- 1Department of Diagnostic Radiology, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
- 2Department of Pathology, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
Background: Epstein-Barr virus (EBV)-positive inflammatory follicular dendritic cell sarcoma (EBV+ IFDCS) represents a low-grade malignancy arising from the proliferation of follicular dendritic cells. This distinct and rare subtype, characterized by abundant lymphoplasmacytic infiltration, is closely linked to EBV infection and is seldom encountered in clinical practice.
Methods: Presented here are three cases of primary EBV+ IFDCS, occurring in the liver and spleen. This study systematically analyzed the clinical presentations, radiological features, and pathological characteristics of our cases. Additionally, we conducted a comprehensive review of the respective characteristics documented in the existing literature.
Results: We present three cases of EBV+ IFDCS, with lesions localized to the spleen (n=2) and liver (n=1). Notably, only one patient developed clinical symptoms secondary to splenic mass rupture and post-embolization sequelae, while the remaining cases were identified incidentally without associated symptomatology. All three patients underwent preoperative contrast-enhanced magnetic resonance imaging (CT) scans demonstrating solitary, well-circumscribed round masses/nodules. The two splenic lesions exhibited necrotic-cystic degeneration and one displayed a capsule, with absence of calcification in all cases. Tumor parenchyma showed mild arterial-phase enhancement and partial delayed-phase washout. The two splenic cases underwent additional magnetic resonance imaging (MRI) evaluation, revealing restricted diffusion in the solid tumor components and apparent diffusion coefficient (ADC) values comparable to the surrounding splenic parenchyma. Complete surgical excision was performed in all patients, and histopathological evaluation confirmed the diagnosis of EBV+ IFDCS through immunohistochemical analysis. As of the latest follow-up, all three patients are alive.
Conclusion: EBV+ IFDCS is a rare condition that primarily arises in the liver and spleen, with prognosis varying among patients with primary tumors in different organs. This study presents three cases of EBV+ IFDCS that occurred in diverse anatomical locations, examines their clinical, radiological, pathological features and differential diagnoses, and aims to deepen the understanding of clinicians and radiologists regarding this form of Mesenchymal dendritic cell neoplasm.
Introduction
EBV-positive inflammatory follicular dendritic cell sarcoma (EBV+ IFDCS) is an exceptionally rare low-grade malignant tumor. According to World Health Organization (WHO) Classification of Tumors of Haematopoietic and Lymphoid Tissues, 5th edition (WHO-HAEM5), it is classified as a distinct subtype under “Mesenchymal dendritic cell neoplasm”, with particular emphasis on its “inflammatory” characteristics (a background of lymphoplasmacytic infiltration) and the central role of EBV infection in its pathogenesis (1). This distinguishes it from traditional non-inflammatory follicular dendritic cell sarcomas. Globally, its annual incidence remains undefined due to its extreme rarity and frequent misdiagnosis. The tumor predominantly occurs in the liver and spleen, while involvement of the lungs, gastrointestinal tract, or lymph nodes is exceedingly uncommon (2, 3).
The three cases reported herein - spanning the spleen and liver - expand the documented spectrum of EBV+ IFDCS and reinforce the centrality of EBV in its pathogenesis.
Case presentation
Case 1
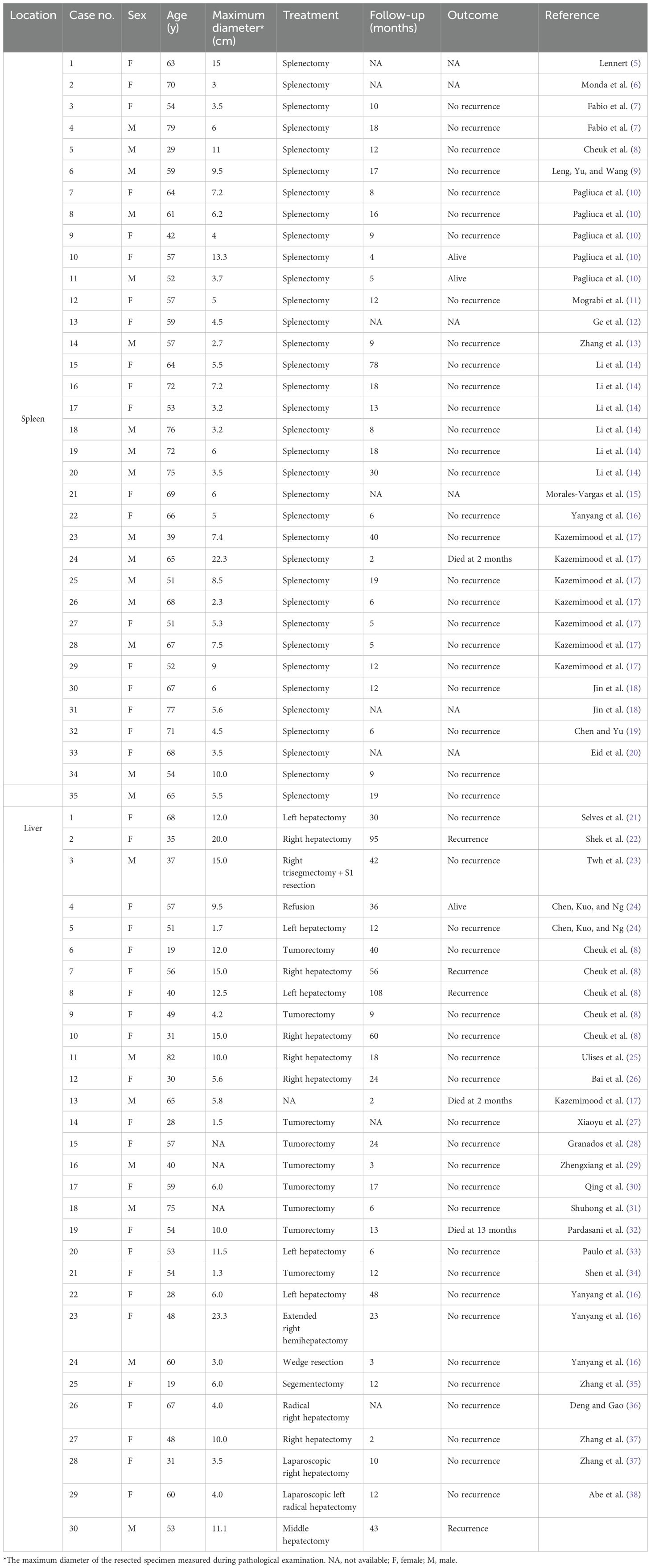
The 54-year-old male patient was referred to our hospital after a splenic puncture rupture and subsequent splenic embolization. The patient had no significant medical or family history of similar conditions. Imaging at our facility showed a well-defined, roundish mass in the spleen with heterogeneous density on computed tomography (CT) and mixed signal intensity on magnetic resonance imaging (MRI), indicative of necrosis and hemorrhage, without evidence of metastasis (Figure 1).
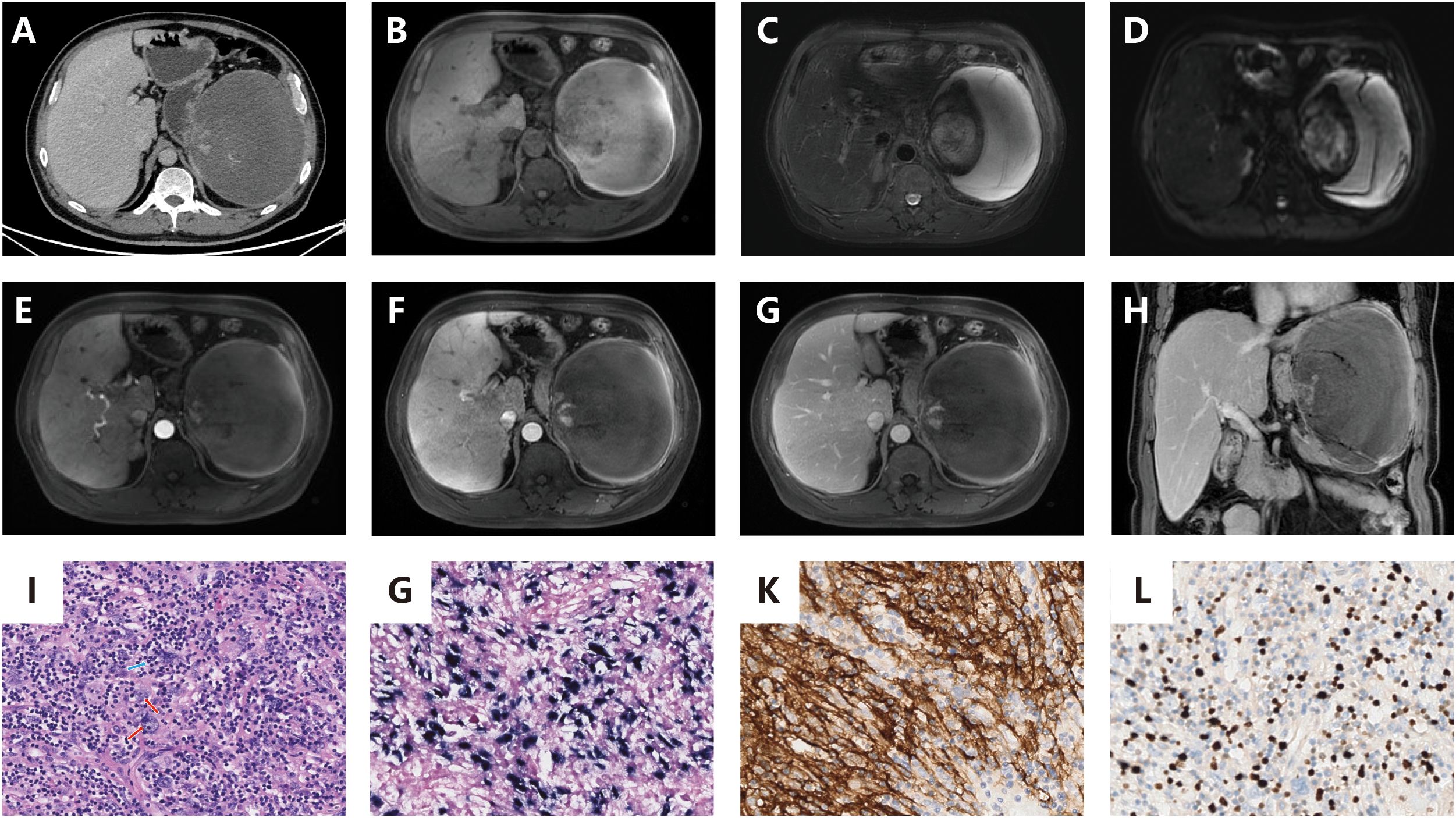
Figure 1. (A): Contrast-enhanced CT reveals a well-defined, rounded splenic mass with mild enhancement of solid components, a visible capsule, absence of calcification, and features of hemorrhage and necrotic cystic degeneration. No evidence of metastatic spread was identified; (B): Contrast-enhanced MRI demonstrates a heterogeneously hypointense signal on pre-contrast T1-weighted imaging; (C): T2-weighted MRI sequences show heterogeneously intermediate-to-hyperintense signal with internal hypointense areas; (D): DWI reveals heterogeneously hyperintense signal; (E–G) (axial): Post-contrast axial scans exhibit minimal enhancement in most regions with focal enhancing areas, showing no delayed phase progression. The tumor is encapsulated and devoid of fatty components; (H) (coronal): Post-contrast coronal scan confirms the encapsulated tumor with minimal and focal enhancement; (I) (x200): Histopathological examination with hematoxylin-eosin (HE) staining displays spindle-shaped neoplastic cells arranged in a storiform pattern, featuring indistinct cellular boundaries, prominent nucleoli, and marked inflammatory infiltrate (red arrow: neoplastic cells; blue arrow: lymphocytes); (J) (x200): EBER in situ hybridization confirms nuclear positivity within tumor cells; (K) (x200): Immunohistochemical analysis reveals CD21 membranous expression in neoplastic cells; (L) (x200): Ki-67 labeling demonstrates a low proliferative index.
The patient underwent a splenectomy. Intraoperative findings included an enlarged, irregular spleen without significant adhesions. Pathological examination of the resected spleen revealed a spindle cell tumor with extensive necrosis and lymphoplasmacytic infiltration, measuring 25×17×6 cm overall, with the solid portion being 10×7×6.5 cm. Immunohistochemical analysis confirmed the presence of CD163, CD21, and EBER positivity, leading to a diagnosis of EBV+ IFDCS.
Postoperatively, the patient recovered well without complications. At the last follow-up on March 17, 2025, there were no signs of recurrence.
Case 2
A 65-year-old male with a history of gastric cancer was admitted for evaluation of a splenic mass found during routine follow-up. He showed no symptoms and there was no family history of similar conditions. Imaging revealed a poorly defined, roundish mass in the spleen with mild enhancement on CT and mixed signal intensity on MRI, indicating necrosis with chronic hemorrhage but no evidence of a capsule, calcification, or metastasis (Figure 2).
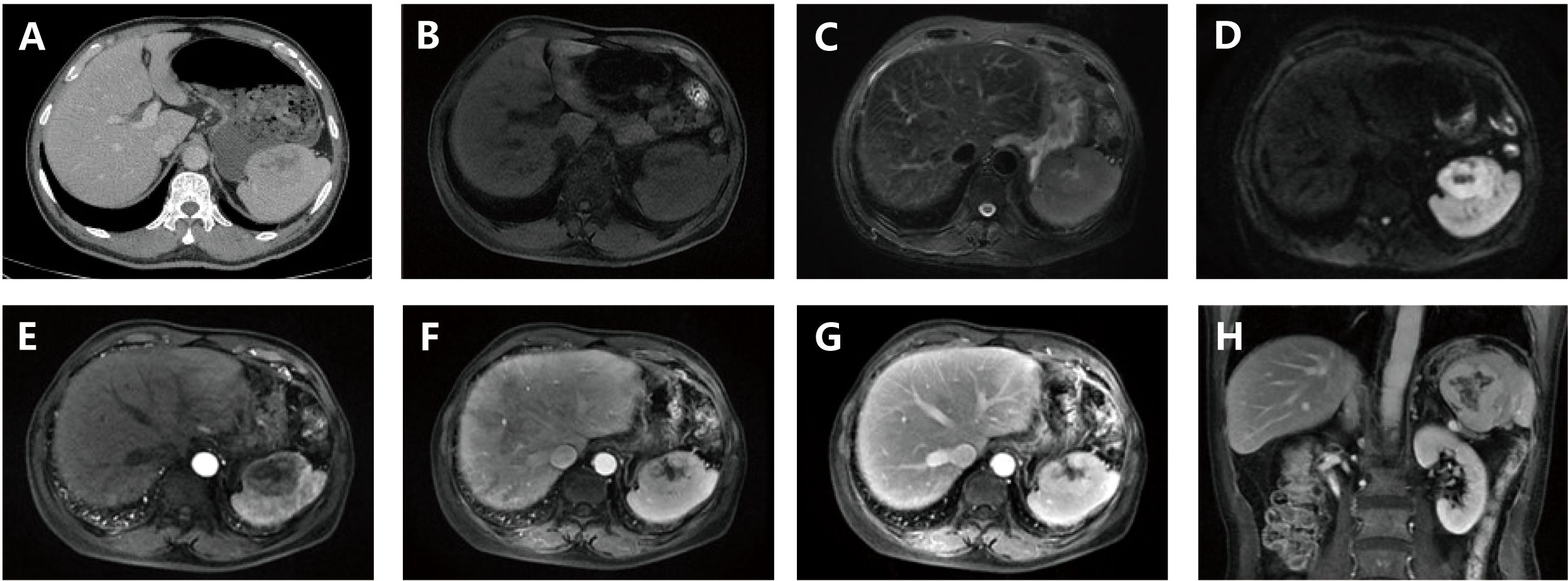
Figure 2. (A) Contrast-enhanced CT demonstrates a splenic space-occupying lesion (mass) exhibiting a rounded morphology with ill-defined margins. The solid components display mild contrast enhancement, while cystic necrosis and poorly-defined hemorrhage are observed. Absence of a capsule, calcification, or metastatic features is noted; (B) Pre-contrast MRI shows the lesion with peripheral isointensity and central hypointensity; (C) T2-weighted MRI demonstrates peripheral isointensity with central intermediate-to-hyperintense signal; (D) DWI) reveals peripheral hyperintensity surrounding a central hypointense region; (E–G) (axial): Post-contrast axial imaging reveals mild-to-moderate heterogeneous enhancement without evidence of encapsulation or fatty components; (H) (coronal): Post-contrast coronal imaging confirms mild-to-moderate heterogeneous enhancement without evidence of encapsulation or fatty components.
The patient underwent a splenectomy. Intraoperatively, an enlarged, irregular spleen without significant adhesions was noted. The resected spleen measured 7.5×6×5 cm, containing a 5.5×4.6×4 cm gray-white, firm tumor with central hemorrhage near the capsule. Pathology diagnosed spindle cell proliferation with lymphoplasmacytic infiltration. Immunohistochemistry confirmed positivity for SMA, Vimentin, Ki-67 (15%), and follicular dendritic cell markers (CD21, CD23, CD35), along with EBER positivity.
The final diagnosis was EBV+ IFDCS. Post-splenectomy, the patient recovered well without complications. At the last follow-up on March 17, 2025, there were no signs of recurrence.
Case 3
A 53-year-old female was admitted for a hepatic mass found in the liver. She had previously undergone three cycles of chemotherapy and had no significant symptoms or family history of similar conditions. Imaging revealed an ill-defined, irregular mass in the liver’s hilar region with low-density on CT and mild-to-moderate enhancement, showing necrotic changes but no capsule, calcification, hemorrhage, or metastasis (Figure 3).
Figure 3. (A, B): Contrast-enhanced CT demonstrates an irregularly shaped, poorly demarcated space-occupying lesion within the hepatic hilar region. The lesion exhibits progressive mild-to-moderate enhancement on post-contrast phases, accompanied by internal cystic necrosis. Absence of encapsulation, calcification, hemorrhage, or metastatic features is noted; (C) (x200): Histopathological evaluation with HE staining displays neoplastic cells arranged in a storiform pattern, characterized by plump spindle-shaped morphology, indistinct cellular boundaries, prominent nucleoli, pale cytoplasm, and marked inflammatory infiltrate (red arrow: neoplastic cells; blue arrow: lymphocytes); (D) (x200): EBER in situ hybridization confirms nuclear positivity within tumor cells; (E) (x200): Immunohistochemical analysis reveals CD21 membranous expression in neoplastic cells; F (x200): Ki-67 labeling demonstrates a low proliferative index.
She underwent a middle hepatectomy. The resected specimen contained a 13×11×6.5 cm gray-white, firm tumor with areas of necrosis. Pathology diagnosed spindle or ovoid cell proliferation with lymphocytic and plasmacytic infiltration, and immunohistochemistry confirmed EBV+ IFDCS.
The final diagnosis was EBV+ IFDCS. Postoperatively, she recovered well without complications. The most recent follow-up occurred on March 17, 2025, at which time the patient was alive with the tumor still present. During the period following the surgery and up to the last follow-up, there was a recurrence of the tumor within the liver. Currently, this recurrence is being managed through ablation therapy.
Discussion
This study provides the analysis of EBV+ IFDCS involving two distinct anatomical sites (spleen, liver), elucidating the organ-specific radiological-pathological correlations and diagnostic challenges within the WHO 5th edition classification framework. According to the WHO-HAEM5 released in 2022, EBV+ IFDCS is formally defined as a distinct stromal-derived dendritic cell tumor (1). As a distinct disease entity, the fifth edition further clarifies the biological distinctions between EBV+ IFDCS and conventional FDCS, while highlighting the central role of EBV in its pathogenesis (1, 4).
EBV+ IFDCS primarily occurs in the spleen and liver and exhibits relatively distinct characteristics, such as a female predominance, prolonged clinical course, presence of a prominent inflammatory background, and association with EBV infection. In our analysis of previously published literature, we identified a total of 35 cases of EBV+ IFDCS in the spleen (5–20), and 30 cases in the liver (8, 16, 17, 21–38), including the case presented in this report (Spleen: NO.34 and 35; Liver: NO.30) (Table 1). Our statistical findings indicate that both splenic and hepatic EBV+ IFDCS cases predominantly affect females, with a male-to-female ratio of approximately 1:1.82, aligning with prior studies. Follow-up data spanning between 2 and 108 months were available for 59 patients. Among the 29 patients with splenic EBV+ IFDCS who had follow-up data, only one death was reported by the last follow-up, with no observed recurrences, yielding an overall mortality rate of 3.4%. Conversely, among the 30 patients with hepatic IFDCS who had follow-up data, two deaths and four recurrences were noted by the last follow-up, resulting in an overall mortality rate of 6.7% and a recurrence rate of 13.3%.
The pathogenesis of this tumor is closely associated with chronic EBV infection, likely attributable to EBV’s tropism for B lymphocytes and its latent genes contributing to tumorigenesis through apoptosis inhibition and immune evasion (38, 39). Specifically, EBV drives malignant transformation of follicular dendritic cells via latent membrane protein 1 (LMP1)-mediated hyperactivation of the nuclear factor kappa B (NF-κB) signaling pathway, resulting in pathological hallmarks including spindle-to-ovoid tumor cells arranged in fascicles or storiform patterns, peritumoral lymphocytic infiltration, thin-walled vascular proliferation, and focal necrosis/hemorrhage. The presence of EBER in situ hybridization positivity can strongly suggest EBV infection within the tumor and is crucial for the diagnosis of EBV+ IFDCS.
Patients with EBV+ IFDCS usually lack clinical symptoms; therefore, radiology examinations are often the first method for detection. The imaging manifestations directly and indirectly reflect the aforementioned pathological features: CT scans of all three patients revealed poorly demarcated, solitary soft tissue masses in our case series, exhibiting heterogeneous density due to necrosis/hemorrhage, demonstrating patchy heterogeneous enhancement (marked enhancement in vascular-rich areas and non-enhancement in necrotic zones); MRI T2-weighted imaging (T2WI) exhibits mixed high-low signal intensities corresponding to cellular-dense regions, collagen fibers, and hemosiderin deposition, while T1-weighted imaging (T1WI) may show focal hyperintensity related to hemorrhage (40). Crucially, EBV+ IFDCS not only presents common radiological characteristics but also manifests distinct organ-specific imaging phenotypes shaped by tissue microenvironment heterogeneity. Hepatic lesions manifest on CT as hypodense masses with central non-enhancing area, pathologically corresponding to LMP1-induced vascular hyalinization and thrombotic ischemic necrosis via excessive vascular endothelial growth factor (VEGF) pathway activation (41). Conversely, splenic lesions demonstrate “snowflake-like” T2WI hyperintensity with delayed enhancement on MRI, reflecting an inflammatory-tumor microenvironment characterized by EBV-driven lymphocyte infiltration and microhemorrhages. These organ-specific imaging variations not only unveil EBV’s divergent molecular mechanisms (vascular dysregulation vs. inflammatory recruitment) but also provide critical diagnostic discriminators. For instance, hepatic EBV+ IFDCS must be differentiated from hepatocellular carcinoma, which typically arises in cirrhotic livers and displays “wash-out” enhancement patterns, and from angiosarcoma, which exhibits aggressive features such as central patchy persistent enhancement with infiltrative margins (42). In the spleen, EBV+ IFDCS requires distinction from sclerosing angiomatoid nodular transformation (SANT), characterized by well-demarcated centripetal progressive enhancement and spoke-wheel patterns, and from splenic lymphoma, which often presents as multifocal coalescing masses with mild enhancement, splenic hilar/retroperitoneal lymphadenopathy, and restricted diffusion on diffusion weighted imaging (DWI).
The heterogeneous nature of microenvironments imparts organ-specific prognostic influences. Our findings demonstrate that patients with primary EBV+IFDCS originating in the liver exhibit a mortality rate nearly twice that of patients with splenic tumors during follow-up. Specifically, within the liver microenvironment, liver sinusoidal endothelial cells (LSECs) exhibit elevated expression of VEGF and intercellular cell adhesion molecule-1 (ICAM-1), facilitating tumor cell adhesion and angiogenesis (43). Concurrently, Kupffer cells (KCs) secrete IL-6 and TGF-β, thereby inducing epithelial-mesenchymal transition (EMT) in tumor cells. In contrast, the splenic microenvironment demonstrates a higher density of CD8+ T cells and NK cells within the red pulp, which suppress tumor proliferation through the PD-1/PD-L1 axis (44). Additionally, splenic tumor cells show heightened susceptibility to ferroptosis due to hypoxic conditions, whereas the oxygen-rich hepatic microenvironment promotes tumor survival (43). These prognostic disparities highlight the necessity for rigorous clinical management strategies in primary hepatic EBV+IFDCS to optimize patient outcomes.
In recent years, as more cases of a similar nature have been documented, our comprehension of EBV+ IFDCS has enhanced. Overall, the three cases presented in this study are consistent with the existing literature pertaining to this disease. When intra-abdominal lesions resembling inflammatory pseudotumor, particularly in the spleen, are suspected, diagnosing EBV+ IFDCS can become comparatively straightforward. Magnetic resonance imaging (MRI), with its high resolution, provides crucial detailed information about the lesion, aiding in a more precise diagnosis.
Conclusion
EBV+ IFDCS is an uncommon and frequently underrecognized neoplasm that can manifest in various anatomical locations. Its rarity, combined with non-specific clinical presentations and imaging characteristics that may resemble those of other inflammatory or neoplastic conditions, makes EBV+ IFDCS a significant challenge for differential diagnosis. Consequently, this leads to low preoperative diagnostic rates and a potential for misinterpretation.
This report details three cases of EBV+ IFDCS, presenting their clinicopathological characteristics and imaging features, including CT and MRI findings. By reviewing relevant literature, the study aims to deepen the understanding of EBV+ IFDCS, highlight key diagnostic indicators, and improve diagnostic accuracy and awareness among medical professionals. Ultimately, this work seeks to reduce the likelihood of misdiagnosis and guide more precise and timely therapeutic interventions.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Cancer Hospital, Chinese Academy of Medical Sciences. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
WB: Investigation, Visualization, Writing – original draft. CH: Investigation, Visualization, Writing – review & editing. ZZ: Conceptualization, Funding acquisition, Investigation, Visualization, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the National Natural Science Foundation of China (grant number 82102029); National High Level Hospital Clinical Research Funding (grant number LC2024A09); and Teaching Research Fund of Cancer Hospital of Chinese Academy of Medical Sciences (grant number E2024015).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2025.1603496/full#supplementary-material
References
1. Alaggio R, Amador C, Anagnostopoulos I, Attygalle AD, Araujo IBO, Berti E, et al. The 5th edition of the world health organization classification of haematolymphoid tumours: lymphoid neoplasms. Leukemia. (2022) 36:1720–48. doi: 10.1038/s41375-022-01620-2
2. Jiang XN, Zhang Y, Xue T, Chen JY, Chan ACL, Cheuk W, et al. New clinicopathologic scenarios of EBV+ Inflammatory follicular dendritic cell sarcoma: report of 9 extrahepatosplenic cases. Am J Surg Pathol. (2021) 45:765–72. doi: 10.1097/PAS.0000000000001632
3. He H, Xue Q, Tan F, Yang L, Wang X, Gao Y, et al. A rare case of primary pulmonary inflammatory pseudotumor-like follicular dendritic cell sarcoma successfully treated by lobectomy. Ann Transl Med. (2021) 9:77. doi: 10.21037/atm-20-4965
4. Jarjour M, Jorquera A, Mondor I, Wienert S, Narang P, Coles MC, et al. Fate mapping reveals origin and dynamics of lymph node follicular dendritic cells. J Exp Med. (2014) 211:1109–22. doi: 10.1084/jem.20132409
5. Lennert K. Malignant lymphomas other than hodgkin’s disease. Berlin, Heidelberg: Springer (1978) p. 51–68.
6. Monda L, Monda L, Roger AW, Roger AW, Juan R, and Juan R. A primary lymph node Malignancy with features suggestive of dendritic reticulum cell differentiation. A report of 4 cases. Am J Pathol. (1986).
7. Fabio F, Luisa L, Fabio F, and Luisa L. Follicular dendritic cells and related sarcoma. Semin Diagn Pathol. (2016). doi: 10.1053/j.semdp.2016.05.002
8. Cheuk W, Chan JK, Shek TW, Chang JH, Tsou MH, Yuen NW, et al. Inflammatory pseudotumor-like follicular dendritic cell tumor: a distinctive low-grade Malignant intra-abdominal neoplasm with consistent Epstein-Barr virus association. Am J Surg Pathol. (2001) 25:721–31. doi: 10.1097/00000478-200106000-00003
9. Leng DN, Yu KJ, and Wang J. Inflammatory pseudotumor-like follicular dendritic cell sarcoma with first clinical manifestation of thrombocytopenia: A case report. Med (Baltimore). (2022) 101:e32528. doi: 10.1097/MD.0000000000032528
10. Pagliuca F, Ronchi A, Auricchio A, Lieto E, and Franco R. Inflammatory pseudotumor-like follicular/fibroblastic dendritic cell sarcoma: focus on immunohistochemical profile and association with Epstein-Barr virus. Infect Agent Cancer. (2022) 17:63. doi: 10.1186/s13027-022-00474-8
11. Mograbi M, Stump MS, Luyimbazi DT, Shakhatreh MH, and Grider DJ. Pancreatic inflammatory pseudotumor-like follicular dendritic cell tumor. Case Rep Pathol. (2019) 2019:2648123. doi: 10.1155/2019/2648123
12. Ge R, Liu C, Yin X, Chen J, Zhou X, Huang C, et al. Clinicopathologic characteristics of inflammatory pseudotumor-like follicular dendritic cell sarcoma. Int J Clin Exp Pathol. (2014) 7:2421–9.
13. Zhang J, He L, Ma X, Wang J, Qiu Y, and Jiang L. Imaging findings of inflammatory pseudotumor-like follicular dendritic cell sarcoma of the spleen: A case report and literature review. Oncol Lett. (2022) 24:399. doi: 10.3892/ol.2022.13519
14. Li X, Shi Z, You R, Li Y, Cao D, Lin R, et al. Inflammatory pseudotumor-like follicular dendritic cell sarcoma of the spleen: computed tomography imaging characteristics in 5 patients. J Comput Assist Tomogr. (2018) 42:399–404. doi: 10.1097/RCT.0000000000000700
15. Morales-Vargas B, Deeb K, and Peker D. Clinicopathologic and molecular analysis of inflammatory pseudotumor-like follicular/fibroblastic dendritic cell sarcoma: A case report and review of literature. Turk Patoloji Derg. (2021) 37:266–72. doi: 10.5146/tjpath.2021.01523
16. Yanyang C, Huijuan S, Yanyang C, Huijuan S, Huijuan S, Hui L, et al. Clinicopathological features of inflammatory pseudotumour-like follicular dendritic cell tumour of the abdomen. Histopathology. (2016) 68:858–65. doi: 10.1111/his.12851
17. Kazemimood R, Saei Hamedani F, Sharif A, Gaitonde S, Wiley E, Giulianotti PC, et al. A rare case of epstein-barr virus negative inflammatory pseudotumor-like follicular dendritic cell sarcoma presenting as a solitary colonic mass in a 53-year-old woman; case report and review of literature. Appl Immunohistochem Mol Morphol. (2017) 25:e30–e3. doi: 10.1097/PAI.0000000000000405
18. Jin J, Zhu X, Wan Y, and Shi Y. Epstein-barr virus (EBV)-positive inflammatory pseudotumor-like follicular dendritic cell sarcoma (IPT-like FDCS) presenting as thrombocytopenia: A case report and literature review. Heliyon. (2024) 10:e32997. doi: 10.1016/j.heliyon.2024.e32997
19. Chen Y and Yu X. A case report: spleen Epstein-Barr virus-positive inflammatory follicular dendritic cell sarcoma. J Gastrointest Oncol. (2024) 15:2706–11. doi: 10.21037/jgo-24-483
20. Eid MK, AlQaqaa AS, Mohammed IJ, and Shahait AD. A rare case of an EBV-positive inflammatory follicular dendritic cell tumor of the spleen. J Surg Case Rep. (2024) 2024:rjae600. doi: 10.1093/jscr/rjae600
21. Selves J, Meggetto F, Brousset P, Voigt JJ, Pradere B, Grasset D, et al. Inflammatory pseudotumor of the liver. Evidence for follicular dendritic reticulum cell proliferation associated with clonal Epstein-Barr virus. Am J Surg Pathol. (1996) 20:747–53. doi: 10.1097/00000478-199606000-00013
22. Shek TW, Ho FC, Ng IO, Chan AC, Ma L, and Srivastava G. Follicular dendritic cell tumor of the liver. Evidence for an Epstein-Barr virus-related clonal proliferation of follicular dendritic cells. Am J Surg Pathol. (1996) 20:313–24. doi: 10.1097/00000478-199603000-00008
23. Twh S, Shek TWH, Tony WHS, Liu CL, Liu CL, Wilfred CGP, et al. Intra-abdominal follicular dendritic cell tumour: a rare tumour in need of recognition. Histopathology. (1998). 33:465–70. doi: 10.1046/j.1365-2559.1998.00547.x
24. Chen TC, Kuo TT, and Ng KF. Follicular dendritic cell tumor of the liver: a clinicopathologic and Epstein-Barr virus study of two cases. Mod Pathol. (2001) 14:354–60. doi: 10.1038/modpathol.3880315
25. Ulises T, Ulises T, William GH, William GH, Cristina RA, Cristina RA, et al. Hepatic follicular dendritic cell sarcoma without Epstein-Barr virus expression. Arch Pathol Lab Med. (2009). doi: 10.5858/2005-129-1480-HFDCSW
26. Bai LY, Kwang WK, Chiang IP, and Chen PM. Follicular dendritic cell tumor of the liver associated with Epstein-Barr virus. Jpn J Clin Oncol. (2006) 36:249–53. doi: 10.1093/jjco/hyl001
27. Xiaoyu T, Wei-qi S, Xiaoyu T, Tu X, Haizhou L, Wei Qi S, et al. Clinicopathologic study of intraabdominal extranodal follicular dendritic cell sarcoma. Chin J Pathol. (2007) 36:660–5.
28. Granados R, Aramburu JA, Rodriguez JM, and Nieto MA. Cytopathology of a primary follicular dendritic cell sarcoma of the liver of the inflammatory pseudotumor-like type. Diagn Cytopathol. (2008) 36:42–6. doi: 10.1002/dc.20744
29. Zhengxiang Z, Zheng-xiang Z, Jun C, Jing C, Qiong S, Qun-li S, et al. Follicular dendritic cell sarcoma: a clinicopathologic study of 8 cases. Chin J Pathol. (2008) 37:395–9. doi: 10.3321/j.issn:0529-5807.2008.06.007
30. Qing L, Li L, Yanhui L, Li L, Qinglong H, Roberto NM, et al. Inflammatory pseudotumor-like follicular dendritic cell tumor of the liver with expression of estrogen receptor suggests a pathogenic mechanism: a case report and review of the literature. J Hematopathol. (2010) 3:109–15. doi: 10.1007/s12308-010-0067-3
31. Shuhong Z, Xiaoge Z, Shu-hong Z, Zhang S, Yuanyuan Z, Xiao-ge Z, et al. Clinicopathological study on the follicular dendritic cell sarcoma. Chin J Oncol. (2010) 32:123–7.
32. Pardasani M, Rajakannu M, Vij M, Rajalingam R, and Rela M. Aggressive variant of hepatic epstein-barr virus-associated inflammatory pseudotumor-like follicular dendritic cell sarcoma with PD-L1 and SSTR2a expression. Diagn (Basel). (2023) 13:2916. doi: 10.3390/diagnostics13182916
33. Paulo NM, Paulo NAM, Sanjay SR, Sanjay R, Sanjay R, Sanjay SR, et al. Follicular dendritic cell sarcoma of the liver: unusual presentation of a rare tumor and literature review. Hepatobil Pancreatic Dis Int. (2011) 10:443–5. doi: 10.1016/s1499-3872(11)60076-3
34. Shen HGD, Zhang Y, and Leow WQ. Uncommon granulomatous manifestation in Epstein-Barr virus-positive follicular dendritic cell sarcoma: a case report. J Pathol Transl Med. (2024) 59:133–8. doi: 10.4132/jptm.2024.09.27
35. Zhang X, Zhu C, Hu Y, and Qin X. Hepatic inflammatory pseudotumour-like follicular dendritic cell tumor: A case report. Mol Clin Oncol. (2017) 6:547–9. doi: 10.3892/mco.2017.1188
36. Deng S and Gao J. Inflammatory pseudotumor-like follicular dendritic cell sarcoma: a rare presentation of a hepatic mass. Int J Clin Exp Pathol. (2019) 12:3149–55.
37. Zhang BX, Chen ZH, Liu Y, Zeng YJ, and Li YC. Inflammatory pseudotumor-like follicular dendritic cell sarcoma: A brief report of two cases. World J Gastrointest Oncol. (2019) 11:1231–9. doi: 10.4251/wjgo.v11.i12.1231
38. Abe K, Kitago M, Matsuda S, Shinoda M, Yagi H, Abe Y, et al. Epstein-Barr virus-associated inflammatory pseudotumor variant of follicular dendritic cell sarcoma of the liver: a case report and review of the literature. Surg Case Rep. (2022) 8:220. doi: 10.1186/s40792-022-01572-w
39. Ise W, Fujii K, Shiroguchi K, Ito A, Kometani K, Takeda K, et al. T follicular helper cell-germinal center B cell interaction strength regulates entry into plasma cell or recycling germinal center cell fate. Immunity. (2018) 48:702–15.e4. doi: 10.1016/j.immuni.2018.03.027
40. Li HL, Liu HP, Guo GW, Chen ZH, Zhou FQ, Liu P, et al. Imaging findings of inflammatory pseudotumor-like follicular dendritic cell tumors of the liver: Two case reports and literature review. World J Gastroenterol. (2019) 25:6693–703. doi: 10.3748/wjg.v25.i45.6693
41. Liu Y, Zheng Y, Zhao X, Dong Z, Zhang M, Fang Y, et al. Targeting JAML promotes normalization of tumour blood vessels to antagonize tumour progression via FAK/SRC and VEGF/VEGFR2 signalling pathways. Life Sci. (2025) 368:123474. doi: 10.1016/j.lfs.2025.123474
42. Razik A, Malla S, Goyal A, Gamanagatti S, Kandasamy D, Das CJ, et al. Unusual primary neoplasms of the adult liver: review of imaging appearances and differential diagnosis. Curr Probl Diagn Radiol. (2022) 51:73–85. doi: 10.1067/j.cpradiol.2020.10.001
43. Li Y, Yang X, Tao L, Zeng W, Zuo M, Li S, et al. Challenges in the diagnosis of epstein-barr virus-positive inflammatory follicular dendritic cell sarcoma: extremely wide morphologic spectrum and immunophenotype. Am J Surg Pathol. (2023) 47:476–89. doi: 10.1097/PAS.0000000000002011
Keywords: EBV-positive inflammatory follicular dendritic cell sarcoma, Epstein-Barr virus, computed tomography, magnetic resonance imaging, case report
Citation: Bai W, Hu C and Zhu Z (2025) EBV-positive inflammatory follicular dendritic cell sarcoma occurring in different organs: a case report and literature review. Front. Oncol. 15:1603496. doi: 10.3389/fonc.2025.1603496
Received: 31 March 2025; Accepted: 11 July 2025;
Published: 31 July 2025.
Edited by:
Lucia Mundo, University of Limerick, IrelandReviewed by:
Siquan Wang, Columbia University, United StatesMengying Hu, University of Pittsburgh, United States
Copyright © 2025 Bai, Hu and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zheng Zhu, ZHJfemh1emhlbmdAc2luYS5jb20=
 Wenhua Bai
Wenhua Bai Chunfang Hu2
Chunfang Hu2