- 1Department of Pathology and Laboratory Medicine, Medstar Georgetown University Hospital, Washington, DC, United States
- 2Medstar Health Urology Oncology, Medstar Georgetown University Hospital, Washington, DC, United States
Papillary Renal Neoplasm with Reverse Polarity (PRNRP) is a rare renal tumor, recently described in 2019 by Al-Obaidy et al. defined by characteristic histology of papillary neoplasm with apically located WHO/ISUP grade 1nuclei and frequent KRAS mutations. Multilocular cystic renal neoplasm of low malignant potential (MC-LMP) is an indolent tumor with a characteristic multicystic appearance with cysts lined by WHO/ISUP nuclear grade1 clear cells and presence of VHL alterations similar to that of clear cell renal cell carcinoma (ccRCC); therefore, considered its variant. Simultaneous occurrence of both these tumor types that are immunophenotypically and genetically distinct within same kidney is extremely rare and this is the first case report to date. Herein, we report a case of a 70-year-old male who was incidentally found to have bilateral renal cysts on imaging follow up for cardiovascular problems. The diagnosis of PRNRP and MC-LMP within the same kidney was made on histology in conjunction with ancillary tests. Awareness of PRNRP and MC-LMP is crucial for accurate diagnosis, as these tumors often resemble some of the aggressive variants of Renal cell carcinoma (RCC), such as Papillary RCC (pRCC) and ccRCC respectively on histology. Ability to correctly identify these indolent tumors is essential for optimal treatment options as they are often amenable to partial nephrectomy. This case underscores the need for further research into the pathogenesis and clinical implications of synchronous renal tumors with distinct immunophenotypes, and genomic profiles within the same kidney.
Introduction
Renal cell carcinoma (RCC) accounts for 4.1% of all new cancers and 2.4% of all cancer-related deaths according to SEER (Surveillance, Epidemiology, and End Results Program) data as of the most recent report (1). RCC has been historically classified based on histomorphologic and cytomorphologic features as tumors with light/clear cell staining cytoplasm, tumors showing papillary or tubulopapillary architecture, tumors with granular/-eosinophilic cytoplasm, tumors with spindle cell morphology, poorly differentiated carcinoma, and tumors featuring distinct genotypic and immunophenotypic profiles. Papillary Renal Neoplasm with Reverse Polarity (PRNRP) is a recently described renal tumor based on its unique histology and molecular profile. Despite its indolent behavior, the World Health Organization (WHO) 2019 RCC classification has not recognized it as a distinct entity and has placed it under the category of Papillary RCC (pRCC), which is a far more aggressive tumor with a worse prognosis. The key defining histologic features include papillary or tubular architecture, WHO/ISUP grade 1 nuclei positioned toward the apex with an oncocytic appearance, a unique immunophenotypic profile with strong diffuse GATA3 expression, and negative Vimentin staining (2–4). Molecularly, these tumors have KRAS mutation in codon 12, distinguishing them from pRCCs, which do not carry these mutations and instead show karyotypic abnormalities characterized by trisomy of chromosomes 7, 17, and loss of chromosome Y (3). Conversely, multilocular cystic renal neoplasm of low malignant potential (MC-LMP) is a WHO-recognized type of renal cell tumor showing a multicystic appearance with cysts lined by WHO/ISUP nuclear grade 1 clear cells. These tumors carry an indolent clinical course. The simultaneous occurrence of renal tumors with distinct histologic types and genetics is rare, though there are a few case reports of such instances. Since recognition of PRNRP, a subset is known to occur synchronously with other renal tumors. However, a case of synchronous PRNRP with MC-LMP has yet to be reported.
To our knowledge, this is the first documented case of incidental synchronous occurrence of PRNRP and MC-LMP within the same kidney that requires combination of morphologic characteristics, immunohistochemistry and molecular analysis to establish diagnosis. This case report further contributes to the diverse presentation of PRNRP and offers insight into molecular findings, prognosis and clinical outcomes of these neoplasms with a review of the literature.
Case description
A 70-year-old male with a notable history of hypertension, hyperlipidemia, and aortic stenosis—who had previously undergone a prosthetic aortic valve replacement—was found to have bilateral renal cysts incidentally during follow-up imaging for his aortic valve replacement. The patient was asymptomatic with the lesions but was further assessed with an abdominal MRI.
The imaging showed a 2.5 cm complex renal mass within the upper to mid pole of the left kidney with irregular, thickened septal enhancement on contrast images, which likely represents a Bosniak type III lesion. There were additional Bosniak type II/IIF lesions within both kidneys. There was no evidence of abdominal adenopathy (Figure 1).
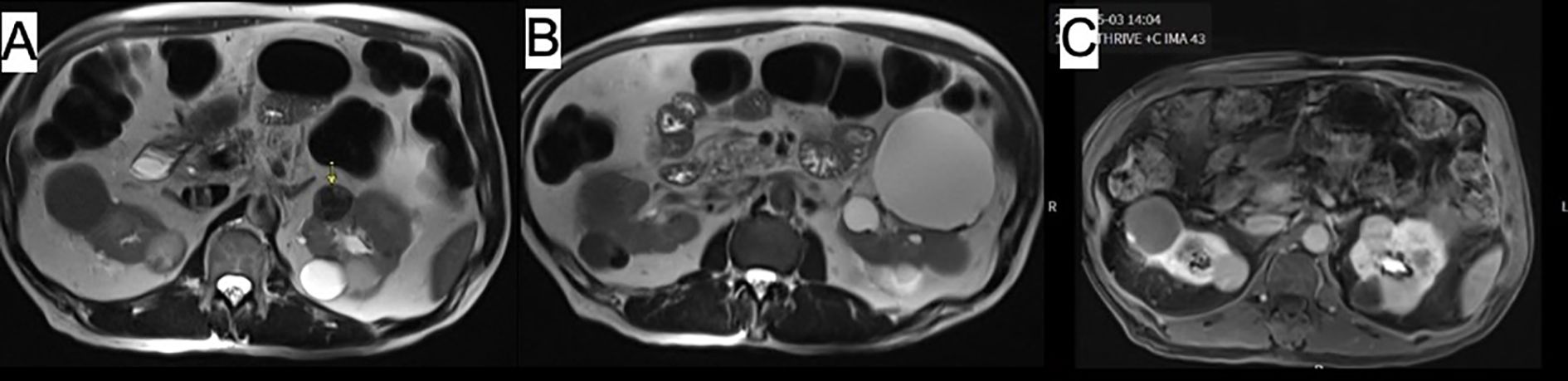
Figure 1. Abdominal MRI images showing (A, B) mass within anterior upper pole of left kidney with thick irregular internal septation (arrow). Image (C) demonstrates additional cysts in both kidneys.
Given the clinical presentation of bilateral renal cystic lesions concerning for primary renal neoplasm, a staged surgical approach was recommended. Intraoperative renal ultrasound was performed, demonstrating a 3 cm cystic anterior renal mass just superior to the renal hilum which corresponded to the position seen on the MRI. Due to the larger solid component in the anterior cystic mass, a robotic partial nephrectomy was advised. In addition, within the same kidney was an anterior middle-pole complex renal cyst. Decortication of that large anterior mid-pole renal cyst was performed to enhance visualization and facilitate the dissection of the cystic lesion, followed by a partial nephrectomy for the mass.
Histology
Gross examination of the left renal partial nephrectomy revealed a well-circumscribed and encapsulated mass measuring 1.7 x 1.7 x 1.0 cm. Upon sectioning, the cut surface appeared tan, brown to tan-pink with a loose papillary architecture. There was no evident necrosis or gross invasion of the capsule. On histological examination, the tumor was solid and cystic, exhibited a papillary architecture, and had WHO ISUP grade 1 nuclei with oncocytic cytoplasm and apically located nuclei (Figures 2A, B). Based on the morphologic characteristics, our differential was broad, ranging from benign to malignant categories. These included pRCC (malignant neoplasm), oncocytoma (benign neoplasm), PRNRP, and eosinophilic variant of ccRCC (malignant neoplasm).
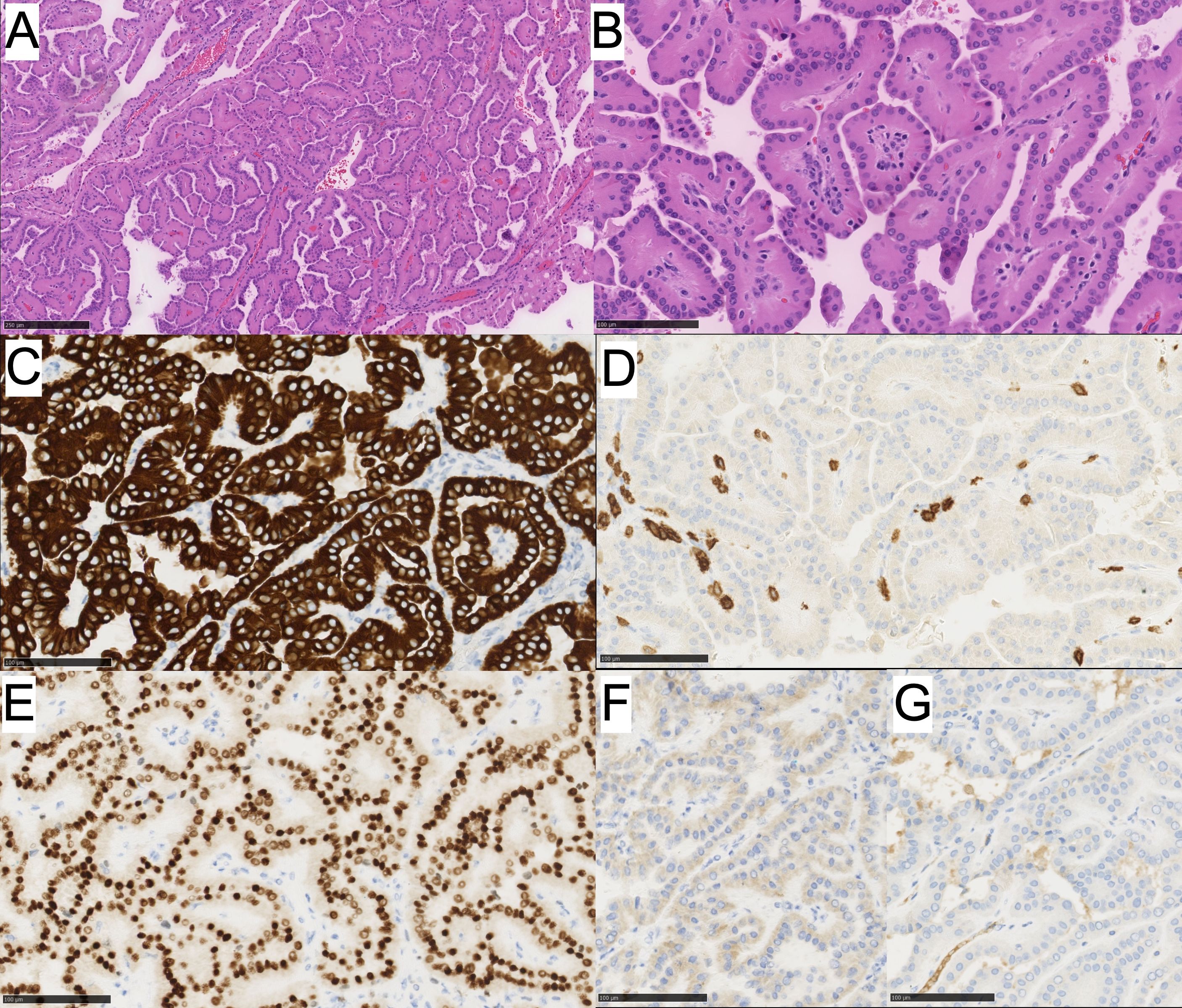
Figure 2. (A) PRNRP showing oncocytic cells with low-grade reversed nuclei polarity and papillary architecture and (B) higher power image. Immunohistochemistry for (C) CK7 is positive, (D) CD117 is negative, (E) GATA3 shows positive nuclear staining, (F) CAIX is negative, and (G) AMCAR is negative.
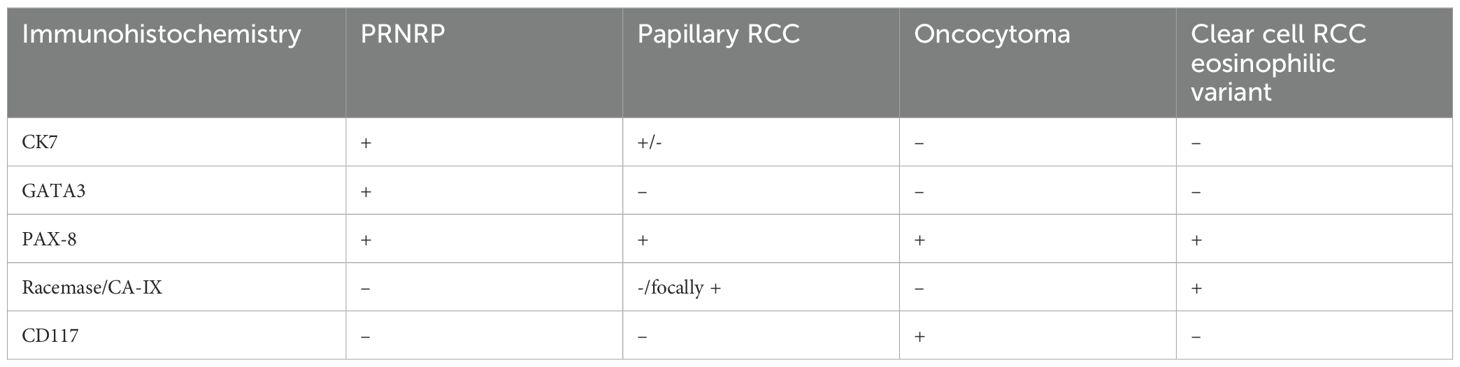
To differentiate these tumors, an immunohistochemical work-up was performed for the following markers: CK7, CK20, GATA3, PAX8, CA-IX, and CD117 (Table 1). The neoplastic cells were diffusely and strongly positive for CK7, GATA3, PAX8 (weak positivity), negative for racemase, CA-IX, CD117, and CK20 (Figures 2C-G). The immunoprofile in conjunction with morphology best classifies this neoplasm as a Papillary Renal Neoplasm with reverse polarity.
To confirm our findings, a targeted KRAS mutation analysis using real-time PCR (RT-PCR ROCHE LSR v2) was performed. KRAS codon G12C (c.34G>T) mutation in exon 2 was detected.
In addition, within the same kidney was an anterior middle-pole complex renal cyst that underwent decortication intraoperatively, and the cyst wall fragments were submitted for pathology evaluation. On histology, these fragments had multiple thin, fibrous septa lined by clear cells with few foci of clear cell clusters. The nuclei were uniformly low grade (WHO/ISUP grade 1) with no nodular expansion (Figures 3A, B). Immunophenotypically, these neoplastic clear cells were positive for CK7 and CA-IX (Figures 3C, D). The morphology and immunophenotype of the cyst were consistent with MC-LMP.
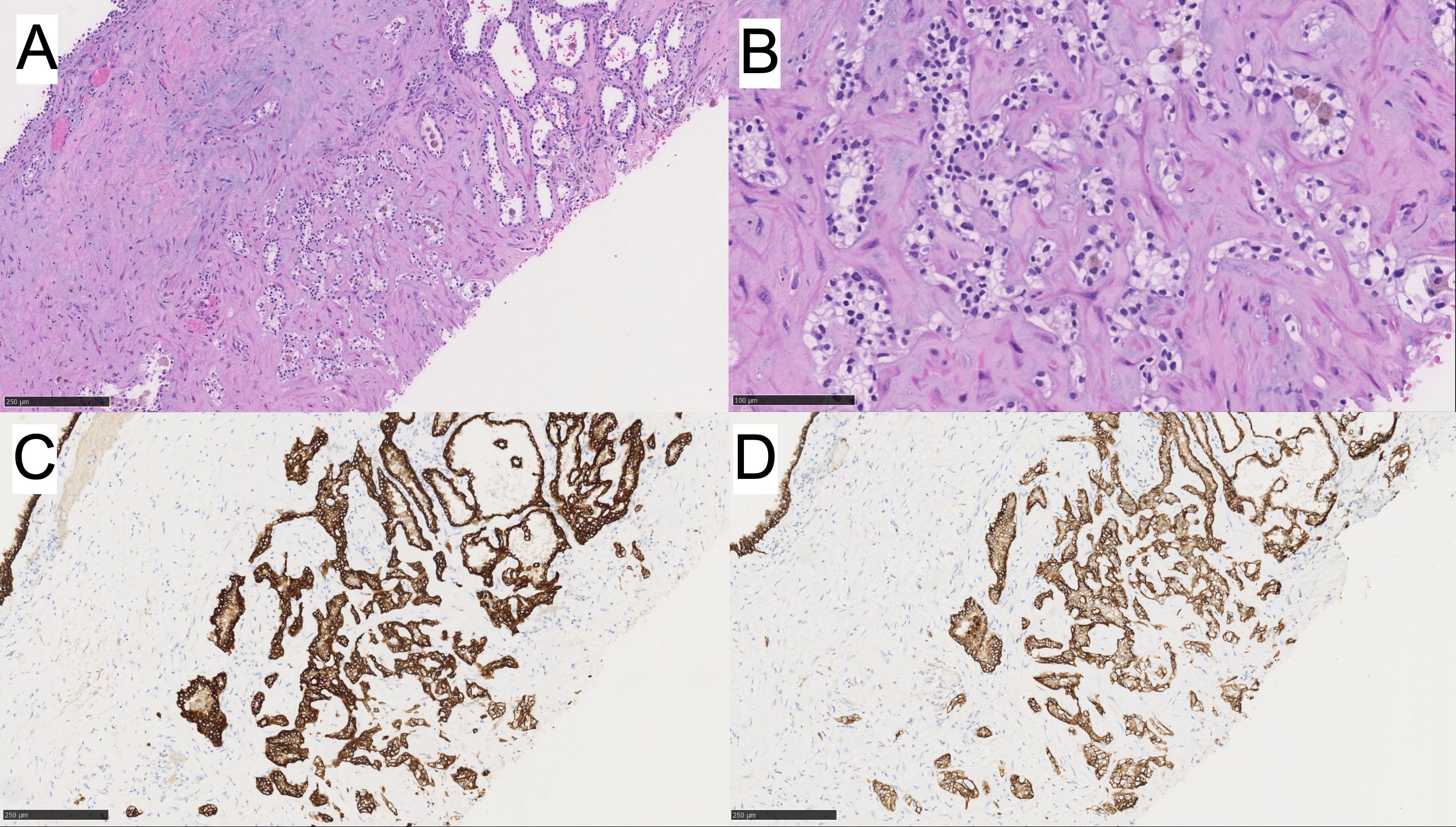
Figure 3. (A, B) MC-LMP with higher power. Immunohistochemistry for (C) CK7 is positive, and (D) CAIX is positive.
Overall, our findings feature two morphologically and immunophenotypically distinct tumor types; PRNRP and MC-LMP within the same kidney.
Molecular analysis
To further confirm the histologic impression and identify a plausible association of simultaneous occurrence of these two tumors in the same kidney, after appropriate patient consent, Next generation sequencing (NGS) and karyotype analysis were performed. A pathogenic variant in KRAS (Exon 2, p.G12C, c.34G>T) was detected with a variant allele frequency (VAF) of 30% in the PRNRP. No mutations in the KRAS gene were found in MC-LMP; instead, the tumor showed a frameshift variant in the BCR gene (c.3275_3278dupCCGG, p.V1094fs) with a VAF of 16%. The BCR gene is one of the candidate genes used to differentiate MC-LMP from ccRCC with cystic change.
No other genetic alterations associated with syndromic or synchronous renal tumors (i.e. VHL, MET, FH, SDH, TSC1/TSC2, BAP1) were identified in the MC-LMP tumor sample. The morphology, immunophenotype, PCR, and NGS findings, indicate that these two tumors are genetically distinct and incidental synchronous tumors with no syndromic association. Based on the clinical follow-up thus far, it appears to carry an indolent prognosis.
On follow up, the patient has recovered well from surgery and is doing well post-surgery. His follow up recent CT chest abdomen pelvis 9 months post resection did not demonstrate any metastatic disease or residual tumor within bilateral renal cysts.
Discussion
The frequency of multifocality in renal tumors is about 5.3% (5). Multifocal renal tumors can be histologically and genetically concordant or discordant and are common in day-to-day practice as well as reported in the literature with a combination of tumor types including pRCC, ccRCC, Chromophobe RCC, MC-LMP, Oncocytoma, etc. The rate of discordant tumor types in multifocal renal tumors is about 6-30%. Their pathogenesis is often multifactorial and yet to be fully elucidated (5–8).
Synchronous and multifocal renal tumors are often seen in association with genetic syndromes like von Hippel-Lindau (VHL) disease or hereditary papillary RCC. About 5-13% of sporadic MC-LMP are found to have concurrent ccRCC, with the likely etiology being the VHL gene mutations. Previously, a subset of PRNRP cases had been noted to occur synchronously with an additional renal tumor in the ipsilateral kidney, including pRCC, ccRCC, chromophobe RCC, and oncocytoma. To our best knowledge, there is no previously reported association between PRNRP and MC-LMP (2). Synchronous PRNPR were typically independent tumors, often physically separate nodules. This reflects the possibility that PRNRP can arise as a sporadic collision tumor alongside others. In a case report with molecular analysis by Lee et al. showed a case of PRNRP harboring a KRAS p.G12V mutation while the synchronous ccRCC carried a distinct PIK3CA mutation (9), suggesting two clonally independent tumors in one kidney. Additionally, a case of PRNRP with KRAS mutation was also reported synchronously with urothelial carcinoma with a distinct FGFR3/KDM6A mutation (10). Similarly, our case demonstrated non-overlapping mutation; KRAS mutation in PRNRP and BCR mutation in MC-LMP, further supporting the concept of genetically separate lesions occurring synchronously.
A striking immunohistochemical feature of PRNRP is it’s consistent GATA3 expression. GATA3 immunohistochemical stain although non-specific can be expressed in a variety of tumor types; in the appropriate clinical setting, it is helpful for confirming urothelial or mammary origins. GATA3 is a transcription factor involved in the development of the urinary tract (11). In a normal kidney, GATA3 is expressed in certain kidney structures (distal nephron tubules and collecting ducts) but not in proximal tubules (12). Although GATA3 expression in kidney tumors is not well documented, there appears to be a pattern of GATA3 expression in tumors believed to originate from the distal tubules and collecting ducts. Among renal tumors, the highest expression is observed in PRNRP and low-grade oncocytic tumors (LOTs), both of which shows 100% GATA3 positivity. Fumarate hydratase-deficient RCC shows GATA3 expression in 33% of cases, though staining may be focal. Clear-cell papillary renal tumors (CCPRT) exhibit 41-67% GATA3 positivity, a characteristic feature, in addition to the “cup-like” CAIX staining pattern, that differentiates them from ccRCC. Collecting duct carcinoma (CDC) shows positivity in 33% of case. Chromophobe RCC expresses GATA3 in 6-51% of cases, while 9% of TF3-translocated RCC shows GATA3 positivity (13). In contrast, ccRCC and pRCC both thought to arise from proximal nephrons are negative for GATA3 expression (14). The consistent expression of GATA3 in PRNRN possibly suggest its origin in distal tubules. While GATA3 can aid in distinguishing specific renal tumor subtypes, its use should be limited to a panel of markers rather than as a sole diagnostic tool.
KRAS mutations are considered a central part of the pathogenesis of adenocarcinomas of many organs including lungs, colorectal, and pancreas (15). KRAS mutations are also frequently reported in various papillary or mucinous precursor lesions such as intraductal papillary mucinous neoplasms of the pancreas or urothelial papilloma/carcinomas. KRAS mutations have also been implicated in approximately 5% of urothelial carcinoma but is not considered a central part of its pathogenesis (16). Upper urothelial tract carcinoma and lower urothelial tract carcinomas have demonstrated mutational differences. RAS associated alterations are seen more commonly in the upper urothelial tract carcinomas (17). KRAS mutations are rare in clear-cell renal cell tumors, except for one case reported in the literature thus far (8). The overall frequency of KRAS mutation in PRNRP is about 85% (2, 18, 19). and the most common mutation being KRAS p.G12V (54%) (18). In contrast our case showed p.G12C (c.34G>T) KRAS mutation.
BCR mutation is a key part of the pathogenesis of chronic myeloid leukemia, the BCR-ABL1 fusion (Philadelphia chromosome), where the upregulation of tyrosine kinase activity drives leukemic cell proliferation. BCR mutations are infrequent in solid tumors; however, its alterations have been linked to certain renal tumors, particularly MC-LMP. A frameshift alteration detected in the BCR gene in MC-LMP and no VHL gene mutation compared to ccRCC suggests likely separate clonal evolutionary mechanisms (19), despite the two neoplasms sharing overlapping histomorphology. MC-LMP often lack the typical VHL alterations (only approximately 25% show VHL alterations) that are seen in ccRCC (20). In fact, MC-LMP often shows other genetic changes in genes such as TCEB1 or other non-VHL-associated. The absence of VHL alterations supports the classification of MC-LMP as a distinct entity from ccRCC and further confirming its indolent nature.
In our case, the absence of end-stage renal disease (ESRD) is notable, as many renal tumors, particularly cystic neoplasms, are frequently identified in patients with ESRD due to alterations in the renal microenvironment (21–23). Acquired cystic kidney disease (ACKD) develops in a significant proportion of ESRD patients – ranging from 8-95% in some studies (24, 25) in dialysis patients – and increases the risk of cystic renal neoplasms. Incidence of renal cancers is approximately 50 times greater in ACKD patients than in the general population (26, 27). Cystic neoplasms observed in ESRD include acquired cystic disease-associated renal cell carcinoma (ACD-RCC), clear cell papillary renal tumor and pRCC (23, 28–30). In contrast, ESRD patients without ACKD are less prone to developing cystic neoplasms. The lack of ESRD in our patient suggests that the coexistence of these two tumors is unlikely driven by the same environmental or systemic factors that contribute to cystic neoplasms in ESRD patients (31).
Awareness of PRNRP and MC-LMP is critical for accurate diagnosis and optimal treatment. PRNRP can be misdiagnosed with other papillary tumors, most importantly pRCC which carries a far worse prognosis than PRNRP. Therefore, awareness of this entity is important to prevent overtreatment. Given its indolent nature, nephron-sparing approaches may be appropriate when feasible. Similarly, MC-LMP should not be confused with more aggressive renal neoplasms such as ccRCC with cystic change as often they are found in association with them. MC-LMP carries an excellent prognosis when completely excised (32–36).
Treatment strategies for both tumors are largely surgical, with no role for adjuvant therapy in the absence of adverse features or metastatic disease. However, the rare coexistence of these tumors emphasizes the importance of thorough histologic evaluation and molecular profiling, which can guide management decisions and improve outcomes. Our case demonstrates absence of recurrence or metastasis during the 9-months follow-up period, which align with the reported indolent nature of these neoplasms (Table 2).
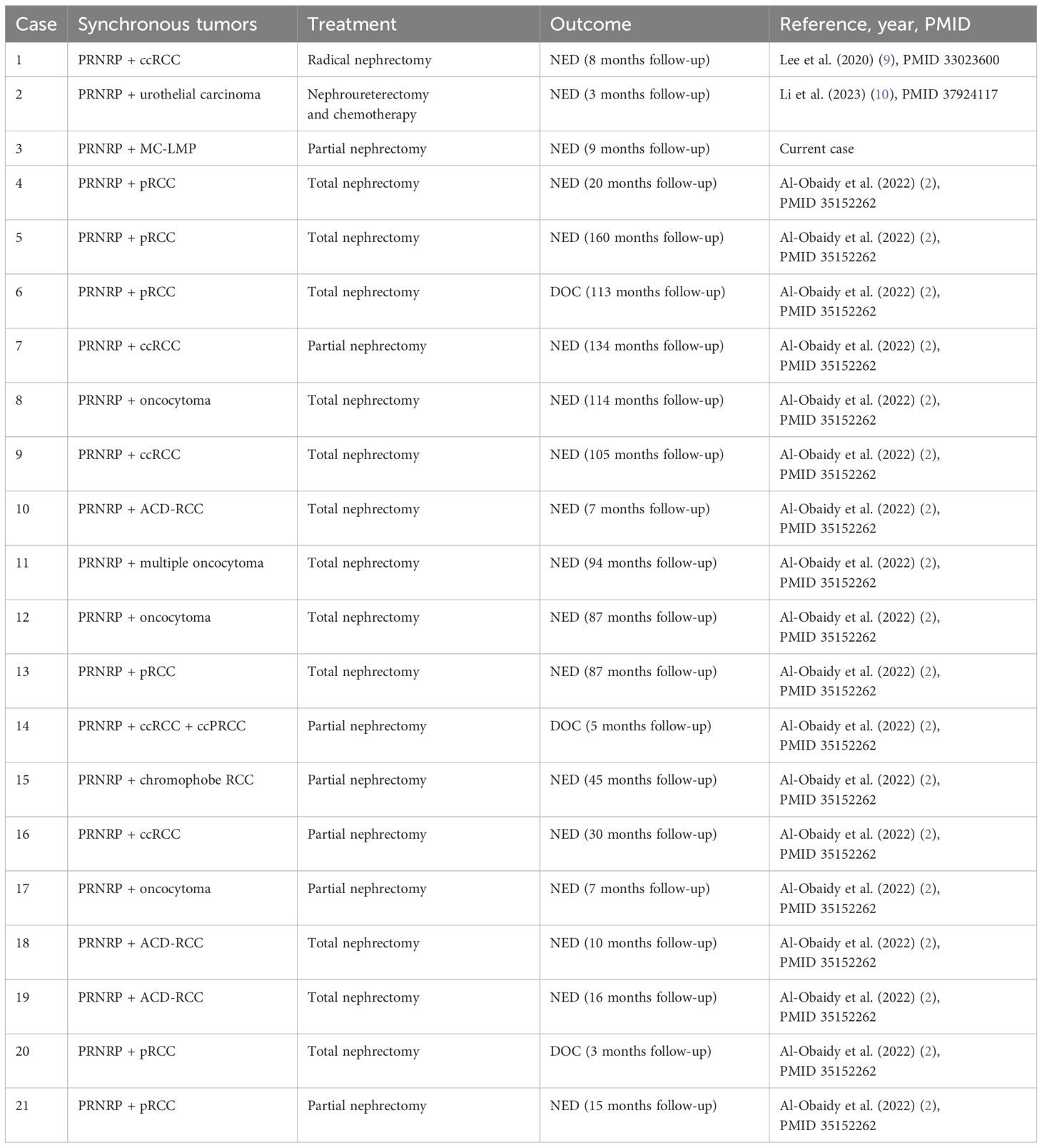
Table 2. Literature review of synchronous PRNRP and other renal neoplasms with treatment approaches and follow-up.
Our case underscores the need for further studies to elucidate the potential biological links between these rare renal neoplasms and refine diagnostic and therapeutic approaches.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
Ethical approval was not required for the studies involving humans because MedStar Health Institutional Review Board has determined that a case report of less than three (3) patients does not meet the DHHS definition of research [45 CFR 46.102(d)(pre-2018)/45 CFR 46.102(l)(1/19/2017)] or the FDA definition of clinical investigation [21 CFR 46.102(c)] and therefore are not subject to IRB review requirements and do not require IRB approval. The studies were conducted in accordance with the local legislation and institutional requirements. The human samples used in this study were acquired from a by- product of routine care or industry. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
PN: Writing – original draft, Writing – review & editing. JC: Writing – original draft, Writing – review & editing. LS: Writing – original draft, Writing – review & editing. RS: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. The funding for the publication of this article was provided by the Department of Pathology and Laboratory, Medstar Georgetown University Hospital.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. SEER Cancer Stat Facts: Kidney and Renal Pelvis Cancer. Bethesda, MD: National Cancer Institute. Available online at: https://seer.cancer.gov/statfacts/html/kidrp.html.
2. Al-Obaidy KI, Saleeb RM, Trpkov K, Williamson SR, Sangoi AR, Nassiri M, et al. Recurrent KRAS mutations are early events in the development of papillary renal neoplasm with reverse polarity. Mod Pathol. (2022) 35:1279–86. doi: 10.1038/s41379-022-01018-6
3. Al-Obaidy KI, Eble JN, Cheng L, Williamson SR, Sakr WA, Gupta N, et al. Papillary renal neoplasm with reverse polarity: A morphologic, immunohistochemical, and molecular study. Am J Surg Pathol. (2019) 43:1099–111. doi: 10.1097/PAS.0000000000001288
4. Wei S, Kutikov A, Patchefsky AS, Flieder DB, Talarchek JN, Al-Saleem T, et al. Papillary renal neoplasm with reverse polarity is often cystic: report of 7 cases and review of 93 cases in the literature. Am J Surg Pathol. (2022) 46:336–43. doi: 10.1097/PAS.0000000000001773
5. Richstone L, Scherr DS, Reuter VR, Snyder ME, Rabbani F, Kattan MW, et al. Multifocal renal cortical tumors: frequency, associated clinicopathological features and impact on survival. J Urol. (2004) 171:615–20. doi: 10.1097/01.ju.0000106955.19813.f6
6. Dimarco DS, Lohse CM, Zincke H, Cheville JC, and Blute ML. Long-term survival of patients with unilateral sporadic multifocal renal cell carcinoma according to histologic subtype compared with patients with solitary tumors after radical nephrectomy. Urology. (2004) 64:462–7. doi: 10.1016/j.urology.2004.04.016
7. Beaugerie A, Audenet F, Verkarre V, Delavaud C, Le Guilchet T, Hurel S, et al. Pathological heterogeneity in sporadic synchronous renal tumors: Is the histological concordance predictable? Urol Oncol. (2018) 36:11.e7–.e2. doi: 10.1016/j.urolonc.2017.09.002
8. Raspollini MR, Castiglione F, Martignoni G, Lapini A, Cheng L, Montironi R, et al. Multiple and bilateral kidney tumors with clear cells of three different histotypes: A case report with clinicopathologic and molecular study. Apmis. (2016) 124:619–23. doi: 10.1111/apm.12536
9. Lee HJ, Shin DH, Park JY, Kim SY, Hwang CS, Lee JH, et al. Unilateral synchronous papillary renal neoplasm with reverse polarity and clear cell renal cell carcinoma: a case report with KRAS and PIK3CA mutations. Diagn Pathol. (2020) 15:123. doi: 10.1186/s13000-020-01042-7
10. Li D, Liu F, Chen Y, Li P, Liu Y, and Pang Y. Ipsilateral synchronous papillary renal neoplasm with reverse polarity and urothelial carcinoma in a renal transplant recipient: a rare case report with molecular analysis and literature review. Diagn Pathol. (2023) 18:120. doi: 10.1186/s13000-023-01405-w
11. Sanchez-Ferras O, Pacis A, Sotiropoulou M, Zhang Y, Wang YC, Bourgey M, et al. A coordinated progression of progenitor cell states initiates urinary tract development. Nat Commun. (2021) 12:2627. doi: 10.1038/s41467-021-22931-5
12. Deebajah M, Qu Z, and Zhang P. GATA3 is a useful immunohistochemical marker to differentiate variants of renal tubular lesions from different segments of renal tubules. Am J Clin Pathol. (2021) 156:S152–S3. doi: 10.1093/ajcp/aqab191.325
13. Akgul M, Sangoi AR, and Williamson SR. GATA3 in renal neoplasms: increased utility and potential pitfalls. Int J Surg Pathol. (2024) 32:365–7. doi: 10.1177/10668969231177883
14. Miettinen M, McCue PA, Sarlomo-Rikala M, Rys J, Czapiewski P, Wazny K, et al. GATA3: a multispecific but potentially useful marker in surgical pathology: a systematic analysis of 2500 epithelial and nonepithelial tumors. Am J Surg Pathol. (2014) 38:13–22. doi: 10.1097/PAS.0b013e3182a0218f
15. Nassar AH, Adib E, and Kwiatkowski DJ. Distribution of KRAS (G12C) somatic mutations across race, sex, and cancer type. N Engl J Med. (2021) 384:185–7. doi: 10.1056/NEJMc2030638
16. Sjödahl G, Lauss M, Gudjonsson S, Liedberg F, Halldén C, Chebil G, et al. A systematic study of gene mutations in urothelial carcinoma; inactivating mutations in TSC2 and PIK3R1. PloS One. (2011) 6:e18583. doi: 10.1371/journal.pone.0018583
17. Audenet F, Isharwal S, Cha EK, Donoghue MTA, Drill EN, Ostrovnaya I, et al. Clonal relatedness and mutational differences between upper tract and bladder urothelial carcinoma. Clin Cancer Res. (2019) 25:967–76. doi: 10.1158/1078-0432.CCR-18-2039
18. Al-Obaidy KI, Eble JN, Nassiri M, Cheng L, Eldomery MK, Williamson SR, et al. Recurrent KRAS mutations in papillary renal neoplasm with reverse polarity. Mod Pathol. (2020) 33:1157–64. doi: 10.1038/s41379-019-0362-1
19. Kim SH, Park WS, and Chung J. SETD2, GIGYF2, FGFR3, BCR, KMT2C, and TSC2 as candidate genes for differentiating multilocular cystic renal neoplasm of low Malignant potential from clear cell renal cell carcinoma with cystic change. Investig Clin Urol. (2019) 60:148–55. doi: 10.4111/icu.2019.60.3.148
20. von Teichman A, Compérat E, Behnke S, Storz M, Moch H, and Schraml P. VHL mutations and dysregulation of pVHL- and PTEN-controlled pathways in multilocular cystic renal cell carcinoma. Mod Pathol. (2011) 24:571–8. doi: 10.1038/modpathol.2010.222
21. Song C, Hong SH, Chung JS, Byun SS, Kwak C, Jeong CW, et al. Renal cell carcinoma in end-stage renal disease: Multi-institutional comparative analysis of survival. Int J Urol. (2016) 23:465–71. doi: 10.1111/iju.13084
22. Åkerlund J, Holmberg E, Lindblad P, Stendahl M, Ljungberg B, Thorstenson A, et al. Increased risk for renal cell carcinoma in end stage renal disease - a population-based case-control study. Scand J Urol. (2021) 55:209–14.
23. Tsuzuki T, Iwata H, Murase Y, Takahara T, and Ohashi A. Renal tumors in end-stage renal disease: A comprehensive review. Int J Urol. (2018) 25:780–6. doi: 10.1111/iju.13759
24. Basile JJ, McCullough DL, Harrison LH, and Dyer RB. End stage renal disease associated with acquired cystic disease and neoplasia. J Urol. (1988) 140:938–43. doi: 10.1016/S0022-5347(17)41893-3
25. Mokhtari GR, Karami H, Ghanbari A, Enshaei A, and Sedaghat S. Prevalence of acquired renal cystic disease in patients with end-stage renal disease receiving hemodialysis. Urotoday Int J. (2011) 04. doi: 10.3834/uij.1944-5784.2011.06.12
26. MacDougall ML, Welling LW, and Wiegmann TB. Renal adenocarcinoma and acquired cystic disease in chronic hemodialysis patients. Am J Kidney Dis. (1987) 9:166–71. doi: 10.1016/S0272-6386(87)80094-X
27. Matson MA and Cohen EP. Acquired cystic kidney disease: occurrence, prevalence, and renal cancers. Med (Baltimore). (1990) 69:217–26. doi: 10.1097/00005792-199007000-00003
28. Ishikawa I and Kovacs G. High incidence of papillary renal cell tumours in patients on chronic haemodialysis. Histopathology. (1993) 22:135–9. doi: 10.1111/j.1365-2559.1993.tb00091.x
29. McDougal WS. Prevalence of renal cell carcinoma in patients with ESRD pre-transplantation: a pathologic analysis. J Urol. (2003) 169:2422. doi: 10.1016/S0022-5347(05)63631-2
30. Truong LD, Choi YJ, Shen SS, Ayala G, Amato R, and Krishnan B. Renal cystic neoplasms and renal neoplasms associated with cystic renal diseases: pathogenetic and molecular links. Adv Anat Pathol. (2003) 10:135–59. doi: 10.1097/00125480-200305000-00003
31. Semjén D, Dénes B, Somorácz Á, Fintha A, Forika G, Jenei A, et al. Renal cell carcinoma in end-stage renal disease: A retrospective study in patients from Hungary. Pathobiology. (2023) 90:322–32. doi: 10.1159/000529276
32. Xiao YM, Yang SK, Wang Y, Mao D, Duan FL, and Zhou SK. Retroperitoneal laparoscopic partial nephrectomy for unilateral synchronous multifocal renal carcinoma with different pathological types: A case report. World J Clin Cases. (2021) 9:6879–85. doi: 10.12998/wjcc.v9.i23.6879
33. Wang B, Gong H, Zhang X, Li H, Ma X, Song E, et al. Bilateral synchronous sporadic renal cell carcinoma: retroperitoneoscopic strategies and intermediate outcomes of 60 patients. PloS One. (2016) 11:e0154578. doi: 10.1371/journal.pone.0154578
34. Sandbergen L, Guven S, and Laguna MP. Can ablation win against partial nephrectomy and become first line therapy in cT1a renal tumours? Curr Opin Urol. (2019) 29:70–7.
35. Wilcox Vanden Berg RN, Basourakos SP, LaRussa S, and McClure TD. Management of the small renal mass: a 2020 update. Curr Oncol Rep. (2020) 22:69. doi: 10.1007/s11912-020-00924-9
Keywords: papillary renal neoplasm with reverse polarity, multiloculate cystic renal neoplasm of low malignancy potential, synchronous renal tumors, KRAS mutation, renal neoplasm pathogenesis
Citation: Nithagon P, Chahine J, Stamatakis L and Samdani R (2025) Synchronous papillary renal neoplasm with reverse polarity and multilocular cystic renal neoplasm of low malignant potential in unilateral kidney: case report with molecular analysis and literature review. Front. Oncol. 15:1605192. doi: 10.3389/fonc.2025.1605192
Received: 03 April 2025; Accepted: 22 July 2025;
Published: 11 August 2025.
Edited by:
John Peter Sfakianos, Icahn School of Medicine at Mount Sinai, United StatesReviewed by:
Peter Julius, University of Zambia, ZambiaTarang Patel, All India Institute of Medical Sciences (AIIMS) Rajkot Gujart India, India
Copyright © 2025 Nithagon, Chahine, Stamatakis and Samdani. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pichayut Nithagon, bnNhbmcxOTk0QGdtYWlsLmNvbQ==
 Pichayut Nithagon
Pichayut Nithagon Joeffrey Chahine1
Joeffrey Chahine1 Lambros Stamatakis
Lambros Stamatakis Rashmi Samdani
Rashmi Samdani