- 1Department of Traditional Chinese Medicine, Guangyuan Central Hospital, Guangyuan, Sichuan, China
- 2Department of Respiratory Medicine,Guangyuan Central Hospital, Guangyuan, Sichuan, China
Background: This meta-analysis systematically evaluated the impact of obesity on perioperative outcomes in patients undergoing lung cancer surgery to provide robust evidence for clinical decision-making and patient management.
Methods: A systematic review was conducted in accordance with Preferred Reporting Items for Systematic Reviews and Meta-analyses guidelines. Comprehensive searches were performed across PubMed, Embase, The Cochrane Library, Web of Science, and Medline. A random-effects model was employed, and the meta-analysis was conducted using Stata software (version 16.0).
Results: Eighteen studies comprising 88,413 participants were included in the meta-analysis. Postoperative mortality was significantly lower in the obese group (odds ratio [OR]: 0.73; 95% confidence interval [CI]: 0.59–0.90; P = 0.003). Statistically non-significant differences were observed between the two groups regarding postoperative complications (OR: 1.14; 95% CI: 0.71–1.84; P = 0.582), pulmonary complications (OR: 1.21; 95% CI: 0.57–2.58; P = 0.621), cardiovascular complications (OR: 1.54; 95% CI: 0.86–2.76, P = 0.150), and the total hospital stays (standardized mean difference [SMD]: −0.00; 95% CI: −0.17 to 0.17; P = 0.995). However, operation time was significantly longer in the obese group (SMD: 0.18; 95% CI: 0.11–0.26; P < 0.001).
Conclusion: The obesity paradox may exist in patients undergoing lung cancer surgery. Although obesity is associated with longer operation duration, it neither increases the risk of complications nor prolongs hospital stays and is generally linked to reduced postoperative mortality.
Systematic review registration: https://www.crd.york.ac.uk/PROSPERO/view/CRD42025648330, identifier CRD42025648330
1 Introduction
Lung cancer remains the leading cause of cancer-related mortality globally. Surgical resection constitutes the primary curative treatment for early-stage disease (1). In 2022, lung cancer accounted for nearly 2.5 million new cases and over 1.8 million deaths worldwide, comprising approximately 12.4% of global cancer diagnoses and 18.7% of cancer-related deaths (2). Given its high incidence and mortality, lung cancer diagnosis and treatment have long been the subject of extensive investigation. Histologically, it is classified into two main types: small-cell lung cancer (SCLC) and non-small-cell lung cancer (NSCLC) (3). SCLC frequently presents as a disseminated disease and is primarily treated with systemic therapy. Surgical intervention is reserved for carefully selected patients with limited-stage disease, typically defined as a solitary pulmonary nodule with regional lymph node involvement and no distant metastases (4). In contrast, surgical resection remains the main treatment for early-stage NSCLC. However, it carries considerable perioperative risks, including respiratory complications, cardiovascular events, and mortality (5).
The World Health Organization (WHO) defines overweight as a body mass index (BMI) greater than 25 kg/m2 and obesity as a BMI exceeding 30 kg/m2 (6). Obesity is regarded as a major global public health concern, with its prevalence having doubled in 70 countries since 1980 and increased in nearly all other countries (7). The global obesity epidemic—driven by sedentary lifestyles, poor dietary habits, genetic predisposition, and environmental influences—has resulted in over 1.9 billion adults being classified as overweight and 650 million as obese (8). Currently, more than one-third of the global population falls into these categories, with projections indicating that by 2030, 38% of adults will be overweight and 20% will be obese (9). This rising burden presents specific challenges for surgeons, particularly in managing the physical, technical, and physiological complexities of thoracic operation in patients with obesity.
Obesity is a major contributor to the development of established cardiovascular risk factors, including diabetes, hypertension, dyslipidemia, and chronic kidney disease (10). It also constitutes a significant risk factor for lung cancer, influencing multiple aspects of the disease. Obesity contributes to tumor onset and progression through mechanisms such as chronic inflammation, hormonal imbalances, and metabolic dysregulation (9). Additionally, obesity complicates clinical outcomes, often resulting in poorer prognoses, increased treatment-related complications, and a higher incidence of comorbidities (9). Despite its established association with comorbid conditions, the impact of obesity on perioperative outcomes in lung cancer surgery remains controversial. Whether patients with obesity experience higher incidences of postoperative complications compared to individuals with normal weight undergoing lung cancer surgery continues to be a subject of investigation.
Some studies suggest that obesity may elevate the risk of postoperative complications, such as respiratory complications, due to physiological stress and technical challenges during surgery (11). Conversely, other studies support the existence of an “obesity paradox,” wherein patients with a BMI of ≥ 30 kg/m2 demonstrate improved survival and fewer complications compared to those of normal weight or underweight following lung cancer resection (12). This discrepancy highlights the need for a comprehensive synthesis of existing evidence.
Li S et al. conducted a systematic review examining the prognostic significance of BMI in patients undergoing lung cancer surgery, with a particular emphasis on the “obesity paradox” (13). This paradox refers to the counterintuitive observation that obese patients may experience improved survival compared to those with normal or low BMI. The review concluded that higher BMI and obesity are associated with favorable in-hospital outcomes and enhanced long-term survival in patients undergoing lung cancer surgery, indicating that the obesity paradox may exist in this patient population. However, the authors noted that potential ethnic variations and confounding variables need to warrant investigation. Notably, the study did not comprehensively evaluate the impact of obesity on key perioperative outcomes, including operation time, postoperative complications, and mortality. Consequently, the conclusions of the previous review offer limited utility for preoperative risk stratification and the optimization of preoperative care. Furthermore, several recent studies published after 2016 have investigated the impact of obesity on perioperative outcomes following lung cancer surgery. To address this, a meta-analysis was conducted to systematically assess the influence of obesity on perioperative outcomes in patients undergoing lung cancer resection, with the objective of providing stronger evidence to inform clinical decision-making and patient management.
2 Methods
2.1 Study registration
This meta-analysis was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) 2020 guidelines (14) and registered with PROSPERO (registration number: CRD42025648330). The PRISMA checklist is provided in Supplementary Material 1.
2.2 Selection criteria
2.2.1 Types of studies
This meta-analysis included observational studies, specifically cross-sectional, case-control, and cohort studies. Case reports, case series, review articles, letters, editorials, and commentaries were excluded.
2.2.2 Types of participants
Included participants were obese patients aged 18 years or older with a diagnosis of lung cancer who underwent surgical resection. Obesity was defined according to WHO criteria: a BMI > 30 kg/m2 for Western populations and > 25 kg/m2 for Asian populations.
2.2.3 Types of interventions and comparisons
Eligible interventions included video-assisted thoracoscopic surgery (VATS), robotic-assisted thoracoscopic surgery, open lobectomy, open pneumonectomy, open wedge resection, and open segmental resection. The control group comprised non-obese patients diagnosed with lung cancer who underwent surgical resection.
2.2.4 Language
Only studies published in English were included in the meta-analysis.
2.3 Exclusion criteria
Studies were excluded if they met any of the following criteria:
1. Published in languages other than English.
2. Did not report standardized incidence ratios, odds ratios (OR), risk ratios, hazard ratios (with 95% confidence interval [CI]), standard errors, or event counts necessary for effect size calculation.
3. Did not include a non-obese comparison group.
4. Contained discrepancies between textual data and tabulated results.
2.4 Search strategy
A comprehensive systematic search was performed across five databases—PubMed, Embase, The Cochrane Library, Web of Science, and Medline—from inception to January 2025 to identify eligible studies. The search strategy employed Medical Subject Headings (MeSH) and relevant keywords: [(lung cancer OR lung neoplasm) AND (VATS OR pulmonary surgical procedures) AND (BMI OR obesity OR obese)]. PubMed search strategy was used as a representative example, with detailed retrieval steps provided in Supplementary Material 2. Additional sources, including journal articles, conference papers, and academic papers, were also examined.
2.5 Data collection and analysis
2.5.1 Study selection
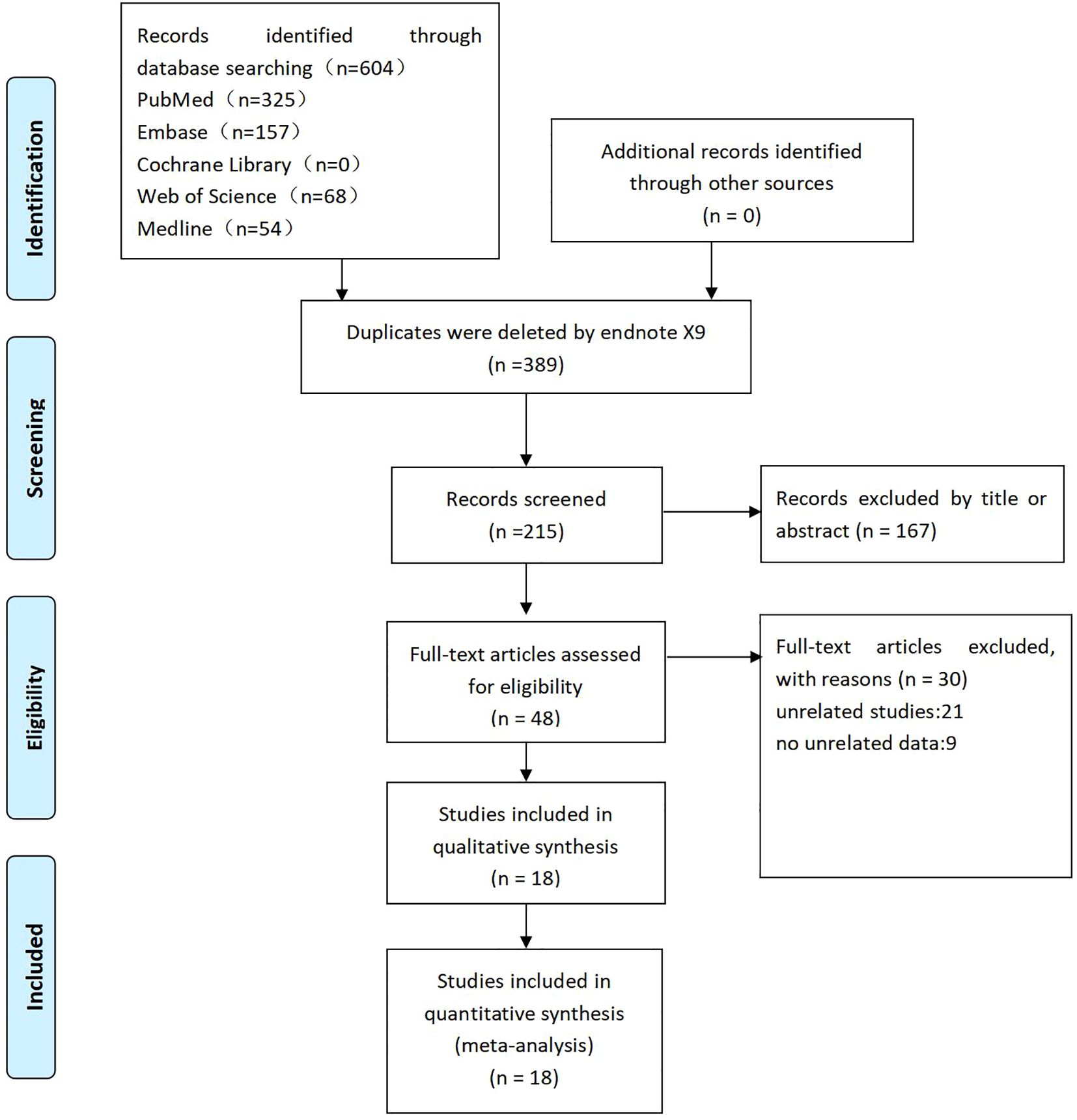
Two independent reviewers (CW and XW) screened the literature based on the predefined inclusion and exclusion criteria. Duplicate records were identified and removed using EndNoteX9 software, and abstracts and keywords were reviewed to eliminate irrelevant studies. Eligible studies were included in the meta-analysis after full-text assessment. Discrepancies were resolved through group discussion, and unresolved conflicts were adjudicated by the corresponding author (YX). The complete study selection process is illustrated in a PRISMA flow diagram (Figure 1).
2.5.2 Data extraction
Two reviewers independently extracted data from the included studies and entered them into a predefined Excel-based data extraction form. Discrepancies were resolved through discussion, and unresolved conflicts were arbitrated by the corresponding author (YX). Extracted data included the following: Study characteristics: first author’s name, year of publication, country, sample size, patient age, surgical approach, and tumor staging. Primary outcomes: postoperative mortality and postoperative complications. Secondary outcomes: pulmonary complications, cardiovascular complications, total hospital stay, and operation time.
2.5.3 Quality assessment
The quality of the included studies was evaluated using the Newcastle–Ottawa Scale (NOS) (15). This tool evaluates the quality of reporting and the risk of bias in included studies using a structured checklist covering three domains: selection, comparability, and exposure or outcomes (15). The scale consists of eight items under three parameters, with a total score of 9. Studies scoring ≤ 3 were classified as low quality, 4–5 as medium quality, and ≥ 6 as high quality (16). Two reviewers (QW and CW) independently assessed the quality of the included studies, and a third reviewer (YX) verified the results. Discrepancies were resolved through discussion.
2.5.4 Data analysis and heterogeneity processing
Meta-analysis and statistical analysis were conducted using Stata software (version 16.0). Given the variability in patients’ risk profiles and surgical selection criteria across different centers, a random effects model was applied to account for heterogeneity and produce more conservative estimates (17, 18). When outcomes were reported as medians with interquartile ranges, the method described by Wan et al. was employed to estimate the mean and standard deviation (19, 20). Dichotomous outcomes are presented as OR with 95% CIs, while continuous variables are expressed as standardized mean difference (SMD) with 95% CIs. Statistical significance was defined as P < 0.05. Heterogeneity was significant when I2 > 50% and/or P < 0.1. Additionally, levels of heterogeneity, assessed by I2 statistic, were interpreted as follows: 0%–25% indicated homogeneity, 25%–50% low heterogeneity, 50%–75% moderate heterogeneity, and > 75% high heterogeneity (21). Sensitivity analysis was performed by omitting one study at a time and recalculating the summary data from the remaining studies to evaluate the impact of each included study on the overall results. Publication bias was assessed using Egger’s test and funnel plots when ≥ 10 studies were available (22).
3 Results
3.1 Literature search
A literature search of five electronic databases was conducted to identify English-language studies examining the effect of obesity on perioperative outcomes following lung cancer surgery. Relevant studies were imported into EndNoteX9 software, and duplicates were removed, resulting in 247 records. Of these, 167 studies were excluded based on titles or abstracts. After a thorough full-text evaluation, 18 studies met the eligibility criteria for inclusion in the meta-analysis.
3.2 Characteristics of studies included in the meta-analysis
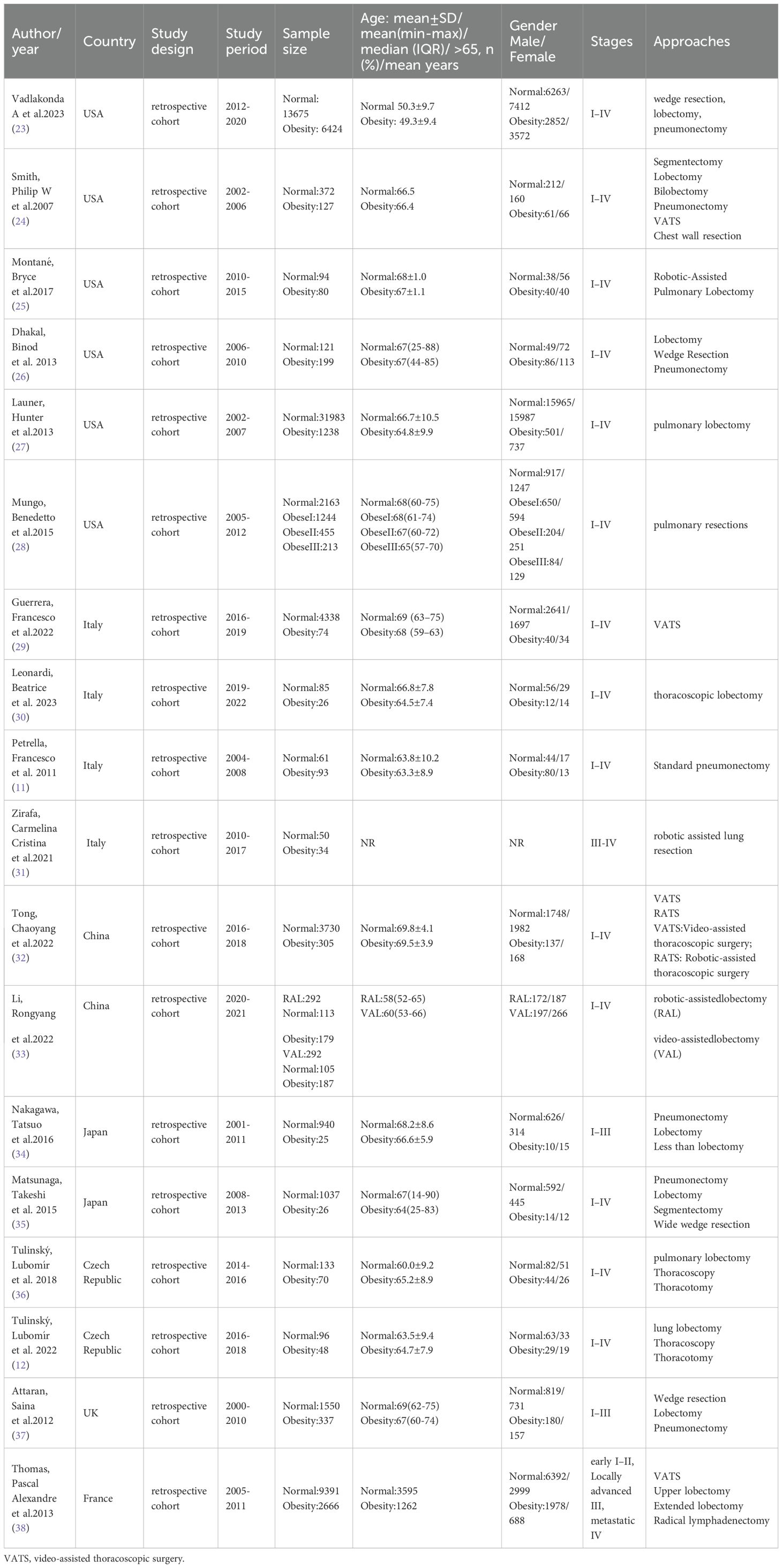
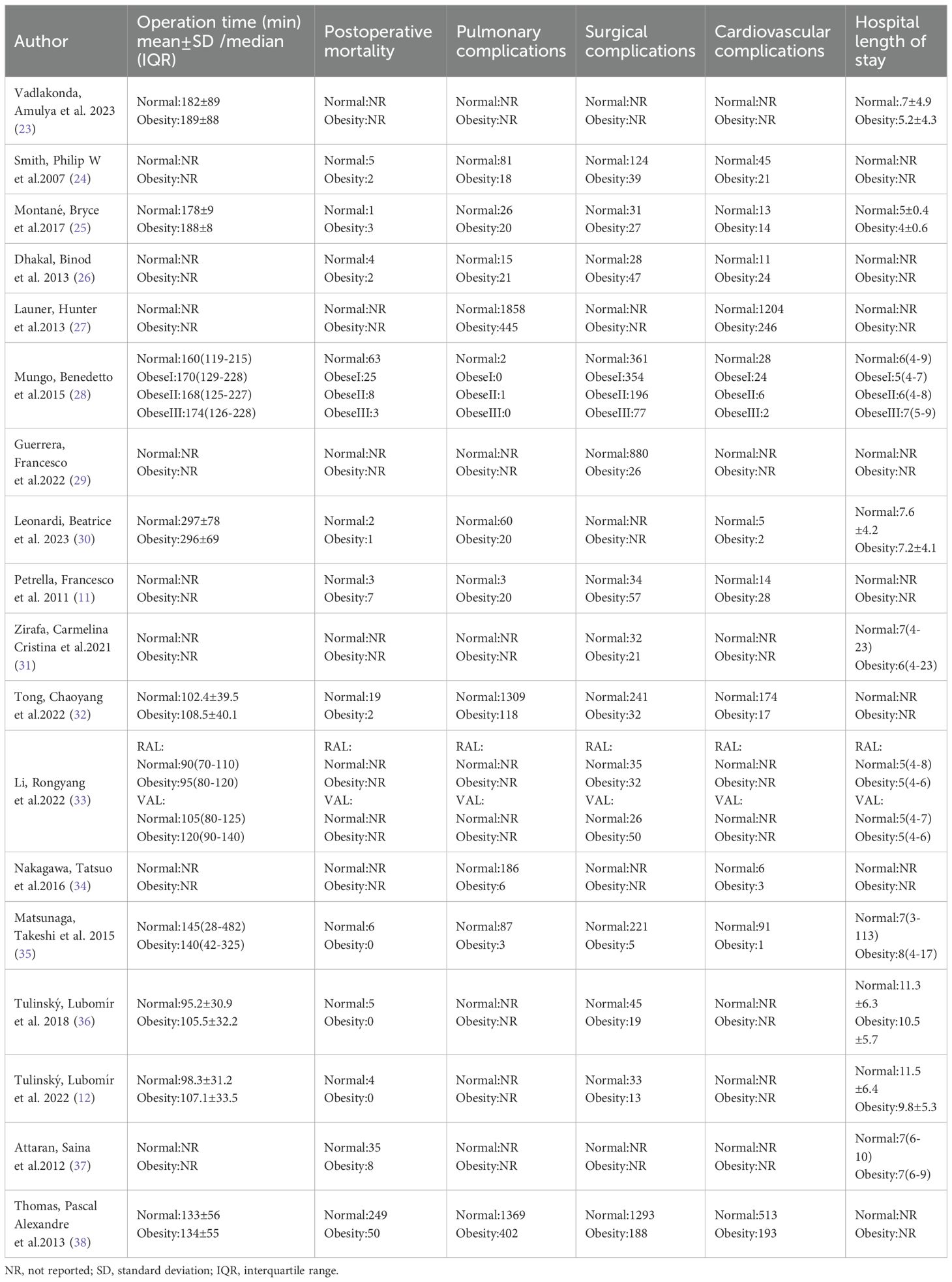
Eighteen studies were included in the meta-analysis, comprising 88,413 participants: 14,050 obese and 74,363 normal-weight patients who underwent lung cancer surgery. Six studies were conducted in the United States of America (23–28), four in Italy (11, 29–31), two in China (32, 33), two in Japan (34, 35), and two in the Czech Republic (12, 36). The remaining studies were performed in the United Kingdom (37) and France (38). Baseline characteristics demonstrated statistically non-significant differences between obese and normal-weight groups. The characteristics of the included studies are summarized in Table 1, and the study outcomes are presented in Table 2.
3.3 Quality assessment of the included studies
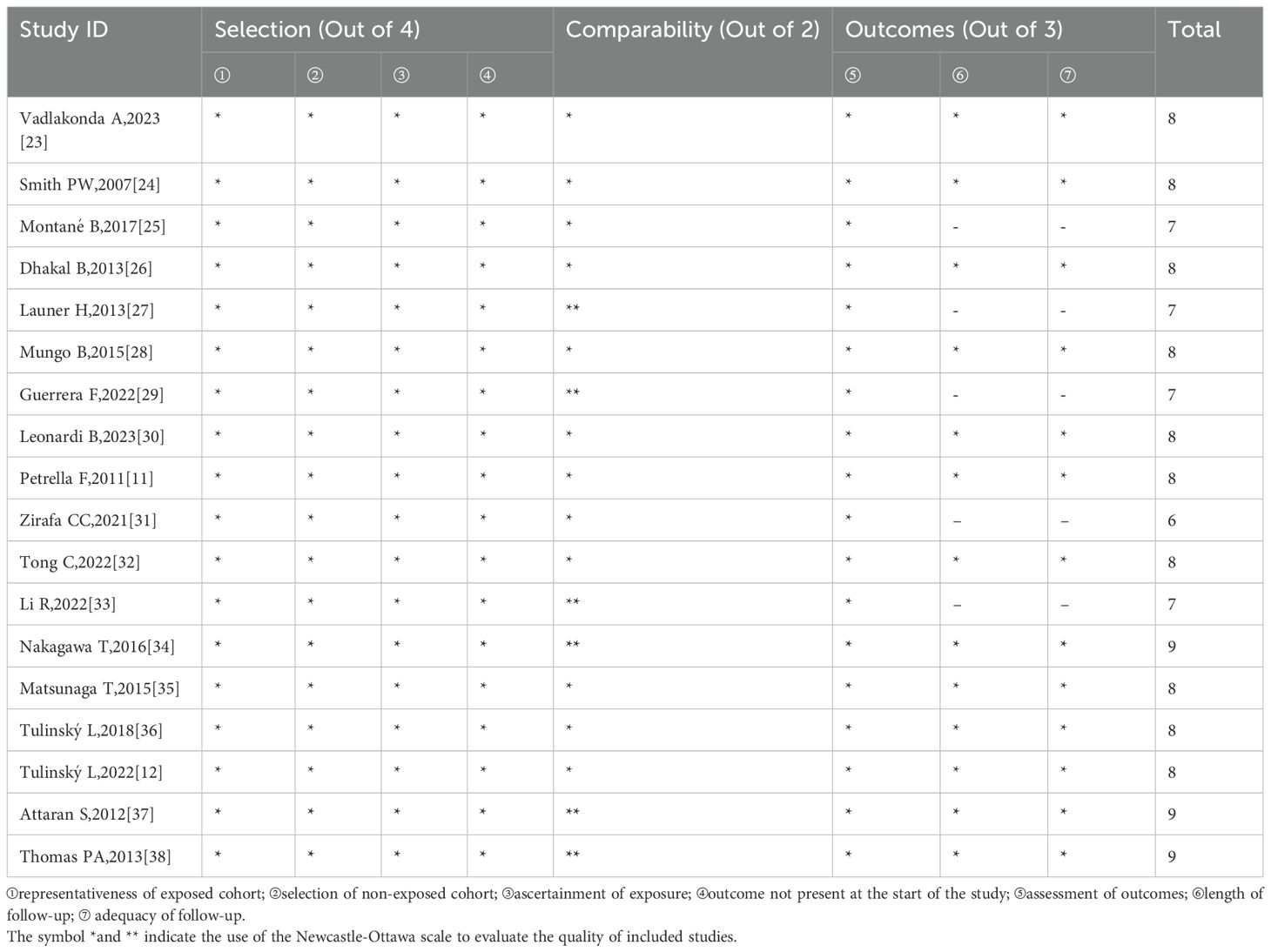
Based on the NOS, one study received six stars, four received seven stars, 10 received eight stars, and the remaining three received nine stars. All included studies were of high quality. Quality assessments according to the NOS are presented in Table 3.
3.4 Primary surgical outcomes
3.4.1 Postoperative 30-day mortality
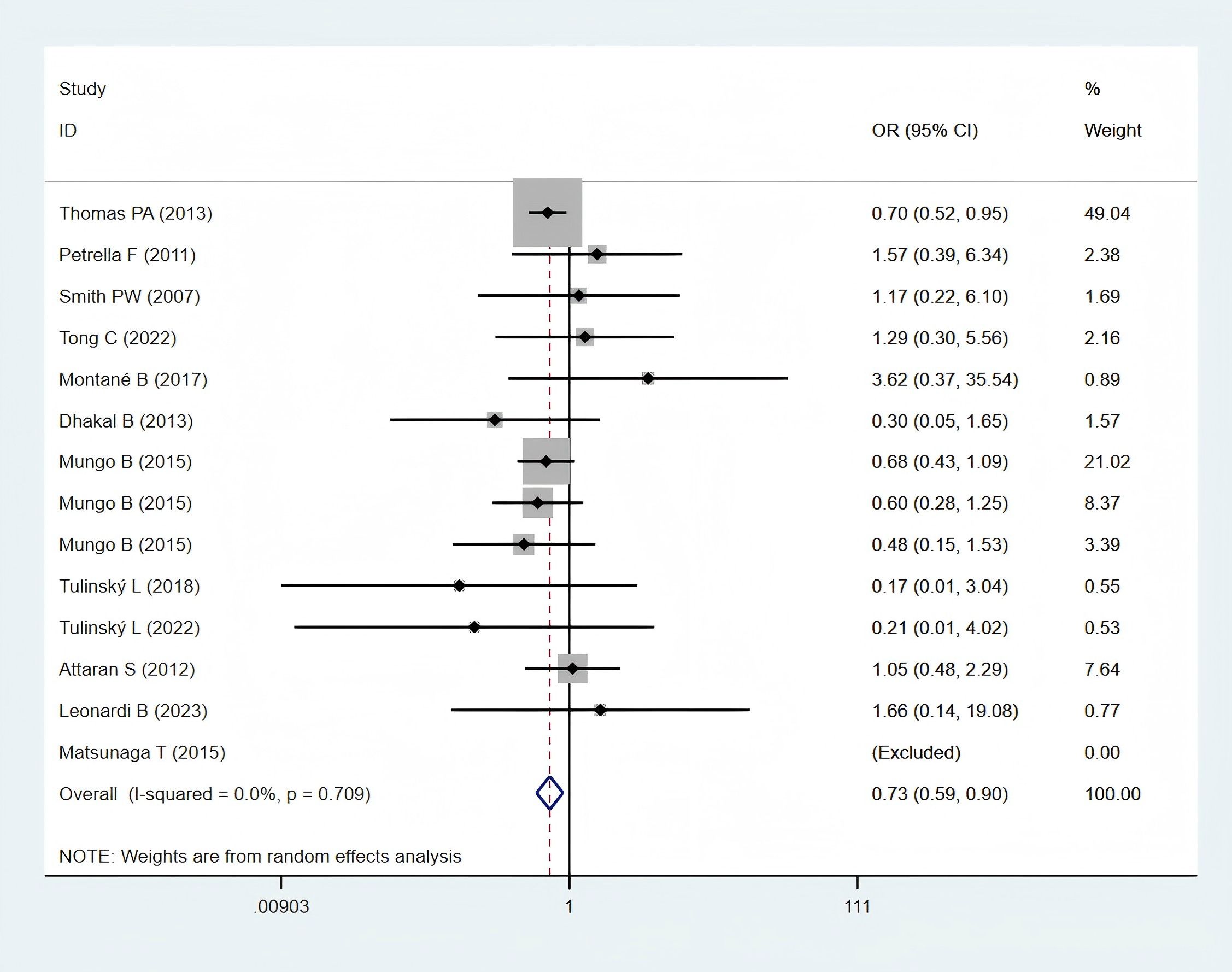
Twelve studies (11, 12, 24–26, 28, 30, 32, 35–38), encompassing 5,889 obese and 23,159 normal-weight patients, reported data on postoperative 30-day mortality and were included in the meta-analysis. A statistically significant difference in postoperative 30-day mortality was observed between the two groups (OR: 0.73; 95% CI: 0.59–0.90; P = 0.003; I² = 0.0%, P = 0.709) (Figure 2), with significantly lower mortality in the obese group.
3.4.2 Postoperative complications
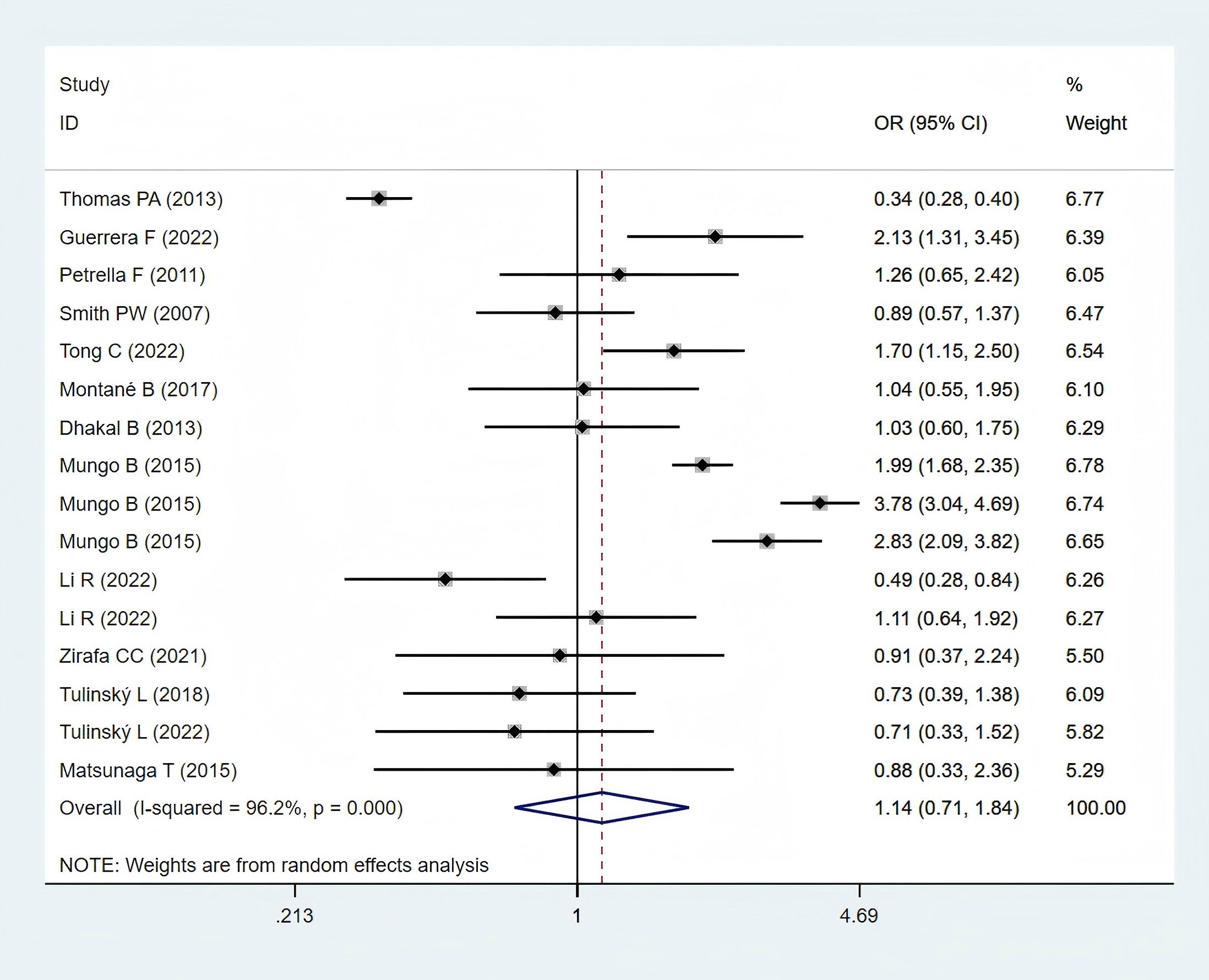
Thirteen studies (11, 12, 24–26, 28, 29, 31–33, 35, 36, 38), comprising 6,000 obese and 26,130 normal-weight patients, reported postoperative complication data and were included in the meta-analysis. A statistically non-significant difference in postoperative complications was observed between the two groups (OR: 1.14; 95% CI: 0.71–1.84; P = 0.582; I² = 96.2%, P < 0.001) (Figure 3).
3.5 Secondary surgical outcomes
3.5.1 Respiratory complications
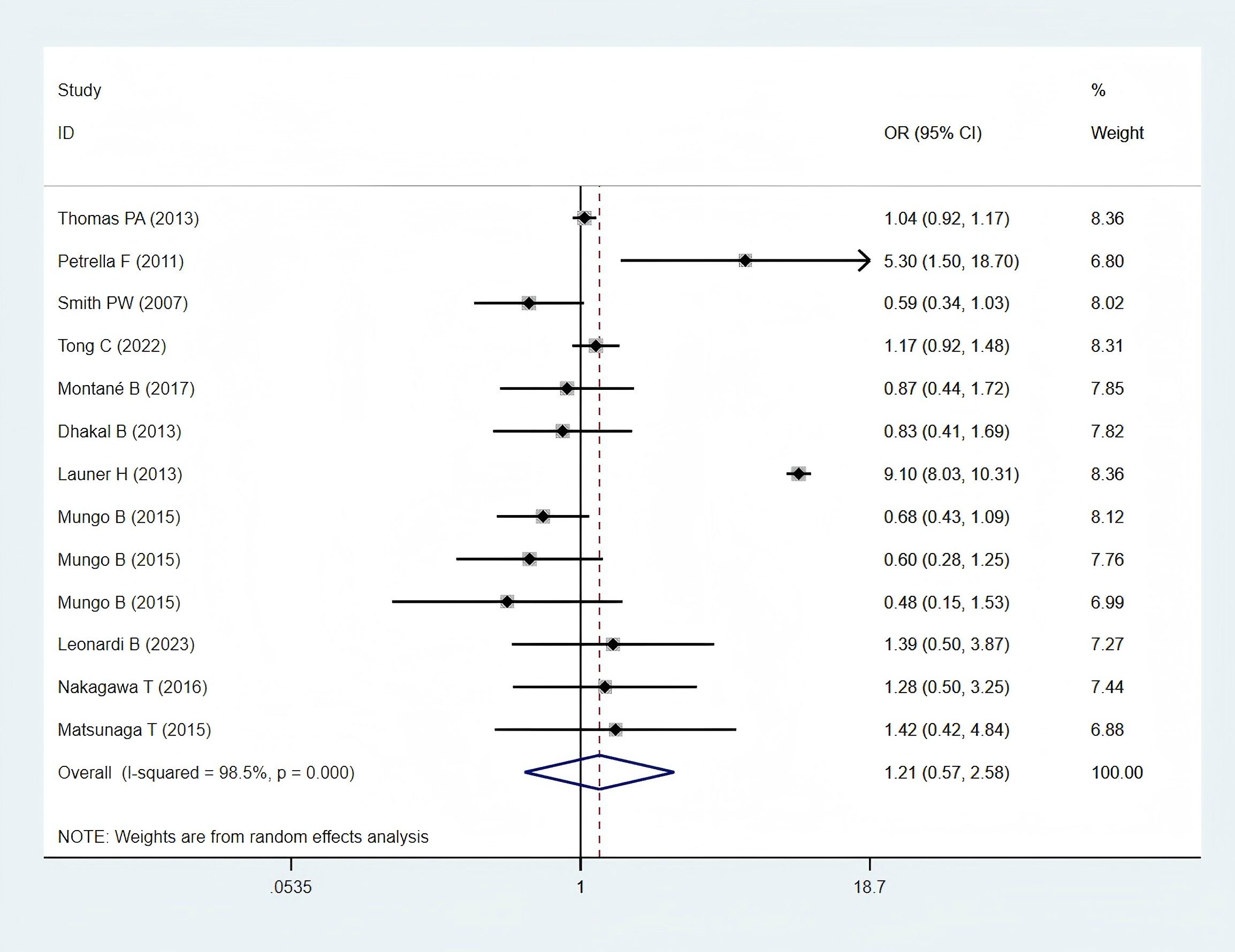
Eleven studies (11, 24–28, 30, 32, 34, 35, 38), including 6,697 patients in the obese group and 54,303 patients in the normal-weight group, provided respiratory complication data and were included in the meta-analysis. Respiratory complications, including lung infection, air leakage, acute respiratory distress syndrome, empyema, pneumonia, pulmonary embolism, pulmonary insufficiency, respiratory failure and other respiratory system complications. A statistically non-significant difference in pulmonary complications was found between the two groups (OR: 1.21; 95% CI: 0.57–2.58; P = 0.621; I² = 98.5%, P < 0.001) (Figure 4).
3.5.2 Cardiovascular complications
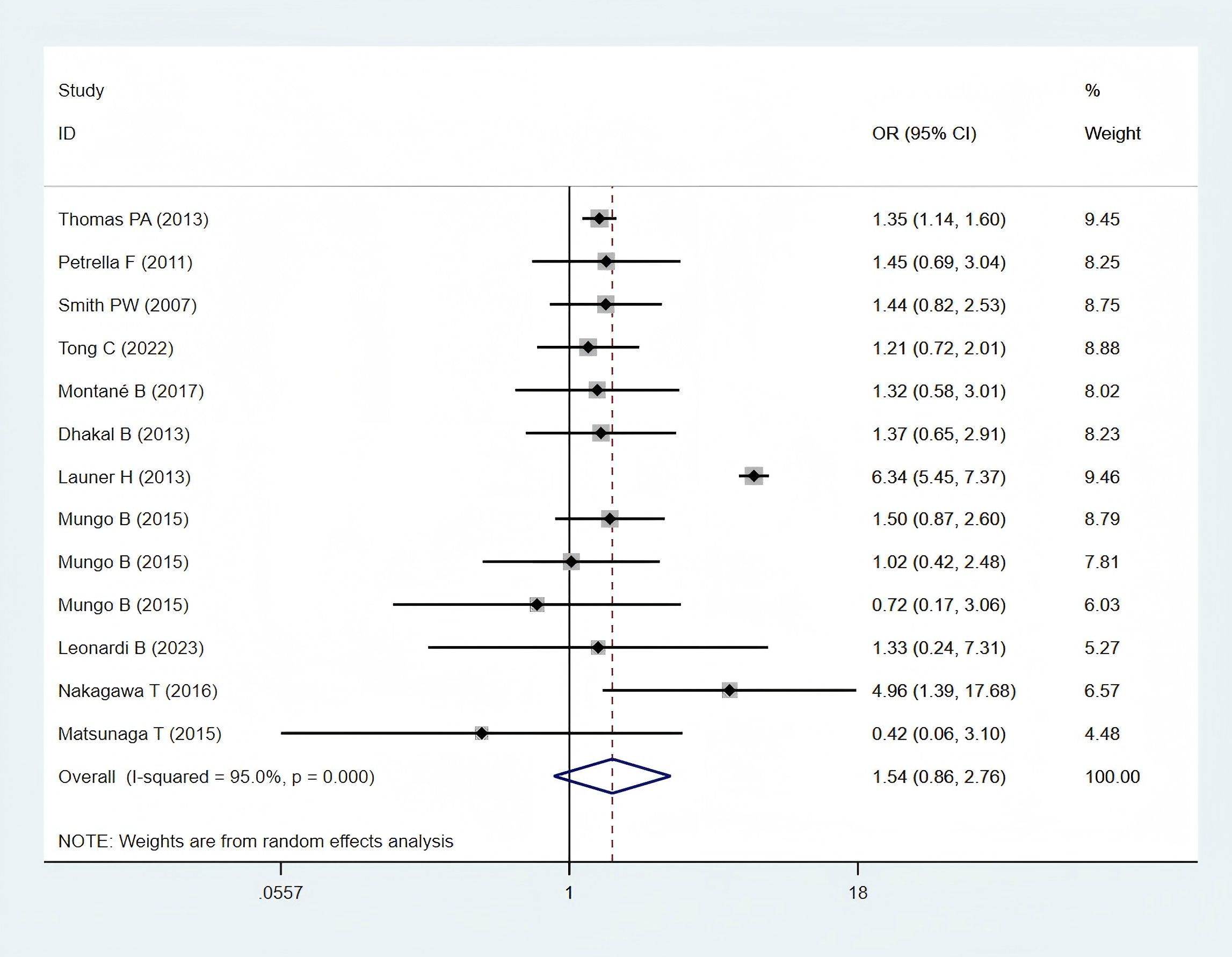
Eleven studies (11, 24–28, 30, 32, 34, 35, 38), comprising 6,697 patients in the obese group and 54,303 patients in the normal-weight group, reported cardiovascular complication data and were included in the meta-analysis. Cardiovascular complications, including arrhythmia, cardiac arrest, myocardial infarction, atrial fibrillation, other arrhythmia and other cardiac complications. A statistically non-significant difference in cardiovascular complications was observed between the two groups (OR: 1.54; 95% CI: 0.86–2.76; P = 0.150; I² = 95.0%, P < 0.001) (Figure 5).
3.5.3 Total hospital stay
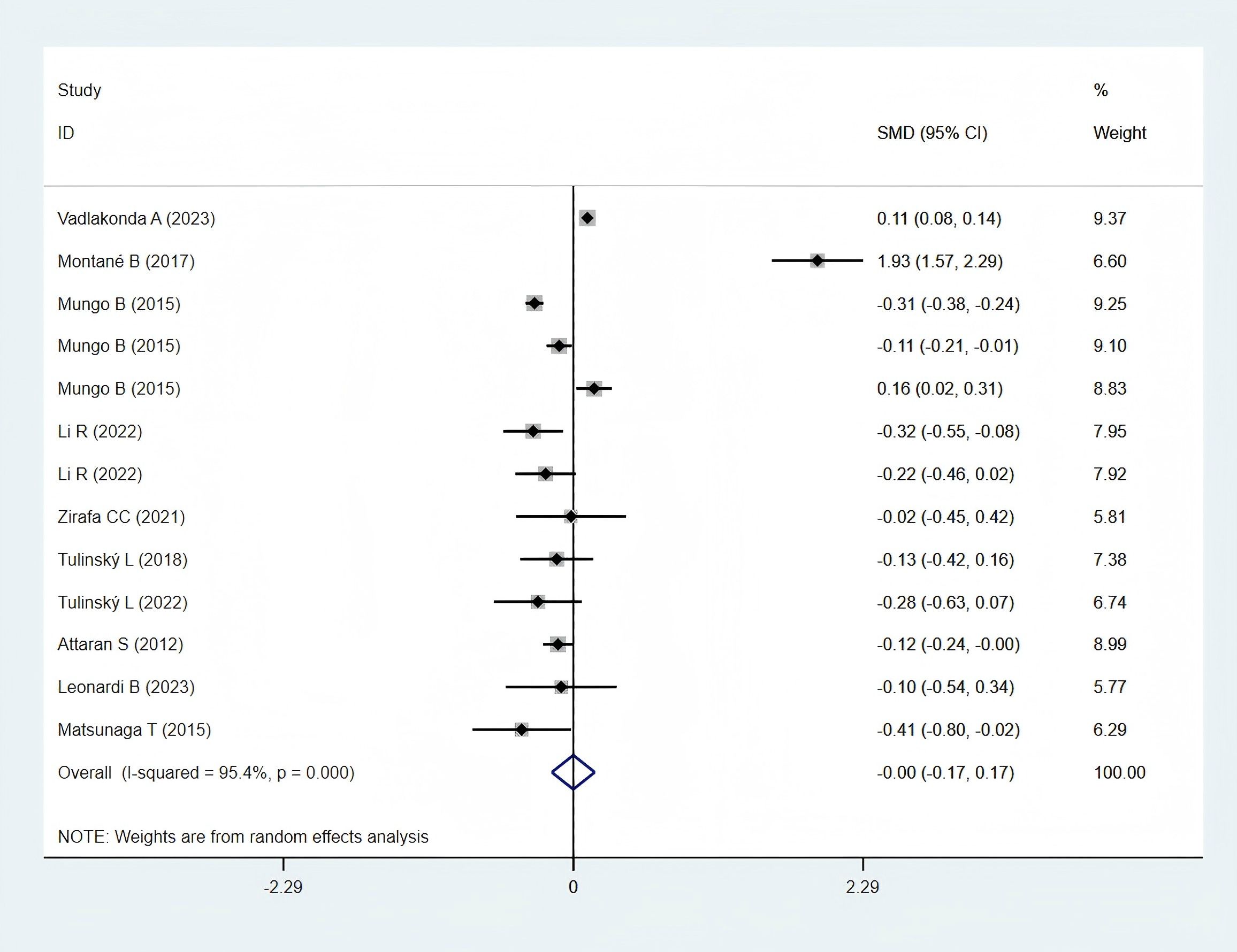
Ten studies (12, 23, 25, 28, 30, 31, 33, 35–37), comprising 9,323 patients in the obese group and 23,427 in the normal-weight group, reported data on total hospital stays and were included in the meta-analysis. A statistically non-significant difference was observed in total hospital stays between the two groups (SMD: −0.00; 95% CI: −0.17 to 0.17; P = 0.995; I² = 95.4%, P < 0.001) (Figure 6).
3.5.4 Operation time
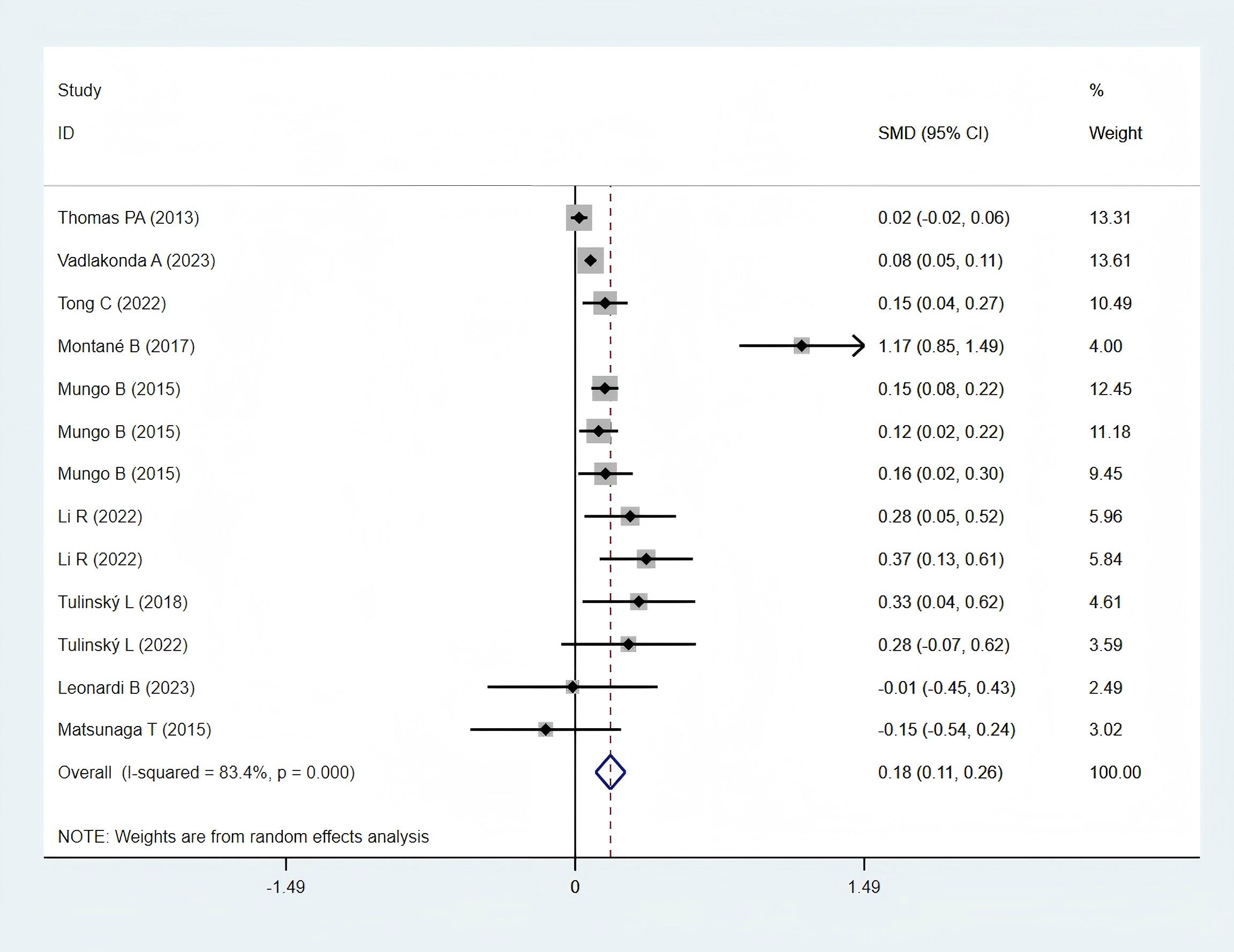
Ten studies (12, 23, 25, 28, 30, 32, 33, 35, 36, 38), including 11,923 patients in the obese group and 32,948 in the normal-weight group, reported operation time data and were included in the meta-analysis. Operation time was significantly longer in the obese group (SMD: 0.18; 95% CI: 0.11–0.26; P < 0.001; I² = 84.3%, P < 0.001) (Figure 7).
3.6 Subgroup analysis
3.6.1 Subgroup analysis of postoperative 30-day mortality
Subgroup analysis demonstrated that postoperative 30-day mortality was significantly lower in the obesity group in the Western populations (OR: 0.72; 95% CI: 0.58–0.89; P = 0.003; I² = 0.0%, P = 0.684). In contrast, no significant difference was observed between the groups in Asian populations (Figure 8A).
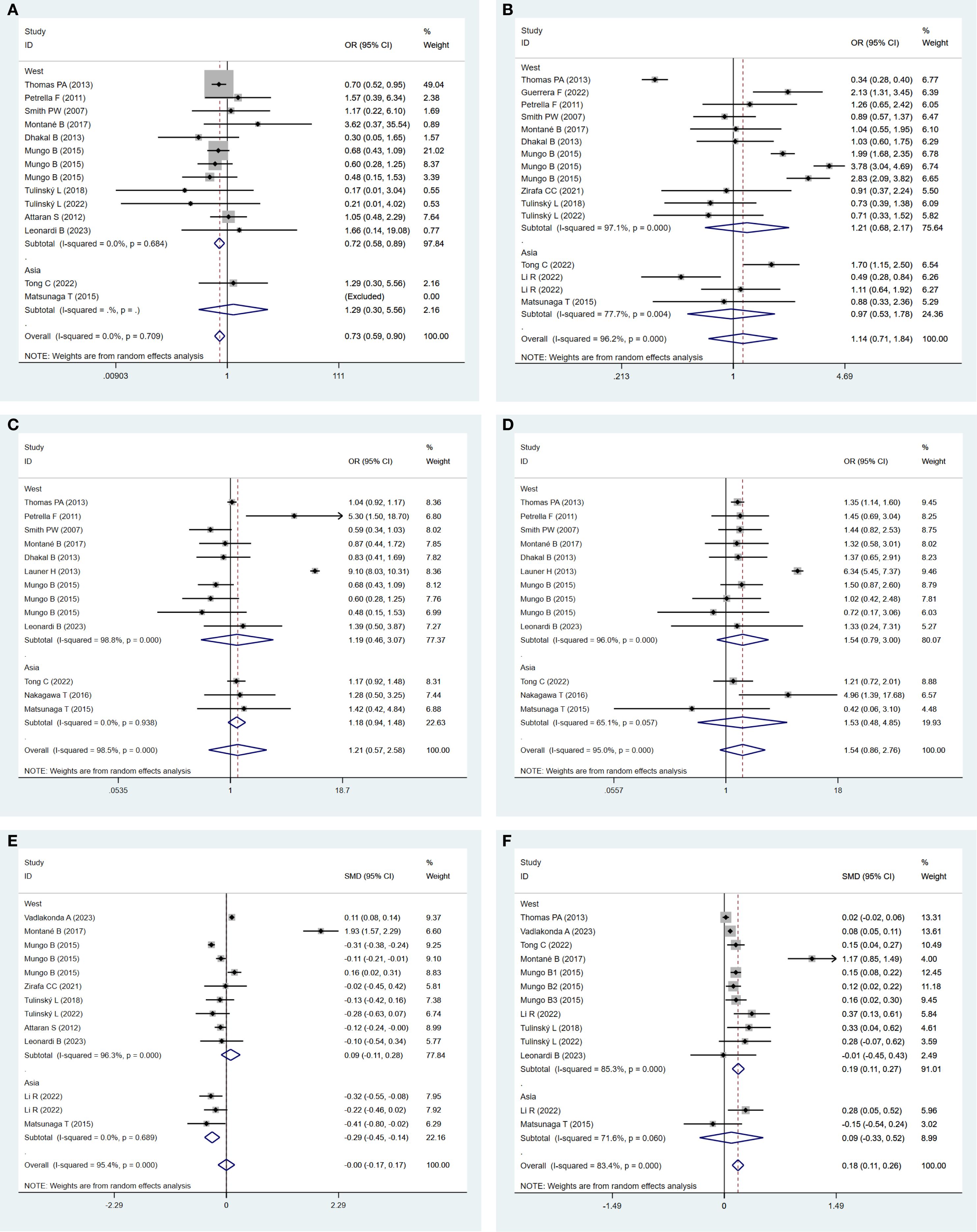
Figure 8. (A) Forest plots for subgroup analysis of postoperative 30-day mortality between obese groups and normal groups. (B) Forest plots for subgroup analysis of postoperative complications between obese groups and normal groups. (C) Forest plots for subgroup analysis of respiratory complications between obese groups and normal groups. (D) Forest plots for subgroup analysis of cardiovascular complications between obese groups and normal groups. (E) Forest plots for subgroup analysis of total hospital stay between obese groups and normal groups. (F) Forest plots for subgroup analysis of operation time between obese groups and normal groups.
3.6.2 Subgroup analysis of postoperative complications
Subgroup analysis indicated a statistically non-significant difference in postoperative complications between the obese and normal-weight groups, either in Western populations (OR: 1.21; 95% CI: 0.68–2.17, P = 0.52; I² = 97.1%, P = 0.000) or in Asian populations (OR: 0.97; 95% CI: 0.53–1.78, P = 0.924; I² = 77.1%, P = 0.004) (Figure 8B).
3.6.3 Subgroup analysis of respiratory complications
Subgroup analysis revealed a statistically non-significant difference in respiratory complications between the obese and normal-weight groups, either in Western populations (OR: 1.19; 95% CI: 0.46–3.07; P = 0.717; I² = 98.8%, P = 0.000) or in Asian populations (OR: 1.18; 95% CI: 0.94–1.48; P = 0.152; I² = 0.0%, P = 0.938) (Figure 8C).
3.6.4 Subgroup analysis of cardiovascular complications
Subgroup analysis indicated a statistically non-significant difference in cardiovascular complications between the obese and normal-weight groups, either in Western populations (OR: 1.54; 95% CI: 0.79–3.00; P = 0.205; I² = 96.0%, P = 0.000) or in Asian populations (OR: 1.53; 95% CI: 0.48–4.85; P = 0.469; I² = 65.1%, P = 0.057) (Figure 8D).
3.6.5 Subgroup analysis of total hospital stay
In Asia, the total hospital stay in the obese group was significantly shorter (SMD: −0.29; 95% CI: −0.45 to −0.14; P = 0.000; I² = 0.0%, P = 0.689). In contrast, a statistically non-significant difference was observed between the two groups in the Western Populations (SMD: 0.09; 95% CI: −0.11 to 0.28; P = 0.378; I² = 96.3%, P = 0.000) (Figure 8E).
3.6.6 Subgroup analysis of operation time
In Western regions, operation time in the obese group was significantly longer (SMD: 0.19; 95% CI: 0.11–0.27; P = 0.000; I² = 85.3%, P = 0.000). In contrast, a statistically non-significant difference was identified between the two groups in Asian populations (SMD: 0.09; 95% CI: −0.33 to 0.52; P = 0.662; I² = 71.6%, P = 0.060) (Figure 8F).
3.7 Sensitivity analysis
Sensitivity analysis demonstrated that excluding studies with larger sample sizes—such as Thomas PA (38)—affected the pooled estimate for operation time. Excluding individual studies one at a time did not significantly alter the pooled estimates for postoperative mortality, postoperative complications, pulmonary complications, cardiovascular complications, or total hospital stays, indicating these results were stable and reliable in the meta-analysis (Supplementary Material 3).
3.8 Publication bias
Funnel plots and Egger’s tests demonstrated no significant publication bias for postoperative mortality, postoperative complications, pulmonary complications, or cardiovascular complications. Due to insufficient data, publication bias was not assessed for other surgical outcomes. Details are provided in Supplementary Material 4.
4 Discussion
This meta-analysis demonstrates that obese patients undergoing lung cancer surgery experience reduced postoperative mortality, prolonged operative duration, and no increase in postoperative complications or total hospital stays compared with individuals of normal weight.
The concept of the “obesity paradox” has garnered considerable attention across various surgical disciplines, including lung cancer surgery. This paradox posits that contrary to conventional expectations, obesity may confer a survival advantage in certain patient populations. In lung cancer surgery, multiple studies have examined this phenomenon, indicating that patients with obesity may experience reduced postoperative mortality compared to their normal-weight individuals. One study evaluating the impact of BMI on surgical outcomes in patients with NSCLC reported that overweight individuals exhibited slightly improved overall survival relative to those with normal BMI. The finding highlighted preoperative BMI as an independent prognostic factor for survival in patients undergoing complete resection for NSCLC, with underweight patients demonstrating notably poorer survival outcomes (39). A study examining the impact of BMI on mortality rates following lung resection surgery provides further evidence supporting the obesity paradox, revealing that low BMI is significantly associated with poorer postoperative outcomes, including higher 90-day mortality. In contrast, obesity was linked to reduced morbidity and mortality, reinforcing the possibility that higher BMI may exert a protective effect in this surgical setting (40). Additionally, research investigating the role of omentin, an adipokine, in lung cancer suggested that the presence of adipocytes and preadipocytes in normal lung tissue may contribute to the enhanced survival observed in patients with obesity. This study identified omentin as a potential prognostic factor, offering a possible explanation for the obesity paradox in lung cancer (41). In conclusion, the obesity paradox in lung cancer surgery is supported by multiple studies indicating that patients with obesity may experience reduced postoperative mortality compared with their normal-weight counterparts. The present meta-analysis observed decreased postoperative mortality among obese individuals following lung cancer surgery in Western populations. These findings emphasize the complexity of the association between BMI and surgical outcomes and highlight the need for further investigation to fully understand the mechanisms underlying this paradox.
In the context of lung cancer surgery, the association between obesity and postoperative complications has been extensively investigated. A study involving elderly patients undergoing thoracoscopic anatomic lung cancer surgery reported that obesity did not significantly elevate the risk of postoperative complications. However, patients with obesity exhibited higher incidences of intraoperative hypoxemia and new-onset arrhythmia compared with non-obese individuals. Despite these intraoperative events, overall postoperative outcomes, including pulmonary complications, length of hospital stay, and hospitalization costs, did not differ significantly between the two groups (32). Similarly, a study evaluating the impact of obesity on perioperative outcomes in patients undergoing minimally invasive anatomical lung resections found that obesity did not confer early outcome benefits. Although the risk of respiratory complications was higher among patients with obesity, overall, cardiovascular and surgical complication rates did not differ significantly between obese and normal-weight individuals (42). Furthermore, research on the impact of obesity on in-hospital and postoperative outcomes following hepatic resection for malignancy—findings that may be extrapolated to thoracic surgery—indicated a greater risk of complications in open surgeries but not in laparoscopic procedures (43). In patients with obesity and colorectal cancer, laparoscopic surgery was associated with significantly reduced postoperative morbidity and improved healthcare resource utilization compared to open surgery (44). These findings suggest that surgical approaches may help mitigate postoperative risks in patients with obesity. In thoracic surgery for lung cancer, a study investigating the impact of BMI on postoperative nausea and vomiting reported a lower incidence of these complications within the first 48 h among patients with a higher BMI (45). This finding suggests that elevated BMI may not necessarily correlate with increased postoperative complications in certain clinical contexts. Additionally, a study on the ‘obesity paradox’ in minimally invasive anatomical lung resection concluded that obesity did not confer early outcome benefits, as patients with obesity exhibited a significantly higher risk of respiratory complications (42). However, overall complication rates did not differ significantly between patients with obese and normal-weight individuals, reinforcing the notion that obesity does not inherently increase postoperative complications in lung cancer surgery (42).
The research found that obesity in Western patients was associated with prolonged operation duration, although it did not significantly influence postoperative complications or total hospital stays. Obesity complicates surgical procedures due to increased adipose tissue, which can obscure anatomical landmarks and hinder surgical access. This challenge is reflected in the increased operation time observed in patients with obesity undergoing lung cancer surgery. It is well established that body habitus in patients with obesity contributes to technical difficulty during surgery (46). St Julien et al. reported a longer operative time in patients with obesity, with an increase of 7.2 min for every 10-unit rise in BMI (47). Although the present meta-analysis indicates that obesity is associated with increased operative duration, potential confounding by comorbidities such as diabetes and cardiovascular disease must be considered. Many of the included studies did not adjust for these variables, and obesity frequently clusters with metabolic dysfunction, which independently increases perioperative risk. Future research should stratify obesity by metabolic status, such as “metabolically healthy obesity” versus “obesity accompanied by diabetes,” to determine whether the increased operative time is driven by BMI alone or associated metabolic conditions.
Several studies have examined the association between obesity and surgical outcomes in patients with lung cancer. For instance, a study investigating the perioperative outcomes in elderly patients undergoing thoracoscopic anatomic lung cancer surgery reported that those with obesity required longer operative time than non-obese individuals (32). Although obesity has been associated with improved postoperative outcomes in older patients, it may still contribute to greater technical complexity and prolonged surgical duration. Similarly, an analysis of data from the American College of Surgeons National Surgical Quality Improvement Program database reported that patients with obesity undergoing pulmonary resections for lung cancer experienced longer operative times compared to their non-obese counterparts (28). The study emphasized that obesity complicates the surgical process and contributes to extended procedural duration. Moreover, the impact of obesity on operative outcomes extends beyond lung cancer surgery; evidence across multiple surgical disciplines consistently demonstrates that obesity is associated with increased operative times and higher rates of postoperative complications. For example, a study comparing laparoscopic and open surgery outcomes in patients with rectal cancer and obesity found that the laparoscopic approach was associated with reduced blood loss and shorter hospital stays; however, operative time remained longer in patients with obesity compared to those with normal weight (48). Prolonged operative time is a recognized risk factor for complications. As operative duration was significantly extended in patients with obesity, this factor alone may elevate perioperative risk, independent of BMI itself. Overall, these studies highlight the surgical challenges associated with obesity, particularly in lung cancer surgery, where increased operative time is a common consequence. The consistent association between obesity and prolonged operative duration highlights the need for tailored surgical strategies and interventions to improve outcomes in this patient population.
This meta-analysis evaluates the immediate impact of obesity on surgical outcomes in patients with lung cancer, focusing on perioperative metrics such as complications, operative time, length of stay, and mortality. In contrast, long-term oncologic outcomes—including disease-free survival and overall survival—are influenced by many factors beyond surgery, such as adjuvant therapy, tumor biology, and comorbidities, making them less directly attributable to obesity’s perioperative effects. Existing literature on obesity in lung cancer surgery often addresses either perioperative or long-term outcomes but rarely both. Perioperative outcomes are more consistently reported in surgical studies, whereas long-term survival data require extended follow-up, which is often unavailable.
This meta-analysis focused on observational studies for several key reasons. Existing randomized controlled trials typically compare surgical techniques or perioperative care protocols rather than obesity as an exposure, making them less relevant to the research objective. Observational studies more accurately reflect routine clinical practice, in which patients with varying BMI levels undergo surgery without randomization. This design aligns with the aim of evaluating the real-life impact of obesity on perioperative outcomes. Most available evidence on obesity and surgical outcomes derives from retrospective or prospective cohort studies. Consequently, most surgical meta-analyses examining patient-specific factors, such as BMI or age, rely on observational data.
This meta-analysis offers several key strengths. First, this is the first meta-analysis to evaluate the effect of obesity on perioperative outcomes in patients undergoing lung cancer surgery, addressing a significant gap in the literature and providing novel insights into the association between obesity and surgical outcomes. Second, this meta-analysis aggregates data from multiple studies, offering a more robust and statistically powerful assessment of the impact of obesity on perioperative outcomes than any individual studies. Third, the findings may inform clinical decision-making by highlighting the potential risks and benefits of lung cancer surgery in patients with obesity, thereby supporting preoperative planning and patient counseling. Moreover, sensitivity analyses were performed to assess the stability of the results, ensuring that no single study or outlier disproportionately influenced the findings. Publication bias was evaluated using Egger’s test and funnel plots, enhancing the credibility of the meta-analysis by addressing potential reporting biases. Due to insufficient reporting of outcomes stratified by surgical approach in the included studies, subgroup analyses could not be performed. However, sensitivity analyses excluding studies dominated by open or minimally invasive techniques were conducted to assess the robustness of pooled estimates. Although VATS and open surgery differ in invasiveness, obesity presents shared challenges in both approaches, including prolonged anesthesia time and technical difficulties related to body habitus. Therefore, the pooled estimates reflect the aggregate risk across real-world practices. Ultimately, the inherent variability in defining postoperative complications across healthcare systems and geographic regions was addressed; therefore, a random-effect model was applied to account for heterogeneity, offering a more conservative estimate.
However, this meta-analysis has some limitations. First, it may not fully account for all relevant factors influencing perioperative outcomes, such as comorbidities, smoking status, or variations in postoperative care, which may confound the results. As a research-level meta-analysis, the analysis is constrained by the variables reported in the original studies. Due to limited data on smoking history, lung function, or nutritional status, these patient-level variables were not included. Second, due to insufficient data, the meta-analysis focused primarily on short-term perioperative outcomes, potentially overlooking the long-term effects of obesity on recovery, survival, and quality of life after lung cancer surgery. Despite these limitations, this meta-analysis provides valuable insights and establishes a foundation for future research into the impact of obesity on perioperative outcomes in lung cancer surgery. Last, combining varying BMI thresholds used for Asians and Western populations into a single “obesity” category increases heterogeneity. Although this binary classification of obesity may oversimplify the association between BMI and surgical risk, it was necessitated by inconsistent reporting across studies. Future investigations should adopt standardized BMI stratification to identify potential threshold effects and better quantify risk gradients.
5 Conclusion
This meta-analysis suggests that the obesity paradox may indeed exist in patients undergoing lung cancer surgery. While obesity is generally associated with prolonged operative duration, it does not appear to increase postoperative complications or total hospital stays and is often linked to reduced mortality. The prognostic role of obesity has been confirmed; however, potential racial differences may limit the generalizability of these findings across clinical settings and remain insufficiently explained. Consequently, these results require validation and refinement through well-designed, international studies. Future research should focus on disentangling obesity phenotypes, such as sarcopenic obesity, refining outcome predictors, and integrating genomic and metabolic data to enhance understanding of individual risk profiles.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Author contributions
QW: Conceptualization, Data curation, Funding acquisition, Writing – original draft. ZL: Investigation, Writing – review & editing. XW: Software, Writing – review & editing. BL: Formal Analysis, Writing – review & editing. CW: Methodology, Writing – review & editing. YX: Resources, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This study is funded by the general project of Sichuan administration of Traditional Chinese Medicine (2024MS606), the subject of Guangyuan administration of Traditional Chinese Medicine (24ZYY0009).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2025.1600503/full#supplementary-material
References
1. Jha SK, De Rubis G, Devkota SR, Zhang Y, Adhikari R, Jha LA, et al. Cellular senescence in lung cancer: Molecular mechanisms and therapeutic interventions. Ageing Res Rev. (2024) 97:102315. doi: 10.1016/j.arr.2024.102315
2. Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2024) 74:229–63. doi: 10.3322/caac.21834
3. Yu KR and Julliard WA. Sublobar resection of non-small-cell lung cancer: wedge resection vs. segmentectomy. Curr Oncol. (2024) 31:2497–507. doi: 10.3390/curroncol31050187
4. Martucci N, Morabito A, La Rocca A, De Luca G, De Cecio R, Botti G, et al. Surgery in small-cell lung cancer. Cancers (Basel). (2021) 13:390. doi: 10.3390/cancers13030390
5. Motono N, Mizoguchi T, Ishikawa M, Iwai S, Iijima Y, and Uramoto H. Analysis of risk factors of postoperative complication for non-small cell lung cancer. BMC Pulm Med. (2024) 24:333. doi: 10.1186/s12890-024-03054-1
6. Slawinski CGV, Barriuso J, Guo H, and Renehan AG. Obesity and cancer treatment outcomes: interpreting the complex evidence. Clin Oncol (R Coll Radiol). (2020) 32:591–608. doi: 10.1016/j.clon.2020.05.004
7. Tsekrekos A, Lovece A, Chrysikos D, Ndegwa N, Schizas D, Kumagai K, et al. Impact of obesity on the outcomes after gastrectomy for gastric cancer: A meta-analysis. Asian J Surg. (2022) 45:15–26. doi: 10.1016/j.asjsur.2021.04.033
8. Hruby A and Hu FB. The epidemiology of obesity: A big picture. Pharmacoeconomics. (2015) 33:673–89. doi: 10.1007/s40273-014-0243-x
9. Georgakopoulou VE, Lempesis IG, Trakas N, Sklapani P, He Y, and Spandidos DA. Lung cancer and obesity: A contentious relationship (Review). Oncol Rep. (2024) 52:158. doi: 10.3892/or.2024.8817
10. Kessler C. Pathophysiology of obesity. Nurs Clin North Am. (2021) 56:465–78. doi: 10.1016/j.cnur.2021.08.001
11. Petrella F, Radice D, Borri A, Galetta D, Gasparri R, Solli P, et al. The impact of preoperative body mass index on respiratory complications after pneumonectomy for non-small-cell lung cancer. Results from a series of 154 consecutive standard pneumonectomies. Eur J Cardiothorac Surg. (2011) 39:738–44. doi: 10.1016/j.ejcts.2010.09.007
12. Tulinský L, Sengul I, Ihnát P, Ostruszka P, Toman D, Guňková P, et al. Obesity in cases undergoing the surgical procedure of lung lobectomy: risk or benefit? Rev Assoc Med Bras (1992). (2022) 68:1090–5. doi: 10.1590/1806-9282.20220526
13. Li S, Wang Z, Huang J, Fan J, Du H, Liu L, et al. Systematic review of prognostic roles of body mass index for patients undergoing lung cancer surgery: does the ‘obesity paradox’ really exist? Eur J Cardiothorac Surg. (2017) 51:817–28. doi: 10.1093/ejcts/ezw386
14. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
15. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. (2010) 25:603–5. doi: 10.1007/s10654-010-9491-z
16. Slagter JS, Outmani L, Tran KTCK, Ijzermans JNM, and Minnee RC. Robot-assisted kidney transplantation as a minimally invasive approach for kidney transplant recipients: A systematic review and meta-analyses. Int J Surg. (2022) 99:106264. doi: 10.1016/j.ijsu.2022.106264
17. Chen K, Pan Y, Zhang B, Maher H, and Cai XJ. Laparoscopic versus open pancreatectomy for pancreatic ductal adenocarcinoma: A systematic review and meta-analysis. Int J Surg. (2018) 53:243–56. doi: 10.1016/j.ijsu.2017.12.032
18. Engels EA, Schmid CH, Terrin N, Olkin I, and Lau J. Heterogeneity and statistical significance in meta-analysis: an empirical study of 125 meta-analyses. Stat Med. (2000) 19:1707–28. doi: 10.1002/1097-0258(20000715)19:13<1707::aid-sim491>3.0.co;2-p
19. Wan X, Wang W, Liu J, and Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. (2014) 14:135. doi: 10.1186/1471-2288-14-135
20. Luo D, Wan X, Liu J, and Tong T. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat Methods Med Res. (2018) 27:1785–805. doi: 10.1177/0962280216669183
21. Higgins JP, Thompson SG, Deeks JJ, and Altman DG. Measuring inconsistency in meta-analyses. BMJ. (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
22. Egger M, Davey Smith G, Schneider M, and Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. (1997) 315:629–34. doi: 10.1136/bmj.315.7109.629
23. Vadlakonda A, Chervu N, Verma A, Sakowitz S, Bakhtiyar SS, Sanaiha Y, et al. Moving beyond frailty: Obesity paradox persists in lung resection. Surgery. (2023) 174:964–70. doi: 10.1016/j.surg.2023.06.035
24. Smith PW, Wang H, Gazoni LM, Shen KR, Daniel TM, and Jones DR. Obesity does not increase complications after anatomic resection for non-small cell lung cancer. Ann Thorac Surg. (2007) 84:1098–106. doi: 10.1016/j.athoracsur.2007.04.033
25. Montané B, Toosi K, Velez-Cubian FO, Echavarria MF, Thau MR, Patel RA, et al. Effect of obesity on perioperative outcomes after robotic-assisted pulmonary lobectomy. Surg Innov. (2017) 24:122–32. doi: 10.1177/1553350616687435
26. Dhakal B, Eastwood D, Sukumaran S, Hassler G, Tisol W, Gasparri M, et al. Morbidities of lung cancer surgery in obese patients. J Thorac Cardiovasc Surg. (2013) 146:379–84. doi: 10.1016/j.jtcvs.2013.02.083
27. Launer H, Nguyen DV, and Cooke DT. National perioperative outcomes of pulmonary lobectomy for cancer in the obese patient: a propensity score matched analysis. J Thorac Cardiovasc Surg. (2013) 145:1312–8. doi: 10.1016/j.jtcvs.2012.10.012
28. Mungo B, Zogg CK, Hooker CM, Yang SC, Battafarano RJ, Brock MV, et al. Does obesity affect the outcomes of pulmonary resections for lung cancer? A National Surgical Quality Improvement Program analysis. Surgery. (2015) 157:792–800. doi: 10.1016/j.surg.2014.10.016
29. Guerrera F, Lyberis P, Lausi PO, Cristofori RC, Giobbe R, Molinatti M, et al. Does morbid obesity influence perioperative outcomes after video-assisted thoracic surgery (VATS) lobectomy for non-small cell lung cancer? Analysis of the Italian VATS group registry. Surg Endosc. (2022) 36:3567–73. doi: 10.1007/s00464-021-08680-y
30. Leonardi B, Forte S, Natale G, Messina G, Rainone A, Opromolla G, et al. One-lung ventilation in obese patients undergoing thoracoscopic lobectomy for lung cancer. Thorac Cancer. (2023) 14:281–8. doi: 10.1111/1759-7714.14747
31. Zirafa CC, Romano G, Sicolo E, Cariello C, Morganti R, Conoscenti L, et al. Robotic surgery for non-small cell lung cancer treatment in high-risk patients. J Clin Med. (2021) 10:4408. doi: 10.3390/jcm10194408
32. Tong C, Li T, Shen Y, Zhu H, Zheng J, and Wu J. Obesity does not increase perioperative outcomes in older patients undergoing thoracoscopic anatomic lung cancer surgery. Front Oncol. (2022) 12:881467. doi: 10.3389/fonc.2022.881467
33. Li R, Ma Z, Li Y, Qu C, Qiu J, Zhang Y, et al. Robotic-assisted thoracoscopic surgery improves perioperative outcomes in overweight and obese patients with non-small-cell lung cancer undergoing lobectomy: A propensity score matching analysis. Thorac Cancer. (2022) 13:2606–15. doi: 10.1111/1759-7714.14597
34. Nakagawa T, Toyazaki T, Chiba N, Ueda Y, and Gotoh M. Prognostic value of body mass index and change in body weight in postoperative outcomes of lung cancer surgery. Interact Cardiovasc Thorac Surg. (2016) 23:560–6. doi: 10.1093/icvts/ivw175
35. Matsunaga T, Suzuki K, Imashimizu K, Banno T, Takamochi K, and Oh S. Body mass index as a prognostic factor in resected lung cancer: obesity or underweight, which is the risk factor? Thorac Cardiovasc Surg. (2015) 63:551–7. doi: 10.1055/s-0035-1554964
36. Tulinský L, Mitták M, Tomášková H, Ostruszka P, Penka I, and Ihnát P. Obesity paradox in patients undergoing lung lobectomy - myth or reality? BMC Surg. (2018) 18:61. doi: 10.1186/s12893-018-0395-2
37. Attaran S, McShane J, Whittle I, Poullis M, and Shackcloth M. A propensity-matched comparison of survival after lung resection in patients with a high versus low body mass index. Eur J Cardiothorac Surg. (2012) 42:653–8. doi: 10.1093/ejcts/ezs135
38. Thomas PA, Berbis J, Falcoz PE, Le Pimpec-Barthes F, Bernard A, Jougon J, et al. National perioperative outcomes of pulmonary lobectomy for cancer: the influence of nutritional status. Eur J Cardiothorac Surg. (2014) 45:652–9. doi: 10.1093/ejcts/ezt452
39. Fukumoto K, Mori S, Shintani Y, Okami J, Ito H, Ohtsuka T, et al. Impact of the preoperative body mass index on the postoperative outcomes in patients with completely resected non-small cell lung cancer: A retrospective analysis of 16,503 cases in a Japanese Lung Cancer Registry Study. Lung Cancer. (2020) 149:120–9. doi: 10.1016/j.lungcan.2020.09.011
40. Ahmed-Issap A, Jain S, Habib A, Mantio K, Spence A, Raseta M, et al. Impact of extremes of BMI on outcomes following lung resection. Thorac Cardiovasc Surg. (2024) 72:379–86. doi: 10.1055/a-2072-9869
41. Parida S, Siddharth S, and Sharma D. Role of omentin in obesity paradox in lung cancer. Cancers (Basel). (2021) 13:275. doi: 10.3390/cancers13020275
42. Gómez-Hernández MT, Fuentes MG, Novoa NM, Rodríguez I, Varela G, and Jiménez MF. Obesity paradox” has not an impact on minimally invasive anatomical lung resection. Cir Esp (Engl Ed). (2022) 100:288–94. doi: 10.1016/j.ciresp.2021.01.018
43. He J, Liu H, Deng L, Wei X, Chen T, Xia S, et al. Influence of obesity on in-hospital and postoperative outcomes of hepatic resection for Malignancy: a 10-year retrospective analysis from the US National Inpatient Sample. BMJ Open. (2019) 9:e029823. doi: 10.1136/bmjopen-2019-029823
44. Logie K, McKechnie T, Talwar G, Lee Y, Parpia S, Amin N, et al. The impact of operative approach for obese colorectal cancer patients: analysis of the National Inpatient Sample 2015-2019. Colorectal Dis. (2024) 26:34–44. doi: 10.1111/codi.16808
45. Zhang N, Feng D, Wu W, Liu J, and Shi H. Influence of higher body mass index on postoperative nausea and vomiting in patients following thoracic surgery for lung cancer: a propensity score-matched cohort study. Sci Rep. (2024) 14:13873. doi: 10.1038/s41598-024-64686-1
46. Liou DZ and Berry MF. Thoracic surgery considerations in obese patients. Thorac Surg Clin. (2018) 28:27–41. doi: 10.1016/j.thorsurg.2017.09.004
47. St Julien JB, Aldrich MC, Sheng S, Deppen SA, Burfeind WR Jr., Putnam JB, et al. Obesity increases operating room time for lobectomy in the society of thoracic surgeons database. Ann Thorac Surg. (2012) 94:1841–7. doi: 10.1016/j.athoracsur.2012.08.006
Keywords: obesity, lung cancer, perioperative outcomes, surgery, meta-analysis
Citation: Wang Q, Li Z, Wang XH, Li B, Wang C and Xiang Y (2025) Effect of obesity on perioperative outcomes following lung cancer surgery: a systematic review and meta-analysis. Front. Oncol. 15:1600503. doi: 10.3389/fonc.2025.1600503
Received: 26 March 2025; Accepted: 08 September 2025;
Published: 25 September 2025.
Edited by:
Denis Fedorinov, Russian Medical Academy of Postgraduate Education, RussiaReviewed by:
Sevindzh Evdokimova, P. Herzen Moscow Oncology Research Institute (MORI), RussiaFidele Kakule Kitaghenda, The Affiliated Hospital of Xuzhou Medical University, China
Anastasia Rays, Russian Cancer Research Center NN Blokhin, Russia
Copyright © 2025 Wang, Li, Wang, Li, Wang and Xiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qiuxiang Wang, OTQzODgyMjkxQHFxLmNvbQ==; Yongguo Xiang, MjUzMzkwNzAwM0BxcS5jb20=
 Qiuxiang Wang
Qiuxiang Wang Zhishu Li
Zhishu Li Xi Huan Wang
Xi Huan Wang Bin Li
Bin Li Chunfeng Wang
Chunfeng Wang Yongguo Xiang
Yongguo Xiang