Abstract
Introduction:
The treatment of B-cell acute lymphoblastic leukemia (ALL) in India involves multi-agent chemotherapy and central nervous system (CNS) prophylaxis, but relapsed/refractory (R/R) B-cell ALL presents with high mortality and limited salvage options. Inotuzumab ozogamicin (InO), a monoclonal antibody targeting cluster of differentiation-22 (CD22) conjugated to calicheamicin, has improved outcomes for R/R B-cell ALL. A phase 3 multicenter trial (INO-VATE) in adult patients with CD22+ B-cell ALL has shown that InO significantly increases response rates and overall survival (OS) compared to standard treatments, with ongoing studies assessing its real-world effectiveness in India.
Methodology:
A multicentric, retrospective, observational study was conducted across five oncology centers in India to evaluate the effectiveness of InO in adult patients with R/R B-cell ALL. The study aimed to assess clinical outcomes, including the rate of complete remission (CR)/CR with incomplete hematologic recovery (CRi), minimal residual disease (MRD) negativity, duration of remission (DOR), OS, and the safety and tolerability of InO in real-world settings.
Results:
The medical records of adult patients (n = 32) aged >18 years with R/R B-cell ALL treated with InO between February 2017 and October 2022 were assessed. Among the total study participants, 59.4% (n = 19) achieved CR/CRi. Of these responders, MRD negativity was achieved in 94.7% (n = 18/19), and 68.4% (n = 13/19) achieved deep responses (MRD negative CR/CRi) after a median of two cycles of InO. The median DOR for those achieving CR/CRi was 6 months. Of the entire cohort, 34.4% (11/32) of the participants proceeded to hematopoietic stem cell transplantation (HSCT). The OS rates at 6 and 12 months in the entire cohort were 46.9% and 28.1%, respectively. The median relapse-free survival (RFS) among the responders was 7 months. Grade 3/4 treatment-related liver toxicity following InO initiation was reported in 37.5% of the participants. Myelosuppression-related adverse events were observed in 87.5% of the recipients.
Conclusion:
The real-world study (RWS) highlights the effectiveness of InO in achieving remission and MRD negativity in R/R B-cell ALL, although treatment-related toxicities remain a concern.
1 Introduction
Adult acute lymphoblastic leukemia (ALL) is the second mostxcommon leukemia in adults (1), with a prevalence of 20% of all cases of ALL (2). The treatment of adult ALL comprises multiple chemotherapy agents and allogeneic hematopoietic stem cell transplantation (HSCT) (3). Although the initial response rates with the conventional chemotherapeutic agents remain approximately 60% to 90%, only 30% to 50% maintain long-term disease-free survival (2).
After intensive multi-agent cytotoxic chemotherapy, the lack of minimal residual disease (MRD) in the marrow is the most significant and predictive indicator of a better clinical result in B-cell ALL patients (4). Cluster of differentiation-22 (CD22) is expressed in ≥90% of leukemic lymphoblasts and >90% of patients with B-cell ALL (3). Therefore, the use of an anti-CD22 antibody to treat B-cell malignancies was introduced (3).
Inotuzumab ozogamicin (InO) is an anti-CD22 antibody conjugated to calicheamicin and has been approved for use in relapsed/refractory (R/R) B-cell ALL based on the results of the INO-VATE trial (3, 4). The rate of complete remission (CR)/CR with incomplete hematologic recovery (CRi) was 73.8% [95% confidence interval (CI), 72%–88%] among patients who received InO monotherapy versus 29.4% (95% CI, 21%–39%) in the standard-of-care (SOC) chemotherapy arm (SC group; p < 0.001). Higher MRD negativity status, progression-free survival (PFS), and 2-year overall survival (OS) were also achieved in patients treated with InO (3). Additionally, a greater number of patients proceeded to HSCT with InO therapy.
In the Indian setting, the management of relapsed/refractory B-cell ALL is often complicated by delayed diagnosis, financial constraints, limited access to novel agents, and delays in drug procurement, particularly in public or resource-limited healthcare systems. These factors can lead to delayed initiation of salvage therapies, resulting in patients often presenting with higher disease burden and having received multiple prior lines of treatment by the time targeted therapies such as InO are introduced (2).
InO treatment for patients with R/R B-cell ALL was introduced in India in 2017 as a part of an early access program but was made commercially available in 2020. A single study of eight patients diagnosed with R/R B-cell ALL who had failed at least two prior lines of therapy (including the persistence of MRD), having received InO under a compassionate use program in India, concluded that InO-based salvage therapy improves the remission status in these patients (2). The real-world data on the use of InO in cases of adult R/R B-cell ALL in India are limited.
2 Objective
A multicentric, retrospective cohort analysis aimed to descriptively evaluate the clinical characteristics of adults diagnosed with CD22+ R/R B-cell ALL, treated with InO, and their associated clinical outcomes, in real-world practice in India.
3 Methods
This multicenter, retrospective study included adults (≥18 years) with R/R B-cell ALL treated with InO monotherapy from five oncology centers in India with confirmed CD22 positivity. Ph+ B-cell ALL patients were included only if they had failed ≥1 tyrosine kinase inhibitor (TKI) in addition to chemotherapy. The study cohort included patients who had been treated with the drug available through the early access program. The patient’s medical records were reviewed and collected by the participating oncologist and the research coordinators. The data were de-identified and sent to the study team for review and analysis. The data were collected until at least 6 months of follow-up were completed, extending to the latest available follow-up records. The primary endpoint was CR or CRi following treatment with InO on the basis of the number of salvage therapies (1 and ≥2) received before the initiation of InO. CR was defined as ≤5% blasts in the bone marrow, no evidence of extramedullary disease (EMD), and full recovery of peripheral blood counts (platelets > 100 × 109/L and absolute neutrophil count > 1 × 109/L). CRi was defined as patients who attained CR but had incomplete recovery of peripheral blood counts (platelets < 100 × 109/L or absolute neutrophil count < 1 × 109/L). The key secondary endpoints were MRD negativity rates, probability of OS and relapse-free survival (RFS) at 6 and 12 months, and analysis of grade 3/4 treatment-emergent adverse events (TEAEs) [including veno-occlusive disease (VOD)].
3.1 Statistical analyses
Mean, median, and standard deviations were provided for continuous variables while performing descriptive analysis of continuous data. Numbers and percentages were provided for dichotomous and polychotomous variables while performing descriptive analysis of categorical data. The proportion of CR/CRi achieved was observed across the number of salvage therapies before InO initiation, with respective 95% CI. Survival outcomes were analyzed using the Kaplan–Meier statistics. No statistical significance testing was performed due to the limited sample size (N = 32) and the retrospective, non-comparative design of the study. Given the heterogeneity in baseline characteristics and treatment history, formal hypothesis testing was considered inappropriate and unlikely to yield statistically meaningful conclusions. The analysis was therefore focused on descriptive evaluation to reflect real-world treatment patterns and outcomes, which may help guide future prospective studies with larger cohorts.
3.2 Ethics committee approval
Approval for this study was obtained from the respective institutional ethics committee (IEC)/institutional review board (IRB) before commencement of the study. The study was conducted in accordance with the approved study protocol and international ethical standards, including the Declaration of Helsinki and Indian regulatory requirements.
4 Results
The data of 32 patients (median age 37.41 ± 15.16 years) treated with InO were included in the final analyses. The patient disposition and follow-up are depicted in Figure 1, while the patient characteristics are detailed in Table 1.
Figure 1
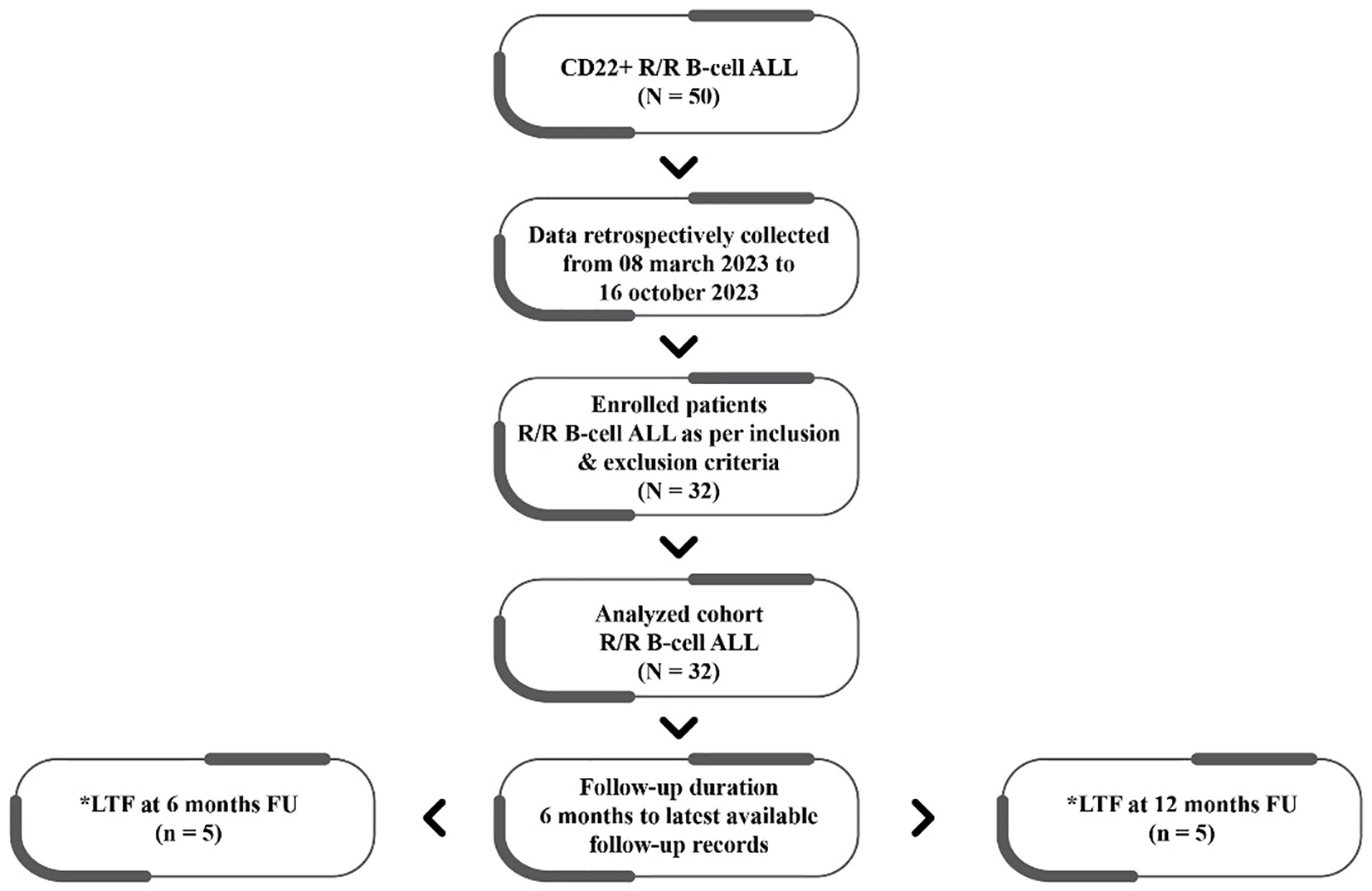
Patient disposition and follow-up. CD, cluster of differentiation-22; R/R, relapsed/refractory; ALL, acute lymphoblastic leukemia; LTF, lost to follow-up; FU, followed up.
Table 1
| Variable | Number of patients, N (%) | |
|---|---|---|
| Participants enrolled | – | 32 |
| Gender | Female | 11 (34.4) |
| Male | 21 (65.6) | |
| Age | 18–65 years | 30 |
| >65 years | 2 | |
| InO therapy received as salvage therapy | 1 | 10 (31.3) |
| 2 | 18 (56.3) | |
| 3 | 4 (12.5) | |
| Number of total InO cycles | 1 | 15 (46.9) |
| 2 | 8 (25) | |
| 3 | 5 (15.6) | |
| 4 | 2 (6.3) | |
| 6 | 2 (6.3) | |
| InO dosage for cycle 1 | 1.8 mg·m−2·cycle−1 | 12 (37.5) |
| <1.8 mg·m−2·cycle−1 | 17 (53.1) | |
| >1.8 mg·m−2·cycle−1 | 3 (9.4) | |
| Blast percentage in bone marrow at baseline | <50% | 16 (50) |
| >50% | 16 (50) | |
Patient characteristics.
InO, inotuzumab ozogamicin.
4.1 Patient characteristics
Prior to the initiation of InO, 28 patients had relapsed, while four patients were refractory to previous chemotherapy regimens. An equal number of patients (n = 16) had high- and low-burden diseases at the time of InO administration. A higher proportion of patients (56.4%; n = 18) had received InO as their second salvage and beyond. Approximately 37.5% (n = 12) had received InO in cycle 1 as per the recommended fractionated dose schedule of 0.8 mg/m2 on day 1, 0.5 mg/m2 on day 8, and 15mg/m2 in a 21–28-day cycle (dose = 1.8 mg·m−2·cycle−1).
4.2 Primary endpoints
4.2.1 Response to InO therapy
Among the total study participants (n = 32), CR or CRi was achieved in 59.4% (19/32; CR = 18, CRi = 1). Most responses occurred after a median of two InO cycles (Table 2). The CR/CRi rate was higher in patients receiving InO as the first (60%) or second (61%) salvage therapy compared to later lines (50%) (Table 3). Remission was not achieved in 37.5% (n = 12/32), and one patient was lost to follow-up.
Table 2
| Number of patients | Median | Range |
|---|---|---|
| 19 | 2 | 1 to 6 |
Median number of cycles of InO needed to attain CR/CRi.
InO, inotuzumab ozogamicin; CR/CRi, complete remission with incomplete hematologic recovery.
Table 3
| Salvage | Number of patients (N = 32) | CR/CRi (n [%]) |
|---|---|---|
| First salvage | 10 | 6 (60) |
| Second salvage | 18 | 11 (61) |
| Third salvage | 4 | 2 (50) |
Number of patients achieving CR/CRi as per the salvage therapy with InO.
InO, inotuzumab ozogamicin; CR/CRi, complete remission with incomplete hematologic recovery.
CR/CRi rates were comparable among the patients with <50% (62.5%) and >50% (56.25%) blasts in baseline bone marrow studies [bone marrow blast (BMB)] (Table 4), indicating that the rate of response was not influenced by the disease burden.
Table 4
| Disease burden at baseline | Number of patients (N [%]) N = 32 |
CR/CRi (N [%]) |
|---|---|---|
| $BMB < 50% | 16 (50) | 10 (62.5) |
| $BMB > 50% | 16 (50) | 9 (56.25) |
Number of patients achieving CR/CRi following treatment with InO classified as per high-burden and low-burden disease.
$BMB, bone marrow blast; InO, inotuzumab ozogamicin; CR/CRi, complete remission with incomplete hematologic recovery.
4.3 Secondary endpoints
4.3.1 MRD negativity
Among 19 patients (59.4%) who achieved CR or CRi, 94.7% (18/19) attained MRD negativity following InO therapy (Table 5).
Table 5
| Number of patients (N = 19) |
Achieved CR N (%) | Achieved CRi N (%) | Patients attaining CR + CRi |
|---|---|---|---|
| 18 | 1 | 19 | |
| Attained MRD negativity | 17 (94.4) | 1 (100%) | 18 (94.8) |
| Not attained MRD negativity | 1 (5.6) | 0 | 1 (5.2) |
Number of patients achieving MRD negativity following initiation of InO among those who had CR/CRi.
MRD, minimal residual disease; InO, inotuzumab ozogamicin; CR/CRi, complete remission with incomplete hematologic recovery.
4.3.2 HSCT and rate of transplantation
Among the responders (CR/CRi), 47.4% (9/19) proceeded to HSCT, and 66.7% (6/9) underwent HSCT after a single cycle of InO.
4.3.3 The median DOR
Based on available data from 17 study participants, the median duration of remission (DOR) was 6 months in the patients who attained CR/CRi.
4.3.4 Overall survival
Overall survival (OS) at 6 and 12 months was 46.9% and 28.1%, respectively. The median OS was 6 months (95% CI: 4.5–7.5) depicted in Figure 2. In patients aged >65 years (n = 2), the 6- and 12-month OS rates were both 50%.
Figure 2
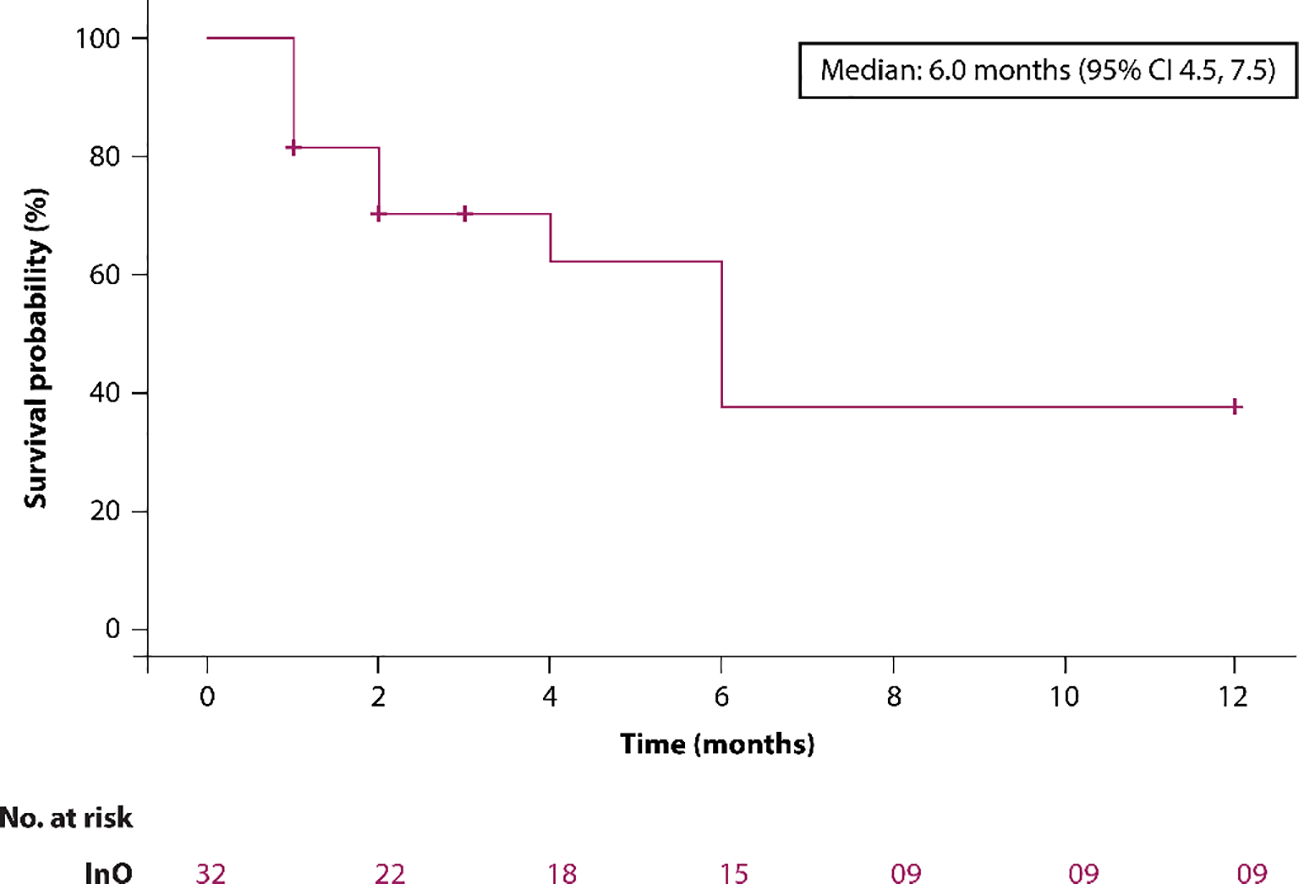
The Kaplan–Meier survival curve.
4.3.5 RFS
The median RFS in patients who achieved CR/CRi was 7.0 months (95% CI: 6.1–8.9 months).
4.3.6 EMD
Of the 32 patients in the cohort, 6.25% (n = 2/32) had EMD at baseline. One of them achieved CR/CRi with InO and had subsequently undergone HSCT. Another patient did not achieve remission.
4.3.7 Safety of InO
In the overall cohort, VOD was observed in 22% of patients (n = 7/32) receiving InO, and 71.4% of these patients (n = 5) developed VOD after HSCT (Table 6). Grade 3/4 hepatotoxicity occurred in 37.5% of patients, while hematologic toxicities were the most frequent adverse events, affecting 87.5% of the cohort (Table 7).
Table 6
| Patient status | Number of patients having undergone HSCT, n (%) | Number of patients not having undergone HSCT, n (%) |
|---|---|---|
| VOD | 5 (45.5) | 2 (10) |
| Grade 3/4 liver toxicities | 4 (36.4) | 8 (40) |
| Hematological toxicities | 9 (81.8) | 19 (95) |
Proportion of patients with adverse events having undergone HSCT and not undergone HSCT.
HSCT, Hematopoietic stem cell transplantation; VOD, veno-occlusive disease.
Table 7
| Patient status | Number of patients | N (%) |
|---|---|---|
| InO as first salvage therapy | 10 | 2 (20) |
| InO as second salvage therapy | 18 | 3 (16.8) |
| InO as third salvage therapy | 4 | 2 (50) |
Proportion of patients with VOD by number of salvage therapies.
InO, inotuzumab ozogamicin; VOD, veno-occlusive disease.
4.3.8 Cause of death
There were 17 deaths in the cohort (53.1%). Disease progression (60.8%) was the major cause of mortality. Septic shock was responsible for 11.8% of deaths. VOD-related complications, including multi-organ failure and VOD-associated pneumonia, each accounted for 5.9% of deaths, both occurring in patients with a history of HSCT. Central nervous system (CNS) relapse contributed to 5.9% of fatalities, while the cause of death was not documented in 11.8% of cases (Table 8).
Table 8
| Hematological toxicities (87.5% [n = 28/32]) | |
|---|---|
| Thrombocytopenia | 27 (84.8) |
| Neutropenia | 20 (62.5) |
| Febrile neutropenia | 14 (43.7) |
| Grade 3/4 treatment-related liver toxicities (37.5% [n = 12/32]) | |
| Increased aspartate aminotransferase | 9 (28.1) |
| Increased alanine aminotransferase | 6 (18.8) |
| Increased bilirubin | 8 (25) |
| Cause of death (53.1% [n = 17/32]) | |
| Progression of disease | 4 (23.5) |
| Relapse | 6 (35.3) |
| Septic shock | 2 (11.8) |
| VOD-related multi-organ failure | 1 (5.9) |
| VOD pneumonia | 1 (5.9) |
| CNS relapse | 1 (5.9) |
| Not mentioned | 2 (11.8) |
Adverse events and cause of death.
VOD, veno-occlusive disease; CNS, central nervous system.
5 Discussion
InO was effective in adults with R/R B-cell ALL, with 59.4% of the patients achieving CR/CRi, and most of these patients achieved MRD negativity (94.7%). Overall, CR/CRi was achieved after a median of two cycles of InO. The global, phase 3 INO-VATE ALL study also concluded that InO achieved higher response rate (CR/CRi [73.8% {95% CI, 72%–88%} versus 29.4% {21%–39%}; p < 0.001]) and MRD-negativity rates (78.4% [68%–87%] versus 28.1% [14%–47%]; p < 0.001) compared to standard chemotherapeutic regimens in patients with R/R B-cell ALL (3). Interestingly, the CR/CRi rate reduced to 66.7% among those receiving InO as salvage 2 in INO-VATE ALL (3). Table 9 summarizes the results of various InO trials.
Table 9
| Study | Phase | Cohort (n) | Dosing | CD22 positivity | CR/CRi | Median OS (months) |
Duration of remission (months) |
|---|---|---|---|---|---|---|---|
| Kantarjian et al., 2012 (12) | II | Adults and children with R/R ALL (49) | 1.3–1.8 mg/m2 q3–4 weeks | >50% | CR 9/49 (18%) CRi 4/49 (8%) |
5.1 (responders) | — |
| Kantarjian et al., 2013 (11) | II | R/R ALL (90) | 1.3–1.8 mg/m2 q3–4 weeks or 0.8 mg/m2 (day 1), then 0.5 mg/m2 (days 8 and 15) q3–4 weeks | >50% | CR 17/90 (19%) CRi 8/90 (19% |
6.2 | 7 |
| Advani et al., 2014 (8) | II | R/R ALL (35) | 0.8 mg/m2 (day 1), then 0.5 mg/m2 (days 8 and 15 | 99% | CR 11/35 (31%) CRi 12/35 (34%) |
7.4 | — |
| Kantarjian et al., 2016 (13) | III | R/R ALL (326) | 0.8 mg/m2 (day 1), then 0.5 mg/m2 (days 8 and 15) q3–4 weeks | 74/109 (68%) >90% CD22 positive |
CR 39/109 (36%) CRi 19/109 (17%) |
7.7 | 4.6 |
| Torrent et al., 2023 (7) | RWS | R/R ALL (34) | CR/CRi 64% |
4 | 3.5 | ||
| Present retrospective | RWS | R/R ALL (32) | 0.8 mg/m2 (day 1), then 0.5 mg/m2 (days 8 and 15) q3–4 weeks | 100% | CR/CRi 59.4% |
— | 6 |
Clinical trials with InO.
InO, inotuzumab ozogamicin; RWS, real-world study; CR/CRi, complete remission with incomplete hematologic recovery; R/R, relapsed/refractory; ALL, acute lymphoblastic leukemia; CD, cluster of differentiation-22; OS, overall survival.
In the present retrospective real-world study (RWS), the remission rate may have been slightly lower compared to that of the phase 3 study due to multiple reasons. The sample size was smaller compared to that of INO-VATE ALL. Therapy with InO was started later in the course of the disease due to logistic constraints, resulting in a more heavily pre-treated cohort of patients. Previous studies have reported a lower response rate with InO when it is started late in 2+ treatment lines. Similarly, in the present RWS, a greater number of patients achieved CR/CRi with InO as first- and second-line salvage treatments as compared to later lines (5–7).
A retrospective study with InO concluded remission in all seven evaluable patients, and six (85.7%) of them also achieved MRD-negative status (2). A phase II study evaluated the safety and efficacy of InO as a standard dose of 1.8 mg·m−2·cycle−1 (0.8 mg/m2 on day 1 and 0.5 mg/m2 on days 8 and 15) in patients with CD22+ R/R B-cell ALL in the second and later salvage settings. The remission rate (CR/CRi) was 65.7%, while 78% (n = 18/23) of patients with CR/CRi achieved MRD negativity (8). In other studies, the CR/CRi was 63%, 53%, and 64% in patients diagnosed with R/R B-cell ALL and treated with InO in the second or later salvage setting (7, 9, 10). The remission rates of the present retrospective RWS are comparable to those of these studies.
The OS rate was 46.9% with a median DOR of 6 months for those achieving CR/CRi in the present retrospective RWS. In the INO-VATE study, the DOR was 5.4 months (11). The RFS period was 7 months in the present retrospective RWS, while PFS was 5.4 months in the INO-VATE study among those achieving CR/CRi (11). A retrospective RWS reported a median DOR of 11.5 months [95% CI, 11.5 to not reached (NR)]. The median OS after InO initiation was 11.6 months (95% CI, 7–15.4). When censored at the time of allo-HSCT, it was 13.6 months (95% CI, 5.5 to NR) (9).
The safety profile of InO was consistent with that of previous studies (2, 11). The VOD was a common non-hematologic toxicity. Higher rates of VOD were seen in the overall cohort compared to the INO-VATE study (21.87% vs. 11%). Higher rates of VOD were observed after HSCT. The use of myeloablative conditioning regimens coupled with a higher percentage of haploidentical HSCTs using the post-transplant cyclophosphamide backbone could have accounted for this. The incidence of non-VOD hepatic derangement was also higher in the current RWS. There is a high prevalence of steatohepatitis in India, which could have predisposed these patients to higher rates of hepatotoxicity with InO.
6 Limitations
This retrospective, multicentric study has inherent limitations of a RWS, including inconsistency in reporting and follow-up, resulting in missing data, particularly on MRD timing and toxicity. The non-randomized design involves potential selection bias, and the limited sample size restricts subgroup analysis and definite findings on comparative safety or efficacy. Despite these limitations, the study provides valuable real-world evidence on InO use in Indian clinical practice and emphasizes the importance of bigger, prospective trials.
7 Conclusion
In this real-world analysis, InO monotherapy was an effective and relatively safe agent for the management of adults with CD22+ R/R B-cell ALL. Larger prospective studies are needed to better characterize long-term outcomes of the patients treated with InO-based therapy.
Statements
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Rajiv Gandhi Cancer Institute & Research Centre, Basavatarakam Indo American Cancer Hospital & Research Institute,Tata Medical Center,Institutional Review Board,Malabar Cancer Center, Institutional Ethics Committee, Fortis Memorial Research Institute. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
NA: Investigation, Supervision, Writing – review & editing, Writing – original draft, Conceptualization. PB: Writing – review & editing, Writing – original draft, Investigation. RB: Writing – review & editing, Investigation, Writing – original draft. CN: Writing – review & editing, Investigation, Writing – original draft. AN: Formal Analysis, Writing – original draft, Data curation, Writing – review & editing, Methodology, Investigation, Validation, Software.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. The authors declare that this study received funding from Pfizer India. The funder was not involved in the study design, collection, analysis, and interpretation of data, the writing of this article or the decision to submit it for publication.
Acknowledgments
The authors would like to thank Dr. Antara Chaudhri for contributing to the study design and Dr Shwetal Sanghvi and Ms. Jyoti Sodha from Insignia Communications, Mumbai, for contributing to the data analysis and manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Radhakrishnan VS Agrawal N Bagal B Patel I . Systematic review of the burden and treatment patterns of adult and adolescent acute lymphoblastic leukemia in India: comprehending the challenges in an emerging economy. Clin Lymphoma Myeloma Leuk. (2021) 21:e85–98. doi: 10.1016/j.clml.2020.08.023
2
Radhakrishnan VS Modak K Bhave SJ Kumar J Roychowdhury M Ghosh M et al . Inotuzumab ozogamicin monotherapy as an outpatient salvage treatment in relapsed refractory B-cell acute lymphoblastic leukemia: compassionate access. Ind J Med Paediatr Oncol. (2021) 42:199–203. doi: 10.1055/s-0041-1731860
3
Kantarjian HM Stock W Cassaday RD DeAngelo DJ Jabbour E O’Brien SM et al . Inotuzumab ozogamicin for relapsed/refractory acute lymphoblastic leukemia in the INO-VATE trial: CD22 pharmacodynamics, efficacy, and safety by baseline CD22. Clin Cancer Res. (2021) 27:2742–54. doi: 10.1158/1078-0432.CCR-20-2399
4
Maffini E Saraceni F Lanza F . Treatment of adult patients with relapsed/refractory B-cell philadelphia-negative acute lymphoblastic leukemia. Clin Hematol Int. (2019) 1:85–93. doi: 10.2991/chi.d.190503.002
5
Kantarjian H Thomas D Jorgensen J Kebriaei P Jabbour E Rytting M et al . Results of inotuzumab ozogamicin, a CD22 monoclonal antibody, in refractory and relapsed acute lymphocytic leukemia. Cancer. (2013) 119:2728–36. doi: 10.1002/cncr.28136
6
Uy N Nadeau M Stahl M Zeidan AM . Inotuzumab ozogamicin in the treatment of relapsed/refractory acute B cell lymphoblastic leukemia. J Blood Med. (2018) 9:67–74. doi: 10.2147/JBM.S136575
7
Torrent A Morgades M García-Calduch O de Llano MPQ Montesinos P Navarro I et al . Results of the compassionate program of inotuzumab ozogamicin for adult patients with relapsed or refractory acute lymphoblastic leukemia in Spain. Eur J Haematol. (2023) 111:485–90. doi: 10.1111/ejh.14031
8
Advani AS Stein AS Kantarjian HM Shustov AR DeAngelo DJ Ananthakrishnan R et al . A phase II study of weekly inotuzumab ozogamicin (InO) in adult patients with CD22-positive acute lymphoblastic leukemia (ALL) in second or later salvage. Blood. (2014) 124:2255. doi: 10.1182/blood.v124.21.2255.2255
9
Badar T Szabo A Wadleigh M Liedtke M Arslan S Siebenaller C et al . Real-world outcomes of adult B-cell acute lymphocytic leukemia patients treated with inotuzumab ozogamicin. Clin Lymphoma Myeloma Leuk. (2020) 20:556–560.e2. doi: 10.1016/j.clml.2020.03.004
10
Espinoza M Rojas J López-Vidal H Castañeda AJ Gazmuri JT . Inotuzumab use: A single-center experience in B-cell acute lymphoblastic leukemia. The 65th ASH annual meeting abstracts. Blood> . (2023) 142:5905. doi: 10.1182/blood-2023-178448
11
Kantarjian HM DeAngelo DJ Stelljes M Liedtke M Stock W Gökbuget N et al . Inotuzumab ozogamicin versus standard of care in relapsed or refractory acute lymphoblastic leukemia: Final report and long-term survival follow-up from the randomized, phase 3 INO-VATE study. Cancer. (2019) 125:2474–87. doi: 10.1002/cncr.32116
12
Kantarjian H Thomas D Jorgensen J Jabbour E Kebriaei P Rytting M et al . Inotuzumab ozogamicin, an anti-CD22-calecheamicin conjugate, for refractory and relapsed acute lymphocytic leukaemia: a phase 2 study. Lancet Oncol. (2012) 13:403–11. doi: 10.1016/S1470-2045(11)70386-2
13
Kantarjian HM DeAngelo DJ Stelljes M Martinelli G Liedtke M Stock W et al . Inotuzumab ozogamicin versus standard therapy for acute lymphoblastic leukemia. N Engl J Med. (2016) 375:740–53. doi: 10.1056/NEJMoa1509277
Summary
Keywords
B-cell acute lymphoblastic leukemia, inotuzumab ozogamicin, CD22, antibody-drug conjugate, HSCT
Citation
Agrawal N, Boyella PK, Bhargava R, Nair CK and Nag A (2025) A retrospective analysis of inotuzumab ozogamicin usage in adult patients with relapsed/refractory B-cell acute lymphoblastic leukemia. Front. Oncol. 15:1601782. doi: 10.3389/fonc.2025.1601782
Received
28 March 2025
Accepted
24 July 2025
Published
10 September 2025
Volume
15 - 2025
Edited by
Mario Tiribelli, University of Udine, Italy
Reviewed by
Erica Brivio, Princess Maxima Center for Pediatric Oncology, Netherlands
Yimei Feng, Xinqiao Hospital, China
Updates
Copyright
© 2025 Agrawal, Boyella, Bhargava, Nair and Nag.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Arijit Nag, arijit.nag@tmckolkata.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.