- 1Comprehensive Cancer Center, Medical Oncology Department, Fondazione Policlinico, Universitario Agostino Gemelli IRCCS, Rome, Italy
- 2Faculty of Medicine and Surgery, Università Cattolica del Sacro Cuore, Rome, Italy
- 3Departmental Unit of Molecular and Genomic Diagnostics, Fondazione Policlinico Universitario Agostino Gemelli IRCCS, Rome, Italy
- 4Pathology Unit, Fondazione Policlinico Universitario Agostino Gemelli IRCCS, Rome, Italy
- 5UOC Radiologia e Neuroradiologia, Dipartimento di Diagnostica per Immagini, Radioterapia Oncologica ed Ematologia, Fondazione Policlinico Universitario A. Gemelli IRCCS, Roma, Italy
- 6Medical Oncology, Ospedale Isola Tiberina – Gemelli Isola, Rome, Italy
Background: Literature evidence reports that RNF43 (ring finger protein 43) gene mutations could serve as predictive biomarkers of response to certain anti-cancer therapies. To delve deeper into the specific role of RNF43 mutations in lung cancer and their relevance to therapy response, we provide the first report of marked efficacy of the dabrafenib and trametinib therapeutic combination in a patient with microsatellite-stable (MSS) non-small-cell lung cancer (NSCLC) with BRAFV600 and RNF43 mutations.
Case description: An 85-year-old patient was diagnosed with NSCLC with the presence of MSS, BRAFV600E and RNF43 mutations. The patient started the combination treatment with dabrafenib and trametinib, soon reporting an overall clinical benefit. A contrast-enhanced cranio–thorax–abdomen CT scan performed after 1 month of therapy reported a sharp reduction in lung cancer and hilo-mediastinal lymphadenomegaly; the central colliquation of the left adrenal metastasis was also reported. After 9 months of therapy, the cranio-thorax-abdomen CT scan with contrast medium confirmed the reduction of the adenocarcinoma, with residual scarring component; the right adrenal lesion was not visible, and the contralateral lesion was stable. At the last follow-up (February 2024), the global clinical condition of the patient was good; she was autonomous, and oxygen therapy was not necessary.
Conclusions: Our clinical case represents the first report of marked efficacy of the dabrafenib–trametinib combination reported in an 85-year-old patient diagnosed with NSCLC with the presence of MSS, BRAFV600E and RNF43 mutations. This supports the hypothesis on the relevance of RNF43 mutations in predicting the clinical benefit of targeted therapies and in modulating the anti-tumor activity of anti-BRAF therapies, suggesting that RNF43 mutations represent a promising biomarker that warrants further validation for its potential to help prioritize therapy combinations in selected lung cancer patients.
Introduction
One of the most important advances of modern oncology is the shift from an organ-centric concept guiding treatment choice towards deep molecular analysis, driving a personalized approach. In particular, the identification of molecular tumor dependencies that can be targeted with available treatments is on the basis of precision medicine in cancer patients (1).
BRAFV600E mutation occurs in 1–2% of lung adenocarcinomas and acts as an oncogenic driver, activating the MAPK/ERK signaling pathway and promoting cancer cell growth and survival (2, 3). In patients with BRAFV600E-mutant non-small-cell lung cancer (NSCLC), dabrafenib (BRAF inhibitor) and trametinib (MEK inhibitor) combination therapy has shown substantial anti-tumor activity and improved treatment outcomes compared with previous observations in patients who primarily received standard-of-care chemotherapy, thanks to its activity on the different components of the MAPK/ERK pathway (4–6). While dabrafenib and trametinib combination therapy represents a valuable treatment option for NSCLC patients with BRAFV600E mutations, further research is needed to identify additional predictive factors and optimize patient selection in this setting.
RNF43 (ring finger protein 43), an E3 ligase with a RING finger motif for protein–protein interaction, can act as a negative regulator of the WNT signaling pathway by promoting the ubiquitination and degradation of WNT receptors (such as Frizzled) on the cell surface. This action reduces the WNT signaling activity, helping to maintain cellular homeostasis and promoting the tumor suppression function of RNF43 (7). The RNF43 gene is often mutated in various types of cancers, including lung cancer (8). These mutations can lead to increased WNT signaling and the consequent dysregulation of important cellular processes, such as proliferation, apoptosis, and cell cycle control, thereby contributing to cancer development and progression (9).
Literature evidence reports that RNF43 mutations could serve as predictive biomarkers of response to certain anti-cancer therapies. For example, there is evidence that some RNF43 mutations may influence the response to targeted therapies, such as anti-EGFR (epidermal growth factor receptor) or anti-BRAF drugs, in specific subsets of patients (10). In particular, Elez and collaborators demonstrated that inactivating RNF43 mutations are predictive of response to anti-BRAF drugs in microsatellite-stable (MSS) colorectal cancer patients (10).
Dabrafenib plus trametinib is currently approved and represents the standard of care for first-line treatment in NSCLC patients with BRAFV600E mutations. To delve deeper into the specific role of RNF43 mutations in lung cancer and their relevance to therapy response, we provide the first report of marked efficacy of the dabrafenib and trametinib therapeutic combination in a patient with MSS NSCLC with BRAFV600 and RNF43 mutations. The study was conducted within the protocol approved by the Ethics Committee of “Agostino Gemelli” University Hospital Foundation (Prot. N. 0001151/24 –28/06/2024). The patient provided written informed consent. We present the following case in accordance with the CARE reporting checklist.
Case presentation
An 85-year-old female patient presented in December 2022 with a worsening dry cough and dyspnea, with severe limitation of daily activities; the Eastern Cooperative Oncology Group (ECOG) performance status (PS) was 2. The patient began oxygen therapy at 6 l/minute during daylight hours, at night rest, and during physical activity. The patient was a former smoker (moderate cigarette consumption, five packs/year) and denied occupational exposures among the risk factors. Some comorbidities were reported, namely arterial hypertension, anxiety-depressive syndrome, and spondylolisthesis.
The patient underwent a comprehensive staging workup that included contrast-enhanced cranio-thorax-abdomen computed tomography (CT), which documented the primary lung lesion (neoformation in the right lower lobe of the lung), hilo-mediastinal lymphadenopathy, and a left adrenal metastasis (Figure 1, T0).
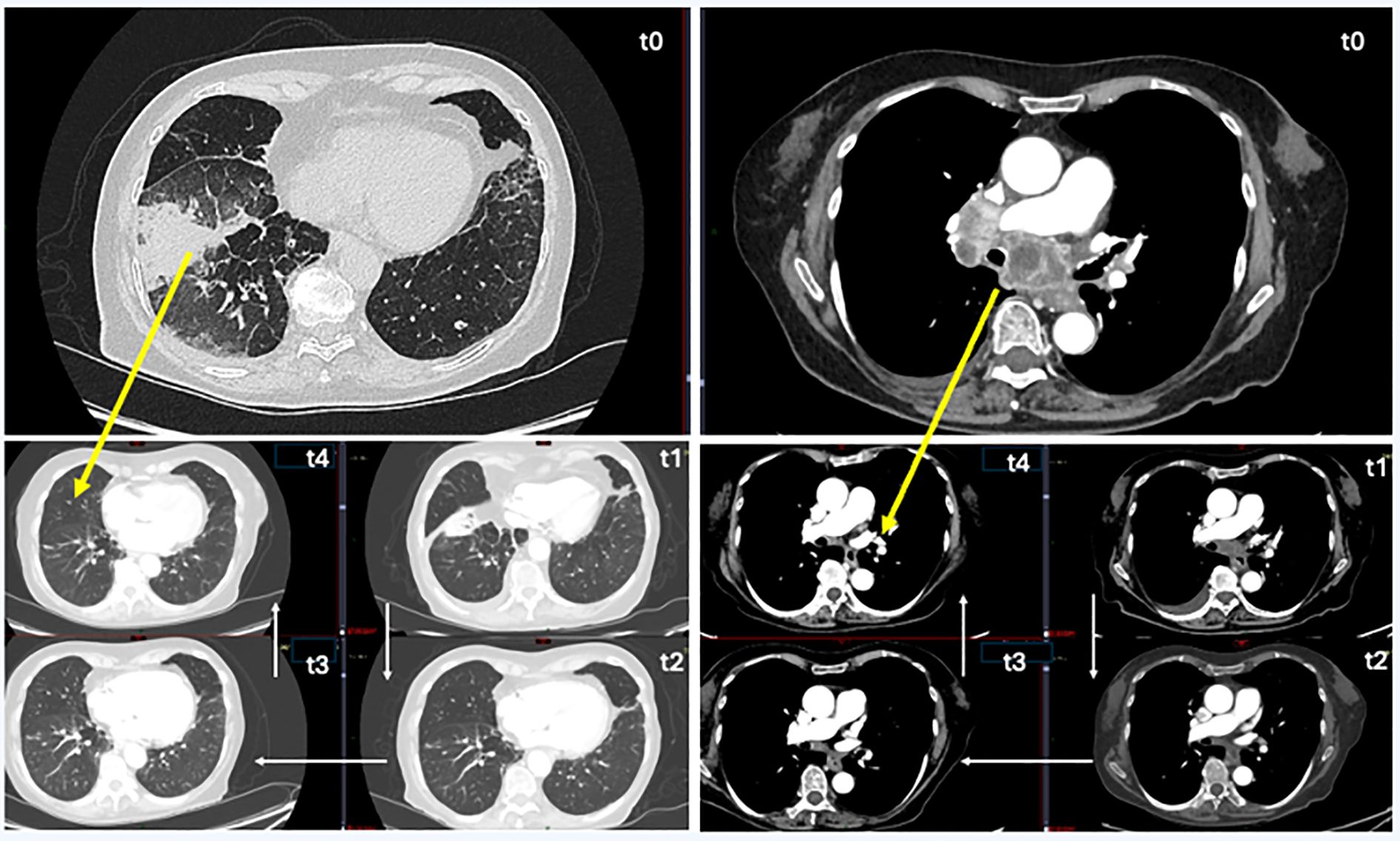
Figure 1. CT chest follow-up images. On the left, images with lung-window CT view, and on the right, images with mediastinal window. T0 (time 0): before therapy, pulmonary neoplasia in the right lung (lower lung lobe) and mediastinal subcarinal lymphatic localizations. T1 (after 3 months): start the reduction of lung parenchymal neoplastic mass and subcarinal lymphatic localizations. T2 (after 5 months): unrecognizable lung parenchymal neoplasm, further reduction of lymphatic localizations and their contact with the airways and pulmonary arteries. T3 (after 9 months): unrecognizable lung parenchymal neoplasm, marked reduction of subcarinal lymphatic localizations. T4 (after 12 months): no radiological evidence of oncological disease, optimal response to treatment.
In January 2023, the patient underwent endobronchial ultrasound-guided transbronchial needle aspiration fibro bronchoscopy on the right paratracheal mediastinal lymph node and on a lung lesion of the anterior segment of the right lower lobe. Histological examination revealed an NSCLC histotype (Figures 2A, B). TNM tumor stage was IV.
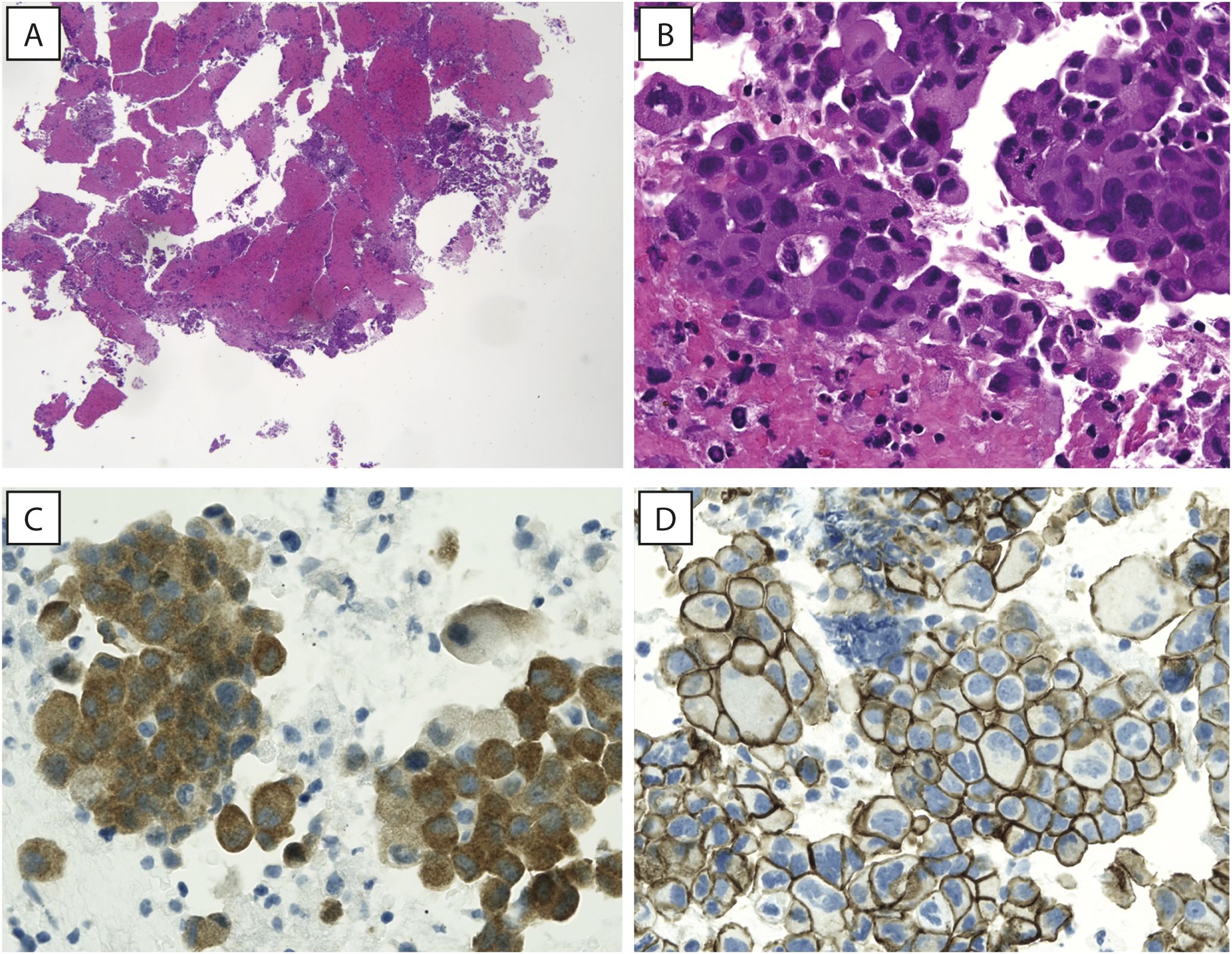
Figure 2. Hematoxylin and eosin images. (A, B) A low magnification view showing tissue fragments embedded in a clot (A, original magnification 40x) and a high magnification image showing papillary structures of adenocarcinoma, with neoplastic cells showing abundant eosinophilic cytoplasm and large, hyperchromatic nuclei (B, original magnification 400x). (C) Neoplastic cells showing strong cytoplasmic staining for BRAF (IHC for BRAFV600E, original magnification 400x). (D) Immunohistochemistry for beta-catenin (Beta-cat4), showing strong and diffuse membrane staining, while no positivity can be appreciated in the nuclei (original magnification 400x).
Comprehensive genomic profiling using next-generation sequencing (NGS; Illumina TruSight™ Oncology 500 High-Throughput assay) allowed the detection of the V600E (missense variant c.1799T>A) mutation in the BRAF gene, supported by immunohistochemical evidence (Figure 2C) with strong clinical significance, a copy number loss in CHEK2 gene, a missense variant of the TP53 gene and a frameshift variant in MAPK3 gene; several variants of unknown significance (VUS) in different genes, including a missense mutation in a microsatellite-stable (MSS) background of RNF43 gene [NM_017763.4(RNF43):c.1112G>T (p.R371L)], were also reported. Immunohistochemistry for β-catenin showed strong and diffuse membrane staining, while no positivity was reported in the nuclei (Figure 2D). PD-L1 expression was assessed by immunohistochemistry using the DAKO assay and revealed a Tumor Proportion Score (TPS) of 95%. The patient was classified as MSS, and a medium TMB (6.3 mut/Mb) was detected.
In February 2023, the patient started the combination treatment with dabrafenib (75 mg tablets; two tablets at 10 a.m. and two tablets at 6 p.m.; 300 mg total daily dosage) and trametinib (2 mg, one tablet per day). From the beginning of the therapy, the patient reported a clinical benefit with gradual resolution of cough episodes and dyspnea, which allowed the suspension of oxygen therapy. A contrast-enhanced cranio-thorax-abdomen CT scan performed after 1 month of therapy reported a sharp reduction in lung cancer and hilo-mediastinal lymphadenomegaly; the central colliquation of the left adrenal metastasis was also reported.
The cranio-thorax-abdomen CT scan of July 2023 (after 5 months of therapy) reported a further sharp reduction in neoformation and hilo-mediastinal lymphadenomegaly and the disappearance of the left adrenal metastasis, while a right adrenal lesion was first identified (Figure 1, T2). In November 2023, after 9 months of therapy, the cranio-thorax-abdomen CT scan with contrast medium confirmed the reduction of the lung lesion, with residual scarring component; the right adrenal lesion was not visible, and the contralateral lesion was stable (Figure 1, T3).
Episodes of fever and hyperpyrexia occurred immediately after starting therapy; treatment with paracetamol was necessary with the periodic and repeated suspension of the dabrafenib and trametinib combination treatment. For this reason, starting from June 2023, the dosage of dabrafenib was gradually reduced as per schedule, up to the daily dosage of 200 mg. With this dosage reduction, the episodes of hyperpyrexia gradually stopped, allowing the patient to continue the treatment. At the last follow-up (February 2024), the clinical condition of the patient was good; the ECOG PS was zero, she was autonomous, and oxygen therapy was not necessary. No radiological evidence of oncological disease was reported; both adrenal lesions (left and right) were no longer detectable on imaging, and the primary lung lesion showed only residual scarring (Figure 1, T4). The clinical timeline of the patient is summarized in Figure 3.
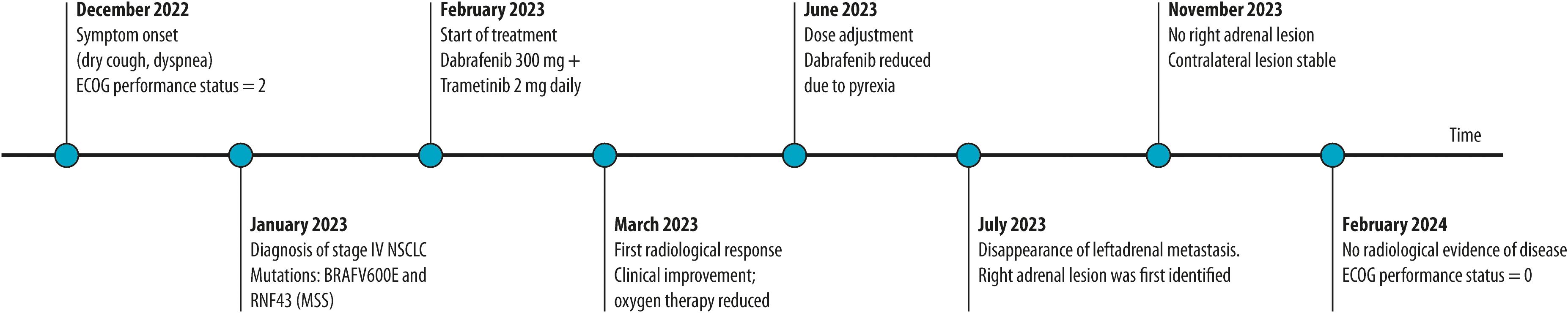
Figure 3. Clinical timeline of the patient. The timeline illustrates symptom onset, diagnosis, initiation of targeted therapy (dabrafenib plus trametinib), radiological and clinical responses, dose adjustment due to pyrexia, and subsequent disease control. Complete radiological remission and performance status improvement were observed by February 2024.
Discussion
Several tools, such as NGS and RNA sequencing, have greatly improved the capacity to detect predictive and prognostic molecular alterations. Therefore, detecting gene mutations, amplifications, and fusions has altered the history of several oncological diseases in both localized and metastatic settings (1).
Our clinical case represents the first report of marked efficacy of the dabrafenib–trametinib combination, reported in an 85-year-old patient diagnosed with NSCLC with the presence of MSS, BRAFV600E and RNF43 mutations. RNF43 mutations include frameshift, nonsense, and missense variants, each with distinct functional implications. In MSI tumors, RNF43 mutations are often frameshift alterations due to defective mismatch repair, leading to loss-of-function. In contrast, our patient had a missense mutation in a microsatellite-stable (MSS) background. This type of mutation may retain partial function or exert context-specific effects. Pyrexia during the combination treatment is the most reported adverse event in clinical trials and was the only adverse event observed in our patient (5). Previous experience with combination therapy in patients with BRAFV600E-mutant melanoma suggests that most grade 3 or 4 adverse events can be managed through dose modification; in our patient, this toxicity was successfully managed through a dose reduction. This case report also attests to the feasibility of the treatment in terms of quality of life, even in older patients.
The analysis of the expression pattern and mutation signature of RNF43 suggests that this gene can be reported as an important prognostic biomarker in pan-cancer (11). Furthermore, RNF43 appears to be a critical modulator in the tumor immune microenvironment, resulting in a promising biomarker for predicting the efficacy of immunotherapeutic regimens (11). Literature data suggest the hypothesis of a possible cross-talk between MAPK and RNF43 pathways in modulating the anti-tumor activity of BRAFV600E-targeted treatments, also related to the state of microsatellites (10). In addition, the results of a recent in vitro study reported that RNF43-mutated pancreatic cancer cells show elevated B-RAF/MEK activity and are highly sensitive to MEK inhibitors (12). Additionally, in colorectal cancer, RNF43 mutations have been shown to predict response to combined anti-BRAF/EGFR therapies (10), further highlighting the therapeutic relevance of this alteration across tumor types. For instance, in colorectal cancer, BRAFV600E mutations often co-occur with RNF43 mutations and infrequently with APC mutations. These characteristics correlate with specific clinical and molecular profiles, such as right-sided tumor localization (10, 13). This highlights the potential relevance of RNF43 as a biomarker in other tumor contexts. Therefore, future research should explore incorporating this biomarker into routine testing, along with BRAF and MSI status, and evaluate their integration with other transcriptomic, microbiome, or microenvironmental indicators to optimize the clinical management of patients with BRAFV600E NSCLC. Of note, we found that β-catenin was expressed only on the cell membrane and not in the cytoplasm and/or nucleus, in agreement with evidence reported by Elez et al. (10); this suggests that also in our patient the response to therapy was independent of β-catenin signaling and involved non-canonical WNT pathways activated by RNF-43 mutations (14–16).
We acknowledge the limitations of our findings due to their derivation from a case report and the consequent need to supplement them with other types of evidence for a more comprehensive understanding of proposed mechanisms. At the same time, our case report proposes for the first time the association between enhanced efficacy of the dabrafenib-trametinib combination and RNF43 mutations in lung cancer, further suggesting the hypothesis on the relevance of RNF43 mutations in predicting the clinical benefit of targeted therapies and on the cross-talk between the MAPK and WNT pathways that may modulate the anti-tumor activity of anti-BRAF therapies (10).
Conclusion
This case report suggests that RNF43 mutations represent a promising biomarker that warrants further validation for its potential in helping prioritize therapy combinations in selected lung cancer patients with BRAFV600E who are most likely to benefit and in identifying those patients for whom alternative treatment approaches are needed.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The study was conducted within the protocol approved by the Ethics Committee of “Agostino Gemelli” University Hospital Foundation (Prot. N. 0001151/24 –28/06/2024). Written informed consent was obtained from the participant/patient(s) for the publication of this case report.
Author contributions
ED: Conceptualization, Data curation, Formal Analysis, Investigation, Writing – original draft, Writing – review & editing. AV: Formal Analysis, Investigation, Writing – original draft, Writing – review & editing. JR: Formal Analysis, Investigation, Writing – original draft, Writing – review & editing. AM: Formal Analysis, Investigation, Writing – original draft, Writing – review & editing. AC: Formal Analysis, Investigation, Writing – original draft, Writing – review & editing. ASt: Formal Analysis, Investigation, Writing – original draft, Writing – review & editing. FM: Formal Analysis, Investigation, Writing – original draft, Writing – review & editing. GH: Formal Analysis, Investigation, Writing – original draft, Writing – review & editing. DO: Formal Analysis, Investigation, Writing – original draft, Writing – review & editing. PT: Formal Analysis, Investigation, Writing – original draft, Writing – review & editing. ASc: Formal Analysis, Investigation, Writing – original draft, Writing – review & editing. SP: Formal Analysis, Investigation, Writing – original draft, Writing – review & editing. FD: Formal Analysis, Investigation, Writing – original draft, Writing – review & editing. MD: Formal Analysis, Investigation, Writing – original draft, Writing – review & editing. EB: Formal Analysis, Investigation, Writing – original draft, Writing – review & editing. GT: Formal Analysis, Investigation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. Editorial assistance was supported by Novartis.
Acknowledgments
Editorial assistance was provided by Simonetta Papa, PhD, Valentina Attanasio, and Aashni Shah (Polistudium SRL, Milan, Italy). This assistance was supported by Novartis.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Gambardella V, Tarazona N, Cejalvo JM, Lombardi P, Huerta M, Roselló S, et al. Personalized medicine: recent progress in cancer therapy. Cancers (Basel). (2020) 12:1009. doi: 10.3390/cancers12041009
2. Kris MG, Johnson BE, Berry LD, Kwiatkowski DJ, Iafrate AJ, Wistuba II, et al. Using multiplexed assays of oncogenic drivers in lung cancers to select targeted drugs. JAMA. (2014) 311:1998–2006. doi: 10.1001/jama.2014.3741
3. Barlesi F, Mazieres J, Merlio JP, Debieuvre D, Mosser J, Lena H, et al. Routine molecular profiling of patients with advanced non-small-cell lung cancer: results of a 1-year nationwide programme of the French Cooperative Thoracic Intergroup (IFCT). Lancet. (2016) 387:1415–26. doi: 10.1016/S0140-6736(16)00004-0
4. Planchard D, Kim TM, Mazieres J, Quoix E, Riely G, Barlesi F, et al. Dabrafenib in patients with BRAF(V600E)-positive advanced non-small-cell lung cancer: a single-arm, multicentre, open-label, phase 2 trial. Lancet Oncol. (2016) 17:642–50. doi: 10.1016/S1470-2045(16)00077-2
5. Planchard D, Smit EF, Groen HJM, Mazieres J, Besse B, Helland Å, et al. Dabrafenib plus trametinib in patients with previously untreated BRAFV600E-mutant metastatic non-small-cell lung cancer: an open-label, phase 2 trial. Lancet Oncol. (2017) 18:1307–16. doi: 10.1016/S1470-2045(17)30679-4
6. Qu J, Shen Q, Li Y, Kalyani FS, Liu L, Zhou J, et al. Clinical characteristics, co-mutations, and treatment outcomes in advanced non-small-cell lung cancer patients with the BRAF-V600E mutation. Front Oncol. (2022) 12:911303. doi: 10.3389/fonc.2022.911303
7. Koo BK, Spit M, Jordens I, Low TY, Stange DE, van de Wetering M, et al. Tumour suppressor RNF43 is a stem-cell E3 ligase that induces endocytosis of Wnt receptors. Nature. (2012) 488:665–9. doi: 10.1038/nature11308
8. Kumar SU, Balasundaram A, Cathryn RH, Varghese RP, R S, R G, et al. Whole-exome sequencing analysis of NSCLC reveals the pathogenic missense variants from cancer-associated genes. Comput Biol Med. (2022) 148:105701. doi: 10.1016/j.compbiomed.2022.105701
9. Li Y, Xiao X, Bossé Y, Gorlova O, Gorlov I, Han Y, et al. Genetic interaction analysis among oncogenesis-related genes revealed novel genes and networks in lung cancer development. Oncotarget. (2019) 10:1760–74. doi: 10.18632/oncotarget.26678
10. Elez E, Ros J, Fernández J, Villacampa G, Moreno-Cárdenas AB, Arenillas C, et al. RNF43 mutations predict response to anti-BRAF/EGFR combinatory therapies in BRAFV600E metastatic colorectal cancer. Nat Med. (2022) 28:2162–70. doi: 10.1038/s41591-022-01976-z
11. Xu Y, Lin Z, Ji Y, Zhang C, Tang X, Li C, et al. Pan-cancer analysis identifies RNF43 as a prognostic, therapeutic and immunological biomarker. Eur J Med Res. (2023) 28:438. doi: 10.1186/s40001-023-01383-1
12. Hsu SH, Tsai YL, Wang YT, Shen CH, Hung YH, Chen LT, et al. RNF43 inactivation enhances the B-RAF/MEK signaling and creates a combinatory therapeutic target in cancer cells. Adv Sci (Weinh). (2024) 11:e2304820. doi: 10.1002/advs.202304820
13. Yaeger R, Chatila WK, Lipsyc MD, Hechtman JF, Cercek A, Sanchez-Vega F, et al. Clinical sequencing defines the genomic landscape of metastatic colorectal cancer. Cancer Cell. (2018) 33:125–136.e3. doi: 10.1016/j.ccell.2017.12.004
14. Tsukiyama T, Zou J, Kim J, Ogamino S, Shino Y, Masuda T, et al. A phospho-switch controls RNF43-mediated degradation of Wnt receptors to suppress tumorigenesis. Nat Commun. (2020) 11:4586. doi: 10.1038/s41467-020-18257-3
15. Radaszkiewicz T, Nosková M, Gömöryová K, Vondálová Blanářová O, Radaszkiewicz KA, Picková M, et al. RNF43 inhibits WNT5A-driven signaling and suppresses melanoma invasion and resistance to the targeted therapy. Elife. (2021) 10:e65759. doi: 10.7554/eLife.65759
Keywords: non-small-cell lung cancer, dabrafenib, trametinib, BRAF V600E, RNF4, case report
Citation: D’Argento E, Vitale A, Russo J, Minucci A, Cancellieri A, Stefani A, Monaca F, Horn G, Occhipinti D, Troisi P, Scala A, Polidori S, D’Argento F, Di Salvatore M, Bria E and Tortora G (2025) Enhanced response to dabrafenib plus trametinib in a patient with BRAFV600E lung cancer harboring an RNF43 variant of unknown significance: a case report. Front. Oncol. 15:1600457. doi: 10.3389/fonc.2025.1600457
Received: 26 March 2025; Accepted: 18 June 2025;
Published: 15 July 2025.
Edited by:
Lucia Anna Muscarella, Home for Relief of Suffering (IRCCS), ItalyReviewed by:
Federico Pio Fabrizio, Kore University of Enna, ItalyMarco Donatello Delcuratolo, IRCCS Casa Sollievo della Sofferenza Hospital, Italy
Copyright © 2025 D’Argento, Vitale, Russo, Minucci, Cancellieri, Stefani, Monaca, Horn, Occhipinti, Troisi, Scala, Polidori, D’Argento, Di Salvatore, Bria and Tortora. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ettore D’Argento, ZXR0b3JlLmRhcmdlbnRvQHBvbGljbGluaWNvZ2VtZWxsaS5pdA==
 Ettore D’Argento
Ettore D’Argento Antonio Vitale1,2
Antonio Vitale1,2 Angelo Minucci
Angelo Minucci Alessio Stefani
Alessio Stefani Francesco D’Argento
Francesco D’Argento Emilio Bria
Emilio Bria