- 1Department of Digestive Surgery, Xijing Hospital of Digestive Diseases, Fourth Military Medical University, Xi'an, Shaanxi, China
- 2Institute of Anal-Colorectal Surgery, The 989th Hospital of the Joint Logistics Support Force of People's Liberation Army (PLA), Luoyang, Henan, China
- 3Department of Gastroenterology, Pingdingshan Medical District, 989 Hospital of People's Liberation Army (PLA) Joint Logistics Support Force, Pingdingshan, Henan, China
Background: Patients with metachronous liver metastasis (MLM) in gastric cancer generally have a poor prognosis. Early detection and accurate prediction of MLM are crucial for improving clinical outcomes. This study aims to identify the risk factors for MLM through clinical pathological parameters and develop a predictive model for MLM in gastric cancer.
Methods: A retrospective analysis of 1248 gastric cancer patients who underwent radical surgery between December 2016 and December 2020 was conducted. Patients were randomly divided into training (70%, n=873) and validation (30%, n=375) datasets. The optimal cutoff values for the continuous variables were determined using the Youden index. Univariate and multivariate logistic regression analyses were used to identify risk factors for MLM. A nomogram was developed based on the results of multivariate analysis. The model’s value was validated through receiver operating characteristic (ROC) curves, calibration curves, and decision curve analysis (DCA).
Results: The incidence of MLM was comparable between the training (10.3%, 90/873) and validation set (9.9%, 37/375). The optimal cutoff value was 3.315ng/ml for preoperative alpha-fetoprotein (AFP) level, 16.275U/ml for preoperative cancer antigen 125 (CA125) level, 0.280×109/L for monocyte count and 1.430×109/L for lymphocyte count, respectively. Univariate analysis showed that age, tumor size, pathological type, surgical method, T stage, N stage, TNM stage, neural invasion, lymphatic vascular invasion, number of lymph nodes harvested (LNH), preoperative total protein (TP), hemoglobin (HB), albumin (ALB), preoperative carcinoembryonic antigen (CEA), preoperative cancer antigen 19-9 (CA19-9), CA125, AFP levels, monocyte count, lymphocyte count, red blood cell (RBC) count and platelet count were considered as potential variables. Multivariate logistic regression analysis indicated that T stage, N stage, monocyte count, lymphocyte count, preoperative AFP and CA125 levels were independent predictive factors for MLM. The identified risk factors were further used to develop a predictive nomogram for MLM. The nomogram exhibited robust discriminatory performance, with an area under the curve (AUC) of 0.859 in the training set and 0.803 in the validation set. Moreover, the nomogram demonstrated excellent calibration and significant clinical utility.
Conclusion: This study successfully developed a predictive nomogram for MLM in gastric cancer. Besides conventional parameters, we identified and incorporated peripheral blood monocyte and lymphocyte counts as novel predictors, demonstrating their independent predictive value. Integrating these factors into nomogram could enhance predictive accuracy of MLM.
Introduction
Gastric cancer ranks as the fifth most prevalent malignancy and third leading cause of global cancer mortality (1). Despite the high incidence of gastric cancer, most patients are diagnosed at advanced stages with unfavorable prognoses due to the lack of distinguishing clinical indications (2). The liver is one of the most frequent sites of distant metastasis in gastric cancer (3–5). Critically, patients developing liver metastasis exhibit extremely poor prognosis, demonstrating a 5-year overall survival rate below 10% (6). Liver metastasis can be classified into two categories based on the timing of its occurrence following surgery: synchronous liver metastasis (SLM) and metachronous liver metastasis (MLM). According to international consensus, MLM refers to the occurrence of liver metastasis more than six months surgery (7). Approximately 2.0% to 9.9% of gastric cancer patients develop SLM, and up to 37% of gastric cancer patients develop MLM (4). Early detection and accurate prediction of MLM are crucial to improving outcomes in gastric cancer. Therefore, exploring the predictive factors of MLM in gastric cancer is very important.
Although extensive researches have been conducted on SLM in gastric cancer (8–10), studies focusing on MLM remain limited. Several clinical factors, including age, gender, T stage, N stage, Lauren classification, tumor size, histological type, and surgical approach, have been identified as potential factors for SLM (11). However, research on MLM, particularly its underlying mechanisms and risk factors, remains in its early stages.
Peripheral blood cells, including neutrophils, lymphocytes, monocytes, etc. could reflect the inflammatory status and immune response capacity in vivo (12), holding significant value in the prognostic assessment of gastric cancer (13). Previous researches have predominantly focused on the analysis of ratio indices, such as neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio (14, 15). However, these composite metrics may obscure independent prognostic contributions of individual cell subsets. Critically, analyses based on absolute blood cell counts for predicting MLM in gastric cancer remain lacking.
Based on this, we aimed to develop a predictive model for MLM in gastric cancer, incorporating clinical pathological features, preoperative tumor markers, and peripheral blood cell counts. This model could provide clinicians with a reliable tool for early identification of patients at high risk for MLM, ultimately enabling more personalized treatment strategies and improving patient outcomes.
Materials and methods
Patients
This retrospective study enrolled patients who received curative gastrectomy in the Department of Digestive Surgery, Xijing Hospital of Digestive Diseases from December 2016 to December 2020.
The inclusion criteria were as follows (1): histologically verified gastric adenocarcinoma (2); no history of prior malignancy; (3) underwent R0 resection; (4) availability of complete clinical data; and (5) a minimum of 36 months of surveillance for non-MLM patients. The exclusion criteria were as follows: (1) liver metastasis occurring within 6 months after surgery; (2) synchronous distant metastasis at diagnosis; (3) subsequent non-liver metastatic progression; (4) incomplete follow-up records. Finally, the cohort (N=1248) comprised two groups: (1) the MLM group, defined as patients with liver metastasis occurring more than six months post-surgery (N=127), and (2) the non-MLM group, defined as patients without distant metastasis within 36 months post-surgery (N=1121). The study was conducted in accordance with the Declaration of Helsinki and received ethical approval (KY20232248-C-1) from the Ethics Committee of the Xijing Hospital of the Fourth Military Medical University.
Clinical information
Clinical data, including gender, age, tumor location, tumor size, pathological type, surgical method, T stage, N stage, TNM stage, neural invasion, lymphatic vascular invasion, LNH, TP, HB, ALB, CEA, AFP, CA125, CA19–9 levels, as well as neutrophil, monocyte, lymphocyte, eosinophil, basophil, RBC, platelet counts from preoperative peripheral blood were collected through a retrospective review of medical records. Perioperative blood samples were obtained within 7 days before surgery.
Clinical follow-up
A standardized clinical surveillance was performed every 3 months throughout the initial 3 years, followed by 6 months surveillance thereafter. Liver metastasis was diagnosed through contrast-enhanced CT. Non-MLM patients were monitored for a minimum of 3 years post-surgery. The follow-up period commenced from the day after surgery until the detection of MLM or the endpoint of the follow-up.
Statistical analysis
Statistical analysis was conducted using R version 4.4.1 and SPSS version 27.0. To simplify the analysis and facilitated interpretation of results, continuous variables were converted to categorical variables. The optimal cutoff values for age, tumor size, LNH, TP, HB, ALB, CA125, CA19-9, CEA, AFP levels, neutrophil, monocyte, lymphocyte, eosinophil, basophil, RBC, platelet counts were determined by maximizing Youden index through ROC curve analysis (16). The thresholds were identified corresponding to the maximum Youden index as the definitive cutoff value. Categorical variables were expressed as frequencies and percentages, with chi-square tests used. Univariate analysis was initially used to evaluate risk factors for MLM. Variables with a p-value < 0.1 in univariate analysis were then applied to forward stepwise multivariable logistic regression to identify independent predictors for MLM. A nomogram was developed based on the multivariate regression model. Its predictive accuracy was assessed using discrimination (AUC) and calibration through internal validation. Bootstrap resampling (1000 resamples) was applied separately to training and validation sets. Calibration plots were used to compare observed and predicted probabilities, while clinical utility was assessed through DCA curves. Statistical significance was set at p<0.05 for two-tailed tests.
Results
Baseline clinicopathological variables of the study population
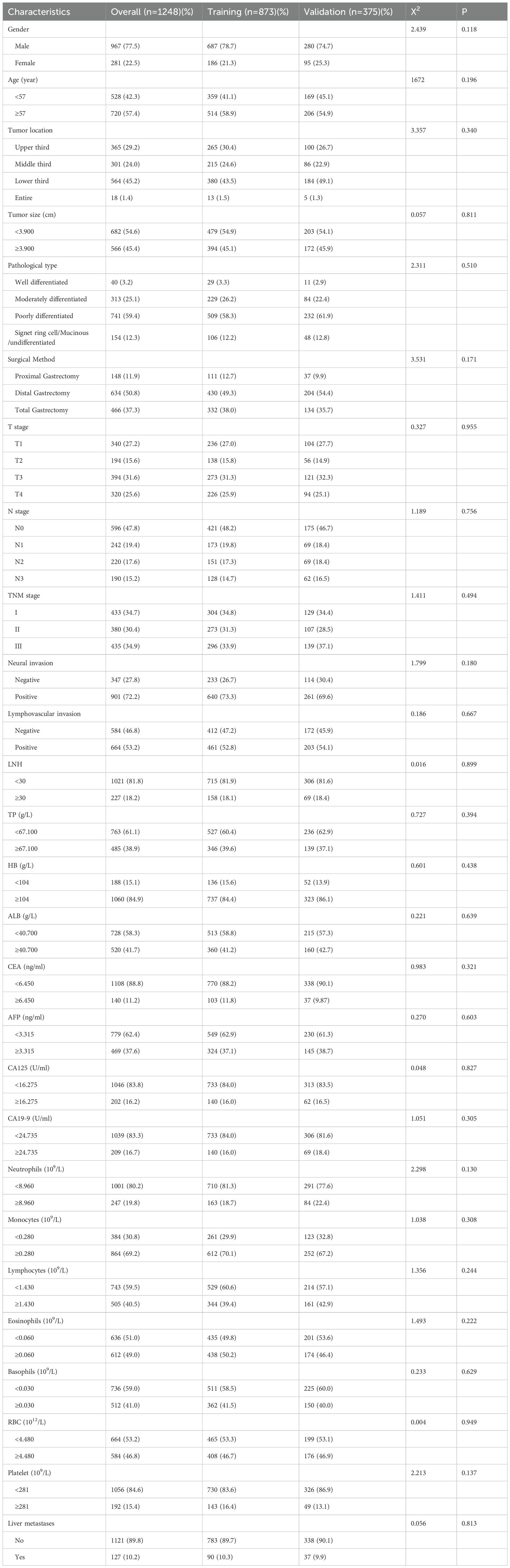
The clinicopathological characteristics were summarized in Table 1. A total of 967 males and 281 females were included in the cohort, with a median age of 59 years. The median follow-up duration was 67 months (range 7-88). The cohort was randomly assigned to a training set (n=873) and a validation set (n=375) at a ratio of 7:3. The incidence of MLM was comparable between the training (10.3%, 90/873) and validation set (9.9%, 37/375). The optimal cutoff values for age, tumor size, LNH, TP, HB, ALB, CEA, AFP, CA125, CA19-9, neutrophil, monocyte, lymphocyte, eosinophil, basophil RBC, and platelet counts could were 57 years, 3.900 cm, 30, 67.100g/L, 104g/L, 40.700 g/L, 6.450ng/ml, 3.315ng/ml, 16.275U/ml, 24.735 U/ml, 8.960×109/L, 0.280×109/L, 1.430×109/L, 0.060×109/L, 0.030×109/L, 4.480×1012/L and 281×109/L, respectively.
Predictive factors selection and development of nomogram
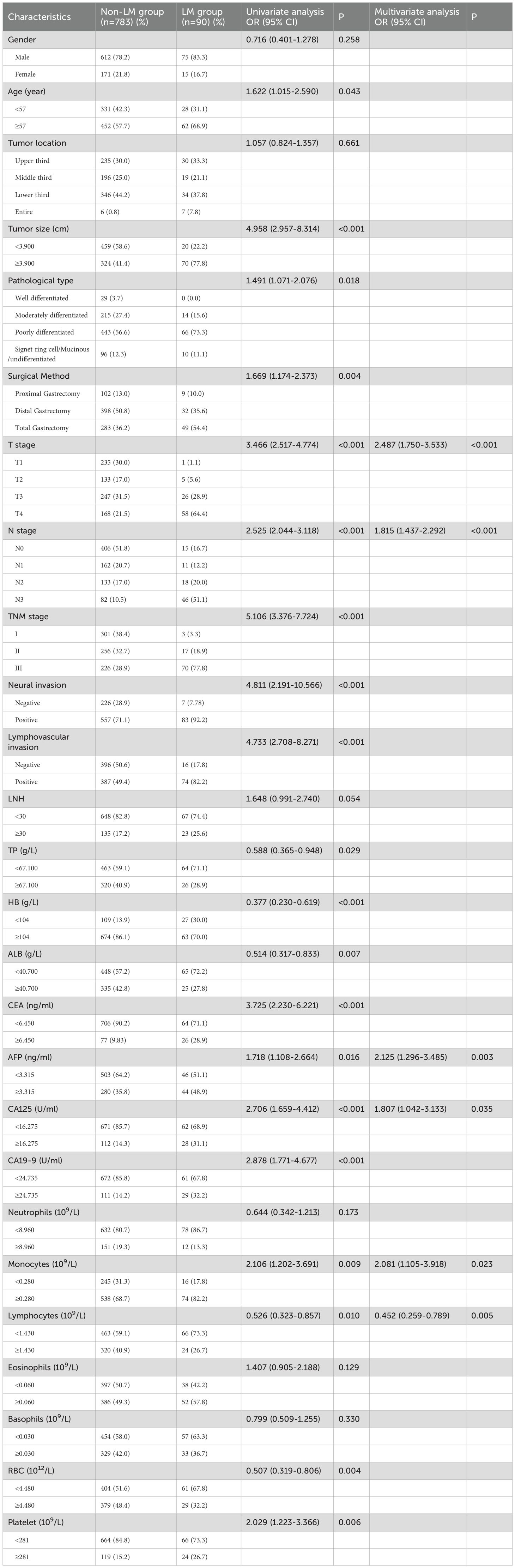
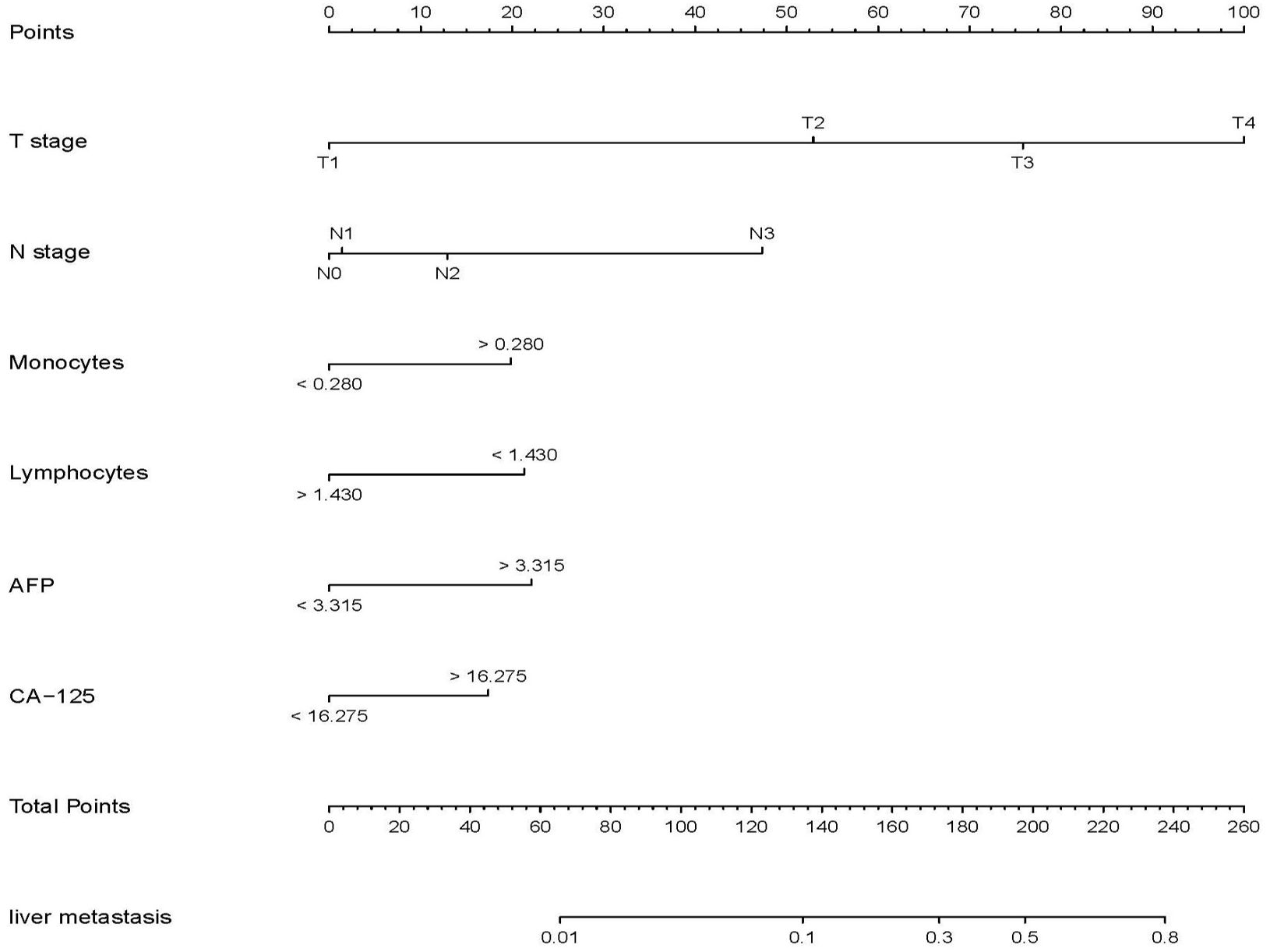
Univariable analysis in the training set was conducted to identify the potential predictors for MLM in gastric cancer patients. Univariate analysis showed that age, tumor size, pathological type, surgical method, T stage, N stage, TNM stage, neural invasion, lymphatic vascular invasion, LNH, TP, HB, ALB, CEA, CA19-9, CA125, AFP levels, monocyte, lymphocyte, RBC and platelet counts were risk factors for MLM in gastric cancer. In all associated features (p<0.1), potential predictors in the training data were selected by multivariable logistic regression. The multivariable logistic regression analysis revealed that T stage, N stage, monocyte count, lymphocyte count, AFP level, and CA125 level served as independent predictors for MLM, as shown in Table 2. Then we developed the nomogram based on the above predictors (Figure 1).
Evaluation and clinical application of the nomogram
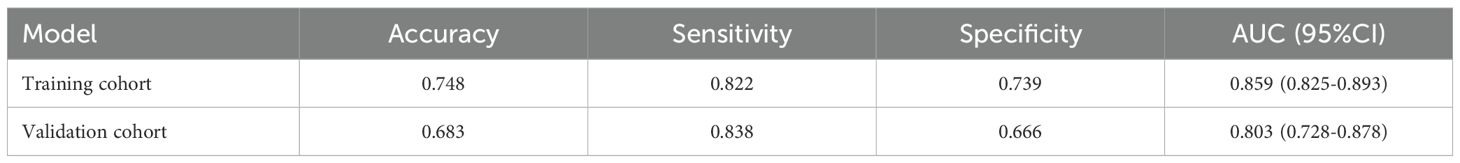
The model exhibited strong predictive performance, with AUCs of 0.859 and 0.803 in the training and validation cohorts, respectively (Figure 2). Calibration plots indicated excellent agreement between predicted and observed results. The curves aligned closely to the 45-degree reference line, confirming reliable calibration (Figure 3). The DCA curves revealed substantial net clinical benefit, highlighting the model’s utility in clinical practice (Figure 4). Furthermore, the training cohort conducted an accuracy of 0.748, sensitivity of 0.822, and specificity of 0.739, while the validation cohort exhibited corresponding metrics of 0.683, 0.838, and 0.666, respectively (Table 3).
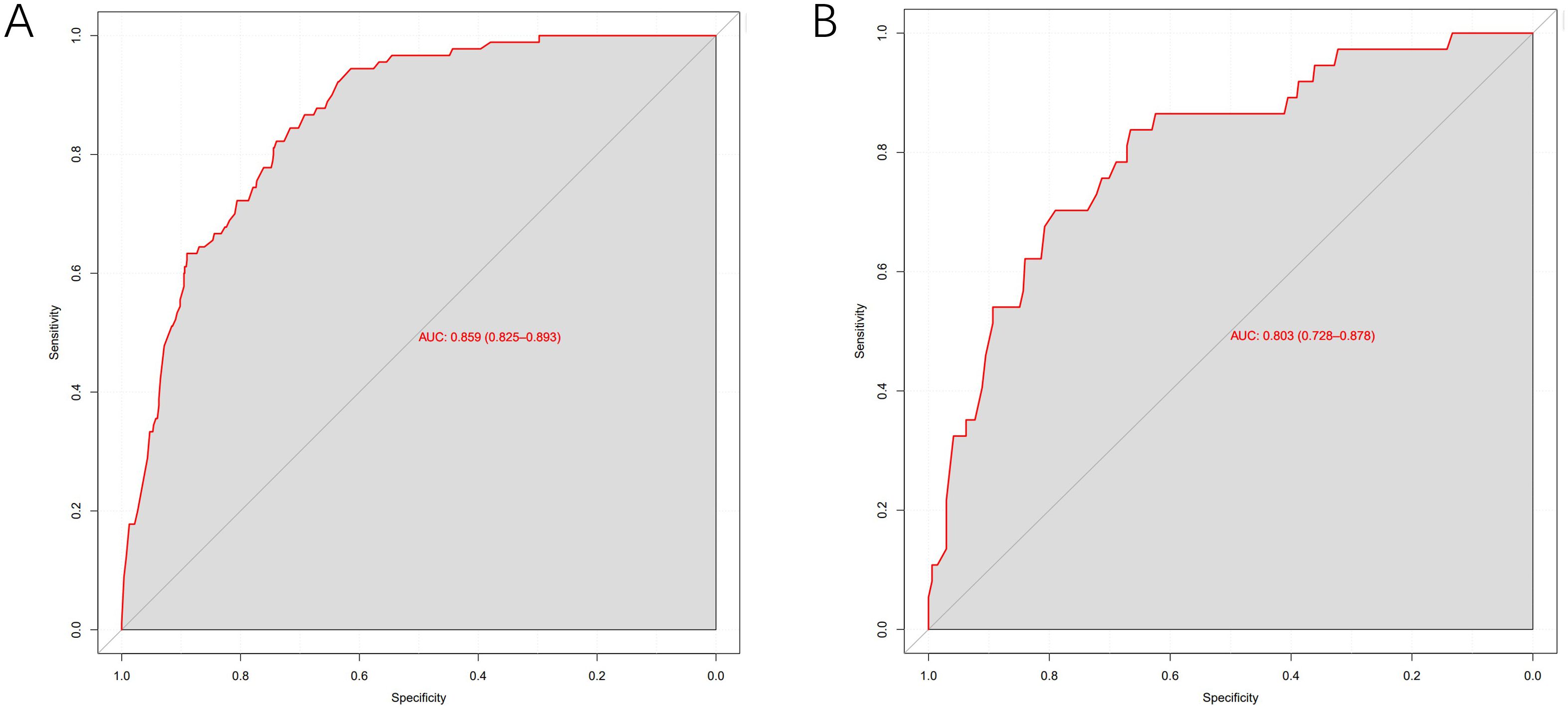
Figure 2. ROC curves of the predictive MLM model in the training cohort (A) and validation cohort (B).
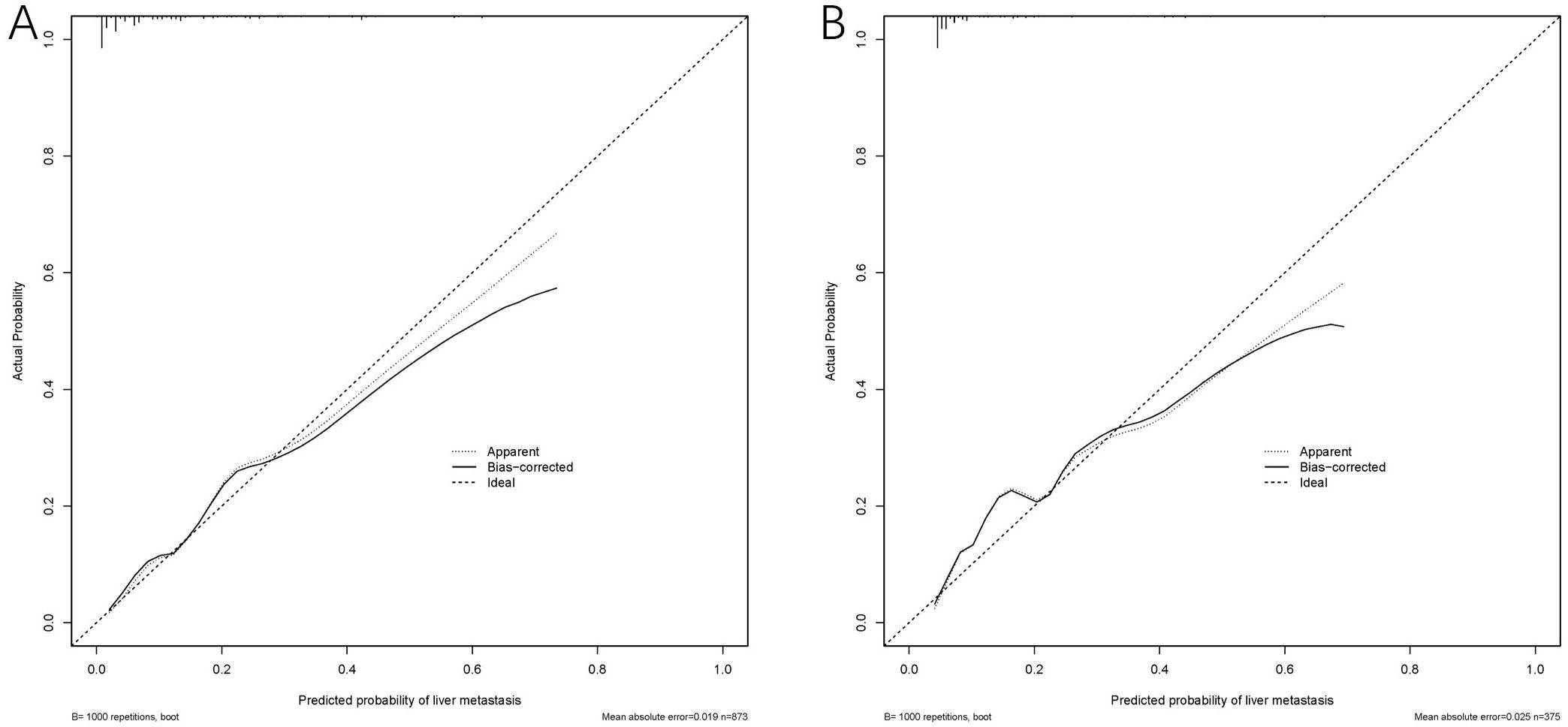
Figure 3. Calibration curves of the MLM nomogram in the cohort. Calibration curve for the training cohort (A) and the validation cohort (B).
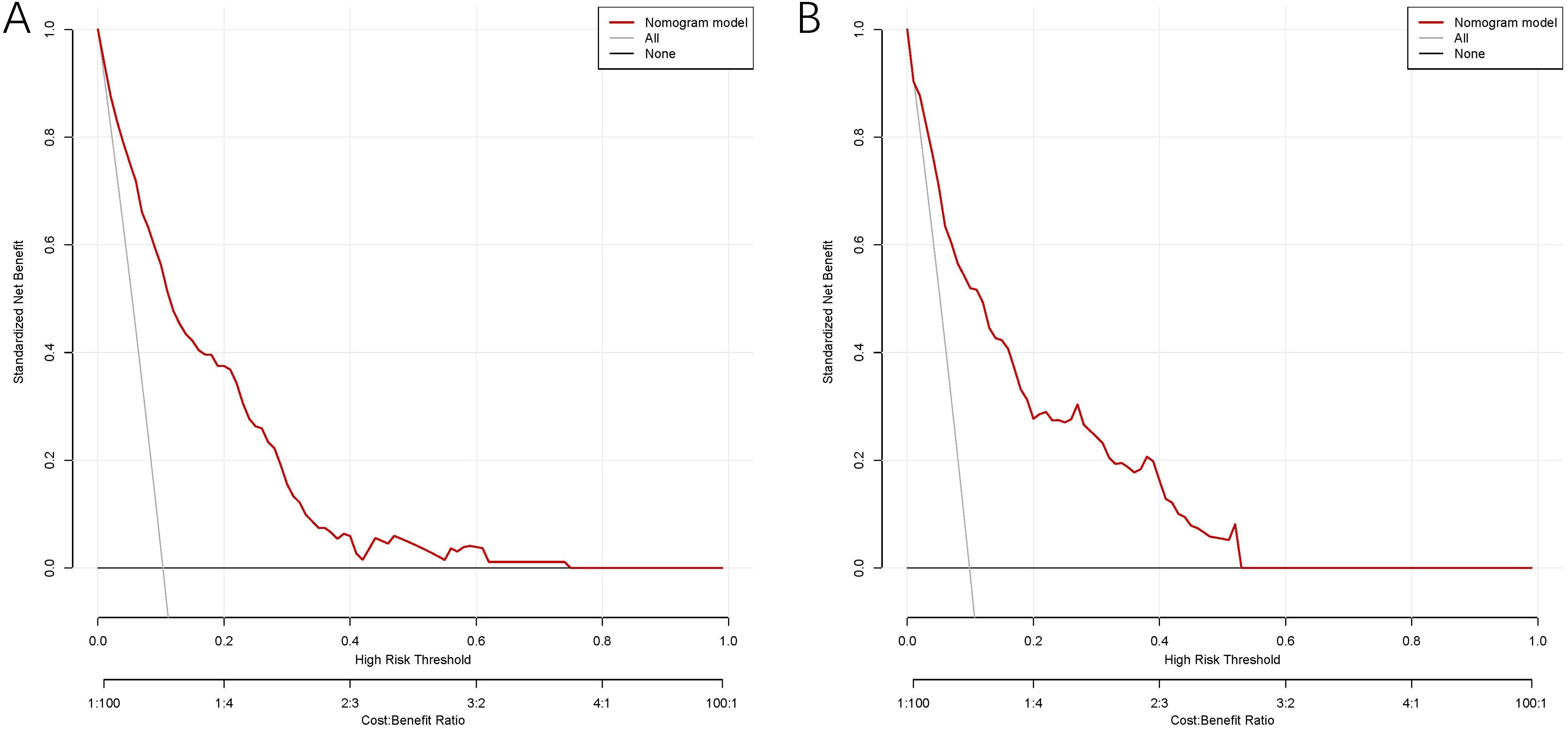
Figure 4. DCA of the nomogram model for predicting MLM in the training cohort (A) and validation cohort (B).
Discussion
MLM is closely associated with poor prognosis in gastric cancer, highlighting its critical role in the clinical management of advanced-stage disease (17). However, current research on MLM in gastric cancer remains limited. In this retrospective study, the results showed that T stage, N stage, AFP level, CA125 level, monocyte count, and lymphocyte count were independent predictors of MLM. Furthermore, we developed a nomogram and validated its high accuracy, reliable calibration, and robust clinical utility. Importantly, all variables are routinely obtained during treatment, emphasizing the nomogram’s cost-effectiveness and practical application.
Tumor markers serve as indispensable tools in oncological practice, providing critical insights for both diagnostic evaluation and prognostic stratification of malignancies (18). Recent studies have highlighted the significant value of tumor markers, particularly CEA, CA125, CA19-9, and CA72-4, in the diagnosis and monitoring of gastrointestinal malignancies (19–21). AFP was initially established as a diagnostic biomarker for primary liver cancer (22). In recent years, AFP was recently recognized as an independent prognostic factor for poor outcomes of gastric cancer (23), particularly in AFP-producing gastric cancer (24). Previous studies found that elevated AFP level in gastric cancer was a risk factor for postoperative liver metastasis (25–27), which were consistent with our findings. Study showed that overexpression of AFP in gastric cancer patients significantly inhibited the infiltration of CD8+T cell, could promote liver metastasis by regulating the PTEN/AKT1/SOX5/CES1 signaling axis (28). Moreover, overexpression of AFP upregulated malignancy-related pathways, such as epithelial-mesenchymal transition (EMT) and angiogenesis (29). These findings suggest a potential mechanism through which AFP facilitates MLM in gastric cancer.
CA125, a high-molecular-weight transmembrane glycoprotein, has been established as the gold-standard biomarker for ovarian cancer (30). Emerging evidence has elucidated its prognostic utility in gastrointestinal malignancies, particularly for risk stratification in gastric (31) and colorectal cancer (32). While multiple studies (18, 33) reported CA125 as a predictive factor for postoperative peritoneal metastasis in gastric cancer, only Yang et al. (26) identified CA125 as an independent risk factor for postoperative liver metastasis in gastric cancer, which is consistent with our findings. Currently, the potential role of CA125 promote MLM in gastrointestinal tumor remains unexplored. Marimuthu et al. (34) discovered that CA125 regulated NRP2 via JAK2/STAT1 signaling and induced liver metastasis in pancreatic ductal adenocarcinoma (PDAC). This mechanism may provide a novel research direction for investigating CA125-mediated MLM in gastric cancer. Based on this, we will conduct a multicenter cohort study to further validate the model’s efficacy and accuracy, while incorporating postoperative tumor markers (AFP and CA125) to test its predictive performance.
Notably, to the best of our knowledge, this was the first study to utilize absolute peripheral blood cell counts for predicting MLM in gastric cancer. Monocytes, as precursors of tumor-associated macrophages (TAMs), reflect changes in systemic immune function (35). Elevated monocyte count has been shown to be associated with poor prognosis in gastrointestinal tumors (36, 37). Additionally, Dou et al. (38) reported that monocytes can be used to predict postoperative liver metastasis in colorectal cancer. In our study, elevated monocyte count was identified for the first time as an independent risk factor for MLM in gastric cancer. As key mediators between innate and adaptive immunity, monocytes significantly influence the tumor microenvironment by facilitating immune tolerance, promoting vascular formation, and enhancing tumor cell spread through diverse biological pathways (35). Furthermore, distinct monocyte subpopulations directly contribute to metastatic progression through CXCL2-mediated interactions with circulating tumor cells (39).
As central effectors of adaptive immunity, lymphocytes play a crucial role in antitumor immune responses by inhibiting the progression of various cancers (40, 41). Previous studies demonstrated that lymphocyte count serves as a significant prognostic indicator associated with poor prognosis in gastric cancer (42, 43). Currently, the predictive potential of lymphocytes for MLM in gastrointestinal cancers remains unexplored. In our study, reduced lymphocyte count was identified as an independent predictor of MLM. Mechanistically, tumor cells promote the production of immunosuppressive molecules, potentially resulting in lymphopenia and facilitating immune evasion (44, 45). This decline provides tumor cells, which would normally be eliminated by the immune system, to evade immune surveillance. Consequently, these cells gain increased opportunities to metastasis.
As demonstrated in our results, our study established a novel nomogram to predict MLM in gastric cancer. Although prior studies (25, 27) have elucidated risk factors associated with postoperative liver metastasis, no dedicated predictive model for MLM has been established. While Yang et al. (26) developed a predictive model for postoperative liver metastasis, it lacks specificity for MLM and relies heavily on complex radiological parameters. Critically, our model innovatively incorporates preoperative peripheral blood parameters to establish a simple yet robust predictor, demonstrating superior discriminative value.
There were some limitations in our study which must be acknowledged. First of all, it was a retrospective, single-center study, and may have been prone to recall bias as well as loss to follow-up bias. Secondly, different methods could be used to calculate cutoff values, including median value, outliers and quartiles. A comparison of predictive model values based on these varying cutoff criteria was not conducted. Thirdly, the predictive value of postoperative tumor markers and peripheral blood cells were not evaluated.
In conclusion, our study developed and validated a novel, cost-effective, and easily accessible nomogram. Besides being predicted with conventional parameters, our model incorporated preoperative peripheral blood monocyte and lymphocyte counts to predict MLM risk in gastric cancer patients, which could enhance the model’s accuracy and predictive value.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The study was conducted in accordance with the Declaration of Helsinki and received ethical approval (KY20232248-C-1) from the Ethics Committee of the Xijing Hospital of the Fourth Military Medical University, following relevant guidelines and regulations. The study followed local legislation and institutional policies. Given the retrospective design of the study, the ethics committee review board waived the requirement for written informed consent for participation from the participants or the participants’ legal guardians/next of kin.
Author contributions
SW: Data curation, Formal Analysis, Investigation, Writing – original draft. GZ: Investigation, Methodology, Project administration, Validation, Writing – original draft. FW: Data curation, Investigation, Writing – original draft. YT: Data curation, Formal Analysis, Writing – original draft. XQ: Data curation, Formal Analysis, Writing – original draft. XD: Formal Analysis, Methodology, Writing – original draft. HD: Formal Analysis, Methodology, Writing – original draft. GR: Methodology, Writing – original draft. LN: Data curation, Writing – original draft. PW: Methodology, Writing – original draft. LD: Data curation, Writing – original draft. YY: Data curation, Writing – original draft. JZ: Funding acquisition, Investigation, Project administration, Resources, Supervision, Validation, Writing – review & editing. FF: Funding acquisition, Investigation, Project administration, Resources, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This study was supported by grants from the 2025 Booster Project of the First Affiliated Hospital of Air Force Medical University (XJZT25CX60); National Natural Science Foundation of China (82072655) and the 989th Hospital Research Project Program(9892023YNKT-08B).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Smyth EC, Nilsson M, Grabsch HI, van Grieken NC, and Lordick F. Gastric cancer. Lancet. (2020) 396:635–48. doi: 10.1016/S0140-6736(20)31288-5
2. Guan W, He Y, and Xu R. Gastric cancer treatment: recent progress and future perspectives. J Hematol Oncol. (2023) 16(1):57. doi: 10.1186/s13045-023-01451-3
3. Cheon SH, Rha SY, Jeung HC, Im CK, Kim SH, Kim HR, et al. Survival benefit of combined curative resection of the stomach (D2 resection) and liver in gastric cancer patients with liver metastases. Ann Oncol. (2008) 19:1146–53. doi: 10.1093/annonc/mdn026
4. Xiao Y, Zhang B, and Wu Y. Prognostic analysis and liver metastases relevant factors after gastric and hepatic surgical treatment in gastric cancer patients with metachronous liver metastases: a population-based study. Irish J Med Sci (1971). (2019) 188:415–24. doi: 10.1007/s11845-018-1864-4
5. Ryu T, Takami Y, Wada Y, Tateishi M, Matsushima H, Yoshitomi M, et al. Oncological outcomes after hepatic resection and/or surgical microwave ablation for liver metastasis from gastric cancer. Asian J Surg. (2019) 42:100–05. doi: 10.1016/j.asjsur.2017.09.005
6. Xie L, Qiu S, Lu C, Gu C, Wang J, Lv J, et al. Gastric cancer-derived LBP promotes liver metastasis by driving intrahepatic fibrotic pre-metastatic niche formation. J Exp Clin Cancer Res. (2023) 42(1):258. doi: 10.1186/s13046-023-02833-8
7. Thelen A, Jonas S, Benckert C, Lopez-Hanninen E, Neumann U, Rudolph B, et al. Liver resection for metastatic gastric cancer. Eur J Surg Oncol. (2008) 34:1328–34. doi: 10.1016/j.ejso.2008.01.022
8. Meng N, Niu X, Wu J, Wu H, Li T, Yang J, et al. Development and validation of nomogram models for predicting overall survival and cancer-specific survival in gastric cancer patients with liver metastases: a cohort study based on the SEER database. Am J Cancer Res. (2024) 14:2272–86. doi: 10.62347/ZPPK5664
9. Granieri S, Altomare M, Bruno F, Paleino S, Bonomi A, Germini A, et al. Surgical treatment of gastric cancer liver metastases: Systematic review and meta-analysis of long-term outcomes and prognostic factors. Crit Rev Oncology/Hematology. (2021) 163:103313. doi: 10.1016/j.critrevonc.2021.103313
10. Fujitani K, Kurokawa Y, Wada R, Takeno A, Kawabata R, Omori T, et al. Prospective single-arm multicenter interventional study of surgical resection for liver metastasis from gastric cancer; 3-year overall and recurrence-free survival. Eur J Cancer. (2024) 213:115080. doi: 10.1016/j.ejca.2024.115080
11. Huang F and Fang M. Prediction model of liver metastasis risk in patients with gastric cancer: A population-based study. Med (Baltimore). (2023) 102:e34702. doi: 10.1097/MD.0000000000034702
12. Garcia-Flores LA, Dawid De Vera MT, Pilo J, Rego A, Gomez-Casado G, Arranz-Salas I, et al. Increased neutrophil counts are associated with poor overall survival in patients with colorectal cancer: a five-year retrospective analysis. Front Immunol. (2024) 15:1415804. doi: 10.3389/fimmu.2024.1415804
13. Yu W, Bao L, Qian Z, and Wang D. Neutrophil-lymphocyte ratio combined with plasma fibrinogen is an useful predictor for the diagnosis of liver metastases from gastric cancer. Asian J Surg. (2022) 45:2378–79. doi: 10.1016/j.asjsur.2022.05.058
14. Zhao G, Liu N, Wang S, Guo J, Song X, Qi Y, et al. Prognostic significance of the neutrophil-to-lymphocyte and platelet-to-lymphocyte ratio in patients with metastatic gastric cancer. Med (Baltimore). (2020) 99:e19405. doi: 10.1097/MD.0000000000019405
15. Zhang L, Wei Z, Xu A, and Zang JH. Can the neutrophil-lymphocyte ratio and platelet-lymphocyte ratio be beneficial in predicting lymph node metastasis and promising prognostic markers of gastric cancer patients? Tumor maker retrospective study. Int J Surg. (2018) 56:320–27. doi: 10.1016/j.ijsu.2018.06.037
16. Youden WJ. Index for rating diagnostic tests. Cancer. (1950) 3:32–5. doi: 10.1002/1097-0142(1950)3:1<32::AID-CNCR2820030106>3.0.CO;2-3
17. Wang C, Zhang Y, Zhang Y, and Li B. A bibliometric analysis of gastric cancer liver metastases: advances in mechanisms of occurrence and treatment options. Int J Surg. (2024) 110:2288–99. doi: 10.1097/JS9.0000000000001068
18. Bao D, Yang Z, Chen S, Li K, and Hu Y. Construction of a nomogram model for predicting peritoneal dissemination in gastric cancer based on clinicopathologic features and preoperative serum tumor markers. Front Oncol. (2022) 12:844786. doi: 10.3389/fonc.2022.844786
19. Shibata C, Nakano T, Yasumoto A, Mitamura A, Sawada K, Ogawa H, et al. Comparison of CEA and CA19–9 as a predictive factor for recurrence after curative gastrectomy in gastric cancer. BMC Surg. (2022) 22(1):213. doi: 10.1186/s12893-022-01667-z
20. Zhao J, Qin R, Chen H, Yang Y, Qin W, Han J, et al. A nomogram based on glycomic biomarkers in serum and clinicopathological characteristics for evaluating the risk of peritoneal metastasis in gastric cancer. Clin Proteomics. (2020) 17:34. doi: 10.1186/s12014-020-09297-4
21. Xu Y, Zhang P, Zhang K, and Huang C. The application of CA72–4 in the diagnosis, prognosis, and treatment of gastric cancer. Biochim Biophys Acta Rev Cancer. (2021) 1876:188634. doi: 10.1016/j.bbcan.2021.188634
22. Galle PR, Foerster F, Kudo M, Chan SL, Llovet JM, Qin S, et al. Biology and significance of alpha-fetoprotein in hepatocellular carcinoma. Liver Int. (2019) 39:2214–29. doi: 10.1111/liv.14223
23. Zhan Z, Chen B, Yu J, Zheng J, Zeng Y, Sun M, et al. Elevated serum alpha-fetoprotein is a significant prognostic factor for patients with gastric cancer: results based on a large-scale retrospective study. Front Oncol. (2022) 12:901061. doi: 10.3389/fonc.2022.901061
24. Kong X, Li X, Tian Y, Ye Q, Xu X, Liu Y, et al. The clinicopathological characteristics of alpha-fetoprotein-producing adenocarcinoma of the gastrointestinal tract—A single-center retrospective study. Front Oncol. (2021) 11:635537. doi: 10.3389/fonc.2021.635537
25. Takayama-Isagawa Y, Kanetaka K, Kobayashi S, Yoneda A, Ito S, and Eguchi S. High serum alpha-fetoprotein and positive immunohistochemistry of alpha-fetoprotein are related to poor prognosis of gastric cancer with liver metastasis. Sci Rep. (2024) 14. doi: 10.1038/s41598-024-54394-1
26. Yang H, Sun J, Liu H, Liu X, She Y, Zhang W, et al. Clinico-radiological nomogram for preoperatively predicting post-resection hepatic metastasis in patients with gastric adenocarcinoma. Br J Radiol. (2022) 95:20220488. doi: 10.1259/bjr.20220488
27. Chen Y, Qu H, Jian M, Sun G, and He Q. High level of serum AFP is an independent negative prognostic factor in gastric cancer. Int J Biol Markers. (2015) 30:387–93. doi: 10.5301/jbm.5000167
28. Li Y, Lin Y, Zhao L, Yang C, Wang B, Gao Z, et al. Characteristics of alpha-fetoprotein-positive gastric cancer revealed by analysis of cancer databases and transcriptome sequencing data. Transl Oncol. (2023) 36:101737. doi: 10.1016/j.tranon.2023.101737
29. Mei Y, Li M, Wen J, Kong X, and Li J. Single-cell characteristics and Malignancy regulation of alpha-fetoprotein-producing gastric cancer. Cancer Med. (2023) 12:12018–33. doi: 10.1002/cam4.5883
30. Han CY, Lu KH, Corrigan G, Perez A, Kohring SD, Celestino J, et al. Normal risk ovarian screening study: 21-year update. J Clin Oncol. (2024) 42:1102–09. doi: 10.1200/JCO.23.00141
31. Lu L, Fang W, Yu J, Gao X, Wang X, Pan Y, et al. Development and validation of serological dynamic risk score to predict outcome in gastric cancer with adjuvant chemotherapy: a multicentre, longitudinal, cohort study. Front Oncol. (2024) 14:1327691. doi: 10.3389/fonc.2024.1327691
32. Magnusson MI, Agnarsson BA, Jonasson JG, Tryggvason T, Aeffner F, le Roux L, et al. Histopathology and levels of proteins in plasma associate with survival after colorectal cancer diagnosis. Br J Cancer. (2023) 129:1142–51. doi: 10.1038/s41416-023-02374-z
33. Shimada H, Noie T, Ohashi M, Oba K, and Takahashi Y. Clinical significance of serum tumor markers for gastric cancer: a systematic review of literature by the Task Force of the Japanese Gastric Cancer Association. Gastric Cancer. (2014) 17:26–33. doi: 10.1007/s10120-013-0259-5
34. Marimuthu S, Lakshmanan I, Muniyan S, Gautam SK, Nimmakayala RK, Rauth S, et al. MUC16 promotes liver metastasis of pancreatic ductal adenocarcinoma by upregulating NRP2-associated cell adhesion. Mol Cancer Res. (2022) 20:1208–21. doi: 10.1158/1541-7786.MCR-21-0888
35. Ugel S, Canè S, De Sanctis F, and Bronte V. Monocytes in the tumor microenvironment. Annu Rev Pathol. (2021) 16:93–122. doi: 10.1146/annurev-pathmechdis-012418-013058
36. Feng F, Zheng G, Wang Q, Liu S, Liu Z, Xu G, et al. Low lymphocyte count and high monocyte count predicts poor prognosis of gastric cancer. BMC Gastroenterol. (2018) 18(1):148. doi: 10.1186/s12876-018-0877-9
37. Jakubowska K, Koda M, Grudzińska M, Kańczuga-Koda L, and Famulski W. Monocyte-to-lymphocyte ratio as a prognostic factor in peripheral whole blood samples of colorectal cancer patients. World J Gastroenterol. (2020) 26:4639–55. doi: 10.3748/wjg.v26.i31.4639
38. Dou X, Xi J, Zheng G, Ren G, Tian Y, Dan H, et al. A nomogram was developed using clinicopathological features to predict postoperative liver metastasis in patients with colorectal cancer. J Cancer Res Clin Oncol. (2023) 149:14045–56. doi: 10.1007/s00432-023-05168-1
39. Qian B, Li J, Zhang H, Kitamura T, Zhang J, Campion LR, et al. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature. (2011) 475:222–25. doi: 10.1038/nature10138
40. Zhai Z, Gao J, Zhu Z, Cong X, Lou S, Han B, et al. The ratio of the hemoglobin to red cell distribution width combined with the ratio of platelets to lymphocytes can predict the survival of patients with gastric cancer liver metastasis. BioMed Res Int. (2021) 2021:1–12. doi: 10.1155/2021/8729869
41. Wu D, Zhong J, Jiang W, Liao Z, Huang S, Sun Y, et al. Preoperative combination score of neutrophils, monocytes, and lymphocytes as a predictor for locally advanced rectal cancer. Int J Colorectal Dis. (2022) 37:1097–106. doi: 10.1007/s00384-022-04143-5
42. Park SJ, Lee J, Kim H, Shin K, Lee M, Park JM, et al. Association between absolute lymphocyte count and overall mortality in patients with surgically resected gastric cancer. Korean J Internal Med. (2021) 36:679–88. doi: 10.3904/kjim.2019.358
43. Saito H, Kono Y, Murakami Y, Shishido Y, Kuroda H, Yamamoto M, et al. Prognostic significance of pre- and postoperative lymphocyte counts in patients with gastric cancer. Dig Surg. (2019) 36:137–43. doi: 10.1159/000486581
44. Huang B, Zhao J, Unkeless JC, Feng ZH, and Xiong H. TLR signaling by tumor and immune cells: a double-edged sword. Oncogene. (2008) 27:218–24. doi: 10.1038/sj.onc.1210904
Keywords: gastric cancer, monocyte, lymphocyte, metachronous liver metastasis, nomogram
Citation: Wang S, Zheng G, Wu F, Tian Y, Qiao X, Dou X, Dan H, Ren G, Niu L, Wang P, Duan L, Yang Y, Zheng J and Feng F (2025) Development of a predictive model for metachronous liver metastasis in gastric cancer. Front. Oncol. 15:1603471. doi: 10.3389/fonc.2025.1603471
Received: 31 March 2025; Accepted: 25 July 2025;
Published: 18 August 2025.
Edited by:
Yu Min, Sichuan University, ChinaReviewed by:
Michela Giulii Capponi, Santo Spirito in Sassia Hospital, ItalyMehmet Torun, Erzurum City Hospital, Türkiye
Copyright © 2025 Wang, Zheng, Wu, Tian, Qiao, Dou, Dan, Ren, Niu, Wang, Duan, Yang, Zheng and Feng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jianyong Zheng, emhqeTY4QDE2My5jb20=; Fan Feng, c3VyZ2VvbmZlbmdmYW5AMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Siyuan Wang
Siyuan Wang Gaozan Zheng1†
Gaozan Zheng1† Ye Tian
Ye Tian Guangming Ren
Guangming Ren Jianyong Zheng
Jianyong Zheng Fan Feng
Fan Feng