- 1Department of Urology, Wuming Hospital of Guangxi Medical University, Nanning, Guangxi, China
- 2Department of Urology, The Second Affiliated Hospital of Guangxi Medical University, Nanning, Guangxi, China
- 3Department of Experimental Research, Guangxi Medical University Cancer Hospital, Nanning, Guangxi, China
- 4Key Laboratory of Early Prevention and Treatment for Regional High-incidence Tumors, Ministry of Education Key Laboratory, Guangxi Medical University, Nanning, Guangxi, China
- 5University Engineering Research Center of Oncolytic & Nanosystem Development, Nanning, Guangxi, China
- 6Department of Urology, Guangxi Medical University Cancer Hospital, Nanning, Guangxi, China
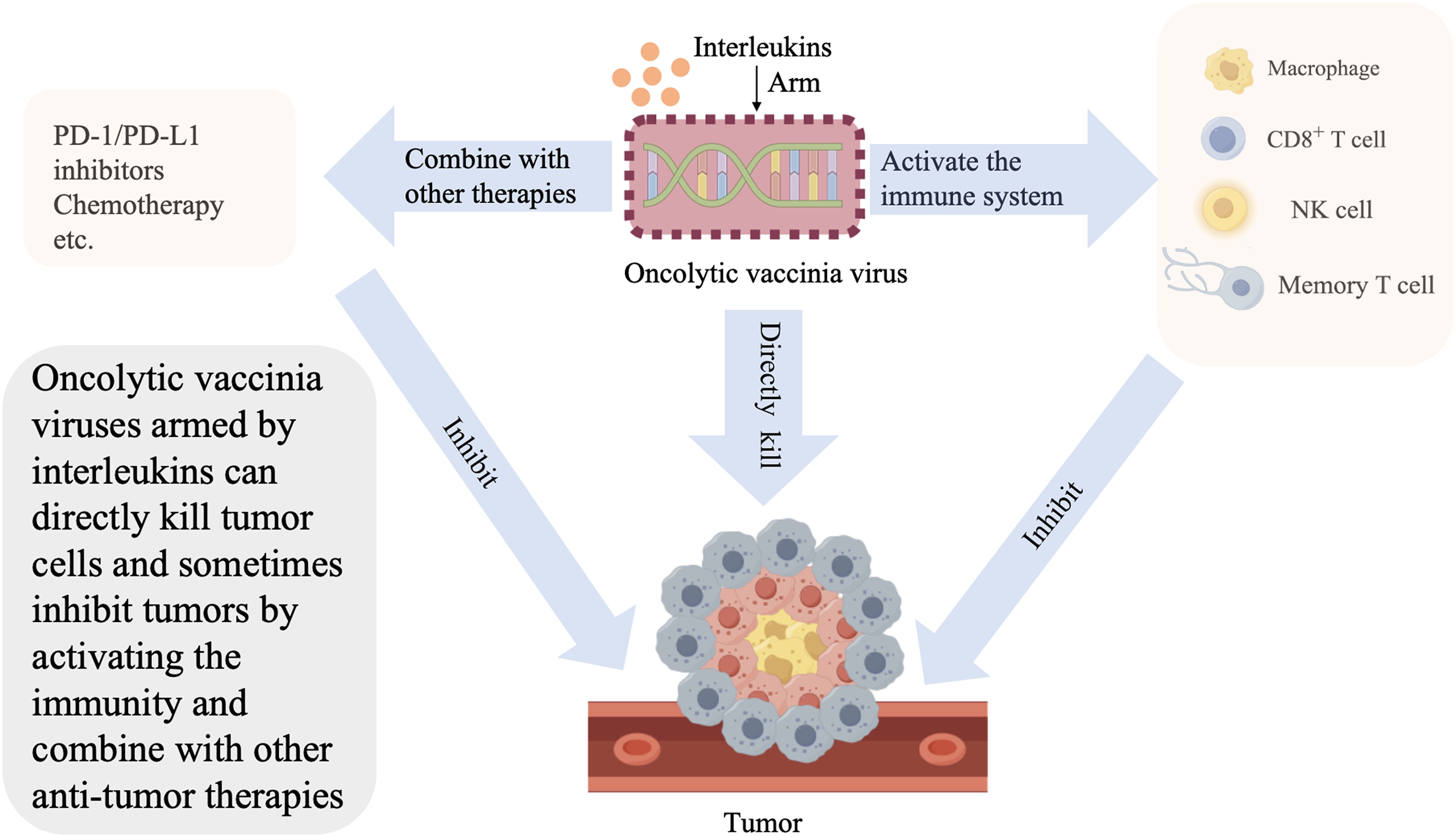
Oncolytic vaccinia viruses armed with interleukins represent a promising frontier in tumor therapy. Oncolytic vaccinia viruses express IL-2, IL-10, IL-12, IL-15, IL-21, IL-23, IL-24, and IL-36γ remodel the tumor microenvironment,enhance immune cell infiltration, suppress immunosuppressive elements and promot systemic antitumor immunity. Combinatorial strategies with chemotherapy, radiotherapy, metabolic modulators, immune checkpoint inhibitors, or natural compounds amplify therapeutic efficacy for tumors. In addition, we review the existing solutions to the problems of the immune clearance of virus, such as the use of inhibitors to prevent neutralizing antibodies from binding to the virus and the use of polymer encapsulation or mesenchymal stem cell loading. We also discussed Current directions include optimizing delivery systems, leveraging Artificial Intelligence for personalized designs of Oncolytic vaccinia virus inserted interleukins to guide the research in the future.
1 Introduction
Gene therapy is currently regarded as a promising strategy for the treatment of advanced malignant tumors. In this treatment modality, tumor cells can be killed directly by the therapeutic genes or cleared through the activation of immunity. Although wild oncolytic viruses (OVs) were initially utilized in cancer treatment in 1950, their use was discontinued because of low infection efficiency and poor anti-tumor effects (1). In 1991, Martuza et al. began to use engineered OVs with modified genomes (2), offering new promise for this type of therapy. The modified strategies of these OVs include the following: deleting unnecessary genes; weakening pathogenicity; enhancing the clearance of tumors; adding costimulatory molecules to the viral genome to improve antigen presentation and T cell activation, thereby enhancing the anti-tumor immune response; adding chemokines to the viral genome to improve the migration and infiltration of immune cells, as well as transform “cold” tumors into “hot” tumors; and adding cytokines to the viral genome to enhance the anti-tumor immune response (3). Therefore, inserting exogenous genes into the genome is an effective way to modify OVs.
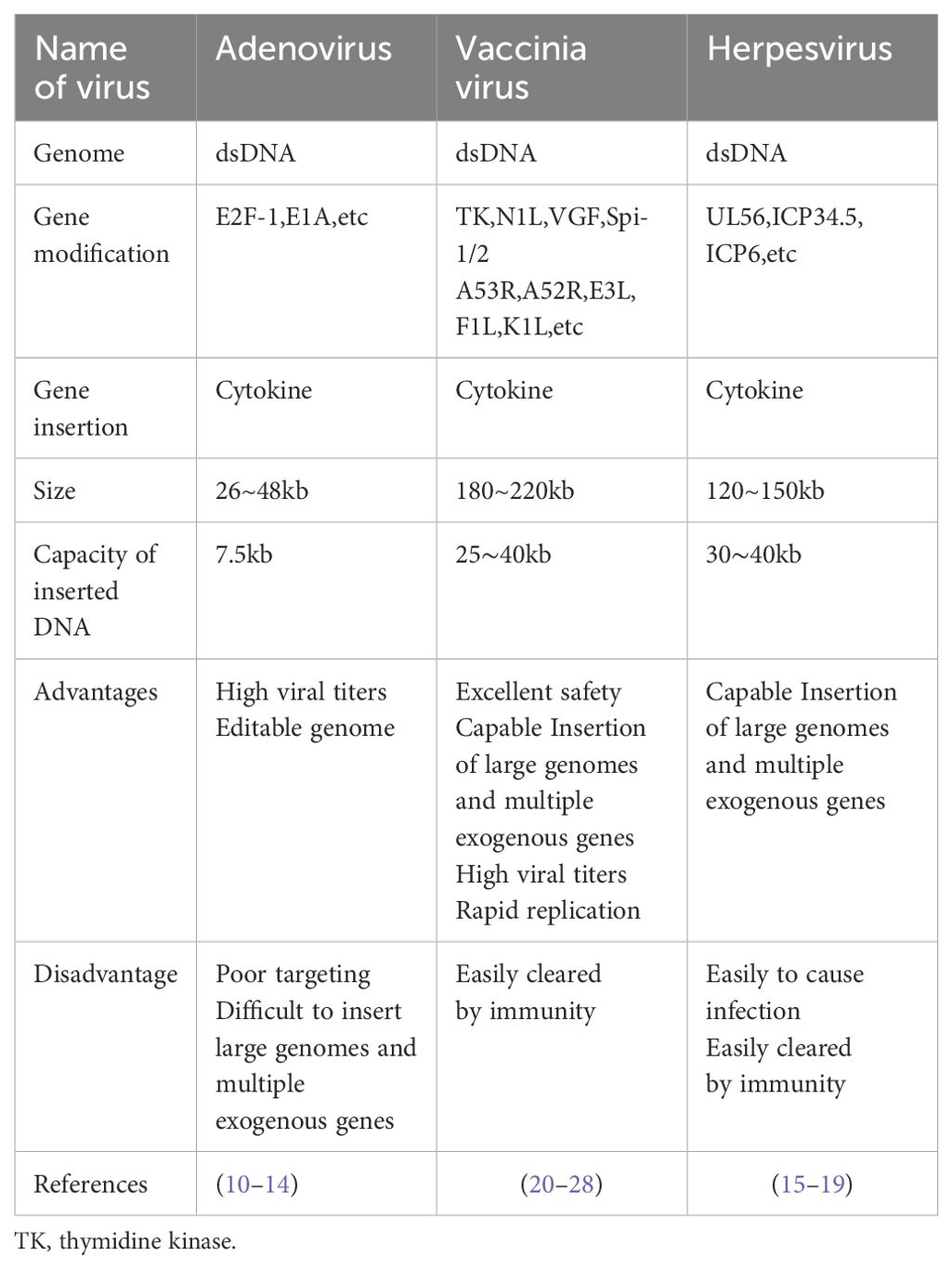
OVs include vaccinia virus, herpes simplex virus, adenovirus, coxsackievirus, measles virus, and vesicular stomatitis virus. Gene insertion into RNA viruses (i.e., coxsackievirus, measles virus, and vesicular stomatitis virus) is difficult (4, 5). DNA viruses (i.e., herpes simplex, adenovirus, and vaccinia) can be modified by exogenous genes (6).Interleukins are widely present in the human body and profoundly affect tumor growth and metastasis. They have been found to remodel the tumor microenvironment (TME), promote the infiltration of immune cells, or directly kill tumor cells (7–9). Consequently, interleukins are used to arm OVs.
1.1 Limitations of herpesvirus/adenovirus modified by interleukins
Oncolytic adenovirus (OAd) is widely used with mature production technology and easy genome editing. Interleukins, along with other cytokines (chemokines, interferons, etc.), are used to modify OAd. However, the OAd genome is unable to accommodate multiple interleukins due to its small size (approximately 26–46 kb) (10). Due to the poor targeting of OAds, the carried interleukin is easily spread to the periphery. Consequently, the application of interleukin-modified OAds is limited. In addition, when OAd is attenuated, it reduces the immune response and virus replication (10–14).
Owing to its large genome, oncolytic herpes simplex virus (oHSV) has apparent advantages with regard to genetic modification. Several oHSVs have been applied to different types of cancer (15). Few studies have been conducted on modifying oHSV with interleukins, mainly focusing on interleukin-2 (IL-2),IL-12, IL-15, etc. (16–18). An oHSV expressing IL-2 (G47Δ-mIL2) enhances anti-tumor immune responses by releasing IL-2 locally. In a glioblastoma (GBM) mouse model, G47Δ-mIL2 significantly prolonged median survival without systemic IL-2 toxicity (18).Another important study by a Chinese team focused on VG161(an engineered oHSV that expresses IL-12, IL-15, IL-15Rα and a PD-1-PD-L1-blocking fusion protein) in the phase I clinical trial of refractory hepatocellular carcinoma showed that there was no dose-limiting toxicity in 44 treated patients with refractory hepatocellular carcinoma, and the main side effect was fever (86.4%). The objective response rate was 18.92% (7/37 cases had significant tumor reduction). The median progression-free survival was 2.9 months, and the overall survival was 12.4 months. The effect was even better in patients who had received prior immune checkpoint inhibitor therapy for more than 3 months, with median overall survival doubling (17.3 vs. 7.4 months). In addition, it was found that the necrosis and shrinkage of tumor lesions in the non-injected area were better than those in the injected area, suggesting that VG161 may exert anti-tumor effects by activating systemic immunity (19), In this study,VG161 was administered by intratumoral injection, but due to the widespread infection of herpes viruses in the population, it is still necessary to focus on the problem of immune clearance during systemic administration of oHSV.
1.2 The advantages of oncolytic vaccinia virus modified by interleukins
Oncolytic vaccinia virus (OVV) can accommodate large fragments of exogenous DNA for insertion without affecting its biological activity. Moreover, it offers genetic stability, rapid iteration with mature progeny produced in 6–8 h, a wide range of infectious forms, a variety of infected tumor cells, no need for a specific cell-surface receptor, great viability, and excellent clinical safety (20–26).OVVs can also directly lyse tumor cells, cause vascular damage, counteract the immune suppressive effects of the TME, and increase the infiltration of lymphocytes in the tumors. However, when used alone, unarmed OVVs have limited impact on tumors. In addition, they cannot effectively deal with tumor recurrence (26). Modification of OVVs involves the deletion of thymidine kinase to reduce its replication in normal cells and improve its targeting of tumor cells, deletion of NIL, VGF, Spi-1/2, A53R, A52R, E3L, F1L, K1L, etc., and insertion of exogenous genes (mainly cytokines) (26, 27), Interleukins are critical cytokines in arming OVVs (28).
The more complex effects of interleukins on immunity are gradually identified in studies. IL-2, IL-10, IL-33, and IL-21 can promote tumor growth and metastasis (29–34).However, the efficacy of IL-15, IL-8, and IL-17A/F, which have been utilized in clinical practice, is limited (35–38), Furthermore, IL-2 may also cause side effects, such as chills, high fever, capillary leakage, respiratory distress, liver and kidney damage, etc. (39).IL-2 demonstrated its therapeutic potential through persistent complete response in melanoma (40). Thus, the trend of research has shifted from interleukin monotherapy to the design of tumor-targeting fusion constructs aimed at improving efficacy and reducing systemic toxicities. Previous studies have shown that constructing a model of OVV expressing interleukins can significantly enhance the effects of anti-tumor immunity and confine interleukins to the TME, thus reducing the dose of interleukins and the unacceptable systemic toxicities. The combination of these two compensates for each other’s shortcomings. However, some problems, such as accelerating virus clearance, activating the signal transducer and activator of transcription 3 (STAT3) pathway, and recruiting regulatory T (Treg) cells, remain to be overcome.
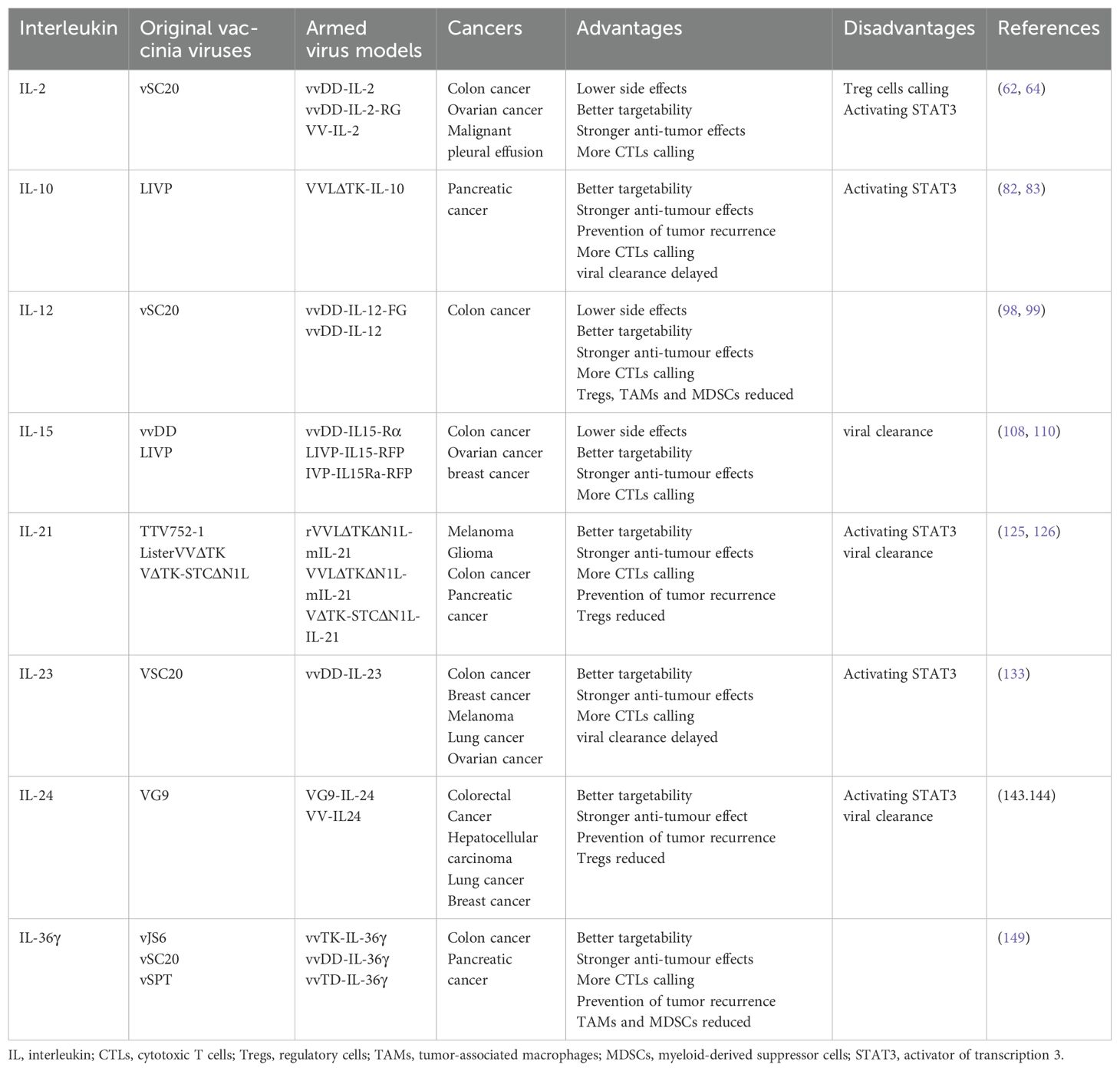
In this article, we reviewed the mechanism underlying the effects of different interleukins, outlined the application of interleukin-armed OVVs, and analyzed the current problems of armed viruses. Moreover, we examined the possibility of combining interleukin-armed OVVs with other immunotherapies to promote the development of such models for clinical application (Table 1).
2 Interleukin-armed oncolytic vaccinia viruses
2.1 IL-2
The pleiotropic cytokine IL-2 is produced mainly by CD4+T cells, while a few are made by CD8+T cells, natural killer (NK) cells, and dendritic cells (DCs). It is a potent T cell mitogen and activator, which can extend the function of T cells and improve the immune clearance of tumors (41, 42). IL-2 binds with its receptor IL-2R, mainly acting on lymphocytes to activate the cytoplasmic adaptors Janus kinase 1 (JAK1) and JAK3, as well as the downstream signal pathways (e.g., STAT5A/B, STAT3/1), which further promote the activation and proliferation of lymphocytes. The differentiation and proliferation of DCs can be upgraded (43). However, IL-2 acts as a double-edged sword in immunity. It is proven to be a highly effective immunosuppressant. Treg cells are incredibly dependent on IL-2. It promotes the maturation of thymic Treg cells and peripheral Treg cells, which play an immunosuppressive role (44–49). Further investigation found that Treg cells express an IL-2 receptor trimer (CD25, CD122, CD132), indicating that Tregs tend to have a higher affinity for IL-2 (50, 51).In contrast, cytotoxic T cells and NK cells express IL-2 receptor dimers with only moderate affinity (52).In other words, low doses of IL-2 tend to induce Treg cell maturation to suppress immunity (53, 54), and only high doses can cause CD8+T cells and NK cells to mature and kill tumor cells (55–58).
Phase 3 clinical trial showed that the median survival time of patients with advanced melanoma who received high doses of intravenous IL-2 reached 25.8 months compared with 18.9 months recorded in the ipilimumab group. Treatment with high-dose IL-2 exerted a better effect. However, grade ≥3 adverse events occurred in all patients who received high-dose intravenous IL-2. High doses of IL-2 may be associated with high rates of side effects, including chills, high fever, capillary leakage, arrhythmias, respiratory distress, and renal insufficiency (59, 60). Therefore, it is desirable to develop an appropriate delivery method for maintaining IL-2 into the TME (61).
Liu et al. used OVV to load IL-12 and constructed a secreted form of vvDD-IL-2 and a membrane-bound form of vvDD-IL-2-RG; vvDD-IL-2 was markedly more toxic than vvDD-IL-2-RG. While the latter shows better efficacy, the survival of mice with colon and ovarian cancer was significantly prolonged, the levels of tumor necrosis factor-α (TNF-α) (a known toxic mediator of IL-2) were reduced, the tumor-infiltrating CD8+ T cell population was increased, and the CD8+/Treg cell ratio was increased significantly. The levels of interferon-γ (IFN-γ), granzyme B perforin, and T helper 1 cell-type (Th1 cell-type) chemokine C-X-C motif chemokine ligand 9 (CXCL9) were increased, whereas those of transforming growth factor-β (TGF-β) and angiogenesis markers (CD105 and vascular endothelial growth factor [VEGF]) were decreased. These results suggested that vvDD-IL-2-RG enhanced anti-tumor immunity and improved survival in mice (62).
IL-2 combined with cisplatin is effective in treating primary and secondary malignant pleural effusion (63). Ekeke et al. constructed an OVV expressing yellow fluorescent protein (VV-YFP) and another expressing IL-2 (VV-IL-2). VV-IL-2 significantly reduced the tumor burden and improved the survival rate of malignant pleural disease mice, had no significant systemic toxicities compared with VV-YFP, and led to an increase in tumor-infiltrating lymphocytes, particularly CD8+T cells (64).
2.2 IL-10
IL-10,secreted by macrophages, T cell subsets and NK cells (65),; its role in tumors is controversial. Evidence indicates that IL-10 can help tumor cells escape immune surveillance (31, 32). High levels of IL-10 are found in the tumors and serum of numerous patients with malignancies (66–70). IL-10 promotes tumor growth and metastasis in many ways, including downregulation of antigen-presenting cells and major histocompatibility complex (MHC) expression in tumors to reduce antigen presentation (32, 71),promote naive CD4+ T cells to Th2 cells (72), and encourage the loss of E-calmodulin and increase of N-calmodulin (73), thus promoting tumor growth. IL-10 binds to the receptor IL-10R expressed by tumor cells, activating JAK1 and tyrosine kinase 2 (TYK2); this is followed by activation of STAT3 (74), which is involved in the growth and metastasis of tumors (75–78).
IL-10 promotes melanoma growth by stimulating angiogenesis and immunosuppression in mice (79). It also encourages the proliferation of glioma cells in vitro (80). Accordingly, IL-10 alone may not be effective against tumors. It has been reported that IL-10 enhances the inhibitory effect of OVV on tumor cells in mice (81). However, the experiment only applied IL-10 after the injection of OVV and did not use OVV as a vector. Inserting IL-10 into the OVV genome may be more promising.
Chard et al. constructed two models of OVV, namely VVLΔTK-IL-10 and VVLΔTK. In a mouse model of pancreatic cancer, 87.5% of mice treated with VVLΔTK-IL-10 produced complete tumor clearance versus 40% of mice treated with VVLΔTK. In the model of advanced pancreatic cancer in situ, the survival time of mice treated with VVLΔTK-IL-10 was significantly improved to 138.5 days, compared with 69.7 days after VVLΔTK treatment (P<0.01). Moreover, IL-10 reduced viral clearance and tumor recurrence, confirming the potential of VVLΔTK-IL-10. Although VVLΔTK-IL-10 treatment reduced the antiviral CD8+ T cell population, an increase in anti-tumor CD8+ T cells was observed at some time. This finding suggested that IL-10 reduced viral clearance, but did not suppress anti-tumor immunity. Research has shown that macrophages present viral antigen and IL-10 downregulates MHC-II of macrophages, inhibits the antigen-presenting ability of macrophages, decreases viral clearance, and increases tumor cell infection and tumor cell antigen release. Therefore, IL-10-armed OVV shows promise as a novel treatment for pancreatic cancer by enhancing tumor inhibition through the regulation of innate and adaptive immune responses (82, 83).
2.3 IL-12
IL-12 is a proinflammatory cytokine secreted by activated antigen-presenting cells (84, 85). IIt is a dimeric factor composed of IL-12B (P40) and IL-12A (P35) (84), which bind to the receptors of IL12 (IL-12Rβ1 and IL-12Rβ2) and activate TYK2 and JAK2, eventually leading to the phosphorylation of STAT4 and the production of IFN-γ. IL-12Rβ1 and IL-12Rβ2 are primarily expressed in NK cells and CD8+T cells, and the activation increases their proliferation (86, 87). In addition, IL-12 can induce Th1 differentiation, reducing the expansion of immunosuppressive cells such as tumor-associated macrophages and myeloid-derived suppressor cells (MDSCs) (88–90).Furthermore, IL-12 promotes the production of more IFN-γ by CD8+ T cells, exerting its inhibitory effect on tumors. It can inhibit tumor angiogenesis while upregulating MHC-I and MHC-II expression in tumor cells, thereby improving the effectiveness of their recognition and elimination (85, 91, 92).
However, even though IL-12 showed potent anti-tumor effects in pre-clinical trials, its impact in clinical trials was negligible at tolerable doses (93–95). In addition, systemic administration of IL-12 is extremely toxic (96, 97). Hence, the application of new delivery modes should be a top priority for research.
Ge et al. constructed membrane-bound OVV vvDD-IL-12-FG and secreted vvDD-IL-12. Mice treated with vvDD-IL-12 had significantly higher levels of IL-12 in tumors than those treated with vvDD-IL-12, and vvDD-IL-12 induced pulmonary and renal edema in mice. In contrast, vvDD-IL-12-FG did not exert these toxic effects and effectively immobilized IL-12 in the TME. Moreover, vvDD-IL-12-FG had the same anti-tumor effect. CD4+ and CD8+ T cells and IFN-γ were increased in tumors of vvDD-IL-12-FG-treated mice. In addition, vvDD caused an increase in MSDCs while vvDD-IL-12-FG inhibited MSDCs and Treg cells, leading to a significant decrease in the expression of TGF-β, cyclooxygenase-2 (COX-2), and angiogenesis markers (VEGF and CD105). This suggests that vvDD-IL-12-FG can deliver IL-12 to the tumor, allowing IL-12 to exert its efficacy without being limited by safety concerns (98).
AZD4820, an OVV that expresses IL-12, showed a complete response in 60% of mice treated with AZD4820 in a mouse model of colon cancer, while no mice showed a complete response (CR),after treatment with the original virus strain. AZD4820 treatment, in combination with PD-L1 blocking antibodies, enhanced tumor-specific T-cell immunity relative to monotherapy. These findings suggest that vaccinia virus delivery of IL-12, combined with immune checkpoint block, induces anti-tumor immunity in tumors that do not respond well to immune checkpoint inhibitors(ICIs) (99).
2.4 IL-15
As a member of the IL-2 family, IL-15 plays a similar role to that of IL-2. Although IL-15 can regulate innate and adaptive immunity, it causes unacceptable toxicity when administered systemically (100). The specific receptor of IL-15 (IL-15Rα) is secreted primarily by NK, T, and B cells (101).Binding of IL-15R to IL-15Rα produces the IL-15R/IL-15Rα complex, which binds to the IL-2Rβγ (IL-15Rβγ) expressed by effector cells (NK, T, and B cells), and further activates them to kill tumor cells (102). IL-15 appears less harmful to the TME than IL-2, as it fails to activate Tregs in a similar manner to IL-2, and occasionally inhibits Tregs (103).IL-15 may be more efficient than IL-2 in tumor suppression. However, IL-15 causes systemic toxicity and is ineffective in head and neck cancer and lung cancer, promoting tumor progression (104, 105). This observation may be related to the short half-life of IL-15 in vivo, elevation of the nflammatory cytokines IL-6 and IFN-γ, etc. (106). Application of IL-15 caused peripheral NK cell activation, which in turn induced IFN-γ expression, leading to systemic toxicity and tumor progression (100). OVVs can immobilize IL-15 in the TME. The short half-life and low biological activity of IL-15 in vivo can be addressed by applying the fully soluble IL-15Rα as an IL-15 agonist (107). Moreover, the effect of the IL-15Rα/IL-15 complex is relatively long-lasting.
Kowalsky et al. inserted the mouse IL-15-IL-15Ra fusion gene into OVV and named it vvDD-IL15-Rα. vvDD-IL15-Rα significantly inhibited colon and ovarian cancer in mice, which progressed rapidly in the vvDD group. Of the mice treated with vvDD-IL15-Rα, 80% were cured with no tumor recurrence; IFN-γ and tumor-specific CD8+T levels were increased. When rechallenged by tumor cells, the mice completely resisted recurrence. These findings indicate that vvDD-IL15-Rα can effectively stimulate anti-tumor adaptive immunity. However, vvDD accumulates more effectively in the tumor than vvDD-IL15-Rα (108). Considering that the researchers did not detect the total number of CD8+T cells and the number of virus-specific CD8+T cells, it is impossible to determine whether the superagonist proteins clear the virus by virus-specific CD8+T cells. This should be investigated in studies in the future. The antiviral effect of IL-15 may be dependent on the presence of IFN during OVV infection (109), and IFN is strongly upregulated during VVDD-IL15-Rα application (108). This may explain the mechanism through which superagonist proteins clear vvDD-IL15-Rα.
Shakiba et al. constructed armed OVVs based on IL-15 and its receptor, respectively, and inserted red fluorescence protein (RFP); the constructs were named LIVP-IL15-RFP and LIVP-IL15Ra-RFP, respectively. By simultaneous use of these two armed viruses, IL-15 can bind to IL-15R in vivo, form an IL-15/IL-15R complex to maintain the effect of IL-15 on the TME, and limit its toxicity. In mice with colon cancer and breast cancer, the combination of LIVP-IL15-RFP and LIVP-IL15Ra-RFP led to the lowest rate of tumor progression and the highest survival rate compared with any monotherapy. The LIVP-IL15-RFP group had an increase in C-C motif chemokine ligand 2 (CCL2), VEGF, and IL-4 inflammatory cytokines, suggesting an IL-15-based side effect. In contrast, these levels were within normal ranges in the combination group. The significant increase in IFN-α, IFN-γ,granulocyte-macrophage colony stimulating factor (GM-CSF), and TNF-α suggested a more effective anti-tumor effect in the combination group (110).
2.5 IL-21
As a member of the IL-2 family, IL-21 is a pleiotropic cytokine secreted by activated CD4+ T cells and NK cells that regulates lymphoid and myeloid cells (111–113). Its receptor IL-21R is expressed by various immune cells, such as T cells, B cells, NK cells, DCs, and intestinal epithelial cells (non-immune cells) (114). Therefore, IL-21 may have a broad and powerful effect on immunity. IL-21R is a trimer consisting of γc, a unique α-chain (CD25), and a β-chain (CD122). Binding of IL-21 to the IL-21Rα/γc complex activates the JAK1 and JAK3 pathways (115),. This is followed by phosphorylation of STAT1/3 downstream, and a slight activation of STAT5 (116). Sustained STAT3 activation leads to the proliferation of many tumor cells (75–78).The widespread expression of IL-21R in immune enables IL-21 to activate macrophage, DCs, NK cells, B cells, NK T cells, CD4+T cells, CD8+ T cells, Treg cells, Th17 cells, follicular helper T cells, etc. (78, 113, 117–122). From this perspective, IL-21 appears to have a complex role in the TME. Although current data show that IL-21 has acceptable toxicity (123, 124), and its efficacy against tumors is fine (112), its ability to recruit cytotoxic T cells alone remains insufficient. Thus, a combination with OVVs may potentiate the effectiveness of IL-21.
Kowalsky et al. constructed an IL-21-armed OVV rTTVΔTK-mIL21, which was more effective in suppressing injected and distant tumors than rTTVΔTK and phosphate-buffered saline (PBS). Treatment with rTTVΔTK-mIL21 prolonged the survival of melanoma and glioma mice and increased NK cells, CD3+ T cells, CD4+ T cells, and CD8+ T cells. In addition, the immune cells of distant tumors were increased, while viral infection was note detected. Tregs were inhibited to an extent which was equal to that noted in the PBS group. This suggests that rTTVΔTK-mIL21 induces more robust systemic immunity with less immune resistance (125).
Another study showed that VVLΔTKΔN1L-mIL-21, an OVV expressing IL-21, exhibited more potent anti-tumor effects than VVLΔTKΔN1L-RFP in a C57 mouse colon cancer model. The rate of complete tumor regression (CR) was 85.7% and 42.9% in the VVLΔTKΔN1L-mIL-21 group and VVLΔTKΔN1L-RFP group, respectively. Compared with the VVLΔTKΔN1L-RFP group, the VVLΔTKΔN1L-mIL-21 group had a slight increase in CD4+ and CD8+T cells and a significant increase in central memory T cells. These results suggested that VVLΔTKΔN1L-mIL-21 had a more potent inhibitory effect on tumor recurrence. Notably, VVLΔTKΔN1L-mIL-21 did not exhibit a more potent anti-tumor effect than VVLΔTKΔN1L-RFP, and replication was significantly reduced in a BALB/c mouse model of colon cancer. Thus, we can speculate the following: 1) VVLΔTKΔN1L has a poor therapeutic effect and affinity for colon cancer; 2) C57BL/6 mice and BALB/c mice have different genetic backgrounds and immune responses to the virus; 3) IL-21 may mediate antiviral immunity and lead to virus clearance; and 4) tumor heterogeneity is an essential factor affecting the efficacy of treatment. Therefore, it is crucial to develop personalized tumor treatment (126).
IL-21-armed OVV VΔTK-STCΔN1L-IL-21 was injected into subcutaneous, orthotopic and disseminated mouse models of pancreatic cancer, and phosphoinositide 3-kinase δ (PI3Kδ) inhibitor CAL-101 was used to inhibit the virus uptake by macrophages. Compared with VΔTK-STCΔN1L, CAL-101, and PBS, treatment with VΔTK-STCΔN1L-IL-21 did not reduce CD4+ cells. Nevertheless, it significantly increased CD8+T cells of circulation and tumors, as well as reduced CD8+T cells in the spleen and mobilized them to the tumors. The effector CD8+ T cells, central memory CD8+ T cells, and NK cells were increased in the circulation, spleen, and tumors. These observations suggested that VΔTK-STCΔN1L-IL-21 has the most apparent inhibitory effect on tumor recurrence and can activate innate and adaptive immunity (127).
2.6 IL-23
IL-23 belongs to the IL-12 cytokine family, which consists of IL-12B (P40) and IL-23P19 or IL-23A subunits. The IL-23 receptor is composed of IL-12Rβ1 and IL-23R. IL-12Rβ1 binds to TYK2 and induces STAT4 phosphorylation, which is critical for increased IFN-γ production and subsequent Th1 cell differentiation (128). IL-23R interacts with JAK2 to induce STAT3 phosphorylation and promote Th17 cell proliferation (129).
The role of IL-23 in the treatment of cancer appears to be controversial. It is generally thought that endogenous IL-23 promotes tumors, while exogenous IL-23 inhibits tumors (130). Endogenous IL-23 is secreted by myeloid cells, and mouse tumor cells do not secrete IL-23 (131)However, human tumor cells secrete a small amount of IL-23 (132). Considering that quantitative polymerase chain reaction and western blotting assays are mainly used, whether human tumors secrete bioactive IL-23 remains to be proven. Chen et al. constructed an OVV expressing IL-23, termed vvDD-IL-23, which has shown a better anti-tumor effect than vvDD in several mouse tumor models. In mouse models of colon cancer, vvDD-IL-23 induced IL-10 expression, inhibited antiviral immunity, upregulated the expression of Th1 chemokine, IFN-γ, TNF-α, perforin, IL-2, and GZMB, and regulated the TME compared with vvDD. The expression of immune checkpoint cytotoxic T-lymphocyte associated protein 4 (CTLA-4), PD-1, and PD-L1 continued to increase after vvDD-IL-23 treatment. This evidence provides a theoretical basis for the next application of armed OVVs in combination with ICIs. In advanced tumors, mice treated with vvDD-IL-23 developed long-lasting systemic anti-tumor immunity, significantly increasing CD4+ and CD8+ T cells in tumors, whereas Treg cells were unaffected (133).
2.7 IL-24
IL-24 is a member of the IL-10 cytokine family (134), produced by IL-4-inducible monocytes and Th2 lymphocytes. Previous studies have primarily focused on the killing effect of IL-24 on tumor cells. However, recent studies have shown that IL-24 also affects immunity. IL-24 has two receptors, namely IL-20R1/IL-20R2 and IL-22R1/IL-20R2 (135). Binding of IL-24 to these two receptors activates the JAK/STAT pathway, primarily STAT3. However, activation of the JAK/STAT pathway is not involved in the apoptotic signaling of tumors by IL-24. IL-24 focuses more on the secretion of its protein, which does not damage the normal cell. Its accumulation leads to an unfolding protein response and endoplasmic reticulum stress in tumor cells, ultimately inducing tumor cell apoptosis (136, 137). Thus, IL-24 has no significant side effects and does not cause a cytokine storm (138). IL-24 is thought to induce the expression of IFN-γ, TNF-α, and IL-6 (139)by lymphocytes, promote Th1-like cytokine secretion and the immunoreactivity of DCs, activate cytotoxic CD8+ T cells (140, 141), induce the proliferation of memory T cells, and reduce the number of Treg cells (140, 142).
Deng et al. constructed the OVV model VG9-IL-24. Compared with VG9-EGFP (the original strain of VG9 with insertion of the enhanced green fluorescent gene), VG9-IL-24 more significantly inhibited tumors and prolonged the survival of mice (143). Nonetheless, the effects of IL-24 on the TME were not thoroughly investigated in that study.
In a bilateral mouse model of colorectal tumor, the investigators focused on the effects of VG9-IL-24 on the TME, injecting VG9-IL-24 into the tumor on one side only. The results showed regression in both sides. IL-24 activated anti-tumor immunity and eliminated primary and distant tumors. Moreover, VG9-IL-24 can promote the secretion of IFN-γ and IL-6 at high levels, and TNF-α and IL-4 at low levels. These levels were significantly higher than those detected in the PBS and VGg-EGFP groups, suggesting that VG9-IL-24 induced specific anti-tumor immunity. In addition, IL-24 activates STAT3, which often predicts tumor progression, and VG9-IL-24 can counteract the tendency of STAT3 phosphorylation (144). However, the effects of VGg-IL-24 on various immune cells have not been clarified, and most of them are limited to the direction in which IL-24 can induce tumor cell apoptosis. The more far-reaching effects of IL-24-arming OVV on the immune system are worthy of further exploration.
2.8 IL-36γ
IL-36γ is a member of the IL-1 gene family. It binds to its receptors IL-36R (IL-1Rrp2) and IL-1RAcP to activate DCs, T cells, and NK cells (145, 146). IL-36γ alters the TME and promotes type 1 lymphocyte-mediated anti-tumor immunity (147). It has also been shown that IL-36γ induces colony formation, migration, and invasion of gastric cancer cell lines. In addition, the expression of IL-36γ was higher in primary gastric tumors compared with normal tissues (148).
Using IL-36γ alone in the treatment of tumors is controversial. The negative effects of IL-36γ on tumor immunity may be avoided by using it as an enhancer of OVVs. By inserting IL-36γ into three OVVs, Yang et al. constructed three models, namely vvTK-IL-36γ, vvDD-IL-36γ, and vvTD-IL-36γ. The results showed that vvTK-IL-36γ has a stronger anti-tumor effect than vvTK in a mouse model of colon cancer. Compared with vvTD, VVTD-IL-36γ significantly prolonged the survival of mice in models of pancreatic cancer and colon cancer. In the mouse model of colon cancer, the greatest numbers of CD8+ and CD4+ T cells were observed in the vvDD-IL-36γ group. Moreover, IL-36γ promoted the differentiation of naive CD8+ T cells into memory and effector T cells, and increased NK cells and DCs. Nevertheless, it decreased myeloid-derived suppressor cells and M2-like tumor-associated macrophages and increased tumor antigen-specific T cells (149). IL-36γ could enhance the activity of a variety of OVVs, and mice cured of colon cancer could cope with the dual challenge of colon cancer and lung cancer cells. These findings suggested that IL-36γ-armed virus could reverse the TME and activate systemic immunity.
3 Progress of interleukins together with other cytokines inserted into OVVs in tumor treatment
The hIL-7-VV (an OVV expressing human IL-7) and mIL-12-VV (an OVV expressing mouse IL-12) were constructed by Nakao et al. These OVVs did not show satisfactory anti-tumor effects when used alone. However, in the combination group, four of seven tumor-bearing mice achieved CR, and a greater number of CD8+ T cells, CD4+ T cells, NK T cells, and NK cells infiltrated the tumor. To reduce the dose of the virus, the researchers simultaneously inserted hIL-17 and mIL-12 into OVV to construct hIL-17/mIL-12-VV. This approach achieved excellent CR in various mouse tumor models. In a bilateral tumor model, injection of hIL-17/mIL-12-VV on one side resulted in tumor regression and immune cell infiltration on the other side with no virus detected. Based on these results, hIL-17/mIL-12-VV can inhibit metastatic tumors by activating systemic immunity, which has a positive significance for treating advanced tumors (150).
In another study, an OVV based on the VG9 strain that co-expressed GM-CSF and IL-24 was constructed, namely VG9-GMCSF-IL24. Compared with VG9 and PBS, VG9-GM-CSF-IL24 showed superior anti-tumor effect in breast cancer, melanoma, and colorectal cancer, and the tumors secreted the highest levels of IFN-γ, TNF-α, IL-4, and IL-6 (151).Thus far, two genes inserted in OVV appear to offer a significant advantage and better tumor suppression effect than a single one. However, previous studies found that VG9-IL-24 could also induce the secretion of IFN-γ, TNF-α, IL-4, and IL-6 (144). Hence, it is important to understand the role that GM-CSF plays in the immune system, the mechanism through which it exert its anti-tumor effect, and whether it impacts the anti-tumor effect of IL-24.In addition, delivery of IL-7 or IL-12 alone is ineffective. This appears to be different from the effect of AZD4820, which also expresses IL-12. AZD4820 resulted in a CR of 60%, while mIL-12-VV led to a CR of only 14%.Be used in two different tumor models certainly should be considered in this situation, but hIL-7-VV only achieved a CR of 0. Furthermore, the combination of IL-7 and IL-12 resulted in a CR of 57%, and the CR linked to hIL-17/mIL-12-VV was even better (150, 152), the synergistic effect of the two genes is surprising, considering that IL-7 does noy play a positive role in the TME (153).The mechanism by which IL-7 and IL-12 interact to reverse their roles in the TME warrants further investigation (Table 2, Figure 1).
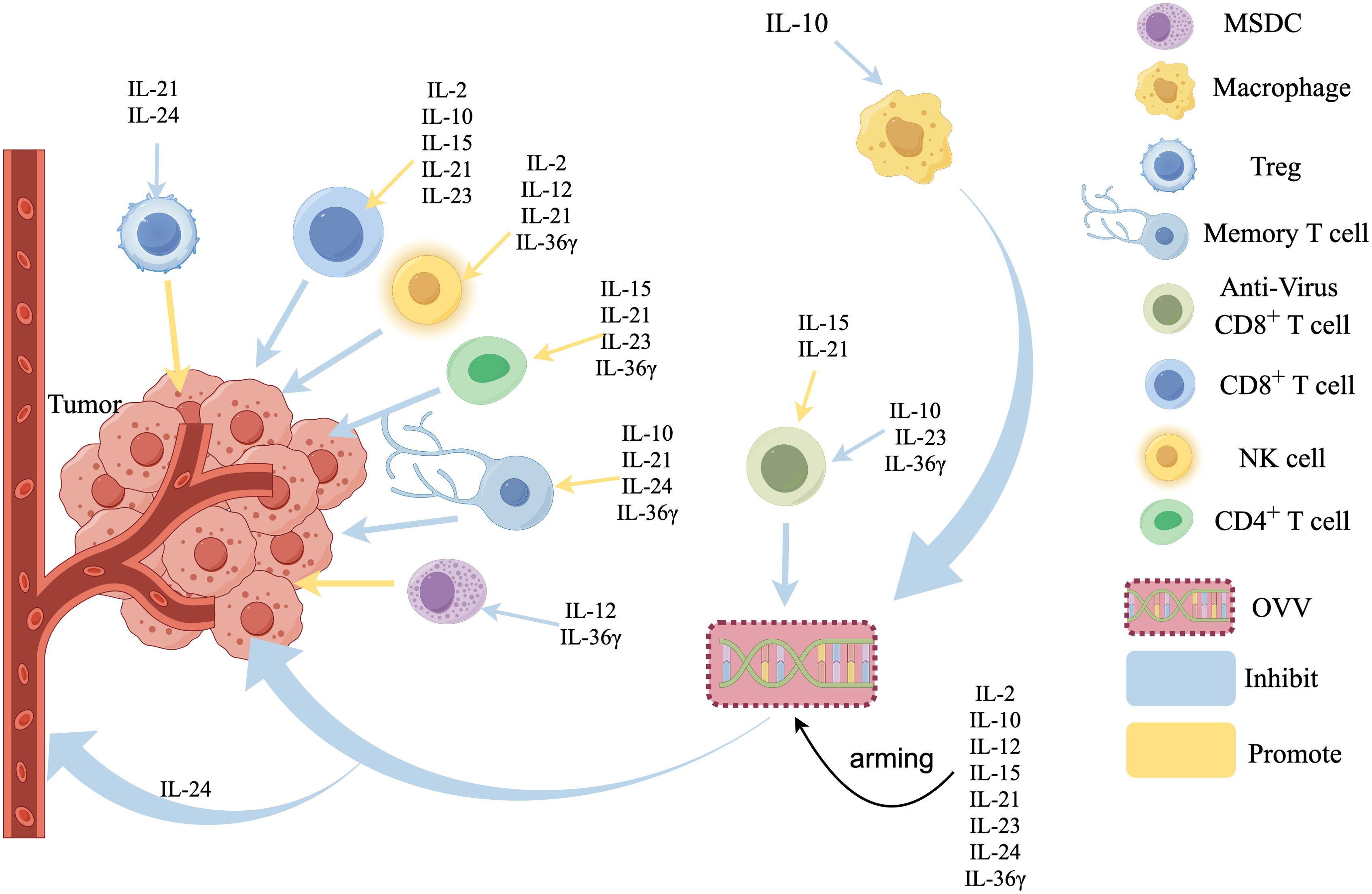
Figure 1. Effects of interleukin-armed OVVs on tumor microenvironment. IL-15, IL-21, IL-23, IL-36γ Promote the infiltration of CD4+ T cells in the tumors, IL-2, IL-12, IL-21, IL-36γ activate Natural Killer (NK) cells, IL-2, IL-10, IL-15, IL-21, IL-23 promote CD8+ T cells infiltration, IL-10, IL-23, IL-36γ inhibited anti-virus CD8+T cells, IL-15, IL-21 promote anti-virus CD8+T cells, IL-10, IL-21, IL-24, IL-36γ promote the production of Memory T cells, IL-21 and IL-24 inhibite regulatory cells (Tregs). IL-2 and IL-36γ inhibited Myeloid-derived suppressor cells (MDSCs), IL-10 inhibits Macrophages,IL-24 can directly inhibit the blood vessels.
4 Progress of interleukin-armed oncolytic vaccinia virus combined with other therapies in tumor treatment
4.1 Progress of interleukin-armed oncolytic vaccinia virus combined with chemotherapy in tumor treatment
Thrombocytopenia is one of the most common side effects of the chemotherapeutic drug mitomycin C, and IL-6 is thought to promote platelet production (154); Thus, IL-6 can alleviate the side effects of mitomycin C and enhance its efficacy. Investigators constructed a hyper-IL-6 fusion protein to enhance the activity and effect of IL-6. The hyper-IL-6 encoded vaccinia virus GLV-1h90 was constructed based on the OVV strain GLV-1h80. GLV-1h90 showed similar anti-tumor effects to those of GLV-1h80 in a mouse model of prostate cancer, indicating that hyper-IL-6 may not have anti-tumor activity. However, the GLV-1H90-vaccinated mice were more active and gained weight, meaning they were healthier. Although it was more effective than any single agent, GLV-1h90 did not exhibit superior anti-tumor effects than GLV-1h80 in combination with mitomycin C. The combination of GLV-1h90 and mitomycin C alleviated the thrombocytopenia and accelerated recovery. These results suggested that GLV-1h90 potentiated mitomycin C and attenuated its side effects (155).
4.2 Progress of oncolytic vaccinia virus expressing interleukin combined with immune checkpoint inhibitors in tumor therapy
Numerous interleukins amplify immune checkpoint blockade. Moreover, OVs are thought to effectively improve the TME, attract immune cells, and upregulate the expression of programmed cell death-ligand 1 (PD-L1) in tumor cells, thereby amplifying the blockade of the programmed cell death-1/PD-L1 (PD-1/PD-L1) monoclonal antibody. Therefore, interleukins, OVs, and immune checkpoint blockade can be combined to maximize anti-tumor effects (9, 108, 156).
Treatment with vvDD-IL-2-RG has a poor therapeutic effect in mice with high tumor burden, but increases the expression of PD-1, PD-L1, and CTLA-4 in tumors. Hence, vvDD-IL-2-RG can be combined with anti-PD-1 and anti-CTLA4 to improve the prognosis of mice with advanced tumors. In the advanced colon cancer model, a combination of vvDD-IL-2-RG and anti-PD-1 cured most of the mice, activated systemic immunity, and suppressed distant tumors. However, the vvDD-IL-2-RG combined with anti-CTLA4 was not effective enough. We hypothesized that because the action of anti-CTLA4 is different from anti-PD-1, anti-CTLA4 mainly acts on CD4+ T cells at the initiation stage of immune response (157). In contrast, anti-PD-1/PD-L1 mainly acts on exhausted T cells within the tumor (158). Thus, vvDD-IL-2-RG recruited T cells to re-infiltrate, possibly amplifying the effect of anti-PD-1 (62).
In a mouse model of pancreatic cancer, the combination of VL-21 and PD-1 blockade significantly inhibited tumor growth and improved the overall survival rate compared with VL-21 alone. Greater numbers of CD8+T cells were also accumulated in the tumors (127).In glioma, VVLΔTK-STCΔN1L-mIL-21 can remarkably increase the expression of PD-L1 in tumor cells and amplify the therapeutic effect of anti-PD-1. In tumor-bearing mice (159).
In another study, vvDD-IL15-Rα combined with anti-PD-1 completely cured all the mice bearing colon tumors and prolonged survival to >200 days (P<0.01). The survival of vvDD-IL15-Rα combined with anti-PD-1 was much more than vvDD-IL15-Rα and vvDD+anti-PD-1 group (108).
In tumor models unresponsive to anti-PD-1 or anti-CTLA4, hIL-17/mIL-12-VV combined with anti-PD-1 or anti-CTLA4 induced a markedly higher CR than viral monotherapy. These data suggest that intratumoral injection of hIL-17/mIL-12-VV has anti-tumor activity in both directly injected and distant tumors and sensitizes tumors to ICIs (150).
In a mouse model of colorectal cancer, AZD4820 combined with anti-PD-L1 increased tumor-specific CD8+T cells and tumor PD-L1 expression compared with the AZD4820 alone group; however, it failed to control tumor volume significantly. It is speculated that AZD4820, which carries IL-12, already has an excellent anti-tumor effect. However, the effect of enhancing the expression of CD8+T cells and tumor PD-L1 has guiding significance for tumor models that do not respond to ICIs (99).
ASP9801, TBio-6517 and TG6050 are three major OVVs expressing interleukins in clinical trials.
ASP9801 is a vaccinia virus that co-expresses IL-7 and IL-12, and the goal of Phase 1 clinical evaluation is to evaluate its safety and tolerability to determine the dosage for Phase 2 clinical trials. The antitumor activity, objective response rate, pharmacokinetics and viral clearance of ASP9801 as a single agent will be evaluated. And in combination with PD-1 monoclonal antibody pembrolizumab, which has not yet published phase 1 results but has completed enrollment in this trial (NCT03954067).
The Phase 1 trial of TBio-6517, a vaccinia virus that expresses IL-12, aims to determine phase 2 doses and evaluate the efficacy of TBio-6517 in patients with solid tumors through intratumoral or intravenous administration and in combination with pembrolizumab. Similarly, the trial (NCT04301011) completed recruitment but did not publish results.
TG6050, a vaccinia virus that expresses IL-12 and a small amount of Anti-CTLA4, is currently being recruited in a Phase 1 clinical trial (NCT05788926) to explore its dose and toxicity in advanced non-small cell lung cancer.
With the rapid development of ICIs, the great prospect of its combination with OVs is more and more concerned, and many preclinical trials have also proved that the effect of combination therapy is revolutionary. Combining interleukin-armed OVVs with ICIs may be the direction of future clinical trials.
4.3 Progress of the combination of interleukin-armed oncolytic vaccinia virus and natural compounds in tumor treatment
Luteolin is known to inhibit tumor growth. Wang et al. applied luteolin to enhance the inhibitory effect of VV-IL-24 on liver cancer. VV-IL-24 combined with luteolin inhibited tumor cells more than either VV-IL-24 or luteolin alone and did not affect normal cells. The tumor volume of mice treated with luteolin and VV-IL-24 was only 105 mm3, which was markedly smaller than that recorded in the VV-IL-24 group (1,088 mm3), luteolin group (3,080 mm3), and PBS group (3,053mm3). Hematoxylin and eosin staining confirmed that the tumor tissue in the combined treatment group were damaged more while the liver, kidney and spleen were not. These findings confirmed the better efficacy and safety of the combined treatment (160) (Table 3).
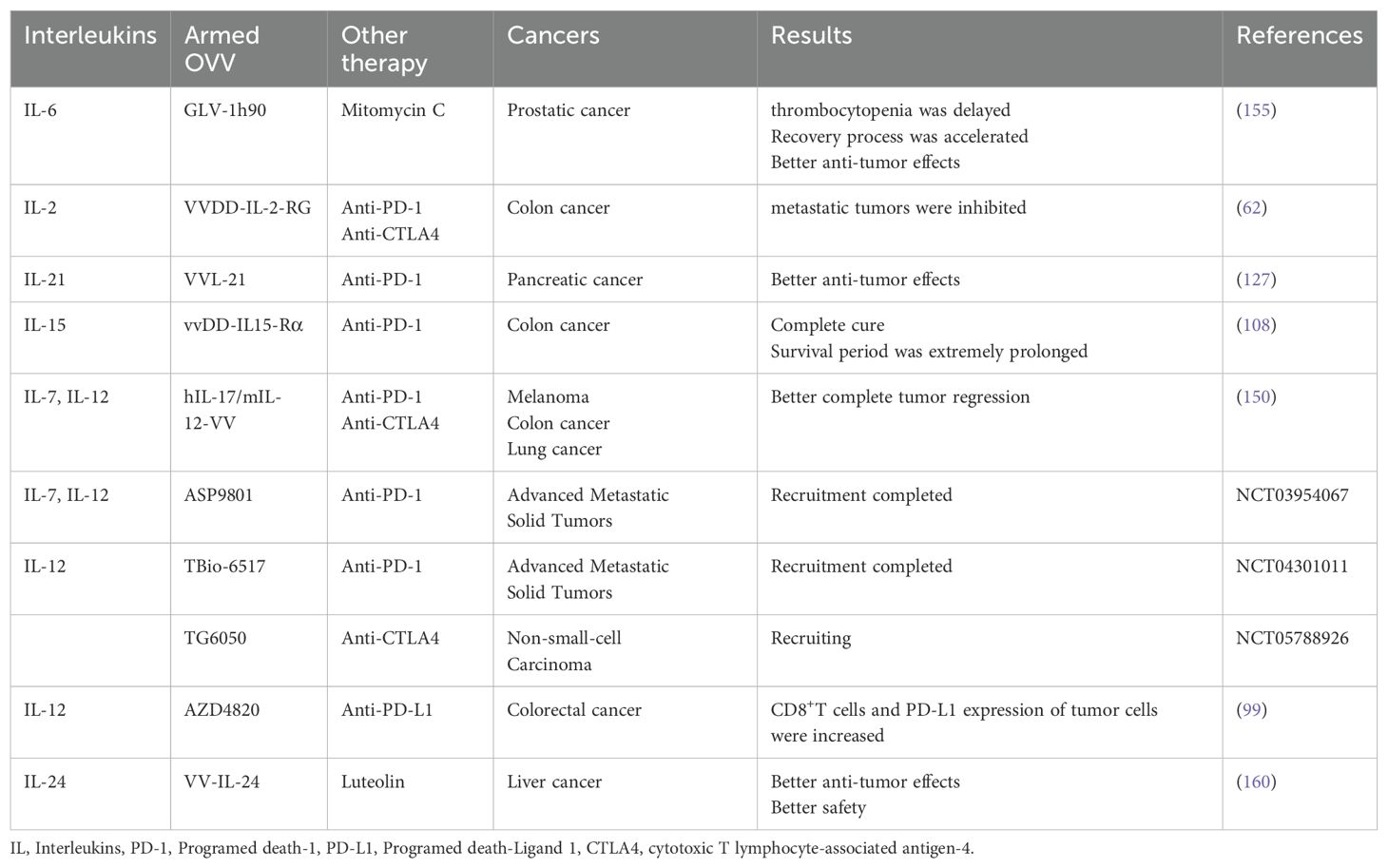
Table 3. Oncolytic vaccinia virus armed with interleukins combined with other therapies in tumour treatment.
5 Future perspective
Since the efficacy of OVs as monotherapy is limited; combination therapy is a key strategy to enhance therapeutic outcomes and represents a future trend. At present, we can use OVV as a suitable vector to deliver interleukins to tumors precisely through membrane binding, gene insertion, and other methods. This approach effectively preserves the influence of interleukins on the TME and limits the extremely strong systemic toxicities of many interleukins (161). Interleukins can promote the infiltration of tumor-infiltrating lymphocytes in tumors and upregulate the MHC expression in tumor cells for better surveillance by the immune system (125, 140, 141), thereby enhancing the effect of OVV on immunity. Interleukin-induced memory T cell properties also suppressed tumor recurrence (126, 127). Some interleukins can inhibit macrophages. Without affecting anti-tumor CD8+T cells, they reduce the expression of MHC on macrophages and inhibit antiviral CD8+T cells. This solves the problem of rapid clearance of OVV by the immunity (82, 83). Furthermore, interleukins can activate immunity to treat distant tumors (125),significantly improving the efficacy of intratumoral injection of OVs in advanced cancer (162–164).
In addition, numerous interleukins have been shown to enhance PD-1/PD-L1 blockade (162–164). Combination of OVVs with PD-1/PD-L1 blockade has also been reported (165, 166). It has been shown that the expression of IL-7 and IL-12 by vaccinia virus can improve the systemic sensitivity to PD-1/PD-L1 blockade (150). Experiments have shown that combining interleukin-armed OVV and PD-1/PD-L1 blockade is more effective and prolongs survival compared with any monotherapy (108, 125). We think that the combination of OVVs expressing interleukins with PD-1/PD-L1 blockade is a promising therapeutic direction.
Moreover, OVV expressing interleukins in combination with chemotherapy is also promising, GLV-1h90 counteracts mitomycin C platelet inhibition by IL-6 to exert a synergistic anti-tumor effect (155). Yu et al. found that cisplatin or gemcitabine potentiated the antitumor effect of GLV-1h68. OVV showed a favorable objective response rate and progression-free survival when combined with platinum-based chemotherapy in patients with platinum-resistant or platinum-refractory ovarian cancer, as shown by the results of a phase II nonrandomized clinical trial (NCT05281471) (167, 168). OVV can further sensitize tumor cells to chemotherapy by promoting the secretion of IFN-I and high-mobility group protein B1 (169).There are few related studies on OVV expressing interleukins combined with chemotherapy, which has huge research prospects.
Another commonly used means of treating tumors is radiotherapy, and studies have shown that radiation combined with OVV has shown considerable antitumor efficacy in several tumor models, such as glioblastoma and pancreatic cancer (170, 171). For the synergistic mechanism, a study by Chen et al. showed that radiotherapy in combination with OVVs can trigger tumor cell necrosis and alter macrophages by releasing damage-associated molecular pattern, generating robust antitumor immunity and enhancing antitumor efficacy (172). And all the above studies demonstrate the potential of radiotherapy and OVVs in combination. We believe that OVVs armed by interleukins combine with radiotherapy deserves further investigation.
Combinations of metabolic modulators with OVs are also promising. It can promote OVs replication and tumor killing by reprogramming tumor cell metabolism, such as enhancing glycolysis inhibition and regulating amino acid and nucleotide metabolism. Simultaneous regulation of immune cell metabolism (such as optimizing T cell glucose and lipid metabolism, NK cell antioxidant pathway) can enhance anti-tumor immune response. Metabolic remodeling combined with oncolytic virotherapy can synergistically enhance the efficiency of viral replication and immune activation, overcome the tumor immunosuppressive microenvironment, and provide a new strategy for cancer immunotherapy. However, it is necessary to balance the relationship between metabolic intervention and antiviral immunity to achieve clinical transformation (173).
A serious problem faced by OVVs is the limited delivery mode just like other OVs. Currently approved OVs are basically given intratumorally, which is undoubtedly not good for patients with advanced systemic metastasis and patients without solid tumors. Intravenous administration of OVs will predictably be necessary. Adenovirus and herpes viruses are widely found in nature, and neutralizing antibodies of both are often present in the human body, so the effect of intravenous administration is greatly reduced. In contrast, OVVs tend to produce neutralizing antibodies after a single intravenous injection which means the development of neutralizing antibodies has limited repeated intravenous administration of OVVs.
To address this problem, inhibitors can be used to block neutralizing antibody production. For example, COX-2 inhibitor can enhance OVVs retention and increase the possibility of repeated dosing by inhibiting neutralizing antibody (174). After prior administration of complement inhibitor,JX-594 (an OVV inserted into GM-CSF),showed an average 10-fold increase in blood infection titers (175).
In addition to the suppression of antiviral immune responses, the implementation of physical shielding strategies through viral encapsulation emerges as a complementary methodology to circumvent rapid immunological clearance and enhance the pharmacokinetic profile of intravenously administered OVVs. Hill et al. employed polyethylene glycol-cholesterol polymer coating to reduce neutralizing antibody binding. Murine studies showed that the polymer coating significantly prolonged the viral circulation time (5-fold increase in plasma drug concentration at 5 minutes), but the improvement in tumor accumulation was limited. This study provides a new strategy for optimizing intravenous delivery of OVV, but it needs to be combined with physical delivery methods to enhance tumor penetration (176). Besides, Nguyen et al. developed a platform based on allogeneic adipose-derived mesenchymal stem cells (AD-MSC). By loading OVVs into AD-MSC successfully protected the virus from inactivation by complement and neutralizing antibodies, and significantly improved virus release efficiency and anti-tumor activity in vitro and in animal models. The experiments showed that AD-MSC was more effective than naked virus in inhibiting tumor growth in both immunodeficient and immunocompetent mouse tumor models. In addition, AD-MSC can be used as a genetic engineering vector to insert therapeutic genes, such as fluorescent protein, into noncoding regions without affecting its ability to replicate. This platform provides a new strategy for overcoming the immune barrier of systemic delivery of OVs and has the potential for clinical translation (177).
6 Discussion
According to the available evidence, key areas of this field require improvement. Firstly, the preparation process and drug delivery system of the virus should be further optimized to improve its efficacy and reduce side effects. Secondly, a deeper understanding of the mechanisms of virus-host cell interactions is needed to more efficiently control their targeting and safety. In addition, challenges remain regarding the application of OVV armed with interleukins to clinical practice. Firstly, additional long-term safety and efficacy data are needed to support its widespread clinical use. Secondly, the high research and production costs are also important factors hindering its widespread application. A multi-faceted collaboration between policymakers, researchers, pharmaceutical companies, and others is necessary to overcome these challenges.
Based on several studies, we think that the combination of interleukin-modified OVVs with other tumor treatment modalities may attract considerable attention in the future. Interleukins have a very wide range of functions, such as recruiting various immune cells in tumors, enhancing PD-1 inhibitors, countering the side effects of chemotherapy, and activating the systemic immunity. When interleukin is limited to the TME by OVV, introducing another treatment (e.g., ICIs, chemotherapies, and other drugs) can be complementary in maximizing tumor inhibition and preventing tumor recurrence. Most currently ongoing clinical trials investigating armed viruses focus on this direction.
Because of the effects of interleukins and OVVs on the immune system, it was impossible to avoid triggering immune surveillance which and leads to clearance of the virus by immunity. This is the main limitation of the current application of this combination. Some delivery methods have been developed to reduce the clearance of armed viruses. The latest solutions are mainly through polymer encapsulation and the use of MSC as a new carrier to solve the problem of OVV system delivery, In addition, although OVVs armed by interleukins combined with a variety of anti-tumor treatments have good effect in different tumors, it needs huge time and economic cost to use traditional methods to screen one by one. We are concerned about the current use of Artificial Intelligence(AI). We believe that the application of AI technology to predict which interleukins is suitable for OVV modification will not only greatly expand the research ideas, but also improve the research efficiency and reduce the research cost, which is worthy of attention in the future.
Author contributions
MZ: Writing – review & editing, Writing – original draft. FH: Visualization, Writing – review & editing, Investigation, Writing – original draft. SL: Investigation, Visualization, Writing – review & editing, Writing – original draft. QW: Writing – review & editing, Funding acquisition, Writing – original draft, Conceptualization, Supervision. YT: Writing – review & editing, Writing – original draft, Supervision, Conceptualization, Funding acquisition.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This article is supported by the funding listed: National Natural Science Foundation of China (82360587, 82260484), Key Projects of Guangxi Natural Science Foundation (2024GXNSFDA010022), Guangxi Science and Technology Program (Key R&D Program) (GK AB25069088), Scientific Research and Technology Development Project, Nanning, China (20183203-3), The “139” Plan for Cultivating High-level and Key Talents in Guangxi Medicine, China (G201903036), Key research and development plan projects of scientific research and technology development plan in Wuming District of Nanning, China (20180120), Guangxi Natural Science Foundation Project, China (2018GXNSFAA138061), Guangxi Science and Technology Program under Grant No.AD25069077.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Kelly E and Russell SJ. History of oncolytic viruses: genesis to genetic engineering. Mol Ther. (2007) 15:651–9. doi: 10.1038/sj.mt.6300108
2. Martuza RL, Malick A, Markert JM, Ruffner KL, and Coen DM. Experimental therapy of human glioma by means of a genetically engineered virus mutant. Science. (1991) 252:854–6. doi: 10.1126/science.1851332
3. Lin D, Shen Y, and Liang T. Oncolytic virotherapy: basic principles, recent advances and future directions. Signal Transduct Target Ther. (2023) 8:156. doi: 10.1038/s41392-023-01407-6
4. Bradley S, Jakes AD, Harrington K, Pandha H, Melcher A, and Errington-Mais F. Applications of coxsackievirus A21 in oncology. Oncolytic Virother. (2014) 3:47–55. doi: 10.2147/ov.S56322
5. Bishnoi S, Tiwari R, Gupta S, Byrareddy SN, and Nayak D. Oncotargeting by vesicular stomatitis virus (Vsv): advances in cancer therapy. Viruses. (2018) 10(2):90. doi: 10.3390/v10020090
6. Cristi F, Gutiérrez T, Hitt MM, and Shmulevitz M. Genetic modifications that expand oncolytic virus potency. Front Mol Biosci. (2022) 9:831091. doi: 10.3389/fmolb.2022.831091
7. Briukhovetska D, Dörr J, Endres S, Libby P, Dinarello CA, and Kobold S. Interleukins in cancer: from biology to therapy. Nat Rev Cancer. (2021) 21:481–99. doi: 10.1038/s41568-021-00363-z
8. Setrerrahmane S and Xu H. Tumor-related interleukins: old validated targets for new anti-cancer drug development. Mol Cancer. (2017) 16:153. doi: 10.1186/s12943-017-0721-9
9. Tripodi L, Sasso E, Feola S, Coluccino L, Vitale M, Leoni G, et al. Systems biology approaches for the improvement of oncolytic virus-based immunotherapies. Cancers (Basel). (2023) 15(4). doi: 10.3390/cancers15041297
10. Mantwill K, Klein FG, Wang D, Hindupur SV, Ehrenfeld M, Holm PS, et al. Concepts in oncolytic adenovirus therapy. Int J Mol Sci. (2021) 22(19). doi: 10.3390/ijms221910522
11. Goradel NH, Mohajel N, Malekshahi ZV, Jahangiri S, Najafi M, Farhood B, et al. Oncolytic adenovirus: A tool for cancer therapy in combination with other therapeutic approaches. J Cell Physiol. (2019) 234:8636–46. doi: 10.1002/jcp.27850
12. Muthukutty P and Yoo SY. Oncolytic virus engineering and utilizations: cancer immunotherapy perspective. Viruses. (2023) 15(8). doi: 10.3390/v15081645
13. Suzuki K, Fueyo J, Krasnykh V, Reynolds PN, Curiel DT, and Alemany R. A conditionally replicative adenovirus with enhanced infectivity shows improved oncolytic potency. Clin Cancer Res. (2001) 7:120–6.
14. Wold WS and Toth K. Adenovirus vectors for gene therapy, vaccination and cancer gene therapy. Curr Gene Ther. (2013) 13:421–33. doi: 10.2174/1566523213666131125095046
15. Ma W, He H, and Wang H. Oncolytic herpes simplex virus and immunotherapy. BMC Immunol. (2018) 19:40. doi: 10.1186/s12865-018-0281-9
16. Nguyen HM, Guz-Montgomery K, and Saha D. Oncolytic virus encoding a master pro-inflammatory cytokine interleukin 12 in cancer immunotherapy. Cells. (2020) 9(2). doi: 10.3390/cells9020400
17. Ma R, Lu T, Li Z, Teng KY, Mansour AG, Yu M, et al. An oncolytic virus expressing IL15/IL15rα Combined with off-the-shelf EGFR-CAR NK cells targets glioblastoma. Cancer Res. (2021) 81:3635–48. doi: 10.1158/0008-5472.Can-21-0035
18. Bommareddy PK, Wakimoto H, Martuza RL, Kaufman HL, Rabkin SD, and Saha D. Oncolytic herpes simplex virus expressing IL-2 controls glioblastoma growth and improves survival. J Immunother Cancer. (2024) 12(4). doi: 10.1136/jitc-2024-008880
19. Shen Y, Bai X, Zhang Q, Liang X, Jin X, Zhao Z, et al. Oncolytic virus VG161 in refractory hepatocellular carcinoma. Nature. (2025) 641:503–11. doi: 10.1038/s41586-025-08717-5
20. Salzman NP. The rate of formation of vaccinia deoxyribonucleic acid and vaccinia virus. Virology. (1960) 10:150–2. doi: 10.1016/0042-6822(60)90015-5
21. Grosenbach DW and Hruby DE. Biology of vaccinia virus acylproteins. Front Biosci. (1998) 3:d354–64. doi: 10.2741/a280
22. Mercer J and Helenius A. Vaccinia virus uses macropinocytosis and apoptotic mimicry to enter host cells. Science. (2008) 320:531–5. doi: 10.1126/science.1155164
23. Smith GL and Moss B. Infectious poxvirus vectors have capacity for at least 25–000 base pairs of foreign DNA. Gene. (1983) 25:21–8. doi: 10.1016/0378-1119(83)90163-4
24. Kirn DH and Thorne SH. Targeted and armed oncolytic poxviruses: A novel multi-mechanistic therapeutic class for cancer. Nat Rev Cancer. (2009) 9:64–71. doi: 10.1038/nrc2545
25. Thorne SH. Immunotherapeutic potential of oncolytic vaccinia virus. Immunol Res. (2011) 50:286–93. doi: 10.1007/s12026-011-8211-4
26. Guo ZS, Lu B, Guo Z, Giehl E, Feist M, Dai E, et al. Vaccinia virus-mediated cancer immunotherapy: cancer vaccines and oncolytics. J Immunother Cancer. (2019) 7:6. doi: 10.1186/s40425-018-0495-7
27. Lv C, Su Q, Liang Y, Hu J, and Yuan S. Oncolytic vaccine virus harbouring the IL-24 gene suppresses the growth of lung cancer by inducing apoptosis. Biochem Biophys Res Commun. (2016) 476:21–8. doi: 10.1016/j.bbrc.2016.05.088
28. Yang X, Huang B, Deng L, and Hu Z. Progress in gene therapy using oncolytic vaccinia virus as vectors. J Cancer Res Clin Oncol. (2018) 144:2433–40. doi: 10.1007/s00432-018-2762-x
29. Tu S, Bhagat G, Cui G, Takaishi S, Kurt-Jones EA, Rickman B, et al. Overexpression of interleukin-1beta induces gastric inflammation and cancer and mobilizes myeloid-derived suppressor cells in mice. Cancer Cell. (2008) 14:408–19. doi: 10.1016/j.ccr.2008.10.011
30. Chabab G, Bonnefoy N, and Lafont V. IL-21 signaling in the tumor microenvironment. Adv Exp Med Biol. (2020) 1240:73–82. doi: 10.1007/978-3-030-38315-2_6
31. Hart KM, Byrne KT, Molloy MJ, Usherwood EM, and Berwin B. IL-10 immunomodulation of myeloid cells regulates a murine model of ovarian cancer. Front Immunol. (2011) 2:29. doi: 10.3389/fimmu.2011.00029
32. Steinbrink K, Jonuleit H, Müller G, Schuler G, Knop J, and Enk AH. Interleukin-10-treated human dendritic cells induce a melanoma-antigen-specific anergy in Cd8(+) T cells resulting in a failure to lyse tumor cells. Blood. (1999) 93:1634–42. doi: 10.1182/blood.V93.5.1634
33. Dwyer GK, D’Cruz LM, and Turnquist HR. Emerging functions of IL-33 in homeostasis and immunity. Annu Rev Immunol. (2022) 40:15–43. doi: 10.1146/annurev-immunol-101320-124243
34. Taniguchi S, Elhance A, Van Duzer A, Kumar S, Leitenberger JJ, and Oshimori N. Tumor-initiating cells establish an IL-33-Tgf-Β Niche signaling loop to promote cancer progression. Science. (2020) 369(6501). doi: 10.1126/science.aay1813
35. Waldmann TA. Cytokines in cancer immunotherapy. Cold Spring Harb Perspect Biol. (2018) 10(12). doi: 10.1101/cshperspect.a028472
36. Zepp JA, Zhao J, Liu C, Bulek K, Wu L, Chen X, et al. IL-17a-induced PLET1 expression contributes to tissue repair and colon tumorigenesis. J Immunol. (2017) 199:3849–57. doi: 10.4049/jimmunol.1601540
37. Vitiello GA and Miller G. Targeting the interleukin-17 immune axis for cancer immunotherapy. J Exp Med. (2020) 217(1). doi: 10.1084/jem.20190456
38. Bakouny Z and Choueiri TK. IL-8 and cancer prognosis on immunotherapy. Nat Med. (2020) 26:650–1. doi: 10.1038/s41591-020-0873-9
39. Vial T and Descotes J. Clinical toxicity of interleukin-2. Drug Saf. (1992) 7:417–33. doi: 10.2165/00002018-199207060-00004
40. Marabondo S and Kaufman HL. High-dose interleukin-2 (IL-2) for the treatment of melanoma: safety considerations and future directions. Expert Opin Drug Saf. (2017) 16:1347–57. doi: 10.1080/14740338.2017.1382472
41. Ma A, Koka R, and Burkett P. Diverse functions of IL-2, IL-15, and IL-7 in lymphoid homeostasis. Annu Rev Immunol. (2006) 24:657–79. doi: 10.1146/annurev.immunol.24.021605.090727
42. Sim GC and Radvanyi L. The IL-2 cytokine family in cancer immunotherapy. Cytokine Growth Factor Rev. (2014) 25:377–90. doi: 10.1016/j.cytogfr.2014.07.018
43. Ross SH and Cantrell DA. Signaling and function of interleukin-2 in T lymphocytes. Annu Rev Immunol. (2018) 36:411–33. doi: 10.1146/annurev-immunol-042617-053352
44. Malek TR. The biology of interleukin-2. Annu Rev Immunol. (2008) 26:453–79. doi: 10.1146/annurev.immunol.26.021607.090357
45. Fehérvari Z, Yamaguchi T, and Sakaguchi S. The dichotomous role of IL-2: tolerance versus immunity. Trends Immunol. (2006) 27:109–11. doi: 10.1016/j.it.2006.01.005
46. Antony PA, Paulos CM, Ahmadzadeh M, Akpinarli A, Palmer DC, Sato N, et al. Interleukin-2-dependent mechanisms of tolerance and immunity in vivo. J Immunol. (2006) 176:5255–66. doi: 10.4049/jimmunol.176.9.5255
47. Pol JG, Caudana P, Paillet J, Piaggio E, and Kroemer G. Effects of interleukin-2 in immunostimulation and immunosuppression. J Exp Med. (2020) 217(1). doi: 10.1084/jem.20191247
48. Hernandez R, Põder J, LaPorte KM, and Malek TR. Engineering IL-2 for immunotherapy of autoimmunity and cancer. Nat Rev Immunol. (2022) 22:614–28. doi: 10.1038/s41577-022-00680-w
49. Dinh TN, Kyaw TS, Kanellakis P, To K, Tipping P, Toh BH, et al. Cytokine therapy with interleukin-2/anti-interleukin-2 monoclonal antibody complexes expands CD4+CD25+Foxp3+ Regulatory T cells and attenuates development and progression of atherosclerosis. Circulation. (2012) 126:1256–66. doi: 10.1161/circulationaha.112.099044
50. Yu A, Zhu L, Altman NH, and Malek TR. A low interleukin-2 receptor signaling threshold supports the development and homeostasis of T regulatory cells. Immunity. (2009) 30:204–17. doi: 10.1016/j.immuni.2008.11.014
51. Cully M. Deal watch: IL-2 focus switches to stimulating T(Regs). Nat Rev Drug Discov. (2017) 16:595. doi: 10.1038/nrd.2017.171
52. Rickert M, Wang X, Boulanger MJ, Goriatcheva N, and Garcia KC. The structure of interleukin-2 complexed with its alpha receptor. Science. (2005) 308:1477–80. doi: 10.1126/science.1109745
53. Klatzmann D and Abbas AK. The promise of low-dose interleukin-2 therapy for autoimmune and inflammatory diseases. Nat Rev Immunol. (2015) 15:283–94. doi: 10.1038/nri3823
54. Yu A, Snowhite I, Vendrame F, Rosenzwajg M, Klatzmann D, Pugliese A, et al. Selective IL-2 responsiveness of regulatory T cells through multiple intrinsic mechanisms supports the use of low-dose IL-2 therapy in type 1 diabetes. Diabetes. (2015) 64:2172–83. doi: 10.2337/db14-1322
55. Fyfe G, Fisher RI, Rosenberg SA, Sznol M, Parkinson DR, and Louie AC. Results of treatment of 255 patients with metastatic renal cell carcinoma who received high-dose recombinant interleukin-2 therapy. J Clin Oncol. (1995) 13:688–96. doi: 10.1200/jco.1995.13.3.688
56. Fyfe GA, Fisher RI, Rosenberg SA, Sznol M, Parkinson DR, and Louie AC. Long-term response data for 255 patients with metastatic renal cell carcinoma treated with high-dose recombinant interleukin-2 therapy. J Clin Oncol. (1996) 14:2410–1. doi: 10.1200/jco.1996.14.8.2410
57. Hernandez R, Toomer KH, Põder J, Santos Savio A, Hsiung S, and Malek TR. Sustained IL-2R signaling of limited duration by high-dose MIL-2/mcd25 fusion protein amplifies tumor-reactive CD8(+) T cells to enhance antitumor immunity. Cancer Immunol Immunother. (2021) 70:909–21. doi: 10.1007/s00262-020-02722-5
58. Busse D, de la Rosa M, Hobiger K, Thurley K, Flossdorf M, Scheffold A, et al. Competing feedback loops shape IL-2 signaling between helper and regulatory T lymphocytes in cellular microenvironments. Proc Natl Acad Sci U S A. (2010) 107:3058–63. doi: 10.1073/pnas.0812851107
59. Rohaan MW, Borch TH, van den Berg JH, Met Ö, Kessels R, Geukes Foppen MH, et al. Tumor-infiltrating lymphocyte therapy or ipilimumab in advanced melanoma. N Engl J Med. (2022) 387:2113–25. doi: 10.1056/NEJMoa2210233
60. Kammula US, White DE, and Rosenberg SA. Trends in the safety of high dose bolus interleukin-2 administration in patients with metastatic cancer. Cancer. (1998) 83:797–805. doi: 10.1002/(SICI)1097-0142(19980815)83:4<797::AID-CNCR25>3.0.CO;2-M
61. Melero I, Berman DM, Aznar MA, Korman AJ, Pérez Gracia JL, and Haanen J. Evolving synergistic combinations of targeted immunotherapies to combat cancer. Nat Rev Cancer. (2015) 15:457–72. doi: 10.1038/nrc3973
62. Liu Z, Ge Y, Wang H, Ma C, Feist M, Ju S, et al. Modifying the cancer-immune set point using vaccinia virus expressing re-designed interleukin-2. Nat Commun. (2018) 9:4682. doi: 10.1038/s41467-018-06954-z
63. Han L, Jiang Q, Yao W, Fu T, and Zeng Q. Thoracic injection of low-dose interleukin-2 as an adjuvant therapy improves the control of the Malignant pleural effusions: A systematic review and meta-analysis base on Chinese patients. BMC Cancer. (2018) 18:725. doi: 10.1186/s12885-018-4581-5
64. Ekeke CN, Russell KL, Murthy P, Guo ZS, Soloff AC, Weber D, et al. Intrapleural interleukin-2-expressing oncolytic virotherapy enhances acute antitumor effects and T-cell receptor diversity in Malignant pleural disease. J Thorac Cardiovasc Surg. (2022) 163:e313–e28. doi: 10.1016/j.jtcvs.2020.11.160
65. Mannino MH, Zhu Z, Xiao H, Bai Q, Wakefield MR, and Fang Y. The paradoxical role of IL-10 in immunity and cancer. Cancer Lett. (2015) 367:103–7. doi: 10.1016/j.canlet.2015.07.009
66. Zhang L, Liu W, Wang X, Wang X, and Sun H. Prognostic value of serum IL-8 and IL-10 in patients with ovarian cancer undergoing chemotherapy. Oncol Lett. (2019) 17:2365–9. doi: 10.3892/ol.2018.9842
67. Mustea A, Könsgen D, Braicu EI, Pirvulescu C, Sun P, Sofroni D, et al. Expression of IL-10 in patients with ovarian carcinoma. Anticancer Res. (2006) 26:1715–8.
68. Mustea A, Braicu EI, Koensgen D, Yuan S, Sun PM, Stamatian F, et al. Monitoring of IL-10 in the serum of patients with advanced ovarian cancer: results from a prospective pilot-study. Cytokine. (2009) 45:8–11. doi: 10.1016/j.cyto.2008.10.019
69. Karstens KF, Kempski J, Giannou AD, Freiwald E, Reeh M, Tachezy M, et al. Systemic interleukin 10 levels indicate advanced stages while interleukin 17a levels correlate with reduced survival in esophageal adenocarcinomas. PloS One. (2020) 15:e0231833. doi: 10.1371/journal.pone.0231833
70. Boyano MD, Garcia-Vázquez MD, López-Michelena T, Gardeazabal J, Bilbao J, Cañavate ML, et al. Soluble interleukin-2 receptor, intercellular adhesion molecule-1 and interleukin-10 serum levels in patients with melanoma. Br J Cancer. (2000) 83:847–52. doi: 10.1054/bjoc.2000.1402
71. Sipak-Szmigiel O, Włodarski P, Ronin-Walknowska E, Niedzielski A, Karakiewicz B, Słuczanowska-Głąbowska S, et al. Serum and peritoneal fluid concentrations of soluble human leukocyte antigen, tumor necrosis factor alpha and interleukin 10 in patients with selected ovarian pathologies. J Ovarian Res. (2017) 10:25. doi: 10.1186/s13048-017-0320-9
72. Dobrzanski MJ, Rewers-Felkins KA, Samad KA, Quinlin IS, Phillips CA, Robinson W, et al. Immunotherapy with IL-10- and IFN-Γ-producing CD4 effector cells modulate “Natural” and “Inducible” CD4 TReg cell subpopulation levels: observations in four cases of patients with ovarian cancer. Cancer Immunol Immunother. (2012) 61:839–54. doi: 10.1007/s00262-011-1128-x
73. Park GB, Chung YH, and Kim D. Induction of galectin-1 by TLR-dependent PI3k activation enhances epithelial-mesenchymal transition of metastatic ovarian cancer cells. Oncol Rep. (2017) 37:3137–45. doi: 10.3892/or.2017.5533
74. Gemelli C, Zanocco Marani T, Bicciato S, Mazza EM, Boraschi D, Salsi V, et al. MafB is a downstream target of the IL-10/STAT3 signaling pathway, involved in the regulation of macrophage de-activation. Biochim Biophys Acta. (2014) 1843:955–64. doi: 10.1016/j.bbamcr.2014.01.021
75. Chen J, Wang J, Lin L, He L, Wu Y, Zhang L, et al. Inhibition of STAT3 signaling pathway by nitidine chloride suppressed the angiogenesis and growth of human gastric cancer. Mol Cancer Ther. (2012) 11:277–87. doi: 10.1158/1535-7163.Mct-11-0648
76. Wang T, Niu G, Kortylewski M, Burdelya L, Shain K, Zhang S, et al. Regulation of the innate and adaptive immune responses by stat-3 signaling in tumor cells. Nat Med. (2004) 10:48–54. doi: 10.1038/nm976
77. Huynh J, Chand A, Gough D, and Ernst M. Therapeutically exploiting STAT3 activity in cancer - using tissue repair as a road map. Nat Rev Cancer. (2019) 19:82–96. doi: 10.1038/s41568-018-0090-8
78. Fletcher JS, Springer MG, Choi K, Jousma E, Rizvi TA, Dombi E, et al. STAT3 inhibition reduces macrophage number and tumor growth in neurofibroma. Oncogene. (2019) 38:2876–84. doi: 10.1038/s41388-018-0600-x
79. García-Hernández ML, Hernández-Pando R, Gariglio P, and Berumen J. Interleukin-10 promotes B16-melanoma growth by inhibition of macrophage functions and induction of tumour and vascular cell proliferation. Immunology. (2002) 105:231–43. doi: 10.1046/j.1365-2567.2002.01363.x
80. Zhang Z, Huang X, Li J, Fan H, Yang F, Zhang R, et al. Interleukin 10 promotes growth and invasion of glioma cells by up-regulating KPNA 2 in vitro. J Cancer Res Ther. (2019) 15:927–32. doi: 10.4103/jcrt.JCRT_284_19
81. Kaufman HL, Rao JB, Irvine KR, Bronte V, Rosenberg SA, and Restifo NP. Interleukin-10 enhances the therapeutic effectiveness of a recombinant poxvirus-based vaccine in an experimental murine tumor model. J Immunother. (1999) 22:489–96. doi: 10.1097/00002371-199911000-00003
82. Chard LS, Lemoine NR, and Wang Y. New role of interleukin-10 in enhancing the antitumor efficacy of oncolytic vaccinia virus for treatment of pancreatic cancer. Oncoimmunology. (2015) 4:e1038689. doi: 10.1080/2162402x.2015.1038689
83. Chard LS, Maniati E, Wang P, Zhang Z, Gao D, Wang J, et al. A vaccinia virus armed with interleukin-10 is a promising therapeutic agent for treatment of murine pancreatic cancer. Clin Cancer Res. (2015) 21:405–16. doi: 10.1158/1078-0432.Ccr-14-0464
84. Vignali DA and Kuchroo VK. IL-12 family cytokines: immunological playmakers. Nat Immunol. (2012) 13:722–8. doi: 10.1038/ni.2366
85. Strasly M, Cavallo F, Geuna M, Mitola S, Colombo MP, Forni G, et al. IL-12 inhibition of endothelial cell functions and angiogenesis depends on lymphocyte-endothelial cell cross-talk. J Immunol. (2001) 166:3890–9. doi: 10.4049/jimmunol.166.6.3890
86. Zaharoff DA, Hance KW, Rogers CJ, Schlom J, and Greiner JW. Intratumoral immunotherapy of established solid tumors with chitosan/IL-12. J Immunother. (2010) 33:697–705. doi: 10.1097/CJI.0b013e3181eb826d
87. PeRussia B, Chan SH, D’Andrea A, Tsuji K, Santoli D, Pospisil M, et al. Natural killer (Nk) cell stimulatory factor or IL-12 has differential effects on the proliferation of TCR-alpha beta+, TCR-gamma delta+ T lymphocytes, and NK cells. J Immunol. (1992) 149:3495–502. doi: 10.4049/jimmunol.149.11.3495
88. Watkins SK, Egilmez NK, Suttles J, and Stout RD. IL-12 rapidly alters the functional profile of tumor-associated and tumor-infiltrating macrophages in vitro and in vivo. J Immunol. (2007) 178:1357–62. doi: 10.4049/jimmunol.178.3.1357
89. Steding CE, Wu ST, Zhang Y, Jeng MH, Elzey BD, and Kao C. The role of interleukin-12 on modulating myeloid-derived suppressor cells, increasing overall survival and reducing metastasis. Immunology. (2011) 133:221–38. doi: 10.1111/j.1365-2567.2011.03429.x
90. Kerkar SP, Goldszmid RS, Muranski P, Chinnasamy D, Yu Z, Reger RN, et al. IL-12 triggers a programmatic change in dysfunctional myeloid-derived cells within mouse tumors. J Clin Invest. (2011) 121:4746–57. doi: 10.1172/jci58814
91. Tugues S, Burkhard SH, Ohs I, Vrohlings M, Nussbaum K, Vom Berg J, et al. New insights into IL-12-mediated tumor suppression. Cell Death Differ. (2015) 22:237–46. doi: 10.1038/cdd.2014.134
92. Boehm U, Klamp T, Groot M, and Howard JC. Cellular responses to interferon-gamma. Annu Rev Immunol. (1997) 15:749–95. doi: 10.1146/annurev.immunol.15.1.749
93. Bajetta E, Del Vecchio M, Mortarini R, Nadeau R, Rakhit A, Rimassa L, et al. Pilot study of subcutaneous recombinant human interleukin 12 in metastatic melanoma. Clin Cancer Res. (1998) 4:75–85.
94. Motzer RJ, Rakhit A, Schwartz LH, Olencki T, Malone TM, Sandstrom K, et al. Phase I trial of subcutaneous recombinant human interleukin-12 in patients with advanced renal cell carcinoma. Clin Cancer Res. (1998) 4:1183–91.
95. Weiss GR, O’Donnell MA, Loughlin K, Zonno K, Laliberte RJ, and Sherman ML. Phase 1 study of the intravesical administration of recombinant human interleukin-12 in patients with recurrent superficial transitional cell carcinoma of the bladder. J Immunother. (2003) 26:343–8. doi: 10.1097/00002371-200307000-00006
96. Del Vecchio M, Bajetta E, Canova S, Lotze MT, Wesa A, Parmiani G, et al. Interleukin-12: biological properties and clinical application. Clin Cancer Res. (2007) 13:4677–85. doi: 10.1158/1078-0432.Ccr-07-0776
97. Cohen J. IL-12 deaths: explanation and a puzzle. Science. (1995) 270:908. doi: 10.1126/science.270.5238.908a
98. Ge Y, Wang H, Ren J, Liu W, Chen L, Chen H, et al. Oncolytic vaccinia virus delivering tethered IL-12 enhances antitumor effects with improved safety. J Immunother Cancer. (2020) 8(1). doi: 10.1136/jitc-2020-000710
99. Kurokawa C, Agrawal S, Mitra A, Galvani E, Burke S, Varshine A, et al. Mediation of antitumor activity by azd4820 oncolytic vaccinia virus encoding IL-12. Mol Ther Oncol. (2024) 32:200758. doi: 10.1016/j.omton.2023.200758
100. Guo Y, Luan L, Rabacal W, Bohannon JK, Fensterheim BA, Hernandez A, et al. IL-15 superagonist-mediated immunotoxicity: role of NK cells and IFN-Γ. J Immunol. (2015) 195:2353–64. doi: 10.4049/jimmunol.1500300
101. Mortier E, Advincula R, Kim L, Chmura S, Barrera J, Reizis B, et al. Macrophage- and dendritic-cell-derived interleukin-15 receptor alpha supports homeostasis of distinct CD8+ T cell subsets. Immunity. (2009) 31:811–22. doi: 10.1016/j.immuni.2009.09.017
102. Thi VAD, Jeon HM, Park SM, Lee H, and Kim YS. Cell-based IL-15:IL-15rα Secreting vaccine as an effective therapy for CT26 colon cancer in mice. Mol Cells. (2019) 42:869–83. doi: 10.14348/molcells.2019.0188
103. Ben Ahmed M, Belhadj Hmida N, Moes N, Buyse S, Abdeladhim M, Louzir H, et al. IL-15 renders conventional lymphocytes resistant to suppressive functions of regulatory T cells through activation of the phosphatidylinositol 3-kinase pathway. J Immunol. (2009) 182:6763–70. doi: 10.4049/jimmunol.0801792
104. Badoual C, Bouchaud G, Agueznay Nel H, Mortier E, Hans S, Gey A, et al. The soluble alpha chain of interleukin-15 receptor: A proinflammatory molecule associated with tumor progression in head and neck cancer. Cancer Res. (2008) 68:3907–14. doi: 10.1158/0008-5472.Can-07-6842
105. Seike M, Yanaihara N, Bowman ED, Zanetti KA, Budhu A, Kumamoto K, et al. Use of a cytokine gene expression signature in lung adenocarcinoma and the surrounding tissue as a prognostic classifier. J Natl Cancer Inst. (2007) 99:1257–69. doi: 10.1093/jnci/djm083
106. Waldmann TA, Dubois S, Miljkovic MD, and Conlon KC. IL-15 in the combination immunotherapy of cancer. Front Immunol. (2020) 11:868. doi: 10.3389/fimmu.2020.00868
107. Van den Bergh JM, Lion E, Van Tendeloo VF, and Smits EL. IL-15 receptor alpha as the magic wand to boost the success of IL-15 antitumor therapies: the upswing of IL-15 transpresentation. Pharmacol Ther. (2017) 170:73–9. doi: 10.1016/j.pharmthera.2016.10.012
108. Kowalsky SJ, Liu Z, Feist M, Berkey SE, Ma C, Ravindranathan R, et al. Superagonist IL-15-armed oncolytic virus elicits potent antitumor immunity and therapy that are enhanced with PD-1 blockade. Mol Ther. (2018) 26:2476–86. doi: 10.1016/j.ymthe.2018.07.013
109. Foong YY, Jans DA, Rolph MS, Gahan ME, and Mahalingam S. Interleukin-15 mediates potent antiviral responses via an interferon-dependent mechanism. Virology. (2009) 393:228–37. doi: 10.1016/j.virol.2009.07.030
110. Shakiba Y, Vorobyev PO, Yusubalieva GM, Kochetkov DV, Zajtseva KV, Valikhov MP, et al. Oncolytic therapy with recombinant vaccinia viruses targeting the interleukin-15 pathway elicits a synergistic response. Mol Ther Oncolytics. (2023) 29:158–68. doi: 10.1016/j.omto.2023.05.002
111. Ren HM, Lukacher AE, Rahman ZSM, and Olsen NJ. New developments implicating IL-21 in autoimmune disease. J Autoimmun. (2021) 122:102689. doi: 10.1016/j.jaut.2021.102689
112. Davis MR, Zhu Z, Hansen DM, Bai Q, and Fang Y. The role of IL-21 in immunity and cancer. Cancer Lett. (2015) 358:107–14. doi: 10.1016/j.canlet.2014.12.047
113. Coquet JM, Kyparissoudis K, Pellicci DG, Besra G, Berzins SP, Smyth MJ, et al. IL-21 is produced by NKT cells and modulates NKT cell activation and cytokine production. J Immunol. (2007) 178:2827–34. doi: 10.4049/jimmunol.178.5.2827
114. Mehta DS, Wurster AL, and Grusby MJ. Biology of IL-21 and the IL-21 receptor. Immunol Rev. (2004) 202:84–95. doi: 10.1111/j.0105-2896.2004.00201.x
115. Asao H, Okuyama C, Kumaki S, Ishii N, Tsuchiya S, Foster D, et al. Cutting edge: the common gamma-chain is an indispensable subunit of the IL-21 receptor complex. J Immunol. (2001) 167:1–5. doi: 10.4049/jimmunol.167.1.1
116. Zeng R, Spolski R, Casas E, Zhu W, Levy DE, and Leonard WJ. The molecular basis of IL-21-mediated proliferation. Blood. (2007) 109:4135–42. doi: 10.1182/blood-2006-10-054973
117. Zeng R, Spolski R, Finkelstein SE, Oh S, Kovanen PE, Hinrichs CS, et al. Synergy of IL-21 and IL-15 in regulating CD8+ T cell expansion and function. J Exp Med. (2005) 201:139–48. doi: 10.1084/jem.20041057
118. Alves NL, Arosa FA, and van Lier RA. IL-21 sustains CD28 expression on IL-15-activated human naive CD8+ T cells. J Immunol. (2005) 175:755–62. doi: 10.4049/jimmunol.175.2.755
119. Parrish-Novak J, Dillon SR, Nelson A, Hammond A, Sprecher C, Gross JA, et al. Interleukin 21 and its receptor are involved in NK cell expansion and regulation of lymphocyte function. Nature. (2000) 408:57–63. doi: 10.1038/35040504
120. Brandt K, Bulfone-Paus S, Jenckel A, Foster DC, Paus R, and Rückert R. Interleukin-21 inhibits dendritic cell-mediated T cell activation and induction of contact hypersensitivity in vivo. J Invest Dermatol. (2003) 121:1379–82. doi: 10.1046/j.1523-1747.2003.12603.x
121. Brandt K, Bulfone-Paus S, Foster DC, and Rückert R. Interleukin-21 inhibits dendritic cell activation and maturation. Blood. (2003) 102:4090–8. doi: 10.1182/blood-2003-03-0669
122. Pelletier M, Bouchard A, and Girard D. In vivo and in vitro roles of IL-21 in inflammation. J Immunol. (2004) 173:7521–30. doi: 10.4049/jimmunol.173.12.7521
123. Davis ID, Skrumsager BK, Cebon J, Nicholaou T, Barlow JW, Moller NP, et al. An open-label, two-arm, phase I trial of recombinant human interleukin-21 in patients with metastatic melanoma. Clin Cancer Res. (2007) 13:3630–6. doi: 10.1158/1078-0432.Ccr-07-0410
124. Thompson JA, Curti BD, Redman BG, Bhatia S, Weber JS, Agarwala SS, et al. Phase I study of recombinant interleukin-21 in patients with metastatic melanoma and renal cell carcinoma. J Clin Oncol. (2008) 26:2034–9. doi: 10.1200/jco.2007.14.5193
125. Chen T, Ding X, Liao Q, Gao N, Chen Y, Zhao C, et al. IL-21 arming potentiates the anti-tumor activity of an oncolytic vaccinia virus in monotherapy and combination therapy. J Immunother Cancer. (2021) 9(1). doi: 10.1136/jitc-2020-001647
126. Wang N, Wang J, Zhang Z, Cao H, Yan W, Chu Y, et al. A novel vaccinia virus enhances anti-tumor efficacy and promotes a long-term anti-tumor response in a murine model of colorectal cancer. Mol Ther Oncolytics. (2021) 20:71–81. doi: 10.1016/j.omto.2020.11.002
127. Marelli G, Chard Dunmall LS, Yuan M, Di Gioia C, Miao J, Cheng Z, et al. A systemically deliverable vaccinia virus with increased capacity for intertumoral and intratumoral spread effectively treats pancreatic cancer. J Immunother Cancer. (2021) 9(1). doi: 10.1136/jitc-2020-001624
128. Floss DM, Schröder J, Franke M, and Scheller J. Insights into IL-23 biology: from structure to function. Cytokine Growth Factor Rev. (2015) 26:569–78. doi: 10.1016/j.cytogfr.2015.07.005
129. Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. (2005) 201:233–40. doi: 10.1084/jem.20041257
130. Ngiow SF, Teng MW, and Smyth MJ. A balance of interleukin-12 and -23 in cancer. Trends Immunol. (2013) 34:548–55. doi: 10.1016/j.it.2013.07.004
131. von Scheidt B, Leung PS, Yong MC, Zhang Y, Towne JE, Smyth MJ, et al. Combined anti-CD40 and anti-IL-23 monoclonal antibody therapy effectively suppresses tumor growth and metastases. Cancer Res. (2014) 74:2412–21. doi: 10.1158/0008-5472.Can-13-1646
132. Baird AM, Leonard J, Naicker KM, Kilmartin L, O’Byrne KJ, and Gray SG. IL-23 is pro-proliferative, epigenetically regulated and modulated by chemotherapy in non-small cell lung cancer. Lung Cancer. (2013) 79:83–90. doi: 10.1016/j.lungcan.2012.10.003
133. Chen L, Chen H, Ye J, Ge Y, Wang H, Dai E, et al. Intratumoral expression of interleukin 23 variants using oncolytic vaccinia virus elicit potent antitumor effects on multiple tumor models via tumor microenvironment modulation. Theranostics. (2021) 11:6668–81. doi: 10.7150/thno.56494
134. Sarkar D, Su ZZ, Lebedeva IV, Sauane M, Gopalkrishnan RV, Dent P, et al. mda-7 (IL-24): signaling and functional roles. Biotechniques. (2002), 30–9. doi: 10.2144/Oct0204
135. Dumoutier L, Leemans C, Lejeune D, Kotenko SV, and Renauld JC. Cutting edge: STAT activation by IL-19, IL-20 and mda-7 through IL-20 receptor complexes of two types. J Immunol. (2001) 167:3545–9. doi: 10.4049/jimmunol.167.7.3545
136. Sauane M, Gopalkrishnan RV, Lebedeva I, Mei MX, Sarkar D, Su ZZ, et al. Mda-7/IL-24 induces apoptosis of diverse cancer cell lines through JAK/STAT-independent pathways. J Cell Physiol. (2003) 196:334–45. doi: 10.1002/jcp.10309
137. Peñaranda Fajardo NM, Meijer C, and Kruyt FA. The endoplasmic reticulum stress/unfolded protein response in gliomagenesis, tumor progression and as a therapeutic target in glioblastoma. Biochem Pharmacol. (2016) 118:1–8. doi: 10.1016/j.bcp.2016.04.008
138. Emdad L, Bhoopathi P, Talukdar S, Pradhan AK, Sarkar D, Wang XY, et al. Recent insights into apoptosis and toxic autophagy: the roles of mda-7/IL-24, a multidimensional anti-cancer therapeutic. Semin Cancer Biol. (2020) 66:140–54. doi: 10.1016/j.semcancer.2019.07.013
139. Caudell EG, Mumm JB, Poindexter N, Ekmekcioglu S, Mhashilkar AM, Yang XH, et al. The protein product of the tumor suppressor gene, melanoma differentiation-associated gene 7, exhibits immunostimulatory activity and is designated IL-24. J Immunol. (2002) 168:6041–6. doi: 10.4049/jimmunol.168.12.6041
140. Ma YF, Ren Y, Wu CJ, Zhao XH, Xu H, Wu DZ, et al. Interleukin (Il)-24 transforms the tumor microenvironment and induces anticancer immunity in a murine model of colon cancer. Mol Immunol. (2016) 75:11–20. doi: 10.1016/j.molimm.2016.05.010
141. Miyahara R, Banerjee S, Kawano K, Efferson C, Tsuda N, Miyahara Y, et al. Melanoma differentiation-associated gene-7 (Mda-7)/interleukin (IL)-24 induces anticancer immunity in a syngeneic murine model. Cancer Gene Ther. (2006) 13:753–61. doi: 10.1038/sj.cgt.7700954
142. Tong AW, Nemunaitis J, Su D, Zhang Y, Cunningham C, Senzer N, et al. Intratumoral injection of INGN 241, a nonreplicating adenovector expressing the melanoma-differentiation associated gene-7 (Mda-7/IL24): biologic outcome in advanced cancer patients. Mol Ther. (2005) 11:160–72. doi: 10.1016/j.ymthe.2004.09.021
143. Deng L, Yang X, Ding Y, Fan J, Peng Y, Xu D, et al. Oncolytic therapy with vaccinia virus carrying IL-24 for hepatocellular carcinoma. Virol J. (2022) 19:44. doi: 10.1186/s12985-022-01779-1
144. Deng L, Yang X, Fan J, Ding Y, Peng Y, Xu D, et al. IL-24-armed oncolytic vaccinia virus exerts potent antitumor effects via multiple pathways in colorectal cancer. Oncol Res. (2021) 28:579–90. doi: 10.3727/096504020x15942028641011
145. Mutamba S, Allison A, Mahida Y, Barrow P, and Foster N. Expression of IL-1Rrp2 by human myelomonocytic cells is unique to DCs and facilitates DC maturation by IL-1F8 and IL-1F9. Eur J Immunol. (2012) 42:607–17. doi: 10.1002/eji.201142035
146. Sun R, Gao DS, Shoush J, and Lu B. The IL-1 family in tumorigenesis and antitumor immunity. Semin Cancer Biol. (2022) 86:280–95. doi: 10.1016/j.semcancer.2022.05.002
147. Wang X, Zhao X, Feng C, Weinstein A, Xia R, Wen W, et al. IL-36γ Transforms the tumor microenvironment and promotes type 1 lymphocyte-mediated antitumor immune responses. Cancer Cell. (2015) 28:296–306. doi: 10.1016/j.ccell.2015.07.014
148. Le N, Luk I, Chisanga D, Shi W, Pang L, Scholz G, et al. IL-36g promotes cancer-cell intrinsic hallmarks in human gastric cancer cells. Cytokine. (2022) 155:155887. doi: 10.1016/j.cyto.2022.155887
149. Yang M, Giehl E, Feng C, Feist M, Chen H, Dai E, et al. IL-36γ-armed oncolytic virus exerts superior efficacy through induction of potent adaptive antitumor immunity. Cancer Immunol Immunother. (2021) 70:2467–81. doi: 10.1007/s00262-021-02860-4
150. Nakao S, Arai Y, Tasaki M, Yamashita M, Murakami R, Kawase T, et al. Intratumoral expression of IL-7 and IL-12 using an oncolytic virus increases systemic sensitivity to immune checkpoint blockade. Sci Transl Med. (2020) 12(526). doi: 10.1126/scitranslmed.aax7992
151. Deng L, Yang X, Fan J, Ding Y, Peng Y, Xu D, et al. An oncolytic vaccinia virus armed with GM-CSF and IL-24 double genes for cancer targeted therapy. Onco Targets Ther. (2020) 13:3535–44. doi: 10.2147/ott.S249816
152. Kurokawa C, Agrawal S, Mitra A, Galvani E, Burke S, Varshine A, et al. Azd4820 oncolytic vaccinia virus encoding IL-12 mediates anti-tumor activity through oncolysis and tumor-specific immunity. J Immunotherapy Cancer. (2022) 10:A872–A. doi: 10.1136/jitc-2022-SITC2022.0835
153. Bednarz-Misa I, Bromke MA, and Krzystek-Korpacka M. Interleukin (IL)-7 signaling in the tumor microenvironment. Adv Exp Med Biol. (2021) 1290:9–49. doi: 10.1007/978-3-030-55617-4_2
154. Kerr R, Stirling D, and Ludlam CA. Interleukin 6 and haemostasis. Br J Haematol. (2001) 115:3–12. doi: 10.1046/j.1365-2141.2001.03061.x
155. Sturm JB, Hess M, Weibel S, Chen NG, Yu YA, Zhang Q, et al. Functional hyper-IL-6 from vaccinia virus-colonized tumors triggers platelet formation and helps to alleviate toxicity of mitomycin C enhanced virus therapy. J Transl Med. (2012) 10:9. doi: 10.1186/1479-5876-10-9
156. Ye J, Chen L, Waltermire J, Zhao J, Ren J, Guo Z, et al. Intratumoral delivery of interleukin 9 via oncolytic vaccinia virus elicits potent antitumor effects in tumor models. Cancers (Basel). (2024) 16(5). doi: 10.3390/cancers16051021
157. Pai CS, Simons DM, Lu X, Evans M, Wei J, Wang YH, et al. Tumor-conditional anti-CTLA4 uncouples antitumor efficacy from immunotherapy-related toxicity. J Clin Invest. (2019) 129:349–63. doi: 10.1172/jci123391
158. Ribas A, Dummer R, Puzanov I, VanderWalde A, Andtbacka RHI, Michielin O, et al. Oncolytic virotherapy promotes intratumoral T cell infiltration and improves anti-PD-1 immunotherapy. Cell. (2017) 170:1109–19.e10. doi: 10.1016/j.cell.2017.08.027
159. Sun Y, Zhang Z, Zhang C, Zhang N, Wang P, Chu Y, et al. An effective therapeutic regime for treatment of glioma using oncolytic vaccinia virus expressing IL-21 in combination with immune checkpoint inhibition. Mol Ther Oncolytics. (2022) 26:105–19. doi: 10.1016/j.omto.2022.05.008
160. Wang C, Li Q, Xiao B, Fang H, Huang B, Huang F, et al. Luteolin enhances the antitumor efficacy of oncolytic vaccinia virus that harbors IL-24 gene in liver cancer cells. J Clin Lab Anal. (2021) 35:e23677. doi: 10.1002/jcla.23677
161. Ladenstein R, Pötschger U, Valteau-Couanet D, Luksch R, Castel V, Yaniv I, et al. Interleukin 2 with anti-GD2 antibody ch14.18/CHO (Dinutuximab beta) in patients with high-risk neuroblastoma (HR-NBl1/SIOPEN): A multicentre, randomised, phase 3 trial. Lancet Oncol. (2018) 19:1617–29. doi: 10.1016/s1470-2045(18)30578-3
162. Moral JA, Leung J, Rojas LA, Ruan J, Zhao J, Sethna Z, et al. ILC2s amplify PD-1 blockade by activating tissue-specific cancer immunity. Nature. (2020) 579:130–5. doi: 10.1038/s41586-020-2015-4
163. Liu C, Liu R, Wang B, Lian J, Yao Y, Sun H, et al. Blocking IL-17a enhances tumor response to anti-PD-1 immunotherapy in microsatellite stable colorectal cancer. J Immunother Cancer. (2021) 9(1). doi: 10.1136/jitc-2020-001895
164. Garris CS, Arlauckas SP, Kohler RH, Trefny MP, Garren S, Piot C, et al. Successful anti-PD-1 cancer immunotherapy requires T cell-dendritic cell crosstalk involving the cytokines IFN-Γ and IL-12. Immunity. (2018) 49:1148–61.e7. doi: 10.1016/j.immuni.2018.09.024
165. Zuo S, Wei M, Xu T, Kong L, He B, Wang S, et al. An engineered oncolytic vaccinia virus encoding a single-chain variable fragment against TIGIT induces effective antitumor immunity and synergizes with PD-1 or LAG-3 blockade. J Immunother Cancer. (2021) 9(12). doi: 10.1136/jitc-2021-002843
166. Jeon YH, Lee N, Yoo J, Won S, Shin SK, Kim KH, et al. Oncolytic vaccinia virus augments T cell factor 1-positive stem-like CD8(+) T cells, which underlies the efficacy of anti-PD-1 combination immunotherapy. Biomedicines. (2022) 10(4). doi: 10.3390/biomedicines10040805
167. Holloway RW, Thaker P, Mendivil AA, Ahmad S, Al-Niaimi AN, Barter J, et al. A phase III, multicenter, randomized study of olvimulogene nanivacirepvec followed by platinum-doublet chemotherapy and bevacizumab compared with platinum-doublet chemotherapy and bevacizumab in women with platinum-resistant/refractory ovarian cancer. Int J Gynecol Cancer. (2023) 33:1458–63. doi: 10.1136/ijgc-2023-004812
168. Holloway RW, Mendivil AA, Kendrick JE, Abaid LN, Brown JV, LeBlanc J, et al. Clinical activity of olvimulogene nanivacirepvec-primed immunochemotherapy in heavily pretreated patients with platinum-resistant or platinum-refractory ovarian cancer: the nonrandomized phase 2 viro-15 clinical trial. JAMA Oncol. (2023) 9:903–8. doi: 10.1001/jamaoncol.2023.1007
169. Huang B, Sikorski R, Kirn DH, and Thorne SH. Synergistic anti-tumor effects between oncolytic vaccinia virus and paclitaxel are mediated by the IFN response and HMGB1. Gene Ther. (2011) 18:164–72. doi: 10.1038/gt.2010.121
170. Storozynsky QT, Agopsowicz KC, Noyce RS, Bukhari AB, Han X, Snyder N, et al. Radiation combined with oncolytic vaccinia virus provides pronounced antitumor efficacy and induces immune protection in an aggressive glioblastoma model. Cancer Lett. (2023) 562:216169. doi: 10.1016/j.canlet.2023.216169
171. Dai MH, Liu SL, Chen NG, Zhang TP, You L, QZ F, et al. Oncolytic vaccinia virus in combination with radiation shows synergistic antitumor efficacy in pancreatic cancer. Cancer Lett. (2014) 344:282–90. doi: 10.1016/j.canlet.2013.11.007
172. Chen WY, Chen YL, Lin HW, Chang CF, Huang BS, Sun WZ, et al. Stereotactic body radiation combined with oncolytic vaccinia virus induces potent anti-tumor effect by triggering tumor cell necroptosis and damps. Cancer Lett. (2021) 523:149–61. doi: 10.1016/j.canlet.2021.09.040
173. Xiong D, Wang Q, Wang WM, and Sun ZJ. Tuning cellular metabolism for cancer virotherapy. Cancer Lett. (2024) 592:216924. doi: 10.1016/j.canlet.2024.216924
174. Chang CL, Ma B, Pang X, Wu TC, and Hung CF. Treatment with cyclooxygenase-2 inhibitors enables repeated administration of vaccinia virus for control of ovarian cancer. Mol Ther. (2009) 17:1365–72. doi: 10.1038/mt.2009.118
175. Evgin L, Acuna SA, Tanese de Souza C, Marguerie M, Lemay CG, Ilkow CS, et al. Complement inhibition prevents oncolytic vaccinia virus neutralization in immune humans and cynomolgus macaques. Mol Ther. (2015) 23:1066–76. doi: 10.1038/mt.2015.49
176. Hill C, Grundy M, Bau L, Wallington S, Balkaran J, Ramos V, et al. Polymer stealthing and mucin-1 retargeting for enhanced pharmacokinetics of an oncolytic vaccinia virus. Mol Ther Oncolytics. (2021) 21:47–61. doi: 10.1016/j.omto.2021.03.011
Keywords: gene therapy, interleukins, tumor microenvironment, oncolytic vaccinia virus, combined therapy
Citation: Zha M, Huang F, Li S, Wang Q and Tang Y (2025) Advances of oncolytic vaccinia viruses armed with interleukin in tumor therapy. Front. Oncol. 15:1594621. doi: 10.3389/fonc.2025.1594621
Received: 16 March 2025; Accepted: 30 April 2025;
Published: 21 May 2025.
Edited by:
Fer. Aranda Vega, Instituto de Investigación Sanitaria de Navarra (IdiSNA), SpainReviewed by:
Shuang Dong, Huazhong University of Science and Technology, ChinaLorella Tripodi, Dipartimento di Medicina Molecolare e Biotecnologie Mediche (MMBM) Università Federico II di Napoli, Italy
Copyright © 2025 Zha, Huang, Li, Wang and Tang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qi Wang, d2FuZ3FpQHN0dS5neG11LmVkdS5jbg==; Yong Tang, eW9uZ190YW5nX21kQGhvdG1haWwuY29t
†These authors have contributed equally to this work and share first authorship
 Mingyong Zha
Mingyong Zha Fei Huang2†
Fei Huang2† Songlin Li
Songlin Li Qi Wang
Qi Wang Yong Tang
Yong Tang