- Department of Gastrointestinal Surgery, Shandong Cancer Hospital and Institute, Shandong First Medical University and Shandong Academy of Medical Sciences, Jinan, Shandong, China
Breast cancer is one of the most common malignant tumors in women. Most early-stage breast cancer does not have the typical symptoms and signs. The common metastatic sites of breast cancer are bones, lymph nodes, soft tissues, lungs, brain, liver. Cases with the gastrointestinal (GI) tract as the first site of metastasis are relatively rare. Their clinical, imaging, and gastroscopic manifestations lack specificity, making them difficult to distinguish from primary gastric cancer. A 45-year-old woman presented with a week-long history of black stools, later diagnosed as gastric metastasis from breast cancer. The patient had undergone surgery, radiotherapy, and chemotherapy 18 years prior for left invasive lobular carcinoma (ILC) of the breast. Current symptoms included black stools, hiccups, fatigue, and decreased appetite, with no nausea, fever, or chills. Gastroscopy revealed a gastric ulcer, and biopsy confirmed poorly differentiated gastric adenocarcinoma. PET-CT indicated high metabolism in the stomach but no distant metastasis. A total gastrectomy with lymph node dissection revealed tumor invasion of the serosal membrane and nerves, confirming metastatic breast cancer. Postoperative treatment and follow-up showed no recurrence or metastasis, and the patient remained stable. Gastric metastasis from breast cancer is an uncommon condition, mostly associated with invasive lobular carcinoma. Accurate diagnosis requires careful consideration of the patient’s medical history and a comprehensive approach utilizing clinical manifestations, imaging, endoscopy, histopathology, and immunohistochemistry (IHC) to minimize the risk of missed or incorrect diagnoses. Treatment remains centered on systemic therapies, including chemotherapy and endocrine therapy.
Introduction
Breast cancer is the malignant tumor with the highest incidence among women in China (1). It is diagnosed by pathologists It is usually diagnosed by pathologists through histopathological examination after physical examination or screening, and comprehensive treatment is carried out by methods such as surgery, radio, and chemotherapy, targeted and endocrine therapy. The prognosis is affected by multiple factors, including pathological type, disease stage at the time of diagnosis, available molecular markers, and so on. Although early-stage localized breast cancers can achieve the best therapeutic effect after comprehensive treatment, patients’ survival time will be shortened rapidly. Common metastatic sites of breast cancer are local and distant lymph nodes, bones, lungs, liver, and brain (2). Invasive lobular carcinoma has a different metastatic pattern compared to invasive breast carcinoma of no special type. ILC metastasizes more frequently in the gastrointestinal tract, genitourinary system and leptomeninges, whereas pulmonary metastases are less common (3). Based on the available case reports, the GI tract as the first metastatic site of breast cancer is still relatively rare. Research conducted by Xu et al. showed that the incidence rate of gastrointestinal (GI) metastases from breast cancer is relatively low, approximately 0.34% (4). Here, we report the diagnosis and treatment processes of a case of breast cancer with gastric metastasis in Shandong Cancer hospital. We reviewed and analyzed published reports to determine the features of breast cancer pathobiology with GI metastasis.
Case presentation
A 45-years-old woman was admitted to our hospital for one-week history of black stool. According to the hospital records, the patient underwent “left breast cancer resection + axillary lymph node dissection” in our hospital 18 years ago (11/19/2002) for “discovering left breast cancer for 3 years”. Postoperative pathology showed the signs of (left) invasive lobular carcinoma of the breast (Figure 1). After the surgery, radiotherapy and 6 cycles of regular chemotherapy were given (medication: pirarubicin, the specific dose is unknown), the postoperative recovery was good. In this instance, the patient had no obvious cause of black stools 1 week ago, with an average of 1 time per day, accompanied by hiccups, decreased appetite, as well as fatigue. But no symptoms of nausea and vomiting, fever, and chills, and any other special discomforts were reported. Electronic gastroscopy at the local hospital revealed a huge ulcer in the upper anterior wall of the stomach (Figure 2). The pathological results of the hospital showed the signs of poorly differentiated (gastric body) adenocarcinoma. Intensified computed tomography (CT) scan of the abdomen and pelvis at the hospital showed consistent results with the manifestations of gastric cancer in the stomach (Figure 3). Pathological results show the poorly differentiated adenocarcinoma (Biopsy of the upper anterior wall of the gastric body). The original unit IHC examinations (Figure 4) showed the following results- cytokeratin (CK), CK8/18 (+), CK19 (+), CK7 (+), GATA-binding protein-3 (GATA-3) (+), gross cystic disease fluid protein 15 (GCDFP-15) (+), estrogen receptor (ER) (+) about 70% cells, progesterone receptor (PR) (-), Ki-67 (+) in 10% cells, synaptophysin (Syn) (-), choriogonadotropin alpha chain CGA (-), CD56 (-), and E-cadherin (+). Refer to Supplementary Table 1 for IHC details. Positron emission tomography-computed tomography (PET-CT) was given to further clarify distant organ metastasis, which showed no abnormal metabolism in the left breast surgery area, while the stomach exhibited high metabolism, indicating a malignant lesion. However, no abnormal metabolism was observed in the abdominal lymph nodes. Laparoscopic-assisted total gastrectomy + abdominal lymph node dissection was performed at Shandong Cancer Hospital on 05/22/2020. Intraoperative exploration showed that the tumor was located in the upper part of the anterior wall of the gastric body, and was ulcer-infiltrated, hard, about 5×4×3 cm in size, penetrated the serosal membrane, and had a clear relationship with the surrounding tissues. There were no obvious swollen lymph nodes around the stomach. Postoperative pathology of total stomach showed the signs of ulcer infiltrating poorly differentiated carcinoma. The tumor invaded the serous membrane and nerve, and tumor thrombus was found in the vessel. The postoperative IHC results further supported the diagnosis of gastric metastasis from breast cancer. Postoperatively, the patient received oral tamoxifen, 1 mg once daily, and tumor control has been good (5). As of the writing of this case report, no recurrence or distant metastasis has been observed during follow-up, and the patient’s overall health condition remained stable. Refer to Supplementary Figure 1 for a detailed timeline.
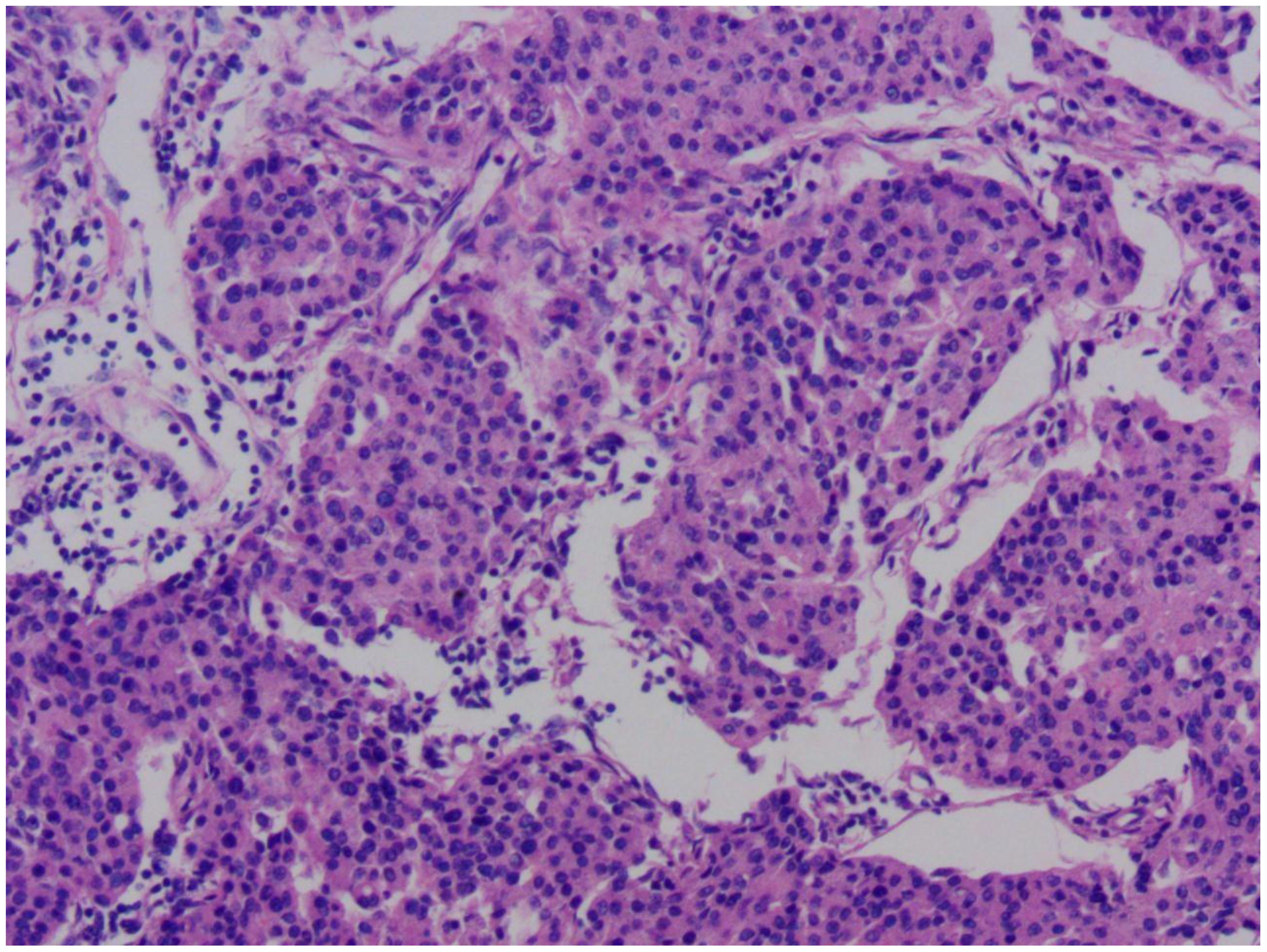
Figure 1. Microscopic pathological image of this patient's breast cancer. The image reveals cancer cells arranged in nest-like structures, with marked nuclear pleomorphism. The size and shape of the cells are inconsistent, demonstrating significant heterogeneity. Normal breast lobular or tubular structures have been disrupted and replaced by disorganized clusters of cancer cells.
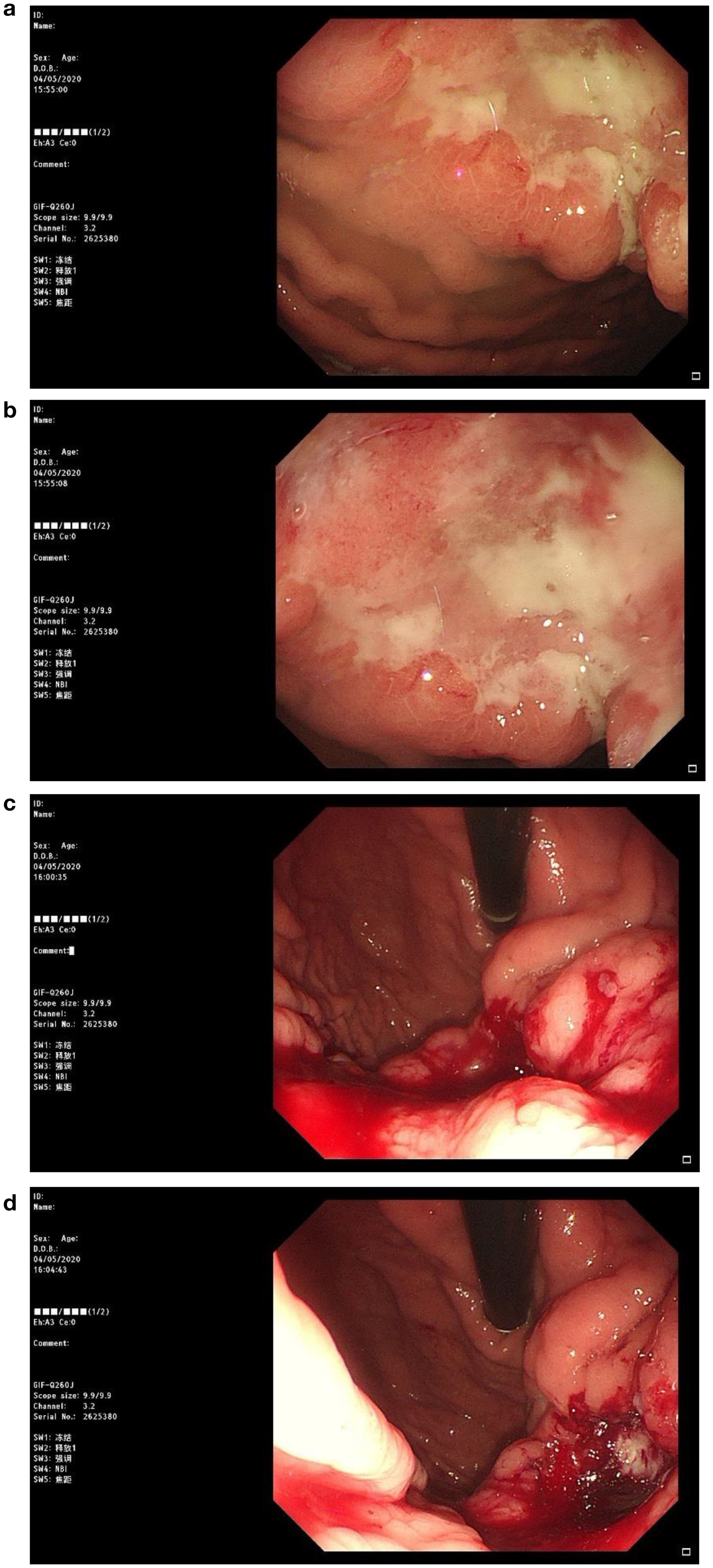
Figure 2. Patient's gastroscopic image. Irregular ulcerative lesions can be observed in figures (a, b), suggesting that the tumor has infiltrated deeper tissues. In figures (c, d), significant active bleeding is evident.
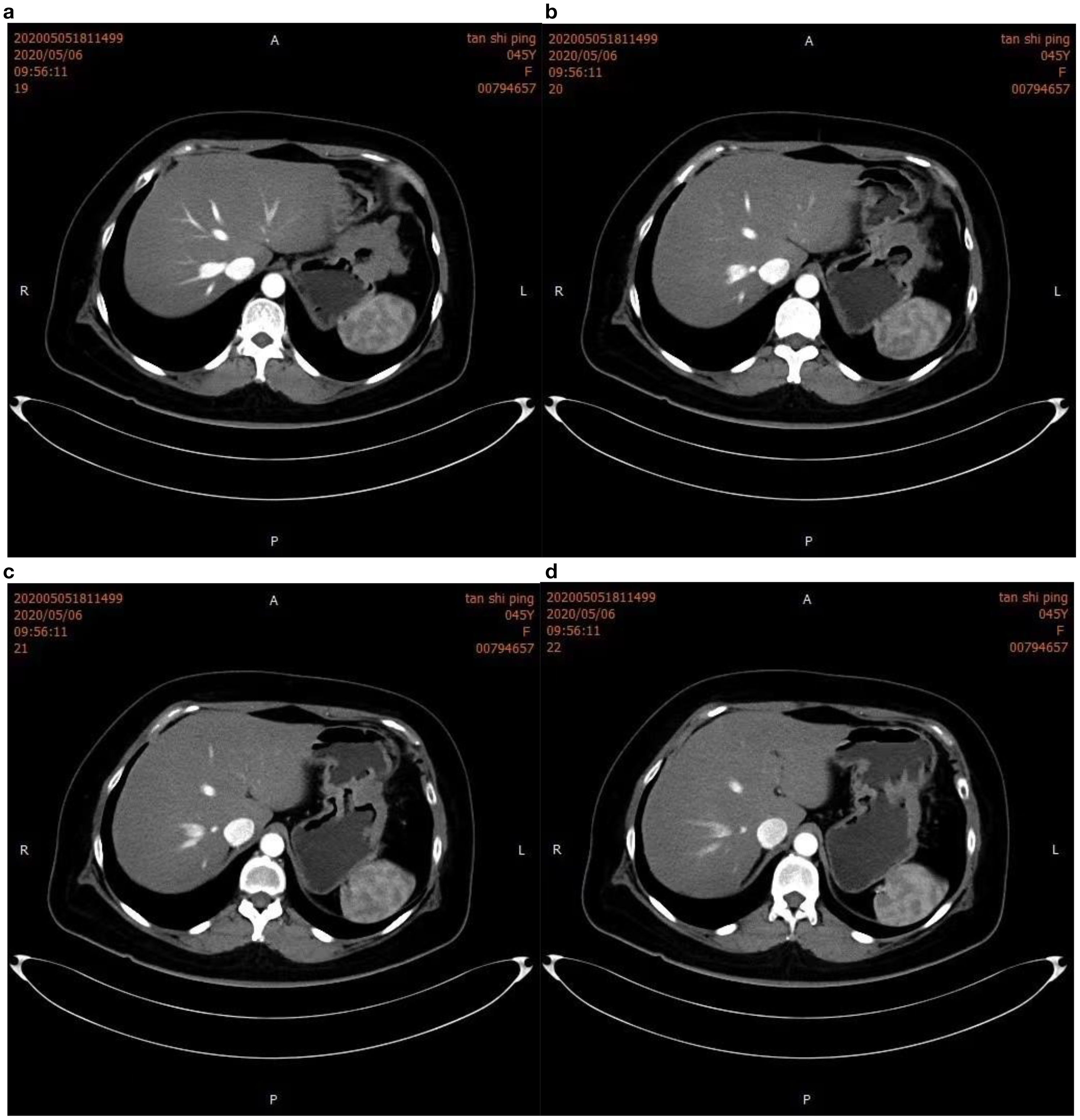
Figure 3. Intensified computed tomography (CT) scan of the abdomen. The image clearly shows gastric wall thickening in figure (a, b), characterized by uneven enhancement. Gastric lumen narrowing is also observed in figure (c, d), suggesting infiltrative growth of the tumor into the gastric cavity.
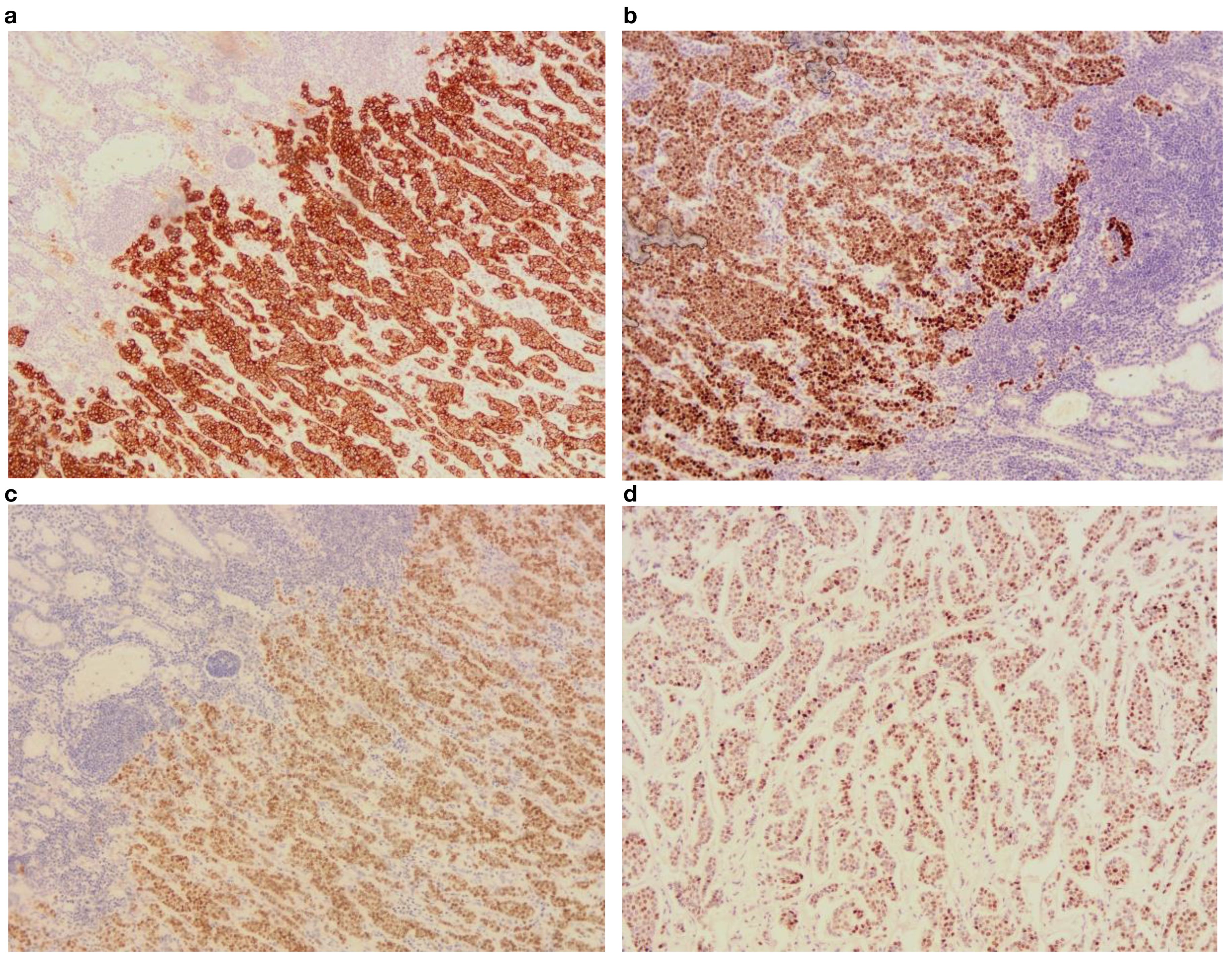
Figure 4. Postoperative immunohistochemical findings under 10x optical microscope. (a) CK(+); (b) ER(+); (c) GATA(+); (d) PR(+).
Discussion
Breast cancer is the malignant tumor with the highest incidence among female-associated cancers in the world. According to global cancer statistics 2022, there are about 2,308,897 new cases of female breast cancer all over the world each year (1, 6). The most common pathological type of breast cancer is invasive ductal carcinoma, followed by invasive lobular carcinoma which accounts for around 10% of breast carcinomas (3). Among them, invasive ductal carcinoma is more prone to metastasis to the liver, lung, and brain. The metastatic sites of lobular breast cancer are more inclined to bones, gynecological organs, peritoneum, retroperitoneum, and GI tract (2, 7, 8). Studies have shown that invasive lobular carcinoma leads to gastric metastases in 65.4% of breast cancer cases. The reason for this feature is currently unclear. But overall, the GI tract is relatively rare as the first metastatic site of breast cancer.
Breast cancer metastasized to the stomach often lacks specific clinical manifestations, such as dyspepsia, anorexia, early satiety, upper abdominal pain, vomiting, and hematemesis, which are roughly like the clinical symptoms of GI reactions or primary gastric cancer, thus making it difficult to be distinguished at the initial diagnosis (4, 9). Its common imaging manifestations are localized or diffused thickening of the stomach wall, causing secondary leathery stomach (10). Therefore, it is difficult to distinguish it from primary gastric cancer, gastric stromal tumor, and gastric lymphoma. Tianyue Li et al. (11) have reported that compared to 18F-FDG, 68Ga-FAPI PET/CT showed a significantly higher level of FAPI activity in the thickened gastric wall, and peritoneum. These imaging findings may be helpful for the diagnosis of gastric metastatic cancer (12). Thus, it has a certain reference value, but there are still few imaging studies on indirect invasive gastric metastatic cancer. The gastroscopic changes mainly have three forms, namely 1) volcanic ulcer, 2) single or multiple localized nodules or polyp-like changes in the gastric wall, and 3) limited or diffused gastric wall involvement, gastric wall stiffness, gastric cavity stenosis. Since most gastric metastatic cancers are submucosal with muscular infiltration, endoscopy results may be normal (11). The histopathological findings of gastric metastatic carcinoma also lack specificity. Cancer cells that metastasize to the stomach from lobular breast tumors are often characterized by poorly differentiated adenocarcinoma and signet ring cell-like carcinoma. It is difficult to detect the primary adenocarcinoma of the stomach by the conventional hematoxylin-eosin (H&E) staining of the tumor tissue. In addition, there are reports of gastric cancer metastasis to the breast to interfere with diagnostic judgment. Therefore, the distinction between primary and metastatic gastric cancer must rely on IHC examinations.
In this report, we present a relatively rare case of the invasive lobular carcinoma of the breast that has been metastasized to the stomach. The pathogenesis of this type of metastatic cancer needs to be further explored. Appropriate detection of the expressions of ER, PR, GATA-3, GCDFP-15, mammaglobin, CK7, and CK20 is crucial for the efficient diagnosis of tumor metastasis. GATA-3 is a member of the GATA family of zinc finger protein transcription factors. Because of its important functions in regulating cell development and differentiation, it has been widely used as the breast metastasis marker. Studies have shown its primary expression in breast epithelial tumors (13). In addition, its expression GATA-3 has also been detected in a variety of cancers, including neuroendocrine tumors (14, 15). Although in breast cancer, GATA-3 positive expression ranges from 60% to 100% of tumor cells (13), its expression in metastatic breast cancer is more sensitive and has significance in the differential diagnosis of breast metastasis (14). Despite its potential diagnostic utility, GATA-3 expression specificity is relatively poor in breast metastasis, and which is why it is being utilized in combination with GCDFP-15 and mammaglobin as a biomarker for diagnosis of metastasis. GCDFP-15, a large molecular weight protein, has been found to be positive in 35-74% of cases of breast cancers. The antibody for the detection of GCDFP-15 has strong specificity. CK7 exhibits positive expression in the breast epithelial cells, while negative expression in the intestinal epithelium. However, a small number of gastric cancers can be positive for CK7 expression. E-cadherin is usually expressed in breast ductal carcinoma but is negative in the case of breast lobular carcinoma. Furthermore, new potential diagnostic markers, such as hepatocyte nuclear factor 4a (HNF4a), for the differentiation between primary gastric tumors and metastatic breast carcinoma are also under study (15).
The IHC results of this patient’s tumor tissue exhibited ER (+), PR (+), CK7 (+), GATA-3 (+), E-cadherin (+), and GCDFP-15 (+) tumor cells, which, in combination with patient’s breast examination records strongly proved that the gastric lesions were not the primary ones, rather they were breast metastasized secondary lesions. Furthermore, since the patient provided the medical history of breast cancer upon admission to our hospital, we suggested breast cancer-related antibody testing by IHC, which greatly helped precise diagnosis at the end. Notably, it is difficult to diagnose metastatic breast cancer correctly at the initial stage, especially when the patient’s medical history is unknown. In such cases, digestive endoscopy to examine the appearance of the gastric tumor or lesions can assist in making an appropriate diagnosis. Therefore, the pathological findings from the gastroscopy biopsy should be combined with tumor IHC and other medical examinations to achieve a precise diagnosis of breast metastasis, thereby avoiding undesired mistakes.
The treatment strategies of breast cancer with gastric metastasis are still based on systemic therapy such as chemotherapy and endocrine therapy according to ESMO guidelines (5, 16). Although surgical treatment is the major option for primary gastric cancer, it is not the principal treatment for metastatic gastric cancer. It is often considered palliative treatment when patients have complications, such as GI bleeding, obstruction, and perforation. However, there are also clinical studies that show patients with good general conditions and only gastric metastases demonstrate no statistically significant difference in overall survival prolongation between surgery and chemotherapy (17). Gastric metastasis of breast cancer usually has a poor prognosis, with an average survival time of 41 months (18). Furthermore, the occurrence of gastric metastasis indicates advanced stage with potential spread through the bloodstream to other organs, which means patients’ prognosis in such cases is typically very poor (19). Despite advancements in medical technology improving overall cancer prognosis, GI metastasis remains a challenge in some breast cancer cases. Thus, conducting detailed endoscopy, biopsy, and gathering complete clinical history is essential for diagnosing gastric metastasis cancer accurately and promptly. Simultaneously, accurately distinguishing between primary and secondary gastric tumors is crucial for implementing timely and effective treatment measures to enhance patients’ quality of life.
Conclusion
Gastric metastasis of breast cancer is a rare occurrence, mostly associated with invasive lobular carcinoma. It is necessary to combine the medical history and comprehensively apply various methods, such as clinical manifestations, imaging, endoscopy, histopathology, and IHC, to prevent missed diagnosis and misdiagnosis. Treatment strategies are still based on systemic treatment such as chemotherapy and endocrine therapy. Palliative surgery may be considered when necessary, but the prognosis remains poor. Therefore, for patients with a history of breast cancer, especially those with invasive lobular carcinoma, who present GI symptoms or are diagnosed with obvious gastric cancer, vigilance regarding possible gastric metastasis from breast tumors is crucial for accurate clinical treatment.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author/s.
Ethics statement
The studies involving humans were approved by Shandong First Medical University, Shandong Cancer Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The human samples used in this study were acquired from the all dates of this case report originate from clinical practices. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
BL: Conceptualization, Investigation, Writing – review & editing, Writing – original draft, Data curation. ZH: Writing – original draft, Data curation. FH: Writing – review & editing, Investigation. YW: Writing – review & editing. XW: Data curation, Writing – review & editing. JZ: Writing – review & editing, Formal Analysis. JC: Conceptualization, Formal Analysis, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2025.1596207/full#supplementary-material
Abbreviations
ILC, Invasive lobular carcinoma; GI, Gastrointestinal tract; FAPI, Fibroblast activation protein inhibitor; PET-CT, Positron emission tomography-computed tomography; H&E, Hematoxylin-eosin staining; IHC, Immunohistochemistry.
References
1. Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2024) 74:229–63. doi: 10.3322/caac.21834
2. Chen S, Navickas A, and Goodarzi H. Translational adaptation in breast cancer metastasis and emerging therapeutic opportunities. Trends Pharmacol Sci. (2024) 45:304–18. doi: 10.1016/j.tips.2024.02.002
3. Kioleoglou Z, Georgaki E, Koufopoulos N, Kostek O, Volakakis N, Dimitriadou A, et al. Gastrointestinal metastases from lobular breast carcinoma: A literature review. Cureus. (2024) 16:e65852. doi: 10.7759/cureus.65852
4. Xu L, Wang C, Yang X, and Dong L. Case report: Cutaneous metastases as a first manifestation from breast cancer with concurrent gastric metastases. Front Pharmacol. (2024) 15:1356167. doi: 10.3389/fphar.2024.1356167
5. Cardoso F, Paluch-Shimon S, Senkus E, Curigliano G, Aapro MS, André F, et al. 5th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 5). Ann Oncol Off J Eur Soc Med Oncol. (2020) 31:1623–49. doi: 10.1016/j.annonc.2020.09.010
6. Wang X and O’Regan RM. Breast cancer therapy in China: Introducing the Special Collection. Cancer. (2024) 130:1368–70. doi: 10.1002/cncr.35288
7. Tan PH, Ellis I, Allison K, Brogi E, Fox SB, Lakhani S, et al. The 2019 World Health Organization classification of tumours of the breast. Histopathology. (2020) 77:181–5. doi: 10.1111/his.14091
8. Ciriello G, Gatza ML, Beck AH, Wilkerson MD, Rhie SK, Pastore A, et al. Comprehensive molecular portraits of invasive lobular breast cancer. Cell. (2015) 163:506–19. doi: 10.1016/j.cell.2015.09.033
9. Fu J-X, Zou Y-N, Long-Li, and Wang X-J. Widespread Metastasis to the Stomach 10 Years After Primary Breast Cancer: A case report and review of the literature. Med (Baltimore). (2020) 99:e22527. doi: 10.1097/MD.0000000000022527
10. Nazareno J, Taves D, and Preiksaitis H-G. Metastatic breast cancer to the gastrointestinal tract: a case series and review of the literature. World J Gastroenterol. (2006) 12:6219–24. doi: 10.3748/wjg.v12.i38.6219
11. Li T, Jiang X, Zhang Z, Chen X, Wang J, Zhao X, et al. Case Report: 68Ga-FAPI PET/CT, a more advantageous detection mean of gastric, peritoneal, and ovarian metastases from breast cancer. Front Oncol. (2022) 12:1013066. doi: 10.3389/fonc.2022.1013066
12. Hathi DK and Jones EF. 68Ga FAPI PET/CT: tracer uptake in 28 different kinds of cancer. Radiol Imaging Cancer. (2019) 1:e194003. doi: 10.1148/rycan.2019194003
13. Tabriz HM, Farmani E, Nazar E, and Javadi AE. GATA Binding Protein 3 (GATA-3) expression evaluation as prognostic factor in breast cancer and its relationship with other immunemarkers. Indian J Pathol Microbiol. (2023) 66:286–90. doi: 10.4103/ijpm.ijpm_453_21
14. Marletta S, Martignoni G, Ghimenton C, Stefanizzi L, Marcolini L, and Caliò A. Well-differentiated neuroendocrine tumor of the urinary bladder expressing GATA 3. Virchows Arch Int J Pathol. (2023) 482:783–8. doi: 10.1007/s00428-022-03478-2
15. Xu L, Yu G, Jiang L, Song X, Qu G, Luo J, et al. HepPar1 and GATA-3 expression in neuroendocrine neoplasms: A potential trap for pathologic diagnosis. Appl Immunohistochem Mol Morphol AIMM. (2023) 31:668–72. doi: 10.1097/PAI.0000000000001160
16. Cardoso F, Kyriakides S, Ohno S, Penault-Llorca F, Poortmans P, Rubio IT, et al. ESMO Guidelines Committee. Early breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol Off J Eur Soc Med Oncol. (2019) 30:1674. doi: 10.1093/annonc/mdz189
17. Gadde R, Tamariz L, Hanna M, Avisar E, Livingstone A, Franceschi D, et al. Metastatic gastric cancer (MGC) patients: Can we improve survival by metastasectomy? A systematic review and meta-analysis. J Surg Oncol. (2015) 112:38–45. doi: 10.1002/jso.23945
18. Kliiger J and Gorbaty M. Metastasis to the pancreas and stomach from a breast cancer primary: a case report. J Community Hosp Intern Med Perspect. (2017) 7:234–7. doi: 10.1080/20009666.2017.1369379
Keywords: breast cancer, gastric metastasis, diagnosis, treatment, prognosis
Citation: Liu B, Han Z, Hu F, Wang Y, Wang X, Zhang J and Chai J (2025) Breast cancer with gastric metastasis in invasive lobular carcinoma: a case report and literature review. Front. Oncol. 15:1596207. doi: 10.3389/fonc.2025.1596207
Received: 19 March 2025; Accepted: 30 June 2025;
Published: 24 July 2025.
Edited by:
Wenzheng Guo, University of Kentucky, United StatesReviewed by:
Nektarios I. Koufopoulos, University General Hospital Attikon, GreeceYuan Tang, University of Toledo, United States
Teodor Florin Georgescu, Carol Davila University of Medicine and Pharmacy, Romania
Copyright © 2025 Liu, Han, Hu, Wang, Wang, Zhang and Chai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jie Chai, amNoYWlAc2RmbXUuZWR1LmNu
†These authors have contributed equally to this work
 Bing Liu†
Bing Liu† Zhifei Han
Zhifei Han Jiazi Zhang
Jiazi Zhang Jie Chai
Jie Chai