Abstract
Pulmonary enteric adenocarcinoma (PEAC) is a rare non-small cell lung cancer subtype characterized by predominant intestinal differentiation (≥50%) and histological resemblance to colorectal adenocarcinoma. We report a 70-year-old male ex-smoker with an incidentally detected 18×11 mm spiculated lung nodule on chest CT, which subsequently demonstrated intense FDG uptake (SUVmax 14.0) on PET-CT. Histopathological evaluation confirmed PEAC. Immunohistochemistry revealed HER2 overexpression (3+) and intestinal differentiation markers (CK7+, CK20+, CDX2+, Villin+), while molecular testing showed wild-type ERBB2 and no actionable mutations. The patient underwent successful R0 resection with no recurrence at 8-month follow-up. This case underscores the critical importance of a multimodal diagnostic approach integrating immunohistochemical markers (notably CK7’s superior specificity), PET-CT imaging, and endoscopic evaluation to reliably differentiate PEAC from metastatic gastrointestinal malignancies. Furthermore, the patient’s favorable outcome following R0 resection without adjuvant therapy reinforces surgical intervention as the cornerstone of treatment for localized PEAC, particularly in early-stage disease. Advanced cases require early multidisciplinary collaboration to develop individualized treatment.
Background
Pulmonary enteric adenocarcinoma (PEAC) is a rare NSCLC subtype defined by predominant (≥50%) intestinal-type differentiation and histological similarity to colorectal adenocarcinoma in primary lung tumors (1). PEAC is relatively rare in clinical practice, with an overall prevalence of approximately 0.6%-0.68% in primary pulmonary adenocarcinoma (2, 3). Literature reports that this disease is more common in middle-aged and elderly people (2). Currently, there are fewer than 500 cases of PEAC described in English scientific literature (4), primarily in the form of case reports or small case series, and literature related to the treatment of PEAC is even scarcer.
Case presentation
A 70-year-old male ex-smoker (30 pack-years) presented with an incidentally detected 18×11 mm spiculated nodule in the anterior segment of the left upper lobe on chest CT (Figure 1). CT-guided biopsy confirmed adenocarcinoma, prompting further evaluation to exclude metastatic disease. Comprehensive endoscopic evaluation including esophagogastroduodenoscopy and colonoscopy showed no evidence of malignancy or other clinically relevant pathology.
Figure 1
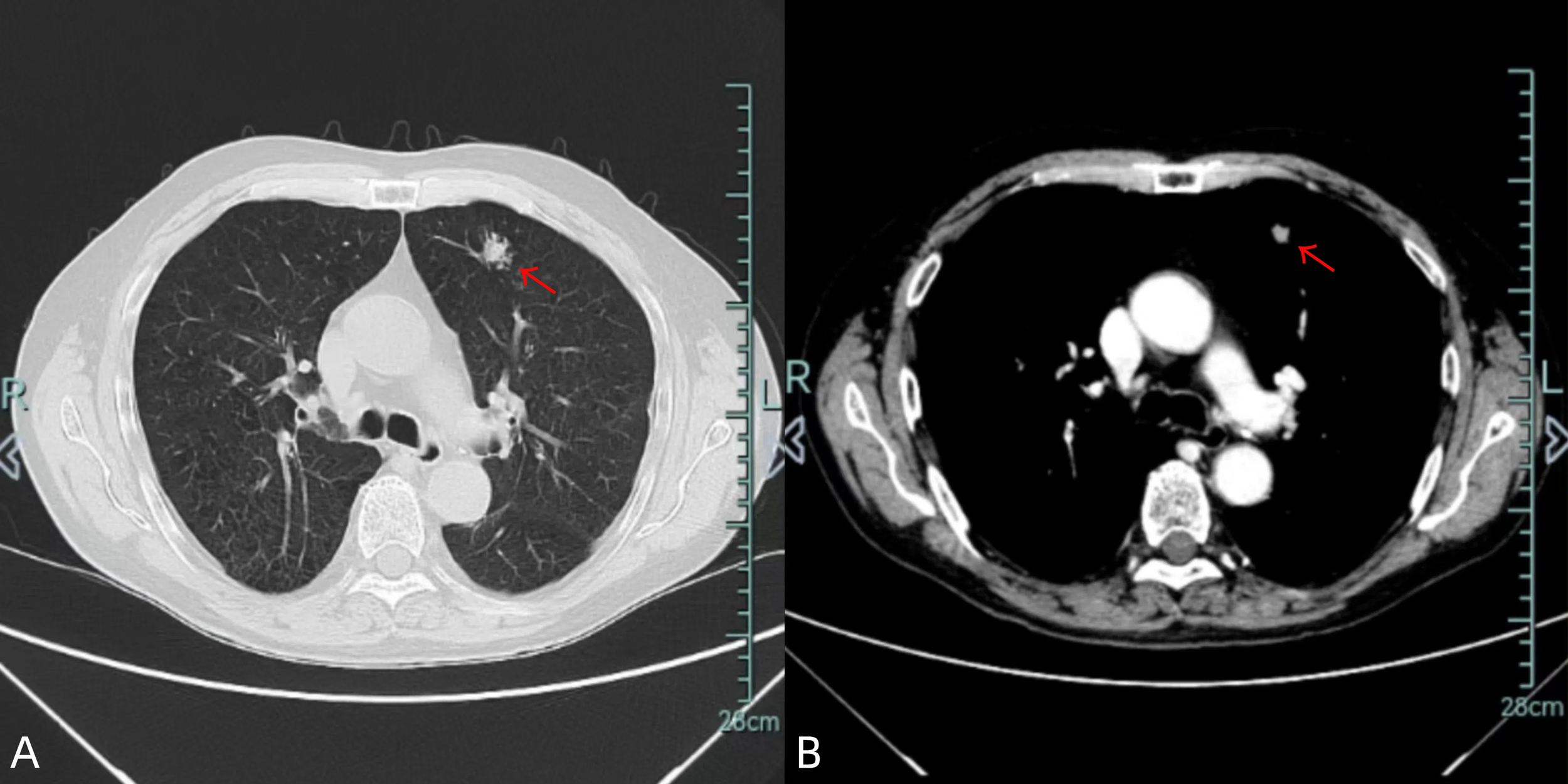
Chest CT images [lung window (A)/mediastinal window (B)] demonstrate an 18×11 mm spiculated nodule in the left upper lobe (red arrow).
PET-CT demonstrated intense FDG uptake (SUVmax 14.0) in the dominant lesion without metabolically active satellite nodules (Figure 2). Serum tumor markers (CEA, CA19-9, CYFRA21-1, NSE) were within normal ranges.
Figure 2
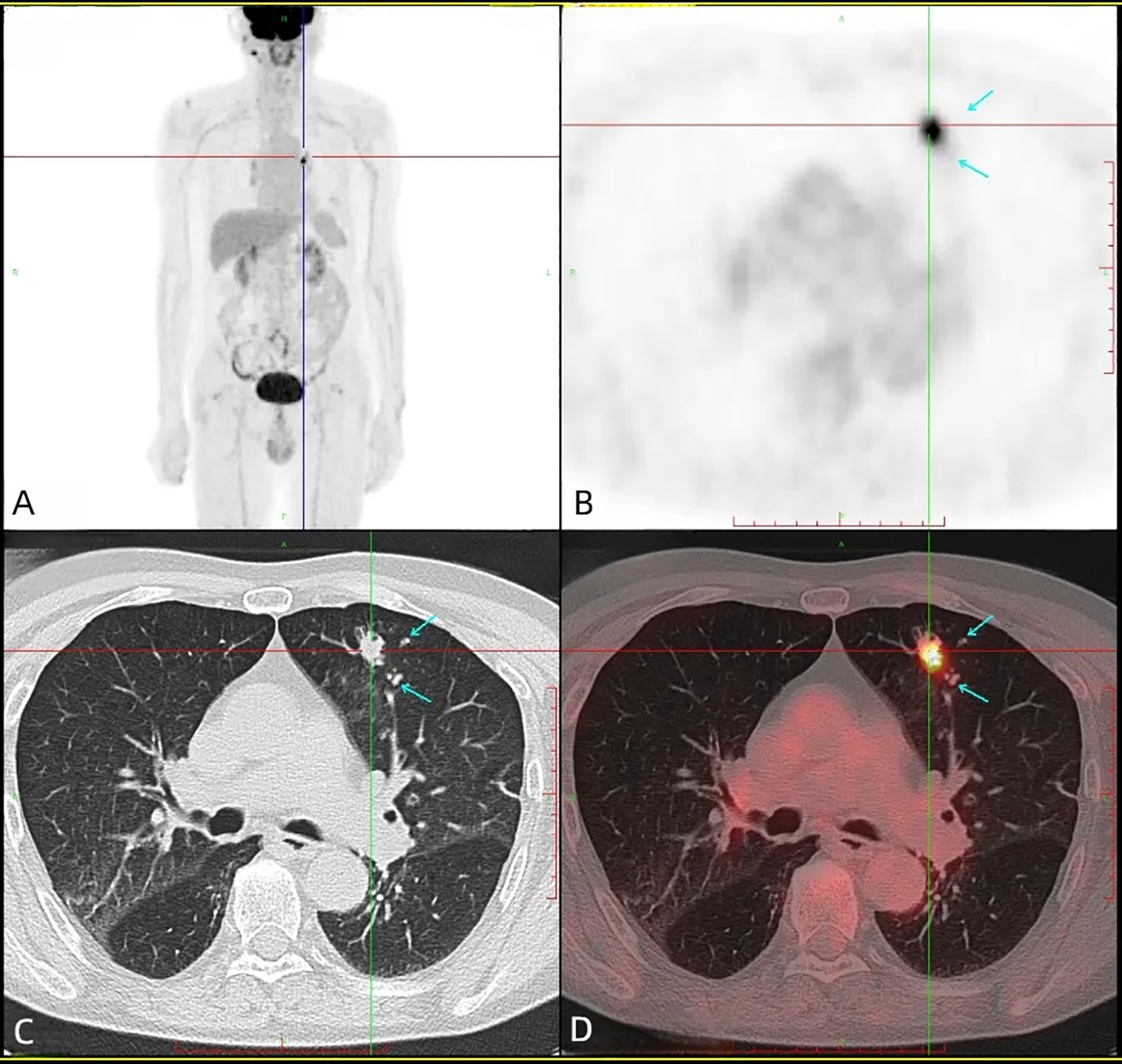
Head + torso 18F-FDG PET/CT revealed a heterogeneous FDG uptake in the left upper lobe (SUVmax = 14.0), with no evidence of hypermetabolic lesions in the gastrointestinal tract. (A) PET maximum intensity projection (MIP) image, (B) axial PET image, (C) axial CT image, (D) axial fusion image.
Thoracoscopic wedge resection of the left upper lobe revealed a 1.5×1.2×1 cm invasive adenocarcinoma with negative surgical margins. Postoperative staging was pT1bNxMx (AJCC 8th edition). Histopathological evaluation demonstrated intestinal differentiation features (Figure 3) with the following characteristics: neural invasion (-), lymphovascular invasion (+), no visceral pleural invasion (PL0), and tumor spread through air spaces (STAS). Immunohistochemical profile: HER-2(3+), CK7(+), TTF-1(-), NapsinA(-), P40(-), CK5/6(-), Ki67(+,60%), ALK(D5F3)(-), ALK-NC(-), ALK-PC(+), P53(+) (mutant type), CDX2(+), CK20(+), Villin(focal+), Muc-2(-), SATB2(-). PD-L1 tumor proportion score was <1%.
Figure 3
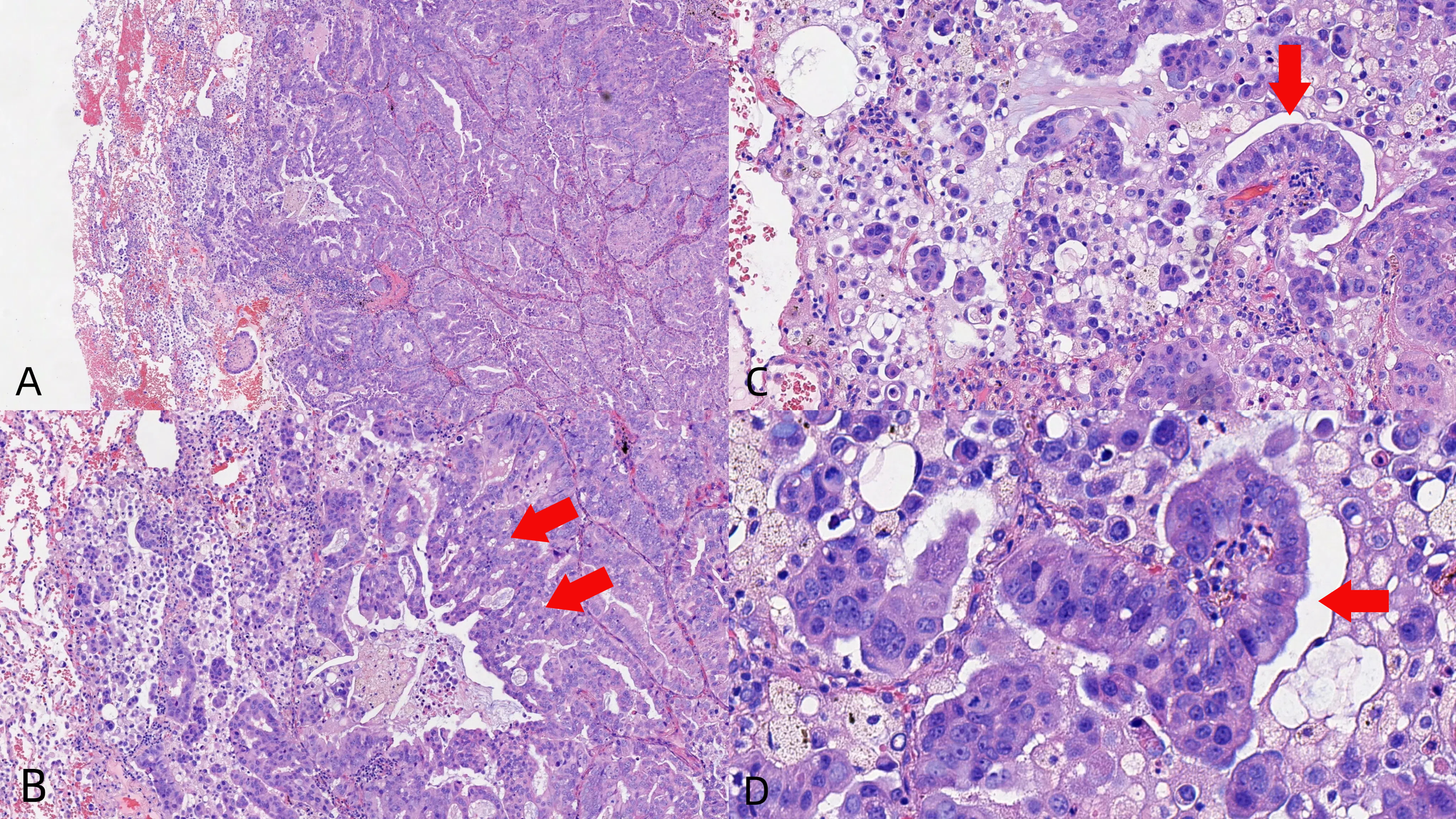
Core biopsies showing the tumor with glandular architecture [H&E stain, power of magnification ×100 (A), ×200 (B, C), and ×400 (D)].
A 10-gene ARMS-PCR panel (EGFR, ALK, ROS1, RET, KRAS, NRAS, BRAF, ERBB2/HER2, PIK3CA, MET) detected no clinically actionable mutations or fusions at a 1% sensitivity threshold. It is noteworthy that HER2 protein overexpression (3+) contradicted the wild-type ERBB2 gene status.
The patient received postoperative surveillance without adjuvant therapy, and no evidence of recurrence was observed at the 8-month follow-up.
Discussion and conclusion
Pulmonary enteric adenocarcinoma (PEAC), also known as the intestinal variant of lung adenocarcinoma, is a very rare subtype of NSCLC. It was first described by Tsao and Fraser in 1991 (5) and was officially classified as a histological type of lung adenocarcinoma in the 2015 World Health Organization histological classification of lung tumors. According to the WHO criteria (1), the diagnosis of PEAC requires: 1.Primary pulmonary origin; 2. ≥50% tumor composition showing intestinal-type differentiation; 3.Morphological overlap with colorectal adenocarcinoma.
Studies have shown that the incidence of PEAC is higher in middle-aged and elderly patients and more common in males (2), though some scholars believe the incidence is similar between sexes (6). Due to the small number of reported cases, sex-specific incidence rates remain controversial. Smokers are more likely to develop PEAC than non-smokers (6), and our patient also had a long-term smoking history, consistent with research findings.
Cough is the most common initial symptom of PEAC, followed by back pain, chest pain, and hemoptysis (2, 7–10). Other symptoms include chest tightness, dyspnea, fever, night sweats, throat discomfort, headache, fatigue, and cervical lymphadenopathy (11). Gastrointestinal symptoms such as nausea, vomiting, abdominal pain, diarrhea, constipation, or bloody stools were not observed (12); thus, PEAC patients are typically first diagnosed in respiratory departments. However, our patient was incidentally found to have a nodule during a physical examination at an external hospital and was asymptomatic upon admission to our institution, which may relate to the tumor’s peripheral location, small diameter, and lack of pleural invasion.
Previous studies have reported varying degrees of elevated serum tumor markers in PEAC patients, particularly CEA and CA19-9. In contrast, CYFRA21–1 and NSE, which are relatively sensitive and specific for lung cancer, showed no significant changes (9, 13). According to statistics from Li H et al., CEA levels were elevated in 68.2% (45/66) of patients, and CA19–9 levels were elevated in 48.4% (15/31) of patients. CYFRA21–1 and NSE were rarely positive, at approximately 10% (2/20) and 0% (0/19), respectively (9). Preoperative tumor marker testing in this patient revealed no abnormally elevated indicators, possibly related to the early stage of the disease.
Observations in existing cases indicate that CEA levels significantly decrease after chemotherapy in advanced PEAC patients with distant metastasis (12, 14), suggesting this marker may help monitor clinical progression. Postoperative re-examination at 3 months showed the patient’s CEA decreased from 4.7 ng/mL preoperatively to 4.4 ng/mL, both within normal ranges. Thus, CEA may not be an ideal marker for monitoring early-stage PEAC.
Since the lung is a common metastatic site for colorectal cancer, clinicians must rule out colorectal-origin malignancies when diagnosing PEAC. Studies show that immunohistochemical markers such as Villin, CK7, CK20, and CDX2 have significantly higher positivity rates in PEAC than in metastatic colorectal cancer (MCC) (15). CK7 is particularly critical for differentiating PEAC from MCC, as it exhibits better specificity than CK20 and CDX2 (6, 16). However, due to morphological, immunohistochemical, and even genetic similarities between PEAC and colorectal adenocarcinoma (15), distinguishing them based solely on histopathology is challenging (17–19). A definitive PEAC diagnosis requires pathological examination of pulmonary lesions combined with systemic multi-organ evaluations to exclude metastatic gastrointestinal tumors. Feng C et al. emphasized that origin cannot be determined by morphological features alone; accurate diagnosis requires comprehensive clinical examinations, immunohistochemical staining, and genetic testing (15). Stojsic J et al. consider colonoscopy one of the most valuable methods for diagnosing PEAC (17). The final diagnosis should integrate the patient’s medical history and clinical evaluations, including CT, fiberoptic endoscopy, and PET-CT, to exclude primary colorectal cancer. In this case, the patient underwent lung biopsy at an external hospital, with pathology results suggesting “adenocarcinoma, metastatic origin to be excluded.” Although initially suspected to be a metastatic lesion, gastrointestinal endoscopy showed no evidence of malignancy or other clinically significant pathology. Ultimately, PET-CT imaging ruled out metastatic disease from other primary sites and confirmed the nodule as a “primary lung malignancy. “After confirming the primary tumor, surgical resection was performed. Based on this patient’s experience, we emphasize the diagnostic value of PET-CT for early-stage PEAC and recommend its use for definitive diagnosis in all PEAC cases.
PEAC is a rare subtype of primary pulmonary adenocarcinoma. Internationally, no specific treatment guidelines exist for PEAC, and most clinicians follow NSCLC protocols. Surgical intervention is the preferred treatment for early-stage patients, supported by data from several studies (7, 9). Advanced patients often receive surgery combined with chemotherapy, typically platinum-paclitaxel regimens. Given the patient’s poor pulmonary function (with severe ventilatory dysfunction and markedly reduced diffusing capacity of the lungs for carbon monoxide [DLCO]) and an ECOG performance status of 2, we opted for thoracoscopic wedge resection instead of anatomical resection and omitted lymph node dissection. Intraoperatively, no enlarged lymph nodes were identified under video-assisted thoracoscopic surgery (VATS), further supporting the rationale for forgoing lymphadenectomy. The postoperative pathology of this patient revealed the presence of lymphovascular invasion (LVI+) and spread through air spaces (STAS). Studies have shown that LVI-positive patients have higher risks of recurrence and mortality compared to LVI-negative patients (20, 21). Furthermore, the 2020 NCCN Guidelines recommended adjuvant chemotherapy for high-risk patients with LVI (22). The 2023 NCCN Guidelines, for the first time, explicitly listed STAS as a high-risk factor in the pathology section and suggested considering adjuvant therapy for stage IB or higher patients with STAS (22). Given that this patient was considered stage IA2 and had poor baseline conditions, adjuvant therapy was not administered. As of the 8-month postoperative follow-up, there has been no evidence of recurrence. However, it should be noted that the follow-up data in this case report is relatively short, which represents a limitation of this study. Although targeted therapy has been proposed as an important approach in recent years, few patients have received such treatments. Fassi E et al. identified high-frequency target gene mutations in PEAC and recommended gene panel analysis for all cases (7). Postoperative molecular profiling in this patient revealed no detectable gene fusions or mutations. Notably, we identified a discordance between HER2 protein overexpression (IHC 3+) and negative molecular findings (wild-type ERBB2 status), which has not been previously documented in published PEAC case reports. This discrepancy has been frequently documented in breast cancer (23, 24). As the existing literature lacks direct evidence explaining this phenomenon in PEAC, the precise underlying mechanisms remain unclear. Additionally, external factors such as limitations in detection methods cannot be disregarded. For instance, the HER2 status may vary across different regions of the lesion, leading to discrepancies between the biopsy sample and the overall tumor characteristics.For early-stage PEAC patients, we recommend prompt surgical intervention to excise lesions, confirm diagnosis, and guide postoperative management. Adjuvant chemotherapy is generally unnecessary for R0-resected early-stage patients. This patient has undergone regular follow-up at our institution with no recurrence. For advanced patients, early multidisciplinary discussions are essential to formulate personalized treatment plans.
In conclusion, PEAC is a rare disease. Preoperative diagnosis should not rely solely on immunohistochemistry but requires comprehensive evaluation of patient history and clinical examinations to exclude gastrointestinal malignancies. Complete surgical resection is the preferred treatment for early-stage patients, while advanced cases necessitate early multidisciplinary collaboration to develop individualized therapies.
Statements
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Ethics Committee of Zhejiang Provincial People’s Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
YB: Writing – original draft, Writing – review & editing. DL: Writing – review & editing. YW: Writing – review & editing. ZW: Writing – review & editing. HW: Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Travis WD Brambilla E Burke AP Marx A Nicholson AG . Introduction to the 2015 world health organization classification of tumors of the lung, pleura, thymus, and heart. J Thorac Oncol. (2015) 10:1240–2. doi: 10.1097/JTO.0000000000000663
2
Zhao L Huang S Liu J Zhao J Li Q Wang HQ . Clinicopathological, radiographic, and oncogenic features of primary pulmonary enteric adenocarcinoma in comparison with invasive adenocarcinoma in resection specimens. Med (Baltimore). (2017) 96:e8153. doi: 10.1097/MD.0000000000008153
3
Inamura K Satoh Y Okumura S Nakagawa K Tsuchiya E Fukayama M et al . Pulmonary adenocarcinomas with enteric differentiation: histologic and immunohistochemical characteristics compared with metastatic colorectal cancers and usual pulmonary adenocarcinomas. Am J Surg Pathol. (2005) 29:660–5. doi: 10.1097/01.pas.0000160438.00652.8b
4
Luo ZH Luo XY Luo XQ Jin AF Zeng QY . Case report: 18F-FDG PET/CT in pulmonary enteric adenocarcinoma. Front Oncol. (2024) 14:1447453. doi: 10.3389/fonc.2024.1447453
5
Tsao MS Fraser RS . Primary pulmonary adenocarcinoma with enteric differentiation. Cancer. (1991) 68:1754–7. doi: 10.1002/1097-0142(19911015)68:8<1754::AID-CNCR2820680818>3.0.CO;2-E
6
Lin D Zhao Y Li H Xing X . Pulmonary enteric adenocarcinoma with villin brush border immunoreactivity: a case report and literature review. J Thorac Dis. (2013) 5:E17–20. doi: 10.3978/j.issn.2072-1439.2012.06.06IF:2.1Q3
7
Fassi E Mandruzzato M Zamparini M Bianchi S Petrelli F Baggi A et al . Clinical presentation and outcome of patients with enteric-type adenocarcinoma of the lung: A pooled analysis of published cases. Lung Cancer. (2023) 179:107176. doi: 10.1016/j.lungcan.2023.107176IF:4.5Q1
8
Kocher F Hilbe W Seeber A Pircher A Schmid T Greil R et al . Longitudinal analysis of 2293 NSCLC patients: a comprehensive study from the TYROL registry. Lung Cancer. (2015) 87:193–200. doi: 10.1016/j.lungcan.2014.12.006
9
Li H Cao W . Pulmonary enteric adenocarcinoma: a literature review. J Thorac Dis. (2020) 12:3217–26. doi: 10.21037/jtd-19-4171IF:2.1Q3
10
Gong J Fan Y Lu H . Pulmonary enteric adenocarcinoma. Transl Oncol. (2021) 14:101123. doi: 10.1016/j.tranon.2021.101123
11
Gu L Wang XZ Wen W Lin J Chen XF Lai GX et al . Clinical analysis of 23 patients pathologically diagnosed with primary and secondary pulmonary enteric adenocarcinoma. Chin Med J (Engl). (2019) 132:1368–9. doi: 10.1097/CM9.0000000000000266IF:7.5Q1
12
Lin LI Xu CW Zhang BO Liu RR Ge FJ Zhao CH et al . Clinicopathological observation of primary lung enteric adenocarcinoma and its response to chemotherapy: A case report and review of the literature. Exp Ther Med. (2016) 11:201–7. doi: 10.3892/etm.2015.2864
13
Chen Q Wang XJ Zhang GJ . Clinicopathological characteristics of pulmonary enteric adenocarcinoma patients [in Chinese. Henan Med Res. (2022) 31:1606–9.
14
Li N Liang Y Li D Tan Q Yin CC . A case report of primary pulmonary enteric adenocarcinoma [in Chinese. J Xiangnan Univ (Med Ed). (2024) 26:57–8. doi: 10.16500/j.cnki.1673-498x.2024.04.013
15
Feng C Feng M Gao Y Zhao X Peng C Yang X et al . Clinicopathologic significance of intestinal-type molecules’ Expression and different EGFR gene status in pulmonary adenocarcinoma. Appl Immunohistochem Mol Morphol. (2019) 27:364–72. doi: 10.1097/PAI.0000000000000632IF:1.3Q3
16
Nicholson AG Tsao MS Beasley MB Borczuk AC Brambilla E Cooper WA et al . The 2021 WHO classification of lung tumors: impact of advances since 2015. J Thorac Oncol. (2022) 17:362–87. doi: 10.1016/j.jtho.2021.11.003IF:21.0Q1
17
Stojsic J Kontic M Subotic D Popovic M Tomasevic D Lukic J . Intestinal type of lung adenocarcinoma in younger adults. Case Rep Pulmonol. (2014) 2014:282196. doi: 10.1155/2014/282196
18
Li HC Schmidt L Greenson JK Chang AC Myers JL . Primary pulmonary adenocarcinoma with intestinal differentiation mimicking metastatic colorectal carcinoma: case report and review of literature. Am J Clin Pathol. (2009) 131:129–33. doi: 10.1309/AJCPB04XWICTFERL
19
Hatanaka K Tsuta K Watanabe K Sugino K Uekusa T . Primary pulmonary adenocarcinoma with enteric differentiation resembling metastatic colorectal carcinoma: a report of the second case negative for cytokeratin 7. Pathol Res Pract. (2011) 207:188–91. doi: 10.1016/j.prp.2010.07.005
20
Ramnefjell M Aamelfot C Helgeland L Akslen LA . Vascular invasion is an adverse prognostic factor in resected non-small-cell lung cancer. APMIS. (2017) 125:197–206. doi: 10.1111/apm.12652IF:2.2Q3
21
Cai JS Wang X Yang F Li Y Qiu MT . Lymphovascular invasion: A non-sized T descriptor for stage IA non-small cell lung cancer. Thorac Cancer. (2022) 13:2413–20. doi: 10.1111/1759-7714.14530IF:2.3Q2
22
Ettinger DS Wood DE Aisner DL Akerley W Bauman JR Bharat A et al . NCCN guidelines® Insights: non-small cell lung cancer, version 2.2023. J Natl Compr Canc Netw. (2023) 21:340–50. doi: 10.6004/jnccn.2023.0020IF:14.8Q1
23
Gheni N Westenberg D . Quantitative real-time PCR assay with immunohistochemical evaluation of HER2/neu oncogene in breast cancer patients and its correlation with clinicopathological findings. Indian J Pathol Microbiol. (2020) 63:S123–8. doi: 10.4103/IJPM.IJPM_136_19IF:0.8Q4
24
El Hadi H Abdellaoui-Maane I Kottwitz D El Amrani M Bouchoutrouch N Qmichou Z et al . Development and evaluation of a novel RT-qPCR based test for the quantification of HER2 gene expression in breast cancer. Gene. (2017) 605:114–22. doi: 10.1016/j.gene.2016.12.027IF:2.6Q2
Summary
Keywords
pulmonary enteric adenocarcinoma (PEAC), PET-CT, multidisciplinary diagnosis, surgical resection, case report
Citation
Bao Y, Liu D, Wang Y, Wu Z and Wang H (2025) Case Report: Controversies in managing pulmonary enteric adenocarcinoma: reflections from an early-stage case. Front. Oncol. 15:1603084. doi: 10.3389/fonc.2025.1603084
Received
31 March 2025
Accepted
27 May 2025
Published
17 June 2025
Volume
15 - 2025
Edited by
James Cm Ho, The University of Hong Kong, Hong Kong SAR, China
Reviewed by
Roland Leung, Queen Mary Hospital, Hong Kong SAR, China
Pengtao Sun, Guangzhou University of Traditional Chinese Medicine, China
Updates
Copyright
© 2025 Bao, Liu, Wang, Wu and Wang.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Haitao Wang, 443292584@qq.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.