- 1Department of Clinical Pharmacy, Affiliated Hospital of Yangzhou University, Yangzhou University, Yangzhou, China
- 2Obstetrics Department, Affiliated Yantai Yuhuangding Hospital of Qingdao University, Yantai, China
- 3Medical Imaging Department, Affiliated Hospital of Yangzhou University, Yangzhou University, Yangzhou, China
- 4Department of Radiology, Affiliated Yantai Yuhuangding Hospital of Qingdao University, Yantai, China
- 5Department of Radiology, Beijing Tiantan Hospital, Capital Medical University, Beijing, China
Purpose: To evaluate the imaging characteristics of central neurocytoma (CN) with different Ki-67 indices and reveal its biological behavior.
Materials and methods: Sixty-nine cases of intraventricular CN confirmed by histopathology were collected retrospectively. According to Ki-67 indices, they were divided into CNs with high Ki-67 (30 cases) and CNs with low Ki-67 (39 cases). Their clinical and imaging findings were compared and analyzed.
Results: Compared to CNs with low Ki-67, CNs with high Ki-67 were larger (P < 0.001) and more solid (P = 0.019). Restricted diffusion (P = 0.020), CT hyperdensity (P < 0.001), abnormal vessels (P = 0.018), and marked MRI enhancement (P < 0.001) were more common in CNs with high Ki-67.
Conclusion: Different Ki-67 indices could affect the imaging characteristics of CNs. Tumor size, radiologic solidity, abnormal vessels, and marked MRI enhancement suggested CNs with high Ki-67. Combining these imaging characteristics and Ki-67 indices offered meaningful insights into their clinical diagnosis and biological behavior.
1 Introduction
Central neurocytoma (CN) is a rare neuronal and mixed neuronal-glial tumor (WHO grade II), accounting for approximately 0.1% - 0.5% of primary brain tumors (1, 2). It is classically located in the lateral ventricle around the foramen of Monro in young adults aged 20–40 years (3, 4). Most of the clinical symptoms are caused by obstructive hydrocephalus and intracranial hypertension, including dizziness, headache, nausea, vomiting, papilledema, and ataxia (5).
Ki-67 index is a reliable marker for cell growth and tissue differentiation (6). Tumors with a low Ki-67 index exhibit good biological behavior, while an elevated Ki-67 index suggests malignant proliferation (6, 7). Most reports indicated that CNs had limited invasiveness, and total resection is an effective treatment with a good prognosis (3, 4, 8). However, the high Ki-67 index may induce atypical histological features and malignant potential (1, 9, 10). These conditions complicate the understanding of CN’s biological behavior, and postoperative radio chemotherapy is suggested (9–11). Accurate preoperative diagnosis is important for clinical intervention. Although atypical CNs can manifest as heterogeneous extra ventricular lesions with parenchymal invasion and cerebral edema, the relationship between Ki-67 index and intraventricular CN has not been systematically evaluated. We hypothesize that different Ki-67 indices may affect imaging characteristics of intraventricular CNs. Therefore, this study aims to provide new insights into their preoperative diagnosis and biological behavior by comparing CNs with different Ki-67 indices.
2 Materials and methods
2.1 Patients and imaging examinations
Eighty-one patients with CN confirmed by pathology from June 2014 to July 2024 were collected. The pathological diagnosis was reached by histomorphological and immunohistochemical features. Inclusion criteria: definite pathological and immunohistochemical diagnoses, definite Ki-67 index, and complete clinical and imaging data. Exclusion criteria: concomitant with other brain tumors, preoperative chemoradiotherapy, poor imaging quality, postoperative recurrence, and extraventricular neurocytoma. All patients underwent magnetic resonance imaging (MRI) using 3.0 T MRI units (GE Signa or GE Discovery, Milwaukee, WI, USA, slice thickness = 5mm, slice interval = 1.5 mm, matrix = 256 × 256 or 512 × 512), including T1-weighted imaging (T1WI, TR = 1750 ms, TI = 720–760 ms), T2-weighted imaging (T2WI, TR = 4000–5000 ms, TE = 100–120 ms), fluid-attenuated inversion recovery (FLAIR, TR = 8000–8400 ms, TI = 2000–2100 ms, TE = 120 ms), and diffusion-weighted imaging (DWI, b values = 0 and 1000s/mm2, TR = 3000–500 ms, TE = 70–100 ms). After intravenous gadolinium injection (0.2 ml/kg), the contrast-enhanced T1WI was obtained. These patients also underwent unenhanced computed tomography (CT, 16 - or 64 - section; GE Hispeed, Milwaukee, WI, USA, tube voltage = 120 kV, tube current = 100–400 mA, slice thickness = 5 mm, slice interval = 5 mm) before treatment. CT imaging is a fast and low-cost preliminary screening before MRI. Especially, some patients came for treatment with intracranial hypertension, and CT was the preferred emergency imaging method. On time-saving and low-cost grounds, X-ray exposure was deemed ethically and clinically acceptable in this study. According to the inclusion and exclusion criteria, 12 cases were ultimately excluded, including 4 cases of incomplete clinical and imaging data, 3 cases of extraventricular neurocytoma, 3 cases of preoperative chemoradiotherapy, and 2 cases of postoperative recurrence. The remaining 69 cases underwent clinical and imaging analyses.
2.2 Clinical and imaging analyses
Based on the Ki-67 indices, 69 patients were divided into CN with high Ki-67 (Ki-67 index > 3%) and CN with low Ki-67 (Ki-67 index ≤ 3%) (12, 13). Their clinical and imaging data were collected and compared. The clinical and imaging indicators included age, sex, intracranial hypertension (dizziness, headache, nausea, or vomiting), size, apparent diffusion coefficient (ADC) value, CT density, cystic-solidity (solid or multi-cystic), intratumoral hemorrhage, intratumoral calcification, marked MRI enhancement, hydrocephalus, periventricular edema, and abnormal vessels. Hyperintensity on DWI and hypointensity on the ADC map suggested restricted diffusion. Avoiding intratumoral calcification, hemorrhage, cystic changes, and abnormal vessels, the CT density and ADC value were measured at the solid part of the lesion. The region of interest (ROI) was determined by the cross-sectional shape of the lesion. ROIs of 20–100 mm2 were suitable for all cases in this study. Furthermore, the mean value of the three ROIs at different positions was selected to ensure the reliability of the measurement. The enhancement degree of the lesion equal to or greater than that of the choroid plexus was defined as marked MRI enhancement. All imaging analyses were performed independently by two neuroradiologists with ten years of experience without knowing the pathological results. In case of different opinions, a consensus should be reached through negotiation.
2.3 Statistical analysis
Statistical analyses were performed to characterize the clinical and imaging features by IBM SPSS 26.0 (SPSS, Chicago, IL, USA). All data were presented as numbers (percentage) or mean ± SD. According to the different data distribution, an independent t-test (two-tailed) or Mann-Whitney U-test was used for the continuous variables, and a chi-square test or Fisher’s exact test (two-tailed) was used for dichotomous variables. P < 0.05 was defined as statistically significant. The interclass correlation coefficient (ICC) or Kappa value was used for the inter-observer variability assessment. ICC > 0.75 or Kappa ≥ 0.8 suggested a better agreement.
3 Results
The Ki-67 index of all 69 cases did not exceed 10%, 39 cases (56.5%) were no more than 3%, and the others (30 cases, 43.5%) were 4% to 10%. The inter-observer agreement was good in clinical and imaging analyses (ICC = 0.87 or Kappa = 0.90). In this study, all CN lesions were located in the lateral ventricle or extended to the contralateral ventricle or the third ventricle, causing hydrocephalus and intracranial hypertension. Compared with CNs with low Ki-67, CNs with high Ki-67 were larger (60.33 ± 35.17 cm3 vs 15.56 ± 9.79 cm3, P < 0.001) and more solid (53.3% vs 25.6%, P = 0.019). Restricted diffusion (0.85 ± 0.14 ×10–3 mm2/s vs 1.48 ± 0.22 ×10–3 mm2/s, P = 0.020), CT hyperdensity (43.20 ± 5.71 Hu vs 34.46 ± 7.49 Hu, P < 0.001), abnormal vessels (33.3% vs 10.3%, P = 0.018) and marked MRI enhancement (66.7% vs 17.9%%, P < 0.001) were more common in CNs with high Ki-67. There were no differences in age, sex, intracranial hypertension, hydrocephalus, intratumoral hemorrhage, intratumoral calcification, and periventricular edema between the two groups. See Table 1, Figures 1, 2 for details.
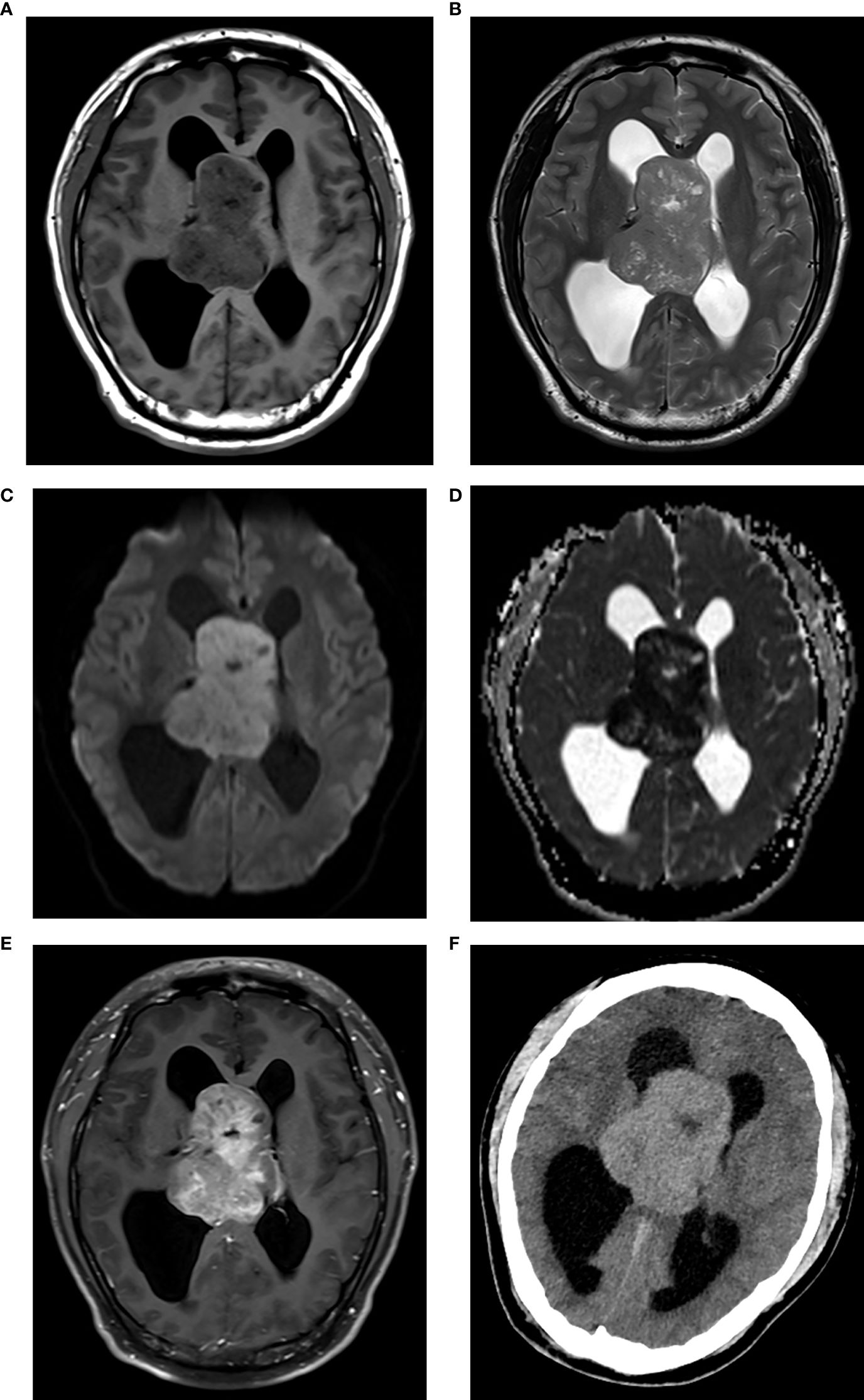
Figure 1. CN with high Ki-67. Axial MRI scans revealed a solid lesion around the septum pellucida, showing isointensity on T1WI (A) isointensity on T2WI (B) hyperintensity on DWI (C) hypointensity on ADC map [(D) the mean ADC = 0.73×10–3 mm2/s], and marked enhancement on contrast-enhanced T1WI (E). The lesion protruded into both lateral ventricles and caused hydrocephalus. The lesion showed hyperdensity on axial CT [(F), CT density = 46 Hu].
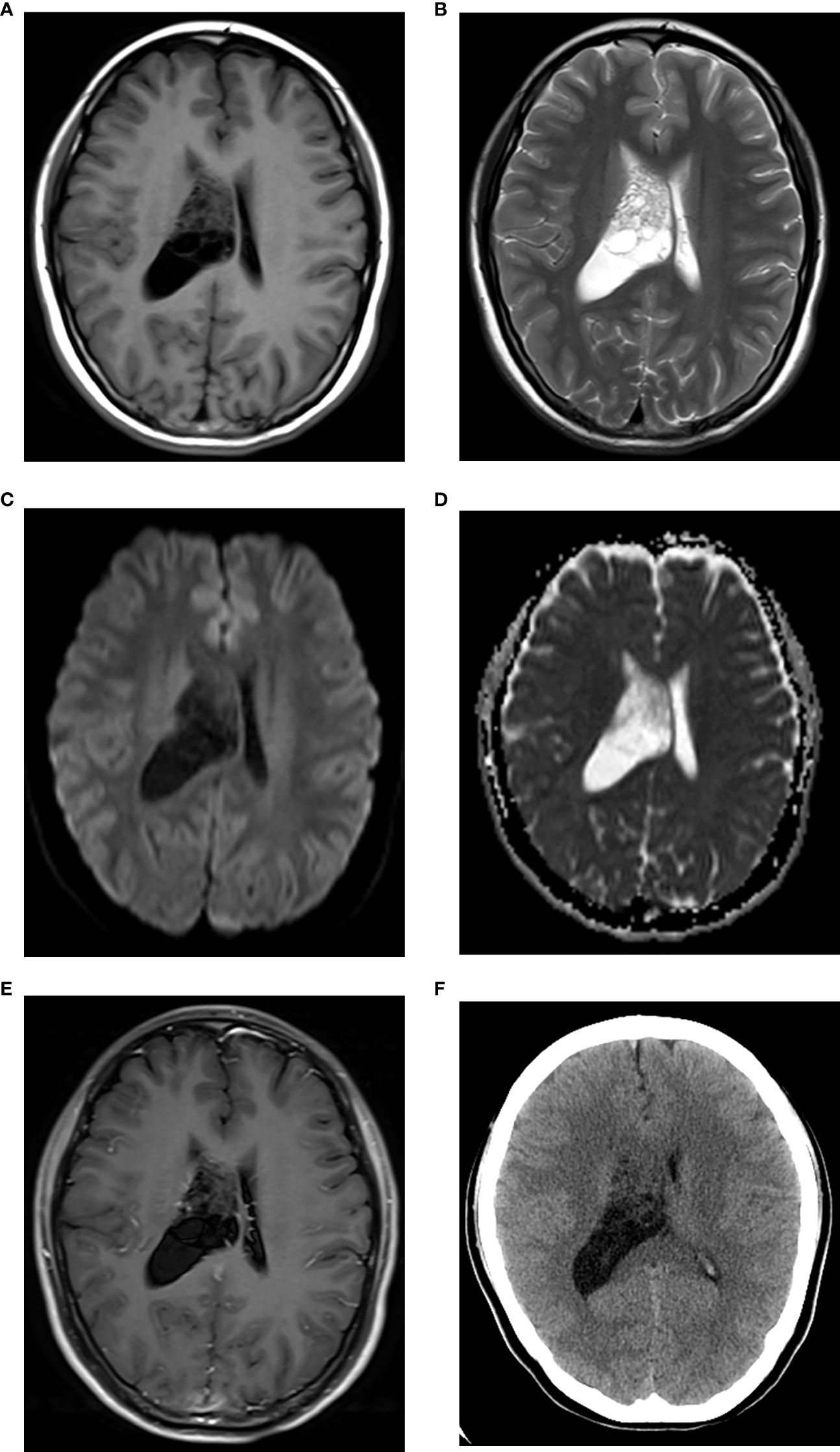
Figure 2. CN with low Ki-67. Axial MRI scans revealed a multi-cystic lesion in the right lateral ventricle, showing iso-hypointensity on T1WI (A) iso-hyperintensity on T2WI (B) iso-hypointensity on DWI (C) iso-hyperintensity on ADC map [(D) the mean ADC = 1.59×10–3 mm2/s], and without marked enhancement on contrast-enhanced T1WI (E). The right lateral ventricle was enlarged. The lesion showed iso-hypodensity on axial CT [(F), CT density = 28 Hu].
4 Discussion
CN originates from bipotential progenitor cells with neuronal and glial differentiation (14, 15). The Ki-67 index is no more than 3% in normal cells and more than 10% in malignant tumors (16, 17). The cut-off value of the Ki-67 index for the atypical CN ranges from 2% to 5% (18, 19). The Ki-67 index > 3% is often used as a threshold for the pathological grading of CN (1, 12, 13). In this study, the Ki-67 indices were 4% to 10% in CNs with high Ki-67. Our results further demonstrated that different Ki-67 indices could affect the imaging characteristics of CN and reveal its biological behavior.
CNs with high Ki-67 indices were often large solid lesions, implying a high tumor burden. The hypercellularity caused by high Ki-67 indices determined the radiologic solidity of CN (20). Limited extracellular space, hypercellularity, and high nucleus-cytoplasm ratio resulted in CT hyperdensity and restricted diffusion (21, 22). The abundant blood supply from the choroidal arteries might also prevent necrosis and cystic changes. However, CNs with low Ki-67 indices were mostly multi-cystic lesions, suggesting a tendency for gangliocyte differentiation (23). The central cystic changes were often caused by tumor necrosis, while the peritumoral cystic changes were due to adhesion, traction, and cerebrospinal fluid infiltration (24). Elevated Ki-67 index also represented active vascularization, bringing about abnormal blood vessels of CN (25). Of course, these abnormal vessels might come from the encapsulation of adjacent blood vessels during CN growth. Susceptibility-weighted imaging (SWI) or T2*WI is sensitive to the vascular components and intratumoral susceptibility signals of CNs with high Ki-67, contributing to a more accurate diagnosis (26–28). The marked enhancement could be explained by hypercellularity and tumor angiogenesis, which were also related to elevated Ki-67 indices (29, 30). The solid lesions with CT hyperdensity and restricted diffusion represented the hypercellularity. Abnormal vessels and marked MRI enhancement meant hypervascularity. Therefore, combining the high Ki-67 index, hypercellularity, and hypervascularity could further reveal the complex biological behavior of CN.
In this study, these patients were young and middle-aged without significant sex differences. Age is a possible influencing factor for the histological grading of CN. Extreme age (>50 years or < 18 years) might tend to atypical features and recurrence (31). The age of our patients did not show this extreme tendency, which needs to be further confirmed by a large sample. The clinical symptoms were associated with the location and mass effect of the lesion (5, 32). Both groups of CN caused obstructive hydrocephalus and intracranial hypertension, although CNs with high Ki-67 were larger. All the lesions near the foramen of Monro were the possible reason. Moreover, the slow growth of CN could compensate for intracranial hypertension and reduce periventricular edema. Intratumoral calcification and hemorrhage were not associated with the Ki-67 indices in this study. Although the blood supply of CN was abundant, intratumoral hemorrhage was uncommon (33). The unbalanced blood supply and vascular degeneration might result in intratumoral hemorrhage and calcification (33, 34).
The imaging assessment of CN based on the Ki-67 index provided clinical implications. The combination of Ki-67 index and imaging characteristics was helpful for preoperative diagnosis and postoperative follow-up of CN. The atypia and hypercellularity related to the high Ki-67 index can lead to postoperative recurrence and poor prognosis (9, 11, 14). Total resection and postoperative radiochemotherapy are recommended. However, the hypervascular CN with high Ki-67 index also increases the difficulty of total resection, and emergency preparation for intraoperative bleeding is necessary (9, 11, 14). In addition, SWI and T2*WI should be used in preoperative diagnosis and postoperative follow-up to better display abnormal blood vessels.
Due to the low incidence of CN, the small sample size limited the statistical power. Indeed, a single Ki-67 index can not fully reflect the complex biological behavior of CN. SWI and T2*WI were not routine sequences in our institution, so they were not included in this retrospective study. In the future, large-scale longitudinal studies with more tumor markers and advanced imaging techniques are necessary.
5 Conclusion
According to the Ki-67 proliferation index, the imaging characteristics of CNs could be affected. Tumor size, radiologic solidity, abnormal vessels, and marked MRI enhancement helped distinguish CNs with different Ki-67 indices. Combining these imaging characteristics with the Ki-67 index offered meaningful insights for clinical diagnosis and biological behavior of CNs.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by The Ethics Committees of Affiliated Yantai Yuhuangding Hospital of Qingdao University. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because it was a retrospective study, the need for written informed consent was waived. Written informed consent was not obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article because it was a retrospective study, the need for written informed consent was waived.
Author contributions
Y-LZ: Writing – original draft, Methodology, Formal analysis, Investigation, Project administration, Conceptualization. LL: Writing – original draft, Methodology, Investigation, Conceptualization, Project administration, Formal analysis. WL: Investigation, Conceptualization, Supervision, Formal analysis, Validation, Writing – review & editing, Methodology. CR: Formal analysis, Conceptualization, Resources, Writing – original draft, Data curation. Y-QL: Writing – original draft, Data curation, Resources, Formal analysis.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, Figarella-Branger D, et al. The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro-oncology. (2021) 23:1231–51. doi: 10.1093/neuonc/noab106
2. Kim DG and Park CK. Central neurocytoma: establishment of the disease entity. Neurosurg Clinics North Am. (2015) 26:1–4. doi: 10.1016/j.nec.2014.09.013
3. Choudhari KA, Kaliaperumal C, Jain A, Sarkar C, Soo MY, Rades D, et al. Central neurocytoma: a multi-disciplinary review. Br J Neurosurg. (2009) 23:585–95. doi: 10.3109/02688690903254350
4. Sander C, Wallenborn M, Brandt VP, Ahnert P, Reuschel V, Eisenlöffel C, et al. Central neurocytoma: SNP array analyses, subtel FISH, and review of the literature. Pathol Res Pract. (2019) 215:152397. doi: 10.1016/j.prp.2019.03.025
5. Yang I, Ung N, Chung LK, Nagasawa DT, Thill K, Park J, et al. Clinical manifestations of central neurocytoma. Neurosurg Clin N Am. (2015) 26:5–10. doi: 10.1016/j.nec.2014.09.011
6. Menon SS, Guruvayoorappan C, Sakthivel KM, and Rasmi RR. Ki-67 protein as a tumour proliferation marker. Clin Chim Acta. (2019) 491:39–45. doi: 10.1016/j.cca.2019.01.011
7. Andrés-Sánchez N, Fisher D, and Krasinska L. Physiological functions and roles in cancer of the proliferation marker Ki-67. J Cell Sci. (2022) 135:jcs258932. doi: 10.1242/jcs.258932
8. Patel DM, Schmidt RF, and Liu JK. Update on the diagnosis, pathogenesis, and treatment strategies for central neurocytoma. J Clin Neurosci. (2013) 20:1193–9. doi: 10.1016/j.jocn.2013.01.001
9. Yang Y, Wadhwani N, Shimomura A, Zheng S, Chandler J, Lesniak MS, et al. Long-term outcomes of central neurocytoma - an institutional experience. J Neurooncol. (2024) 169:195–201. doi: 10.1007/s11060-024-04713-3
10. Sweiss FB, Lee M, and Sherman JH. Extraventricular neurocytomas. Neurosurg Clin N Am. (2015) 26:99–104. doi: 10.1016/j.nec.2014.09.004
11. Dutta SW, Kaleem TA, Muller DA, Peterson J, Harrell AC, Quinones-Hinojosa A, et al. Central neurocytoma: Clinical characteristics, patterns of care, and survival. J Clin Neurosci. (2018) 53:106–11. doi: 10.1016/j.jocn.2018.04.015
12. Sugita Y, Furuta T, Komaki S, Ohshima K, Sakata K, and Morioka M. Malignant progression of an extraventricular neurocytoma arising from the VIIIth cranial nerve: A case report and literature review. Neuropathology. (2019) 39:120–6. doi: 10.1111/neup.12533
13. Tish S, Habboub G, Jones J, Ostrom QT, Kruchko C, Barnholtz-Sloan JS, et al. The epidemiology of central and extraventricular neurocytoma in the United States between 2006 and 2014. J Neurooncol. (2019) 143:123–7. doi: 10.1007/s11060-019-03144-9
14. Lee SJ, Bui TT, Chen CH, Lagman C, Chung LK, Sidhu S, et al. Central neurocytoma: A review of clinical management and histopathologic features. Brain Tumor Res Treat. (2016) 4:49–57. doi: 10.14791/btrt.2016.4.2.49
15. Romano N, Federici M, and Castaldi A. Imaging of extraventricular neurocytoma: a systematic literature review. Radiol Med. (2020) 125:961–70. doi: 10.1007/s11547-020-01198-8
16. Sun X and Kaufman PD. Ki-67: more than a proliferation marker. Chromosoma. (2018) 127:175–86. doi: 10.1007/s00412-018-0659-8
17. Sobecki M, Mrouj K, Colinge J, Gerbe F, Jay P, Krasinska L, et al. Cell-cycle regulation accounts for variability in Ki-67 expression levels. Cancer Res. (2017) 77:2722–34. doi: 10.1158/0008-5472.CAN-16-0707
18. Kalawi AZ, Malicki DM, Abdullaev Z, Pratt DW, Quezado M, Aldape K, et al. The role of methylation profiling in histologically diagnosed neurocytoma: a case series. J Neurooncol. (2022) 159:725–33. doi: 10.1007/s11060-022-04117-1
19. AbdelBari Mattar M, Shebl AM, and Toson EA. Atypical central neurocytoma: an investigation of prognostic factors. World Neurosurg. (2021) 146:e184–93. doi: 10.1016/j.wneu.2020.10.068
20. Ma Z, Yan H, Shi H, Li Y, Song J, Huang J, et al. The typical and atypical MR imaging findings of central neurocytomas: Report on eighteen cases and review of the literature. Clin Neurol Neurosurg. (2016) 146:18–23. doi: 10.1016/j.clineuro.2016.04.012
21. Donoho D and Zada G. Imaging of central neurocytomas. Neurosurg Clin N Am. (2015) 26:11–9. doi: 10.1016/j.nec.2014.09.012
22. Sun PF, Ma L, Ye BQ, and Pei YY. Application of diffusion-weighted imaging combined with apparent diffusion coefficient in differential diagnosis between central neurocytoma and ependymoma. Neuroradiology. (2020) 62:439–45. doi: 10.1007/s00234-019-02342-6
23. Sato D, Takami H, Takayanagi S, Ikemura M, Matsuura R, Tanaka S, et al. Intraventricular central neurocytoma molecularly defined as extraventricular neurocytoma: a case representing the discrepancy between clinicopathological and molecular classifications. Brain Tumor Pathol. (2023) 40:230–4. doi: 10.1007/s10014-023-00469-2
24. Ramsahye H, He H, Feng X, Li S, and Xiong J. Central neurocytoma: radiological and clinico-pathological findings in 18 patients and one additional MRS case. J Neuroradiol. (2013) 40:101–11. doi: 10.1016/j.neurad.2012.05.007
25. González A, Pietrani M, Álvarez S, Mosquera C, Liotard T, and Ajler P. Intraventricular neurocytoma: A diagnostic challenge with prognostic value. Radiol Case Rep. (2024) 19:4151–7. doi: 10.1016/j.radcr.2024.06.055
26. Yu Y, Zhang H, Xiao Z, She D, Xing Z, Yang X, et al. Diffusion-weighted MRI combined with susceptibility-weighted MRI: added diagnostic value for four common lateral ventricular tumors. Acta Radiol. (2018) 59:980–7. doi: 10.1177/0284185117738562
27. Haller S, Haacke EM, Thurnher MM, and Barkhof F. Susceptibility-weighted imaging: technical essentials and clinical neurologic applications. Radiology. (2021) 299:3–26. doi: 10.1148/radiol.2021203071
28. Tang MY, Chen TW, Zhang XM, and Huang XH. GRE T2∗-weighted MRI: principles and clinical applications. BioMed Res Int. (2014) 2014:312142. doi: 10.1155/2014/312142
29. Li X, Guo L, Sheng S, Xu Y, Ma L, Xiao X, et al. Diagnostic value of six MRI features for central neurocytoma. Eur Radiol. (2018) 28:4306–13. doi: 10.1007/s00330-018-5442-y
30. Niiro T, Tokimura H, Hanaya R, Hirano H, Fukukura Y, Sugiyma K, et al. MRI findings in patients with central neurocytomas with special reference to differential diagnosis from other ventricular tumours near the foramen of Monro. J Clin Neurosci. (2012) 19:681–6. doi: 10.1016/j.jocn.2011.06.030
31. Patil AS, Menon G, Easwer HV, and Nair S. Extraventricular neurocytoma, a comprehensive review. Acta Neurochir (Wien). (2014) 156:349–54. doi: 10.1007/s00701-013-1971-y
32. Andour H, Rostoum S, Cherraqi A, Fikri M, Ech-Cherif El Kettani N, Jiddane M, et al. Central neurocytoma-positive and differential diagnosis: An example through a case report. SAGE Open Med Case Rep. (2023) 11:2050313X231164280. doi: 10.1177/2050313X231164280
33. Zhang D, Wen L, Henning TD, Feng XY, Zhang YL, Zou LG, et al. Central neurocytoma: clinical, pathological and neuroradiological findings. Clin Radiol. (2006) 61:348–57. doi: 10.1016/j.crad.2006.01.002
Keywords: central neurocytoma, Ki-67, MRI, CT, imaging diagnosis
Citation: Zhang Y-l, Liu L, Li W, Ran C and Luo Y-q (2025) Imaging characteristics of central neurocytomas according to Ki-67 proliferation index. Front. Oncol. 15:1601817. doi: 10.3389/fonc.2025.1601817
Received: 28 March 2025; Accepted: 19 May 2025;
Published: 03 June 2025.
Edited by:
Emma Gangemi, Sapienza University of Rome, ItalyCopyright © 2025 Zhang, Liu, Li, Ran and Luo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Li, bGl3ZWlxZDgzMDEyN0AxNjMuY29t
†These authors have contributed equally to this work
 Yan-li Zhang1†
Yan-li Zhang1† Wei Li
Wei Li Yu-qi Luo
Yu-qi Luo