Abstract
Background:
FLT3-ITD mutations are among the most common genetic alterations in acute myeloid leukemia (AML) and are associated with poor clinical outcomes. However, data from low- and middle-income countries remain limited. This study aimed to investigate the prevalence, clinical characteristics, treatment patterns, and outcomes of adult AML patients with FLT3-ITD mutations in Thailand.
Methods:
We analyzed data from 360 adult patients with newly diagnosed AML, prospectively collected from 11 institutions nationwide between 2016 and 2023. FLT3-ITD mutational status, clinical features, response to therapy, and survival outcomes were compared between FLT3-ITD and FLT3-wild-type patients.
Results:
FLT3-ITD mutations were detected in 28.1% of patients. FLT3-ITD patients had higher white blood cell counts, bone marrow blast percentages, and NPM1 co-mutations compared to wild-type FLT3. Induction chemotherapy rates were similar, but FLT3 inhibitor use was nearly absent. Complete remission was achieved in 55.7% of FLT3-ITD patients versus 66.5% in wild-type FLT3. Median overall survival was significantly shorter in the FLT3-ITD group (8.8 vs. 13.2 months, p=0.039), while relapse-free survival was not significantly different. Multivariable analysis confirmed FLT3-ITD mutation as an independent predictor of poor overall survival.
Conclusions:
In this nationwide real-world study, FLT3-ITD AML was associated with inferior outcomes despite comparable induction therapy. Limited access to FLT3-targeted treatments and stem cell transplantation may contribute to these disparities. Our findings highlight the urgent need for expanding access to molecular testing and targeted therapies in resource-limited settings.
Introduction
Acute myeloid leukemia (AML) is a heterogeneous hematological malignancy characterized by rapid proliferation of myeloid progenitor cells in the bone marrow, leading to impaired hematopoiesis and subsequent clinical manifestations. The prognosis for AML patients is influenced by various factors, including age, cytogenetic abnormalities, and specific genetic mutations. Among these, the FLT3-ITD (Fms-like tyrosine kinase 3 internal tandem duplication) mutation has emerged as a critical prognostic factor. This mutation occurs in approximately 18-30% of AML cases and is associated with poor prognosis, characterized by higher rates of relapse and reduced overall survival compared to patients without this mutation (1–4).
FLT3 is a receptor tyrosine kinase that plays a significant role in hematopoiesis and cell survival. The presence of FLT3-ITD mutation leads to constitutive activation of the FLT3 signaling pathway, promoting cell proliferation and survival while inhibiting apoptosis. Consequently, FLT3-ITD mutations are linked to a more aggressive disease course, with patients often presenting with higher white blood cell counts and increased percentages of bone marrow blasts. Studies have consistently shown that FLT3-ITD mutations correlate with inferior outcomes in patients with AML, including lower complete remission rates and shorter overall survival (4, 5).
The significance of FLT3-ITD indicates prognosis and alters treatment approaches, making it a mutational test for classifying AML subtypes according to the World Health Organization’s definition of the disease in 2022 (WHO classification of hematolymphoid tumors, 5th edition: Myeloid and Histiocytic/Dendritic neoplasms) (6). A study in Thailand by the Thai Acute Leukemia Working Group (TALWG), which compiles patient data from multicenter medical institutions across Thailand, reported that information on adult AML patients, focusing only on risk group classification based on chromosomal abnormalities, showed 1-year and 2-year overall survival rates of 31.9% and 29.6%, respectively (7). There is yet to be a report on gene mutations used for classification and prognostic indications. Additionally, there has been no analysis of AML patient data regarding the prevalence of FLT3 mutations, clinical characteristics, other co-occurring chromosomal abnormalities, treatment regimens, or detailed survival rates.
FLT3 mutations can be detected at diagnosis and upon disease relapse. Two types of FLT3 mutations have been identified: internal tandem duplication (FLT3-ITD), found in 20-30% of cases, and mutations in the kinase domain (FLT3-TKD), found in 7-12% (2, 8–10). Targeted therapies for patients with FLT3 mutations, such as FLT3 tyrosine kinase inhibitor (TKI) drugs, have clear benefits in terms of increasing response rates and overall survival. Studies involving AML patients with FLT3 mutations at diagnosis, using standard high-dose chemotherapy in combination with FLT3 TKIs such as midostaurin (RATIFY study) (11), sorafenib (SORAML study) (12), and quizartinib (QuANTUM-First study) (13), have shown significantly improved outcomes in terms of relapse-free survival and overall survival compared to standard chemotherapy alone. In cases of relapsed or refractory (R/R) AML with FLT3 mutations, the use of TKI compared to high-dose chemotherapy alone, such as gilteritinib (ADMIRAL study) (14) and quizartinib (QuANTUM-R study) (15), has also shown significant improvements in overall survival. This demonstrates the critical role of targeted therapy in treatment. However, access to these therapies remains limited in many regions, including Thailand, where healthcare disparities may affect treatment availability and patient outcomes.
The present study aimed to assess the prevalence and clinical outcomes of FLT3-ITD mutations in adult AML patients in Thailand using data from the Thai Acute Leukemia Working Group (TALWG) registry. By analyzing real-world data, this study sought to provide insights into the clinical characteristics, treatment patterns, and survival outcomes of FLT3-ITD AML patients in a setting with limited access to targeted therapies. Furthermore, this study contributes to the growing body of evidence regarding the impact of FLT3-ITD mutations on clinical outcomes in AML, particularly in the context of real-world practice in Thailand. By elucidating the prevalence and implications of this mutation, our findings may serve as a foundation for future research and policy development aimed at improving the management of AML in the region.
Methods
Study participants and definitions
This study conducted a multicenter prospective observational analysis using data from the Thai Acute Leukemia Working Group (TALWG) registry, covering the period from January 1, 2016, to December 31, 2023. The study included adult patients aged ≥ 18 years with newly diagnosed AML and available FLT3-ITD status. The clinical characteristics, cytogenetics, mutational status, risk stratification based on the European Leukemia Net (ELN) 2022 AML risk classification (4), treatment regimens (according to guideline for diagnosis and management of adult acute leukemia on behalf of the Thai Society of Hematology) (16), response rates, percentage of allogeneic stem cell transplantation, and survival outcomes, including relapse-free survival (RFS) and overall survival (OS), were collected and analyzed. RFS was defined as the time from AML diagnosis to relapse and OS was defined as the time from diagnosis to death from any cause. Patients with acute promyelocytic leukemia (APL), mixed phenotypic acute leukemia (MPAL), or lacking clinical information including FLT3 mutational status and survival outcomes were excluded from the study. FLT3-ITD detection techniques vary across institutions, including polymerase chain reaction (PCR) based on capillary electrophoresis and next-generation sequencing (NGS) of myeloid panels. NGS was used to determine other genetic mutation testing for AML risk classification. High-intensity treatment consisted of standard induction chemotherapy using the 7 + 3 regimen, which included a seven-day continuous intravenous infusion of cytarabine at 100 mg/m² over 24 h, combined with a three-day bolus of idarubicin at 12 mg/m². Alternatively, a modified 5 + 2 regimen was used (17), consisting of a five-day continuous infusion of cytarabine at 100 mg/m² over 24 hours and a two-day bolus of idarubicin at 12 mg/m². This was followed by 3–4 cycles of consolidation chemotherapy, involving intermediate-dose cytarabine (IDAC) at 1.5 g/m² or high-dose cytarabine (HiDAC) at 3 g/m², administered intravenously every 12 hours for three days. Low-intensity treatment included hypomethylating agents, such as a seven-day course of subcutaneous azacitidine at 75 mg/m², hydroxyurea, or transfusion support, as needed. Palliative care in this study was the only transfusion support as needed. Some AML patients with relapsed or refractory disease may receive salvage treatment with MEC (a five-day regimen consisting of intravenous Mitoxantrone 8 mg/m², Etoposide 100 mg/m², and Cytarabine 1 g/m²) or FLAG-Idarubicin (Fludarabine 30 mg/m² and Cytarabine 2 g/m² intravenously for five days, along with Idarubicin 8 mg/m² intravenously for three days, and G-CSF).
Objectives
The primary endpoint of this study was the prevalence of the FLT3-ITD mutation in adult patients with AML in Thailand. The secondary endpoints included the clinical characteristics, treatment patterns, response rates, and survival outcomes of AML patients with and without the FLT3-ITD mutation in a real-world setting in Thailand.
Statistical analysis
Continuous variables are summarized using median values and range or interquartile range (IQR), while categorical variables are presented as percentages. The Chi-square test or Fisher’s exact test was used to compare categorical variables between FLT3-ITD AML and wild-type FLT3 AML patients, whereas the Mann-Whitney U test was used for continuous variables. Survival analysis was performed using the Kaplan-Meier method with the log-rank test to determine differences between groups. The Cox proportional hazard model was used to evaluate the hazard ratio (HR) with 95% confidence interval (95% CI) in the time-to-event analysis of the survival outcomes for univariable analyses (p-value <0.1 were included in the multivariable analyses. The sample size calculation was based on the primary outcome of the expected prevalence of the FLT3-ITD mutation in adult AML patients, which was estimated at 30% (1). A sample size of 360 patients was required to achieve a margin of error of 5% in estimating the prevalence with a 95% CI, accounting for a potential 10% loss. With this sample size, the anticipated 95% CI was 25-35% (18).
Results
Clinical characteristics
A total of 360 adult patients with newly diagnosed AML were included in this study. FLT3-ITD detection methods consisted of PCR in 140 cases (38.9%) and NGS in 220 cases (61.1%). Among these, 101 patients (28.1%) had FLT3-ITD mutations, whereas 259 patients (71.9%) had wild-type FLT3. The median age of patients with FLT3-ITD AML was 52 years (IQR 41-59) of compared to 51 years (IQR 36-61) for those with wild-type FLT3 AML (p=0.247). The proportion of male patients was similar between the FLT3-ITD (41.5%) and wild-type FLT3 (48.3%) groups (p=0.254). Regarding the Eastern Cooperative Oncology (ECOG) performance status, a higher proportion of patients with FLT3-ITD AML had an ECOG score of 3–4 compared than those with wild-type FLT3 AML (6.9% vs. 1.9%, p=0.018). Other baseline characteristics, such as the AML subtype, showed no significant differences between the two groups. Patients with FLT3-ITD AML had significantly higher median white blood cell counts (44.5 x 109/L, IQR 12.0-110.1) compared to those with wild-type FLT3 AML (23.5 x 109/L, IQR 7.1-62.1) (p=0.002). The median bone marrow blast percentage was also higher in the FLT3-ITD group (85%, IQR 68.7-90.0) compared than in the wild-type FLT3 group (80%, IQR 50.0-90.0) (p=0.011). Normal cytogenetics was more prevalent in the FLT3-ITD group (69.3%) than in the wild-type FLT3 group (52.1%) (p=0.003). There were significant differences in the distribution of certain mutations between the two groups. The FLT3-ITD group had a higher prevalence of NPM1 mutations (45.6% vs. 12.3%, p<0.001) and a lower incidence of biallelic CEBPA mutations (0 vs. 8.5%, p=0.043) (Table 1). There were two types of NPM1 mutations: type A (c.863_864insTCTG) was found in 71.7% and 65.6% of FLT3-ITD and wild-type FLT3 while type B (c.863_864insCATG) was found in 28.3% of FLT3-ITD and 34.4% of wild-type FLT3 AML.
Table 1
| Characteristics | Total (n = 360) | p-value | |
|---|---|---|---|
| FLT3-ITD AML (n = 101) | Wild-type FLT3 AML (n = 259) | ||
| Age, years | 52 (41-59) | 51 (36-61) | 0.247 |
| Age range, n (%) | |||
| <30 years | 12 (11.9) | 42 (16.2) | 0.301 |
| 30–60 years | 65 (64.3) | 151 (58.3) | 0.292 |
| >60 years | 24 (23.8) | 66 (25.5) | 0.735 |
| Male sex, n (%) | 42 (41.5) | 125 (48.3) | 0.254 |
| ECOG performance status, n (%) | |||
| 0-1 | 73 (72.3) | 206 (79.5) | 0.138 |
| 2 | 21 (20.8) | 48 (18.5) | 0.625 |
| 3-4 | 7 (6.9) | 5 (1.9) | 0.018 |
| Subtype of AML, n (%) | |||
| de novo AML | 86 (85.1) | 208 (80.3) | 0.286 |
| Secondary AML | 15 (14.9) | 51 (19.7) | 0.363 |
| AML-MR | 9 (8.9) | 32 (12.4) | 0.355 |
| t-AML | 1 (0.9) | 2 (0.7) | 0.838 |
| Previous diagnosed MDS or MPN | 5 (4.9) | 17 (6.6) | 0.566 |
| White blood cell count, x 109/L | 44.5 (12.0-110.1) | 23.5 (7.1-62.1) | 0.002 |
| Hemoglobin, g/dL | 7.4 (6.0-9.4) | 7.9 (6.7-9.4) | 0.074 |
| Platelets, x 109/L | 55 (34-94) | 50 (24-91) | 0.265 |
| Bone marrow blasts, % | 85 (68.7-90.0) | 80 (50-90) | 0.011 |
| Cytogenetics, n (%) | |||
| Normal | 70 (69.3) | 135 (52.1) | 0.003 |
| Other intermediate* | 26 (25.7) | 54 (20.9) | 0.316 |
| 11q23-rearranged | 1(0.9) | 7 (2.7) | 0.322 |
| -5/5q- | 0 | 2 (0.7) | 0.376 |
| -7/7q- | 1 (0.9) | 13 (5.0) | 0.076 |
| Monosomal karyotype | 0 | 13 (5.0) | 0.022 |
| Complex | 2 (1.9) | 30 (11.6) | 0.004 |
| t(8;21) | 1 (0.9) | 17 (6.6) | 0.029 |
| inv(16) | 0 | 4 (1.5) | 0.209 |
| Mutations, n (%) | |||
| NPM1 | 46 (45.6) | 32 (12.3) | <0.001 |
| biallelic CEBPA | 0/32 | 5/59 (8.5) | 0.043 |
| TP53 | 0/23 | 7/51 (13.7) | 0.127 |
| European LeukemiaNet 2022 risk, n (%) | |||
| Favorable | 9 (8.9) | 53 (20.5) | 0.009 |
| Intermediate | 87 (86.1) | 151 (58.3) | <0.001 |
| Adverse | 5 (4.9) | 55 (21.2) | <0.001 |
Clinical characteristics between FLT3-ITD AML and wild-type FLT3 AML.
Continuous variables are reported as median (interquartile range). AML-MR, Acute myeloid leukemia with myelodysplasia; t-AML, therapy-related acute myeloid leukemia; MDS, Myelodysplastic neoplasm; MPN, Myeloproliferative neoplasm.*Other intermediate: FLT3-ITD AML (n = 26): +8 (6 cases)/+13 (6 cases)/t(7;11)(p15;p15) (2 cases)/and one case each of: +1q, t(3;13), +8 + 22, +8 inv(9), -9q, +11, t(12;17), -13, -16, +21, +22, -22 and Wild-type FLT3 AML (n = 54): +8 (14 cases)/+21 (5 cases)/+4 (4 cases)/-Y (4 cases)/t(7;11) (3 cases)/and one case each of: -1q, t(1;12)(q21;q24.3) with t(16;21), -2, +3, t(3;5), +5, +5 + 8, +5 inv(9), -5-12, -6, -7, inv(8), +8-9q, -9q, -12-20, t(13;14), t(x;15)(p22.3;q15), +11, t(11;18), inv(21), +13, inv(17), -16-20, -X.
Treatment allocation and response rates
Treatment intensity was guided by physician discretion, largely based on age and performance status. Patients receiving high-intensity therapy were significantly younger (median age: 44 years; IQR 32–54) than those receiving low-intensity therapy (median age: 65 years; IQR 59–70) (p < 0.001). The proportion of patients with ECOG performance status 0–2 was higher in the high-intensity group (98.9%) compared to the low-intensity group (90.9%) (p = 0.001). Among FLT3-ITD patients, 69.3% received high-intensity induction chemotherapy, resulting in a complete remission (CR) rate of 55.7%. In the wild-type FLT3 group, 73.8% received high-intensity therapy, with a CR rate of 66.5%. These differences were not statistically significant (p = 0.397 and p = 0.109, respectively). Primary refractory disease occurred in 27.1% of FLT3-ITD patients and 16.7% of wild-type patients (p = 0.061). Among those receiving low-intensity regimens, non-response was common: 74.2% in FLT3-ITD and 60.3% in wild-type FLT3 (p = 0.180). In this study consisted of 45 patients who underwent allogeneic stem cell transplantation in the real-world data setting in Thailand. Of these, 26 patients (57.8%) received upfront allogeneic stem cell transplantation after first complete remission (CR1) while 19 patients (42.2%) underwent transplantation after achieving second complete remission (CR2) salvage therapy for relapse or refractory disease. Among those transplanted in CR1, there were 5 patients with FLT3-ITD AML and 21 with wild-type FLT3 AML. For those transplanted in CR2, 9 patients had FLT3-ITD AML and 10 had wild-type FLT3 AML.
Overall survival
Median follow-up was shorter in FLT3-ITD patients (8.1 months, IQR 3.4–14.8) than in wild-type FLT3 patients (11.4 months, IQR 4.6–21.5) (p = 0.006). Overall survival (OS) was significantly lower in FLT3-ITD patients (median 8.8 months) compared to wild-type FLT3 (13.2 months, log-rank test p < 0.001) (Table 2, Figure 1A). When stratified by treatment intensity, the median OS in the high-intensity treatment group was 10.8 months for FLT3-ITD and 15.5 months for wild-type FLT3 patients (p = 0.002). In the low-intensity group, the median OS was 4.1 months for FLT3-ITD and 5.9 months for wild-type FLT3 patients (p = 0.169). In both mutational groups, high-intensity therapy resulted in significantly longer OS than low-intensity treatment (p < 0.001 for each comparison) (Figure 1B). When analyzed by age, a significant OS difference was observed among patients aged 30–60 years, with median OS of 13.9 months in wild-type FLT3 compared to 8.1 months in FLT3-ITD (p = 0.001) (Figure 1C). Among patients who underwent allogeneic stem cell transplantation, OS was longer in the wild-type FLT3 group than in the FLT3-ITD group (21.5 vs. 14.7 months, p < 0.001). Transplantation was associated with significantly improved OS in both subgroups: in FLT3-ITD patients, median OS was 14.7 months with transplantation versus 7.8 months without (p < 0.001); in wild-type FLT3 patients, the corresponding OS was 21.5 vs. 12.0 months (p < 0.001) (Figure 1D).
Table 2
| Characteristics | Total (n = 360) | p-value | |
|---|---|---|---|
| FLT3-ITD AML (n = 101) | Wild-type FLT3 AML (n = 259) | ||
| High-intensity treatment | 70 (69.3) | 191 (73.8) | 0.397 |
| 7 + 3 | 64/70 (91.4) | 165/191 (86.4) | 0.388 |
| 5 + 2 | 6/70 (8.6) | 26/191 (13.6) | 0.422 |
| CR rate | 39/70 (55.7) | 127/191 (66.5) | 0.109 |
| Refractory | 19/70 (27.1) | 32/191 (16.7) | 0.061 |
| Low-intensity treatment | 31 (30.7) | 68 (26.3) | 0.431 |
| HMAs | 12/31 (38.7) | 25/68 (36.7) | 0.686 |
| Palliative care | 19/31 (61.3) | 43/68 (63.3) | 0.657 |
| CR rate | 3/31 (9.7) | 10/68 (14.7) | 0.492 |
| Refractory | 23/31 (74.2) | 41/68 (60.3) | 0.180 |
| Ara-C Consolidation | 44 (62.9) | 130 (68.1) | 0.429 |
| IDAC | 25/44 (56.8) | 86/130 (66.2) | 0.281 |
| HiDAC | 19/44 (43.2) | 44/130 (33.9) | 0.265 |
| Allogeneic stem cell transplantation | 14 (13.9) | 31 (11.9) | 0.626 |
| Relapse | 36 (35.6) | 81 (31.3) | 0.132 |
| Follow up time, months | 8.1 (3.4-14.8) | 11.4 (4.6-21.5) | 0.006 |
| Overall survival (OS), months | 8.8 (4.2-16.2) | 13.2 (6.5-33.2) | <0.001 |
| Treatment | |||
| High-intensity treatment | 10.8 (4.7-20.9) | 15.5 (8.8-39.7) | 0.002 |
| Low-intensity treatment | 4.1 (1-9.7) | 5.9 (2.1-13.4) | 0.169 |
| Age group | |||
| <30 years | 13.3 (9.5-16.2) | 19.9 (12.2-35.6) | 0.137 |
| 30–60 years | 8.1 (4.3-20.7) | 13.9 (7.9-36.0) | 0.001 |
| >60 years | 4.2 (0.7-10.3) | 6.7 (2.2-14.8) | 0.148 |
| Allogeneic stem cell transplantation | |||
| Yes | 14.7 (7-22.9) | 21.5 (19.1-80.7) | 0.019 |
| No | 7.8 (3.1-13.6) | 12 (4.6-28) | <0.001 |
| Relapse-free survival (RFS), months | 7.3 (5.2-11.7) | 7.8 (4.9-11.3) | 0.704 |
| Treatment | |||
| High-intensity treatment | 7.4 (5.3-11.7) | 7.6 (4.9-11.4) | 0.764 |
| Low-intensity treatment | 5.2 (4.7-6.2) | 7.8 (3.2-9.1) | 0.717 |
| Age group | |||
| <30 years | 7.5 (6.5-11.7) | 8 (5.8-14.9) | 0.823 |
| 30–60 years | 7.4 (5.3-11.8) | 7.2 (4.6-10.8) | 0.829 |
| >60 years | 5.2 (4.7-6.2) | 7.8 (7.2-11) | 0.033 |
| Allogeneic stem cell transplantation | |||
| Yes | 5.6 (4.7-6.4) | 10.3 (7.6-12) | 0.013 |
| No | 7.5 (5.3-11.7) | 7.4 (4.8-11) | 0.456 |
Treatment patterns and survival outcomes between patients with FLT3-ITD AML and wild-type FLT3 AML.
Continuous variables were reported as median (interquartile range). CR, complete remission; HMAs, Hypomethylating agents.
Figure 1
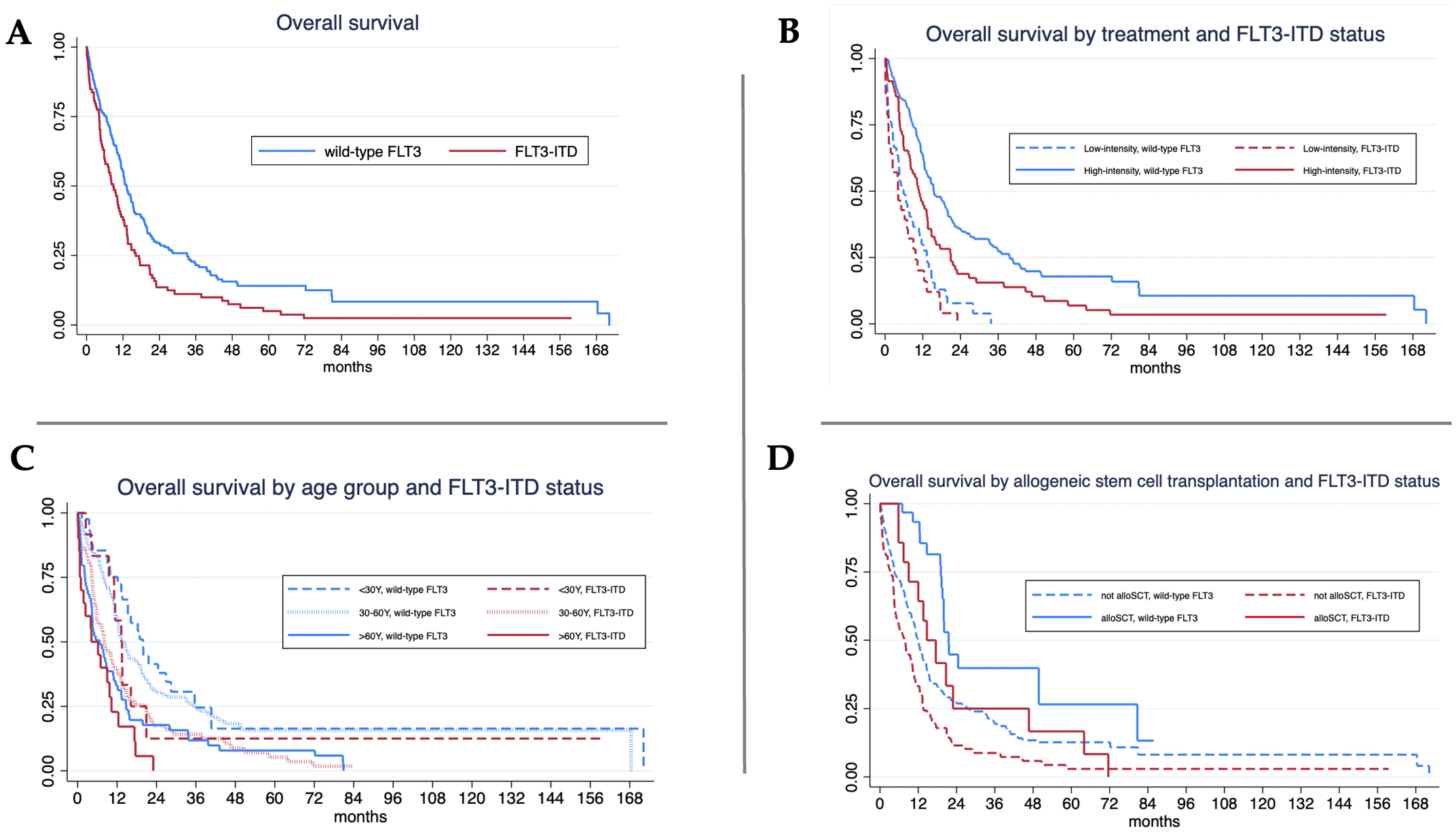
Overall survival (OS) analysis. (A)FLT3-ITD mutant showed significant lower in median OS 8.8 vs. 13.2 months (log-rank test, p < 0.001).; (B) For high-intensity treatment, FLT3-ITD mutant showed significant lower in median OS 10.8 vs. 15.5 months (log-rank test, p = 0.002) while the median OS of FLT3-ITD and wild-type were 4.1 vs. 5.9 months (log-rank test, p = 0.169) in patients with low-intensity treatment.; (C) For age < 30 years, median OS of FLT3-ITD and wild-type were 13.3 vs. 19.9 months (log-rank test, p = 0.137) while FLT3-ITD mutant showed significant lower in median OS 8.1 vs. 13.9 months (log-rank test, p = 0.001) in patients aged 30–60 years and 4.2 vs. 6.7 months (log-rank test, p = 0.148) in patients aged older than 60 years.; (D) For allogeneic stem cell transplantation (alloSCT), median OS of FLT3-ITD and wild-type were 14.7 vs. 21.5 months (log-rank test, p = 0.019). FLT3-ITD mutant also showed significant lower in median OS 7.8 vs. 12 months (log-rank test, p<0.001) in those without alloSCT (log-rank test, p = 0.148).
Relapse-free survival
Relapse occurred in 117 patients (32.5%): 35.6% in FLT3-ITD and 31.3% in wild-type FLT3 (p = 0.132). Median relapse-free survival (RFS) was comparable between the two groups: 7.3 months for FLT3-ITD and 7.8 months for wild-type FLT3 (log-rank test, p = 0.704) (Figure 2A). When stratified by treatment intensity, RFS was 7.4 months in FLT3-ITD and 7.6 months in wild-type FLT3 among patients receiving high-intensity therapy (p = 0.764), and 5.2 months versus 7.8 months, respectively, among those receiving low-intensity treatment (p = 0.717). No significant differences in RFS were observed within each intensity group (Figure 2B). In the subgroup of patients aged over 60 years, FLT3-ITD AML was associated with significantly shorter RFS compared to wild-type FLT3 (5.2 vs. 7.8 months, p = 0.033) (Figure 2C). Among patients who underwent allogeneic stem cell transplantation, wild-type FLT3 patients had a significantly longer median RFS than those with FLT3-ITD (10.3 vs. 5.6 months, p = 0.013). However, when assessed within each mutational subgroup, the impact of transplantation on RFS did not reach statistical significance (Figure 2D).
Figure 2
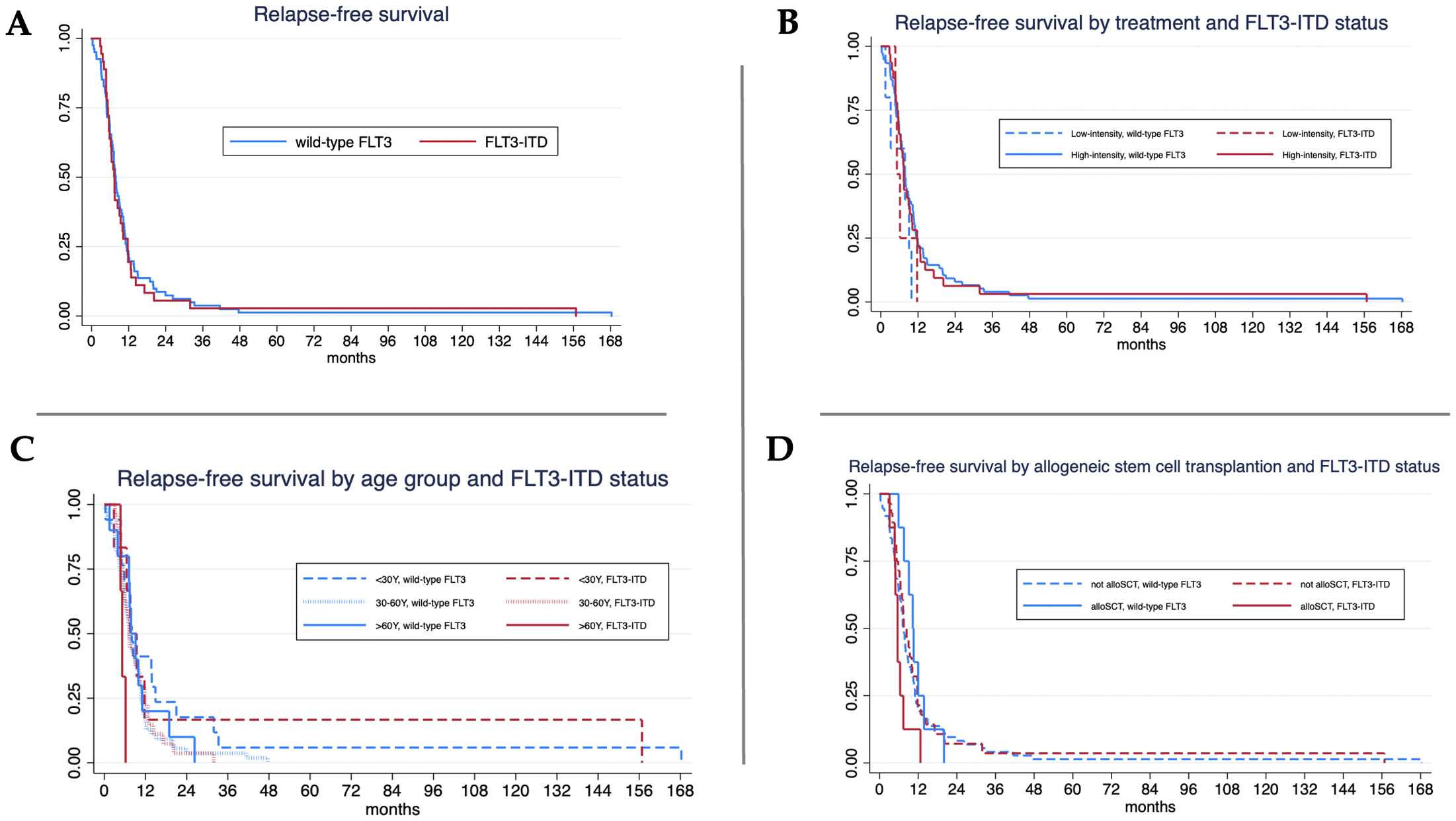
Relapse-free survival (RFS) analysis. (A) Median RFS in FLT3-ITD vs. wild-type FLT3 was 7.3 vs. 7.8 months (log-rank test, p = 0.704).; (B) For high-intensity treatment, FLT3-ITD mutant and wild-type FLT3 AML showed median OS 7.4 vs. 7.6 months (log-rank test, p = 0.764) and the median RFS of FLT3-ITD and wild-type were 5.2 vs. 7.8 months (log-rank test, p = 0.717) in patients with low-intensity treatment.; (C) For age < 30 years, median RFS of FLT3-ITD and wild-type were 7.5 vs. 8 months (log-rank test, p = 0.823) and 7.4 vs. 7.2 months (log-rank test, p = 0.829) in patients aged 30–60 years. FLT3-ITD mutant showed significant lower in median RFS 5.2 vs. 7.8 months (log-rank test, p = 0.033) in patients aged older than 60 years.; (D) For allogeneic stem cell transplantation (alloSCT), median RFS of wild-type FLT3 was longer than FLT3-ITD of 10.3 vs. 5.6 months (log-rank test, p = 0.013).
Subgroup and multivariable analyses
Subgroup analyses showed that FLT3-ITD conferred a significantly higher risk of death across all age groups (Figure 3A). ECOG 3–4 was associated with a worse prognosis (HR 2.71, 95% CI: 1.25–5.87), as was secondary AML (HR 1.59, 95% CI: 1.17–2.18) and 11q23 rearrangement (HR 1.97, 95% CI: 0.97–4.02). Favorable outcomes were observed in patients with co-mutated NPM1 (HR 0.69, 95% CI: 0.53–0.90), while TP53 mutations trended toward poorer OS (HR 1.97, 95% CI: 0.87–4.46). For RFS, complex cytogenetics (HR 2.77, 95% CI: 1.37–3.59) and adverse ELN risk (HR 2.01, 95% CI: 1.23–3.28) were associated with increased relapse risk (Figure 3B). Multivariable analysis was only performed for OS because the p-value for RFS did not meet the criteria (p-value <0.1) for all parameters. Multivariable analysis confirmed worse OS with FLT3-ITD (HR 1.51), ECOG 3–4 (HR 2.47), and age >60 years (HR 1.89), while better OS with age <30 (HR 0.45), age 30–60 (HR 0.56), and ELN favorable-risk classification (HR 0.66).
Figure 3
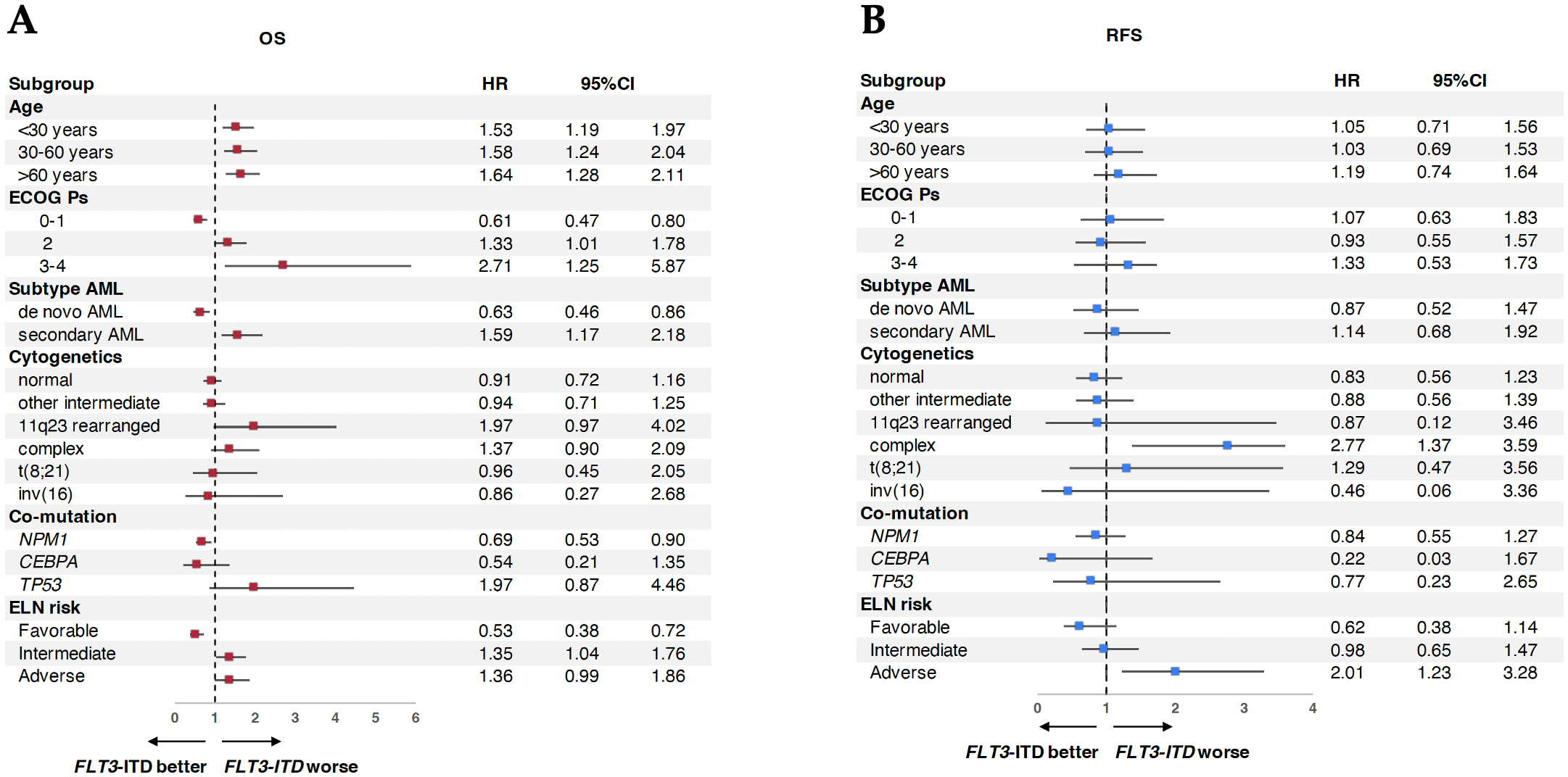
Subgroup analysis of AML patients between FLT3-ITD vs. wild-type FLT3 on survival. (A) Subgroup analysis of overall survival (OS). (B) Subgroup analysis of relapse-free survival (RFS).
Use of FLT3 inhibitor therapy and treatment in relapse/refractory AML
Two patients with newly diagnosed FLT3-ITD AML received midostaurin in combination with intensive chemotherapy as frontline therapy. Both were classified as intermediate-risk AML with normal cytogenetics and concurrent type A NPM1 mutations. The first patient, a 27-year-old woman, was treated with a 7 + 3 induction regimen plus midostaurin, followed by four cycles of high-dose cytarabine (HiDAC) combined with midostaurin. She remained in complete remission on midostaurin maintenance after 11.1 months of follow-up. The second patient, a 52-year-old man, also received 7 + 3 plus midostaurin and achieved CR. He proceeded to consolidation with a single cycle of HiDAC and underwent allogeneic transplantation from a matched unrelated donor. Midostaurin maintenance was not continued post-transplant due to financial constraints. At 13.6 months of follow-up, he remained in remission. These two cases represent the only instances in this cohort where FLT3-targeted therapy was incorporated into first-line treatment.
Among 232 patients (64.4%) with relapsed or refractory AML, 77.2% of FLT3-ITD and 59.5% of wild-type FLT3 patients had relapsed or failed initial therapy. FLT3-ITD mutations were redetected at relapse in all previously mutated patients and newly emerged in 58.2% of initially wild-type cases. Only 21.5% of relapsed/refractory patients received salvage chemotherapy. MEC was administered to 13 FLT3-ITD and 25 wild-type patients, yielding CR rates of 23.1% and 32.0%, respectively (p = 0.425). FLAG-Idarubicin achieved CR in 1/5 FLT3-ITD (20.0%) and 3/7 wild-type FLT3 (42.9%) (p = 0.092). Notably, FLT3 inhibitors were not administered in the relapsed/refractory setting.
Discussion
The prevalence of FLT3-ITD mutation in our study (28.1%) is consistent with previous reports from Western countries, which range from 18-30% (1–4). Our findings confirm that FLT3-ITD AML is associated with distinct clinical and molecular characteristics such as higher white blood cell counts, bone marrow blast percentages, and co-occurrence with NPM1 mutations. These features contribute to the aggressive nature of FLT3-ITD AML and underscore the need for more effective therapeutic strategies. Despite comparable use of induction chemotherapy and complete remission rates between FLT3-ITD and wild-type FLT3 AML patients, the significantly shorter overall survival in the FLT3-ITD group highlights the dismal prognosis associated with this mutation. This is particularly concerning in the context of limited access to FLT3 inhibitors and allogeneic stem cell transplantations in Thailand. Socioeconomic factors played a significant role in the decision to proceed with allogeneic stem cell transplantation in Thailand, particularly due to the limited number of transplant centers and challenges in healthcare reimbursement. Furthermore, a proportion of AML patients in our study received palliative care due to treatment limitations (18.8% of FLT3-ITD AML and 16.6% of wild-type FLT3 AML), which was associated with poor survival outcomes.
Subgroup analysis further elucidated the prognostic impact of FLT3-ITD, with age, performance status, AML subtype, cytogenetics, and co-mutations influencing survival outcomes. The interaction between FLT3-ITD and other molecular abnormalities such as NPM1 and TP53 mutations highlights the complex biology of AML. A favorable prognosis was associated with the co-mutation of NPM1 and FLT3-ITD. In contrast, the trend towards poorer outcomes in FLT3-ITD AML with TP53 mutations emphasizes the need for targeted therapies that can overcome the resistance conferred by these co-occurring mutations.
FLT3-ITD mutation is a well-established adverse prognostic factor in AML, which confers a higher risk of relapse and shorter overall survival. Several studies have consistently demonstrated a negative impact of FLT3-ITD on the clinical outcomes of patients with AML. FLT3-ITD mutations are associated with a significantly lower complete remission rate, shorter relapse-free survival, and overall survival in patients with AML treated with intensive chemotherapy (4). Several studies have reported that FLT3-ITD mutations are independent predictors of inferior overall survival in AML patients, regardless of age or cytogenetic risk group, even in the context of a normal karyotype (5, 19). The presence of FLT3-ITD mutations, particularly those with a high allelic ratio, is a strong adverse prognostic factor for overall survival in AML patients receiving allogeneic stem cell transplantation (20, 21).
The findings of this study are largely consistent with previous reports on the clinical characteristics and outcomes of FLT3-ITD AML patients. The prevalence of FLT3-ITD mutation in our cohort (28.1%) is within the range reported in Western countries, which typically vary between 25-35% (1). Similar to other studies, we found that FLT3-ITD AML patients were more likely to have normal cytogenetics and NPM1 mutations. Despite the comparable use of induction chemotherapy and complete remission rates between FLT3-ITD and wild-type FLT3 AML patients, the significantly shorter overall survival in the FLT3-ITD group is consistent with the dismal prognosis associated with this mutation reported in previous studies (4, 5). The comparable relapse-free survival between FLT3-ITD and wild-type FLT3 AML patients in our study differs from some previous reports that have shown a higher risk of relapse in FLT3-ITD AML (4, 5). However, our findings suggest that the survival disadvantage of FLT3-ITD AML may be driven by factors other than relapse, such as primary refractory disease or early mortality. Although allogeneic stem cell transplantation improved OS in FLT3-ITD AML patients, it did not significantly prolong RFS. This finding may be explained by the high relapse risk associated with FLT3-ITD disease, the absence of post-transplant FLT3 inhibitor maintenance, lack of measurable residual disease (MRD) monitoring, and the limited sample size which may have reduced the statistical power to detect the difference. This finding underscores the need for novel therapies to overcome the inherent resistance of FLT3-ITD AML to conventional chemotherapy. The interaction between FLT3-ITD and other molecular abnormalities such as NPM1 and TP53 mutations has been reported in several studies. The favorable prognosis associated with the co-mutation of NPM1 and FLT3-ITD in our study is consistent with previous findings that NPM1 mutation may mitigate the adverse impact of FLT3-ITD (5, 19). In contrast, the trend towards poorer outcomes in FLT3-ITD AML with TP53 mutations in our study emphasizes the need for targeted therapies that can overcome the resistance conferred by these co-occurring mutations (5, 22). The subgroup analysis in our study further elucidated the prognostic impact of FLT3-ITD, with age, performance status, AML subtype, cytogenetics, and co-mutations influencing the survival outcomes.
This study has several strengths and limitations that provide valuable insights into the real-world clinical practice of managing FLT3-ITD AML in Thailand. A key strength is the nationwide scope of the study, which included data from multiple institutions across the country, thereby enhancing the generalizability of the findings. The real-life information gathered reflects actual clinical practices and treatment outcomes in Thailand, highlighting the importance of mutational testing in guiding treatment decisions. These data can inform national policies regarding the implementation of precision medicine approaches, particularly in the context of FLT3-ITD mutations. However, several limitations should be acknowledged. The limited use of FLT3 inhibitors in newly diagnosed and relapsed/refractory FLT3-ITD AML may contribute to the poor survival outcomes observed. Furthermore, only approximately 10% of patients underwent allogeneic stem cell transplantation, which may also explain the inferior survival rates in this cohort. The absence of allelic ratio measurement and measurable residual disease (MRD) monitoring limits the depth of prognostic assessment. Variability in mutation testing methods across institutions may also have affected the consistency of molecular data.
Conclusions
In summary, this real-world nationwide registry confirms the poor prognosis of FLT3-ITD AML in settings with limited access to FLT3 inhibitors and allogeneic transplantation. The findings highlight the need for broader access to targeted therapies and standardization in diagnostic practices. Future studies should integrate MRD tracking and comprehensive genomic profiling to enhance treatment strategies and improve outcomes for AML patients in resource-constrained regions.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Research Ethics Committee Panel 5 of the Faculty of Medicine, Chiang Mai University, Thailand. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
TR: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Validation, Writing – original draft, Writing – review & editing. ER: Validation, Writing – review & editing. CW: Writing – review & editing. SK: Writing – review & editing. WO: Writing – review & editing. CC: Writing – review & editing. CP: Writing – review & editing. TP: Writing – review & editing. PN: Writing – review & editing. SS: Writing – review & editing. WL: Writing – review & editing. PSi: Writing – review & editing. KP: Writing – review & editing. CS: Writing – review & editing. JJ: Writing – review & editing. PSa: Writing – review & editing. AS: Writing – review & editing. DJ-U: Writing – review & editing. CN: Writing – review & editing. AT: Validation, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, and/or publication of this article.
Acknowledgments
The authors thank the Thai acute leukemia working group (TALWG) members on behalf of the Thai Society of Hematology (TSH), who contributed to patient care in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Daver N Schlenk RF Russell NH Levis MJ . Targeting FLT3 mutations in AML: review of current knowledge and evidence. Leukemia. (2019) 33:299–312. doi: 10.1038/s41375-018-0357-9
2
Niparuck P Limsuwanachot N Pukiat S Chantrathammachart P Rerkamnuaychoke B Magmuang S et al . Cytogenetics and FLT3-ITD mutation predict clinical outcomes in non transplant patients with acute myeloid leukemia. Exp Hematol Oncol. (2019) 8:3. doi: 10.1186/s40164-019-0127-z
3
Owattanapanich W Herzig J Jahn N Panina E Ruchutrakool T Kungwankiattichai S et al . Genetic alterations in Thai adult patients with acute myeloid leukemia and myelodysplastic syndrome-excess blasts detected by next-generation sequencing technique. Ann Hematol. (2021) 100:1983–93. doi: 10.1007/s00277-021-04513-z
4
Dohner H Wei AH Appelbaum FR Craddock C DiNardo CD Dombret H et al . Diagnosis and management of AML in adults: 2022 recommendations from an international expert panel on behalf of the ELN. Blood. (2022) 140:1345–77. doi: 10.1182/blood.2022016867
5
Patel JP Gonen M Figueroa ME Fernandez H Sun Z Racevskis J et al . Prognostic relevance of integrated genetic profiling in acute myeloid leukemia. N Engl J Med. (2012) 366:1079–89. doi: 10.1056/NEJMoa1112304
6
Khoury JD Solary E Abla O Akkari Y Alaggio R Apperley JF et al . The 5th edition of the world health organization classification of haematolymphoid tumours: myeloid and histiocytic/dendritic neoplasms. Leukemia. (2022) 36:1703–19. doi: 10.1038/s41375-022-01613-1
7
Wanitpongpun C Utchariyaprasit E Owattanapanich W Tantiworawit A Rattarittamrong E Niparuck P et al . Types, clinical features, and survival outcomes of patients with acute myeloid leukemia in Thailand: A 3-year prospective multicenter study from the thai acute leukemia study group (TALSG). Clin Lymphoma Myeloma Leuk. (2021) 21:e635–e43. doi: 10.1016/j.clml.2021.03.004
8
Frohling S Schlenk RF Breitruck J Benner A Kreitmeier S Tobis K et al . Prognostic significance of activating FLT3 mutations in younger adults (16 to 60 years) with acute myeloid leukemia and normal cytogenetics: a study of the AML Study Group Ulm. Blood. (2002) 100:4372–80. doi: 10.1182/blood-2002-05-1440
9
Thiede C Steudel C Mohr B Schaich M Schakel U Platzbecker U et al . Analysis of FLT3-activating mutations in 979 patients with acute myelogenous leukemia: association with FAB subtypes and identification of subgroups with poor prognosis. Blood. (2002) 99:4326–35. doi: 10.1182/blood.V99.12.4326
10
Whitman SP Maharry K Radmacher MD Becker H Mrozek K Margeson D et al . FLT3 internal tandem duplication associates with adverse outcome and gene- and microRNA-expression signatures in patients 60 years of age or older with primary cytogenetically normal acute myeloid leukemia: a Cancer and Leukemia Group B study. Blood. (2010) 116:3622–6. doi: 10.1182/blood-2010-05-283648
11
Stone RM Mandrekar SJ Sanford BL Laumann K Geyer S Bloomfield CD et al . Midostaurin plus chemotherapy for acute myeloid leukemia with a FLT3 mutation. N Engl J Med. (2017) 377:454–64. doi: 10.1056/NEJMoa1614359
12
Rollig C Serve H Huttmann A Noppeney R Muller-Tidow C Krug U et al . Addition of sorafenib versus placebo to standard therapy in patients aged 60 years or younger with newly diagnosed acute myeloid leukaemia (SORAML): a multicentre, phase 2, randomised controlled trial. Lancet Oncol. (2015) 16:1691–9. doi: 10.1016/S1470-2045(15)00362-9
13
Erba HP Montesinos P Kim HJ Patkowska E Vrhovac R Zak P et al . Quizartinib plus chemotherapy in newly diagnosed patients with FLT3-internal-tandem-duplication-positive acute myeloid leukaemia (QuANTUM-First): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. (2023) 401:1571–83. doi: 10.1016/S0140-6736(23)00464-6
14
Perl AE Martinelli G Cortes JE Neubauer A Berman E Paolini S et al . Gilteritinib or chemotherapy for relapsed or refractory FLT3-mutated AML. N Engl J Med. (2019) 381:1728–40. doi: 10.1056/NEJMoa1902688
15
Cortes JE Khaled S Martinelli G Perl AE Ganguly S Russell N et al . Quizartinib versus salvage chemotherapy in relapsed or refractory FLT3-ITD acute myeloid leukaemia (QuANTUM-R): a multicentre, randomised, controlled, open-label, phase 3 trial. Lancet Oncol. (2019) 20:984–97. doi: 10.1016/S1470-2045(19)30150-0
16
Thai Acute Leukemia Working Group (TALWG) on behalf of the Thai Society of Hematology . Guidelines for diagnosis and management of adult acute leukemia (2023). Available online at: https://www.tsh.or.th/file_upload/files/eBook%20Guideline%20Acute%20Leukemia%20in%20Adults%202023.pdf (Accessed July 30, 2025).
17
Wiernik PH Banks PL Case DC Jr. Arlin ZA Periman PO Todd MB et al . Cytarabine plus idarubicin or daunorubicin as induction and consolidation therapy for previously untreated adult patients with acute myeloid leukemia. Blood. (1992) 79:313–9. doi: 10.1182/blood.V79.2.313.313
18
Naing L Nordin RB Abdul Rahman H Naing YT . Sample size calculation for prevalence studies using Scalex and ScalaR calculators. BMC Med Res Methodol. (2022) 22:209. doi: 10.1186/s12874-022-01694-7
19
Gale RE Green C Allen C Mead AJ Burnett AK Hills RK et al . The impact of FLT3 internal tandem duplication mutant level, number, size, and interaction with NPM1 mutations in a large cohort of young adult patients with acute myeloid leukemia. Blood. (2008) 111:2776–84. doi: 10.1182/blood-2007-08-109090
20
Schlenk RF Kayser S Bullinger L Kobbe G Casper J Ringhoffer M et al . Differential impact of allelic ratio and insertion site in FLT3-ITD-positive AML with respect to allogeneic transplantation. Blood. (2014) 124:3441–9. doi: 10.1182/blood-2014-05-578070
21
Bornhauser M Illmer T Schaich M Soucek S Ehninger G Thiede C et al . Improved outcome after stem-cell transplantation in FLT3/ITD-positive AML. Blood. (2007) 109:2264–5. doi: 10.1182/blood-2006-09-047225
22
Papaemmanuil E Gerstung M Bullinger L Gaidzik VI Paschka P Roberts ND et al . Genomic classification and prognosis in acute myeloid leukemia. N Engl J Med. (2016) 374:2209–21. doi: 10.1056/NEJMoa1516192
Summary
Keywords
FLT3-ITD mutation, acute myeloid leukemia, real-world data, Thailand, treatment outcomes
Citation
Rattanathammethee T, Rattarittamrong E, Wanitpongpun C, Kungwankiattichai S, Owattanapanich W, Chanswangphuwana C, Polprasert C, Piyajaroenkij T, Niparuck P, Saengboon S, Limvorapitak W, Silpsamrit P, Prayongratana K, Sriswasdi C, Julamanee J, Saelue P, Sasakul A, Jit-ueakul D, Nakhakes C and Tantiworawit A (2025) Real-world data on adult AML with FLT3-ITD mutation from the Thai acute leukemia working group. Front. Oncol. 15:1606943. doi: 10.3389/fonc.2025.1606943
Received
06 April 2025
Accepted
15 August 2025
Published
29 August 2025
Volume
15 - 2025
Edited by
Alexandra Smith, University of York, United Kingdom
Reviewed by
Kazuhito Suzuki, Jikei University Hospital, Japan
Muhammad Rafie Raza, Indus Hospital, Pakistan
Updates
Copyright
© 2025 Rattanathammethee, Rattarittamrong, Wanitpongpun, Kungwankiattichai, Owattanapanich, Chanswangphuwana, Polprasert, Piyajaroenkij, Niparuck, Saengboon, Limvorapitak, Silpsamrit, Prayongratana, Sriswasdi, Julamanee, Saelue, Sasakul, Jit-ueakul, Nakhakes and Tantiworawit.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Thanawat Rattanathammethee, thanawat.r@cmu.ac.th
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.