- Department of Medical Oncology, Tianjin Medical University General Hospital, Tianjin, China
Background: Aumolertinib is a novel third-generation epidermal growth factor receptor tyrosine kinase inhibitor (EGFR-TKI) with proven efficacy and safety for untreated non-small-cell lung cancer (NSCLC) patients with EGFR sensitizing mutations (EGFRm) in China. The progression-free survival (PFS) improvement of the combination of first-generation EGFR-TKIs and bevacizumab was confirmed by CTONG1509, JO25567, and NEJ026 studies, however, the effect of third-generation EGFR-TKIs plus bevacizumab remains under debate. This study aimed to investigate the efficacy and safety of aumolertinib plus bevacizumab in untreated EGFRm advanced NSCLC.
Methods: We conducted a phase II single-arm prospective clinical trial for advanced EGFRm NSCLC treated with aumolertinib combined with bevacizumab. Treatment continued until disease progression, occurrence of unacceptable toxicities, or the patient withdrew consent. The study was stratified according to sex, smoking history, stage, EGFR mutation status, and central nervous system (CNS) metastasis. The primary endpoint was the 12–month progression-free survival rate (PFS%), and secondary endpoints included the objective response rate (ORR), overall survival (OS), and progression-free survival (PFS).
Results: Between September 16, 2020, and November 11, 2021, a total of 21 patients were enrolled in the study. The median follow-up was 36.8 months (ranging from 33.2 to 40.4 months), and all 21 patients were included in the evaluation. The PFS% at 12-month was 81% (95% confidence interval (CI): 64.1–97.9%), the median PFS was 26 months (95% CI: 16.5-35.5) and the ORR reached 85.7%, with an average reduction of the target lesions of 48.2%. Among patients with CNS metastasis, the ORR was 92.9% (13/14), and for TP53 co-mutation patients, the ORR was 86.6% (12/14). Grade 3 adverse events were observed in 4 patients (19.2%), and no grade 4 or 5 adverse events reported.
Conclusion: The combination of aumolertinib and bevacizumab in patients with advanced EGFRm NSCLC achieved the study’s primary endpoint. This study indeed extended PFS compared with previous literature, and it was deemed safe and tolerable.
Introduction
Approximately 20-40% of non-small-cell lung cancer (NSCLC) patients harbor epidermal growth factor receptor mutations (EGFRm), primarily consisting of exon 19 deletions and exon 21 p.L858R (L858R) mutations (1). Epidermal growth factor receptor tyrosine kinase inhibitors (EGFR-TKIs), including gefitinib, erlotinib, and osimertinib, are the preferred first-line treatment for patients with EGFR mutations (2–4). Notably, when employed as the initial treatment for EGFRm NSCLC patients, osimertinib has demonstrated prolonged progression-free survival (PFS) and overall survival (OS) compared to erlotinib and gefitinib (2, 5). Furthermore, studies have indicated its superior brain permeability compared to first- and second-generation treatments (6, 7). Nevertheless, despite these advancements, disease progression and acquired resistance remain inevitable.
To address this challenge, integrated treatment strategies have been proposed. Bevacizumab, a recombinant anti-vascular endothelial growth factor (VEGF) monoclonal antibody, functions by inhibiting angiogenesis and impeding tumor growth (8). In numerous clinical phase 2/3 trials, the combination of EGFR-TKIs and bevacizumab has consistently shown a significant enhancement in progression-free survival (PFS) compared to EGFR-TKIs monotherapy when used as the primary treatment for patients with EGFRm NSCLC (9–11). Various phase 3 studies, such as FLAURA2 and AENEAS, which compare third-generation TKIs with first-generation TKIs as the initial therapy for patients with advanced EGFRm NSCLC, have reported the superior PFS associated with third-generation agents. Consequently, the exploration of combining third-generation EGFR-TKIs with bevacizumab in the first-line treatment of EGFRm NSCLC represents a current focal point of research.
Aumolertinib (formerly known as almonertinib; HS-10296) is an oral, third-generation EGFR-TKI designed to selectively target mutant EGFR rather than wild-type EGFR. It has been specifically formulated for the treatment of advanced EGFRm NSCLC. The APPOLO study demonstrated the remarkable efficacy and safety profile of aumolertinib in patients with EGFR T790M-positive NSCLC, showcasing its effectiveness, particularly in brain metastases (BM) (13). In the AENEAS trial, a phase 3 study conducted among Chinese patients, aumolertinib, as a first-line treatment, significantly extended progression-free survival (PFS) and duration of response (DOR) when compared to gefitinib in patients with advanced EGFRm NSCLC. However, the overall survival (OS) data from this study are currently immature (12).
A phase 1/2 single-arm study evaluating the initial treatment of osimertinib plus bevacizumab for advanced EGFRm NSCLC patients successfully achieved its primary endpoint, which was the 12-month progression-free survival rate (PFS%) (14). Building upon this foundation, we propose an investigation into the combination of aumolertinib and bevacizumab as a first-line therapy for advanced EGFRm NSCLC. Our objective is to explore the efficacy and safety of aumolertinib plus bevacizumab in untreated EGFRm advanced NSCLC.
Methods
Patient selection
Patients were enrolled between September 2020 to November 2021, and followed up until August 2024. The eligibility criteria were as follows: (1) individuals aged 18 years or older, (2) NSCLC confirmed histologically/cytologically or diagnosed clinically as advanced peripheral lung cancer, (3) EGFR-sensitizing mutation identified through next-generation sequencing (NGS) analysis, (4) clinical stage IIIB, IIIC, or IV disease not suitable for radical radiation therapy (based on the eighth edition of lung cancer TNM staging system), (5) presence of measurable lesion according to the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1; (6) adequate hematologic and organ function; (7) Eastern Coop-erative Oncology Group performance status 0 or 1, (8) signed informed consent form.
The exclusion criteria were: (1) previous exposure to EGFR inhibitors or VEGF receptor inhibitors, (2) coexistence or history of interstitial lung disease, (3) a high susceptibility to bleeding or embolism, (4) unmanaged hypertension, (5) Patients who received radiotherapy to the brain can participate, but are required to have an interval ≥14 days between the last days of radiotherapy and study treatment.
Treatment
The patients received oral aumolertinib (110 mg) once daily and bevacizumab (Avastin, 7.5 mg/kg) on day 1 intravenously every 3 weeks. Treatment persisted until disease progression, occurrence of unacceptable toxicities, or withdrawal of patient consent.
Evaluation of efficacy and safety
Eight weeks following the initiation of aumolertinib and bevacizumab, routine chest/abdominal computed tomography and brain magnetic resonance imaging (MRI) were conducted, with subsequent assessments every 2-4 months. Tumor lesion response was evaluated based on the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1. The Objective Response Rate (ORR) was defined as the proportion of patients achieving complete response and partial response (CR+PR). The Disease Control Rate (DCR) was calculated as the proportion of patients demonstrating objective response or stable disease for a minimum of four weeks. PFS was delineated as the duration from the initiation of the first medication to disease progression or death. The primary endpoint focused on 12-month PFS%, while secondary endpoints encompassed ORR, PFS, and safety parameters.
Adverse events (AEs) were systematically evaluated throughout the study, graded in accordance with the National Cancer Institute Common Terminology Criteria for Adverse Events version 5.0.
Sample size calculation
To consider combined aumolertinib and bevacizumab a promising treatment strategy, we aimed for an improvement in 12-month PFS rates from a historical 60% to 80%, which would require a sample size of 24 patients to provide 80% power while controlling the type 1 error at 10%. Ultimately, we achieved 80% of our projected enrollments.
Statistical analysis
Survival analyses were performed according to the Kaplan-Meier method, and confidence intervals (CIs) were calculated at a 95% confidence level. All analyses were performed using IBM SPSS software, version 24.0.
Ethics
This study adhered to the rules and regulations of clinical studies with respect to human subject protection, and it was approved by the Tianjin Medical University of General Hospital of Ethics Committee and conducted in accordance with the principles of the Declaration of Helsinki. Informed consent was obtained from all enrolled patients.
Results
Patient characteristics
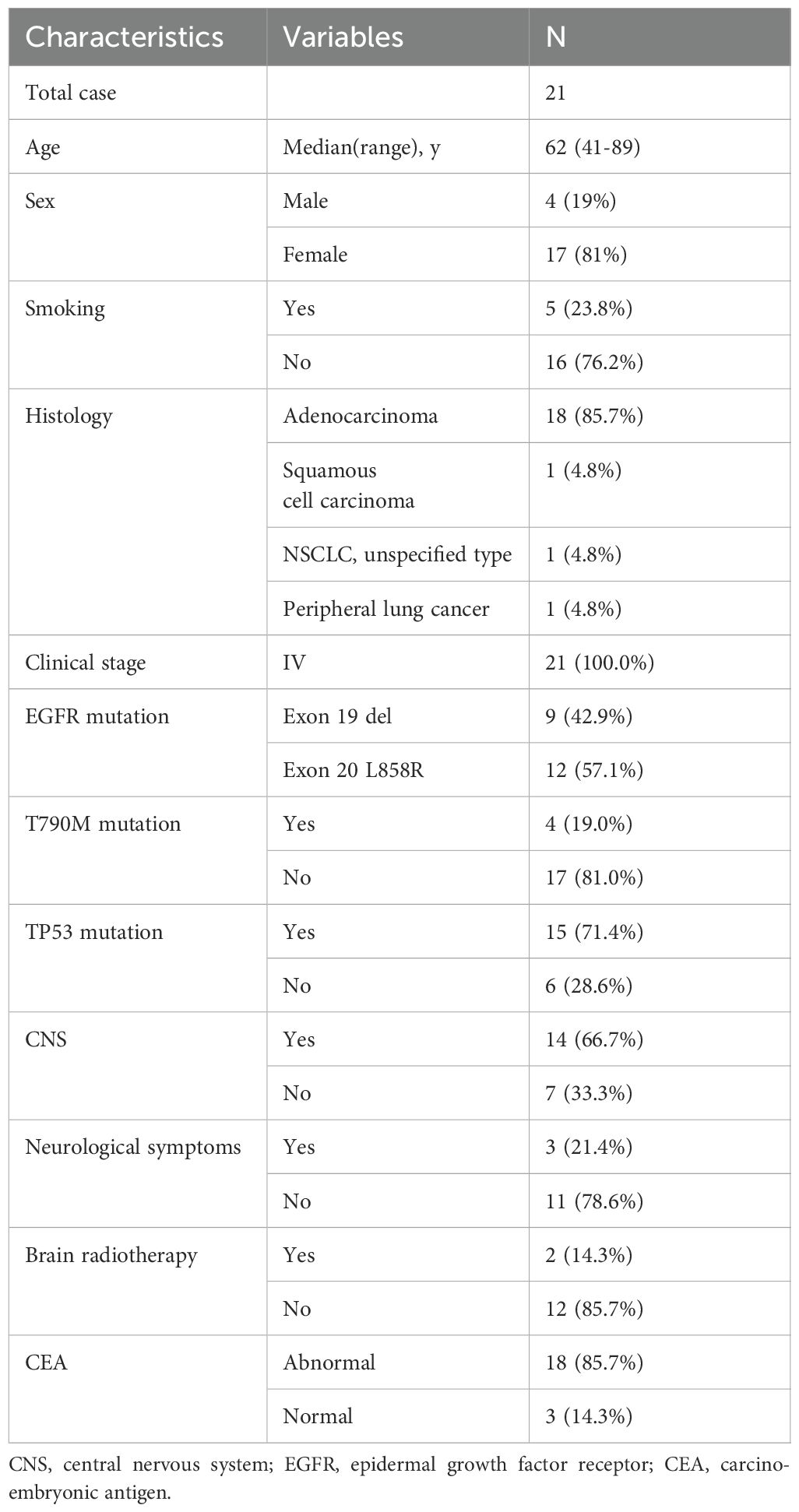
A total of 21 patients, comprising four men and fifteen women, were included in this study conducted between September 16, 2020, and November 11, 2021. The detailed characteristics of these enrolled patients are presented in Table 1. The median age of the participants was 62 years, with an age range spanning from 41 to 89 years. The majority of patients presented with adenocarcinoma (85.7%), while one had squamous cell carcinoma, another had NSCLC (undetermined pathological subtype), and one patient clinically manifested advanced peripheral lung cancer. All patients were diagnosed at clinical stage IV and received primary treatment. The EGFR mutations at diagnosis were located in exons 19 deletion (42.9%) and 21 L858R (57.1%). TP53 mutation and primary T790M occurred in 71.4% and 19.0% of cases, respectively. 14 patients had concomitant CNS involvement, with 21.4% (3/14) presenting symptomatic brain metastases at enrollment. Brain radiotherapy was administered to two patients, one undergoing whole-brain radiotherapy (WBRT), and the other receiving gamma knife radiosurgery.
Clinical responses
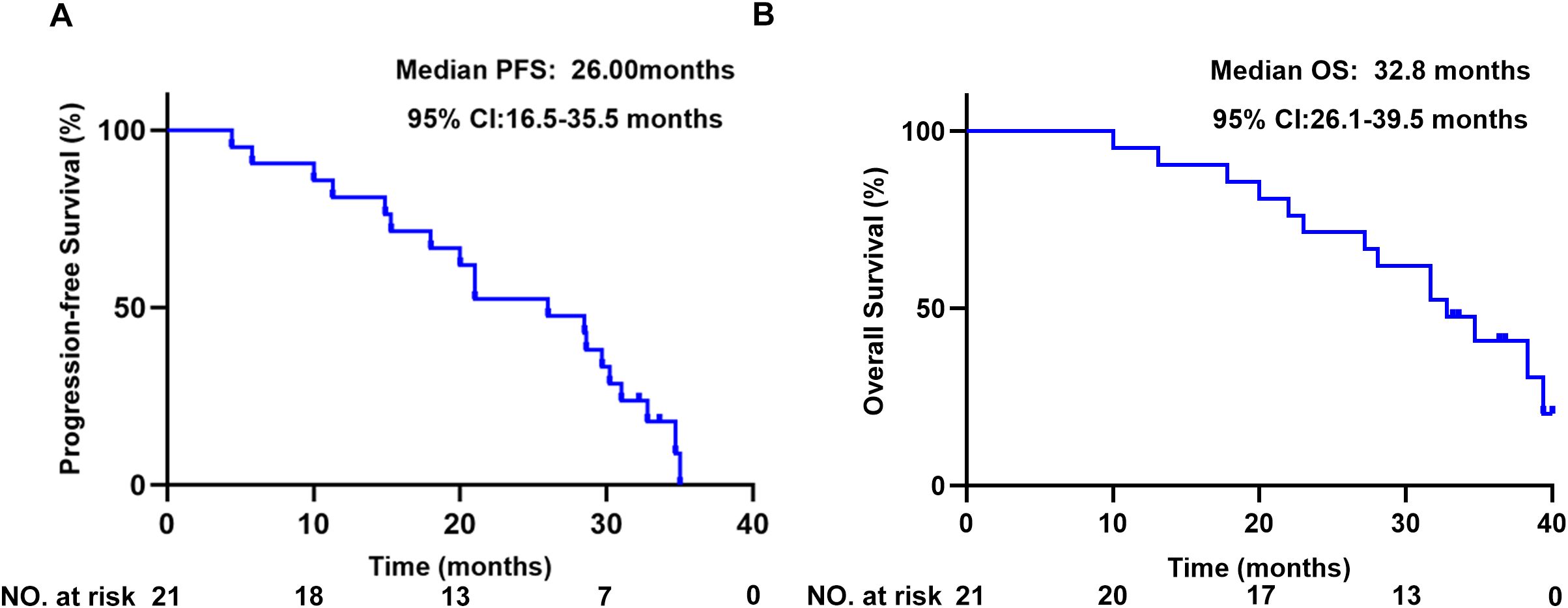
All 21 patients were ultimately evaluated. The primary endpoint, the 12-month PFS%, reached 81% (95% CI: 64.1–97.9%). The patients exhibited a median PFS of 26 months (95% CI: 16.5-35.5) (Figure 1A), with a median follow-up for PFS of 36.8 months (33.2m- 40.4m). The median OS was 32.8 months (95% CI: 26.1-39.5) (Figure 1B).
Within this cohort, seventeen patients experienced disease progression, whereas two withdrew from treatment without progression. Two patients continue to receive ongoing treatment (Figure 2A). Notably, 95.2% (20/21) of patients demonstrated a clinical response, marked by an average reduction of 48.2% in target lesions (Figure 2B).
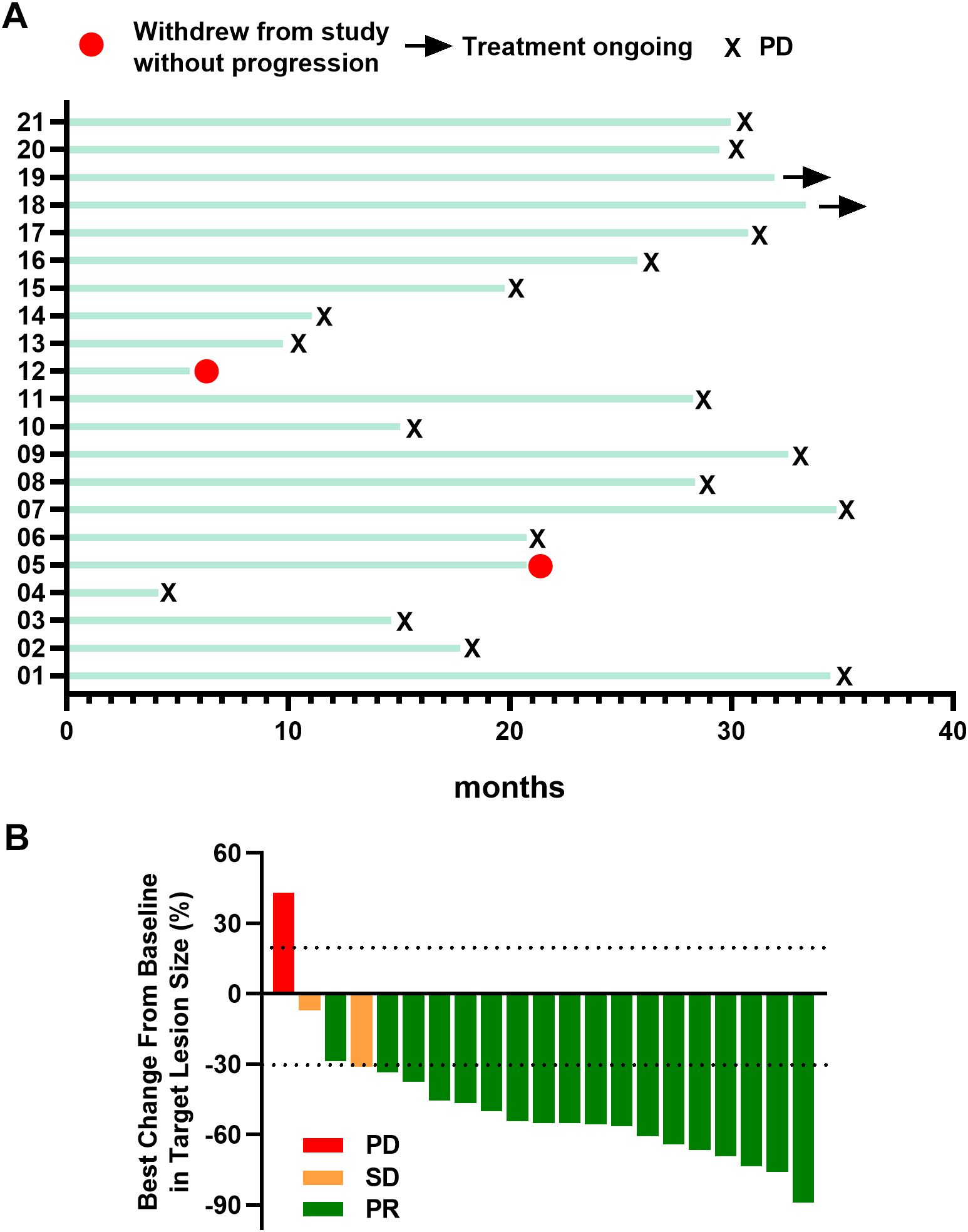
Figure 2. (A) Swimming plot of patients. (B) Best percentage change from baseline in target lesion size Responders were confirmed by Response Evaluation Criteria in Solid Tumors guidelines. PR, partial response; SD, stable disease; PD, progressive disease.
Subgroup analysis further explored various parameters. The median PFS for patients with CNS metastases (n=14) or without (n=7) were 21 (95% CI: 13.7-28.3) and 31 months (95% CI: 0–64.4), respectively, with a Hazard Ratio (HR) of 1.77(95% CI: 0.62–5.08) (Figure 3A). For patients with exon 19 deletion (n=9) or exon 21 p.L858 (n=12), the median PFS was 26 (95% CI: 11.4-40.6) and 20 months (95% CI: 2.2-37.8), respectively, with an HR of 0.57 (95% CI: 0.21–1.56) (Figure 3B). In patients with (n=15) and without (n=6) TP53 mutation, the median PFS was 21 months (95% CI: 4.34–37.67) and 26 months (95% CI: 15.68–36.32), respectively, with an HR of 0.86 (95% CI: 0.32–2.35) (Figure 3C). For patients with (n=4) and without (n=17) T790M mutation, the median PFS was 26 months (95% CI: 9.05–42.95) and 21 months (95% CI: 9.57–32.43), respectively, with an HR of 0.71 (95% CI: 0.20–2.48) (Figure 3D).

Figure 3. Subgroup analysis of PFS. (A): 19Del and L858R. (B): With or without CNS. (C): with or without TP53 mutant. (D): with or without T790M mutant. PFS, progression-free survival; 19 Del, EGFR 19 Del; L858R, EGFR 21 Exon L858R; CNS, central nervous system; TP53m, with TP53 mutant; TP53WT, without TP53 mutant; NE, not estimable; CI, confidence interval; HR, hazard ratio.
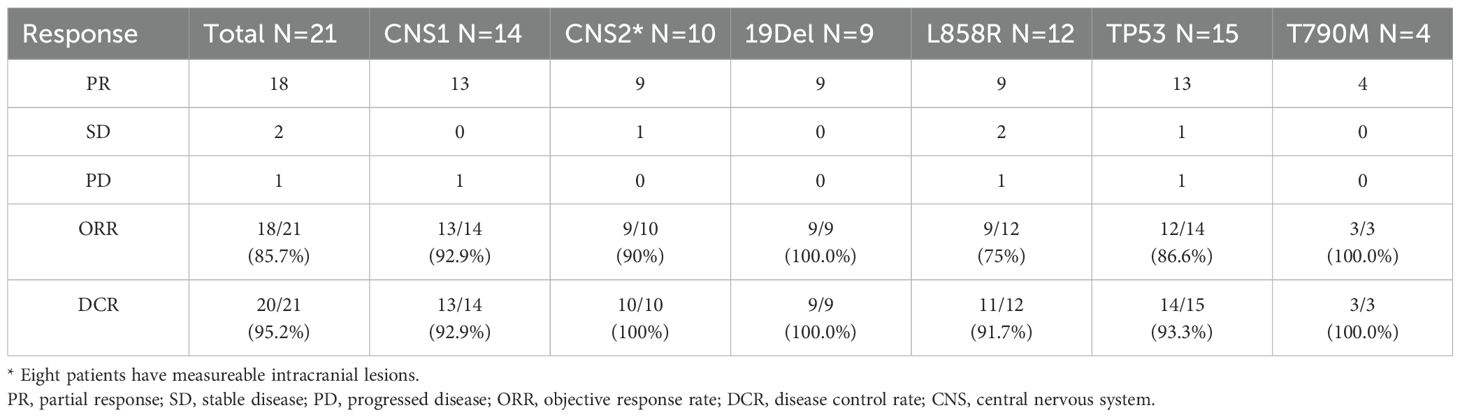
The ORR was 85.7% (18/21) (Table 2). Noteworthy ORRs were observed in patients with CNS metastasis (92.9%), intracranial lesions (90%), EGFR 19del (100.0%), EGFR L858R (75%), TP53 comutation (86.6%), and primary T790M comutation (100.0%) (Table 2).
Safety
The AEs encountered by patients during treatment with aumolertinib and bevacizumab are delineated in Table 3. The predominant AEs included increased creatine phosphokinase (28.6%), proteinuria (19.0%), elevated AST/ALT levels (19.0%), and weakness (14.3%). AEs of grade 3 or higher severity were noted in four patients (19%), with no occurrences of grade 4 or 5 events. Bevacizumab discontinuation was necessitated in four patients (23.5%) due to bleeding risk or creatine phosphokinase elevation accompanied by chest pain and arrhythmia.
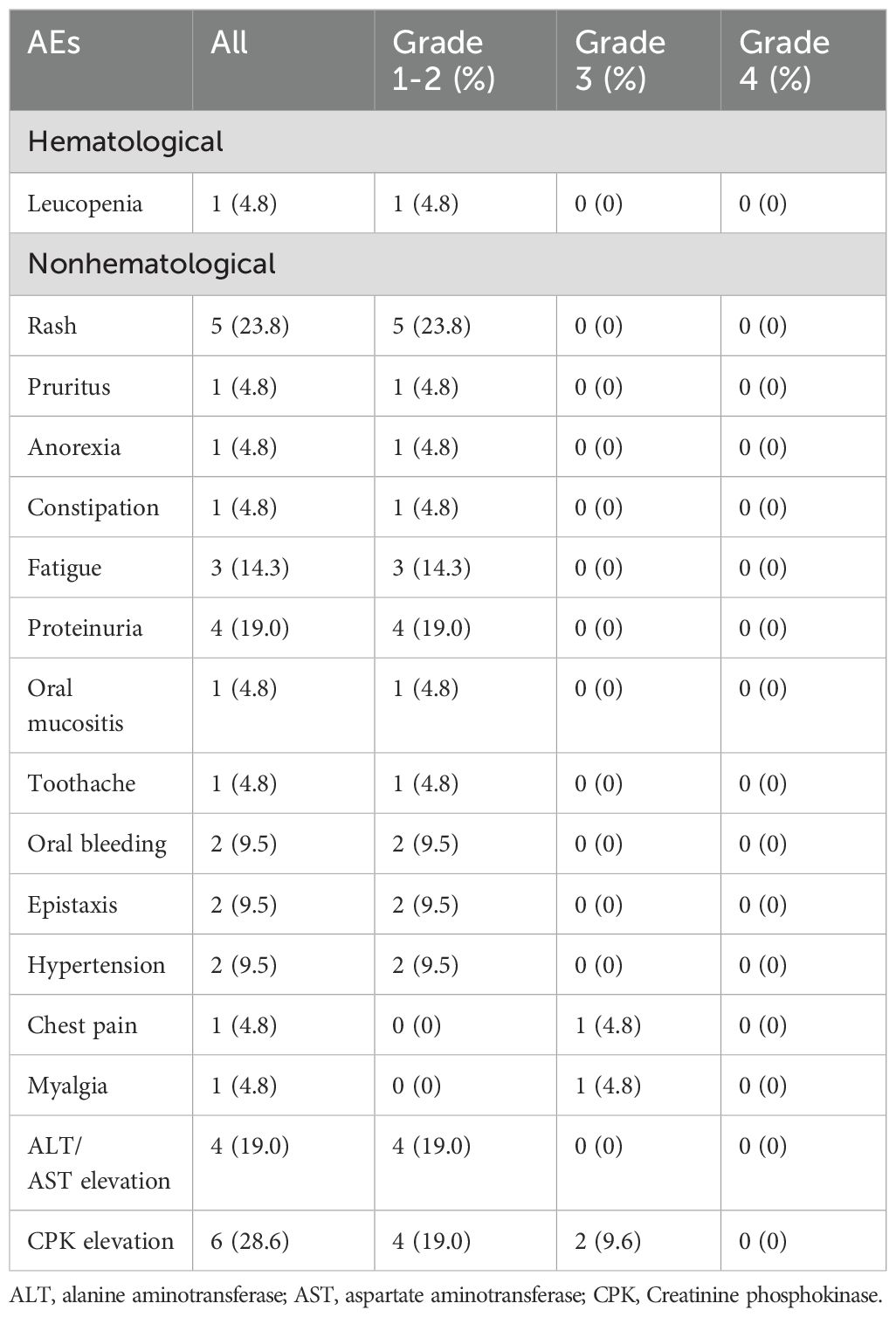
Table 3. Adverse events (AEs) graded according to the common toxicity criteria for adverse events (CTCAE).
Discussion
This phase 2 trial investigated the combined administration of aumolertinib and bevacizumab in previously untreated stage IV EGFRm NSCLC patients. This prospective Phase II clinical trial met the primary study endpoint. Moreover, in comparison with previously published literature, it demonstrated favorable treatment efficacy and exhibited good safety and tolerability. The adverse events align with the known characteristics of each agent.
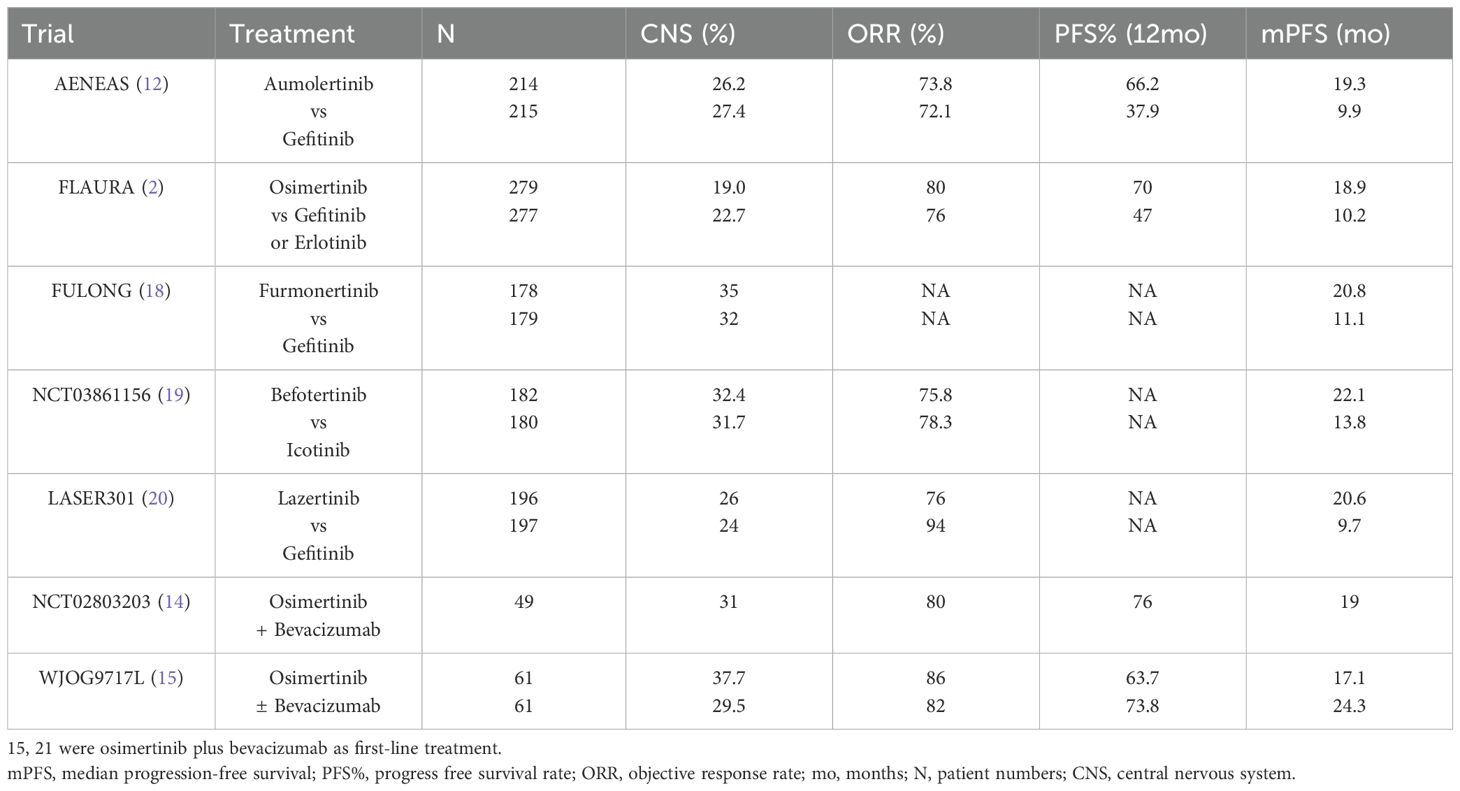
The study design is rooted in prior trials showing improved efficacy and safety when combining first-generation EGFR-TKIs with VEGF inhibitors for EGFRm NSCLC patients (9–11, 16, 17). Previous trials revealed the significant improvement in PFS with third-generation TKIs as first-line treatment (2) (Table 4). Our study, notably, emulated the structure of a phase 1/2 trial investigating osimertinib and bevacizumab. The results showcased a 12-month PFS of 81%, surpassing the outcomes observed with aumolertinib alone and the osimertinib-bevacizumab combination. Compared with aumolertinib alone in AENEAS (19.3 months), the median PFS (26 months) in this study seemed to be more beneficial (12). During the period of our study, data from phase 3 studies of third-generation EGFR-TKIs and studies investigating osimertinib in combination with bevacizumab have been published and are summarized in Table 4 (12, 14, 18–20). The WJOG9717L study, however, indicated no improvement in PFS with osimertinib-bevacizumab as first-line treatment (15). The mPFS and 1-year PFS rate in our study are higher than those in these studies, heralding the potential for the combination treatment regimen in this study to become a frontline therapeutic approach. It may require more refined stratification to identify specific populations that may precisely benefit from the combination therapy.
Our study’s patient characteristics differed, notably with a higher percentage of patients having preexisting CNS metastases (66.7%, Table 4) (2, 12, 18–20). CNS metastases associated with a more aggressive disease phenotype and significantly shorter TKI treatment-related PFS compared to those without CNS metastases (21). The established vascular normalization effect of antiangiogenic drugs in the central nervous system has therapeutic implications. The BRAIN study demonstrated that bevacizumab could delay brain metastases, showcasing its potential value (22). A retrospective study on EGFRm NSCLC patients with brain metastases revealed a 100% ORR and a 2-year survival rate of 62.5%, suggesting the efficacy of first-generation EGFR-TKI combined with bevacizumab as first-line therapy (23). Presently, third-generation EGFR-TKIs like osimertinib exhibit superior CNS efficacy in patients with EGFR-mutant brain metastases (24, 25). Our study aligns with the AENEAS analysis at ASCO 2022 (NO.9096), indicating that combining aumolertinib and bevacizumab may be effective and protective against CNS progression, as suggested by a 92.9% ORR in our study.
In subgroup analysis, patients with EGFR exon 19 deletions exhibited better PFS trends, contrasting with worse trends in those with EGFR L858R. This is consistent with the trend of subgroup analysis in the WJOG9717L study (15). The relationship between TP53 mutation and EGFR-TKI efficacy remains debated, our data indicate better PFS trends in patients without TP53 mutation. Additionally, previous studies indicated that the occurrence of a primary T790M mutation was associated with worse response and poor prognosis in patients with advanced EGFR-m NSCLC treated with first-generation (26–29), and second-generation (30, 31) EGFR-TKIs. In recent studies, primary T790M mutation showed some sensitivity towards osimertinib with the ORR fluctuating between 10% and 72.2% (32–37). In our study, four patients who had concurrent EGFR T790M at baseline benefited from the treatment strategy combining bevacizumab with aumolertinib. The best therapeutic outcome was PR, and the ORR reached 100%. This suggests that in cases of primary resistance to first-generation EGFR TKIs mediated by the primary EGFR T790M mutation, the treatment strategy adopted in this study is capable of enhancing the clinical efficacy.
Despite these therapeutic implications, our study has limitations. A small sample size from a single institution may impact result conclusiveness. Being a single-arm study lacks a control group, and future studies should expand the sample size and consider randomized controlled trials.
In conclusion, our study suggests potential benefits of aumolertinib and bevacizumab combination treatment for EGFRm advanced NSCLC compared to aumolertinib monotherapy. Nonetheless, it cannot be concluded arbitrarily that aumolertinib combined with bevacizumab is the first-line treatment of EGFRm NSCLC. Next, we should focus on subgroup analysis to select the population more suitable for the combination of aumolertinib and bevacizumab, further expand the sample size, and conduct randomized controlled studies. In the future, whether aumolertinib combined with bevacizumab can significantly improve PFS as a first-line treatment strategy may be answered.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Tianjin Medical University of General Hospital of Ethics Committee. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
LK: Data curation, Formal Analysis, Writing – original draft, Funding acquisition, Writing – review & editing. LP: Data curation, Writing – original draft. XY: Data curation, Software, Writing – original draft. QM: Data curation, Investigation, Methodology, Resources, Writing – original draft. LZ: Data curation, Formal Analysis, Investigation, Writing – original draft. XL: Project administration, Software, Visualization, Writing – original draft. DZ: Conceptualization, Supervision, Funding acquisition, Writing – review & editing. FM: Conceptualization, Data curation, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was funded by grants from the National Natural Science Foundation of China (grant number:81702678, 82272672), and the China Postdoctoral Science Foundation (Certificate Number:2023M742622).
Acknowledgments
We thank the study participants for their invaluable cooperation.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Liu L, Liu J, Shao D, Deng Q, Tang H, Liu Z, et al. Comprehensive genomic profiling of lung cancer using a validated panel to explore therapeutic targets in East Asian patients. Cancer Sci. (2017) 108:2487–94. doi: 10.1111/cas.2017.108.issue-12
2. Soria JC, Ohe Y, Vansteenkiste J, Reungwetwattana T, Chewaskulyong B, Lee KH, et al. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med. (2018) 378:113–25. doi: 10.1056/NEJMoa1713137
3. Rosell R, Carcereny E, Gervais R, Vergnenegre A, Massuti B, Felip E, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. (2012) 13:239–46. doi: 10.1016/S1470-2045(11)70393-X
4. Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. (2010) 362:2380–8. doi: 10.1056/NEJMoa0909530
5. Ramalingam SS, Vansteenkiste J, Planchard D, Cho BC, Gray JE, Ohe Y, et al. Overall survival with osimertinib in untreated, EGFR-mutated advanced NSCLC. N Engl J Med. (2020) 382:41–50. doi: 10.1056/NEJMoa1913662
6. Ballard P, Yates JW, Yang Z, Kim DW, Yang JC, Cantarini M, et al. Preclinical comparison of osimertinib with other EGFR-TKIs in EGFR-mutant NSCLC brain metastases models, and early evidence of clinical brain metastases activity. Clin Cancer Res. (2016) 22:5130–40. doi: 10.1158/1078-0432.CCR-16-0399
7. Ahn MJ, Chiu CH, Cheng Y, Han JY, Goldberg SB, Greystoke A, et al. Osimertinib for patients with leptomeningeal metastases associated with EGFR T790M-positive advanced NSCLC: the AURA leptomeningeal metastases analysis. J Thorac Oncol. (2020) 15:637–48. doi: 10.1016/j.jtho.2019.12.113
8. Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. (2006) 355:2542–50. doi: 10.1056/NEJMoa061884
9. Seto T, Kato T, Nishio M, Goto K, Atagi S, Hosomi Y, et al. Erlotinib alone or with bevacizumab as first-line therapy in patients with advanced non-squamous non-small-cell lung cancer harbouring EGFR mutations (JO25567): an open-label, randomised, multicentre, phase 2 study. Lancet Oncol. (2014) 15:1236–44. doi: 10.1016/S1470-2045(14)70381-X
10. Saito H, Fukuhara T, Furuya N, Watanabe K, Sugawara S, Iwasawa S, et al. Erlotinib plus bevacizumab versus erlotinib alone in patients with EGFR-positive advanced non-squamous non-small-cell lung cancer (NEJ026): interim analysis of an open-label, randomised, multicentre, phase 3 trial. Lancet Oncol. (2019) 20:625–35. doi: 10.1016/S1470-2045(19)30035-X
11. Zhou Q, Xu CR, Cheng Y, Liu YP, Chen GY, Cui JW, et al. Bevacizumab plus erlotinib in Chinese patients with untreated, EGFR-mutated, advanced NSCLC (ARTEMIS-CTONG1509): A multicenter phase 3 study. Cancer Cell. (2021) 39:1279–1291.e1273. doi: 10.1016/j.ccell.2021.07.005
12. Lu S, Dong X, Jian H, Chen J, Chen G, Sun Y, et al. AENEAS: A randomized phase III trial of aumolertinib versus gefitinib as first-line therapy for locally advanced or metastaticNon-small-cell lung cancer with EGFR exon 19 deletion or L858R mutations. J Clin Oncol. (2022) 40:3162–71. doi: 10.1200/JCO.21.02641
13. Lu S, Wang Q, Zhang G, Dong X, Yang CT, Song Y, et al. Efficacy of aumolertinib (HS-10296) in patients with advanced EGFR T790M+ NSCLC: updated post-national medical products administration approval results from the APOLLO registrational trial. J Thorac Oncol. (2022) 17:411–22. doi: 10.1016/j.jtho.2021.10.024
14. Yu HA, Schoenfeld AJ, Makhnin A, Kim R, Rizvi H, Tsui D, et al. Effect of osimertinib and bevacizumab on progression-free survival for patients with metastatic EGFR-mutant lung cancers: A phase 1/2 single-group open-label trial. JAMA Oncol. (2020) 6:1048–54. doi: 10.1001/jamaoncol.2020.1260
15. Kenmotsu H, Wakuda K, Mori K, Kato T, Sugawara S, Kirita K, et al. Randomized phase 2 study of osimertinib plus bevacizumab versus osimertinib for untreated patients with nonsquamous NSCLC harboring EGFR mutations: WJOG9717L study. J Thorac Oncol. (2022) 17:1098–108. doi: 10.1016/j.jtho.2022.05.006
16. Ichihara E, Hotta K, Nogami N, Kuyama S, Kishino D, Fujii M, et al. Phase II trial of gefitinib in combination with bevacizumab as first-line therapy for advanced non-small cell lung cancer with activating EGFR gene mutations: the Okayama Lung Cancer Study Group Trial 1001. J Thorac Oncol. (2015) 10:486–91. doi: 10.1097/JTO.0000000000000434
17. Nakagawa K, Garon EB, Seto T, Nishio M, Ponce Aix S, Paz-Ares L, et al. Ramucirumab plus erlotinib in patients with untreated, EGFR-mutated, advanced non-small-cell lung cancer (RELAY): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. (2019) 20:1655–69. doi: 10.1016/S1470-2045(19)30634-5
18. Shi Y, Chen G, Wang X, Liu Y, Wu L, Hao Y, et al. Furmonertinib (AST2818) versus gefitinib as first-line therapy for Chinese patients with locally advanced or metastatic EGFR mutation-positive non-small-cell lung cancer (FURLONG): a multicentre, double-blind, randomised phase 3 study. Lancet Respir Med. (2022) 10:1019–28. doi: 10.1016/S2213-2600(22)00168-0
19. Shun Lu JZ, Jian H, Wu L, Chen Y, Fan Y, Fang J, et al. Befotertinib (D-0316) versus icotinib as first-line therapy for patients with EGFR-mutated locally advanced or metastatic non-small cell lung cancer: A multicentre, open-label, randomized phase III study. ESMO ASIA. (2022) 11:905-15.
20. Cho BC, Ahn MJ, Kang JH, Soo RA, Reungwetwattana T, Yang JC, et al. A randomized, double-blind, multinational phase III study to assess the efficacy and safety of lazertinib versus gefitinib in the first-line treatment of patients with EGFR mutation (EGFRm), advanced NSCLC. ESMO ASIA. (2022) 20:4208-17. doi: 10.1200/JCO.23.00515
21. Schuler M, Wu YL, Hirsh V, O’Byrne K, Yamamoto N, Mok T, et al. First-line afatinib versus chemotherapy in patients with non-small cell lung cancer and common epidermal growth factor receptor gene mutations and brain metastases. J Thorac Oncol. (2016) 11:380–90. doi: 10.1016/j.jtho.2015.11.014
22. Besse B, Le Moulec S, Mazieres J, Senellart H, Barlesi F, Chouaid C, et al. Bevacizumab in patients with nonsquamous non-small cell lung cancer and asymptomatic, untreated brain metastases (BRAIN): A nonrandomized, phase II study. Clin Cancer Res. (2015) 21:1896–903. doi: 10.1158/1078-0432.CCR-14-2082
23. Chikaishi Y, Kanayama M, Taira A, Nabe Y, Shinohara S, Kuwata T, et al. Effect of erlotinib plus bevacizumab on brain metastases in patients with non-small cell lung cancer. Ann Transl Med. (2018) 6:401. doi: 10.21037/atm.2018.09.33
24. Reungwetwattana T, Nakagawa K, Cho BC, Cobo M, Cho EK, Bertolini A, et al. CNS response to osimertinib versus standard epidermal growth factor receptor tyrosine kinase inhibitors in patients with untreated EGFR-mutated advanced non-small-cell lung cancer. J Clin Oncol. (2018) 36:JCO2018783118. doi: 10.1200/JCO.2018.78.3118
25. Wu YL, Ahn MJ, Garassino MC, Han JY, Katakami N, Kim HR, et al. CNS efficacy of osimertinib in patients with T790M-positive advanced non-small-cell lung cancer: data from a randomized phase III trial (AURA3). J Clin Oncol. (2018) 36:2702–9. doi: 10.1200/JCO.2018.77.9363
26. Yu HA, Arcila ME, Hellmann MD, Kris MG, Ladanyi M, and Riely GJ. Poor response to erlotinib in patients with tumors containing baseline EGFR T790M mutations found by routine clinical molecular testing. Ann Oncol. (2014) 25:423–8. doi: 10.1093/annonc/mdt573
27. Ding D, Yu Y, Li Z, Niu X, and Lu S. The predictive role of pretreatment epidermal growth factor receptor T790M mutation on the progression-free survival of tyrosine-kinase inhibitor-treated non-small cell lung cancer patients: a meta-analysis. Onco Targets Ther. (2014) 7:387–93. doi: 10.2147/OTT.S58870
28. Liu Y, Sun L, Xiong ZC, Sun X, Zhang SL, Ma JT, et al. Meta-analysis of the impact of de novo and acquired EGFR T790M mutations on the prognosis of patients with non-small cell lung cancer receiving EGFR-TKIs. Onco Targets Ther. (2017) 10:2267–79. doi: 10.2147/OTT.S133082
29. Costa C, Molina MA, Drozdowskyj A, Gimenez-Capitan A, Bertran-Alamillo J, Karachaliou N, et al. The impact of EGFR T790M mutations and BIM mRNA expression on outcome in patients with EGFR-mutant NSCLC treated with erlotinib or chemotherapy in the randomized phase III EURTAC trial. Clin Cancer Res. (2014) 20:2001–10. doi: 10.1158/1078-0432.CCR-13-2233
30. Yang JC, Schuler M, Popat S, Miura S, Heeke S, Park K, et al. Afatinib for the treatment of NSCLC harboring uncommon EGFR mutations: A database of 693 cases. J Thorac Oncol. (2020) 15:803–15. doi: 10.1016/j.jtho.2019.12.126
31. Yang JC, Schuler M, Popat S, Miura S, Park K, Passaro A, et al. Afatinib for the treatment of non-small cell lung cancer harboring uncommon EGFR mutations: an updated database of 1023 cases brief report. Front Oncol. (2022) 12:834704. doi: 10.3389/fonc.2022.834704
32. Wang S, Yan B, Zhang Y, Xu J, Qiao R, Dong Y, et al. Different characteristics and survival in non-small cell lung cancer patients with primary and acquired EGFR T790M mutation. Int J Cancer. (2019) 144:2880–6. doi: 10.1002/ijc.v144.11
33. Zhang B, Xu J, Zhang X, Gu P, Wang H, Wang S, et al. Coexistence of sensitive and resistant epidermal growth factor receptor (EGFR) mutations in pretreatment non-small cell lung cancer (NSCLC) patients: First or third generation tyrosine kinase inhibitors (TKIs)? Lung Cancer. (2018) 117:27–31. doi: 10.1016/j.lungcan.2018.01.006
34. Si J, Gu X, Wang W, Ying S, and Song Z. Clinical outcomes of lung adenocarcinoma patients harboring uncommon epidermal growth factor receptor (EGFR) mutations treated with EGFR-tyrosine kinase inhibitors (TKIs). Ann Palliat Med. (2022) 11:1624–34. doi: 10.21037/apm-21-2828
35. Zeng Y, Guo T, Zhou Y, Zhao Y, Chu L, Chu X, et al. Clinical outcomes of advanced non-small cell lung cancer patients harboring distinct subtypes of EGFR mutations and receiving first-line tyrosine kinase inhibitors: brain metastasis and. novo T790M matters. BMC Cancer. (2022) 22:198. doi: 10.1186/s12885-022-09245-5
36. Chang JW, Huang CY, Fang YF, Chang CF, Yang CT, Kuo CS, et al. Epidermal growth factor receptor tyrosine kinase inhibitors for de novo T790M mutation: A retrospective study of 44 patients. Thorac Cancer. (2022) 13:1888–97. doi: 10.1111/1759-7714.14272
Keywords: aumolertinib, bevacizumab, EGFR-TKI, non-small cell lung cancer, progression-free survival
Citation: Kong L, Peng L, Yang X, Ma Q, Zhang L, Liu X, Zhong D and Meng F (2025) Aumolertinib plus bevacizumab for untreated advanced NSCLC with EGFR sensitive mutation. Front. Oncol. 15:1595812. doi: 10.3389/fonc.2025.1595812
Received: 18 March 2025; Accepted: 16 May 2025;
Published: 04 June 2025.
Edited by:
Massimo Broggini, Mario Negri Institute for Pharmacological Research (IRCCS), ItalyReviewed by:
Jing Wang, Mass General Brigham, United StatesClaudia Scimone, University of Naples Federico II, Italy
Copyright © 2025 Kong, Peng, Yang, Ma, Zhang, Liu, Zhong and Meng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Diansheng Zhong, ZHpob25nQHRtdS5lZHUuY24=; Fanlu Meng, bWVuZ2Zhbmx1MTEwMUAxNjMuY29t
†These authors have contributed equally to this work
 Lingping Kong
Lingping Kong Lina Peng†
Lina Peng† Linlin Zhang
Linlin Zhang Xia Liu
Xia Liu Diansheng Zhong
Diansheng Zhong