- 1Department of Neurooncology and Radiosurgery, Franciszek Lukaszczyk Oncology Center, Bydgoszcz, Poland
- 2Department of Radiotherapy, Franciszek Lukaszczyk Oncology Center, Bydgoszcz, Poland
- 3Department of Clinical Medicine, Jan and Jędrzej Śniadecki University of Science and Technology in Bydgoszcz, Bydgoszcz, Poland
- 4Department of Clinical Oncology, Franciszek Lukaszczyk Oncology Center, Bydgoszcz, Poland
Radiation treatment with modern techniques is frequently used in the complex interdisciplinary management of glioblastoma, both in primary and recurrent setting. However, standard imaging limits the ability to precisely distinguish treatment-related changes from tumor progression and to accurately assess the outcomes of radiotherapy. Given the challenges, there is growing need for advanced imaging modalities that can enhance diagnostic precision and guide therapeutic decision. One such modality is FET-PET, whose role in glioblastoma radiotherapy is increasingly recognized - from target definition to response assessment and differentiation between progression and post-irradiation changes. Recently, PET RANO criteria have been published, providing an optimal strategy for evaluating treatment response using amino-acid PET. Earlier, contributions from the PET/RANO group also supported the integration of PET imaging in radiotherapy planning and monitoring of glioma patients. Increasing evidence highlights the advantages of amino-acid PET over standard RANO MRI in predicting overall survival. However, its value in case of patient selection and monitoring of reirradiation is less clear. In this narrative review, we aimed to summarize the published data on FET-PET in patients after initial treatment from the perspective of radiation oncologist. We focused on the role of FET-PET in accurate diagnosis of recurrence and in treatment response after reirradiation.
1 Introduction
Glioblastoma is the most common malignant brain tumor in adults. Standard treatment includes maximal safe resection followed by radiotherapy with concomitant and adjuvant temozolomide. Despite advances in oncological therapies, glioblastoma remains associated with tremendously poor prognosis – median overall survival (OS) ranges from 14 to 18 months and median progression-free survival (PFS) is approximately 10 to 12 months (1–4).
Early diagnosis of treatment failure to initiate salvage therapy remains controversial due to limited therapeutic options and the low accuracy of MRI in reliably determining the true extent of disease. Moreover, conventional MRI sequences often fail to distinguish post-treatment changes - such as radionecrosis and pseudoprogression - and actual tumor progression, which may lead to inappropriate treatment decisions.
Posttreatment changes on conventional MRI vary depending on the type of previous treatment. Radiotherapy-induced changes are typically located within areas that received the highest radiation dose – the most often near resection cavity or tumor bed. These post-radiotherapy changes may include Swiss-like and bubble-like patterns, oedema and mass effect. On T2-weighted images, radiation-induced alternations are characterized by low signal intensity with a centrally increased signal corresponding to necrosis. Additional findings may include enhancement of the white matter and cortex (5). Changes after treatment with bevacizumab with or without lomustine are commonly manifested by the reduction in contrast enhancement and oedema (6, 7). Response assessment criteria based on MRI findings of adult gliomas have been described in RANO 2.0 (8).
In recent years, PET imaging has gained prominence for evaluating treatment response (9–11). The most commonly used PET tracer in oncology is 18F-fluorodeoxyglucose ([18F] FGD). However, its diagnostic utility in brain tumors is limited due to high physiological glucose uptake in normal brain tissue (12, 13). Gliomas are characterized by the overexpression of L-amino acid transporters compared to normal brain cells (14). It enables the use of amino acid tracers such as 11 C-methionine ([11C] MET), 18 F-dihydroxyphenylalanine ([18 F] F-DOPA) and 18 F-fluoroethyl-L-tyrosine ([18 F] FET) in neuro-oncology.
These tracers have the unique ability to cross blood-brain barrier (BBB) and to visualize tumor extent beyond the areas of contrast enhancement seen on MRI or even beyond FLAIR (15, 16). Compared to conventional MRI, PET offers higher sensitivity and specificity for detecting neoplastic infiltration, superior assessment of metabolic response to treatment, greater accuracy in differentiating tumor progression from radiation-induced changes and response to treatment (9, 17, 18). The recently published ESTRO/EANO recommendations on reirradiation of glioblastoma endorse the addition of amino acid PET to assess recurrence after primary treatment (19). FET-PET demonstrates excellent sensitivity of 0.82 and specificity of 0.76 in the diagnosis of primary brain tumors (20). FET is characterized by high in vivo stability and easy synthesis (21). Its concentration is significantly increased in neoplastic lesions compared to healthy or infected tissues (22, 23). The high intracellular accumulation of FET is attributed to elevated LAT1–3 expression on glioma cells as well as its molecular weight of approximately 220 g/mol (24, 25). Another advantage of FET is its half-life of 110min, which makes is suitable for clinical use (26). FET uptake and kinetics in glioma are generally stable, but tumor-to-background ratio (TBR) might slightly decrease due to dexamethasone administration (25). General recommendations for the use of PET in glioma patients have been summarized by the PET/RANO working group and in EANM/EANO/RANO/SNMMI guidelines (27, 28). More recently, PET RANO 1.0 criteria have been introduced to standardize treatment response assessment based on PET imaging (29). In response to growing interest in amino acid PET tracers in neuro-oncology, PET RANO BM 1.0 criteria were also published to evaluate the metabolic response of brain metastases (30).
For radiation oncologists, accurate diagnosis of recurrence is particularly crucial and requires advanced imaging. Recently published recommendations on the use of FET-PET focus only on treatment assessment after first-line therapy and do not address other challenges such as post-treatment changes, selection criteria for retreatment and response assessment after re-irradiation. Therefore, we aimed to summarize the available evidence on the use of FET-PET in clinical scenarios following initial treatment: in patients with residual tumor, patients with enlarging lesions, patients with new, small areas of contrast enhancement and after retreatment.
2 Methods
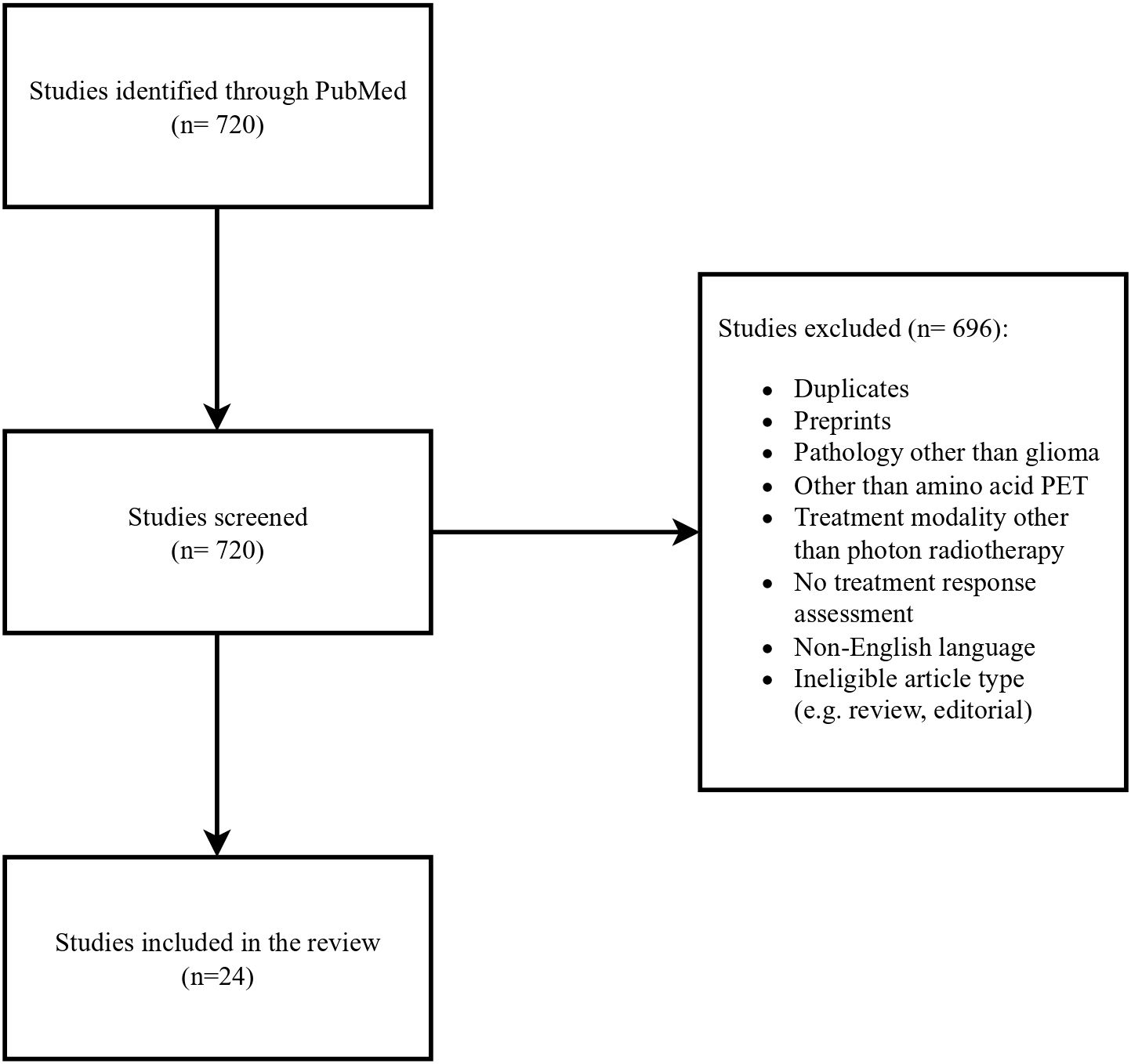
The authors conducted a literature search of published journal articles written in English between January and May 2025 in PubMed. Keywords such as:” glioblastoma”, “primary brain tumors”, “FET”, “PET”, “recurrent glioblastoma”, “treatment-related changes”, “PET/RANO” and” RANO” were used. Retrospective studies, prospective studies and systematic reviews were analyzed. A workflow of the literature selection process is illustrated in Figure 1.
3 FET-PET in patients with residual tumor after initial treatment
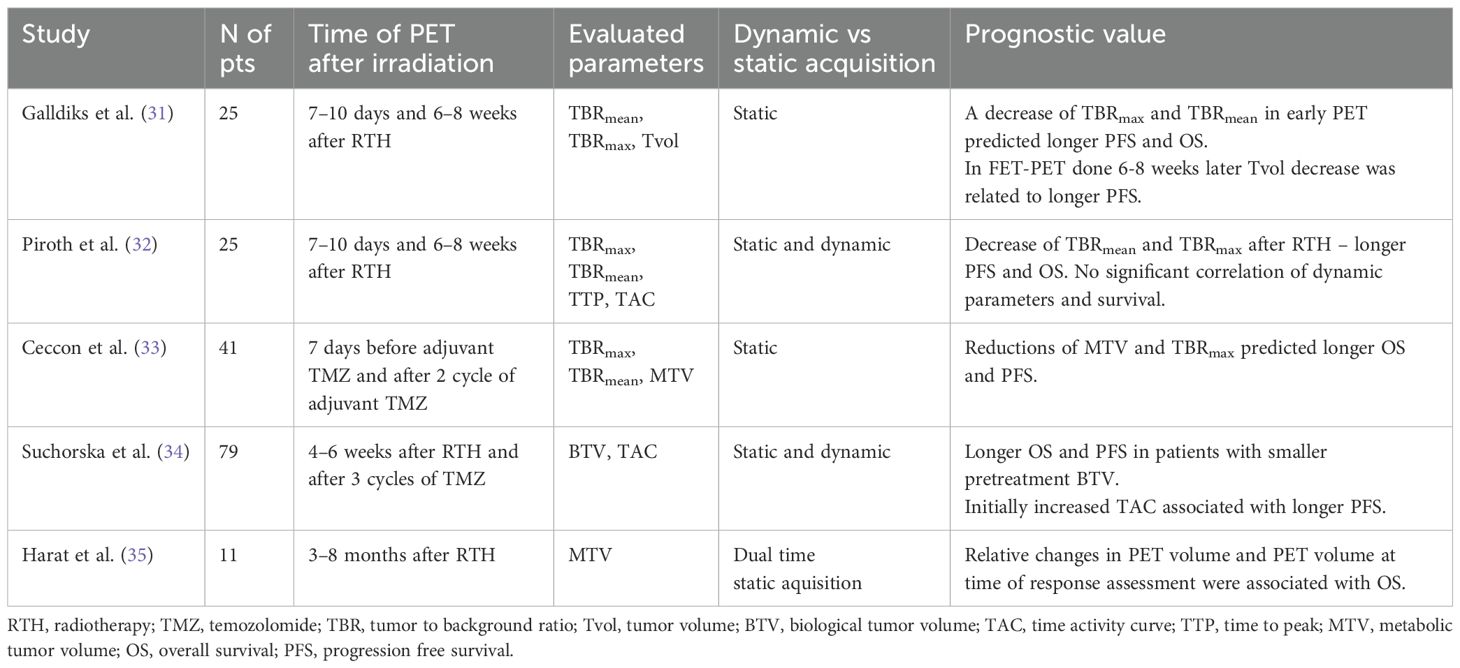
The optimal management and prognosis of patients with residual, stable tumor on MRI after radiochemotherapy remain unclear. The association between prognostic factors such as OS and PFS and both static (e.g. TBRmean/max – tumor-to-brain ratio; MTV- metabolic tumor volume; BTV - biological tumor volume) and dynamic (TTP – time to peak; TAC – time-activity curve) FET-PET parameters after glioblastoma treatment has been evaluated in five prospective studies.
In a study by Galldiks et al., 25 patients with glioblastoma were evaluated with MRI and FET-PET at different timepoints following standard treatment – after surgery, 7–10 days after radiochemotherapy and 6–8 weeks later. The results showed that a ≥ 10% decrease in both TBRmax and TBRmean on FET-PET done 7–10 days after radiochemotherapy was a favorable prognostic factor for PFS (TBRmax 9.3 vs. 4.7 months; p= 0.002; TBRmean 10.3 vs. 5.1 months p <0.001) and OS (TBRmax 15.4 vs. 8.5 months; p = 0.001; TBRmean 16.1 vs. 9.3 months, p<0.001). FET-PET performed 6–8 weeks later had a lower predictive value for TBRmax/mean but a reduction in TVOL1.6 (tumor volume with TBR > 1.6) was associated with improved PFS (9.3 vs. 5.1 months; p = 0.002). MRI-based tumor volume changes were not associated with survival (31).
Another study by Piroth et al. analyzed static and dynamic FET-PET parameters in 25 glioblastoma patients at the same timepoints. They found that a ≥ 10% decrease in TBRmax on FET-PET after radiochemotherapy (7–10 days) was associated with longer median PFS (9.3 vs 4.7 months; p =0.002) and OS (18.0 vs 8.5 months; p <0.01). Similarly, a ≥5% reduction in TBRmean (cutoff 5%) was associated with longer PFS (10.3 vs 5.1 months) and OS (22.8 vs 9.3 months), with p < 0.001 for both. However, changes in TTP and the slope of the TAC were not associated with survival outcomes (32).
A study by Ceccon et al. included 41 glioma patients (90% diagnosed with WHO grade 4 glioblastoma) who received surgery, radiochemotherapy and adjuvant temozolomide. FET-PET performed after two cycles of adjuvant temozolomide showed that a reduction in TBRmax and MTV compared to baseline was associated with longer OS (24 vs. 12 months; p =0.032, and 29 vs. 12 months; p = 0.005) and PFS (both 11 vs. 8 months; p = 0.031 and 0.007, respectively). No significant correlations were observed between MRI findings and OS or PFS (33).
A prospective study by Suchorska et al. assessed both static and dynamic FET-PET in 79 patients with newly diagnosed glioblastoma treated with surgery, radiochemotherapy with temozolomide and adjuvant temozolomide. FET-PET imaging was performed before and after surgery, 4–6 weeks after radiochemotherapy and after four cycles of adjuvant temozolomide. A smaller BTV (below 9.5cm3, sensitivity 64%, specificity 70%) before radiochemotherapy was identified as a prognostic factor. Median OS and PFS for BTV below 9.5 cm3 were 17.5 and 8.8 months (p<0,002), respectively, compared to 10.7 and 3.9. months for BTV above 9.5cm3 (p <0.08). Additionally, patients with initially increased TACs had longer OS (29.7 vs 12.5 months; p <0.02, HR 2.1) and longer PFS (11.9 vs 5.8 months; p < 0.05, HR 1.8) (34).
Finally, a prospective study by Harat et al. evaluated the use of a simultaneous integrated boost planned on FET-PET in the postoperative treatment of 17 patients with newly diagnosed glioblstoma. The study demonstrated that FET-PET-based treatment response assessment enables accurate evaluation of biological response. In 81% of analyzed cases, there was discordance between PET and MRI-based RANO criteria for response assessment (35). In summary, FET-PET may be considered as a reliable tool in early treatment response evaluation and survival prediction. Abovementioned studies have been summarized in Table 1.
4 FET-PET in patients with progressive enlarging of a mass lesion
After radiochemotherapy a progression of contrast enhancement on MRI might represent both tumor progression and radionecrosis.
Radionecrosis is a local tissue response to radiotherapy and typically develops within regions exposed to the highest dose of radiation. The risk of radionecrosis increases with higher total dose, larger fraction size and concurrent chemotherapy. Treatment-related necrosis can occur up to 30% of patients. In contrast to pseudoprogression, radionecrosis generally develops more than three months after treatment, although it can also manifest years or even decades later. On MRI images, radionecrosis can be characterized by contrast enhancement, which may be accompanied by clinical symptoms (34–36). Symptomatic radionecrosis can be managed with steroids, bevacizumab and surgery (36, 37). While biopsy can distinguish necrosis from recurrence, it carries surgical risks and might be limited due to sampling errors that do not represent the full pathological profile of the tumor (38–40). FET-PET might help to distinguish post-treatment necrotic tissue from tumor progression. However, most studies are retrospective and include relatively small patient cohorts with heterogenous histopathology.
A systematic review analyzing three studies on the use of FET-PET for the differential diagnosis of radionecrosis and glioma recurrence demonstrated higher diagnostic accuracy compared to FDG-PET (specificity 78–95% versus 70–88% for FDG and sensitivity 82–91% for FET 70–84% for FDG) (41).
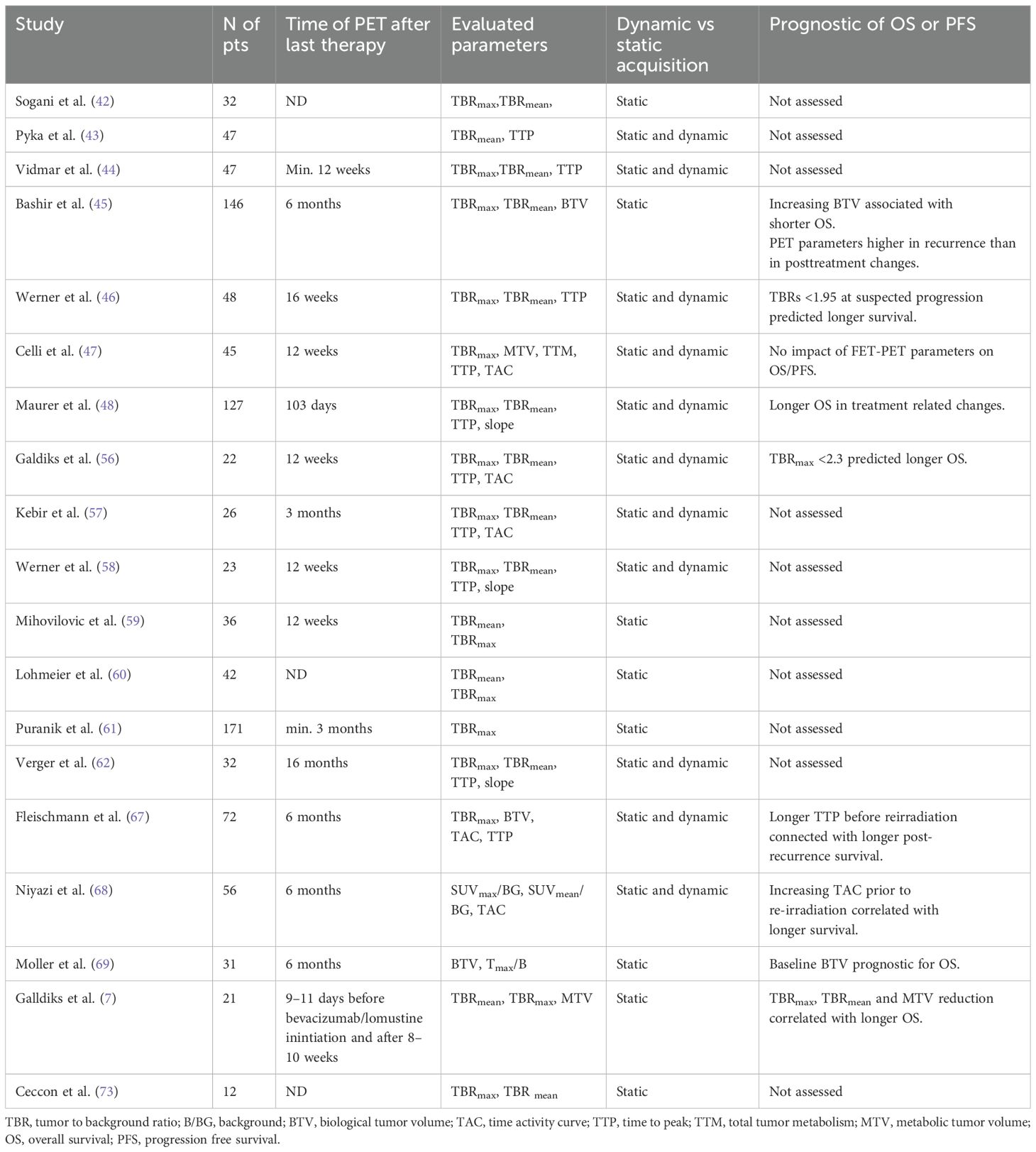
The diagnostic accuracy, sensitivity and specificity of MRI were reported as 93.75%, 96%, and 85.7%, respectively. When both MR and FET-PET were evaluated, these values increased to 96.87%, 100%, and 85.7%, respectively. TBRmax cut-off (threshold) of 2.09 provided sensitivity of 100% and specificity of 72% for distinguishing recurrence from radionecrosis. A TBRmean cut-off of 1.517 yielded a slightly lower specificity but higher sensitivity – 89% and 86% (42).
Another study showed that similar TBRmax cut-off 2.07 (measured 30–40 min. postinjection) resulted in sensitivity and specificity of 80% and 84.6%, respectively (43).
In contrast, a study by Vidmar reported higher value TBRmax cut-off - 3.03 (sensitivity and specificity of 77% and 82%) (44). The discrepancies in TBR tresholds might be attributed to small, heterogenous study populations, the lack of histopathological examination, and differences in PET protocols and interpretation.
A retrospective study by Bashir et al. involving 146 glioblastoma patients showed that FET-PET parameters measured at least 6 months post-treatment were significantly higher in recurrent glioblastoma than in post-treatment changes (TBRmax 3.2 vs 1.6; TBRmean 2.0 vs 1.6; and BTV 14.8 cm3 vs. 0.01 cm3; p < 0.0001). Optimal thresholds were identified as 2.0 for TBRmax and 1.8 for TBRmean and 0.55 cm3 for BTV to accurately differentiate tumor progression from treatment-related changes. Increasing TBRmax (HR 1.328, 95% CI: 1.116–1.582; p = 0.001) and BTV during follow-up (HR 1.303, 95% CI: 1.179–1.439; p < 0.0001) were associated with shorter OS (45).
Another retrospective study by Werner et al. evaluated 48 patients with high grade glioma and suspicious MRI findings. FET-PET demonstrated significantly superior diagnostic performance (threshold for both TBRmax and TBRmean: 1.95; accuracy: 83%; p < 0.001) compared to apparent diffusion coefficient (ADC) values (threshold:1.09 × 10−3 mm2/s; accuracy, 69%; p = 0.13). The optimal TTP cut-off of 32.5 min was optimal for the differentiation (accuracy 72%; sensitivity 80%; specificity 69%; p< 0.01) and for slope - 0.32 SUV/h (change of SUV per hour) with an accuracy of 74%, sensitivity of 70% and specificity of 75% (p= 0.02). Combining static FET-PET parameters with ADC increased diagnostic accuracy to 89%, while combining both static and dynamic FET-PET parameters yielded the highest accuracy (93%). TBRmax and TBRmean values <1.95 were associated with longer OS (p = 0.01) (46).
Celli et al. retrospectively analyzed 45 glioma patients (26 with WHO grade 4 glioblastoma) and found similar sensitivity (86.2%) and specificity (81.31%) for FET-PET in the differential diagnosis performed at least 12 weeks after radiotherapy. The optimal cut-offs for recurrence were consistent with previous findings (TBRmax ≥ 2.1, SUVmax ≥ 3.5, and TTP ≤ 29 min). However, no FET-PET parameters significantly impacted overall survival (47).
Maurer et al. conducted a retrospective study of 147 glioma patients (67 with WHO grade 4 glioblastoma). The diagnostic performance of TBRmax and TBRmean thresholds of 1.95 was slightly lower (sensitivity 70%; specificity 71%; accuracy 70%; for TBRmax and sensitivity 56%; specificity 79%; accuracy 62%; for TBRmean). The optimal cut-off for slope was lower – 0.2SUV/h (sensitivity 54%; specificity 86%; accuracy 63%);. Nonetheless, TBRmax > 1.95 combined with a slope < 0.2 SUV/h achieved a sensitivity of 86%, a specificity of 67%, and an accuracy of 81% in detecting progression (48).
In summary, static (TBRmax/mean < 2) and dynamic (TTP >30min) FET-PET parameters can differentiate necrosis from tumor progression. Diagnostic accuracy can be further improved by combining FET-PET with MRI-derived parameters, such as ADC. However, these improvements do not appear to translate into significantly longer OS.
5 FET-PET in patients with new, small areas of contrast enhancement
Management of asymptomatic patients with new, small, multicentric lesions remains challenging, as such MRI findings might indicate either tumor progression or pseudoprogression.
The pathogenesis of pseudoprogression remains unclear, and no universally accepted definition currently exists. However, it is often described as at least 25% increase in tumor size according to Macdonald criteria, followed by either partial response or stable disease lasting for at least six months after radiochemotherapy (49).
Pseudoprogression is more commonly observed in patients with MGMT promoter methylation and in those treated with radiochemotherapy including temozolomide, compared to radiotherapy alone (50, 51).
Similar to necrosis, pseudoprogression can mimic tumor progression on MRI. Histopathological examination may reveal residual, stable tumor tissue within areas of pseudoprogession and necrosis, potentially leading to a wrong diagnosis of progression (39, 52–55).
Studies of FET-PET in patients with suspected MRI findings showed promising results.
A retrospective study by Galldiks et al. included 22 glioblastoma patients with suspicious MRI findings within the first 12 weeks after radiochemotherapy. In patients with pseudoprogression, FET uptake was significantly lower than in those with true progression (TBRmax 1.9 ± 0.4 vs. 2.8 ± 0.5, TBRmean 1.8 ± 0.2 vs. 2.3 ± 0.3; p<0.001). TTP was shorter in progression than in pseudoprogression (mean TTP 26 ± 10 vs. 35 ± 9 min, P = 0.05). TACs type II (uptake peaking at a mid-point; >20–40 min) or III (early uptake peak ≤20 min followed by a constant decline) were more frequently observed in progression (p=0.04). The optimal TBRmax cut-off for identifying pseudoprogression was 2.3 (sensitivity 100%, specificity 91%, accuracy 96%, p<0.001) (56).
Another study by Kebir et al. reported comparable results in 26 glioblastoma patients with MRI changes observed at least 12 weeks post-radiotherapy. TBRmax and TBRmean were significantly higher in patients with progression compared to those with late pseudoprogression (TBRmax 2.4 ± 0.1 vs. 1.5± 0.2, P =0.003; TBRmean 2.1 ± 0.1 vs. 1.5 ± 0.2, p =0.012). The optimal cut-off for both parameters was 1.9 (TBRmax: sensitivity 84%, specificity 86%, accuracy 85%, p =0.015; TBRmean: sensitivity 74%, specificity 86%, accuracy 77%, p= 0.023). The authors suggested to diagnosing late progression when TBRmax exceeds 2.4 and late pseudoprogression when it is below 1.0; values between 1.0 and 2.4 should be interpreted with caution. TTP was significantly shorter in progression (mean TTP 25 ± 2 vs. 40 ± 2 min, p< 0.001). TACs type II or III were more frequently observed in progression, with a sensitivity of 84%, specificity of 100%, and an accuracy of 89% (p< 0.001) (57).
A retrospective study by Werner et al. analyzed 23 glioblastoma patients with progressive MRI findings after chemoradiation with lomustine and temozolomide. FET-PET were consistent with previous studies and significantly contributed to diagnosing pseudoprogression. Lower TBRmean/max values were observed in pseudoprogression compared to progression (TBRmean 1.9 ± 0.2 vs. 2.1 ± 0.2; P = 0.023 and TBRmax 2.8 ± 0.6 vs. 3.2 ± 0.5; P = 0.045), while TTP was higher in pseudoprogression (36.6 ± 8.3 vs. 24.8 ± 9.4 minutes; p = 0.005). The optimal cut-off values were: TBRmean 1.95 (sensitivity 82%; specificity 92%; accuracy 87%; P = 0.029), TBRmax 2.85 (sensitivity 64%; specificity 92%; accuracy 78%; P = 0.046) and TTP 35 minutes (sensitivity, 64%; specificity, 83%; accuracy 74 P = 0.010). Combining TBRmean with TTP improved the specificity and positive predictive value to 100% for detecting pseudoprogression (sensitivity 55%; accuracy 78%; P = 0.005) (58).
In contrast, a study by Mihovilovic et al. reported higher FET-PET parameters in 36 glioblastoma patients with suspected recurrence. The optimal tresholds for differentiating progression from late pseudoprogression were: TBRmax of 3.52 (sensitivity 89%, specificity 75%, area under the curve (AUC) 0.87 ± 0.07; p=0.0020) and TBRmean of 2.98 (sensitivity 82%, specificity 87.5%, AUC 0.84 ± 0.08, p=0.004) (59).
In conclusion, current evidence supports the utility of FET-PET in distinguishing progression from pseudoprogression. Static and dynamic parameters appear similar in necrosis and pseudoprogression. However, the benefit of FET-PET lies in identifying patients requiring immediate therapeutic intervention due to progression, rather than in distinguishing between begin post-treatment changes – such as pseudoprogression and radionecrosis - that can be monitored. Some studies did not clearly differentiate between necrosis and pseudoprogression, focusing instead on distinguishing recurrence from general treatment-related changes. A retrospective study by Lohmeier et al. involving 42 patients with low- and high-grade gliomas found that a TBRmax threshold of 2 provided a sensitivity of 81% and specificity of 60% in differentiating glioma recurrence from post-treatment changes (60). In a retrospective study by Puranik et al., TBRmax cutoff value of 2.5 in patients with grade 3 and 4 gliomas yielded a sensitivity of 91.6% and a specificity of 76.9% in distinguishing tumor recurrence from post-treatment changes (61). Verger et al., in a study of 31 low- and high-grade glioma patients reported that a TBRmax of 2.61 yielded a sensitivity of 80%, and a specificity of 86% in distinguishing progression from treatment-related changes (62).
These varying results may stem from methological differences between centers, including how background activity is defined (63). A retrospective study comparing five methods for differentiating pseudoprogression from true progression more than 12 weeks post-radiotherapy found similar diagnostic performance across approaches, with AUCs ranging from 0.80 to 0.88 (64). Another study demonstrated variability in background activity measurement depending on the region-of-interest method (2D-ROI vs. VOI 3cm diameter vs. crescent-shaped VOI), with the crescent-shaped VOI yielding the most consistent results (65). Most of these studies were conducted before the establishment of the PET RANO 1.0 criteria or EANM/EANO/RANO/SNMMI guidelines, which recommended using a crescent-shaped VOI in the contralateral frontal lobe including healthy white and gray matter for background activity assessment (28, 29).
6 Treatment response assessment based on FET-PET after reirradiation
Most patients with glioblastoma experience tumor recurrence shortly after initial treatment. However, there is scarce evidence regarding the use of FET-PET in the context of reirradiation.
A systematic review demonstrated that amino acid PET, including FET-PET, has superior prognostic value compared to MRI using RANO criteria in predicting OS in patients treated with bevacizumab for recurrent glioma. FET-PET predicted 9-month OS with a sensitivity of 76% (95% CI 60–87) and specificity of 71% (95% CI 53–83), whereas MRI yielded a sensitivity of 32% (95% CI 19–48) and specificity of 82% (95% CI 66–92) (66).
Several studies have showed that changes in static and dynamic FET-PET parameters can predict prognosis. However, the results are inconsistent. This variability is likely due to small patient populations and retrospective nature of most studies.
In a retrospective study by Fleischmann et al., 72 patients with recurrent high-grade glioma underwent FET-PET prior to re-irradiation with or without bevacizumab (at least 6 months after the initial radiotherapy). A shorter TTPmin prior to treatment was associated with shorter post-recurrence survival (PRS): 6 months for TTPmin <12,5min, 7 months for TTPmin 12.5–25 min and 11 months for TTPmin >25 min (p=0.027). Early TBRmax was not predictive of PRS (67).
Another retrospective study by Niyazi et al. included 56 patients with recurrent malignant glioma who underwent FET-PET before re-irradiation with or without bevacizumab. The study reported a significant decrease in median TBRmax (3.3 vs 2.6, p <0.001) and BTV (13.7 cc vs 7.3 cc, p = 0.006) after re-irradiation, but without significant impact on PFS or survival. TBRmean values also did not correlate with survival. However, patients with pretherapeutic decreasing FET kinetics had worse survival compared to those with other kinetics (p = 0.01) (68).
A phase I clinical trial evaluated the prognostic value of FET-PET in 31 patients undergoing re-irradiation for high grade glioma. Baseline BTV and MRI volume were both prognostic for OS (HR = 1.3 p < 0.01 and HR = 1.3 p < 0.01, respectively). However, changes in BTV and TBRmax were not correlated with survival (69).
In a prospective study by Galldiks et al., 21 glioblastoma patients with recurrent glioblastoma undergoing bevacizumad with lomustine therapy (without re-irradiation) were monitored using FET-PET. A reduction in TBRmax > 27% and TBR mean > 17% predicted improved OS > 9 months (sensitivity 92%, specificity 63% for both; p = 0.036 and p=0.02, respectively) Additionally, an absolute MTV below 5 ml at follow-up was associated with improved OS (12 vs. 6 months, sensitivity 85%; specificity, 88%; p < 0.001). MRI-based response assessment did not predict OS (7).
The role of FET-PET in the assessing response to re-irradiation remains limited due to the lack of significant correlation reported in current studies.
In a phase I trial, Moller et al. used FET-PET to monitor results of stereotactic radiotherapy for recurrent glioblastoma. While pre-treatment TBRmax and BTV were associated with better outcomes, changes in this parameter did not predict survival. Similar findings were reported by Niyazi in patients undergoing re-irradiation with bevacizumab. These findings indicate the need for further studies. Recent data suggest that early-phase FET-PET imaging may reflect the most aggressive tumor regions (70, 71). High uptake in early acquisition is more commonly observed in IDH-wildtype gliomas, and longer TTP appears to have a favorable prognostic impact (72). A retrospective study in patients treated with TTFields (tumor treating fields) in recurrent tumors demonstrated the value FET-PET monitoring. Increased uptake was observed in time of further progression compared to baseline (TBRmax 3.5 ± 0.6, range 2.5–4.4; TBRmean 2.7 ± 0.7, range 2.0–4.0). In patients treated with TTFields as a maintenance therapy without progression, FET-PET showed either reduced metabolic activity or no increased uptake at follow-up (73).
Abovementioned studies have been summarized in Table 2.
7 Conclusions
Growing evidence supports the efficacy of FET-PET in accurate selection for reirradiation. Based on current data, FET-PET can reliably distinguish post-treatment changes from true tumor progression and may enhance target definition by identifying areas of tumor infiltration beyond contrast enhancement on MRI. Most studies suggest that TBRmean and TBRmax above 2.0 should be considered indicative of progression or metabolically active disease following primary radiotherapy. In the PET RANO classification, stable disease corresponds to stable uptake after treatment. However, it may still reflect a metabolically active tumor. Careful evaluation is crucial, and optimal management for this subgroup remains unclear. Future research should explore whether additional therapies for metabolically stable glioblastoma could lead to improved outcomes. The utility of FET PET in monitoring patients after re-irradiation also warrants further investigation.
Author contributions
IZ: Writing – original draft, Writing – review & editing. MB: Writing – original draft, Writing – review & editing. MM: Writing – original draft, Writing – review & editing. MH: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Miller KD, Ostrom QT, Kruchko C, Patil N, Tihan T, Cioffi G, et al. Brain and CNS tumor statistics 382. CA: A Cancer J Clin. (2021) 71:381–406 doi: 10.3322/caac.21693
2. Stupp R, Mason WP, Van Den Bent MJ, Weller M, Fisher B, Taphoorn MJB, et al. Radiotherapy plus Concomitant and Adjuvant Temozolomide for Glioblastoma . Available online at: www.nejm.org (Accessed January 19, 2024).
3. Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, Figarella-Branger D, et al. The 2021 WHO classification of tumors of the central nervous system: A summary. Neuro Oncol. (2021) 23:1231–51. doi: 10.1093/neuonc/noab106
4. Blumenthal DT, Gorlia T, Gilbert MR, Kim MM, Nabors LB, Mason WP, et al. Is more better? The impact of extended adjuvant temozolomide in newly diagnosed glioblastoma: a secondary analysis of EORTC and NRG Oncology/RTOG. Neuro Oncol. (2017) 19:1119–26. doi: 10.1093/neuonc/nox025
5. Kumar AJ, Leeds NE, Fuller GN, Van Tassel P, Maor MH, Sawaya RE, et al. Malignant gliomas: MR imaging spectrum of radiation therapy– and chemotherapy-induced necrosis of the brain after treatment. Radiology. (2000) 217:377–84. doi: 10.1148/radiology.217.2.r00nv36377
6. Liao G, Khan M, Zhao Z, Arooj S, Yan M, and Li X. Bevacizumab treatment of radiation-induced brain necrosis: A systematic review. Front Oncol. (2021) 11:593449. doi: 10.3389/fonc.2021.593449
7. Galldiks N, Dunkl V, Ceccon G, Tscherpel C, Stoffels G, Law I, et al. Early treatment response evaluation using FET PET compared to MRI in glioblastoma patients at first progression treated with bevacizumab plus lomustine. Eur J Nucl Med Mol Imaging. (2018) 45:2377–86. doi: 10.1007/s00259-018-4082-4
8. Wen PY, van den Bent M, Youssef G, Cloughesy TF, Ellingson BM, Weller M, et al. RANO 2.0: update to the response assessment in neuro-oncology criteria for high- and low-grade gliomas in adults. J Clin Oncol. (2023) 41:5187–99. doi: 10.1200/JCO.23.01059
9. Lohmann P, Stavrinou P, Lipke K, Bauer EK, Ceccon G, Werner J-M, et al. FET PET reveals considerable spatial differences in tumour burden compared to conventional MRI in newly diagnosed glioblastoma. Eur J Nucl Med Mol Imaging. (2019) 46:591–602. doi: 10.1007/s00259-018-4188-8
10. Hygino Da Cruz LC, Rodriguez I, Domingues RC, Gasparetto EL, and Sorensen AG. Pseudoprogression and pseudoresponse: imaging challenges in the assessment of posttreatment glioma. AJNR Am J Neuroradiol. (2011) 32:1978–85. doi: 10.3174/ajnr.A2397
11. Van Den Bent MJ, Dhermain FG, Hau P, Lanfermann H, and Jacobs AH. Advanced MRI and PET imaging for assessment of treatment response in patients with gliomas. Lancet Neurol. (2010) 9:906–26. doi: 10.1016/S1474
12. Fletcher JW, Djulbegovic B, Soares HP, Siegel BA, Lowe VJ, Lyman GH, et al. Recommendations on the use of 18 F-FDG PET in oncology. J Nucl Med. (2008) 49:480–508. doi: 10.2967/jnumed.107.047787
13. Dunet V, Pomoni A, Hottinger A, Nicod-Lalonde M, and Prior JO. Performance of 18 F-FET versus 18 F-FDG-PET for the diagnosis and grading of brain tumors: systematic review and meta-analysis. Neuro Oncol. (2016) 18:426–34. doi: 10.1093/neuonc/nov148
14. Nawashiro H, Otani N, Uozumi Y, Ooigawa H, Toyooka T, Suzuki T, et al. High expression of L-type amino acid transporter 1 in infiltrating glioma cells. Brain Tumor Pathol. (2005) 22:89–91. doi: 10.1007/s10014-005-0188-z
15. Najjar AM, Johnson JM, and Schellingerhout D. The emerging role of amino acid PET in neuro-oncology. Bioengineering. (2018) 5. doi: 10.3390/BIOENGINEERING5040104
16. Harat M, Miechowicz I, Rakowska J, Zarębska I, and Małkowski B. A biopsy-controlled prospective study of contrast-enhancing diffuse glioma infiltration based on FET-PET and FLAIR. Cancers (Basel). (2024) 16. doi: 10.3390/cancers16071265
17. Roelcke U, Wyss MT, Nowosielski M, Rudà R, Roth P, Hofer S, et al. Amino acid positron emission tomography to monitor chemotherapy response and predict seizure control and progression-free survival in WHO grade II gliomas. Neuro Oncol. (2016) 18:744–51. doi: 10.1093/neuonc/nov282
18. Rachinger W, Goetz C, Pöpperl G, Gildehaus FJ, Kreth FW, Holtmannspötter M, et al. Positron emission tomography with O-(2-[18F]flouroethyl)-L- tyrosine versus magnetic resonance imaging in the diagnosis of recurrent gliomas. Neurosurgery. (2005) 57:505–11. doi: 10.1227/01.NEU.0000171642.49553.B0
19. Andratschke N, Heusel A, Albert NL, Alongi F, Baumert BG, Belka C, et al. ESTRO/EANO recommendation on reirradiation of glioblastoma. Radiotherapy Oncol. (2025) 204. doi: 10.1016/j.radonc.2024.110696
20. Dunet V, Rossier C, Buck A, Stupp R, and Prior JO. Performance of 18F-fluoro-ethyl-tyrosine ( 18F-FET) PET for the differential diagnosis of primary brain tumor: A systematic review and metaanalysis. J Nucl Med. (2012) 53:207–14. doi: 10.2967/jnumed.111.096859
21. Wester HJ, Herz M, Weber W, Heiss P, Senekowitsch-Schmidtke R, Schwaiger M, et al. Synthesis and radiopharmacology of O-(2-[18F]fluoroethyl)-L-tyrosine for tumor imaging. J Nucl Med. (1999) 40:205–12.
22. Rapp M, Heinzel A, Galldiks N, Stoffels G, Felsberg J, Ewelt C, et al. Diagnostic performance of 18F-FET PET in newly diagnosed cerebral lesions suggestive of glioma. J Nucl Med. (2013) 54:229–35. doi: 10.2967/jnumed.112.109603
23. Kaim AH, Weber B, Kurrer MO, Westera G, Schweitzer A, Gottschalk J, et al. 18F-FDG and 18F-FET uptake in experimental soft tissue infection. Eur J Nucl Med. (2002) 29:648–54. doi: 10.1007/s00259-002-0780-y
24. Habermeier A, Graf J, Sandhöfer BF, Boissel JP, Roesch F, and Closs EI. System l amino acid transporter LAT1 accumulates O-(2-fluoroethyl)-l-tyrosine (FET). Amino Acids. (2015) 47:335–44. doi: 10.1007/s00726-014-1863-3
25. Langen KJ, Hamacher K, Weckesser M, Floeth F, Stoffels G, Bauer D, et al. O-(2-[18F]fluoroethyl)-l-tyrosine: uptake mechanisms and clinical applications. Nucl Med Biol. (2006) 33:287–94. doi: 10.1016/j.nucmedbio.2006.01.002
26. Langen KJ, Stoffels G, Filss C, Heinzel A, Stegmayr C, Lohmann P, et al. Imaging of amino acid transport in brain tumours: Positron emission tomography with O-(2-[18F]fluoroethyl)-L-tyrosine (FET). Methods. (2017) 130:124–34. doi: 10.1016/j.ymeth.2017.05.019
27. Galldiks N, Niyazi M, Grosu AL, Kocher M, Langen K-J, Law I, et al. Contribution of PET imaging to radiotherapy planning and monitoring in glioma patients-a report of the PET/RANO group. Neuro Oncol. (2021) 23:881–93. doi: 10.1093/neuonc/noab013
28. Law I, Albert NL, Arbizu J, Boellaard R, Drzezga A, Galldiks N, et al. Joint EANM/EANO/RANO practice guidelines/SNMMI procedure standards for imaging of gliomas using PET with radiolabelled amino acids and [ 18 F]FDG: version 1.0. Eur J Nucl Med Mol Imaging. (2019) 46:540–57. doi: 10.1007/s00259-018-4207-9
29. Albert NL, Galldiks N, Ellingson BM, van den Bent MJ, Chang SM, Cicone F, et al. PET-based response assessment criteria for diffuse gliomas (PET RANO 1.0): a report of the RANO group. Lancet Oncol. (2024) 25:e29–41. doi: 10.1016/S1470-2045(23)00525-9
30. Albert NL, Galldiks N, Ellingson BM, van den Bent MJ, Chang SM, Cicone F, et al. RANO criteria for response assessment of brain metastases based on amino acid PET imaging. Nat Med. (2025). 31:1424–30 doi: 10.1038/s41591-025-03633-7
31. Galldiks N, Langen K-J, Holy R, Pinkawa M, Stoffels G, Nolte KW, et al. Assessment of treatment response in patients with glioblastoma using O-(2–18 F-fluoroethyl)-L-tyrosine PET in comparison to MRI. J Nucl Med. (2012) 53:1048–57. doi: 10.2967/jnumed.111.098590
32. Piroth MD, Liebenstund S, Galldiks N, Stoffels G, Shah NJ, Eble MJ, et al. Monitoring of radiochemotherapy in patients with glioblastoma using O-(2-[18F]fluoroethyl)-L-tyrosine positron emission tomography: Is dynamic imaging helpful? Mol Imaging. (2013) 12(6):388–95. doi: 10.2310/7290.2013.00056
33. Ceccon G, Lohmann P, Werner J-M, Tscherpel C, Dunkl V, Stoffels G, et al. Early treatment response assessment using 18 F-FET PET compared with contrast-enhanced MRI in glioma patients after adjuvant temozolomide chemotherapy. J Nucl Med. (2021) 62:918–25. doi: 10.2967/jnumed.120.254243
34. Suchorska B, Jansen NL, Linn J, Kretzschmar H, Janssen H, Eigenbrod S, et al. Biological tumor volume in 18FET-PET before radiochemotherapy correlates with survival in GBM. Neurology. (2015) 84:710–9. doi: 10.1212/WNL.0000000000001262
35. Harat M, Blok M, Miechowicz I, Wiatrowska I, Makarewicz K, and Małkowski B. Safety and efficacy of irradiation boost based on 18F-FET-PET in patients with newly diagnosed glioblastoma. Clin Cancer Res. (2022) 28:3011–20. doi: 10.1158/1078-0432.CCR-22-0171/698881/AM/SAFETY-AND-EFFICACY-OF-IRRADIATION-BOOST-BASED-ON
36. Furuse M, Nonoguchi N, Kuroiwa T, Miyamoto S, Arakawa Y, Shinoda J, et al. A prospective, multicentre, single-arm clinical trial of bevacizumab for patients with surgically untreatable, symptomatic brain radiation necrosis. Neurooncol Pract. (2016) 3:272–80. doi: 10.1093/nop/npv064
37. Bernhardt D, König L, Grosu AL, Rieken S, Krieg SM, Wick W, et al. DEGRO practical guideline for central nervous system radiation necrosis part 2: treatment. Strahlentherapie und Onkologie. (2022) 198:971–80. doi: 10.1007/s00066-022-01973-8
38. Rahmathulla G, Marko NF, and Weil RJ. Cerebral radiation necrosis: A review of the pathobiology, diagnosis and management considerations. J Clin Neurosci. (2013) 20:485–502. doi: 10.1016/J.JOCN.2012.09.011
39. Verma N, Cowperthwaite MC, Burnett MG, and Markey MK. Differentiating tumor recurrence from treatment necrosis: a review of neuro-oncologic imaging strategies. Neuro Oncol. (2013) 15:515–34. doi: 10.1093/NEUONC/NOS307
40. Siu A, Wind JJ, Iorgulescu JB, Chan TA, Yamada Y, and Sherman JH. Radiation necrosis following treatment of high grade glioma-a review of the literature and current understanding. Acta Neurochir (Wien). (2012) 154:191–201. doi: 10.1007/s00701-011-1228-6
41. Ninatti G, Pini C, Gelardi F, Sollini M, and Chiti A. The role of PET imaging in the differential diagnosis between radiation necrosis and recurrent disease in irradiated adult-type diffuse gliomas: A systematic review. Cancers (Basel). (2023) 15. doi: 10.3390/cancers15020364
42. Sogani SK, Jena A, Taneja S, Gambhir A, Mishra AK, D’Souza MM, et al. Potential for differentiation of glioma recurrence from radionecrosis using integrated 18F-fluoroethyl-L-tyrosine (FET) positron emission tomography/magnetic resonance imaging: A prospective evaluation. Neurol India. (2017) 65:293–301. doi: 10.4103/NEUROINDIA.NI_101_16
43. Pyka T, Hiob D, Preibisch C, Gempt J, Wiestler B, Schlegel J, et al. Diagnosis of glioma recurrence using multiparametric dynamic 18F-fluoroethyl-tyrosine PET-MRI. Eur J Radiol. (2018) 103:32–7. doi: 10.1016/J.EJRAD.2018.04.003
44. Vidmar MS, Doma A, Smrdel U, Zevnik K, and Studen A. The value of FET PET/CT in recurrent glioma with a different IDH mutation status: the relationship between imaging and molecular biomarkers. Int J Mol Sci. (2022) 23. doi: 10.3390/ijms23126787
45. Bashir A, Jacobsen SM, Henriksen OM, Broholm H, Urup T, Grunnet K, et al. Recurrent glioblastoma versus late posttreatment changes: Diagnostic accuracy of O-(2-[18F]fluoroethyl)-L-tyrosine positron emission tomography (18F-FET PET). Neuro Oncol. (2019) 21:1595–606. doi: 10.1093/neuonc/noz166
46. Werner JM, Stoffels G, Lichtenstein T, Borggrefe J, Lohmann P, Ceccon G, et al. Differentiation of treatment-related changes from tumour progression: a direct comparison between dynamic FET PET and ADC values obtained from DWI MRI. Eur J Nucl Med Mol Imaging. (2019) 46:1889–901. doi: 10.1007/s00259-019-04384-7
47. Celli M, Caroli P, Amadori E, Arpa D, Gurrieri L, Ghigi G, et al. Diagnostic and prognostic potential of 18F-FET PET in the differential diagnosis of glioma recurrence and treatment-induced changes after chemoradiation therapy. Front Oncol. (2021) 11:721821. doi: 10.3389/fonc.2021.721821
48. Maurer GD, Brucker DP, Stoffels G, Filipski K, Filss CP, Mottaghy FM, et al. 18F-FET PET imaging in differentiating glioma progression from treatment-related changes: A single-center experience. J Nucl Med. (2020) 61:506–11. doi: 10.2967/jnumed.119.234757
49. Brandsma D, Stalpers L, Taal W, Sminia P, and van den Bent MJ. Review Clinical features, mechanisms, and management of pseudoprogression in Malignant gliomas. Lancet Oncol. (2008) 9:453. doi: 10.1016/S1470-2045(08)70125-6
50. Van Mieghem E, Wozniak A, Geussens Y, Menten J, De Vleeschouwer S, Van Calenbergh F, et al. Defining pseudoprogression in glioblastoma multiforme. Eur J Neurol. (2013) 20:1335–41. doi: 10.1111/ene.12192
51. Balaña C, Capellades J, Pineda E, Estival A, Puig J, Domenech S, et al. Pseudoprogression as an adverse event of glioblastoma therapy. Cancer Med. (2017) 6:2858–66. doi: 10.1002/cam4.1242
52. Rowe LS, Butman JA, Mackey M, Shih JH, Cooley-Zgela T, Ning H, et al. Differentiating pseudoprogression from true progression: analysis of radiographic, biologic, and clinical clues in GBM. J Neurooncol. (2018) 139:145–52. doi: 10.1007/s11060-018-2855-z
53. Abdulla S, Saada J, Johnson G, Jefferies S, and Ajithkumar T. Tumour progression or pseudoprogression? A review of post-treatment radiological appearances of glioblastoma. Clin Radiol. (2015) 70:1299–312. doi: 10.1016/j.crad.2015.06.096
54. Melguizo-Gavilanes I, Bruner JM, Guha-Thakurta N, Hess KR, and Puduvalli VK. Characterization of pseudoprogression in patients with glioblastoma: is histology the gold standard? J Neurooncol. (2015) 123:141–50. doi: 10.1007/s11060-015-1774-5
55. Sanghera P, Rampling R, Haylock B, Jefferies S, McBain C, Rees JH, et al. The concepts, diagnosis and management of early imaging changes after therapy for glioblastomas. Clin Oncol. (2012) 24:216–27. doi: 10.1016/j.clon.2011.06.004
56. Galldiks N, Dunkl V, Stoffels G, Hutterer M, Rapp M, Sabel M, et al. Diagnosis of pseudoprogression in patients with glioblastoma using O-(2-[ 18 F]fluoroethyl)-L-tyrosine PET. J Nucl Med Mol Imaging. (2015) 42:685–95. doi: 10.1007/s00259-014-2959-4
57. Kebir S, Fimmers R, Galldiks N, Schafer N, Mack F, Schaub C, et al. Late pseudoprogression in glioblastoma: diagnostic value of dynamic O-(2-[18 F]fluoroethyl)-L-tyrosine PET. Clin Cancer Res. (2016) 22:2190–6. doi: 10.1158/1078-0432.CCR-15-1334/268068/AM/LATE-PSEUDOPROGRESSION-IN-GLIOBLASTOMA-DIAGNOSTIC
58. Werner JM, Weller J, Ceccon G, Schaub C, Tscherpel C, Lohmann P, et al. Diagnosis of pseudoprogression following lomustine–temozolomide chemoradiation in newly diagnosed glioblastoma patients using FET-PET. Clin Cancer Res. (2021) 27:3704–13. doi: 10.1158/1078-0432.CCR-21-0471
59. Mihovilovic MI, Kertels O, Hänscheid H, Löhr M, Monoranu C-M, Kleinlein I, et al. O-(2-((18)F)fluoroethyl)-l-tyrosine PET for the differentiation of tumour recurrence from late pseudoprogression in glioblastoma. J Neurology Neurosurg & Psychiatry. (2019) 90:238. doi: 10.1136/jnnp-2017-317155
60. Lohmeier J, Bohner G, Siebert E, Brenner W, Hamm B, and Makowski MR. Quantitative biparametric analysis of hybrid 18F-FET PET/MR-neuroimaging for differentiation between treatment response and recurrent glioma. Sci Rep. (2019) 9. doi: 10.1038/s41598-019-50182-4
61. Puranik AD, Dev ID, Rangarajan V, Jain Y, Patra S, Purandare NC, et al. FET PET to differentiate between post-treatment changes and recurrence in high-grade gliomas: a single center multidisciplinary clinic controlled study. Neuroradiology. (2025). 67(2):363–39. doi: 10.1007/s00234-024-03495-9
62. Verger A, Filss CP, Lohmann P, Stoffels G, Sabel M, Wittsack HJ, et al. Comparison of O-(2-18F-fluoroethyl)-L-tyrosine positron emission tomography and perfusion-weighted magnetic resonance imaging in the diagnosis of patients with progressive and recurrent glioma: A hybrid positron emission tomography/magnetic resonance study. World Neurosurg. (2018) 113:e727–37. doi: 10.1016/j.wneu.2018.02.139
63. Filss CP, Albert NL, Böning G, Kops ER, Suchorska B, Stoffels G, et al. O-(2-[18F]fluoroethyl)-l-tyrosine PET in gliomas: influence of data processing in different centres. EJNMMI Res. (2017) 7. doi: 10.1186/s13550-017-0316-x
64. Kertels O, Mihovilovic MI, Linsenmann T, Kessler AF, Tran-Gia J, Kircher M, et al. Clinical utility of different approaches for detection of late pseudoprogression in glioblastoma with O-(2-[18F]Fluoroethyl)-l-tyrosine PET. Clin Nucl Med. (2019) 44:695–701. doi: 10.1097/rlu.0000000000002652
65. Unterrainer M, Vettermann F, Brendel M, Holzgreve A, Lifschitz M, Zähringer M, et al. Towards standardization of18F-FET PET imaging: Do we need a consistent method of background activity assessment? EJNMMI Res. (2017) 7:1–8. doi: 10.1186/s13550-017-0295-y
66. Chaban A, Waschulzik B, Bernhardt D, Delbridge C, Schmidt-Graf F, Wagner A, et al. Amino acid PET vs. RANO MRI for prediction of overall survival in patients with recurrent high grade glioma under bevacizumab therapy. Eur J Nucl Med Mol Imaging. 51(6):1698–702. doi: 10.1007/s00259-024-06601-4
67. Fleischmann DF, Unterrainer M, Bartenstein P, Belka C, Albert NL, and Niyazi M. 18F-FET PET prior to recurrent high-grade glioma re-irradiation—additional prognostic value of dynamic time-to-peak analysis and early static summation images? J Neurooncol. (2017) 132:277–86. doi: 10.1007/s11060-016-2366-8
68. Niyazi M, Jansen N, Ganswindt U, Schwarz SB, Geisler J, Schnell O, et al. Re-irradiation in recurrent Malignant glioma: Prognostic value of [ 18F]FET-PET. J Neurooncol. (2012) 110:389–95. doi: 10.1007/s11060-012-0980-7
69. Moller S, Law I, Costa J, Poulsen HS, Engelholm SA, and Engelholm S. Prognostic value of 18 F-FET PET imaging in re-irradiation of high-grade glioma: Results of a phase I clinical trial. Radiotherapy Oncol. (2016) 121:132–7. doi: 10.1016/j.radonc.2016.08.014
70. Harat M, Rakowska J, Harat M, Szylberg T, Furtak J, Miechowicz I, et al. Combining amino acid PET and MRI imaging increases accuracy to define Malignant areas in adult glioma. Nat Commun. (2023) 14:4572. doi: 10.1038/s41467-023-39731-8
71. Albert NL, Winkelmann I, Suchorska B, Wenter V, Schmid-Tannwald C, Mille E, et al. Early static 18 F-FET-PET scans have a higher accuracy for glioma grading than the standard 20–40 min scans. Eur J Nucl Med Mol Imaging. (2016) 43:1105–14. doi: 10.1007/s00259-015-3276-2
72. Mittlmeier LM, Suchorska B, Ruf V, Holzgreve A, Brendel M, Herms J, et al. 18f-fet pet uptake characteristics of long-term idh-wildtype diffuse glioma survivors. Cancers (Basel). (2021) 13. doi: 10.3390/cancers13133163
Keywords: glioblastoma, amino acid PET, post-treatment changes, tumor progression, response assessment
Citation: Zarębska I, Blok M, Marjański M and Harat M (2025) The role of FET-PET in patient selection and response assessment for reirradiation in recurrent glioblastoma. Front. Oncol. 15:1604448. doi: 10.3389/fonc.2025.1604448
Received: 01 April 2025; Accepted: 21 July 2025;
Published: 07 August 2025.
Edited by:
Anouk van der Hoorn, University Medical Centre Groningen, NetherlandsReviewed by:
Harshan Ravi, University of Texas MD Anderson Cancer Center, United StatesHannes Becker, Tübingen University Hospital, Germany
Chris W. Govaerts, University Medical Center Groningen, Netherlands
Copyright © 2025 Zarębska, Blok, Marjański and Harat. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Maciej Harat, aGFyYXRtQGNvLmJ5ZGdvc3pjei5wbA==
 Izabela Zarębska
Izabela Zarębska Maciej Blok1,2,3
Maciej Blok1,2,3 Maciej Harat
Maciej Harat