhang Y, Kou G, and Li Y. Jejunum hemolymphangioma causing refractory anemia in a young woman. Am J Gastroenterol. (2020) 115:810. doi: 10.14309/ajg.0000000000000438
14. Zhao JJ, Syn NL, Chong C, Tan HL, Ng JYX, Yap A, et al. Comparative outcomes of needlescopic, single-incision laparoscopic, standard laparoscopic, mini-laparotomy, and open cholecystectomy: A systematic review and network meta-analysis of 96 randomized controlled trials with 11,083 patients. Surgery. (2021) 170:994–1003. doi: 10.1016/j.surg.2021.04.004
15. Davis M, Fenoglio-Preiser C, and Haque AK. Cavernous lymphangioma of the duodenum: case report and review of the literature. Gastrointest Radiol. (1987) 12:10–2. doi: 10.1007/BF01885092
16. Hanagiri T, Baba M, Shimabukuro T, Hashimoto M, Takemoto H, Inoue A, et al. Lymphangioma in the small intestine: report of a case and review of the Japanese literature. Surg Today. (1992) 22:363–7. doi: 10.1007/BF00308747
17. Morris-Stiff G, Falk GA, El-Hayek K, Vargo J, Bronner M, and Vogt DP. Jejunal cavernous lymphangioma. BMJ Case Rep. (2011), bcr0320114022 2011:bcr0320114022. doi: 10.1136/bcr.03.2011.4022
18. Zhao N, Fu Y, Wang Z, An Q, and Jia W. Case report: Submucosal cavernous lymphangioma causing jejuno-jejunal intussusception in an adult. Front Surg. (2022) 9:953840. doi: 10.3389/fsurg.2022.953840
Keywords: cavernous lymphangioma, wireless capsule endoscopy, small intestinal, pediatric, single-incision laparoscopic
Citation: Yang X, Guan J and Zhang Y (2025) Pediatric small intestine cavernous lymphangioma detected via wireless capsule endoscopy: a rare case report. Front. Oncol. 15:1594332. doi: 10.3389/fonc.2025.1594332
Received: 15 March 2025; Accepted: 22 July 2025;
Published: 11 August 2025.
Edited by:
Chi-Kong Li, The Chinese University of Hong Kong, ChinaReviewed by:
Akira Umemura, Iwate Medical University, JapanHany Ariffin, University of Malaya, Malaysia
Copyright © 2025 Yang, Guan and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yi Zhang, WmhhbmdZaTI3NTNAMTYzLmNvbQ==
 Xiaoyu Yang1,2
Xiaoyu Yang1,2 Junwen Guan
Junwen Guan Yi Zhang
Yi Zhang