- 1Department of Pharmacy, The Sixth Affiliated Hospital, Sun Yat-Sen University, Guangzhou, China
- 2Department of Graceland Medical Center, The Sixth Affiliated Hospital, Sun Yat-Sen University, Guangzhou, China
- 3Biomedical Innovation Center, The Sixth Affiliated Hospital, Sun Yat-sen University, Guangzhou, China
- 4Department of Gastroenterology, The Sixth Affiliated Hospital, Sun Yat-Sen University, Guangzhou, China
- 5Department of General Practice, The Sixth Affiliated Hospital of Sun Yat-Sen University, Guangzhou, China
Objective: To explore a practical complications related Medication Therapy Management (MTM) service for colorectal cancer patient which based on take home cancer drugs (THCDs), and minimize the occurrence of unexpected events by reducing complications and adverse reactions in home therapy.
Method: A total of 144 patients with colorectal cancer (CRC) who underwent home cancer drugs treatment for the first time met the include criteria from July 1, 2023 to July 31, 2024. They were divided into control group and MTM intervention group randomly, MTM intervention group conducted with three courses of MTM intervention, and control group adapt with three times of conventional follow up. We compared patient characteristics, complications, adverse effects, and knowledge-practice-attitude (KPA) results.
Results: Among them, 119 patients were enrolled. There were significant differences regard of cancer pain, insomnia, anxiety, and defecation disorder (p<0.05); Multivariate analysis results showed that pain, chemotherapy-induced nausea or vomiting (CINV), and defecation disorder were independent factors for unscheduled hospital admission (p<0.05); There were significant differences regard of adverse effects for home medication patient which include jaundice, hypo leukocytosis, limb edema, and fatigue (p<0.05); MTM intervention group showed better feedback than control group in Attitudes and practice Toward screening (p<0.05).
Conclusion: MTM demonstrates significant clinical benefits in colorectal cancer (CRC) patients by effectively reducing the incidence of treatment-related complications, including nausea and vomiting (CINV), abdominal pain, and insomnia. Furthermore, it contributes to decreased rates of unplanned hospitalization and enhances key patient outcomes (KPA), warranting further investigation and clinical application in CRC management.
Introduction
Colorectal cancer (CRC) stands as one of the most prevalent malignant tumors of the digestive system, ranking third in global incidence and second in mortality rates. According to the latest statistics from the International Agency for Research on Cancer (IARC) under the World Health Organization, the global burden of CRC reached 1,933,600 new cases in 2020 (1), with China alone reporting 517,100 new cases in 2022 (2). Over the past decades, significant advancements in treatment modalities have led to a steady improvement in survival rates (3, 4), particularly through the development of novel therapeutic agents and optimized chemotherapy regimens. The current standard of care for CRC involves a multidisciplinary approach, combining surgical intervention with adjuvant therapies including radiotherapy, chemotherapy, targeted therapy, and integrated traditional Chinese medicine. Clinical evidence demonstrates that patients undergoing chemotherapy achieve significantly higher 3- and 5-year overall survival (OS) and progression-free survival (PFS) rates compared to non-chemotherapy groups (5, 6)). However, the cytotoxic nature of chemotherapeutic agents not only targets malignant cells but also adversely affects healthy tissues, leading to a spectrum of treatment-related complications (7, 8). This is particularly concerning for home-based care, as studies indicate that over 50% of terminal cancer patients require hospital readmission due to acute complications arising during home treatment (9, 10).
The paradigm of cancer management has evolved with medical advancements, establishing take-home cancer drugs (THCDs) as an integral component of CRC treatment protocols. Modern pharmacotherapy for CRC has become increasingly sophisticated, encompassing not only antineoplastic agents but also comprehensive supportive care medications. While existing research in China has predominantly focused on clinical characteristics, healthcare utilization patterns, and intravenous chemotherapy regimens (11), there remains a notable gap in understanding the management of gastrointestinal complications during home-based treatment. Specifically, insufficient attention has been given to critical issues such as cancer-related pain, nutritional deficiencies, CINV, defecation disorder, and sleep disturbances. These unaddressed complications frequently necessitate hospital readmissions for symptom management, significantly compromising patients’ quality of life and treatment adherence.
Emerging evidence highlights the efficacy of medication therapy management (MTM) in optimizing patient outcomes (12). This approach, particularly when implemented through home-based pharmaceutical care programs, has demonstrated significant benefits in medication safety evaluation and adverse drug reaction management (13, 14). Notably, systematic management of THCDs has been associated with reduced 90-day readmission rates (15). Despite these advancements, there remains a global knowledge gap regarding the implementation of MTM services for CRC patients and the optimization of THCDs programs. Therefore, this study aims to investigate home-based medication strategies to effectively manage treatment-related complications, mitigate adverse drug reactions, enhance treatment compliance, and prevent unexpected clinical events through comprehensive drug therapy management services within the THCDs framework.
Methods
Study design and patients
A total of 144 patients who came to the Sixth Affiliated Hospital of Sun Yat-sen University for the treatment of colorectal cancer from January 2023 to Aug 2023 were selected. The trial protocol was approved by the Ethics Committee of the Sixth Affiliated Hospital of Sun Yat-sen University, approval number 2022ZSLYEC-616. all patients provided written informed consent prior to initiation of any study treatment. The inclusion criteria were: (1) Patients aged 18 or above were diagnosed by pathological examination as colorectal cancer patients in TNM stages II to IV (American Cancer Federation 8th Edition); (2) Colorectal cancer patients first time received THCDs therapy; (3) Complete clinical data and postoperative follow-up data. Exclusion criteria: (1) Patients with other malignant tumors; (2) End-stage patients dead or give up drug treatment midway; (3) Patients with communication difficulties and difficulty in obtaining relevant data. They were divided into control group and MTM intervention group according to random number table method, with 72 cases in each group. The control group was included in general discharge guidance, and the observation group was included in MTM service of take-home cancer drugs.
MTM procedure
Medication therapy management
Medication therapy management (MTM) intervention strategies were developed by integrating core MTM elements with disease characteristics (Figure 1). Three rounds of MTM intervention were conducted after the first, third, and sixth course of chemotherapy, each including three sections.
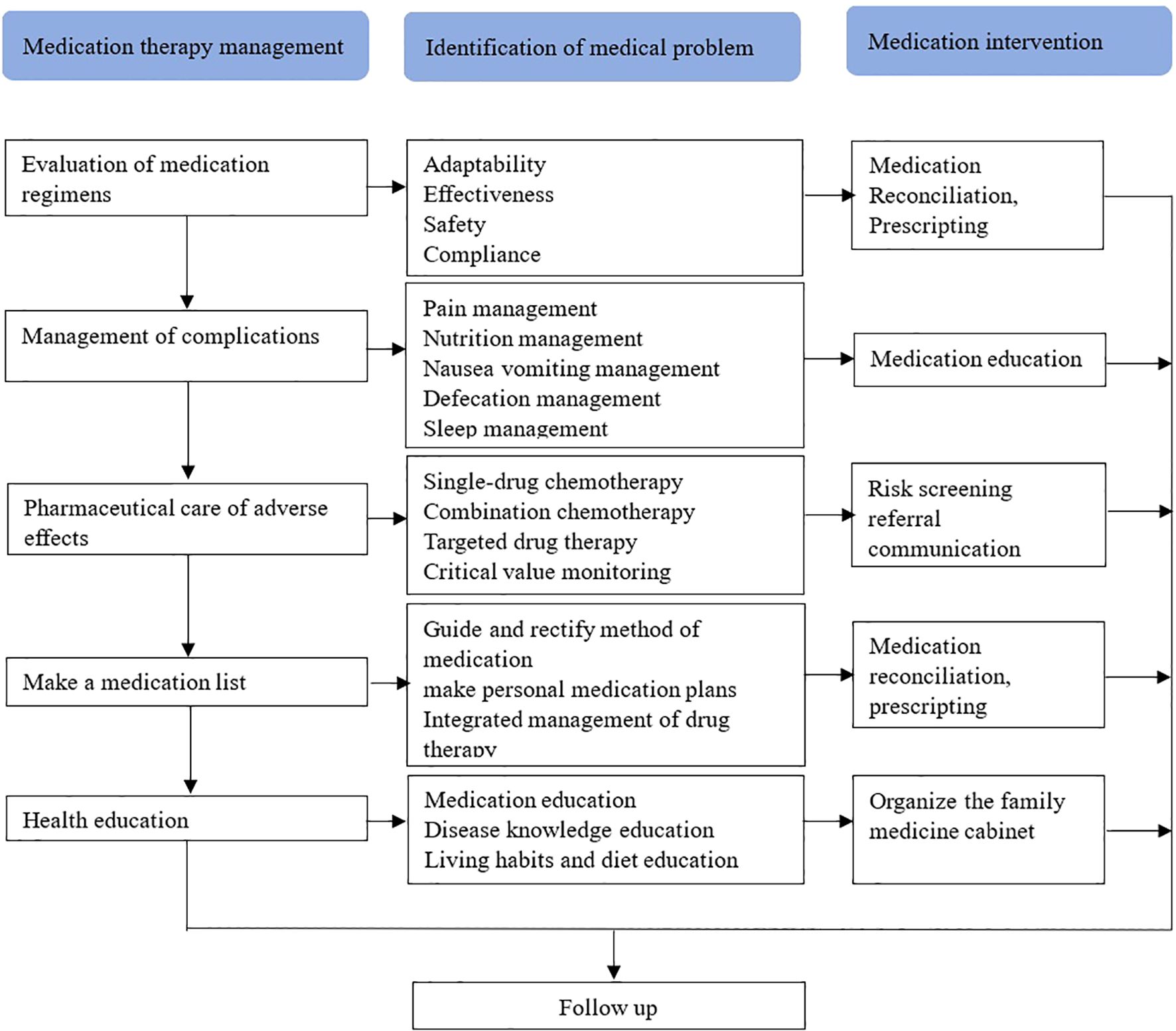
Figure 1. Intervention strategies include the evaluation of medication regimen, management of complications, pharmaceutical care of adverse effects, make a medication list, and health education, aims to identify the medical problems of take-home cancer drugs, thereby improving medication compliance and preventing medication errors.
Evaluation of medication regimens
Section 1 involves the comprehensive documentation of patients’ current drug use, including chemotherapy drugs, targeted drugs, and immune drugs. The Screening Tool of Older Person’s Prescriptions (STOPP) and Screening Tool to Alert doctors to Right Treatment (START) are utilized to evaluate the indications, effectiveness, safety, and compliance of these medications based on evidence-based practice and individual patient circumstances.
Management of complications
In Section 2, the focus is on evaluating potential issues related to chemotherapy for colorectal cancer and associated complications such as cancer pain, nutrition, nausea/vomiting, bowel movements, and insomnia. This includes providing health education, medication guidance, and psychological counseling for patients in order to manage complications effectively.
Pharmaceutical care of adverse effects
Section 3 details the recording of adjusted medication regimens for patients with colorectal cancer along with management plans for disease complications and concurrent conditions. It also involves categorizing the patient’s personal drug plan list according to drug purpose while providing suggestions for proper medication use. For patients with improper drug utilization, recommendations are made for reorganization of their medication regimen through communication with specialists or attending doctor.
Following three rounds of Medication Therapy Management (MTM) intervention for colorectal cancer patients comes follow-up management.
Conventional follow-up
Conventional follow up included the explanation of the treatment duration, hospitalization appointment, and therapy attention, also education of living habits and dietary precautions, Health & Nutrition suggestion, and reminded the patient of the return time. Three times of conventional follow up was adapt after each course of chemotherapy at first visit, third visit, and sixth visit.
Statistical analysis
All statistical analyses were performed using SPSS statistical software system (Version 25.0, SPSS, Chicago). Frequency analysis was conducted to identify the general characteristics of the study patients, and a descriptive statistical analysis was conducted to identify the level of research variables. A paired sample t-test was conducted to verify the difference in the perception of the importance of MTM in oral drugs. The chi-square test was used to evaluate the percentage and difference between the two groups, and the Kruskal–Wallis test was used to compare the categorical data. Values of p<0.05 were considered statistically significant.
Results
Clinical demographics
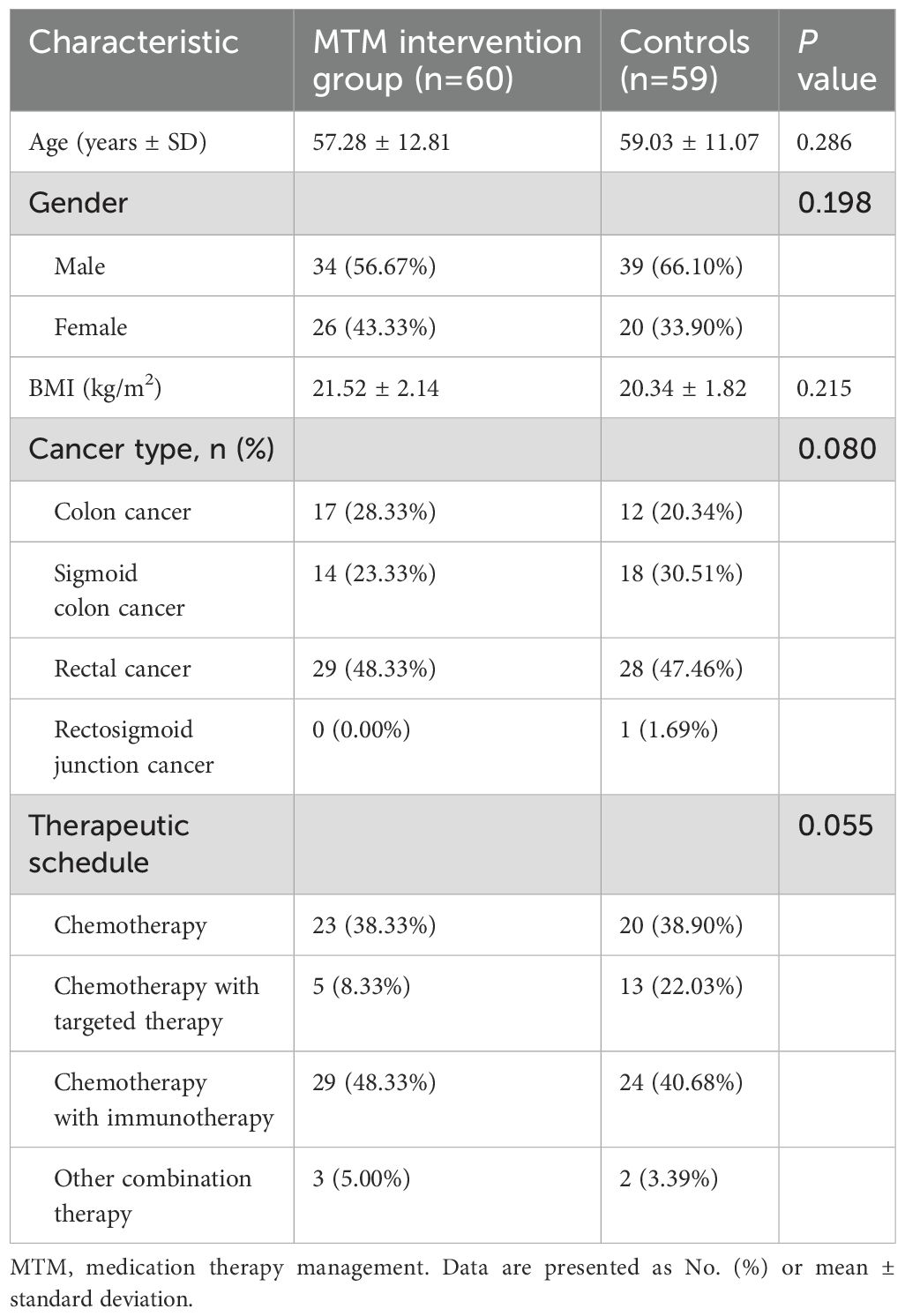
A total of 119 patients with colorectal cancer were eligible among 144 participants met the inclusion criteria who underwent home cancer drugs treatment from January 1, 2023 to August 31, 2023, with 60 participants allocated to MTM intervention group (34 men, 26 women) and 59 participants allocated to Control group (39 men, 20 women), and the mean ages of the patients from MTM intervention group and Control group were 57.28 ± 12.81 and 59.03 ± 11.07 years respectively. There were no significant differences between the two groups in terms of age, BMI, cancer type, or therapeutic schedule (p>0.05; Table 1).
Complication symptoms for home medication patient with colorectal cancer
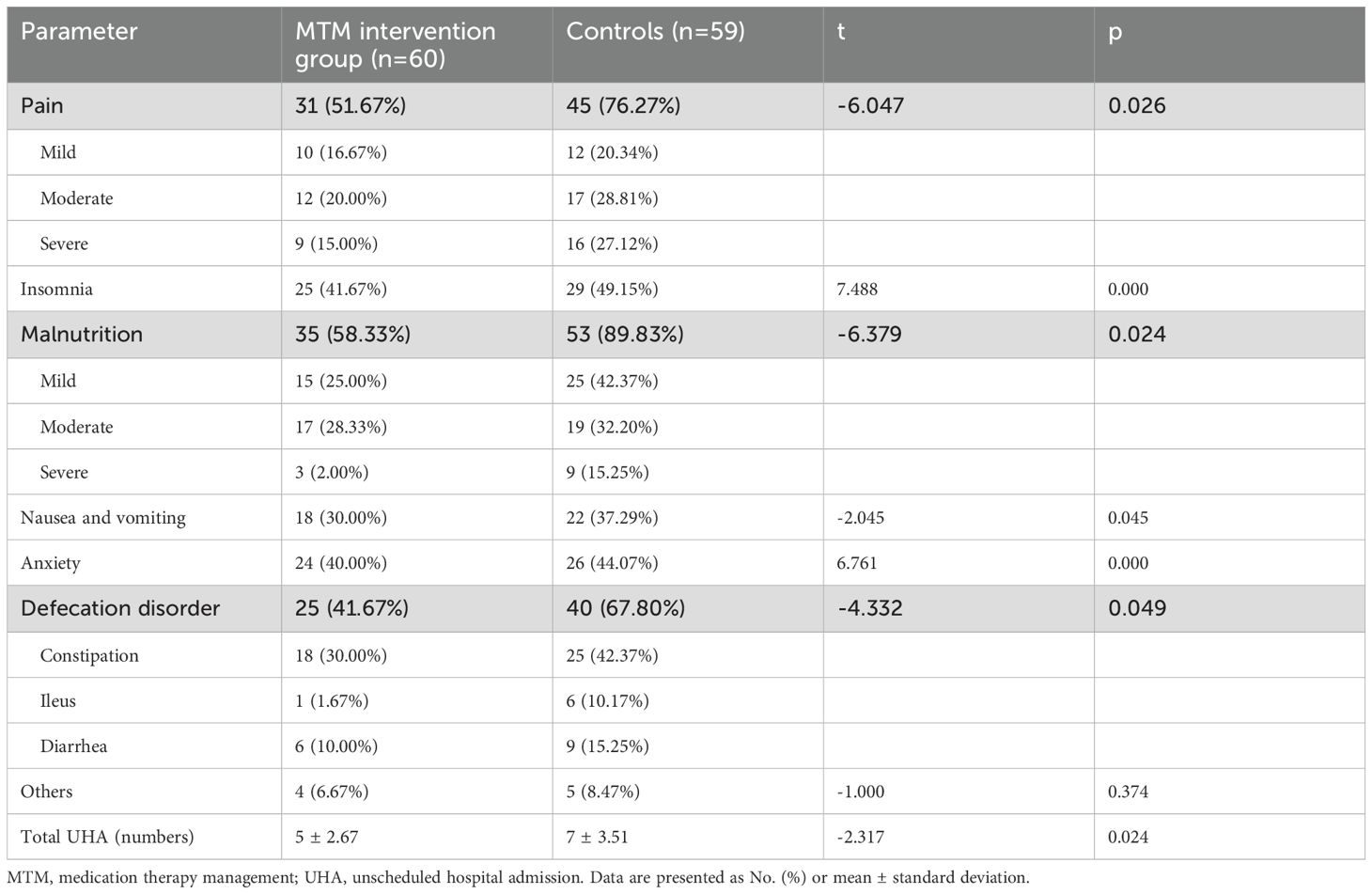
Patients with colorectal cancer experience a range of complications primarily affecting the gastrointestinal tract throughout disease progression and the post-surgical period. Significant differences were observed in terms of cancer pain, insomnia, malnutrition, CINV, anxiety, and defecation disorder (p<0.05; Table 2). In the MTM intervention group, a lower percentage of patients experienced pain (51.67% vs 76.27%), insomnia (41.67% vs 49.15%), malnutrition (58.33% vs 89.83%), CINV (30% vs 37.29%), anxiety (40% vs 44.07%), and defecation disorder (41.67% vs 67.80%) compared to the control group. There was significantly difference between the two groups in terms of unscheduled hospital admission (UHA), which 21.67% of the patients in the MTM intervention group and 30.51% in the control group (p<0.05).
Multivariate analysis of complications during home medication treatment
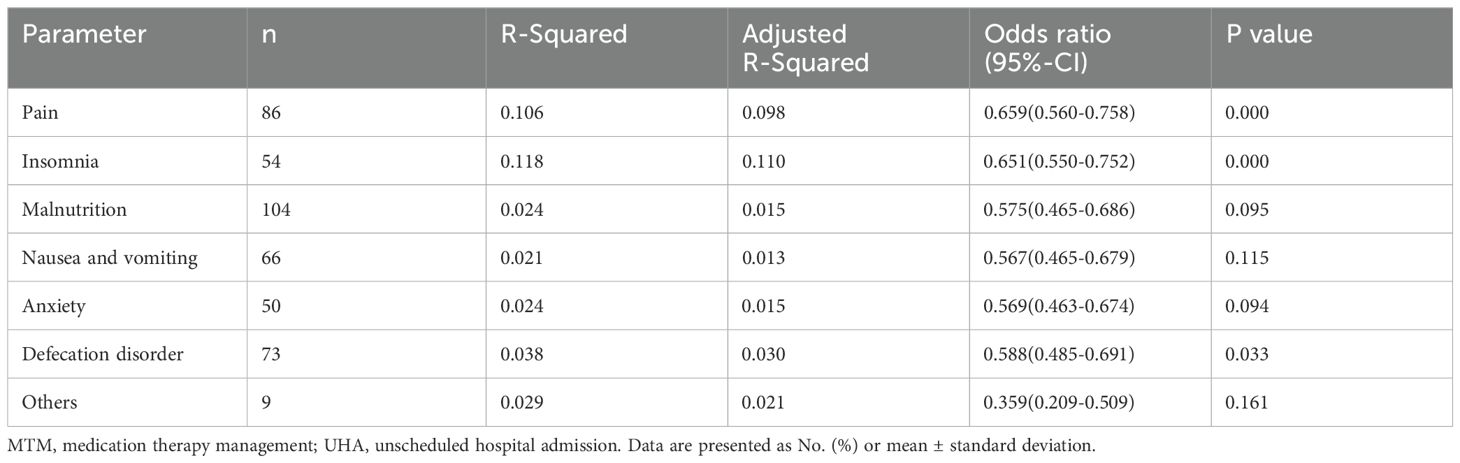
Relationship between unscheduled hospital admission and complications were conducted by multivariate analysis. Pain, insomnia, malnutrition, CINV, anxiety and defecation disorder were selected as significant risk factors of UHA (Table 3). Multivariate analysis results showed that pain, CINV, and defecation disorder were independent factors for unscheduled hospital admission (p<0.05).
The adverse effects between two groups
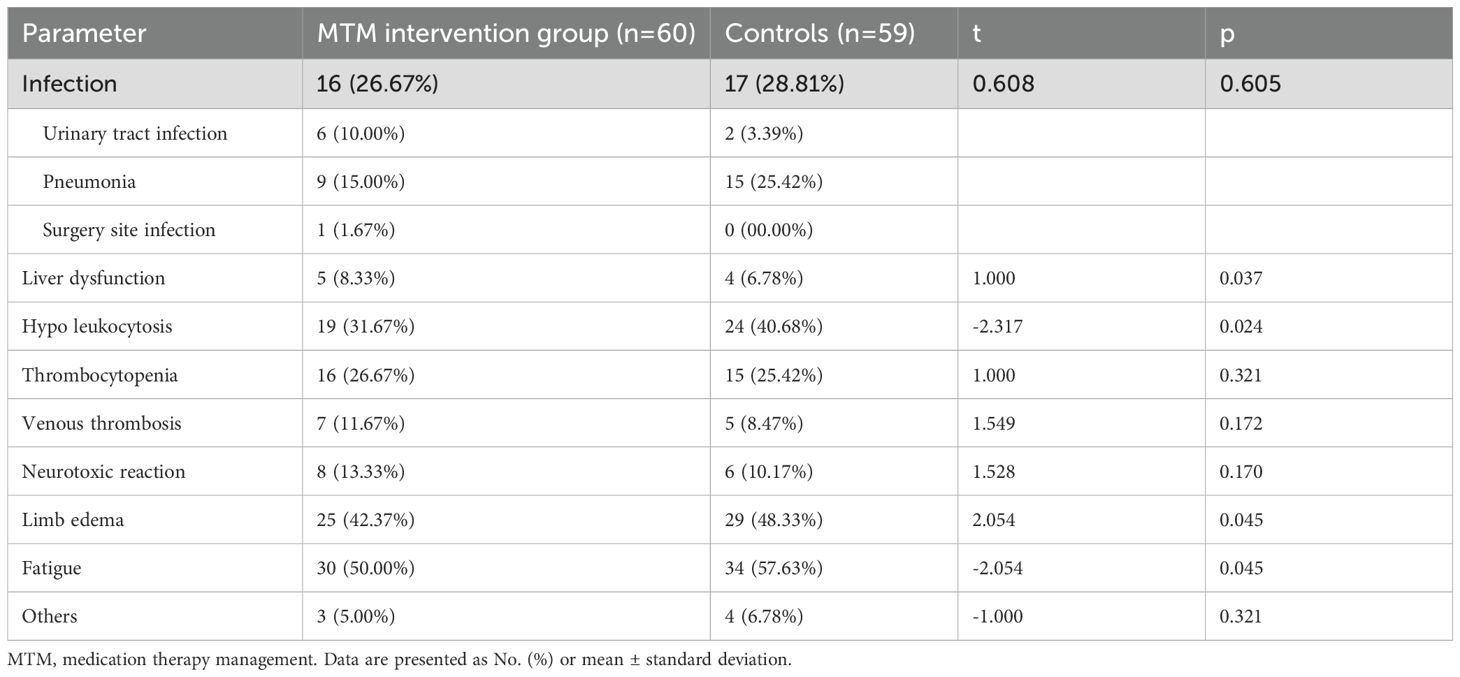
Colorectal cancer patients commonly receive various drug combinations that frequently lead to adverse drug reactions such as infection(urinary tract infection, pneumonia, surgery site infection), decreased liver function(jaundice), bone marrow suppression (primarily decreased white blood cells, platelets), venous thrombosis, limb edema, fatigue, and gastrointestinal reactions (inability to eat properly along with symptoms like nausea or vomiting), neurotoxic reactions (sensory disorders in extremities or abnormal sensations accompanied by painful cramps or cold sensitivity), skin manifestations oral mucositis impairment, the adverse effects. The most common adverse effects were fatigue, limb edema, hypo leukocytosis, and occurred 57.63%, 48.33%, and 40.68% in control group, and significantly decreased in MTM intervention group which were 50.00%, 42.37%, and 31.67%. There were significantly difference between the two groups in terms of liver dysfunction, hypo leukocytosis, limb edema, and fatigue (p<0.05; Table 4; Figure 2).
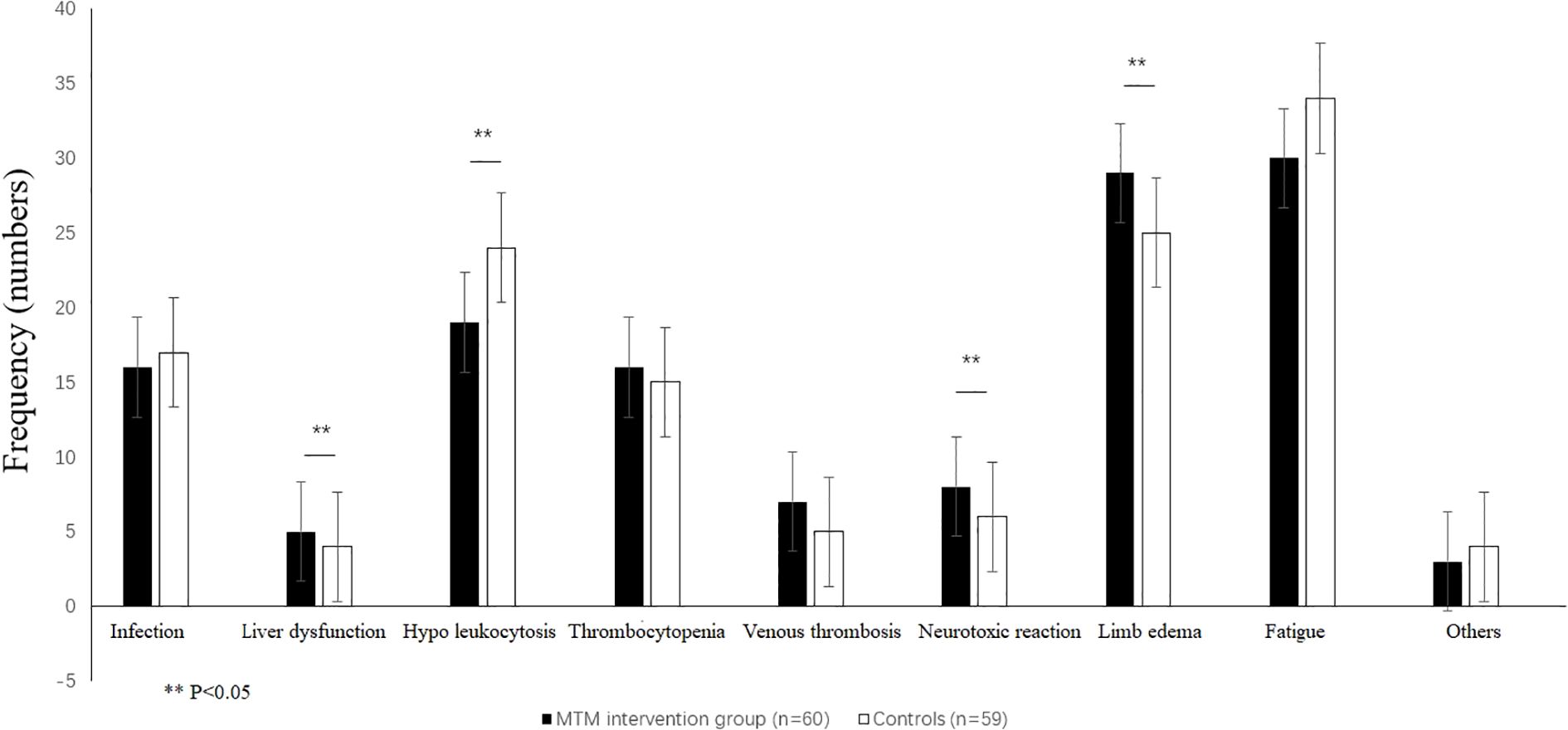
Figure 2. Patient reported adverse reactions included treatment infections, jaundice, hypo leukocytosis, thrombocytopenia, venous thrombosis, peripheral neuritis, limb edema, and purpose unclear. **p<0.05, by a t-test.
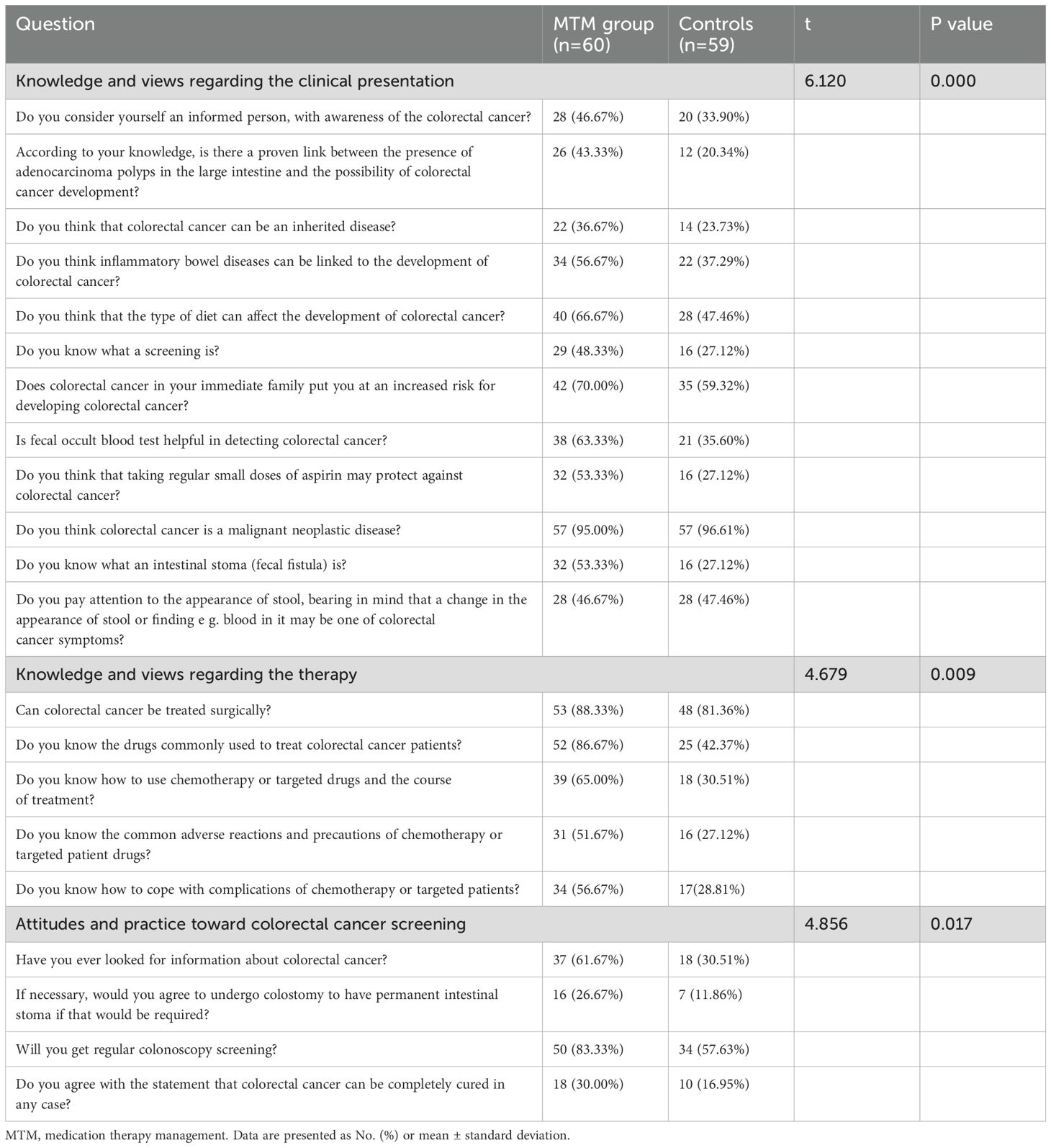
Questionnaire results of KPA from participants
A total of 119 questionnaires were sent out and returned, we counted “Yes” responses n (%). The Knowledge and views regarding the colorectal cancer and therapy rate in Group A was higher than group B (p<0.001). MTM intervention group showed better feedback than control group in Attitudes and practice Toward screening (p<0.05; Table 5).
Discussion
The implementation of Take-Home Cancer Drugs (THCDs) has become indispensable in the management of various malignancies (16). However, patients undergoing home-based treatment frequently encounter challenges due to insufficient medical support and limited understanding of their therapeutic regimens, potentially leading to medication errors or inappropriate drug use. Medication Therapy Management (MTM) interventions encompass a comprehensive approach, including the management of comorbidities, monitoring of additional medications, surveillance for adverse events, assessment of potential drug interactions, patient education, guidance provision, adherence evaluation, and management of treatment-related toxicities (17, 18). Pain represents a predominant symptom in the CRC population, with epidemiological studies indicating a prevalence exceeding 70% (19–21). Chemotherapy-induced peripheral neuropathy frequently manifests through characteristic symptoms such as seizures and neuropathic pain (22). The constipating effects of opioid analgesics are well-documented, with literature reporting constipation incidence rates ranging from 40% to 90% among chemotherapy patients (23, 24). These findings are consistent with our observational data, which revealed constipation rates of 42.37% in the control group versus 30% in the MTM intervention group. The pathophysiology is further complicated by reduced oral intake secondary to chemotherapy-induced anorexia, nausea, and vomiting.
The MTM intervention demonstrated significant clinical efficacy in symptom management. Pain severity was markedly reduced, with 51.67% of intervention group patients reporting improvement compared to 76.27% in controls. Sleep disturbances and bowel dysfunction showed notable improvement, with 30% fewer patients experiencing insomnia (versus 37.29% in controls) and 41.67% fewer patients reporting defecation disorders (compared to 67.8% in controls). The intervention group also demonstrated reduced consultation rates for surgical complications including anastomotic leakage, hemorrhage, wound infections, and pulmonary infections. Statistical analysis revealed significantly lower incidence rates of pain, sleep disturbances, weight loss, nausea/vomiting, and anxiety in the MTM group (p<0.05), accompanied by reduced unscheduled hospital admissions. Multivariate regression analysis identified significant correlations between unscheduled admissions and the presence of pain, insomnia, or defecation disorders (p<0.05). The intricate interplay between pain-induced insomnia and gastrointestinal symptoms creates a complex clinical syndrome that substantially impairs quality of life (25, 26). This cyclical relationship suggests that therapeutic strategies should prioritize the simultaneous management of these interconnected symptoms while minimizing treatment-related adverse effects (19).
Common challenges in home medication management include dosing errors, non-adherence (manifesting as under- or over-medication), severe toxicities, and postoperative complications (27, 28). Adverse drug reactions can be objectively assessed through laboratory parameters and clinical manifestations. For instance, leukopenia predisposes patients to infections, thrombocytopenia may cause hemorrhagic complications, hepatic dysfunction can lead to jaundice, and hypoalbuminemia may present as peripheral edema, while immunological disturbances can manifest as cutaneous pruritus. Alarmingly, medication non-adherence contributes to approximately 125,000 annual deaths (29), with cancer patients demonstrating particularly low adherence rates to oral therapies (46%) (30). However, targeted interventions have shown efficacy in improving medication adherence among high-risk populations (31, 32). Our findings indicate that structured medication management significantly reduced the incidence of hepatic dysfunction, leukopenia, peripheral edema, and fatigue in home-treated patients (p<0.05).
Patients with colorectal cancer received personalized education on disease management and medication protocols, tailored to their educational background and comprehension abilities. The educational program encompassed a comprehensive range of topics, including risk factors, clinical manifestations, treatment options, drug indications and dosages, optimal timing of medication administration, necessary precautions, management of adverse drug reactions, handling of overdoses or missed doses, lifestyle recommendations, and monitoring frequency for symptoms and signs during medication use (33). To assess the impact of the intervention, surveys were conducted using key performance indicators (KPA), which included 12 statements related to clinical presentation and risk factors, 5 statements evaluating participants’ confidence and behavior, and 5 statements assessing attitudes toward colorectal cancer screening. The results revealed statistically significant differences between the MTM intervention group and the control group in terms of knowledge and understanding of clinical presentation and therapy, as well as attitudes toward colorectal cancer screening practices. These findings suggest that MTM intervention enhances patients’ disease knowledge, boosts confidence in rehabilitation, and improves treatment cooperation, ultimately leading to better prognosis (p<0.05).
MTM is designed to provide individualized pharmaceutical care throughout the entire course of home drug therapy. It aims to promote health literacy and improve medication compliance by offering evidence-based recommendations combined with practical application (34). The services include a holistic evaluation of all medications used by the patient, rather than focusing solely on drugs for a single condition. Additionally, MTM provides health education, manages complications, monitors for adverse reactions in colorectal cancer patients, and addresses concurrent symptoms during treatment. These issues are prioritized because they are more urgent and directly impact patients’ quality of life (35). The results demonstrate that this pharmaceutical care model not only reduces the incidence of adverse reactions but also minimizes complication rates, while emphasizing patient-centered care through personalized services.
Conclusion
This study demonstrates that the application of MTM significantly reduces the incidence of THCDs-related complications such as CINV, abdominal pain, and insomnia, while also decreasing the rate of unscheduled hospital admissions. Furthermore, MTM enhances the KPA of colorectal cancer patients. However, the study has some limitations. First, the data were collected from a single center, this observation limits their applicability in a more heterogeneous international context, and future research should involve multi-center studies with larger populations to validate these findings. Additionally, due to the limited study duration, the relationship between complications and survival rates was not explored. Nevertheless, this study enrolled a substantial number of cases and included detailed follow-up parameters, enabling a comprehensive analysis. Therefore, the results and insights derived from this study remain valuable for advancing patient care in colorectal cancer management.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Ethics Committee of the Sixth Affiliated Hospital of Sun Yat-sen University (2022ZSLYEC-616). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants' legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
QW: Data curation, Formal analysis, Conceptualization, Writing – review & editing, Writing – original draft. YZ: Methodology, Writing – original draft, Data curation, Formal analysis. HL: Methodology, Data curation, Writing – original draft, Investigation. YO: Writing – original draft, Investigation, Formal analysis, Methodology. JF: Writing – original draft, Software, Investigation, Methodology. XW: Supervision, Writing – original draft, Investigation, Software. JC: Software, Writing – original draft, Project administration, Supervision. XL: Methodology, Project administration, Conceptualization, Writing – original draft, Software, Investigation, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by grants received from action plan for popularizing science at the community of Guangdong Provincial Science and Technology Association (GDKP2023-3-056), Clinical pharmaceutical Research Foundation of China International Medical Exchange Foundation (Z-2021-46-2101-2023), Wu Jieping Medical Foundation Clinical Research Fund (320.6750.2023-06-21). Supported by the program of Guangdong Provincial Clinical Research Center for Digestive Diseases (2020B1111170004).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer LT declared a shared parent affiliation with the authors to the handling editor at the time of review.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
THCDs, take home cancer drugs; MTM, medication treatment management; UHA, unscheduled hospital admission; CRC, colorectal cancer; CINV, chemotherapy-induced nausea or vomiting; STOPP, Screening Tool of Older Person’s Prescriptions; START, Screening Tool to Alert doctors to Right Treatment; ROC, Receiver Operator Characteristic; AUC, area under the curve; KPA, Knowledge-practice-attitude.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
2. Han B, Zheng R, Zeng H, Wang S, Sun K, Chen R, et al. Cancer incidence and mortality in China, 2022. J Natl Cancer Center. (2024) 4:47–53. doi: 10.1016/j.jncc.2024.01.006
3. Coleman MP, Quaresma M, Berrino F, Lutz J-M, De Angelis R, Capocaccia R, et al. Cancer survival in five continents: a worldwide population-based study (CONCORD). Lancet Oncol. (2008) 9:730–56. doi: 10.1016/S1470-2045(08)70179-7
4. Jiang Y, Yuan H, Li Z, Ji X, Shen Q, Tuo J, et al. Global pattern and trends of colorectal cancer survival: a systematic review of population-based registration data. Cancer Biol Med. (2021) 19:175–86. doi: 10.20892/j.issn.2095-3941.2020.0634
5. Holleczek B, Rossi S, Domenic A, Innos K, Minicozzi P, Francisci S, et al. On-going improvement and persistent differences in the survival for patients with colon and rectum cancer across Europe 1999-2007-Results from the EUROCARE-5 study. Eur J Cancer. (2015) 51:2158 68. doi: 10.1016/j.ejca.2015.07.024
6. Fang L, Yang Z, Zhang M, Meng M, Feng J, and Chen C. Clinical characteristics and survival analysis of colorectal cancer in China: a retrospective cohort study with 13,328 patients from southern China. Gastroenterol Rep (Oxf). (2021) 9:571–82. doi: 10.1093/gastro/goab048
7. Hauner K, Maisch P, and Retz M. Nebenwirkungen der Chemotherapie [Side effects of chemotherapy. Urologe A. (2017) 56:472–9. doi: 10.1007/s00120-017-0338-z
8. Beaver CC and Magnan MA. Managing chemotherapy side effects: achieving reliable and equitable outcomes. Clin J Oncol Nurs. (2016) 20:589–91. doi: 10.1188/16.CJON.589-591
9. Qu R, Ma Y, Zhang Z, and Fu W. Increasing burden of colorectal cancer in China. Lancet Gastroenterol Hepatol. (2022) 7:700. doi: 10.1016/S2468-1253(22)00156-X
10. Gamblin V, Prod’homme C, Lecoeuvre A, Bimbai A-, Luu J, Hazard PA, et al. Home hospitalization for palliative cancer care: factors associated with unplanned hospital admissions and death in hospital. BMC Palliat Care. (2021) 20:24. doi: 10.1186/s12904-021-00720-7
11. Pardhan A, Vu K, Gallo-Hershberg D, Forbes L, Gavura S, and Kukreti V. Evolving best practice for take-home cancer drugs. JCO Oncol Pract. (2021) 17:e526–36. doi: 10.1200/OP.20.00448
12. Austin Z. Medication therapy management - it’s complicated. Am J Pharm Educ. (2017) 81:6820. doi: 10.5688/ajpe8176820
13. Alqudah MAY, Al-Samman RM, Mukattash TL, and Abu-Farha RK. Knowledge and attitudes of pharmacists towards colorectal cancer health education in Jordan: A cross-sectional study. Int J Clin Pract. (2021) 75:e13986. doi: 10.1111/ijcp.13986
14. Chisholm-Burns MA, Kim Lee J, Spivey CA, Slack M, Herrier RN, Hall-Lipsy E, et al. US pharmacists’ effect as team members on patient care: systematic review and meta-analyses. Med Care. (2010) 48:923–33. doi: 10.1097/MLR.0b013e3181e57962
15. Wiegmann LE, Belisle MS, Alvarez KS, and Kale NJ. Aiming beyond: A pharmacist impact on 90-day readmissions and clinical outcomes within a family medicine service. J Pharm Pract. (2020) 33:738–44. doi: 10.1177/0897190019825970
16. Egerton NJ. In-office dispensing of oral oncolytics: a continuity of care and cost mitigation model for cancer patients. Am J Manag Care. (2016) 22:s99–s103.
17. Tartarone A, Gallucci G, Lazzari C, Lerose R, Lombardi L, Aieta M, et al. Crizotinib-induced cardiotoxicity: The importance of a proactive monitoring and management. Future Oncol. (2015) 11:2043–8. doi: 10.2217/fon.15.47
18. Kendall M, Mason B, Momen N, Barclay S, Munday D, Lovick R, et al. Proactive cancer care in primary care: A mixed-methods study. Fam Pract. (2013) 30:302–12. doi: 10.1093/fampra/cms085
19. Zielińska A, Włodarczyk M, Makaro A, Sałaga M, and Fichna J. Management of pain in colorectal cancer patients. Crit Rev Oncol Hematol. (2021) 157:103122. doi: 10.1016/j.critrevonc.2020.103122
20. Fallon M, Giusti R, Aielli F, Hoskin P, Rolke R, Sharma M, et al. Management of cancer pain in adult patients: ESMO Clinical Practice Guidelines. Ann Oncol. (2018) 29:iv166–91. doi: 10.1093/annonc/mdy152
21. Glare PA, Davies PS, Finlay E, Gulati A, Lemanne D, Moryl N, et al. Pain in cancer survivors. J Clin Oncol. (2014) 32:1739–47. doi: 10.1200/JCO.2013.52.4629
22. Derksen TM, Bours MJ, Mols F, and Weijenberg MP. Lifestyle-related factors in the self-management of chemotherapy-induced peripheral neuropathy in colorectal cancer: A systematic review. Evid Based Complement Alternat Med. (2017) 2017:7916031. doi: 10.1155/2017/7916031
23. Larkin PJ, Cherny NI, La Carpia D, Guglielmo M, Ostgathe C, Scotté F, et al. Diagnosis, assessment and management of constipation in advanced cancer: ESMO clinical practice guidelines. Ann Oncol. (2018) 29:iv111–25. doi: 10.1093/annonc/mdy148
24. Staats PS, Markowitz J, and Schein J. Incidence of constipation associated with long-acting opioid therapy: a comparative study. South Med J. (2004) 97:129–34. doi: 10.1097/01.SMJ.0000109215.54052.D8
25. Gatta G, Ciccolallo L, Faivre J, Bouvier AM, Berrino F, Gerard JP, et al. Late outcomes of colorectal cancer treatment: a FECS-EUROCARE study. J Cancer Surviv. (2007) 1:247–54. doi: 10.1007/s11764-007-0030-1
26. Schoormans D, van Es B, Mols F, Wasowicz D, Beijer S, and Ezendam NPM. The relation between sleep quality, sleep quantity, and gastrointestinal problems among colorectal cancer survivors: result from the PROFILES registry. Support Care Cancer. (2022) 30:1391–8. doi: 10.1007/s00520-021-06531-z
27. Butt F and Ream E. Implementing oral chemotherapy services in community pharmacies: A qualitative study of chemotherapy nurses’ and pharmacists’ views. Int J Pharm Pract. (2016) 24:149–59. doi: 10.1111/ijpp.12237
28. Goodin S, Griffith N, Chen B, Chuk K, Daouphars M, Doreau C, et al. Safe handling of oral chemotherapeutic agents in clinical practice: Recommendations from an international pharmacy panel. J Oncol Pract. (2011) 7:7–12. doi: 10.1200/JOP.2010.000068
29. Greer JA, Amoyal N, Nisotel L, Fishbein JN, MacDonald J, Stagl J, et al. A systematic review of adherence to oral antineoplastic therapies. Oncologist. (2016) 21:354–76. doi: 10.1634/theoncologist.2015-0405
30. Spoelstra SL, Given BA, Given CW, Grant M, Sikorskii A, You M, et al. An intervention to improve adherence and management of symptoms for patients prescribed oral chemotherapy agents: an exploratory study. Cancer Nurs. (2013) 36:18–28. doi: 10.1097/NCC.0b013e3182551587
31. Mathes T, Antoine SL, Pieper D, and Eikermann M. Adherence enhancing interventions for oral anticancer agents: a systematic review. Cancer Treat Rev. (2014) 40:102–8. doi: 10.1016/j.ctrv.2013.07.004
32. Viswanathan M, Kahwati LC, Golin CE, Blalock SJ, Coker-Schwimmer E, Posey R, Lohr KN, et al. Medication therapy management interventions in outpatient settings: A systematic review and meta-analysis. JAMA Intern Med. (2015) 175:76–87. doi: 10.1001/jamainternmed.2014.5841
33. Pallan A, Dedelaite M, Mirajkar N, Newman PA, Plowright J, and Ashraf S. Postoperative complications of colorectal cancer. Clin Radiol. (2021) 76:896–907. doi: 10.1016/j.crad.2021.06.002
34. Benjamin RM. Medication adherence: helping patients take their medicines as directed. Public Health Rep. (2012) 127:2–3. doi: 10.1177/003335491212700102
35. Ali K, Al-Quteimat O, Naseem R, Malhi SM, Wajdi M, Jahan N, et al. Incorporating a clinical oncology pharmacist into an ambulatory care pharmacy in pediatric hematology-oncology and transplant clinic: Assessment and significance. J Oncol Pharm Pract. (2021) 27:815–20. doi: 10.1177/1078155220934167
Keywords: colorectal cancer, MTM, complication, adverse effect, KPA
Citation: Wang Q, Zhou Y, Li H, Ou Y, Fei J, Wu X, Chen J and Li X (2025) Description and disposition of home patients with colorectal cancer accessing a practical, complications related medication therapy management service. Front. Oncol. 15:1595010. doi: 10.3389/fonc.2025.1595010
Received: 17 March 2025; Accepted: 30 June 2025;
Published: 17 July 2025.
Edited by:
Xiaodong Chu, Jinan University, ChinaReviewed by:
Yanfang Chen, Guangzhou Medical University, ChinaLiu Tao, Sun Yat-sen University Cancer Center (SYSUCC), China
Copyright © 2025 Wang, Zhou, Li, Ou, Fei, Wu, Chen and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xia Wu, d3V4aWEyQE1haWwuc3lzdS5lZHUuY24=; Junrong Chen, Y2hlbmpyNUBtYWlsLnN5c3UuZWR1LmNu; Xiaoyan Li, bGl4eWFuNUBtYWlsLnN5c3UuZWR1LmNu
†These authors have contributed equally to this work and share first authorship
 Qinbo Wang
Qinbo Wang Yuan Zhou3,4†
Yuan Zhou3,4† Xiaoyan Li
Xiaoyan Li