- 1Department of Radiology, Affiliated Cancer Hospital of Zhengzhou University & Henan Cancer Hospital, Zhengzhou, China
- 2Department of Cancer Epidemiology, Affiliated Cancer Hospital of Zhengzhou University & Henan Cancer Hospital, Zhengzhou, China
Background: The application performance of the Breast Imaging-Reporting and Data System (BI-RADS) in primary healthcare settings remains uncertain. The normativity of BI-RADS classification and the efficacy of breast cancer detection guided by BI-RADS classification were evaluated here.
Methods: All data used in the current study were derived from a breast cancer screening cohort baseline database, which consists of 8,996 women aged 35–64 years from Central China. Participants aged 35–44 underwent automated breast ultrasound (ABUS) and handheld ultrasound (HHUS), while those aged 45–64 were screened with ABUS, HHUS, and mammography (MG). All imaging diagnoses were made by radiologists according to the BI-RADS 5th edition classification system published by the ACR in 2013. The distribution of malignant imaging findings and inter-modality agreement on BI-RADS classifications were assessed. Based on pathological results, the area under the receiver operating characteristic (ROC) curve (AUC) was used to assess the performance of breast cancer screening according to BI-RADS-guided referrals.
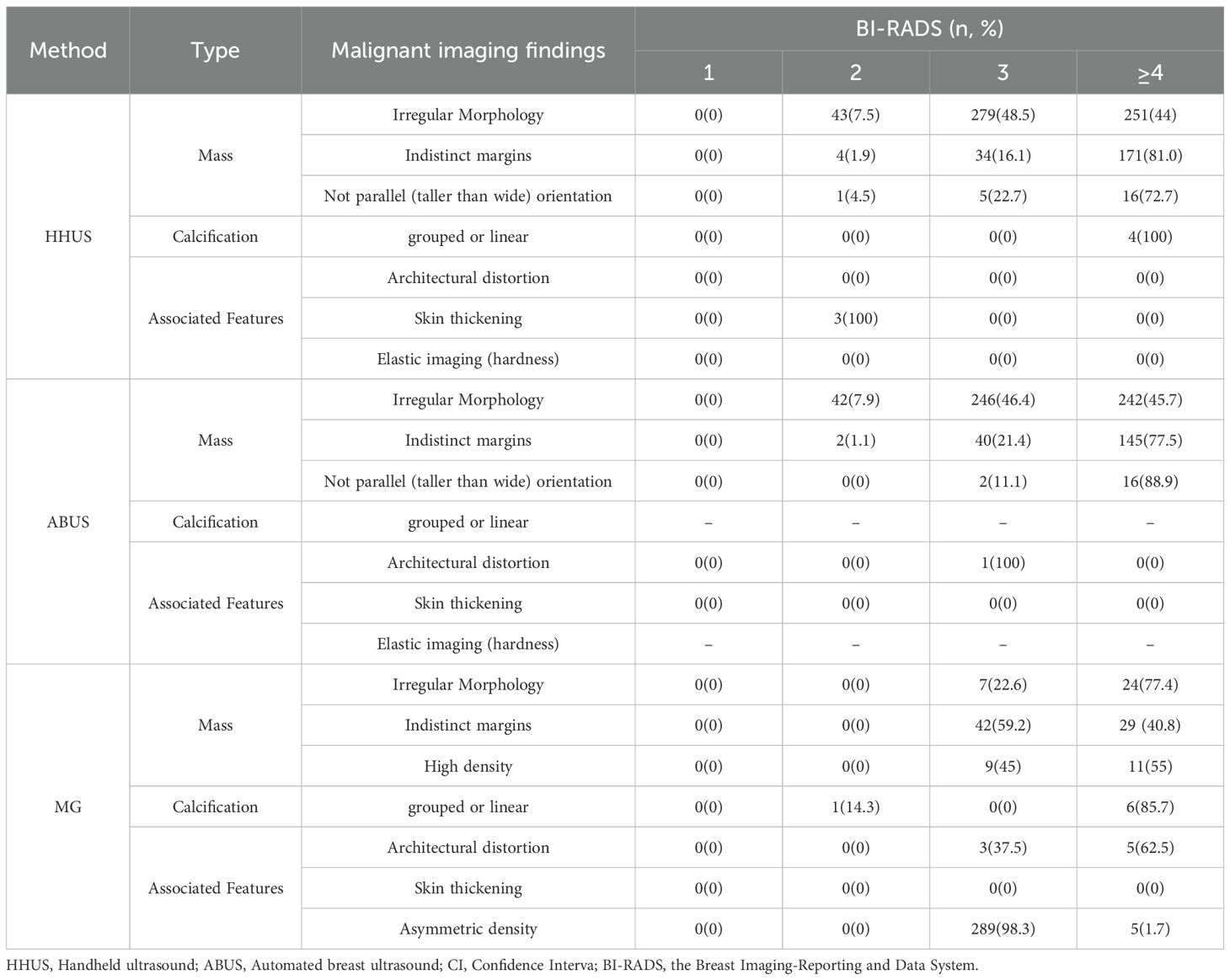
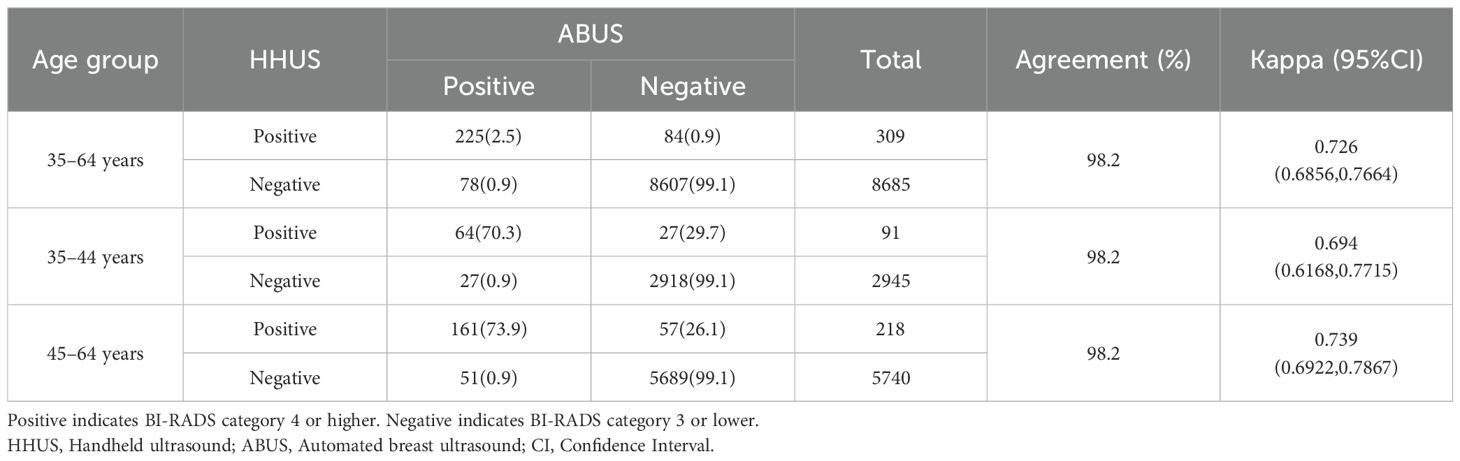
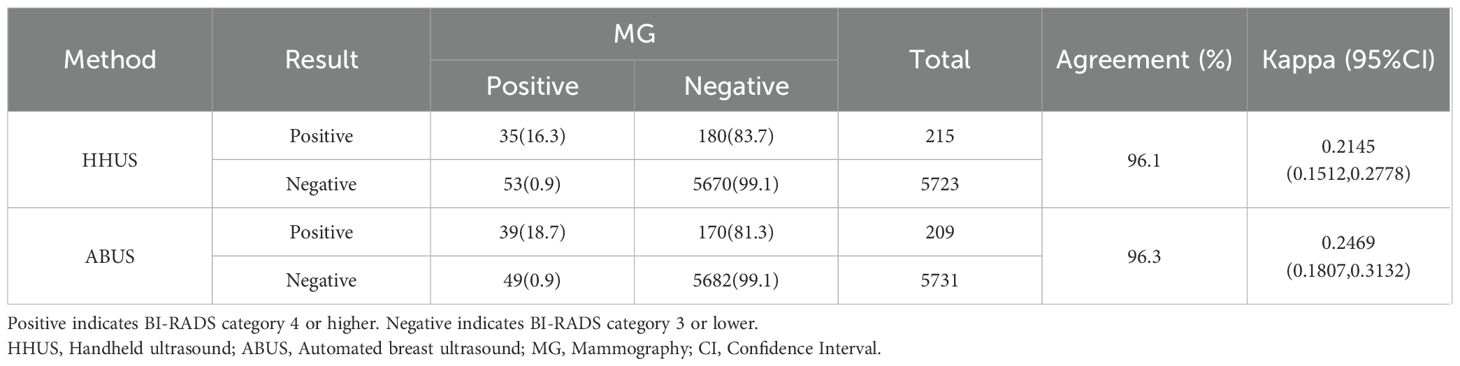
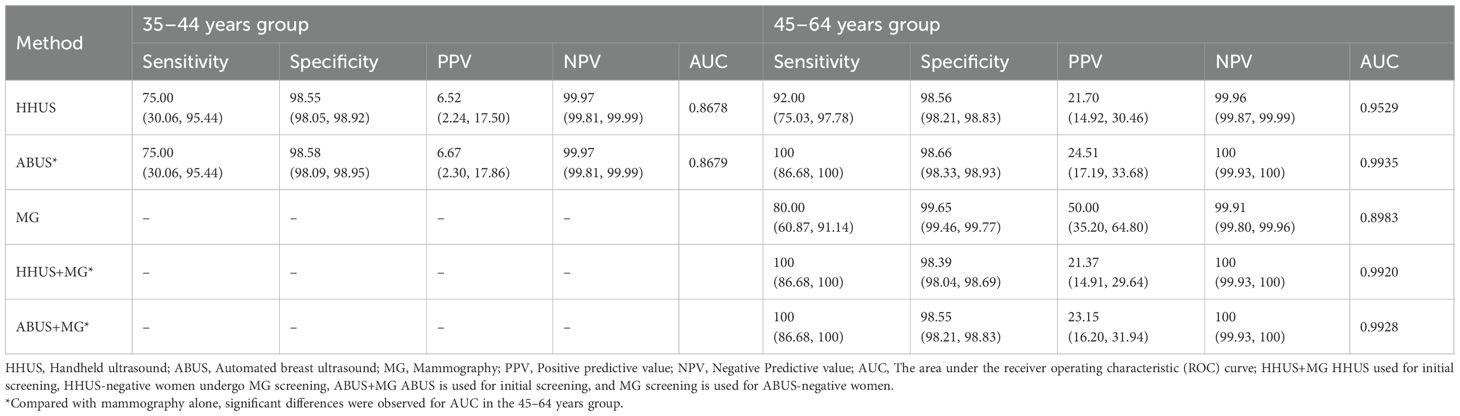
Results: Of individuals found with irregular morphology, 44%, 45.7% and 77.4% were classified as BI-RADS category 4 or higher for HHUS, ABUS and MG, respectively; For those with indistinct margins, the proportion was 81%, 77.5% and 40.8%, correspondingly; For grouped calcifications, they were 100% for HHUS and 85.7% for MG; Meanwhile, 72.7% and 88.9% not parallel (taller than wide) masses were categorized as BI-RADS category 4 for HHUS and ABUS. The concordance of BI-RADS classification was as high as 98.2% between HHUS and ABUS (Kappa = 0.726), whereas it was about 96% between ultrasound and MG (Kappa ranged from 0.21 to 0.25). The BI-RADS guided screening performance for breast cancer showed AUC values of 0.9935 for ABUS, 0.9529 for HHUS, 0.8983 for MG. If the BI-RADS diagnosis of MG was considered in ultrasound-negative women, only the HHUS’s performance was boosted, achieving an AUC of 0.9920.
Conclusions: Radiologists at primary hospitals can effectively apply BI-RADS based on the malignant features they found. BI-RADS can provide a reliable framework for guiding breast cancer screening in primary healthcare settings.
Background
The goal of breast cancer screening is to detect cancer at an early stage, thereby improving treatment outcomes and survival rates (1–3). The imaging modalities usually used in breast cancer screening include handheld ultrasound (HHUS), and mammography (MG). HHUS is widely used in primary healthcare settings due to its ease of operation, low cost, and effectiveness in detecting breast masses and cystic lesions (4). Technological advancements have led to the incorporation of automated breast ultrasound (ABUS) in breast cancer screening, providing higher resolution and precise spatial localization by volume imaging for clearer breast imaging and improved mass detection rates (5, 6). MG, which utilizes low-dose X-rays, is vital for early breast cancer detection, especially in women with less dense breast glandular tissue (7, 8).
However, imaging diagnostic accuracy is modulated by radiologists experience (9). To enhance diagnostic accuracy, the Breast Imaging-Reporting and Data System (BI-RADS) is a widely adopted classification system for interpreting breast imaging findings (10, 11). BI-RADS integrates imaging features, risk assessment, and clinical information to inform diagnostic and treatment decisions (12, 13). The BI-RADS classification ranges from 0 to 6, with BI-RADS category 4 and 5 indicating a higher likelihood of malignancy (14). BI-RADS was built on previous work focused on the positive predictive value of imaging features, by clarifying previous terms with an aim toward risk stratification. The malignancy indicators emphasized within BI-RADS encompass grouped or linear calcifications (15–17) and mass-related signs such as irregular shape, indistinct margin, not parallel (taller than wide) (18–21), along with other accompanying signs such as architectural distortion (22, 23), asymmetry (24, 25), and harder textures in tumors through ultrasound elastography (26, 27).
Despite advancements in imaging technology, the variance in clinical expertise, imaging equipment, and training levels across different levels of hospitals persists, leading to an uncertain efficacy of identifying and interpreting malignant signs and radiological diagnostic capabilities in breast cancer screening within the primary healthcare setting (12, 28). Therefore, this study aims to assess the application of BI-RADS classification in primary hospitals by evaluating the relationship between imaging features, BI-RADS classification outcomes, and pathological findings in breast cancer screening.
Materials and methods
Study design and population
Data used in the current study were extracted from a breast cancer screening cohort which consists of 8,996 women aged 35–64 years from Central China. BI-RADS diagnoses, pathological results and imaging characteristics were retrospectively analyzed. Participants enrolled in this screening cohort were local general women aged 35–64 years. The exclusion criteria included being pregnant, lactating, or planning to become pregnancy; had a history of breast tumor resection, contralateral breast surgery, breast augmentation, or percutaneous biopsy within the past 12 months; had a prior tumor diagnosis or treatment within the last 12 months; or exhibited suspicious signs without an imaging indication. This study was approved by the independent ethic committee of Henan Cancer Hospital (Approval Number: 19/109-1893).
Imaging screening
In the current study, breast cancer imaging screening modalities included HHUS, ABUS and MG. Based on current screening guidelines (29, 30), which recommended MG for women aged ≥45 years, participants were stratified into two age groups: 35–44 years (screened with ABUS and HHUS) and 45–64 years (screened with ABUS, HHUS and MG).
HHUS was performed using the EADN U50 ultrasound device (frequency range: 7.0-16.0 MHz; EADN, Shenzhen, China), with detailed documentation of breast lesion characteristics. ABUS was operated using the SIUI IBUS BE3 (frequency: 5–12 MHz; Shantou Institute of Ultrasonic Instruments, Shantou, China) and Invenia ABUS (C15-6XW Reverse Curve™, frequency: 6–15 MHz; GE Healthcare, Hatfield, UK) devices for scanning. MG was conducted using the Hologic Selenia Dimensions system (Hologic, Massachusetts, USA). Ultrasound and MG images were interpreted by radiologists according to the BI-RADS 5th edition classification system published by the ACR in 2013.
The key imaging findings serving as the basis for the BI-RADS classification were collected as follows: the size, shape, margin, orientation of the lesion, the presence of calcifications, and other associated signs of suspicious lesions (including architectural distortion, skin thickening, and hardness in elasticity-imaging) for HHUS and ABUS; The size, shape, and density of lesion, the presence of calcifications, and other associated signs (including architectural distortion, skin thickening, and the presence of asymmetries) for MG. Mass shape was categorized as regular(including round, oval) or irregular; Orientation was classified as parallel (long axis of lesion is parallel to the skin, also referred to as wider than tall) or not parallel (long axis of lesion is not parallel to the skin, also referred to as taller than wide); The margin was described as circumscribed or indistinct; Calcifications were classified as grouped, linear or benign-appearing. Architectural distortion was defined as a localized distorted breast parenchyma with no definite mass visible; asymmetries was defined as an asymmetric dense shadow of fibro glandular tissue without a clear three-dimensional outline or distinct margin when compared with the corresponding location on the contralateral breast.
Pathological examination
Pathological diagnosis was carried out by pathologists at the screening units in accordance with uniform standards. Women with BI-RADS category 4 or higher underwent biopsy, and confirmed cases were staged according to the eighth-edition Breast Cancer Staging System published by the American Joint Committee on Cancer (AJCC).
Mammography screenings were carried out employing the Hologic Selenia Dimensions system (Hologic, Inc., Marlborough, MA, USA), a system renowned for its high-resolution imaging capabilities, thereby contributing to the accuracy of the diagnostic process.
Statistical analysis
The distribution of malignant imaging findings across the BI-RADS categories from different imaging screening modalities was calculated to assess the prevalence of high classifications that would warrant clinical biopsy. The inter-modality agreement on BI-RADS category 4 or higher was assessed to evaluate diagnostic characteristics of each imaging method. The efficacy of breast cancer screening based on BI-RADS-guided referrals for pathological biopsy was evaluated using the diagnostic indicators of sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and the area under the receiver operating characteristic (ROC) curve. SAS 9.4 software was utilized for all statistical analyses, with a significance level set at α = 0.05.
Results
Breast cancer detection
Of 8996 women, 362 individuals were classified as BI-RADS category 4 or higher, and 117 biopsies were performed in the breast cancer screening program. Ultimately, 29 breast cancer cases were identified, with 4 cases in the 35–44 years group and 25 cases in the 45–64 years group.
Imaging findings and BI-RADS classification
Table 1 delineates the distribution of malignant imaging findings across the BI-RADS categories in HHUS, ABUS and MG. A minority of mass-related malignant signs were BI-RADS category 2 in both HHUS (48 women) and ABUS (44 women), while the majority were BI-RADS category 3 (318 for HHUS and 288 for ABUS) or BI-RADS category 4 or higher (438 for HHUS and 403 for ABUS). Notably, among mass-related malignant signs, indistinct margins and not parallel (taller than wide) orientation showed a high proportion of lesions being classified as BI-RADS category 4 or higher (81% and 72.7% in HHUS, 77.5% and 88.9% in ABUS, respectively). MG less frequently identified mass-related malignant signs, such as 31 for irregular morphology and 71 for indistinct margins, compared to HHUS (573 vs. 209) and ABUS (530 vs. 187). However, MG detected more calcification than HHUS (7 vs. 4). Among individuals with calcification, 100% were BI-RADS category 4 or higher for HHUS, whereas 85.7% for MG. Meanwhile, all eight individuals with architectural distortion detected by MG were BI-RADS category 3 or higher, of whom 62.5% were BI-RADS category 4 or higher. This diagnostic pattern is similar to that of the characteristics observed with calcifications, indicates that both architectural distortion and calcifications are strong indicators of malignancy for radiologists. Asymmetric density in MG was largely concentrated in BI-RADS category 3 (98.3%).
Agreement of BI-RADS classification across different imaging modalities
The agreement on BI-RADS classifications of category 4 or higher between HHUS and ABUS was 98.2%, with a Kappa coefficient of 0.726, indicative of a substantial agreement between the two imaging modalities. When stratified by age groups, the agreement for both 35–44 and 45–64 subgroups was found to be 98.2%. This suggests no significant variation in consistency between the two ultrasound techniques across different age groups (Table 2).
In contrast, the agreement between ultrasound and MG within the 45–64 age group was comparatively lower, with agreement rates of 96.1% for HHUS and MG, and 96.3% for ABUS and MG, respectively. This discrepancy primarily stemmed from a higher proportion of cases where ultrasound flagged as positive while MG flagged as negative (77.3% for HHUS and 77.6% for ABUS), versus fewer instances where ultrasound flagged as negative while MG flagged as positive (22.7% for HHUS and 22.4% for ABUS) (Table 3).
Efficacy of BI-RADS classification in cancer screening
The efficacy of different image-based BI-RADS classification systems in breast cancer screening was evaluated across age groups. In the 35–44 age group, both HHUS and ABUS independently detected all 4 breast cancer cases of, exhibiting comparable performance metrics in sensitivity(75% vs 75%), specificity(98.55% vs 98.58%), PPV(6.52% vs 6.67%), and NPV(99.97% vs 99.97%). Among the 45–64 years group, ABUS detected all 25 confirmed breast cancer cases, HHUS detected 23 cancer cases, and MG only identified 20 cancer cases. The sensitivities were 100.0% for ABUS, 92.0% for HHUS, and 80.0% for MG, with specificities ranging from 98.6% to 99.7% (Table 4). Incorporating MG BI-RADS diagnoses into assessments of women with negative ultrasound findings (BI-RADS category 3 or lower) increased the sensitivity of HHUS from 92% to 100%, albeit with a minor decrease in specificity from 98.56% to 98.39%. A similar drop in specificity was observed for ABUS, from 98.66% to 98.55%. The evaluation of AUC yielded values of 0.9529 for HHUS, 0.9935 for ABUS, and 0.8983 for MG. The synergistic application of HHUS and MG, following the aforementioned combined assessment rules, significantly enhanced the AUC of HHUS, elevating it from 0.9529 to 0.9920. Notably, the AUC for ABUS combined with MG was slightly lower than when using ABUS alone.
Discussion
This study found that almost all malignant signs identified by radiologists at primary healthcare hospitals were diagnosed as BI-RADS category 3 or higher, which alert clinical attention. Signs with high malignancy indications, such as not parallel (taller than wide) orientation and grouped calcifications, were categorized as higher, namely BI-RADS category 4 or higher, to prompt timely biopsies in clinical practice. Ultrasound is sensitive in detecting mass-related malignant signs, while MG is sensitive in detecting malignant calcifications and architectural distortion. The efficacy of BI-RADS classification used in breast cancer screening is promising in primary healthcare settings.
In this study, it was observed that irregularly shaped masses were similarly categorized as BI-RADS category 3 and category 4 or higher by HHUS and ABUS. However, in MG, these masses tended to be assigned higher BI-RADS levels. Considering the significantly lower detection of mass lesions by MG compared to ultrasound, it can be hypothesized that this difference may be related to the lower sensitivity of MG in detecting isodense lesions. Therefore, irregularly shaped masses visualized on MG often draw high attention from radiologists (31, 32). This study also indicated that in ultrasound examinations, features of indistinct mass margins were strongly associated with a categorization of BI-RADS category 4 or higher. This contrasted starkly with the findings of MG, which did not show a distinct preference for indistinct mass margins between BI-RADS category 3 and 4. This may be due to the fact that during the MG imaging process, the edges of many benign lesions become blurred due to the surrounding breast tissue, complicating the evaluation of these lesions (33, 34). Furthermore, regarding the orientation observed in the images, masses with a parallel (wider-than-tall) orientation are less likely to be malignant on ultrasound compared to those with a not parallel (taller-than-wide) orientation (35). In this study, masses with a not parallel (taller-than-wide) orientation were classified as BI-RADS category 4 or higher in 72.7% and 88.9% of HHUS and ABUS diagnoses, respectively.
In the context of breast cancer screening, calcifications are pivotal imaging findings, especially in MG examinations. Coarse or popcorn-like calcifications are often associated with benign lesions, while small and grouped, branching, or linear calcifications may indicate malignancy (15). The distribution and morphology of calcifications are more distinctly observable on MG than ultrasound, which is consistent with the findings of other studies. This is possibly because MG can more intuitively present calcifications (36).
The study demonstrated a high diagnostic consistency between HHUS and ABUS, indicating that ABUS can be effectively utilized for BI-RADS classification like HHUS. However, when comparing HHUS and ABUS to MG, the consistency was lower with 96.1% and 96.3% respectively. Discrepancies predominantly manifested as a higher proportion of cases (77.3% for HHUS and 77.6% for ABUS) where both HHUS and ABUS detected abnormalities while MG did not, as opposed to fewer instances (22.7% for HHUS and 22.4% for ABUS) where ultrasound flagged as negative while MG flagged as positive. The differences may be due to the varying abilities of ultrasound and MG to detect different types of malignancy-related pathological features observed in this study. Ultrasound may be more effective in identifying mass-related signs, while MG may be more excellent at detecting calcifications and architectural distortions. The reason why MG detects fewer mass-related signs compared to ultrasound may be related to the high-density glandular tissue that can obscure isodense lesions, while ultrasound is not affected by such tissue density (37). Our findings align with those of previous studies, which indicate that MG identifies fewer mass-related malignancies compared to ultrasound but detects a higher prevalence of malignancies associated with calcifications and architectural distortions (38–41), which highlights the complementary nature of ultrasound and MG in breast cancer screening.
This study also evaluated the performance of BI-RADS classification system in breast cancer screening, highlighting notable differences across age groups and screening methods. In the screening program for individuals aged 35-44, both HHUS and ABUS showed similar diagnostic performance, with sensitivities of 75% and specificities of 98.55% and 98.58%, respectively. For individuals aged 45-64, ABUS showed slightly higher sensitivity (100% vs 92%) and specificity (98.66% vs 98.56%). This aligns with a similar finding in a previous study that ABUS has statistically significant higher diagnostic accuracy than HHUS in detecting breast cancer (42). This difference may be attributed to ABUS providing volume and more comprehensive breast imaging, thereby enhancing the detection rate of lesions (43). When using MG as a supplementary examination to the ultrasound-negative women, the BI-RADS classification system can increase the sensitivity from 92% to 100% in the 45–64 age group, underscoring the benefits of a comprehensive screening approach. However, a minor decrease in specificity may result in an increase in false-positive results, imposing a psychological burden on patients and increasing the cost of follow-up examinations. Despite achieving a sensitivity of 100% when used alone, ABUS showed a decrease in AUC if using MG as a supplementary examination, suggesting that adding additional screening methods may not confer additional benefits to ABUS (44).
Nevertheless, this study has several limitations that should be considered when interpreting its results. Firstly, our study was conducted at a single center, which may limit the breadth of our findings. The practices of BI-RADS in primary healthcare in our study may not be fully representative of those found in other settings. Secondly, while our study evaluated the implementation of the BI-RADS classification system in primary healthcare hospitals, it did not involve a quality control assessment by highly experienced radiologists to directly compare and more accurately evaluate the standardization and compliance of BI-RADS application at these facilities.
Conclusion
Radiologists at primary hospitals can effectively adhere to BI-RADS guidelines to provide clinical indications of malignant risks. The differences in BI-RADS classification diagnoses between ultrasound and MG reflect the characteristics of each imaging technique. Based on the BI-RADS findings, HHUS, ABUS, and MG have good efficacy in breast cancer screening. In conclusion, the application of BI-RADS is acceptable in primary healthcare hospitals.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by ethic committee of Henan Cancer Hospital (Approval Number: 19/109-1893). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
XL: Methodology, Writing – original draft, Project administration, Writing – review & editing, Conceptualization. HW: Methodology, Writing – review & editing, Data curation, Formal analysis, Funding acquisition. H-FX: Data curation, Formal analysis, Methodology, Funding acquisition, Writing – review & editing. S-KZ: Conceptualization, Formal analysis, Resources, Writing – review & editing, Methodology, Project administration, Data curation. B-JZ: Formal analysis, Writing – review & editing, Methodology, Validation. H-LL: Writing – review & editing, Supervision, Conceptualization, Project administration.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This project was supported by the Henan Key Project of Science and Technology (Grant number: 202102310107), the Henan Provincial Natural Science Foundation (Grant number: 242300420416), the Open Project of the Key Laboratory of Cancer Invasion and Metastasis, Ministry of Education (grant no. 2024KFKT009), and the Henan Province Key R&D and Promotion Project (Grant number: 232102310242).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that Generative AI was used in the creation of this manuscript. AI only used for academic translation of manuscripts and grammar correction.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Houssami N. Can population breast screening programs be leveraged to reduce the burden of breast cancer? Breast. (2021) 60:245–6. doi: 10.1016/j.breast.2021.11.011
2. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
3. Saslow D, Solomon D, Lawson HW, Killackey M, Kulasingam SL, Cain JM, et al. American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. J lower genital tract Dis. (2012) 16:175–204. doi: 10.1097/LGT.0b013e31824ca9d5
4. Iacob R, Iacob ER, Stoicescu ER, Ghenciu DM, Cocolea DM, Constantinescu A, et al. Evaluating the role of breast ultrasound in early detection of breast cancer in low- and middle-income countries: A comprehensive narrative review. Bioengineering. (2024) 11:262. doi: 10.3390/bioengineering11030262
5. Zhang J, Wu F, Meng F, Zhang G, Wang R, Yang Y, et al. A High-Resolution 3D Ultrasound Imaging System Oriented towards a Specific Application in Breast Cancer Detection Based on a 1 x 256 Ring Array. Micromachines. (2024) 15:209. doi: 10.3390/mi15020209
6. Xiao Y, Zhou Q, and Chen Z. Automated breast volume scanning versus conventional ultrasound in breast cancer screening. Acad Radiol. (2015) 22:387–99. doi: 10.1016/j.acra.2014.08.013
7. Gastounioti A, Cohen EA, Pantalone L, Ehsan S, Vasudevan S, Kurudi A, et al. Changes in mammographic density and risk of breast cancer among a diverse cohort of women undergoing mammography screening. Breast Cancer Res Treat. (2023) 198:535–44. doi: 10.1007/s10549-023-06879-2
8. Pisano ED, Gatsonis C, Hendrick E, Yaffe M, Baum JK, Acharyya S, et al. Diagnostic performance of digital versus film mammography for breast-cancer screening. New Engl J Med. (2005) 353:1773–83. doi: 10.1056/NEJMoa052911
9. Berg WA, Zhang Z, Lehrer D, Jong RA, Pisano ED, Barr RG, et al. Detection of breast cancer with addition of annual screening ultrasound or a single screening MRI to mammography in women with elevated breast cancer risk. Jama. (2012) 307:1394–404. doi: 10.1001/jama.2012.388
10. Kim D, Kim J, Jung HK, and Kim S. Assessment of Malignant risk stratification for microcalcifications interpreted as “amorphous” morphology on mammography: A study based on the 5th edition of breast Imaging Reporting and Data System. Eur J Radiol. (2023) 162:110795. doi: 10.1016/j.ejrad.2023.110795
11. Merjane V, Perin DMP, Bacha P, Miranda BMM, Bitencourt AGV, and Iared W. Breast Imaging Reporting and Data System (BI-RADS(R)): a success history and particularities of its use in Brazil. Rev Bras ginecologia e obstetricia: Rev da Federacao Bras das Sociedades Ginecologia e Obstetricia. (2024) 46:e-rbgo6. doi: 10.61622/rbgo/2024AR06
12. Wang P, Xia H, Liu L, Wang X, Yan L, Kong Z, et al. Improving the diagnostic performance and breast imaging reporting and data system category agreement of less experienced radiologists by utilizing computer-aided diagnosis software for breast ultrasound. Ultrasound Q. (2024) 40:e00695. doi: 10.1097/RUQ.0000000000000695
13. Lian W, Lian K, and Lin T. Breast Imaging Reporting and Data System evaluation of breast lesions improved with virtual touch tissue imaging average grayscale values. Technol Health Care. (2024) 32:925–36. doi: 10.3233/THC-230306
14. Choi JS. Breast imaging reporting and data system (BI-RADS): advantages and limitations. J Korean Soc Radiol. (2023) 84:3–14. doi: 10.3348/jksr.2022.0142
15. Berg WA, Arnoldus CL, Teferra E, and Bhargavan M. Biopsy of amorphous breast calcifications: pathologic outcome and yield at stereotactic biopsy. Radiology. (2001) 221:495–503. doi: 10.1148/radiol.2212010164
16. Burnside ES, Ochsner JE, Fowler KJ, Fine JP, Salkowski LR, Rubin DL, et al. Use of microcalcification descriptors in BI-RADS 4th edition to stratify risk of Malignancy. Radiology. (2007) 242:388–95. doi: 10.1148/radiol.2422052130
17. Bent CK, Bassett LW, D’Orsi CJ, and Sayre JW. The positive predictive value of BI-RADS microcalcification descriptors and final assessment categories. AJR. Am J roentgenol. (2010) 194:1378–83. doi: 10.2214/AJR.09.3423
18. Liberman L, Abramson AF, Squires FB, Glassman JR, Morris EA, and Dershaw DD. The breast imaging reporting and data system: positive predictive value of mammographic features and final assessment categories. AJR. Am J roentgenol. (1998) 171:35–40. doi: 10.2214/ajr.171.1.9648759
19. Xu Y, Sun J, Guo F, Nanding A, Li Q, and Jiang D. Focus on the predictive value of subclassification of extratumoral structural abnormalities for Malignant nonspiculate and noncalcified masses on digital mammography. Front Genet. (2022) 13:822858. doi: 10.3389/fgene.2022.822858
20. Ryu MJ, Kim YS, and Lee SE. Association between imaging features using the BI-RADS and tumor subtype in patients with invasive breast cancer. Curr Med Imaging. (2022) 18:648–57. doi: 10.2174/1573405617666210520155157
21. Ferre R, Pare M, and Mesurolle B. Ultrasound features of retroareolar breast carcinoma. Diagn interventional Imaging. (2017) 98:409–13. doi: 10.1016/j.diii.2017.02.009
22. Chen X, Zhang Y, Zhou J, Wang X, Liu X, Nie K, et al. Diagnosis of architectural distortion on digital breast tomosynthesis using radiomics and deep learning. Front Oncol. (2022) 12:991892. doi: 10.3389/fonc.2022.991892
23. Wadhwa A, Majidi SS, Cherian S, Dykstra DS, Deitch SG, Hansen C, et al. Architectural distortion on screening digital breast tomosynthesis: pathologic outcomes and indicators of Malignancy. J Breast Imaging. (2021) 3:34–43. doi: 10.1093/jbi/wbaa099
24. Chopier J, Roedlich MN, and Mathelin C. Breast imaging of mass, architectural distortion and asymmetry: Clinical practice guidelines. J gynecologie obstetrique biologie la Reprod. (2015) 44:947–59. doi: 10.1016/j.jgyn.2015.09.056
25. Gurando AV, Babkina TM, Dykan IM, Kozarenko TM, Gurando VR, and Telniy VV. Digital breast tomosynthesis and full-field digital mammography in breast cancer detection associated with four asymmetry types. Wiadomosci lekarskie. (2021) 74:842–8. doi: 10.36740/WLek202104106
26. Du Y, Ma J, Wu T, Li F, Pan J, Du L, et al. Downgrading Breast Imaging Reporting and Data System categories in ultrasound using strain elastography and computer-aided diagnosis system: a multicenter, prospective study. Br J Radiol. (2024) 97:1653–60. doi: 10.1093/bjr/tqae136
27. Deeg J, Swoboda M, Egle D, Wieser V, Soleiman A, Ladenhauf V, et al. Shear-wave elastography gradient analysis of newly diagnosed breast tumours: A critical analysis. Diagnostics. (2024) 14:1657. doi: 10.3390/diagnostics14151657
28. Sun Y, Wang Y, Zhang H, Hu Z, Ma Y, and He Y. What breast cancer screening program do rural women prefer? A discrete choice experiment in Jiangsu, China. patient. (2024) 17:363–78. doi: 10.1007/s40271-024-00684-9
29. Zhang B. Reflections on the controversy surrounding international breast cancer screening guidelines. Oncol Prog. (2016) 14:109–11. doi: 10.11877/j.issn.1672-1535.2016.14.02.05
30. Oeffinger KC, Fontham ET, Etzioni R, Herzig A, Michaelson JS, Shih YC, et al. Breast cancer screening for women at average risk: 2015 guideline update from the American cancer society. Jama. (2015) 314:1599–614. doi: 10.1001/jama.2015.12783
31. Brem RF, Lenihan MJ, Lieberman J, and Torrente J. Screening breast ultrasound: past, present, and future. AJR. Am J roentgenol. (2015) 204:234–40. doi: 10.2214/AJR.13.12072
32. Mendelson EB, Berg WA, and Merritt CR. Toward a standardized breast ultrasound lexicon, BI-RADS: ultrasound. Semin roentgenol. (2001) 36:217–25. doi: 10.1053/sroe.2001.25125
33. O’Connell AM, Marini TJ, and Kawakyu-O’Connor DT. Cone-beam breast computed tomography: time for a new paradigm in breast imaging. J Clin Med. (2021) 10:5135. doi: 10.3390/jcm10215135
34. AL Mousa DS, Ryan EA, Mello-Thoms C, and Brennan PC. What effect does mammographic breast density have on lesion detection in digital mammography? Clin Radiol. (2014) 69:333–41. doi: 10.1016/j.crad.2013.11.014
35. Gity M, Jafari M, Olfatbakhsh A, Rezaei Kalantari K, Hashemi E, and Sari F. Assessment of ultrasound features and BI-RADS categories of Malignant breast masses in women </=40. Arch Iranian Med. (2021) 24:383–9. doi: 10.34172/aim.2021.55
36. Mordang JJ, Gubern-Merida A, Bria A, Tortorella F, Mann RM, Broeders MJM, et al. The importance of early detection of calcifications associated with breast cancer in screening. Breast Cancer Res Treat. (2018) 167:451–8. doi: 10.1007/s10549-017-4527-7
37. Tomazelli J, Dias MBK, Ribeiro CM, Assis M, Pla MAS, Canella EO, et al. Evaluation of breast cancer screening indicators in the female population using the National Health System, Brazil, 2018-2019: a descriptive study. Epidemiologia e servicos saude: Rev do Sistema Unico Saude do Brasil. (2023) 32:e2022567. doi: 10.1590/s2237-96222023000200009
38. Chen HL, Zhou JQ, Chen Q, and Deng YC. Comparison of the sensitivity of mammography, ultrasound, magnetic resonance imaging and combinations of these imaging modalities for the detection of small (</=2 cm) breast cancer. Medicine. (2021) 100:e26531. doi: 10.1097/MD.0000000000026531
39. Kelly KM, Dean J, Comulada WS, and Lee SJ. Breast cancer detection using automated whole breast ultrasound and mammography in radiographically dense breasts. Eur Radiol. (2010) 20:734–42. doi: 10.1007/s00330-009-1588-y
40. Kolb TM, Lichy J, and Newhouse JH. Comparison of the performance of screening mammography, physical examination, and breast US and evaluation of factors that influence them: an analysis of 27,825 patient evaluations. Radiology. (2002) 225:165–75. doi: 10.1148/radiol.2251011667
41. Corsetti V, Ferrari A, Ghirardi M, Bergonzini R, Bellarosa S, Angelini O, et al. Role of ultrasonography in detecting mammographically occult breast carcinoma in women with dense breasts. La Radiologia Med. (2006) 111:440–8. doi: 10.1007/s11547-006-0040-5
42. Choi WJ, Cha JH, Kim HH, Shin HJ, Kim H, Chae EY, et al. Comparison of automated breast volume scanning and hand- held ultrasound in the detection of breast cancer: an analysis of 5,566 patient evaluations. Asian Pacific J Cancer prevention: APJCP. (2014) 15:9101–5. doi: 10.7314/APJCP.2014.15.21.9101
43. Depretto C, Liguori A, Primolevo A, Di Cosimo S, Cartia F, Ferranti C, et al. Automated breast ultrasound compared to hand-held ultrasound in surveillance after breast-conserving surgery. Tumori. (2021) 107:132–8. doi: 10.1177/0300891620930278
Keywords: BI-RADS, breast cancer screening, primary healthcare setting, automated breast ultrasound, handheld ultrasound, mammography
Citation: Li X, Wang H, Xu H-F, Zhang S-K, Zheng B-J and Li H-L (2025) BI-RADS application for breast cancer screening in primary healthcare settings: assessing protocol adherence and diagnostic validity. Front. Oncol. 15:1599759. doi: 10.3389/fonc.2025.1599759
Received: 25 March 2025; Accepted: 26 September 2025;
Published: 16 October 2025.
Edited by:
Karolina Osowiecka, University of Warmia and Mazury in Olsztyn, PolandReviewed by:
Rajendra A. Badwe, Tata Memorial Hospital, IndiaGiovanna Trapani, University of Palermo, Italy
Copyright © 2025 Li, Wang, Xu, Zhang, Zheng and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hai-Liang Li, bGloYWlsaWFuZ2d5QDE2My5jb20=
†These authors have contributed equally to this work and share first authorship
 Xiang Li
Xiang Li Hong Wang
Hong Wang Hui-Fang Xu
Hui-Fang Xu Shao-Kai Zhang2
Shao-Kai Zhang2 Hai-Liang Li
Hai-Liang Li