- 1Radiation Oncology and Therapy Department, First Hospital of Jilin University, Changchun, China
- 2Gastric and Colorectal Surgery Department, General Surgery Center, First Hospital of Jilin University, Changchun, China
- 3Oncology Department, First Hospital of Jilin University, Changchun, China
- 4Department of Radiology, First Hospital of Jilin University, Changchun, China
- 5Department of Pathology, First Hospital of Jilin University, Changchun, China
Background: Neoadjuvant chemoradiotherapy has become the standard treatment for mid-to-low location LARC. Recently, total neoadjuvant therapy (TNT) has been used in patients with ‘high-risk’ or ‘very high-risk’ LARC according to ESMO guideline (2017). TNT not only increases the pathological complete response (pCR) rate, but also provides patients with more opportunities to preserve organ function. However, TNT mode seems to have reached a plateau. Some clinical studies published in recent years have also confirmed that different modes of neoadjuvant chemoradiotherapy combined with ICIs can further improve the pCR rate to varying degrees. It is necessary to explore an appropriate treatment mode for patients with mid-to-low location, who are unresectable or difficult to achieve R0 resection at the initial stage of surgery and have a strong desire to preserve anal cavity. Numerous basic researches have demonstrated that radiotherapy can remodel the tumor immune microenvironment and play a potential synergistic effect on ICIs. Therefore, this study will aim to explore whether TNT combined with ICIs could improve pCR rates in patients with ‘high-risk’ or ‘very high-risk’ LARC.
Methods: This prospective, single-center, single-arm phase II trial aims to assess the pCR rate of neoadjuvant long-course concurrent chemoradiotherapy sequential 6 cycles CapeOX regimen combined with sintilimab in ‘high-risk/very high-risk’ LARC patients with mid-to-low location and with pMMR phenotype. The primary endpoint for this study is the pCR rate. Secondary endpoints include 2-year sustained of clinical complete response (cCR) rate; CR rate (the rate of patients with sustained cCR for 2 years and pCR); major pathological remission; neoadjuvant rectal score; 3-year non-regrowth disease free survival; 3-year disease-free survival; 3-year overall survival; 3-year localized recurrence-free survival; 3-year distant metastasis free survival; 3-year stoma-free survival, anal sphincter preservation rate; surgical R0 resection rate and safety (adverse events during neoadjuvant therapy and 30 days after surgery, as well as tolerance).
Discussion: This study will investigate whether neoadjuvant long-course concurrent chemoradiotherapy sequential total neoadjuvant chemotherapy combined with immunotherapy could further enhances tumor pCR rate in ‘high-risk/very high-risk’ LARC patients with mid-to-low location and with pMMR phenotype and is expected to improve prognosis.
Trial registration: ClinicalTrials.gov NCT05998122.
Background
Among all cancers, colorectal cancer ranks third in incidence and second in mortality worldwide (1). In China, the incidence of rectal cancer is on the rise, and most patients are diagnosed with locally advanced rectal cancer (LARC) at initial diagnosis (2), characterized by low rates of R0 resection and anal preservation (3). Preoperative neoadjuvant chemoradiotherapy (nCRT) has emerged as the standard treatment for mid-to-low location LARC (4). Statistical data indicates that nCRT achieves pathological complete response (pCR) rate of 8% - 24%, offering patients who achieved clinical complete response (cCR) potential ‘Watch and Wait’ opportunities (5–8). Preserving anal sphincter function while maintaining efficacy has become a key issue for LARC. Clinical trials demonstrate that total neoadjuvant therapy (TNT) can improve both pCR rates and organ preservation. However, in most trials, the pCR rate related to TNT was still lower than 30%, highlighting the need for novel strategies to enhance tumor response (9). The European Society for Medical Oncology (ESMO) guideline classify patients into different subgroups based on digital rectal examination (DRE), rectal high-resolution magnetic resonance imaging (HRMRI), and rectal intraluminal ultrasound (10). The guideline recommends adjusting treatment regimens for patients in different risk levels to achieve better efficacy and lower risk in precise treatment. While PD-1/PD-L1 inhibitors revolutionized care of colorectal cancer with deficient mismatch repair (dMMR) or microsatellite instability-high (MSI-H) phenotype, these represent only 8% - 10% of the cases. The remaining cases of proficient mismatch repair (pMMR) or microsatellite stabilization (MSS) phenotype could hardly benefit from immune checkpoint inhibitors (ICIs) treatment. Interestingly, it has been demonstrated that radiotherapy has the effect of stimulating the release of neoantigens and activating immunity (11). Building on this, the VOLTAGE study demonstrated that adding ICIs after nCRT could increase the pCR rate of MSS phenotype rectal cancer to 30% (12). At present, studies have shown that TNT combined with immunotherapy demonstrates better tumor regression and pCR rates, but its survival benefits such as DFS and OS need longer follow-up (13). Crucially, no solid evidence yet confirms whether adding immunotherapy significantly boosts pCR rates for mid-to-low location LARC patients with ‘high-risk/very high-risk’ according to ESMO guideline. Our center conducted a prospective, single-arm, observational study of long course concurrent chemoradiotherapy consolidation chemotherapy combined with PD-1 inhibitors, and the results are being submitted (STARS-RC03, NCT04906044). A total of 29 pMMR LARC patients received neoadjuvant long-course concurrent chemoradiotherapy followed by at least 3 cycles of CapeOX chemotherapy combined with PD-1 inhibitor. One patient discontinued treatment and was not effectively evaluated for efficacy. Our results showed that this regimen was safe and tolerated. Grade III toxicity was mainly associated with chemotherapy-induced hematological toxicity (51.71%, 15/29), and was reversed through active symptomatic treatment. No grade III or higher immunotherapy-related toxicity was observed. The pCR rate was 17.86% (5/28), and the overall complete response rate (cCR and pCR) was 50% (14/28). In 19 patients who undergone radical surgery, the major pathological remission (MPR) rate was 73.58% (14/19), and the R0 resection rate was 89.47% (17/19). Subgroup analysis found that the CR rate was positively correlated with the number of consolidation therapy, but the incidence of grades III or higher adverse events did not increase with the number of consolidation therapy. Building on these results, we propose the hypothesis that neoadjuvant long-course concurrent chemoradiotherapy sequential 6 cycles CapeOX regimen combined with PD-1 inhibitor further enhances the tumor pCR rate and has the potential to improve prognosis in mid-to-low location LARC patients with ‘high-risk/very high-risk’ and with pMMR phenotype.
Methods and analysis
Trial organization, ethical approval, and drug supply
The trial is co-sponsored by the Department of Gastric and Colorectal Surgery and the Department of Radiotherapy of the First Hospital of Jilin University. Scientific review was obtained from the Review Board of the First Hospital of Jilin University on August 16, 2023 (2023-HS-084). Ethical approval was obtained from the Ethics Committee (Approval No.23K162-002) of the First Hospital of Jilin University on October 18, 2023. All relevant materials have been registered on the official ClinicalTrials.gov website (NCT05998122). The PD-1 inhibitor selected for this study is sintilimab from Innovent Biologics, Inc.
Study population
All included patients required pathological immunohistochemical confirmation of rectal adenocarcinoma with intact expressions of all 4 MMR proteins, had mid-to-low location tumors (distal margin ≤10 cm from anal verge) verified by HIMRI, and were risk-stratified according to ESMO rectal cancer guidelines (2017) as ‘high-risk’ (cT3c/d or very low localization levators threatening, MRF-negative cT3c/d mid-rectum, cN1-N2 with extranodal involvement, EMVI-positive, or limited cT4aN0) or ‘very high-risk’ (cT3 with MRF involved, any cT4a/b, or lateral lymph node metastasis) (10). Participants willingly volunteered for this clinical study and provided written informed consent. Detailed inclusion and exclusion criteria are available in Table 1. The study commenced in October 2023 and is expected to end before December 2028. If all enrolled patients complete all treatment plans during this period, the study will be terminated. Study enrollment is ongoing, with the first patient recruited on October 31, 2023.
Study design
This prospective, single-center, single-arm phase II trial aims to assess the pCR rate of neoadjuvant long-course concurrent chemoradiotherapy sequential 6 cycles CapeOX regimen combined with sintilimab in mid-to-low location LARC with ‘high-risk/very-high-risk’ and with pMMR phenotype. The treatment process is showed in Figure 1. Enrolled patients will initially undergo long-course concurrent chemoradiotherapy, targeting the primary tumor area and regional lymphatic drainage area: 50 Gy/25 fractions/5 weeks with intensity-modulated radiation therapy or volumetric modulated arc therapy, alongside simultaneous capecitabine 825 mg/m2, twice daily, throughout the radiotherapy period. Following the completion of neoadjuvant concurrent chemoradiotherapy (7–10 days later), the subjects will receive 6 cycles of CapeOX combined with sintilimab treatment: sintilimab 200 mg intravenously on the day 1, oxaliplatin 130 mg/m2 intravenously on the day 1, and capecitabine 1000 mg/m2 orally twice daily from day 1 to day 14. Efficacy evaluations will occur every three cycles using DRE, HRMRI and endoscopy. Patients with progressive disease (PD) will undergo multidisciplinary team (MDT) discussion for alternative treatment strategies, while those with tumor regression and good tolerance will continue therapy. Patients whose tumors have not completely regressed after 6 cycles of consolidation treatment will directly receive surgical treatment. For patients evaluated as cCR, ‘Watch and Wait’ or surgery can be chosen based on MDT discussion and patient preference. The evaluation criteria of cCR according to ESMO guideline (2017), including no palpable tumor on DRE, no residual tumor or white scar under endoscopy, negative scar biopsy, and no residual tumor or residual fibrosis or residual wall thickening because of edema on HRMRI, and no suspicious lymph nodes on T2-weighted images (10, 14).
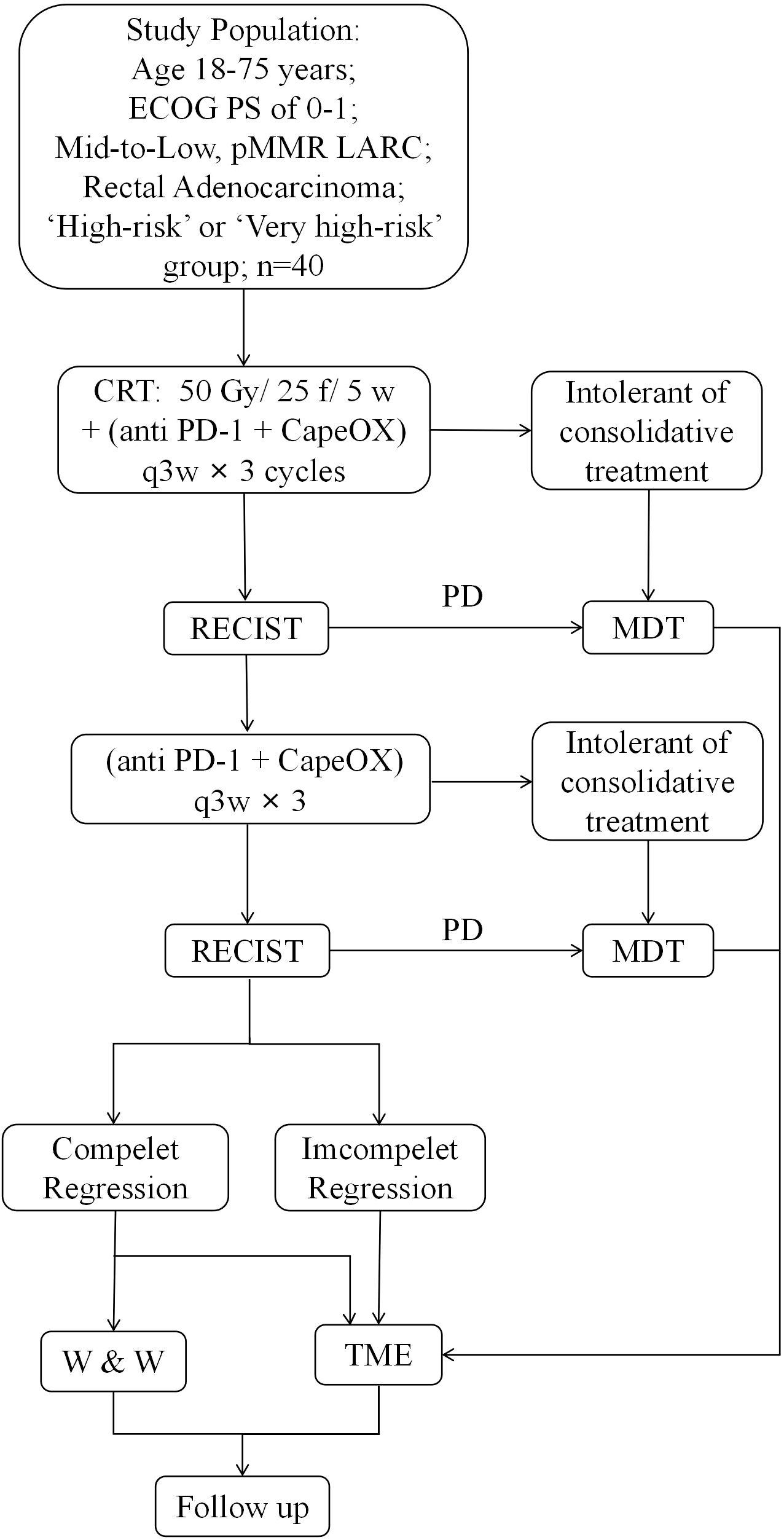
Figure 1. Study design. Recruitment pathway. All patients who are not eligible the STARS-RC06 trial will progress through these steps. pMMR, proficient mismatch repair. MDT, multidisciplinary team.
Additionally, we will collect biological samples from all participants during treatment, The time points are shown in Figure 2. Fecal samples will be stored at -80°C for 16S rRNA sequencing. Tumor tissue samples will be used for the detection of PD-L1 expression level, MSI and TMB in the central laboratory. And blood samples will be used for MSI and TMB detection. Freshly collected specimens, resection, needle aspiration biopsy, punch or clamp biopsy will be all acceptable.
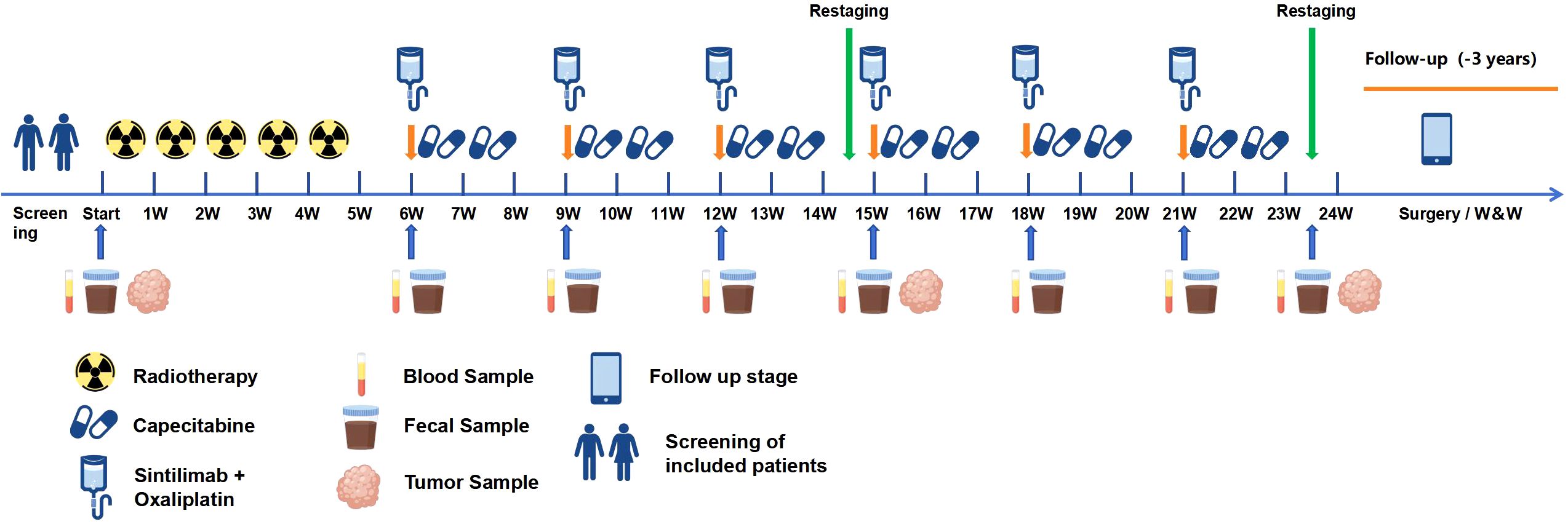
Figure 2. Sample collection procedure. Blood and fecal samples will be used for next generation sequencing (NGS), and tumor samples will be used for immunohistochemical staining and NGS.
Criteria for discontinuing or modifying interventions
If patients cannot tolerate consolidation therapy or experiences progression in efficacy evaluation, the subsequent treatment plan will be decided after discussion by the MDT and included in the follow-up. The specific treatment process is outlined in Figure 1. For grades III and IV chemotherapy-related adverse events (AEs), reduce the dose by 20% to 25%, as appropriate. All of immunotherapy-related adverse events (irAEs) were managed according to the NCCN Guidelines for Rectal Cancer (2022) for the management of immunotherapy toxicity (4). Drugs such as antihypertension, antidiabetes, painkillers and other symptomatic treatment are allowed. If a patient has hepatic injury or myelosuppression, hepatoprotectant, granulocyte colony-stimulating factor, and thrombopoietin may be used. The use of other anti-cancer drugs and traditional Chinese medicine is prohibited.
Study endpoints and assessment
The primary endpoint of this trial is the pCR rate, defined as the percentage of patients who achieved pCR after nCRT in the eligible population. Secondary endpoints include 2-year sustained of cCR rate; CR rate (the rate of patients with sustained cCR for 2 years and pCR); MPR, which is defined as residual viable tumor (RVT) ≤10%; neoadjuvant rectal (NAR) score, which were calculated based on pathological and clinical staging (NAR=[5pN - 3(cT - pT) + 12]2/9.61); 3-year non⁃regrowth disease free survival; 3-year disease-free survival; 3-year overall survival; 3-year localized recurrence-free survival; 3-year distant metastasis free survival; 3-year stoma-free survival, anal sphincter preservation rate; surgical R0 resection rate and safety (adverse events during neoadjuvant therapy and 30 days after surgery, as well as tolerance). In addition, the study also has exploratory endpoints to further investigate potential factors related to antitumor therapy, such as infiltrating lymphocytes in tumor tissue, the structure of gut microbiome, and RAS/BRAF status.
The per-protocol set (PP) is defined as subjects who strictly adhered to the study protocol without major violations, completed all treatment and follow-up, and had measurable data for the primary endpoint (pCR). The modified intention-to-treat set (mITT) excludes participants who received no intervention or provided absolutely no post-baseline data. Patients achieving cCR who opt for Watch & Wait management are included in the mITT set for efficacy analysis.
Sample size
Based on evidence of the results of previous clinical trials summarized in the systematic review, the pCR rate of nCRT alone is approximately 16% (5), and the pCR rate of nCRT combined with ICIs is approximately 36% (13). Therefore, this study sets the null hypothesis (H0) at P0 = 0.16 and alternative hypothesis (H1) at P1 = 0.36. Using a two-sided test with α = 0.05 and β = 0.2, and accounting for a 20% dropout rate, the calculated total sample size is 40 patients. Computations were performed using PASS 2021 v21.0.3.
Follow-up
The follow-up methods include outpatient visits and telephone follow-ups. The follow-up schedule consists of assessments every 3 months for the first 2 years, followed by visits once every 6 months in the 3rd year, and subsequently, visits once every 1 year for the 4th–5th years. Procedure refer to ESMO Rectal Cancer Guidelines (2017) (10). Follow-up evaluations include, but are not limited to DRE, carcinoembryonic antigen measurements, endoscopy and biopsy, abdominal enhanced computed tomography, HRMRI, and anal cavity ultrasound.
Discussion
Currently, China faces a substantial proportion of patients with mid-to-low LARC, emphasizing the importance of achieving R0 resection and preserving anal function. Neoadjuvant therapy has become the standard treatment for LARC, and the pCR rate of neoadjuvant long-course concurrent chemoradiotherapy alone is approximately 16% (5). Consequently, optimizing the neoadjuvant treatment approach for patients with LARC has emerged as a central scientific concern in this field.
In recent years, the application of the TNT mode has gained traction, particularly in patients with refractory LARC. Phase III randomized controlled clinical studies have reported that TNT mode elevates the pCR rate of patients with LARC to 28%, the organ function preservation rate to 50%, and reduced the distant metastasis rate to 17% (15). TNT offers the advantage of more substantial tumor shrinkage, a significant increase in the cCR rate, and more opportunities for ‘Watch and Wait’. This approach preserves the structure and function of organs, ultimately enhancing the quality of life for patients with LARC (16). Moreover, the 7-year follow-up results of the PRODIGE-23 study (7), presented at the 2023 ASCO Annual Meeting, highlighted that the TNT regimen, consisting of three-agent, high-intensity induction chemotherapy combined with long-course concurrent chemoradiotherapy, not only enhanced the pCR rate (28% vs. 13%) but also conferred a long-term survival benefit. However, 43% of the study’s patient population had T2–T3b stage and 13% had high location rectal cancer (10–15 cm from the anal verge). This raises a cautious consideration regarding the necessity of high-intensity preoperative neoadjuvant therapy for patients with LARC without high-risk factors. In response to this question, the OPRA study specifically enrolled only patients with low LARC (17). The results hinted that the TNT mode, comprising neoadjuvant long-course concurrent chemoradiotherapy plus sequential full cycles consolidation chemotherapy, improved the de-operation survival rate for patients. The CAO/ARO/AIO-12 study further affirmed that consolidation of full cycles of chemotherapy after long-course concurrent chemoradiotherapy produced a higher pCR rate than induction approach (18). The precise stratification of neoadjuvant therapy for patients with LARC has become a pivotal topic in clinical multidisciplinary consultations. Currently, ICIs has been demonstrated to be effective in dMMR/MSI-H LARC, avoiding the toxicity of nCRT and radical surgery (19). This underscores the importance of precise adjustments in neoadjuvant therapy for patients with LARC.
To further refine the stratification of patients with LARC, the RAPIDO study (8) enrolled patients with LARC with at least one high-risk factor (cT4, EMVI+, cN2, MRF+, and lateral lymph node+). The results demonstrated that a short course of radiotherapy combined with a full-cycles of neoadjuvant chemotherapy had advantages in increasing the pCR rate and reducing the 3-year treatment failure rate and distant conversion in the enrolled population. However, it is apparent from the reported data that the efficacy of tumor shrinkage with the TNT modality seems to have reached a plateau. Nevertheless, from a safety perspective, this modality appears feasible for the majority of patients with LARC and has received recommendations in clinical guidelines (4).
It is well known that radiation therapy helps to remodel the tumor immune microenvironment and exerts a potential synergistic effect on ICIs. On this basis, the VOLTAGE study first revealed that nCRT sequential Nivolumab could increase the pCR rate of LARC (12). More recently, four studies from China reported that adding PD-1 inhibitors during neoadjuvant therapy in LARC patients could further improve the pCR or cCR rates (20–23). However, current research has primarily focused on the combination modality of short-course radiotherapy and immunotherapy. Short-course radiotherapy (25 Gy/5 fractions) is disadvantaged by underdosing compared with long-course concurrent radiotherapy (50 - 50.4 Gy/25–28 fractions). Previous studies suggest that short-course radiotherapy lacks a significant downstaging effect, has a lower rate of anal preservation, and exhibits a higher rate of local recurrence compared with long-course concurrent chemoradiotherapy, especially in patients with lower margins are within 5 cm of the anal verge (24). Consequently, clinical guidelines for rectal cancer diagnosis and treatment, such as those from National Comprehensive Cancer Network, ESMO, and Chinese Society of Clinical Oncology, recommend long-course concurrent chemoradiotherapy for such patients. For patients with subperitoneal LARC, who are not initially resectable or face challenges in achieving R0 resection, and express a strong desire for anal preservation, further exploration is needed to assess the feasibility of full-cycles nCRT combined with immunotherapy.
Ethics statement
Scientific review was obtained from the Review Board of the First Hospital of Jilin University on August 16, 2023 (2023-HS-084). Ethical approval was obtained from the Ethics Committee (Approval No.23K162-002) of the First Hospital of Jilin University on October 18, 2023. Informed consent will be obtained from all subjects and/or their legal guardians.
Author contributions
MG: Writing – original draft, Data curation. YG: Writing – original draft, Data curation. LH: Writing – original draft, Data curation. XS: Writing – original draft, Data curation. TL: Writing – original draft, Data curation. ZW: Writing – original draft, Data curation. YL: Data curation. ZL: Writing – original draft, Data curation. JW: Writing – original draft, Data curation. XQ: Writing – original draft, Data curation. LG: Writing – original draft, Data curation. PC: Writing – review & editing, Investigation, Funding acquisition, Supervision, Data curation. QW: Writing – review & editing, Supervision, Investigation, Data curation.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was funded by National Natural Science Foundation of China, (Grant No. 82272738), and by Scientific and Technological Developing Scheme Foundation of Jilin Province (Grant No. YDZJ202501ZYTS139).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
2. Xia C, Dong X, Li H, Cao M, Sun D, He S, et al. Cancer statistics in China and United States, 2022: profiles, trends, and determinants. Chin Med J. (2022) 135:584–90. doi: 10.1097/CM9.0000000000002108
3. Gezen C, Kement M, Altuntas YE, Okkabaz N, Seker M, Vural S, et al. Results after multivisceral resections of locally advanced colorectal cancers: an analysis on clinical and pathological t4 tumors. World J Surg Oncol. (2012) 10:39. doi: 10.1186/1477-7819-10-39
4. Benson AB, Venook AP, Al-Hawary MM, Azad N, Chen YJ, Ciombor KK, et al. Rectal cancer, version 2.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. (2022) 20:1139–67. doi: 10.6004/jnccn.2022.0051
5. Maas M, Nelemans PJ, Valentini V, Das P, Rödel C, Kuo LJ, et al. Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: a pooled analysis of individual patient data. Lancet Oncol. (2010) 11:835–44. doi: 10.1016/S1470-2045(10)70172-8
6. Jin J, Tang Y, Hu C, Jiang LM, Jiang J, Li N, et al. Multicenter, randomized, phase III trial of short-Term radiotherapy plus chemotherapy versus long-Term chemoradiotherapy in locally advanced rectal cancer (STELLAR). J Clin Oncol. (2022) 40:1681–92. doi: 10.1200/JCO.21.01667
7. Conroy T, Bosset JF, Etienne PL, Rio E, François É, Mesgouez-Nebout N, et al. Neoadjuvant chemotherapy with FOLFIRINOX and preoperative chemoradiotherapy for patients with locally advanced rectal cancer (UNICANCER-PRODIGE 23): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. (2021) 22:702–15. doi: 10.1016/S1470-2045(21)00079-6
8. Bahadoer RR, Dijkstra EA, van Etten B, Marijnen CAM, Putter H, Kranenbarg EM, et al. Short-course radiotherapy followed by chemotherapy before total mesorectal excision (TME) versus preoperative chemoradiotherapy, TME, and optional adjuvant chemotherapy in locally advanced rectal cancer (RAPIDO): a randomised, open-label, phase 3 trial. Lancet Oncol. (2021) 22:29–42. doi: 10.1016/S1470-2045(20)30555-6
9. Kasi A, Abbasi S, Handa S, Al-Rajabi R, Saeed A, Baranda J, et al. Total neoadjuvant therapy vs standard therapy in locally advanced rectal cancer: A systematic review and meta-analysis. JAMA Netw Open. (2020) 3:e2030097. doi: 10.1001/jamanetworkopen.2020.30097
10. Glynne-Jones R, Wyrwicz L, Tiret E, Brown G, Rödel C, Cervantes A, et al. Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. (2017) 28:iv22–40. doi: 10.1093/annonc/mdx224
11. Sharabi AB, Lim M, DeWeese TL, and Drake CG. Radiation and checkpoint blockade immunotherapy: radiosensitisation and potential mechanisms of synergy. Lancet Oncol. (2015) 16:e498–509. doi: 10.1016/S1470-2045(15)00007-8
12. Bando H, Tsukada Y, Inamori K, Togashi Y, Koyama S, Kotani D, et al. Preoperative Chemoradiotherapy plus Nivolumab before Surgery in Patients with Microsatellite Stable and Microsatellite Instability-High Locally Advanced Rectal Cancer. Clin Cancer Res. (2022) 28:1136–46. doi: 10.1158/1078-0432.CCR-21-3213
13. Yang L, Cui X, Wu F, Chi Z, Xiao L, Wang X, et al. The efficacy and safety of neoadjuvant chemoradiotherapy combined with immunotherapy for locally advanced rectal cancer patients: a systematic review. Front Immunol. (2024) 15:1392499. doi: 10.3389/fimmu.2024.1392499
14. Martens MH, Maas M, Heijnen LA, Lambregts DM, Leijtens JW, Stassen LP, et al. Long-term outcome of an organ preservation program after neoadjuvant treatment for rectal cancer. J Natl Cancer Inst. (2016) 108:djw171. doi: 10.1093/jnci/djw171
15. Cercek A, Goodman KA, Hajj C, Weisberger E, Segal NH, Reidy-Lagunes DL, et al. Neoadjuvant chemotherapy first, followed by chemoradiation and then surgery, in the management of locally advanced rectal cancer. J Natl Compr Cancer Network: JNCCN. (2014) 12:513–9. doi: 10.6004/jnccn.2014.0056
16. Custers PA, van der Sande ME, Grotenhuis BA, Peters FP, van Kuijk SMJ, Beets GL, et al. Long-term quality of life and functional outcome of patients with rectal cancer following a watch-and-wait approach. JAMA Surg. (2023) 158:e230146. doi: 10.1001/jamasurg.2023.0146
17. Garcia-Aguilar J, Patil S, Gollub MJ, Kim JK, Yuval JB, Thompson HM, et al. Organ preservation in patients with rectal adenocarcinoma treated with total neoadjuvant therapy. J Clin Oncol. (2022) 40:2546–56. doi: 10.1200/JCO.22.00032
18. Fokas E, Allgäuer M, Polat B, Klautke G, Grabenbauer GG, Fietkau R, et al. Randomized phase II trial of chemoradiotherapy plus induction or consolidation chemotherapy as total neoadjuvant therapy for locally advanced rectal cancer: CAO/ARO/AIO-12. J Clin oncology: Off J Am Soc Clin Oncol. (2019) 37:3212–22. doi: 10.1200/JCO.19.00308
19. Cercek A, Lumish M, Sinopoli J, Weiss J, Shia J, Lamendola-Essel M, et al. PD-1 blockade in mismatch repair-deficient, locally advanced rectal cancer. New Engl J Med. (2022) 386:2363–76. doi: 10.1056/NEJMoa2201445
20. Yang Z, Gao J, Zheng J, Han J, Li A, Liu G, et al. Efficacy and safety of PD-1 blockade plus long-course chemoradiotherapy in locally advanced rectal cancer (NECTAR): a multi-center phase 2 study. Signal Transduct Target Ther. (2024) 9:56. doi: 10.1038/s41392-024-01762-y
21. Li Y, Pan C, Gao Y, Zhang L, Ji D, Cui X, et al. Total neoadjuvant therapy with PD-1 blockade for high-risk proficient mismatch repair rectal cancer. JAMA Surg. (2024) 159:529–37. doi: 10.1001/jamasurg.2023.7996
22. Xia F, Wang Y, Wang H, Shen L, Xiang Z, Zhao Y, et al. Randomized phase II trial of immunotherapy-based total neoadjuvant therapy for proficient mismatch repair or microsatellite stable locally advanced rectal cancer (TORCH). J Clin Oncol. (2024) 42:3308–18. doi: 10.1200/JCO.23.02261
23. Lin ZY, Zhang P, Chi P, Xiao Y, Xu XM, Zhang AM, et al. Neoadjuvant short-course radiotherapy followed by camrelizumab and chemotherapy in locally advanced rectal cancer (UNION): early outcomes of a multicenter randomized phase III trial. Ann Oncol. (2024) 35:882–91. doi: 10.1016/j.annonc.2024.06.015
24. Ngan SY, Burmeister B, Fisher RJ, Solomon M, Goldstein D, Joseph D, et al. Randomized trial of short-course radiotherapy versus long-course chemoradiation comparing rates of local recurrence in patients with T3 rectal cancer: Trans-Tasman Radiation Oncology Group trial 01.04. J Clin oncology: Off J Am Soc Clin Oncol. (2012) 30:3827–33. doi: 10.1200/JCO.2012.42.9597
Keywords: locally advanced rectal cancer, total neoadjuvant therapy, immunotherapy, immune checkpoint inhibitor, pathological complete response
Citation: Gu M, Guo Y, He L, Sun X, Liang T, Wang Z, Li Y, Liu Z, Wang J, Qiu X, Guo L, Chang P and Wang Q (2025) Total neoadjuvant chemoradiotherapy plus anti PD-1 for mid-to-low locally advanced rectal cancer: study protocol of a prospective, single arm, phase II study (STARS - RC06). Front. Oncol. 15:1594927. doi: 10.3389/fonc.2025.1594927
Received: 17 March 2025; Accepted: 15 July 2025;
Published: 08 August 2025.
Edited by:
Ammad Ahmad Farooqi, Institute of Biomedical and Genetic Engineering (IBGE), PakistanReviewed by:
Min Jung Kim, Seoul National University, Republic of KoreaZhengyang Yang, Beijing Friendship Hospital, China
Andrea Pretta, University of Cagliari, Italy
Copyright © 2025 Gu, Guo, He, Sun, Liang, Wang, Li, Liu, Wang, Qiu, Guo, Chang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Quan Wang, d3F1YW5Aamx1LmVkdS5jbg==; Pengyu Chang, Y2hhbmdwZW5neXVAamx1LmVkdS5jbg==
 Meichen Gu
Meichen Gu Yuchen Guo
Yuchen Guo Liang He2
Liang He2 Xuan Sun
Xuan Sun Tingting Liang
Tingting Liang Liang Guo
Liang Guo Pengyu Chang
Pengyu Chang Quan Wang
Quan Wang