- 1Department of Oncology, The Second Xiangya Hospital, Central South University, Changsha, Hunan, China
- 2Department of Hematology, Institute of Molecular Hematology, The Second Xiangya Hospital, Central South University, Changsha, Hunan, China
- 3Department of Hematology, The Affiliated Changsha Central Hospital, Hengyang Medical School, University of South China, Changsha, Hunan, China
Background: To explore the potential association between long-term exposure to raw lacquer and the development of chronic myelomonocytic leukemia (CMML).
Methods: We analyzed the clinical and hematological characteristics of an elderly couple with CMML. Whole-exome sequencing (WES) was performed to identify relevant gene variants, with a focus on TET2 mutation status.
Results: Two unrelated CMML patients within the same family, both with over 40 years of raw lacquer exposure, developed CMML. WES revealed that both patients harbored multi-hit TET2 gene mutations and lacked ASXL1 mutations. Both demonstrated relative sensitivity to hydroxyurea or hypomethylating agent (HMA) therapy. Unaffected family members lacked significant raw lacquer exposure.
Conclusions: Long-term exposure to raw lacquer may be associated with the onset of familial CMML. CMML patients with multi-hit TET2 mutations in the absence of ASXL1 mutations may have a favorable prognosis.
Introduction
A familial cluster of chronic myelomonocytic leukemia (CMML) is presented, involving a non-consanguineous couple diagnosed with CMML at approximately 75 years of age, with diagnoses occurring 2 years apart. Both patients exhibited identical clinical and morphological features. A significant environmental factor shared by the couple was chronic exposure to raw Chinese lacquer for over 40 years, suggesting that it may be an extrinsic pathogenic factor for familial CMML. Whole-exome sequencing (WES) identified multipoint mutations in the TET2 gene in both patients, along with mutations in transcription factors RUNX1 and NRAS. This suggests that TET2 may be a molecular target of urushiol and brenzcatechin, the main components of raw lacquer. As no other specific extrinsic etiologic factors were identified, chronic raw lacquer exposure may be associated with an increased risk of CMML and its familial aggregation.
Case reports
Case 1
A 75-year-old woman was referred to the Department of Hematology, The Second XiangYa Hospital, Changsha, Hunan, for evaluation of leukocytosis [white blood cell (WBC) count 26.15 × 109/L] persisting for 3 weeks. The initial presentation involved abdominal pain and weakness. Peripheral blood analysis revealed monocytosis (24.2%, absolute monocyte count 6.33 × 109/L), anemia (hemoglobin 79 g/L), and thrombocytopenia (platelets 10 × 109/L). Bone marrow (BM) aspiration showed hypercellularity with 25% monocytes (23% mature monocytes and 2% monoblasts). Flow cytometry immunophenotyping was positive for CD13, CD33, CD14, CD16, CD64, HLA-DR, CD4, CD11b, and CD36, but negative for CD34, CD117, CD15, and CD56. Cytogenetic analysis revealed a normal karyotype (Figures 1C–E). She was diagnosed with myeloid proliferative CMML (MP-CMML) and classified as high-risk [Mayo Molecular Model (MMM) = 3 risk factors] (1). Treatment with subcutaneous azacitidine (100 mg/day for 7 days per month) was initiated. Hematological complete remission was achieved after four cycles.
The patient’s medical history was significant only for occasional contact dermatitis. Her husband worked as a raw lacquer producer and painter. They were exposed to raw lacquer for more than 40 years. Physical examination at presentation revealed moderate anemia without palpable superficial lymphadenopathy or hepatosplenomegaly. Laboratory findings included hemoglobin 77 g/L, WBC 7.79 × 109/L with monocytes 31.6% (absolute count 2.46 × 109/L), and platelets 22 × 109/L. Virological screening (Epstein-Barr Virus DNA (EBV-DNA), Cytomegalovirus (CMV), coxsackievirus, rubella, Herpes simplex virustype 1 (HSV-1), Toxoplasma, and HIV) was negative. Serum levels of trace elements and heavy metals (Zn, Pb, As, Ti, Cd, Cu, Se, Fe, Ni, Co, Pt, and Mn) were within normal ranges. Exclusionary testing was negative for BCR::ABL1, PDGFRα, and PDGFRβ fusion genes, as well as JAK2 V617F, MPL, and CALR mutations.
Case 2 (husband of case 1)
A 77-year-old man presented on May 17, 2021, with pallor, weakness, and exertional dyspnea. Peripheral blood analysis showed leukocytosis (WBC 34.43 × 109/L) with monocytosis (35.9%, absolute monocyte count 12.36 × 109/L), anemia (hemoglobin 96 g/L), and a platelet count of 136 × 109/L. Bone marrow morphology was consistent with CMML. WES covering 242 hematological malignancy-related genes was performed on peripheral blood mononuclear cells (PBMCs) (Chigene Medical Laboratory, Beijing, China). His past medical history was unremarkable except for his occupation as a raw lacquer producer and painter for over 40 years in a private workshop, during which he experienced several episodes of lacquer contact dermatitis. He cohabited with his wife (Case 1) for over 55 years, sharing the lacquer exposure for >40 years. His diet and other living conditions were comparable to those of nearby residents. His two sons and one daughter had lived with them for less than 15 years and showed no hematological abnormalities on examination. The family pedigree is shown in Figure 1F. He was diagnosed with MP-CMML and managed with low-dose hydroxyurea for leukocyte control. His most recent blood tests showed hemoglobin 73 g/L, WBC 22.83 × 109/L, monocytes 38.2% (absolute count 8.71 × 109/L), and platelets 80 × 109/L. He remains alive despite having the disease.
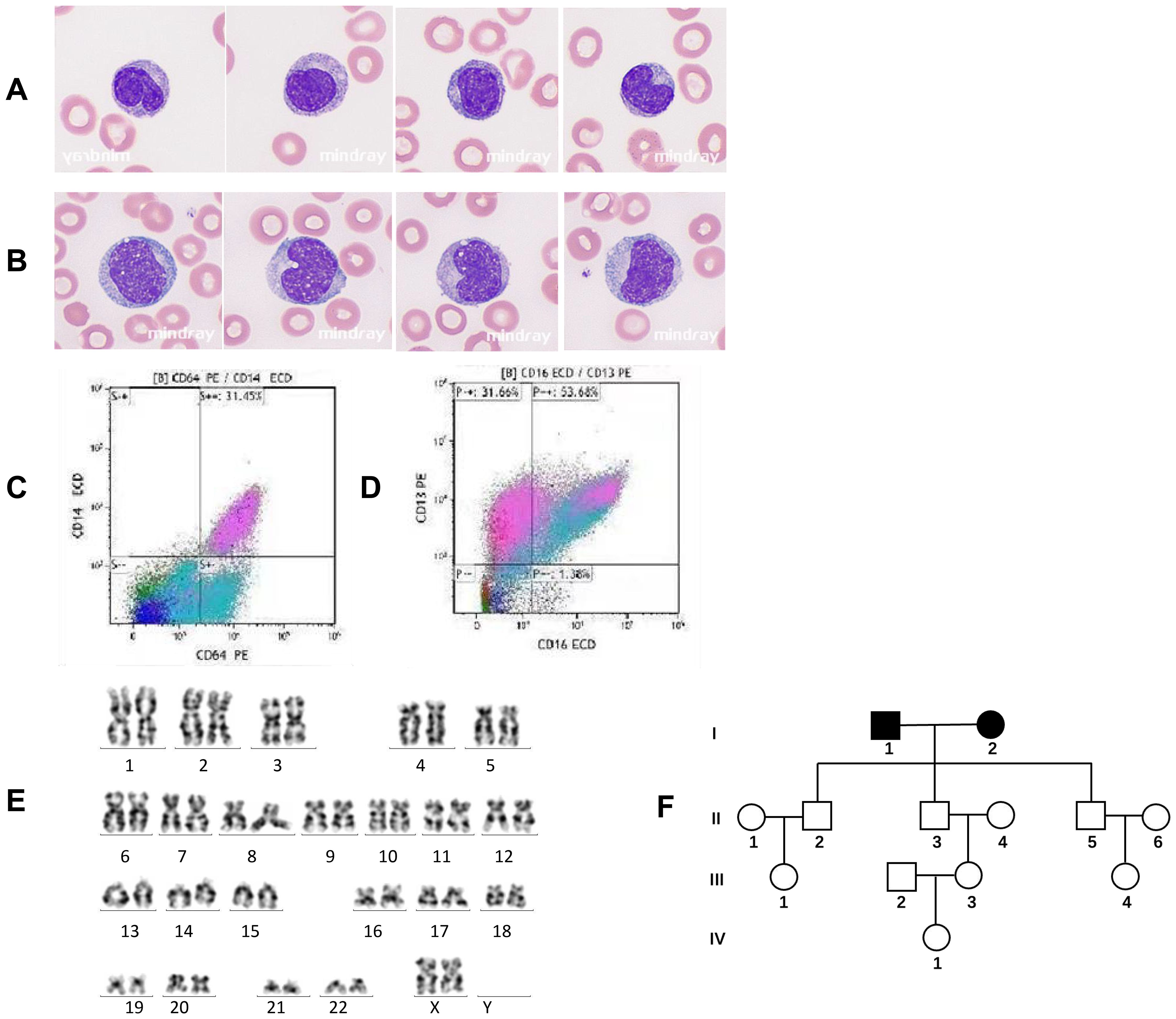
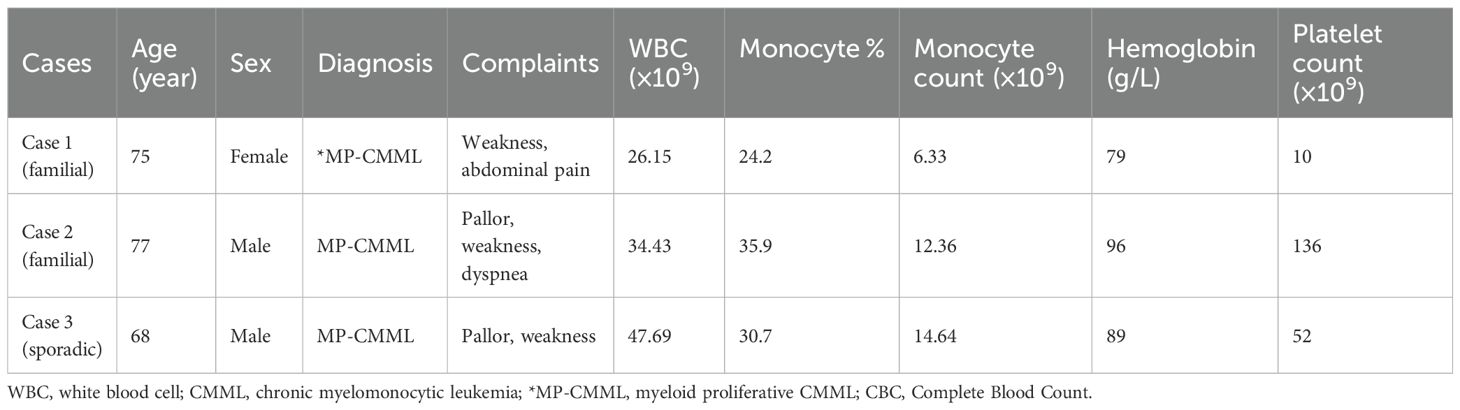
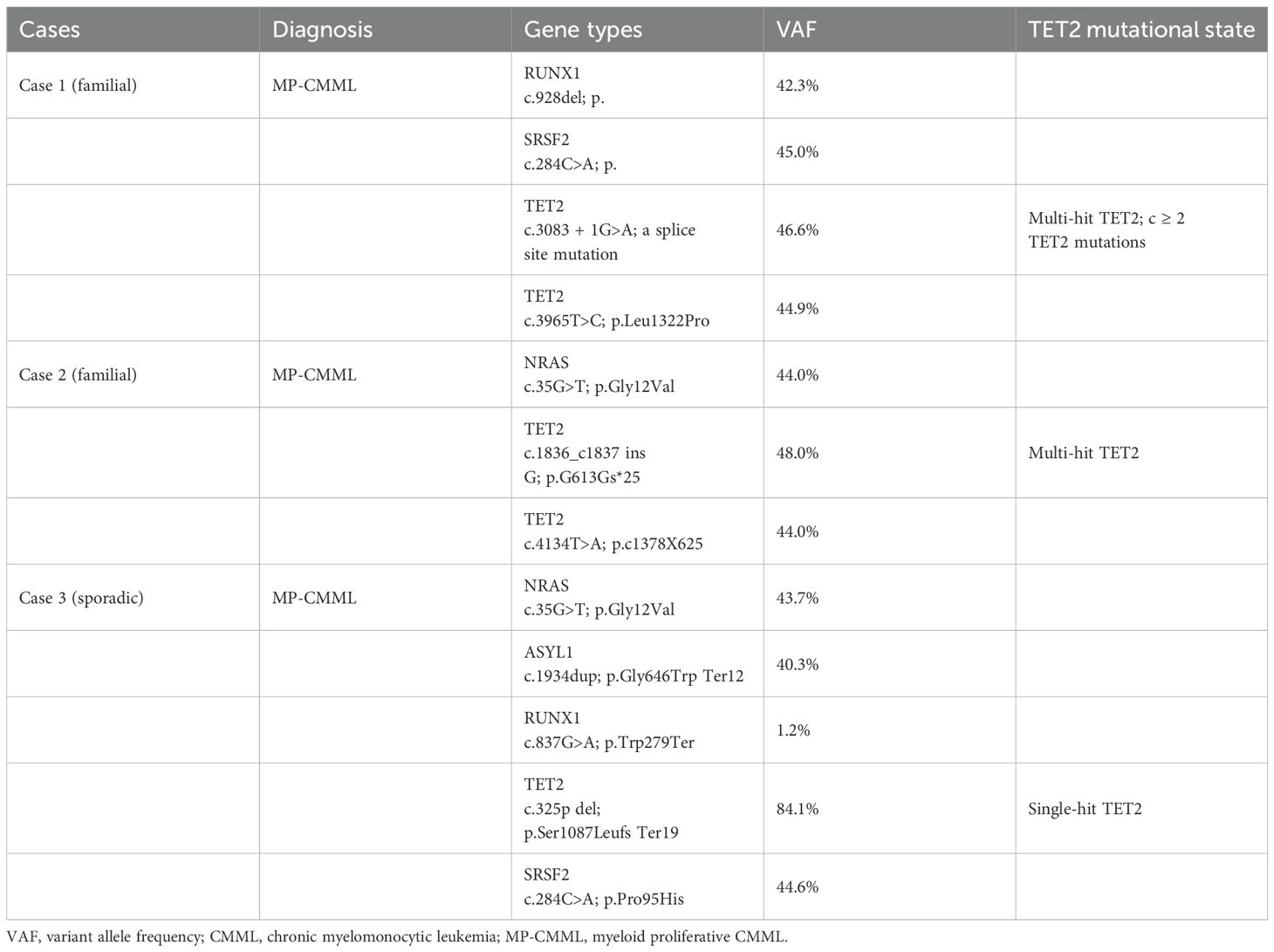
Figure 1. Peripheral blood morphology, cytogenetics, and flow cytometry immunophenotyping in chronic myelomonocytic leukemia (CMML). (A) Peripheral blood smear of Case 1 with familial CMML indicating mature or immature monocytes with smaller nuclei and a nuclear/plasma ratio Wright–Giemsa, ×1,000. (B) Peripheral blood smear of a control case with sporadic CMML showing immature or mature monocytes with larger nuclei and the nuclear/plasma ratio. Wright–Giemsa, ×1,000. (C, D) Immunophenotyping of monocytes by flow cytometry demonstrating monocyte fractions [CD14+CD16+ (non-classical)] in Case 1 with familial CMML. (E) The karyotype of Case 1 (46,XX) with familial CMML. (F) Pedigree of the familial CMML. Apart from the married couple (I1 and I2) diagnosed with CMML, all other members in the pedigree were unaffected.
Control case (sporadic CMML)
For comparison, a 68-year-old retired man with no history of raw lacquer or related chemical exposure was evaluated on August 26, 2023, for a 3-month history of pallor and weakness. Blood tests revealed leukocytosis (WBC 47.69 × 109/L) with monocytosis (30.7%, absolute monocyte count 14.64 × 109/L), anemia (hemoglobin 89 g/L), and thrombocytopenia (platelets 52 × 109/L). Bone marrow morphology confirmed CMML. He was diagnosed with MP-CMML. Clinical features and hematological parameters of this sporadic case and the familial cases are summarized in Table 1. He received standard hypomethylating agent (HMA) therapy (azacitidine: 100 mg/day, days 1–7/month), achieving incomplete hematological remission after five cycles. Due to developing thrombocytopenia with HMA, low-dose hydroxyurea was used for leukocyte control.
Methods
Two familial CMML patients and one sporadic CMML patient were included after providing informed written consent in accordance with the Declaration of Helsinki. PBMNCs were obtained at diagnosis. The study was approved by the Institutional Ethics Committee of the Second Xiang-Ya Hospital, Central South University.
Genomic DNA was extracted from fresh PBMNCs. WES was performed by Kindstar Global Company (Wuhan, China). Sequencing achieved 99.59% coverage at 20x depth, with an average sequencing depth ≥1,000x. Captured libraries were sequenced on an Illumina NextSeq 550 platform.
Results
WES of Case 1 (familial CMML) revealed three somatic mutations: RUNX1 c.928del [p.Met310Ter; variant allele frequency (VAF), 42.3%], SRSF2 c.284C>A (p.Pro95His; VAF, 45%), and multi-hit TET2 mutations [TET2 c.3803 + 1G>A, a splice site mutation (VAF, 46.6%) and c.3965T>C (p.Leu1322Pro), a missense mutation (VAF, 44.9%)]. The mutant TET2 loci were located in the Cys-rich domain and double-stranded β-helix (DSBH) domain (Figure 2A, top panel), consistent with previous reports (2).
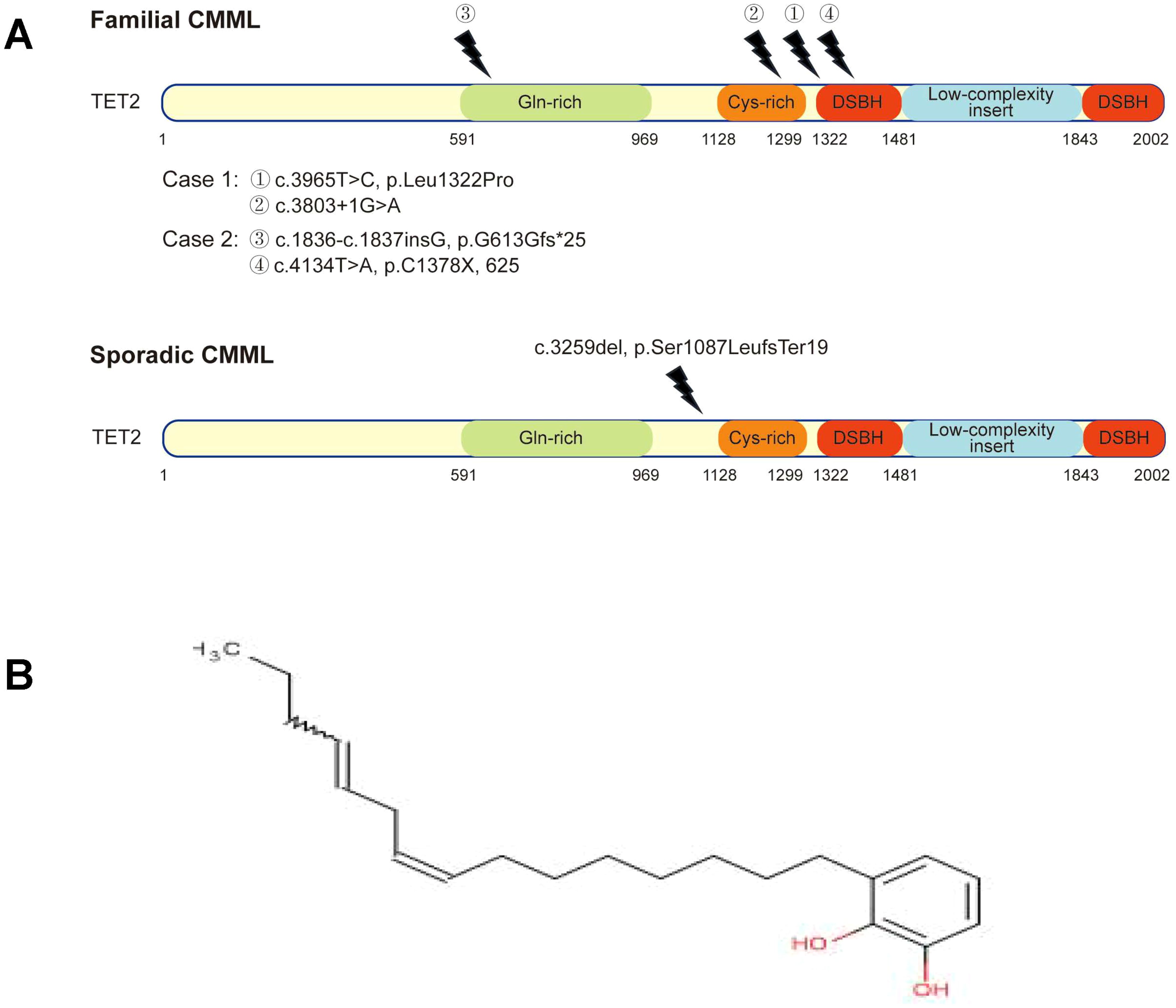
Figure 2. Schematic representation of TET2 mutations. (A) Top panel: mutant sites on TET2 in Case 1 and Case 2 with familial chronic myelomonocytic leukemia (CMML). DSBH, double-stranded β-helix domain. (A) Bottom panel: control case with sporadic CMML shows a novel mutation in the TET2 gene, which is not different from that of TET2 mutation in familial CMML. (B) The chemical structure of urushiol.
WES of Case 2 (covering 242 genes) identified an NRAS mutation (c.35G>T, p.Gly12Val; VAF, 44.0%) and multi-hit TET2 mutations [c.1836_1837insG, p.Gly613fs*25 (frameshift); VAF, 48%; and c.4134T>A, p.Cys1378*; VAF, 44%]. Both TET2 mutation sites represent novel loci in the context of CMML (Figure 2A, top panel).
WES of the sporadic CMML control case identified five mutations: ASXL1, NRAS, RUNX1, SRSF2, and TET2 (c.3259del, p.Ser1087LeufsTer19; VAF, 84.1%). The TET2 mutation site is also novel (Figure 2A, bottom panel) (2). Genetic mutations and VAFs for all three patients are summarized in Table 2.
Recent analyses of large CMML cohorts indicate that TET2 mutations without concurrent ASXL1 mutations (ASXL1WT/TET2mut) confer a favorable impact on overall survival (OS) (2, 3). Both familial CMML cases exhibited ASXL1WT/TET2mut status, while the control case was ASXL1mutTET2mut, suggesting a potentially poorer prognosis for the latter. This difference is also reflected in the initial response to HMA; the control case was intolerant to HMA and developed therapy-related thrombocytopenia. Morphologically, monocytes from the control case displayed a larger nucleus and a higher nuclear–cytoplasmic ratio (Figure 1B) compared to those of Case 1 (Figure 1A), suggesting higher proliferative activity.
Discussion
While certain chemotherapeutic agents are well-established causes of therapy-related leukemia, exposure to environmental agents like benzene and petroleum products has been linked to an increased risk of Acute Myelogenous Leukemia (AML) (4, 5). However, the etiology of CMML remains poorly defined. A case–control study specifically examining CMML did not find an association with benzene exposure (6). Associations between CMML and other chemical substances have not been previously reported.
In this familial cluster of two non-consanguineous individuals developing CMML within 2 years, long-term occupational exposure to raw lacquer emerged as a plausible major risk factor after excluding other potential causes (specific dietary habits, heavy metal poisoning, and viral infection). This hypothesis is supported by 1) a shared 40-year history of occupational raw lacquer exposure; 2) recurrent raw lacquer contact dermatitis in both individuals; 3) the absence of hematological abnormalities in their children, who lacked significant exposure; and 4) lack of evidence for genetic predisposition, viral triggers, or heavy metal poisoning.
Raw lacquer’s primary component is urushiol, a mixture of brenzcatechin derivatives with unsaturated side chains (Figure 2B). Urushiol is a well-known cause of contact dermatitis (7, 8), but its association with CMML, particularly in a familial aggregation pattern, has not been reported. Contact dermatitis involves the activation of monocytes or Langerhans-like cells and inflammation (9, 10). It is conceivable that chronic, long-term stimulation of the monocyte system by such agents could promote clonal monocyte proliferation and potentially leukemic evolution.
Reports of familial AML are rare and often syndromic (e.g., Down syndrome). Familial platelet disorder with predisposition to AML (FPD/AML), caused by germline RUNX1 mutations (RUNX1-FPD), has been described in approximately 11 families (11). Case 1 harbored a somatic RUNX1 mutation (c.928del, p.Met310Ter) distinct from the 62 mutations reported in FPD/AML (12). As her husband lacked germline or somatic RUNX1 mutations and had no history of thrombocytopenia, a diagnosis of RUNX1-FPD or RUNX1-related CMML was not supported. In Case 2, we identified a mutation in the N-RAS gene. It has been reported that approximately 35% of patients with CMML have point mutations in the K-RAS or N-RAS gene, which can result in leukemic transformation events (13). Patel BJ et al. demonstrated that the disease progression of CMML is associated with TET2 and RAS mutations (14). Whether the somatic RUNX1 mutation in Case 1 or the NRAS mutation in Case 2 resulted from DNA damage induced by chronic urushiol exposure warrants further investigation.
TET2 is the most frequently mutated gene in CMML (~61%) (1, 3, 15), and multiple TET2 mutations per patient are common at diagnosis (2, 15, 16). The clinical significance lies in the association of multiple TET2 mutations with older age and improved survival in the absence of ASXL1 mutations (3, 17). Multihit TET2 mutations (≥2 mutations) have been proposed as a molecular marker for differentiating oligomonocytic CMML (OM-CMML) from classical CMML (17). The current model suggests that initiating driver mutations often involve TET2 in hematopoietic stem cells (HSCs) (18), followed by secondary mutations (including additional TET2 or SRSF2 mutations) at the myeloid progenitor level, accelerating differentiation toward granulocyte–monocyte progenitors (GMPs) and clonal monocytosis (18). Furthermore, early clonal dominance of TET2 has been described as a unique finding in CMML (19). Loss-of-function TET2 mutations lead to DNA hypermethylation, altered gene expression, and aberrant monocytic differentiation (19, 20).
In our cases, both patients exhibited multi-hit TET2 mutations with identical mutation numbers but distinct sites. The VAF of these TET2 mutations (44%–48%, mean 45.88%) was significantly lower than that observed in the sporadic control case (84.1%). In the control case, although there was a single-hit TET2 mutation, the VAF was significantly increased. This increase of more than 55% could also be classified as multi-hit TET2 mutations (20), which meant that a biallelic alteration due to loss of heterozygosity also reflected the increase of clonal tumor load. Because of the concomitant ASXL1 mutation and high mutant TET2 VAF in the control case, he showed insensitivity to demethylating treatment. The clinical implications of these findings may include 1) advanced age at onset for familial CMML (75 and 77 years old); 2) the absence of ASXL1 mutation, suggesting a relatively favorable prognosis; and 3) multi-hit TET2 mutations combined with relatively low VAF values, potentially correlating with good sensitivity to HMA or hydroxyurea therapy observed in both patients. Whether chronic urushiol exposure directly drives TET2 mutations requires further experimental validation and additional case studies.
Conclusion
We report the first familial cluster of CMML and preliminarily identify long-term raw lacquer exposure as a potential pathogenic factor. Both affected individuals harbored multi-hit TET2 mutations and demonstrated a favorable response to HMA or hydroxyurea therapy.
Data availability statement
All date generated or analyzed during this study are included in this published article.
Ethics statement
The studies involving humans were approved by the Medical Ethics Committee of The Second XiangYa Hospital of Central South University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin. Written informed consent was obtained from the individual(s), and minor(s)’ legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Author contributions
YZ: Writing – original draft, Conceptualization, Investigation, Methodology. YL: Investigation, Writing – review & editing, Project administration, Methodology. HP: Methodology, Writing – review & editing, Investigation. YY: Data curation, Methodology, Conceptualization, Writing – review & editing. GZ: Investigation, Conceptualization, Funding acquisition, Methodology, Writing – original draft, Formal Analysis.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. The National Natural Science Foundation of China (No. 81470323 and No. 81500171).
Acknowledgments
We thank the patients and their family members for their participation in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Patnaik MM and Tefferi A. Chronic myelomonocytic leukemia: 2022 update on diagnosis, risk stratification, and management. Am J Hematol. (2022) 97:352–72. doi: 10.1002/ajh.26455
2. Coltro G, Mangaonkar AA, Lasho TL, Finke CM, Pophali P, Carr R, et al. Clinical, molecular, and prognostic correlates of number, type, and functional localization of TET2 mutations in chronic myelomonocytic leukemia(CMML)-a study of 1084 patients. Leukemia. (2020) 34:1407–21. doi: 10.1038/s41375-019-0690-7
3. Patnaik MM, Lasho TL, Vijayvargiya P, Finke CM, Hanson CA, Ketterling RP, et al. Prognostic interaction between ASXL1 and TET2 mutation in chronic myelomonocytic leukemia. Blood Cancer J. (2016) 6:e385. doi: 10.1038/bcj.2015.113
4. Brandt L, Nilsson P, and Mitelman F. Occupational exposure to petroleum products in men with acute nonlymphocytic leukemia. Br Med J. (1978) 4:553. doi: 10.1136/bmj.1.6112.553
5. Austin A, Delzell E, and Cole P. Benzene and leukemia: a review of the literature and a risk assessment. Am J Epidemiol. (1988) 127:419. doi: 10.1093/oxfordjournals.aje.a114820
6. Gross SA, Irons RD, Scott PK, Galbraith D, Wang XQ, Chen Y, et al. A case-control study of chronic myelomonocytic leukemia (CMML) in Shanghai, China: evaluation of risk factors for CMML, with special focus on benzene. Arch Environ Occup Health. (2012) 67:206–18. doi: 10.1080/19338244.2011.627892
7. Mizuta T, Kasami S, Shigehara Y, and Kato M. Urushiol-induced airborne and systemic pustular dermatitis from Japanese lacquer. Contact Dermat. (2022) 86:62–4. doi: 10.1111/cod.13976
8. Motz VA, Bowers CP, Kneubehl AR, Lendrum EC, Young LM, and Kinder DH. Efficacy of the saponin component of Impatiens capensis Meerb.in preventing urushiol-induced contact dermatitis. J Ethnopharmacol. (2015) 162:163–7. doi: 10.1016/j.jep.2014.12.024
9. Donglang G, Tongtong L, Dan C, Chan Z, Changming W, Guang Y, et al. Comparative study on different skin pruritus mouse models. Front Med (Lausanne). (2021) 8:630237. doi: 10.3389/fmed.2021.630237
10. Otsuka M, Egawa G, and Kabashima K. Uncovering the mysteries of langerhans cells, inflammatory dendritic epidermal cells, and monocyte-derived langerhans cell-like cells in the epidermis. Front Immunol. (2018) 9:1768. doi: 10.3389/fimmu.2018.01768
11. Song WJ, Sullivan MG, Legare RD, Hutchings S, Tan X, Kufrin D, et al. Haploinsufficiency of CBFA2 causes familial thrombocytopenia with propensity to develop acute myelogenous leukemia. Nat Genet. (1999) 23:166–75. doi: 10.1038/13793
12. Sood R, Kamikubo Y, and Liu P. Role of RUNX1 in hematological Malignancies. Blood. (2017) 129:2070–82. doi: 10.1182/blood-2016-10-687830
13. Such E, Germing U, Malcovati L, Cervera J, Kuendgen A, Della Porta MG, et al. Development and validation of a prognostic scoring system for patients with chronic myelomonocytic leukemia. Blood. (2013) 121:3005–15. doi: 10.1182/blood-2012-08-452938
14. Caraffini V, Geiger O, Rosenberger A, Hatzl S, Perfler B, Berg JL, et al. Loss of RAF kinase inhibitor protein is involved in myelomonocytic differentiation and aggravates RAS-driven myeloid leukemogenesis. Haematologica. (2020) 105:375–86. doi: 10.3324/haematol.2018.209650
15. Patel BJ, Przrychodzen B, Thota S, Radivoyevitch T, Visconte V, Kuzmanovic T, et al. Genomic determinants of chronic myelomonocytic leukemia. Leukemia. (2017) 31:2815–23. doi: 10.1038/leu.2017.164
16. Itzykson R, Kosmider O, Renneville A, Gelsi-Boyer V, Meggendorfer M, Morabito M, et al. Prognostic score including gene mutation in chronic myelonocytic leukemia. J Clin Oncol. (2013) 31:2428–36. doi: 10.1200/JCO.2012.47.3314
17. Garcia-Gisbert N, Arenillas L, Roman-Bravo D, Rodriguez-Sevilla JJ, Fernández-Rodríguez C, Garcia-Avila S, et al. Multi-hit TET2 mutations as a differential molecular signature of oligomonocytic and overt chronic myelomonocytic leukemia. Leukemia. (2022) 36:2922–6. doi: 10.1038/s41375-022-01733-8
18. Itzykson R, Kosmider O, Renneville A, Morabito M, Preudhomme C, Berthon C, et al. Clonal architecture of chronic myelomonocytic leukemias. Blood. (2013) 121:2186–98. doi: 10.1182/blood-2012-06-440347
19. Awada H, Nagata Y, Goyal A, Asad MF, Patel B, Hirsch CM, et al. Invariant phenotype and molecular association of biallelic TET2 mutant myeloid neoplasia. Blood Adv. (2019) 3:339–49. doi: 10.1182/bloodadvances.2018024216
Keywords: raw lacquer, chronic myelomonocytic leukemia, familial, TET2 mutation, Runx1, N-ras
Citation: Zhang Y, Luo Y, Peng H, Yin Y and Zhang G (2025) Raw lacquer-associated familial chronic myelomonocytic leukemia with multi-hit TET2 mutations. Front. Oncol. 15:1605369. doi: 10.3389/fonc.2025.1605369
Received: 03 April 2025; Accepted: 30 July 2025;
Published: 22 August 2025.
Edited by:
Noritaka Yamaguchi, Chiba University, JapanReviewed by:
Erica Bresciani, National Human Genome Research Institute (NIH), United StatesKiran Kumar Chitluri, Vellore Institute of Technology, India
Copyright © 2025 Zhang, Luo, Peng, Yin and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yafei Yin, c3VubGlnaHQyMjM1QDEyNi5jb20=; Guangsen Zhang, emdzbGx6eUAxNjMuY29t
 Yang Zhang1
Yang Zhang1 Yujiao Luo
Yujiao Luo Hongling Peng
Hongling Peng Guangsen Zhang
Guangsen Zhang