- The Affiliated Yueqing Hospital of Wenzhou Medical University, Yueqing, Zhejiang, China
Meningeal carcinomatosis (MC) is a distinct form of brain metastasis (BM) that occurs in patients with advanced triple-negative breast cancer (TNBC). The occurrence of BM typically indicates a poor prognosis. Spatial heterogeneity in HER2 expression is relatively common in breast cancer cases; however, the emergence of both temporal and spatial heterogeneity within the brain parenchyma and cerebrospinal fluid (CSF) is exceedingly rare. Thus, the phenomenon warrants further investigation. Herein, we report a case of advanced TNBC with BM and MC. HER2 was expressed in the CSF and exhibited spatial and temporal heterogeneity. The CSF was analyzed using immunohistochemistry and flow cytometry, confirming the presence of HER2-positive tumor cells in the patient’s CSF. MC was effectively controlled after treatment with trastuzumab deruxtecan (T-DXd). Relevant literature was reviewed to analyze the reasons for this phenomenon. In this case, the spatial and temporal heterogeneity of the HER2 receptors observed in the CSF suggests that BM may be driven by the synergistic interaction of multiple subclonal tumor cells.
1 Introduction
Breast cancer has a very heterogeneous disease presentation. The most common classification is based on four major biomarkers, namely the estrogen receptor (ER), the progesterone receptor (PR), Ki67, and human epidermal growth factor receptor 2 (HER2), which are divided into four subtypes based on their immunohistochemical expression. Breast cancer that does not express ER, PR, and HER2 is defined as triple-negative breast cancer (TNBC) (1). Gene signatures differ greatly between breast cancer subtypes, depending on their signaling pathways, exhibiting differences in metastatic site preference (2). Brain metastases (BMs) are common metastatic sites in advanced breast cancer and are most common in HER2-positive and TNBC subtypes, with an incidence of 20%–30% (3). The median time from diagnosis to BM development for these two subtypes is 28–36 months. Once BM occurs, it often presages an extremely poor prognosis (4). Meningeal carcinomatosis (MC) is a specific site of BM. Approximately 2%–5% of patients with breast cancer develop MC, which can result in a severely poor prognosis (5). Currently, there are four common treatments for BM: intrathecal chemotherapy, radiotherapy, surgery, and systemic treatment with anticancer drugs (6).
Herein, we report a case of advanced TNBC with BM and MC. HER2 expression was not detected in the primary mammary tumor or brain parenchyma but was identified in cerebrospinal fluid (CSF) tumor cells, demonstrating spatial and temporal heterogeneity. MC was effectively controlled after treatment with trastuzumab deruxtecan (T-DXd).
2 Case presentation
2.1 Chief complaints
A 42-year-old woman presented to the emergency department with meningeal metastases from breast cancer, headache, vomiting, and paroxysmal confusion.
2.2 History of the present illness
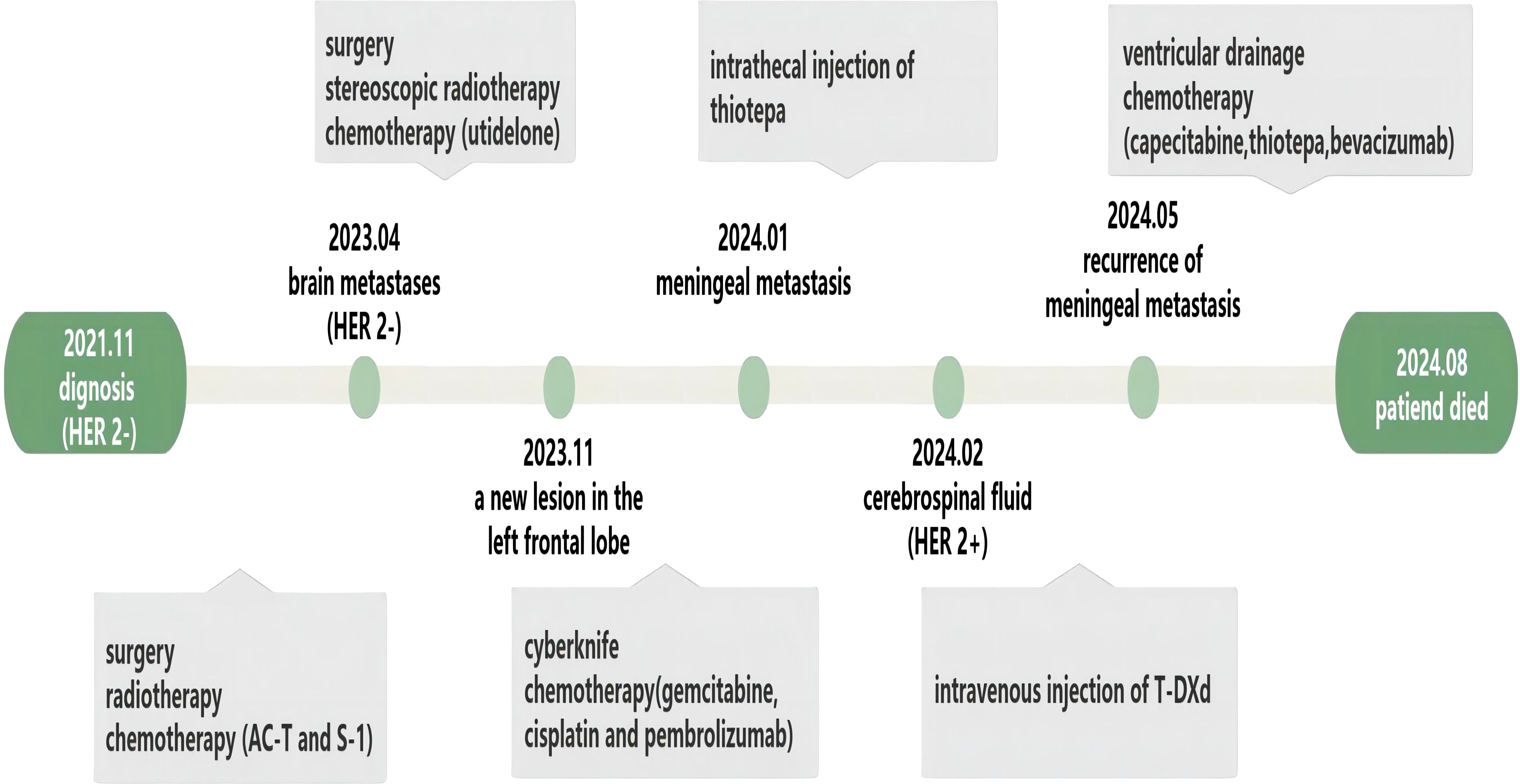
In January 2024, the patient developed a gradually aggravated headache, and a lumbar puncture was performed at another hospital. At that time, the CSF pressure increased to 260 mmH2O, indicating meningeal metastasis of the breast cancer. The patient was dehydrated to reduce the intracranial pressure, and the treatment effect was poor. The disease progressed rapidly and gradually resulted in decreased muscle strength in the extremities up to grade IV.
2.3 History of past illness
In November 2021, the patient underwent right mastectomy and lymph node dissection of a breast mass found during a physical examination. Postoperative pathology revealed TNBC; the histological type was invasive ductal carcinoma, the tumor size was 5 cm × 4 cm × 4 cm with four axillary lymph node metastases, the resection margin of the tumor was negative, and a cancer thrombus was visible in the vessel. As the patient’s tumor stage reached pT3N2M0, she received four courses of epirubicin combined with cyclophosphamide, followed by four courses of docetaxel (AC-T). After the completion of chemotherapy, local radiotherapy was continued, and S-1 was used as a consolidation regimen for 6 months. During follow-up in April 2023, the patient was found to have BM in both the left and right frontal lobes. The left lesion was surgically resected, and the right metastatic lesion was treated with stereoscopic radiotherapy. Immunohistochemistry was used to detect the expression of HER2 in the resected brain tissue, and the result was considered zero. The patient received seven courses of utidelone (UTD1) from May 2023 to October 2023. In November 2023 and January 2024, the patient underwent CyberKnife therapy due to the recurrence of a new lesion in the left frontal lobe. During the two periods of CyberKnife treatment, the patient received one course of chemotherapy with gemcitabine, cisplatin, and pembrolizumab. In January 2024, the patient presented with clinical symptoms of meningeal metastasis. As her condition continued to deteriorate, she was referred to our hospital.
2.4 Personal and family history
The patient received long-term entecavir tablets for chronic viral hepatitis B infection and had no family history of tumor-related diseases.
2.5 Physical examination
The patient presented with paroxysmal confusion and weakness of the extremities, with normal muscle tone.
2.6 Laboratory examinations
In February 2024, the patient’s CSF was tested after the symptoms of MC developed; the results showed proliferating and infiltrating atypical cells, and 6% of the cells were HER2(+++). Flow cytometry testing of the CSF, using the epithelial cell-specific marker CD326 and the leukocyte marker CD45, suggested that the proportion of tumor cells in the CSF was 20% (Figure 1). The proportion decreased to zero after treatment with T-DXd.
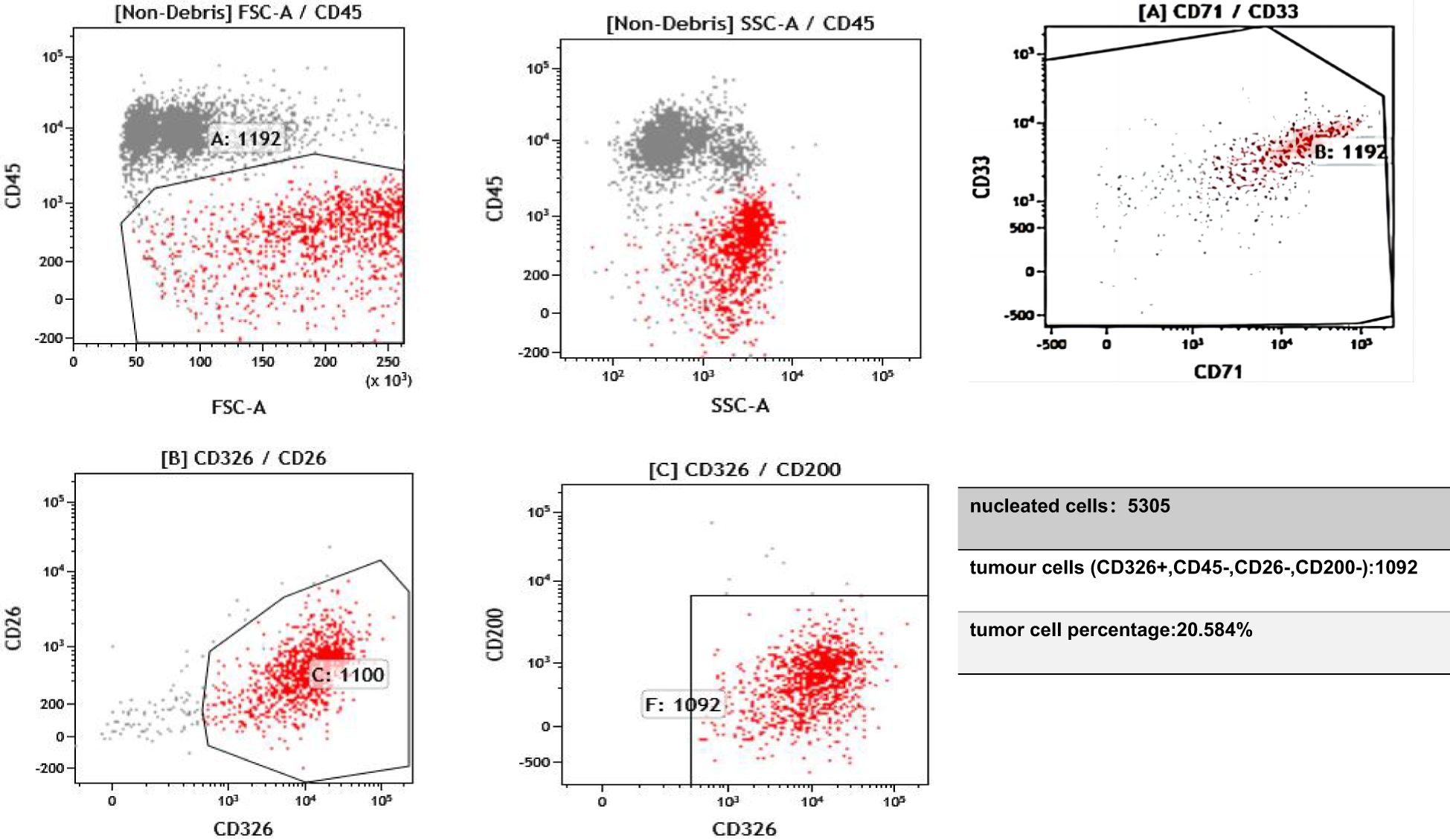
Figure 1. On 11 February 2024, flow cytometry analysis of the cerebrospinal fluid identified a population of epithelial cells positive for CD326 and negative for CD45. CD45 is expressed in hematopoietic cells but is absent in tumor cells. CD33 and CD71 serve to exclude myeloid cells and nucleated red blood cells. Epithelial-derived tumor cells typically express CD326 (EpCAM). CD26 and CD200 are expressed on mesothelial cells and can help distinguish mesothelial cells from tumor cells.
2.7 Pathological diagnosis
Histopathology of the right breast revealed invasive ductal carcinoma, histological grade III, with tumor size 5 cm × 4 cm × 4 cm. Immunohistochemical results were as follows: CD31 (vascular endothelial +), CK5/6 (neoplastic cells +), calponin (−), D2-40 (lymphatic +), E-cd (+), ER (−), HER2(0), Ki67 (70%), P63 (−), and PR (−).
2.8 Imaging examinations
Magnetic resonance imaging revealed postoperative changes in the skull, patchy heterogeneous enhancement of the left frontoparietal junction, and meningeal thickening (Figure 2).
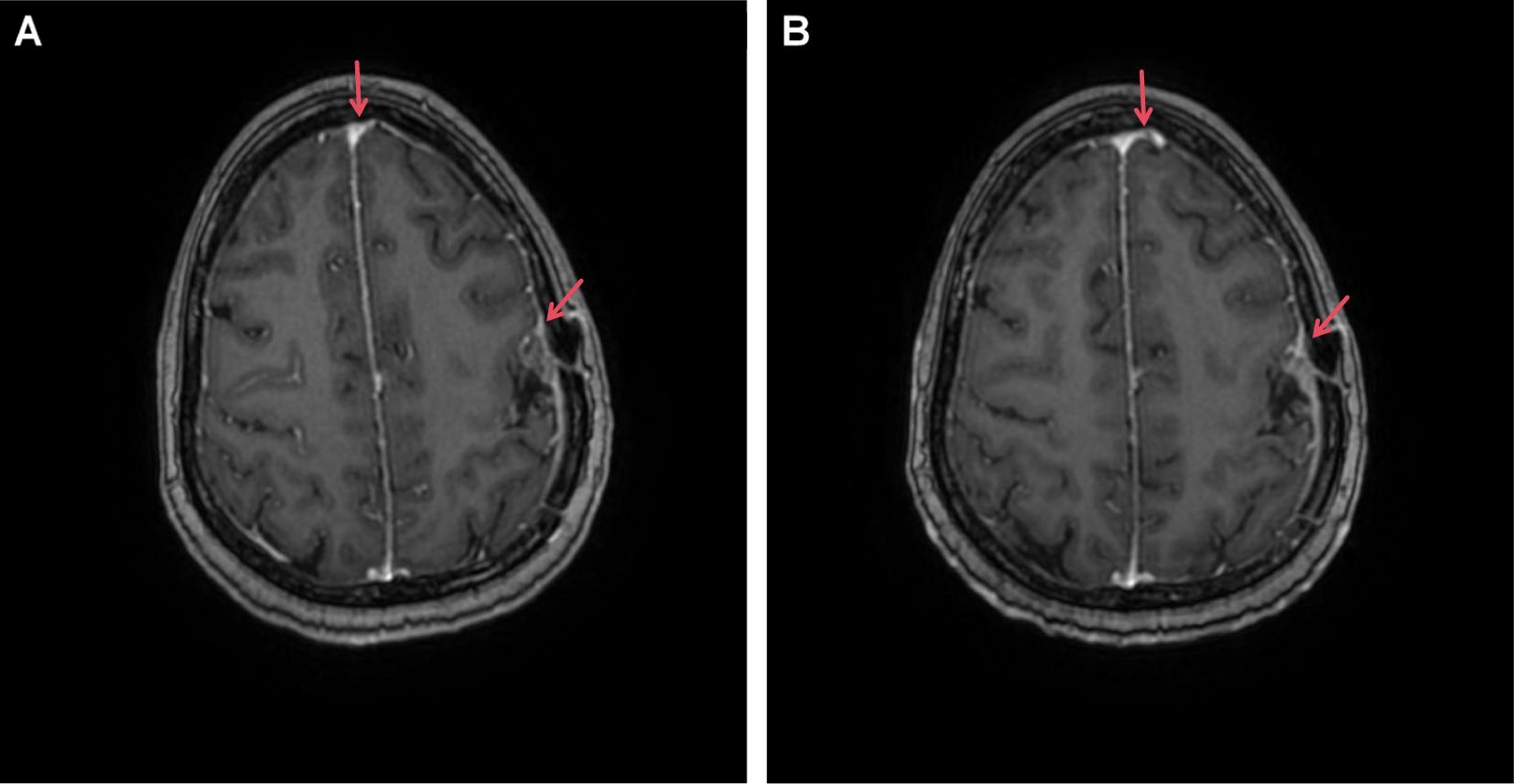
Figure 2. Enhanced MRI image of our patient with brain metastasis undergoing T-DXd treatment. (A) T1 image (20 February 2024); (B) T1 image (14 April 2024). Red arrows highlight areas of tumor presence along with meningeal thickening and enhancement.
2.9 Final diagnosis
Based on the available information, the patient was diagnosed with a triple-negative breast malignancy (pT3N2M0). The stage after recurrence was rTxNxM1 (brain and meningeal metastases).
2.10 Treatment
The patient declined whole-brain radiation and, after ruling out any contraindications, intrathecal thiotepa was administered twice weekly for disease control. Based on the findings from the DESTINY-Breast04 trial, which demonstrated the clinical benefit of T-DXd in patients with low HER2 expression, we adjusted the treatment strategy for this case. Following detailed discussion with the patient and confirmation of HER2 expression (6% of tumor cells were HER2+++, as per immunohistochemistry of the CSF), the patient consented to off-label therapy with T-DXd at a dose of 300 mg every 3 weeks that was initiated in February 2024.
2.11 Outcome and follow-up
After one course of T-DXd, the patient’s headache significantly improved, autonomous consciousness was recovered, and limb muscle strength returned to normal. After three courses of T-DXd, we reviewed the flow cytometry examination of the CSF, and the results showed that the proportion of tumor cells had decreased to 0. For a period of 3 months, the patient reported good quality of life and normal daily functioning. However, this state was not sustained, and the patient’s condition rapidly deteriorated 1 month later. Thiotepa was then administered, but it only delayed disease progression. In August 2024, after 3 months of palliative treatment, the patient chose to refuse further treatment due to aggravation of the disease (Figure 3).
3 Discussion
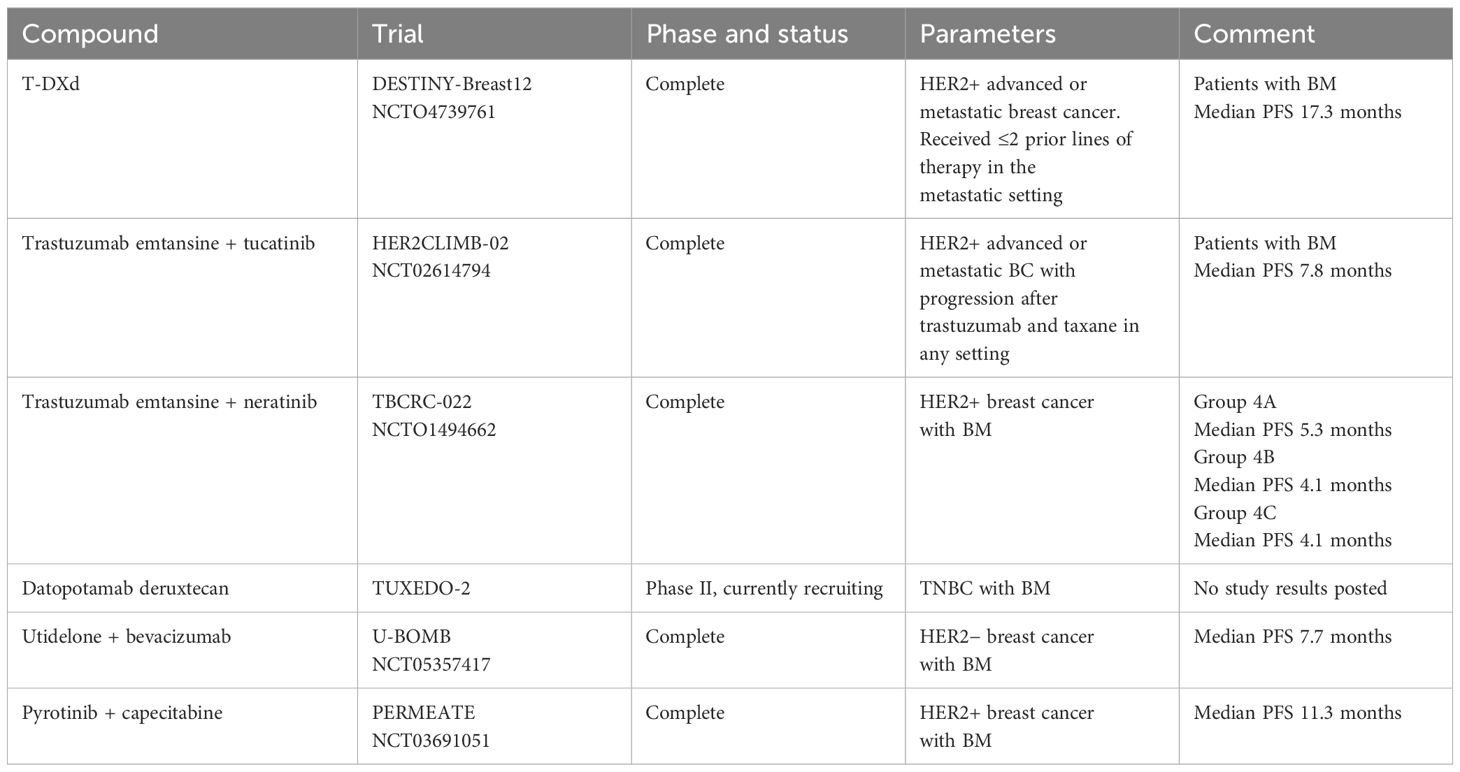
MC is a devastating metastatic site of cancer that is common in advanced breast cancer, and with the improvements in patient survival rate, this proportion will gradually increase (7, 8). The mean overall survival of patients with MC is 2–4 months, with rapid deterioration occurring within 4–6 weeks in untreated patients (9). TNBC is associated with an elevated risk of MC, and its prognosis is worse than that of other types of breast cancer (7). Given the poor MC prognosis, enabling early diagnosis could optimize the timing of therapeutic interventions for patients. Current clinical diagnosis of MC primarily relies on MRI and CSF cytology, yet these methods often fail to detect the disease at early stages (10–12). For patients with high-risk MC, advanced liquid biopsy technologies, such as CellSearch® technology, modified fast aneuploidy screening test-sequencing system (mFAST-SeqS), and ultra-low-pass whole-genome sequencing (ulpWGS), applied to CSF analysis may improve diagnostic sensitivity and ultimately enhance patient survival outcomes (13–15). Currently, systemic therapy remains critically important in the management of breast cancer with BM. Notably, investigative chemotherapy, such as antibody–drug conjugates (ADCs) and tyrosine kinase inhibitors (TKIs), has demonstrated particularly encouraging efficacy in improving survival outcomes for these patients (Table 1). Our patient exhibited a unique pathological characteristic. The patient’s primary tumor site and BM site were HER2(0); however, when the patient had MC, 6% of the cells in the CSF were positive for HER2 (+++). This rare phenomenon reflects the spatiotemporal heterogeneity of tumors.
To date, knowledge of the mechanism of BM from breast cancer is still limited, but the process can be roughly summarized as follows: gene expression activates certain signaling pathways that allow tumor extravasation after the dynamic remodeling of the extracellular matrix (16); tumor cells infiltrate into peripheral blood vessels; the interaction between the microenvironment of the blood–brain barrier (BBB) and metastatic breast cancer cells establishes a metastatic niche that promotes BM; and cells with a particular genetic profile colonize the new soil of the brain (17). MC, as a distinct subtype of BM, exhibits a lower incidence rate and demonstrates highly diverse metastatic routes. However, different cancer types may exhibit a predilection for specific pathways (18). The pathways of cancer cell metastasis in MC include the following: 1) cancer cells invade arachnoid granulations through venous sinuses; 2) tumors within the brain parenchyma breach the glia limitans to enter perivascular (Virchow–Robin) spaces; 3) tumor cells in the dura mater invade via meningeal lymphatic vessels; 4) cancer cells in arterial circulation cross through fenestrated vessels and choroid plexus epithelial tight junctions; 5) cancer cells migrate along nerve bundles to reach the leptomeninges; 6) cancer cells invade via retrograde flow through Batson’s valveless venous plexus; and 7) cancer cells from bone metastatic lesions infiltrate through bridging veins. Interestingly, the last pathway has been demonstrated and widely discussed in breast cancer (19). The leptomeningeal environment is nutrient-deprived and hypoxic. Upon metastasizing to the leptomeninges, cancer cells must adapt to these harsh conditions. Examples include remodeling the local microenvironment by upregulating complement component C3 (C3) or lipocalin-2 (LCN2) to promote survival (20, 21). Metabolic adaptation is also critical, such as leveraging glycolysis under hypoxia and oxidative phosphorylation (12, 22). Discordance in HER2 receptor expression exists between primary breast cancer and BM. Some tissues show subtype switching, and the proportion is up to one-third in BM (23, 24). This phenomenon is not difficult to understand when considering BM formation as a process of tumor cell selection and evolution. At the same time, attention must be paid to tumor heterogeneity.
Spatial heterogeneity refers to phenotypic and genomic differences in different regions of a tumor, whereas temporal heterogeneity refers to the emergence of new biological characteristics of metastatic lesions after tumor progression (25). The former reflects the coexistence of multiple subclones of tumor cells (26, 27), while the latter is often more complex and usually related to the pattern of tumor evolution and the seeding pattern of metastasis. The most prevalent form of spatial heterogeneity observed in breast cancer is the expression of HER2 receptors, accounting for 23% of cases (28). Intratumor HER2 heterogeneity has been linked to a shorter duration of disease-free survival, particularly in patients with TNBC (29, 30). Temporal heterogeneity between primary and metastatic tumors can be explained by two models of tumor cell evolution. These two evolutionary models exhibit linear and parallel progressions (31). The linear progression of metastatic tumors is driven by dominant clones selectively derived from the primary tumor in response to therapeutic pressure. Genetic disparities between primary and metastatic tumors are minimal (32). A parallel progression model often occurs in cases where the primary tumor has multiple subclones that disseminate early during treatment and undergo a quiescent phase before undergoing separate clonal evolution, resulting in significant genetic divergence between primary and metastatic tumors (33).
Before the implementation of single-cell and multiregion sequencing techniques, it was widely acknowledged that metastasis was predominantly driven by a single subclone (34). However, technological advances have led to new theories, including polyclonal seeding and metastatic cross-seeding, in which clusters of tumor cells composed of multiple subclones may be more effective than monoclonal cells in the formation of metastatic tumors (34).
A key limitation of this study was the inability to perform single-cell sequencing, thereby restricting our capacity to fully characterize the observed tumor heterogeneity. However, when HER2 receptor heterogeneity is present in the brain tissue and CSF, it is reasonable to believe that BM arises from the synergistic interaction of multiple subclonal tumor cells.
4 Conclusion
With the increasing adoption of rapid and affordable sequencing technology and the enrichment of tumor treatment methods, we will gain a deeper understanding of the heterogeneity of metastatic tumors. The development of personalized treatment strategies for each patient will most likely become the norm in the future.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
Written informed consent was obtained from the participant/patient(s) for the publication of this case report.
Author contributions
XW: Writing – original draft. ZW: Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Wenzhou Medical and Health Research Project (a Study on the Accuracy of EPCAM and CD45 in the Diagnosis of Malignant Serous Cavity Effusions and Their Role as Indicators for Efficacy Evaluation Based on Flow Cytometry, No. 2024028).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Allison KH. Prognostic and predictive parameters in breast pathology: a pathologist’s primer. Mod Pathol. (2021) 34:94–106. doi: 10.1038/s41379-020-00704-7
2. Bode AM and Dong Z. Recent advances in precision oncology research. NPJ Precis Oncol. (2018) 2:11. doi: 10.1038/s41698-018-0055-0
3. Kennecke H, Yerushalmi R, Woods R, Cheang MC, Voduc D, Speers CH, et al. Metastatic behavior of breast cancer subtypes. J Clin Oncol. (2010) 28:3271–7. doi: 10.1200/JCO.2009.25.9820
4. Sperduto PW, Kased N, Roberge D, Chao ST, Shanley R, Luo X, et al. The effect of tumor subtype on the time from primary diagnosis to development of brain metastases and survival in patients with breast cancer. J Neurooncol. (2013) 112:467–72. doi: 10.1007/s11060-013-1083-9
5. Chamberlain MC, Glantz M, Groves MD, and Wilson WH. Diagnostic tools for neoplastic meningitis: detecting disease, identifying patient risk, and determining benefit of treatment. Semin Oncol. (2009) 36:S35–45. doi: 10.1053/j.seminoncol.2009.05.005
6. Franzoi MA and Hortobagyi GN. Leptomeningeal carcinomatosis in patients with breast cancer. Crit Rev Oncol Hematol. (2019) 135:85–94. doi: 10.1016/j.critrevonc.2019.01.020
7. Huppert LA, Fisch S, Tsopurashvili E, Somepalle SS, Salans M, Vasudevan HN, et al. Demographic and clinical characteristics of patients with metastatic breast cancer and leptomeningeal disease: a single center retrospective cohort study. Breast Cancer Res Treat. (2024) 206:625–36. doi: 10.1007/s10549-024-07339-1
8. Goyal G, Singh A, Avaronnan M, Raut NV, Talreja V, Chandrasekharan A, et al. Treatment pattern and outcomes of leptomeningeal carcinomatosis in India - a retrospective study. Lancet Reg Health Southeast Asia. (2024) 24:100331. doi: 10.1016/j.lansea.2023.100331
9. Beauchesne P. Intrathecal chemotherapy for treatment of leptomeningeal dissemination of metastatic tumours. Lancet Oncol. (2010) 11:871–9. doi: 10.1016/S1470-2045(10)70034-6
10. Clarke JL, Perez HR, Jacks LM, Panageas KS, and Deangelis LM. Leptomeningeal metastases in the MRI era. Neurology. (2010) 74:1449–54. doi: 10.1212/WNL.0b013e3181dc1a69
11. Malani R, Fleisher M, Kumthekar P, Lin X, Omuro A, Groves MD, et al. Cerebrospinal fluid circulating tumor cells as a quantifiable measurement of leptomeningeal metastases in patients with HER2 positive cancer. J Neurooncol. (2020) 148:599–606. doi: 10.1007/s11060-020-03555-z
12. Bönig L, Möhn N, Ahlbrecht J, Wurster U, Raab P, Puppe W, et al. Leptomeningeal metastasis: the role of cerebrospinal fluid diagnostics. Front Neurol. (2019) 10:839. doi: 10.3389/fneur.2019.00839
13. Torre M, Lee EQ, Chukwueke UN, Nayak L, Cibas ES, and Lowe AC. Integration of rare cell capture technology into cytologic evaluation of cerebrospinal fluid specimens from patients with solid tumors and suspected leptomeningeal metastasis. J Am Soc Cytopathol. (2020) 9:45–54. doi: 10.1016/j.jasc.2019.09.001
14. Angus L, Deger T, and Jager A. Detection of aneuploidy in cerebrospinal fluid from patients with breast cancer can improve diagnosis of leptomeningeal metastases. Clin Cancer Res. (2021) 27:2798–806. doi: 10.1158/1078-0432.ccr-20-3954
15. Fitzpatrick A and Iravani M. Assessing CSF ctDNA to improve diagnostic accuracy and therapeutic monitoring in breast cancer leptomeningeal metastasis. Clin Cancer Res. (2022) 28:1180–91. doi: 10.1158/1078-0432.ccr-21-3017
16. Winkler J and Abisoye-Ogunniyan A. Concepts of extracellular matrix remodelling in tumour progression and metastasis. Nat Commun. (2020) 11:5120. doi: 10.1038/s41467-020-18794-x
17. Tomasik B, Bienkowski M, Gorska Z, Gutowska K, Kumiega P, Jassem J, et al. Molecular aspects of brain metastases in breast cancer. Cancer Treat Rev. (2023) 114:102521. doi: 10.1016/j.ctrv.2023.102521
18. Remsik J and Boire A. The path to leptomeningeal metastasis. Nat Rev Cancer. (2024) 24:448–60. doi: 10.1038/s41568-024-00700-y
19. Whiteley AE and Ma D. Breast cancer exploits neural signaling pathways for bone-to-meninges metastasis. Science. (2024) 384. doi: 10.1126/science.adh5548
20. Boire A, Zou Y, Shieh J, Macalinao DG, Pentsova E, and Massagué J. Complement component 3 adapts the cerebrospinal fluid for leptomeningeal metastasis. Cell. (2017) 168:1101–1113.e13. doi: 10.1016/j.cell.2017.02.025
21. Chi Y and Remsik J. Cancer cells deploy lipocalin-2 to collect limiting iron in leptomeningeal metastasis. Science. (2020) 369:276–82. doi: 10.1126/science.aaz2193
22. Remsik J and Chi Y. Leptomeningeal metastatic cells adopt two phenotypic states. Cancer Rep (Hoboken). (2022) 5:e1236. doi: 10.1002/cnr2.1236
23. Sperduto PW, Mesko S, Li J, Cagney D, Aizer A, Lin NU, et al. Estrogen/progesterone receptor and HER2 discordance between primary tumor and brain metastases in breast cancer and its effect on treatment and survival. Neuro Oncol. (2020) 22:1359–67. doi: 10.1093/neuonc/noaa025
24. Hulsbergen AFC, Claes A, Kavouridis VK, Ansaripour A, Nogarede C, Hughes ME, et al. Subtype switching in breast cancer brain metastases: a multicenter analysis. Neuro Oncol. (2020) 22:1173–81. doi: 10.1093/neuonc/noaa013
25. Vogelstein B, Papadopoulos N, Velculescu VE, Zhou S, Diaz LA Jr., and Kinzler KW. Cancer genome landscapes. Science. (2013) 339:1546–58. doi: 10.1126/science.1235122
26. Nik-Zainal S, Alexandrov LB, Wedge DC, Van Loo P, Greenman CD, Raine K, et al. Mutational processes molding the genomes of 21 breast cancers. Cell. (2012) 149:979–93. doi: 10.1016/j.cell.2012.04.024
27. Ahmed M and Kim DR. Life history dynamics of evolving tumors: insights into task specialization, trade-offs, and tumor heterogeneity. Cancer Cell Int. (2024) 24:364. doi: 10.1186/s12935-024-03538-4
28. Seol H, Lee HJ, Choi Y, Lee HE, Kim YJ, Kim JH, et al. Intratumoral heterogeneity of HER2 gene amplification in breast cancer: its clinicopathological significance. Mod Pathol. (2012) 25:938–48. doi: 10.1038/modpathol.2012.36
29. Kurozumi S, Padilla M, Kurosumi M, Matsumoto H, Inoue K, Horiguchi J, et al. HER2 intratumoral heterogeneity analyses by concurrent HER2 gene and protein assessment for the prognosis of HER2 negative invasive breast cancer patients. Breast Cancer Res Treat. (2016) 158:99–111. doi: 10.1007/s10549-016-3856-2
30. Narusawa E, Kurozumi S, Katayama A, Koibuchi Y, Ogawa A, Takata D, et al. Utility of human epidermal growth factor 2 heterogeneity as a prognostic factor in triple-negative breast cancer. Med Mol Morphol. (2024) 57:177–84. doi: 10.1007/s00795-024-00386-z
31. Caswell DR and Swanton C. The role of tumour heterogeneity and clonal cooperativity in metastasis, immune evasion and clinical outcome. BMC Med. (2017) 15:133. doi: 10.1186/s12916-017-0900-y
32. Marusyk A, Almendro V, and Polyak K. Intra-tumour heterogeneity: a looking glass for cancer? Nat Rev Cancer. (2012) 12:323–34. doi: 10.1038/nrc3261
33. Klein CA. Parallel progression of primary tumours and metastases. Nat Rev Cancer. (2009) 9:302–12. doi: 10.1038/nrc2627
Keywords: human epidermal growth factor receptor 2, trastuzumab deruxtecan, case report, meningeal carcinomatosis, triple-negative breast cancers
Citation: Wang X and Weng Z (2025) Case Report: Triple-negative breast cancer with brain and meningeal metastases exhibits spatiotemporal heterogeneity in terms of HER2 expression. Front. Oncol. 15:1599148. doi: 10.3389/fonc.2025.1599148
Received: 24 March 2025; Accepted: 30 May 2025;
Published: 19 June 2025.
Edited by:
Jorge Morales-Montor, National Autonomous University of Mexico, MexicoReviewed by:
Brandon Peter Lucke-Wold, University of Florida, United StatesRocío García-Becerra, National Autonomous University of Mexico, Mexico
Giulia Pericoli, Bambino Gesù Children’s Hospital (IRCCS), Italy
Copyright © 2025 Wang and Weng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhiyun Weng, V2VuZ196aGlfeXVuQDE2My5jb20=
 Xubin Wang
Xubin Wang Zhiyun Weng
Zhiyun Weng