- 1Department of Genetic Medicine, Kanagawa Cancer Center, Yokohama, Japan
- 2Cancer Prevention and Control Division, Kanagawa Cancer Center Research Institute, Yokohama, Japan
- 3Graduate School of Health Innovation, Kanagawa University of Human Services, Kawasaki, Japan
- 4Center for Innovation Policy, Kanagawa University of Human Services, Kawasaki, Japan
Personalized cancer screening guided by genomic information holds the potential for effective cancer prevention. However, current approaches face challenges, including the low risk associated with most genetic variants and limited experience in genetic counseling for healthy individuals. We are initiating a feasibility study that employs a germline cancer genomic panel of 30–80 high-risk cancer genes, such as BRCA1 and BRCA2, to identify individuals with pathogenic variants. Genetic counseling will be conducted by certified medical doctors and genetic counselors utilizing the Kanagawa Prospective “ME-BYO” Cohort Study. This initiative seeks to adapt hereditary cancer counseling practices to preventive medicine, addressing the differences in counseling between clinical and preventive settings. It aims to establish a framework for personalized cancer screening, contributing to future guidelines for identifying individuals at high risk of hereditary cancers. This research will help bridge the gap between genomic research and practical preventive medicine, paving the way for personalized cancer screening strategies based on genetic information.
1 Introduction
Personalized medicine guided by genomic information holds great promise for advancing cancer prevention. By elucidating the constitution defined by the human genome, we can perform personalized cancer screening based on personal genomic information. According to this strategy, intensive cancer screening, including magnetic resonance imaging or risk reduction treatments, could be provided. Currently, some examinations and treatments are already being provided for patients with hereditary cancers as standard medical practice. However, despite recent advances in genomic research, personalized cancer prevention has not been utilized widely for several reasons. First, recent advances in genomic research, such as genome-wide association studies (1), have led to the availability of direct-to-consumer genetic testing (2) for the general population. However, most identified genetic variants carry a low risk of developing cancer, and the supporting evidence is often insufficient. Consequently, the clinical utility of these findings for cancer prevention remains limited. Therefore, large genome cohort studies in Japan are being conducted to simultaneously obtain genetic and detailed environmental information and analyze their interactions (3, 4). However, a period of more than 10 years will be needed to clarify the relationship with actual cancer incidence. Moreover, practical strategies for personalized cancer prevention using genomic information remain unclear. Second, limited knowledge and practical experience hinder the effective delivery of genetic counseling to cancer-free individuals undergoing genomic testing results for preventive screening. Third, genetic testing for cancer screening of the general population is costly.
2 Novel initiatives for personalized cancer screenings
A framework for personalized cancer screenings has recently been established, and the cost of genomic analysis has decreased dramatically due to recent technological innovations. We focused on the germline cancer genome panel, which has recently become widely used in genomic medicine. This panel test can comprehensively analyze approximately 30–80 genes with a high risk of cancer development, such as BRCA1 and BRCA2. The cost of this test is approximately 150,000–200,000 yen (about 1,000–1,300 USD) (5). Although prices have dropped considerably in recent years, it remains more expensive than other optional cancer screening methods, such as blood tests and imaging examinations, which are typically conducted annually or biennially. In contrast, because this test is performed only once in a lifetime, individual with a family history of cancer may be willing to bear the costs. Personalized measures centered on surveillance after diagnosis have been established, with clear medical benefits for those who undergo testing; individuals identified as having a genetic predisposition can opt for intensive surveillance, such as frequent magnetic resonance imaging. Those at particularly high risk, such as carriers of pathogenic variants in BRCA1 or BRCA2, may also consider risk-reducing surgeries as part of their preventive care. This is a clear difference from the direct-to-consumer genetic tests currently available on the market. However, to widely implement this approach in clinical practice, determining the optimal genetic counseling system remains a major challenge. Currently, genetic counseling, provided by specialist doctors and certified genetic counselors in Japan, is mainly targeted toward patients with cancer; moreover, physicians have limited experience in providing such counseling in the field of preventive medicine.
3 Barriers to implementation
Several differences exist in genetic counseling between clinical and preventive medicine. First, the target individual may be a cancer patient in clinical settings or a healthy person in preventive contexts. However, this difference can be overcome by applying genetic counseling methods being used at specialist medical institutions for unaffected relatives of patients with hereditary tumors. Second, the prior probability of hereditary cancers in patients who receive genetic counseling may differ significantly from that in patients who receive genetic counseling at cancer hospitals. Cancer hospital visitors are either patients with cancer or unaffected relatives of patients diagnosed with hereditary cancers, and the likelihood of undergoing testing is high. For example, the probability of hereditary breast-ovarian cancer has been reported to be approximately 10% (6).
Conversely, the proportion of people with pathogenic variants of cancers is much lower; the percentage of BRCA1 or BRAC2 pathogenic variants in the general population is reported to be 0.2–0.3% (7, 8), and even if the frequency of pathogenic variants in other genes covered by panel testing is considered, it is unlikely to be very high. Therefore, individuals are required to be targeted for testing by pre-screenings, selecting a high-risk group of pathogenic variants. For example, it is necessary to understand the National Comprehensive Cancer Network (NCCN) guidelines and conduct medical interviews with people. The guideline presents criteria for genetic testing, including individuals diagnosed with breast cancer at age 50 years or younger, and those with triple-negative breast cancer. For individuals without cancer diagnosis, testing is also recommended if they have close blood relatives with breast cancer diagnosed at age 50 years or younger, male breast cancer, ovarian cancer, or pancreatic cancer. Further, specialized medical professionals, such as genetic counselors or physicians whose subspecialty is genetic medicine, are required, which currently remains a major obstacle. However, this can be solved by utilizing an AI Chatbot system that we have recently developed (9, 10).
4 Design of our study
We have planned to conduct a feasibility study, titled “Implementation of a Cancer Risk Assessment Program Using Genetic Testing in the General Female Population (UMIN000056304) (Figure 1).” This study will utilize the know-how of hereditary tumor counseling, which is routinely provided to patients with cancer at medical institutions. Further, we will use the research platform of the Kanagawa Prospective “ME-BYO” Cohort Study (the ME-BYO Cohort) (11) in this study. Participants have been enrolled in advance for this population-based cohort study. The certified medical doctors and certified genetic counselors of the Department of Genetic Medicine, Kanagawa Cancer Center, will provide genetic counseling for the participants with pathogenic variants.
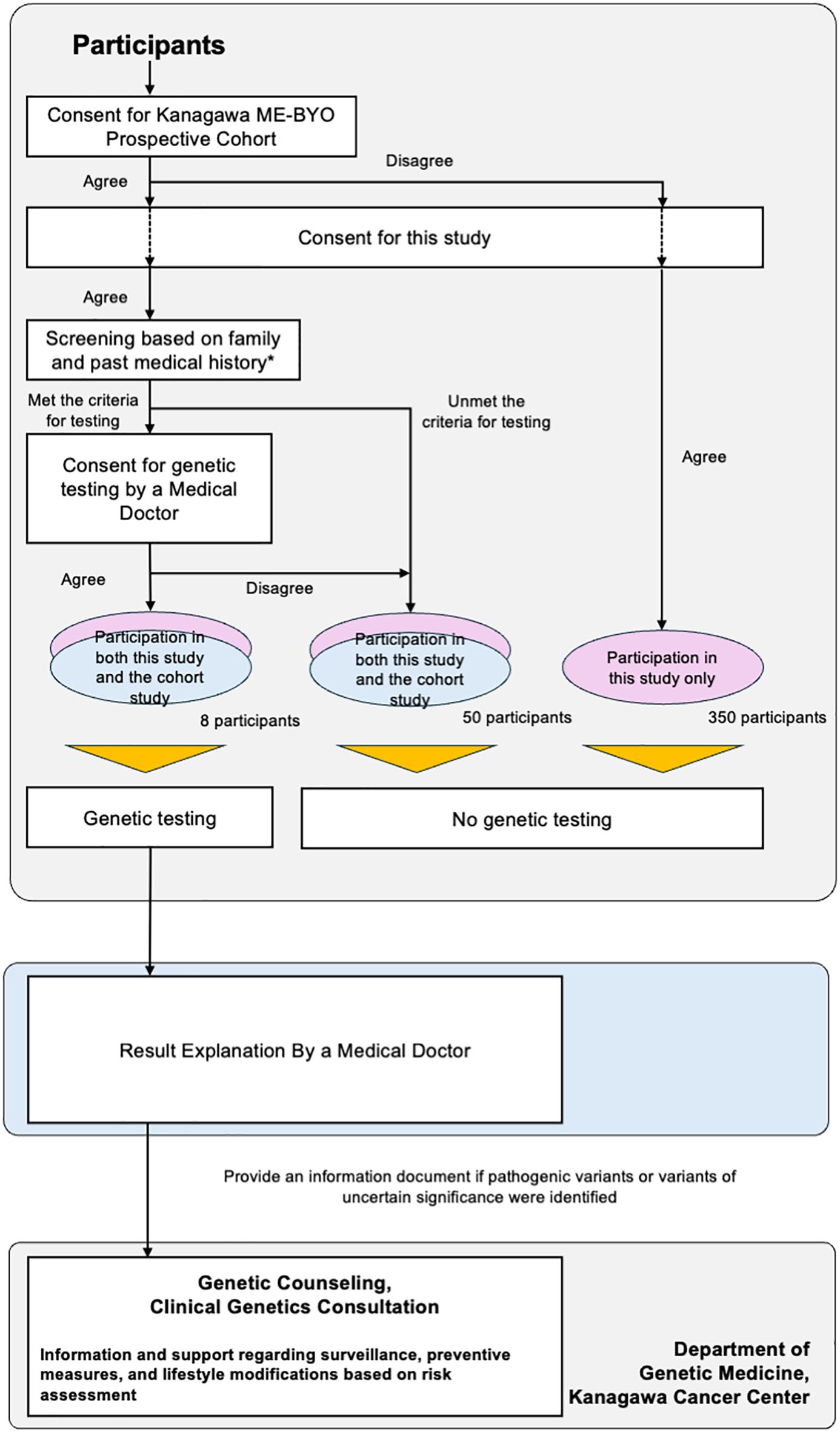
Figure 1. Study design of “Implementation of a Cancer Risk Assessment Program Using Genetic Testing in the General Female Population”.
5 Discussion
The model of the personalized cancer screening program developed in this study can be used to realize tailor-made cancer screening strategies that utilize genetic information obtained from whole-genome analysis, which will become available in the near future. The model developed in this study targets the causative genes of hereditary cancers with a high risk of cancer development.
Essential next steps include collaborative activities with other projects and institutions in Japan. In 2023, Tohoku Medical Megabank Organization announced that it had started reporting germline findings, including those related to hereditary breast and ovarian cancer and Lynch syndrome, to their study participants (12). In addition to experience in the high-volume center of genetic medicine, the accumulated experiences from population-based genomic cohort studies would greatly benefit from these collaborative activities. Finally, we can create guidelines for identifying patients at a high risk of hereditary cancers, significantly contributing to the development of personalized cancer screening.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author contributions
HiN: Conceptualization, Funding acquisition, Writing – review & editing, Writing – original draft. KW: Conceptualization, Writing – review & editing. AS: Conceptualization, Writing – review & editing. EH: Conceptualization, Writing – review & editing. MO: Conceptualization, Writing – review & editing. HaN: Conceptualization, Writing – review & editing. SN: Conceptualization, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The study, “Implementation of a Cancer Risk Assessment Program Using Genetic Testing in the General Female Population,” is partly supported by Kanagawa Institute of Industrial Science and Technology. The genetic testing in this study is partly supported by Actmed Co., Ltd. (Tokyo, Japan). The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article, or the decision to submit it for publication. The ME-BYO Cohort is partly supported by the Japan Society for the Promotion of Science (JSPS) KAKENHI Grant [no. 22H04923 (CoBiA)] and Kanagawa Prefecture.
Acknowledgments
We would like to thank Editage (https://www.editage.jp) for English language editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Abdellaoui A, Yengo L, Verweij KJH, and Visscher PM. 15 years of gwas discovery: realizing the promise. Am J Hum Genet. (2023) 110:179–94. doi: 10.1016/j.ajhg.2022.12.011
2. Oh B. Direct-to-consumer genetic testing: advantages and pitfalls. Genomics Inform. (2019) 17:e33. doi: 10.5808/GI.2019.17.3.e33
3. Takeuchi K, Naito M, Kawai S, Tsukamoto M, Kadomatsu Y, Kubo Y, et al. Study profile of the Japan multi-institutional collaborative cohort (J-micc) study. J Epidemiol. (2021) 31:660–8. doi: 10.2188/jea.JE20200147
4. Kuriyama S, Yaegashi N, Nagami F, Arai T, Kawaguchi Y, Osumi N, et al. The tohoku medical megabank project: design and mission. J Epidemiol. (2016) 26:493–511. doi: 10.2188/jea.JE20150268
5. Home Page of Act Med Corporation (2024). Available online at: https://www.actmed.jp/company.html (Accessed March 24, 2025).
6. Haneda E, Sato A, Suganuma N, Sebata Y, Okamoto S, Toda S, et al. Efficacy of clinical guidelines in identifying all Japanese patients with hereditary breast and ovarian cancer. Int J Environ Res Public Health. (2022) 19:6182. doi: 10.3390/ijerph19106182
7. Brca Gene Changes: Cancer Risk and Genetic Testing, Fact Sheet by National Cancer Institute (2024). Available online at: https://www.cancer.gov/about-cancer/causes-prevention/genetics/brca-fact-sheet (Accessed March 24, 2025).
8. Ohneda K, Hamanaka Y, Kawame H, Fuse N, Nagami F, Suzuki Y, et al. Returning individual genomic results to population-based cohort study participants with brca1/2 pathogenic variants. Breast Cancer. (2023) 30:110–20. doi: 10.1007/s12282-022-01404-7
9. Sato A, Haneda E, Suganuma N, and Narimatsu H. Preliminary screening for hereditary breast and ovarian cancer using a chatbot augmented intelligence genetic counselor: development and feasibility study. JMIR Form Res. (2021) 5:e25184. doi: 10.2196/25184
10. Sato A, Haneda E, Hiroshima Y, and Narimatsu H. Preliminary screening for hereditary breast and ovarian cancer using an ai chatbot as a genetic counselor: clinical study. J Med Internet Res. (2024) 26:e48914. doi: 10.2196/48914
11. Nakamura S, Watanabe R, Saito Y, Watanabe K, Chung UI, and Narimatsu H. The me-byo index: A development and validation project of a novel comprehensive health index. Front Public Health. (2023) 11:1142281. doi: 10.3389/fpubh.2023.1142281
Keywords: hereditary tumor, artificial intelligence, breast cancer, germ-line cells, cancer screening
Citation: Narimatsu H, Watanabe K, Sato A, Haneda E, Okamoto M, Nakada H and Nakamura S (2025) Personalized preventive medicine using genomic information: future perspective and corresponding research plan. Front. Oncol. 15:1599753. doi: 10.3389/fonc.2025.1599753
Received: 26 March 2025; Accepted: 12 May 2025;
Published: 09 June 2025.
Edited by:
Sharon R Pine, University of Colorado Anschutz Medical Campus, United StatesReviewed by:
Song Tang, The First Affiliated Hospital of Guangdong Pharmaceutical University, ChinaCopyright © 2025 Narimatsu, Watanabe, Sato, Haneda, Okamoto, Nakada and Nakamura. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hiroto Narimatsu, aGlyb3RvLW5hcmltYXRzdUB1bWluLm9yZw==
 Hiroto Narimatsu
Hiroto Narimatsu Kaname Watanabe
Kaname Watanabe Ann Sato1,3
Ann Sato1,3 Sho Nakamura
Sho Nakamura