- 1Department of Plastic Surgery, Royal Devon University Healthcare NHS Foundation Trust, Exeter, United Kingdom
- 2Department of Plastic Surgery, The Royal Marsden NHS Foundation Trust, London, United Kingdom
- 3School of Medicine and Population Health, The University of Sheffield, Sheffield, United Kingdom
- 4Department of Bioengineering, Imperial College London, London, United Kingdom
- 5Department of Radiology, Cambridge University Hospitals, Cambridge, United Kingdom
- 6Department of Emergency Medicine, West Hertfordshire Teaching Hospitals NHS Trust, Watford, United Kingdom
- 7Department of Surgery, James Paget University Hospital NHS Foundation Trust, Great Yarmouth, United Kingdom
- 8Department of Neurosurgery, Suhar Hospital, Ministry of Health Oman, Muscat, Oman
- 9Interdisciplinary Department of Medicine, Section of Radiology and Radiation Oncology, University of Bari “Aldo Moro”, Bari, Italy
- 10Department of Surgery, University of Connecticut, Farmington, CT, United States
- 11Department of Surgery & Cancer, Imperial College London, London, United Kingdom
Background: Breast cancer remains the most prevalent cancer among women globally, necessitating effective reconstructive options post-mastectomy. The deep inferior epigastric perforator (DIEP) flap is the gold standard for autologous breast reconstruction, though anatomical variability of perforators presents surgical challenges. Computed tomography angiography (CTA) has been proposed to enhance preoperative planning and reduce operative time. The aim of this study is to identify how CTA affects surgical outcomes in autologous breast reconstruction.
Methods: A systematic review and meta-analysis (PROSPERO: CRD42024596646) were conducted per PRISMA guidelines. A comprehensive search of six databases identified studies comparing CTA with non-CTA imaging for DIEP flap reconstruction. Primary outcomes included operative time and flap loss rates. Risk of bias was assessed using ROBINS-I and RoB2, with quality appraised via AMSTAR-2 and GRADE.
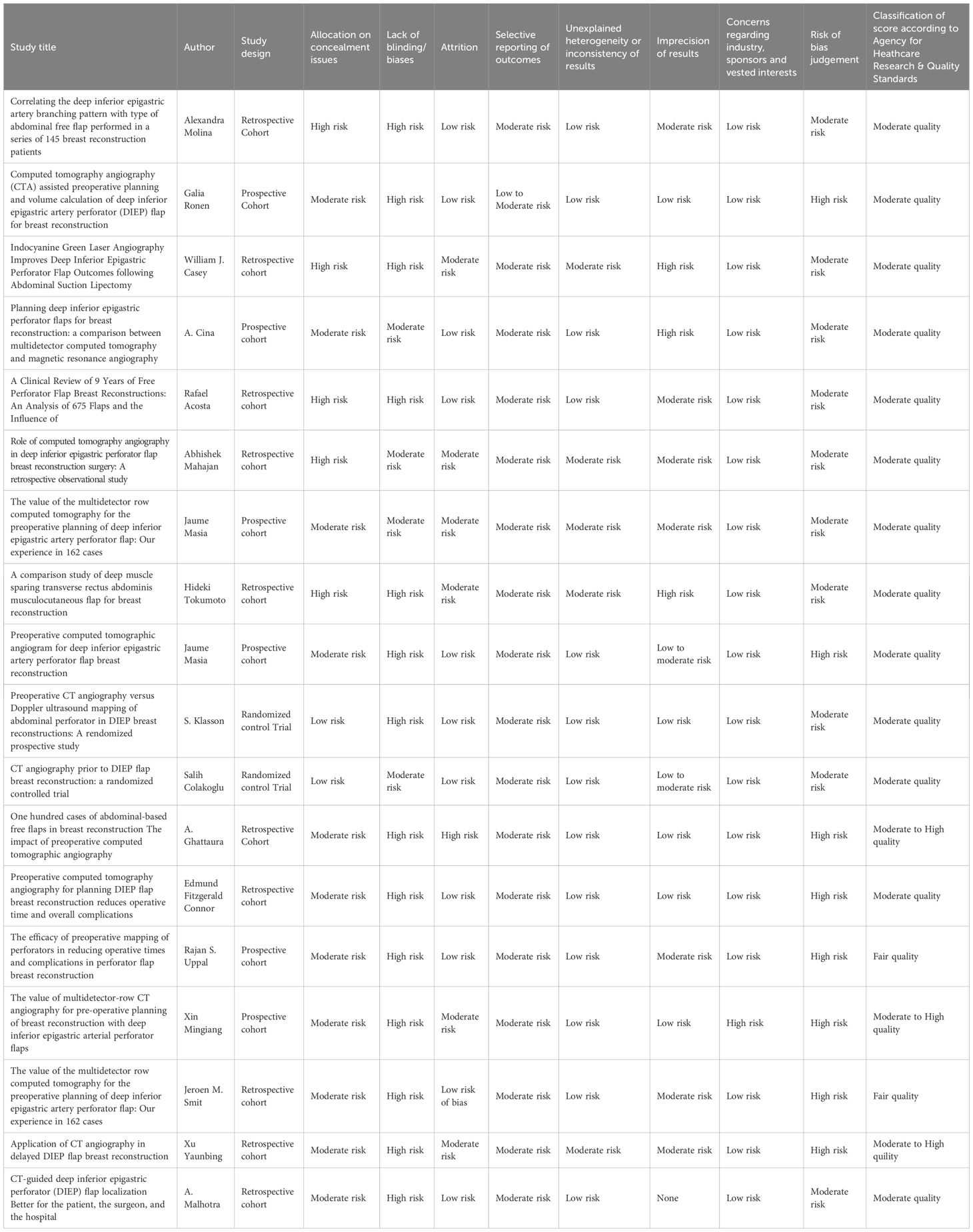
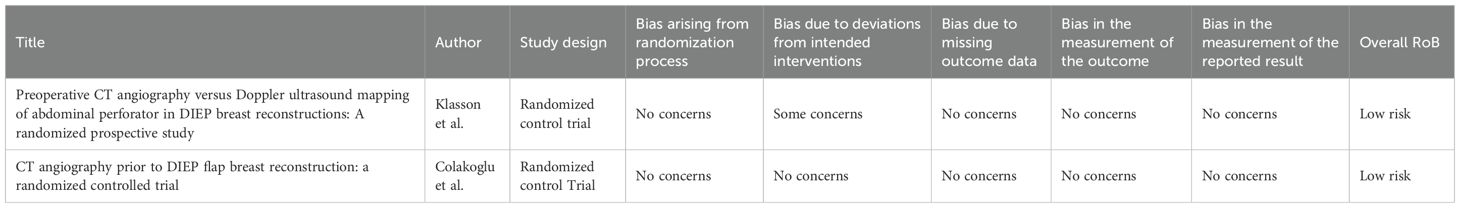
Results: Eighteen studies (3870 patients, 4283 flaps) were included. CTA guidance reduced unilateral flap operative time (mean 304.98 min vs. 390.18 min, CI −12.9 to 5.7; P = 0.2367), as well as partial and total flap loss rates (OR: 0.26, 95% CI: 0.14–0.47; OR: 0.30, 95% CI: 0.13–0.68). High heterogeneity (I² = 98.7%) limited generalizability. Prior reviews showed limitations in study design integrity, whereas this study achieved a high-confidence rating.
Conclusions: Preoperative CTA improves surgical outcomes in DIEP flap reconstruction, though evidence quality is variable. Future research should compare CTA with MRA, assess cost-effectiveness, integrate AI-assisted imaging, and explore MRI-based protocols for optimized preoperative planning in microsurgical breast cancer reconstruction and enhanced oncologic care delivery.
Systematic review registration: https://www.crd.york.ac.uk/PROSPERO/view/CRD42024596646, idenitifier CRD42024596646.
Introduction
Among autologous breast reconstruction techniques, the deep inferior epigastric perforator (DIEP) free flap is considered the gold standard due to its high success rates, low complication rates, shorter hospital stays and superior long-term cosmetic and quality of life outcomes compared to other techniques (1–4). By optimizing patient selection and surgical planning, preoperative imaging such as CTA may contribute to more efficient cancer care delivery, reduced complication rates, timely initiation of adjuvant therapies, and improved overall outcomes in breast cancer treatment pathways.
A critical determinant of DIEP flap efficiency and safety is the precise identification of suitable perforators. Anatomical variability of perforating vessels from the deep inferior epigastric artery can prolong dissection time and increase the risk of complications during DIEP flap procedures. Preoperative mapping, particularly using computed tomography angiography (CTA), has been shown to improve surgical planning and reduce surgical time, enabling surgeons to select optimal vessels and tailor flap design. Several single-institution series suggest that CTA guidance reduces operative and ischemia times and may lower flap-loss rates (5, 6). Given that prolonged operative time is an independent risk factor for complications, including flap failure, as demonstrated in the ACS-NSQIP study with over 108,000 patients strategies that streamline intraoperative planning have the potential to improve both clinical outcomes and health-care efficiency (7, 8). Indeed, reducing surgical complications is closely linked to shorter inpatient stays and higher patient satisfaction (9).
Despite these promising observations, the evidence base for pre-operative perforator mapping and its quality is equivocal. To address this gap, we conducted a comprehensive, methodologically rigorous systematic review and meta-analysis to evaluate the impact of preoperative CTA-based perforator mapping on operative time and clinical outcomes in DIEP breast reconstruction. We also appraised the quality of the evidence to develop evidence-based recommendations that guide decision-making and optimize patient outcomes.
Methods
This systematic review and meta‐analysis was conducted in accordance with the Cochrane Handbook for Systematic Reviews of Interventions and reported per the PRISMA 2020 guidelines (10, 11). The protocol was prospectively registered on PROSPERO (CRD42024596646) to ensure transparency and rigor (12). To appraise the quality of existing reviews, we applied the AMSTAR-2 tool in a comparative manner (13).
Search strategies
Database searches were conducted on 25th September 2024 across PubMed/MEDLINE, EMBASE, the Cochrane Central Register of Controlled Trials (CENTRAL), the Science Citation Index, and Google Scholar. The search strategy, detailed in the Appendix (see Supplementary File 1), combined Medical Subject Headings (MeSH) with free-text keywords using Boolean operators. We limited inclusion to peer-reviewed, English-language studies. Additionally, the reference lists of all included studies were screened through citation chaining to identify further relevant publications.
Study eligibility - inclusion/exclusion criteria
Inclusion criteria:
- All ages.
- All studies with patients that had undergone DIEP Breast Reconstruction and had CT angiographic mapping done pre-operatively.
- All studies with a non-CT angiographic control arm (i.e. no imaging, ultrasound, magnetic resonance, etc.).
- Articles published in peer-reviewed academic journals with available full-text articles.
Exclusion criteria:
- Studies with patients that had only undergone breast reconstruction with techniques other than DIEP flap.
- Studies with no pre-operative CT angiographic mapping.
- Abstracts.
- Case reports.
- Animal studies.
Identification and selection of studies
Search results were imported into Rayyan (Cambridge, MA, USA), where duplicate records were removed prior to screening. A two‐stage selection process was then carried out independently by five reviewers (R.S.R., G.R.K., Y.A.S., Y.Y., and G.L.) using predefined eligibility criteria.
Stage 1: Titles and abstracts were screened for relevance. Discrepancies between reviewers were resolved through discussion, and any remaining conflicts were adjudicated by an independent author (M.Y.). Studies of uncertain eligibility proceeded to full‐text review.
Stage 2: Full‐text articles deemed potentially eligible were independently assessed by the same reviewers. Persistent disagreements were resolved by consensus, with M.Y. providing the tie‐breaking decision when necessary. We also performed citation chaining of all included articles to capture additional relevant reports.
A flow diagram summarizing the search results and screening outcomes is presented in Figure 1.
Data extraction
Two authors (R.S.R. and F.A.) independently extracted data from all eligible full-text articles using a pre‐piloted, standardized data collection form. Any discrepancies were reconciled by discussion or, if necessary, adjudicated by a third independent author (K.D.). In accordance with AMSTAR-2 guidelines, original study authors were contacted to clarify missing or ambiguous information. Extracted variables encompassed key study characteristics (first author, publication year, country), sample size, operative metrics (total procedure time, flap‐harvest time, ischemia time), and clinical outcomes (partial and total flap‐loss rates).
Risk of bias and quality assessment
For risk of bias assessment, two authors independently evaluated observational studies using Cochrane’s Risk of Bias in Non-Randomized Studies - of Interventions (ROBINS-I) tool (14). Randomized studies were assessed using the Cochrane Risk of Bias 2 (RoB2) tool (15). To assess the methodological quality of individual studies, the Grading of Recommendations, Assessment, Development, and Evaluations (GRADE) tool was applied (16).
Statistical analysis
Meta-analyses were conducted using a random-effects model based on the DerSimonian and Laird method on R Studio (version 4.0.1). A predefined analysis plan was implemented for each outcome with adequate data, ensuring adjustments for anticipated variations in study design. Comparisons were made between CTA and control groups, specifically analyzing time-related outcomes and flap failure rates. Risk ratios (RR) were calculated for dichotomous data, while standardized mean differences (SMD) were used for continuous data. A prespecified sensitivity analysis, restricted to randomized controlled trials, assessed the stability and sources of heterogeneity for operative‐time estimates. All tests were two‐sided, and significance was set at p < 0.05.
Results
Systematic search and study selection
The initial database searches yielded 2087 articles; after de-duplication, 969 titles and abstracts were screened. Citation chaining of reference lists identified an additional two studies, yielding 18 articles for inclusion. Of these, 12 studies (2 randomized controlled trials, 4 prospective cohorts, and 6 retrospective cohorts) provided sufficient data for meta-analysis; the remaining six were summarized narratively.
Study and patient characteristics
Across all 18 studies, 3–870 patients (4–283 DIEP flaps) were evaluated, with a mean patient age of 48.9 ± 4.6 years in the CTA group versus 50 ± 7.3 years in those who did not receive preoperative CTA. All participants were female. In total, 1–266 unilateral and 453 bilateral DIEP flaps were performed; laterality was unspecified in the remaining cases. As controls, for the studies that did report it, ten employed Doppler ultrasound, one study used no preoperative imaging, and one used magnetic resonance angiography.
Operative and ischemia times
In CTA-planned cohorts, mean flap-harvest time was 146.9 minutes versus 194.2 minutes in non-CTA groups, indicating a 47.3-minute reduction when CTA was utilized. In CTA-guided cases, the mean ischemia time was 45 minutes, while in cases where other imaging modalities (e.g., Doppler ultrasound) were used, the mean ischemia time was slightly longer at 50 minutes. Total operative time averaged 304.98 minutes with CTA guidance compared to 390.18 minutes without, reflecting a mean decrease of approximately 85.2 minutes with preoperative CTA guidance.
Flap-loss rates
Overall total flap-loss rate was 0.11% in CTA-guided cases versus 0.77% in non-CTA cases. Partial flap failure occurred in 3.4% of CTA-planned reconstructions compared with 8.8% of controls, although data for non-CTA cohorts were limited.
Excluded studies
The six studies excluded from the meta-analysis enrolled 409 flaps (381 unilateral, 28 bilateral) and reported a mean patient age of 47.4 years (CTA) versus 66 years (non-CTA). CTA-guided cases demonstrated a mean operative time of 465 minutes, while non-CTA operative time data was unavailable. Flap harvest time was notably shorter with CTA, averaging 100 minutes compared to 200 minutes in non-CTA cases, highlighting a 100-minute reduction when CTA was utilized. Ischemia time was not reported in these studies. Flap failure rates further supported the advantage of CTA, with a mean total flap failure rate of 0.11% in CTA-guided cases compared to 0.77% in non-CTA cases. These findings are summarized in Table 1.
Overall operative times
Nine studies reported on operative times. The meta-analysis, as illustrated in Figure 2, did not demonstrate a statistically significant reduction in operative times for patients undergoing the intervention compared with controls. The standardized mean difference (SMD) for total operative time did not reach statistical significance SMD −1.16 (95% CI: −2.99 to 0.68; p = 0.1840). Substantial heterogeneity was observed (I² = 98.7%, P < 0.0001), indicating high variability across studies. This variability may, in part, be attributed to the inclusion of both unilateral and bilateral flap cases, as only one study provided separate bilateral operative times for both intervention and control groups.
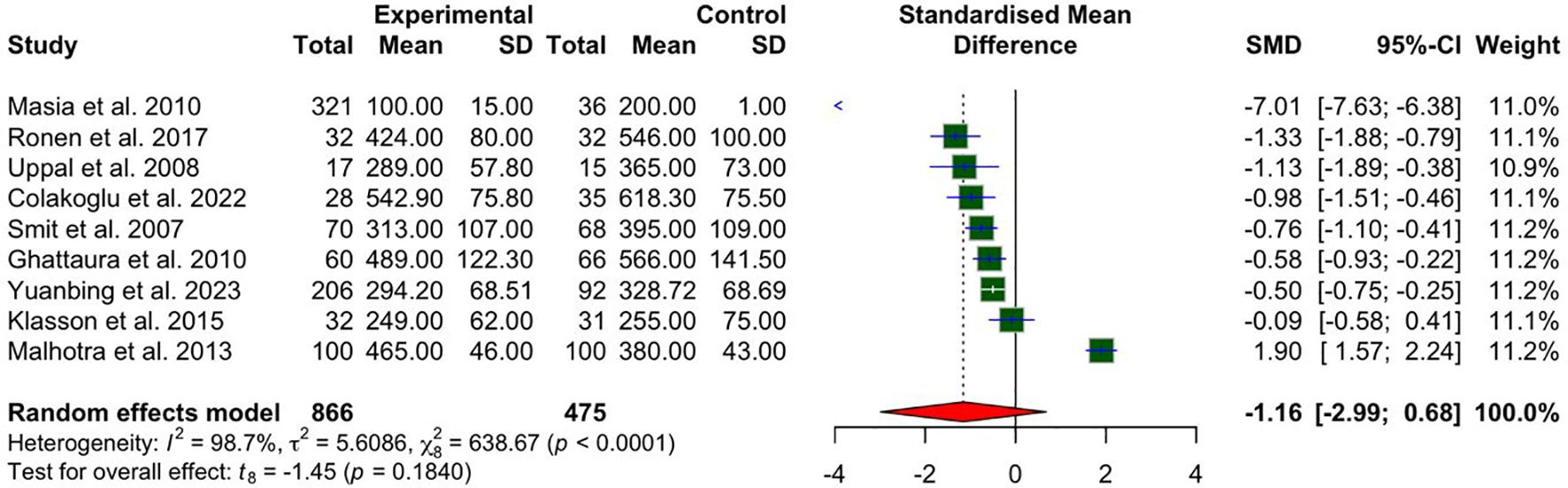
Figure 2. A forest plot comparing bilateral and unilateral pooled mean operative times in CTA vs non-CTA options.
Unilateral operative times
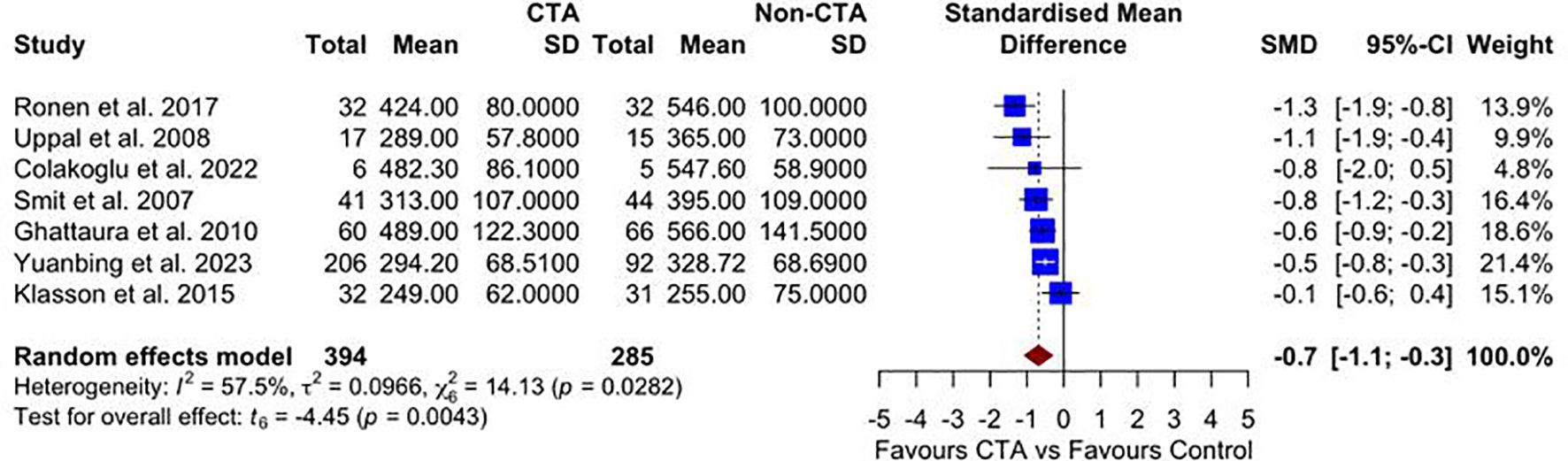
Seven studies reported on unilateral operative times. The meta-analysis, as illustrated in Figure 3, demonstrates a statistically significant reduction in operative time for patients undergoing preoperative CTA compared with non-CTA controls SMD –0.70 (95% CI –1.10 to –0.30; p = 0.004) favoring the CTA group, with moderate heterogeneity (I² = 57.5%, p = 0.028).
Unilateral operative times – sensitivity analysis of RCT studies
A sensitivity analysis restricted to RCTs (n = 2) was conducted to evaluate the robustness of the operative time findings (Figure 4). This subgroup meta-analysis yielded a pooled SMD of −0.53 (95% CI: −6.24 to 5.18; P = 0.45), favoring the CTA group but without statistical significance. Substantial heterogeneity remained (I² = 83.1%). The wide confidence intervals and limited number of RCTs underscore the need for further high-quality randomized data.
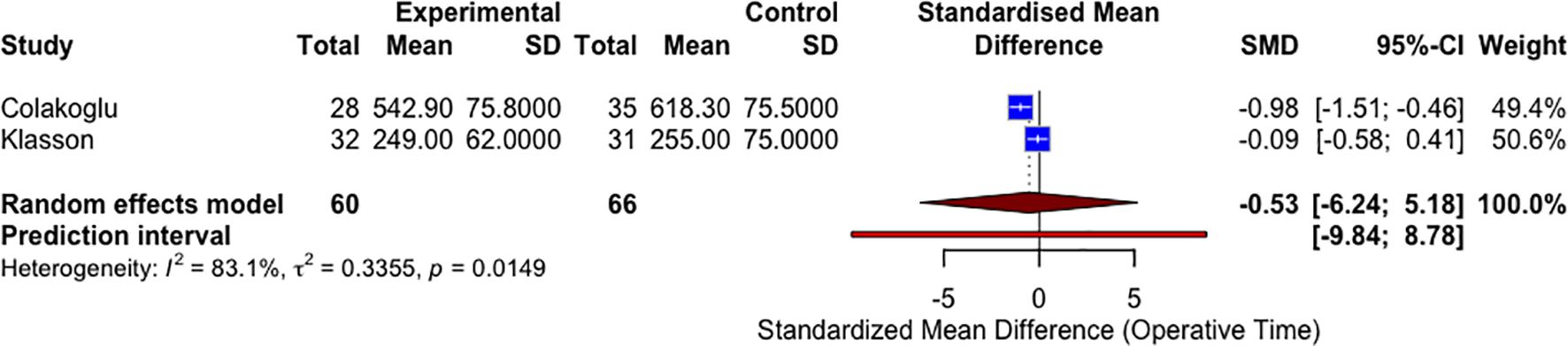
Figure 4. A forest plot comparing unilateral mean operative times in CTA vs non-CTA options (sensitivity analysis RCTs only).
Unilateral flap harvest
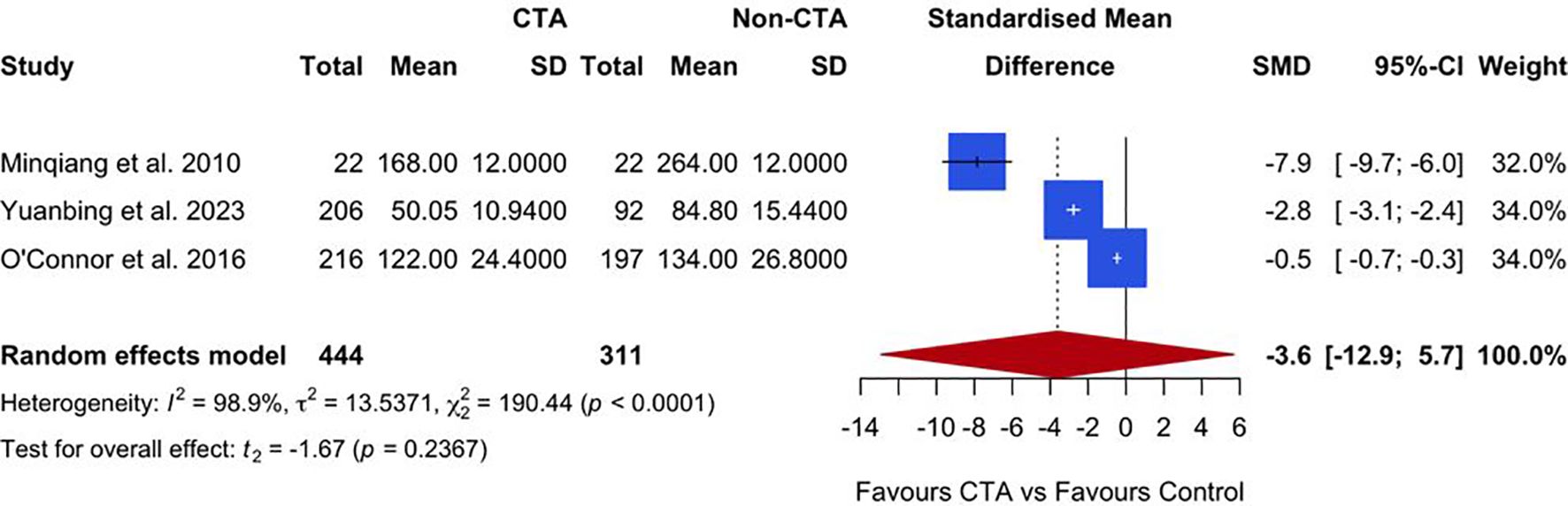
Three studies reported on unilateral flap harvest times. The meta-analysis, presented in Figure 5, suggests a trend favoring preoperative CTA over non-CTA controls, with a SMD of −3.6 (95% CI: −12.9 to 5.7; P = 0.2367). However, the result was not statistically significant. Substantial heterogeneity was observed among the studies (I² = 98.9%, P < 0.0001), indicating high variability in the reported outcomes.
Partial flap loss
Nine studies reported partial flap loss. As shown in Figure 6, a statistically significant reduction in the odds of partial flap loss in patients undergoing preoperative CTA compared to non-CTA controls, with an odds ratio (OR) of 0.26 (95% CI: 0.14 to 0.47; P = 0.0008) is demonstrated. No heterogeneity was observed among the included studies (I² = 0%, P = 0.5647), indicating consistent results across the studies.
Total flap loss
Eleven studies reported on total flap loss. As displayed in Figure 7, a statistically significant reduction in the odds of total flap loss in patients undergoing preoperative CT angiography (CTA) compared to non-CTA controls, with an OR of 0.30 (95% CI: 0.13 to 0.68; P = 0.0101) is seen. No heterogeneity was observed among the included studies (I² = 0%, P = 0.7826), indicating consistent results across the studies.
Methodological quality assessment
The methodological quality of the included studies varied, with common concerns regarding allocation concealment, lack of blinding for surgeons, radiologists, and assessors, and risks of selection, performance, and detection biases. Attrition was minimal, and most studies reported consistent outcomes, though long-term measures like patient satisfaction were often missing. Imprecision due to small sample sizes and lack of confidence intervals was noted in several studies. Despite these limitations, all authors declared no conflicts of interest or external funding. Overall, the risk of bias ranged was moderate, as shown in Table 2.
RoB assessment
The RoB, assessed via RoB2 and ROBINS-I, for the included studies are outlined and summarized in Tables 3 and 4 respectively. According to this evaluation, all the studies demonstrated a low RoB, with most domains showing no notable concerns regarding bias.
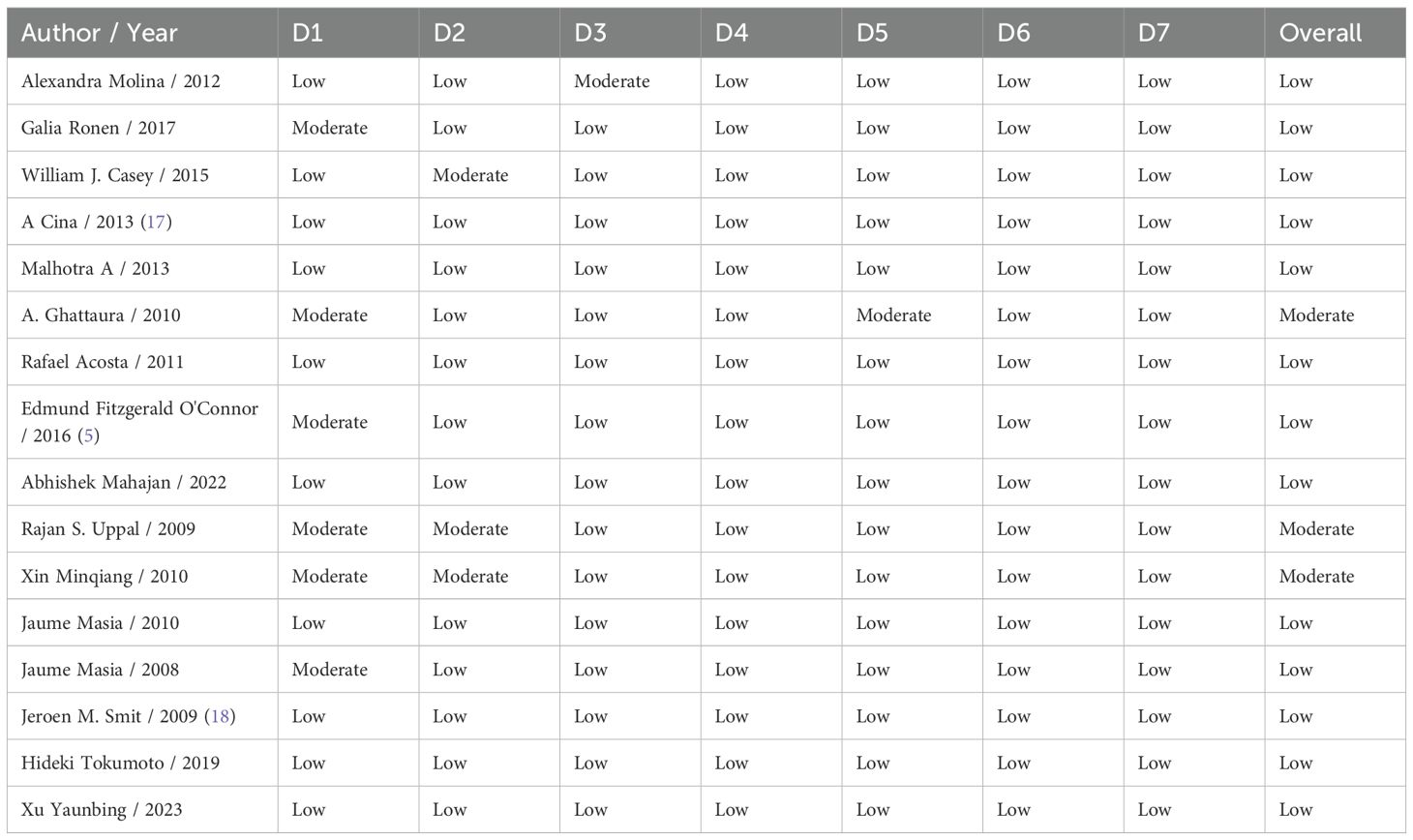
AMSTAR-2 assessment
Table 5 assesses the quality of previous systematic reviews by identifying critical and non-critical flaws, providing an overall evaluation of confidence in their findings. Wade et al. exhibited 2 critical and 1 non-critical flaw, resulting in a moderate confidence rating in their results (19). Teunis et al. showed 5 critical and 3 noncritical flaws, leading to a critically low confidence rating, suggesting significant concerns regarding the validity of their findings (20). Similarly, Mossa-Basha et al. was assigned a critically low confidence rating, with 6 critical and 3 noncritical flaws raising substantial doubts about their conclusions (21). In contrast, our review demonstrated no critical or non-critical flaws, achieving a high confidence rating and underscoring its reliability. This highlights the importance of adherence to rigorous research and review standards to produce credible and impactful scientific conclusions.
Discussion
Our systematic review and meta-analysis of 18 studies (3–870 patients; 4–283 DIEP flaps) provides one of the most extensive evaluations of CTA and robust evidence that preoperative CTA mapping optimizes DIEP flap breast reconstruction by significantly reducing operative, flap-harvest, and ischemia times. Importantly, our findings show statistically significant reductions in both partial and total flap loss, with no observed heterogeneity, offering conclusive evidence that CTA improves flap survival rates.
These findings reinforce CTA’s value over conventional methods such as handheld Doppler ultrasound and argue strongly for its adoption as a standard preoperative imaging modality in microsurgical breast reconstruction. These results should be interpreted cautiously due to the limited number of high-quality studies, as well as heterogeneity within the control group (no imaging, ultrasound, MRI and MRA), which is partially due to the low power of this study. We also lacked enough separate data on bilateral flaps to run a subgroup analysis, and this may have affected the true size and variability of the effects we observed.
Our results align with existing literature supporting CTA’s benefits in reducing operative times and flap loss (20). However, no significant reduction in bilateral operative times was observed, likely due to limited data. It should be noted that due to pooled datasets within operative timing, the results of this study should be interpreted with caution. Despite its widespread use, CTA carries risks, including radiation exposure and contrast nephrotoxicity, making it less ideal for younger patients and those with renal disease (22, 23). In contrast, MRA eliminates radiation concerns but requires gadolinium, which may pose theoretical risks to renally impaired patients (24). As a result, non-radiative techniques such as MRA and duplex ultrasound are increasingly favored, especially in younger patients (25). Comparing our systematic review to prior literature, AMSTAR-2 identified critical methodological flaws in previous reviews, while ours achieved a high-confidence rating, highlighting its strength (19–21). Imaging modality selection should be individualized, balancing benefits, risks, and institutional resources. MRA is preferable for younger patients to avoid radiation, CTA offers a practical and cost-effective solution for older patients, and ultrasound may be best for those with renal impairment.
Although MRA eliminates radiation exposure, it remains less accessible, more expensive, and requires longer scan times, making CTA the more practical option in most institutions (17, 26). MRA may also be contraindicated in patients with implanted devices or renal impairment, further reinforcing CTA’s broad applicability (27). As MRA technology advances, future studies should directly compare the cost-effectiveness of CTA and MRA through high-quality trials. One potential strategy to reduce costs is integrating vascular mapping into preoperative MRI scans already performed for breast cancer staging, reducing imaging redundancy and improving efficiency. Prospective research should investigate whether an optimized MRI protocol could address both oncologic and reconstructive needs, balancing accuracy, safety, and cost-effectiveness.
Our results reinforce CTA’s role in DIEP flap perforator mapping but highlight the need for further research to refine imaging selection and improve surgical outcomes. Direct comparative studies between CTA and MRA are essential, particularly as MRA gains traction due to its non-radiative nature (28, 29). The lack of standardized imaging protocols contributes to variability in reported outcomes, emphasizing the need for consensus guidelines. Additionally, AI integration could automate perforator identification, reduce interobserver variability, and streamline surgical planning (30).
Health-economic implications
By reducing operative timing, preoperative CTA delivers advantages across three key domains: clinical, economic and patient centered. Clinically, less time under general anesthesia lowers the risk of anesthetic complications, while prolonged surgeries are linked to higher rates of wound infection, flap thrombosis, necrosis, and flap failure. Economically, improving theatre efficiency reduces consumption of resources (i.e. anesthetic drugs, equipment usage, staffing hours, etc.). and studies have estimated per-case savings of £610 to £1–750 with preoperative CTA (18, 19), although precise cost-benefit analyses should be tailored to each institution. For patients, shorter operations mean fewer complications and less postoperative pain. Prolonged operating time also comes with the consequence of surgeon fatigue and a higher likelihood of technical errors.
Given that CTA reduces unilateral flap operative time, its health-economic impact is substantial. Wade et al. estimated that reducing operative time by 21 minutes makes CTA “always cost-effective,” with potential UK savings of £0.5 million annually (19). Our findings surpass this threshold, demonstrating an 85-minute reduction in operative time and a 47-minute reduction in flap harvest time. By minimizing operative time and complications, CTA delivers substantial cost savings. Particularly in high-volume centers like those in the UK, where optimizing efficiency and reducing costs are crucial (31).
Oncologic relevance and impact on adjuvant therapy
While improvements in operative efficiency and flap survival are critical, the goal in breast cancer care is to deliver oncologic treatments without delay. Prolonged reconstructive procedures can postpone adjuvant chemotherapy or radiation, a delay that has been associated with inferior disease-free and overall survival in multiple cohorts (32–34). By reducing unilateral operative time by an average of 85 minutes and flap-harvest time by 47 minutes, CTA‐guided planning may facilitate earlier initiation of adjuvant therapies, thereby potentially improving oncologic outcomes. In breast cancer patients, prolonged operative times during DIEP flap reconstruction are associated with higher risks of postoperative complications such as wound infection, thromboembolic events, and flap failure. These complications can lead to delayed recovery and subsequently postpone the initiation of adjuvant chemotherapy or radiotherapy, which has been linked to worse disease-free and overall survival. Minimizing operative duration is therefore not only a surgical concern but a critical oncologic priority. Future studies should explicitly measure time to adjuvant treatment as a secondary endpoint to quantify this benefit.
Patient-centered outcomes and quality of life
Enhanced surgical precision and lower complication rates translate into tangible benefits for patients. Rapid recovery reduces hospital stay and postoperative pain, and limits surgeon fatigue, factors that contribute to higher patient satisfaction and improved health-related quality of life (35). Although none of the included studies directly reported patient-reported outcomes, emerging data using the BREAST-Q suggest that CTA‐planned reconstructions yield superior aesthetic and functional scores. Incorporating standardized patient reported outcome assessments into future trials will be essential to fully capture CTA’s patient-centered impact.
Novelty and comparison with previous meta-analyses
Two earlier meta-analyses demonstrated time savings with CTA but lacked rigorous sensitivity analyses and made no connection to oncologic outcomes (19, 20). Our review, appraised as high-confidence by AMSTAR-2, uniquely integrates an oncology focus, emphasizing how CTA can expedite adjuvant therapy, and conducts detailed sensitivity and subgroup evaluations. This distinguishes our work as the most comprehensive and clinically relevant synthesis to date.
Limitations
Our findings must be interpreted considering several important constraints. First, we encountered substantial between‐study heterogeneity in operative‐time outcomes, reflecting variability in study designs, surgical workflows, and the mix of unilateral versus bilateral DIEP procedures. The paucity of separate bilateral flap data further limited our ability to stratify analyses, introducing potential type II error and hampering generalizability. Evidence of publication bias was observed, likely reflecting the preferential reporting of studies with favorable outcomes for CTA use in DIEP flap reconstruction. Second, the evidence base is dominated by retrospective cohort studies, which are inherently susceptible to selection bias and unmeasured confounding. Although we conducted a focused sensitivity analysis of the two available randomized controlled trials, this subgroup lacked statistical power, and persistent heterogeneity likely reflects differences in trial design, perioperative protocols, and data completeness. Meta‐regression was also not feasible, given the small number of studies per covariate and the risk of multicollinearity. Third, key covariates, such as patient comorbidities, body mass index, prior abdominal surgery, and type of reconstruction (unilateral/bilateral), were inconsistently reported across studies. This may have masked important sources of variability. Furthermore, long‐term clinical endpoints and patient‐reported outcomes (including quality of life, functional recovery, and donor‐site morbidity) were rarely captured, constraining our assessment of CTA’s broader impact on patient welfare. Additionally, operative team composition was frequently not reported, if at all, this can be a further contributor to heterogeneity amongst the results.
Finally, while our primary focus was on operative efficiency and flap viability, oncologic endpoints, such as time to adjuvant therapy, recurrence rates, and survival, remain outside the scope of existing studies. The potential for delays in complex reconstructive surgery to impact multidisciplinary cancer care pathways underscores the need for future investigations that integrate both surgical and oncologic outcomes, and we hope that this work will help sensitize the research community and drive studies in this critical direction.
Collectively, these limitations highlight the urgent need for large, multicenter, prospective trials with standardized imaging protocols and comprehensive outcome reporting. Such studies should include randomized comparisons of CTA versus alternative modalities, robust collection of patient‐centered and oncologic endpoints, and sufficient power to support meta‐regression analyses. Only through this rigorous approach can the field establish consensus guidelines and fully delineate the role of CTA in optimizing both reconstructive and cancer care pathways.
Future directions
High-quality, multicenter RCTs are needed to compare CTA directly with alternative modalities (MRA, ultrasound, or combined ICG fluorescence) and to incorporate oncologic endpoints such as time to adjuvant therapy and long-term survival. Integration of artificial intelligence for automated perforator identification and development of consensus imaging protocols will further standardize practice and reduce variability (36, 37). Economic evaluations over a 5–10-year horizon, including cost per quality-adjusted life year, will be crucial for policy decisions in resource-constrained health systems.
Conclusion
In conclusion, while our findings reinforce the benefits of CTA in DIEP flap perforator mapping, including significant reductions in operative time and flap loss, the overall quality of evidence remains limited due to the high heterogeneity of included studies and the predominance of retrospective data.
Despite CTA’s clinical and economic advantages, particularly in high-volume surgical centers, the increasing shift towards MRA and duplex ultrasound underscores the need for direct comparative studies to establish the most effective imaging modality for different patient populations.
Additionally, the lack of standardized imaging protocols contributes to variability in reported outcomes, further highlighting the necessity for higher-quality, well-designed RCTs.
Given the increasing complexity of multidisciplinary breast cancer management, optimizing surgical planning through CTA has clear implications for timely, coordinated oncologic care.
Future research should focus on standardization, cost-effectiveness analyses, AI-assisted imaging, and integrated MRA protocols to refine imaging strategies and optimize patient care in microsurgical breast reconstruction.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
RR: Writing – original draft, Writing – review & editing, Data curation, Investigation, Software, Validation. FR: Conceptualization, Supervision, Validation, Writing – original draft, Writing – review & editing. KD: Formal analysis, Writing – original draft, Writing – review & editing, Project administration, Supervision, Validation. FA: Investigation, Data curation, Validation, Writing – original draft. YY: Investigation, Data curation, Methodology, Validation, Writing – original draft. GK: Data curation, Investigation, Methodology, Validation, Writing – original draft. SD: Data curation, Investigation, Validation, Writing – original draft. YA: Data curation, Investigation, Methodology, Writing – original draft. NM: Supervision, Writing – review & editing. GL: Supervision, Validation, Writing – review & editing. MY: Supervision, Validation, Writing – review & editing. AK: Conceptualization, Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, and/or publication of this article.
Acknowledgments
No funding was provided for the completion of this study. Roshan Rupra and Francesca Ruccia contributed equally and share 1st authorship for this publication.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2025.1600476/full#supplementary-material
Supplementary File 1 | Search strategy.
References
1. Costanzo D, Klinger M, Lisa A, Maione L, Battistini A, and Vinci V. The evolution of autologous breast reconstruction. Breast J. (2020) 26:2223–5. doi: 10.1111/tbj.14025
2. Rozen WM and Ashton MW. Improving outcomes in autologous breast reconstruction. Aesthetic Plast Surg. (2009) 33:327–35. doi: 10.1007/s00266-008-9272-1
3. Schaverien MV and Butler CE. Complications in DIEP flap breast reconstruction after mastectomy for breast cancer: A prospective cohort study comparing unilateral and bilateral reconstructions. Ann Surg Oncol. (2017) 24:1451–3. doi: 10.1245/s10434-017-5809-3
4. Shah JK, Amakiri UO, Cevallos P, Yesantharao P, Ayyala H, Sheckter CC, et al. Updated trends and outcomes in autologous breast reconstruction in the United States, 2016-2019. Ann Plast Surg. (2024) 92:e1–e13. doi: 10.1097/SAP.0000000000003764
5. Fitzgerald O’Connor E, Rozen WM, Chowdhry M, Band B, Ramakrishnan VV, and Griffiths M. Preoperative computed tomography angiography for planning DIEP flap breast reconstruction reduces operative time and overall complications. Gland Surg. (2016) 5:93–8.
6. Casey WJ 3rd, Chew RT, Rebecca AM, Smith AA, Collins JM, and Pockaj BA. Advantages of preoperative computed tomography in deep inferior epigastric artery perforator flap breast reconstruction. Plast reconstructive surgery. (2009) 123:1148–55. doi: 10.1097/PRS.0b013e31819e23e1
7. Arellano JA, Comerci AJ, Liu HY, Alessandri Bonetti M, Nguyen VT, Parent B, et al. Complications in prolonged intraoperative ischemia time in free flap breast reconstruction: A systematic review and meta-analysis. Aesthetic Plast Surg. (2024). doi: 10.1007/s00266-024-04382-7
8. Wan M, Zhang JX, Ding Y, Jin Y, Bedford J, Nagarajan M, et al. High-risk plastic surgery: an analysis of 108,303 cases from the American college of surgeons national surgical quality improvement program (ACS NSQIP). Plast Surg (Oakv). (2020) 28:57–66. doi: 10.1177/2292550319880921
9. Kwok AC and Agarwal JP. An analysis of free flap failure using the ACS NSQIP database. Does flap site and flap type matter? Microsurgery. (2017) 37:531–8.
10. Cumpston M, Li T, Page MJ, Chandler J, Welch VA, Higgins JP, et al. Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev. (2019) 10:ED000142. doi: 10.1002/14651858.ED000142
11. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71.
12. Yousif Y RF, Khajuria A, Aftab F, Rupra RS, Khan GR, AlSaidi Y, et al. The impact of CT angiographic mapping in DIEP flap breast reconstruction on operative time and clinical outcomes: A systematic review (2024). Available online at: https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=596646 (Accessed January 26, 2025).
13. Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. (2017) 358:j4008. doi: 10.1136/bmj.j4008
14. Sterne JA, Hernan MA, Reeves BC, Savovic J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. (2016) 355:i4919. doi: 10.1136/bmj.i4919
15. Sterne JAC, Savovic J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. (2019) 366:l4898. doi: 10.1136/bmj.l4898
16. Phi L, Ajaj R, Ramchandani MH, Brant XM, Oluwadara O, Polinovsky O, et al. Expanding the grading of recommendations assessment, development, and evaluation (Ex-GRADE) for evidence-based clinical recommendations: validation study. Open Dent J. (2012) 6:31–40. doi: 10.2174/1874210601206010031
17. Cina A, Barone-Adesi L, Rinaldi P, Cipriani A, Salgarello M, Masetti R, et al. Planning deep inferior epigastric perforator flaps for breast reconstruction: a comparison between multidetector computed tomography and magnetic resonance angiography. Eur Radiol. (2013) 23:2333–43. doi: 10.1007/s00330-013-2834-x
18. Smit JM, Dimopoulou A, Liss AG, Zeebregts CJ, Kildal M, Whitaker IS, et al. Preoperative CT angiography reduces surgery time in perforator flap reconstruction. J plastic reconstructive aesthetic surgery: JPRAS. (2009) 62:1112–7. doi: 10.1016/j.bjps.2007.12.090
19. Wade RG, Watford J, Wormald JCR, Bramhall RJ, and Figus A. Perforator mapping reduces the operative time of DIEP flap breast reconstruction: A systematic review and meta-analysis of preoperative ultrasound, computed tomography and magnetic resonance angiography. J Plast Reconstr Aesthet Surg. (2018) 71:468–77. doi: 10.1016/j.bjps.2017.12.012
20. Teunis T, Heerma van Voss MR, Kon M, and van Maurik JF. CT-angiography prior to DIEP flap breast reconstruction: a systematic review and meta-analysis. Microsurgery. (2013) 33:496–502. doi: 10.1002/micr.22119
21. Mossa-Basha M and Lee C. Impact of preoperative computed tomography angiogram on abdominal flap breast reconstruction outcomes: A systematic review. J Reconstr Microsurg. (2017) 33:328–35. doi: 10.1055/s-0037-1598617
22. Khaw MS, Yap CW, Lee P, and Ong SJ. What you need to know about: imaging in patients with renal failure. Br J Hosp Med (Lond). (2023) 84:1–9. doi: 10.12968/hmed.2022.0544
23. Harris E. Radiation from CT scans in young people tied to higher cancer risk. JAMA. (2023) 330:2146. doi: 10.1001/jama.2023.22935
24. Weinreb JC, Rodby RA, Yee J, Wang CL, Fine D, McDonald RJ, et al. Use of intravenous gadolinium-based contrast media in patients with kidney disease: consensus statements from the American college of radiology and the national kidney foundation. Radiology. (2021) 298:28–35. doi: 10.1148/radiol.2020202903
25. Newman TM, Vasile J, Levine JL, Greenspun DT, Allen RJ, Chao MT, et al. Perforator flap magnetic resonance angiography for reconstructive breast surgery: a review of 25 deep inferior epigastric and gluteal perforator artery flap patients. J Magn Reson Imaging. (2010) 31:1176–84. doi: 10.1002/jmri.22136
26. Cevik J, Seth I, Hunter-Smith DJ, and Rozen WM. A history of innovation: tracing the evolution of imaging modalities for the preoperative planning of microsurgical breast reconstruction. J Clin Med. (2023) 12. doi: 10.3390/jcm12165246
27. Sam AD 2nd, Morasch MD, Collins J, Song G, Chen R, and Pereles FS. Safety of gadolinium contrast angiography in patients with chronic renal insufficiency. J Vasc Surg. (2003) 38:313–8. doi: 10.1016/S0741-5214(03)00315-X
28. Mishra A, Jain N, and Bhagwat A. CT angiography of peripheral arterial disease by 256-slice scanner: accuracy, advantages and disadvantages compared to digital subtraction angiography. Vasc Endovascular Surg. (2017) 51:247–54. doi: 10.1177/1538574417698906
29. Wagner RD, Doval AF, Mehra NV, Le HB, Niziol PA, Ellsworth WA, et al. Incidental findings in CT and MR angiography for preoperative planning in DIEP flap breast reconstruction. Plast Reconstr Surg Glob Open. (2020) 8:e3159. doi: 10.1097/GOX.0000000000003159
30. Lim B, Cevik J, Seth I, Sofiadellis F, Ross RJ, Rozen WM, et al. Evaluating artificial intelligence’s role in teaching the reporting and interpretation of computed tomographic angiography for preoperative planning of the deep inferior epigastric artery perforator flap. JPRAS Open. (2024) 40:273–85. doi: 10.1016/j.jpra.2024.03.010
31. Offodile AC 2nd, Chatterjee A, Vallejo S, Fisher CS, Tchou JC, and Guo L. A cost-utility analysis of the use of preoperative computed tomographic angiography in abdomen-based perforator flap breast reconstruction. Plast reconstructive surgery. (2015) 135:662e–9e. doi: 10.1097/PRS.0000000000001133
32. Kupstas AR, Hoskin TL, Day CN, Habermann EB, and Boughey JC. Effect of surgery type on time to adjuvant chemotherapy and impact of delay on breast cancer survival: A national cancer database analysis. Ann Surg Oncol. (2019) 26:3240–9. doi: 10.1245/s10434-019-07566-7
33. Pomponio MK, Keele LJ, Fox KR, Clark AS, Matro JM, Shulman LN, et al. Does time to adjuvant chemotherapy (TTC) affect outcomes in patients with triple-negative breast cancer? Breast Cancer Res Treat. (2019) 177:137–43.
34. Yu KD, Fan L, Qiu LX, Ling H, Jiang YZ, and Shao ZM. Influence of delayed initiation of adjuvant chemotherapy on breast cancer survival is subtype-dependent. Oncotarget. (2017) 8:46549–56. doi: 10.18632/oncotarget.10551
35. Smith JM, Boukovalas S, Chang EI, Liu J, Selber JC, Hanson SE, et al. Analysis of breast aesthetic revision procedures after unilateral abdominal-based free-flap breast reconstruction: A single-center experience with 1251 patients. Plast Reconstr Surg Glob Open. (2023) 11:e4861. doi: 10.1097/GOX.0000000000004861
36. Araujo RJ, Garrido V, Baracas CA, Vasconcelos MA, Mavioso C, Anacleto JC, et al. Computer aided detection of deep inferior epigastric perforators in computed tomography angiography scans. Computerized Med Imaging Graphics. (2019) 77:101648. doi: 10.1016/j.compmedimag.2019.101648
Keywords: DIEP, CTA, operative time, complication rate, flap loss
Citation: Rupra RS, Ruccia F, Daneshi K, Aftab F, Yousif YF, Khan GR, Dehnadi S, AlSaidi YH, Maggialetti N, Lorusso G, Yan M and Khajuria A (2025) A systematic review and meta-analysis on computed tomography angiography mapping for deep inferior epigastric perforator flap breast reconstruction. Front. Oncol. 15:1600476. doi: 10.3389/fonc.2025.1600476
Received: 26 March 2025; Accepted: 21 July 2025;
Published: 17 September 2025.
Edited by:
Yousef Tanas, Houston Methodist Hospital, United StatesReviewed by:
Gioacchino De Sario Velasquez, University of Maryland Medical Center, United StatesAbdelrahman Zidan, Emory University, United States
Copyright © 2025 Rupra, Ruccia, Daneshi, Aftab, Yousif, Khan, Dehnadi, AlSaidi, Maggialetti, Lorusso, Yan and Khajuria. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Francesca Ruccia, RnJhbmNlc2NhLlJ1Y2NpYUBybWgubmhzLnVr
†These authors have contributed equally to this work and share first authorship
 Roshan S. Rupra1†
Roshan S. Rupra1† Kian Daneshi
Kian Daneshi Yousif F. Yousif
Yousif F. Yousif Ankur Khajuria
Ankur Khajuria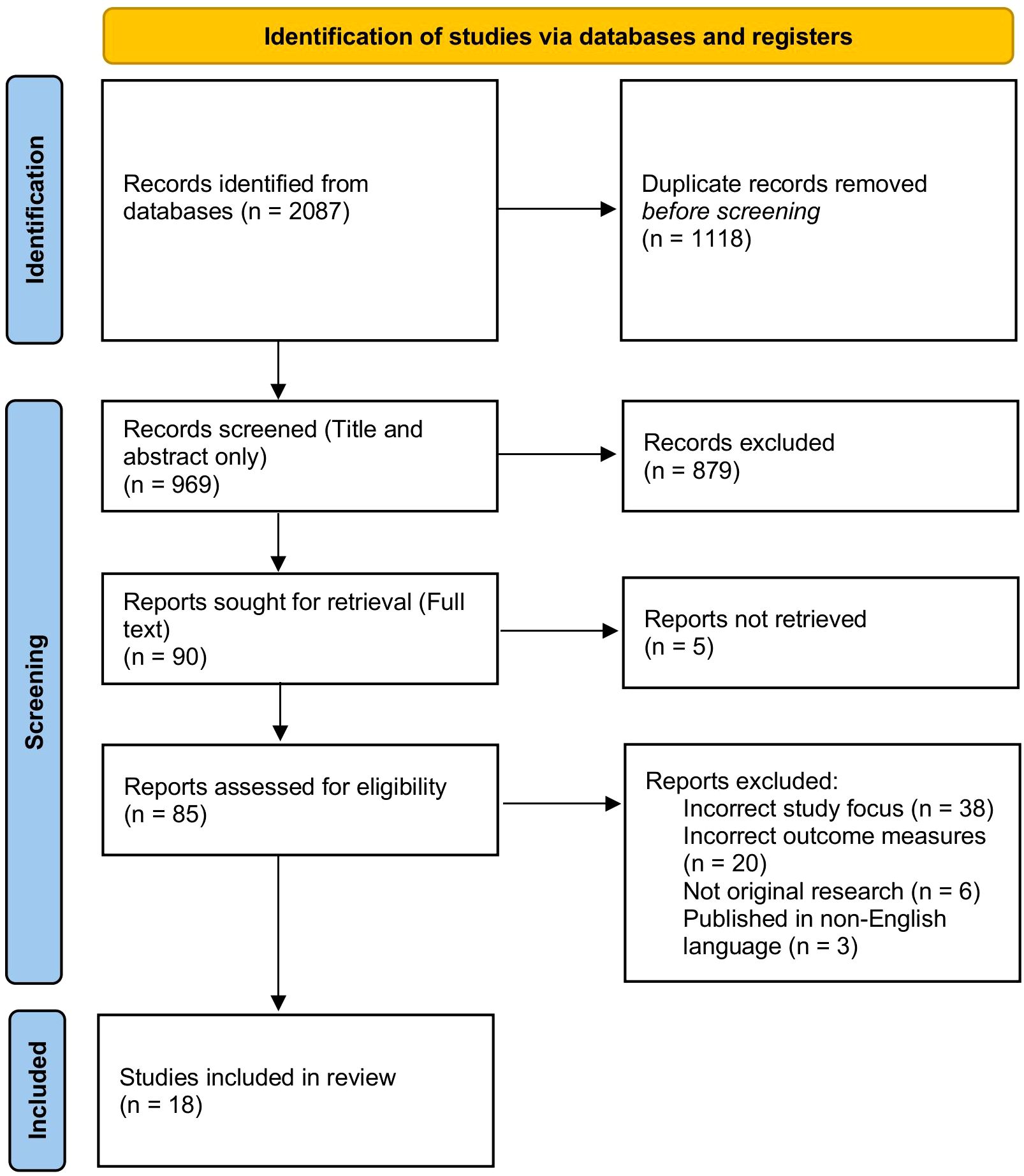
![Forest plot showing odds ratios for studies comparing CTA and Non-CTA groups. Each study lists events and totals for both groups, alongside calculated odds ratios (OR), confidence intervals (CI), and weight. Overall OR is 0.26 with a 95% CI of [0.14; 0.47]. The random effects model indicates no heterogeneity.](https://www.frontiersin.org/files/Articles/1600476/fonc-15-1600476-HTML/image_m/fonc-15-1600476-g006.jpg)
![Forest plot showing a meta-analysis of studies comparing CTA and Non-CTA events. Each study lists events, totals, odds ratios (OR), 95% confidence intervals, and weights. Diamonds represent pooled estimates, and horizontal lines indicate confidence intervals. The combined OR is 0.30 with a 95% CI of [0.13; 0.68]. Heterogeneity statistics are also included.](https://www.frontiersin.org/files/Articles/1600476/fonc-15-1600476-HTML/image_m/fonc-15-1600476-g007.jpg)