- 1Department of Clinical Research Design and Evaluation, Samsung Advanced Institute for Health Sciences & Technology, Sungkyunkwan University, Seoul, Republic of Korea
- 2Center for Clinical Epidemiology, Samsung Medical Center, Seoul, Republic of Korea
- 3Patient-Centered Outcomes Research Institute, Samsung Medical Center, Seoul, Republic of Korea
- 4Departments of Health, Behavior, and Society and Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, United States
- 5Department of Thoracic and Cardiovascular Surgery, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Republic of Korea
Although esophageal cancer survivors experience lower health-related quality of life (HRQoL), it is hard to provide proper supportive care due to difficulties to find potential target population. This study aims to develop a trial-ready cohort (TRC) to assess the unmet needs and HRQoL of survivors of esophageal cancer. This prospective, multicenter TRC study will include 600 patients diagnosed with primary esophageal cancer who have undergone curative treatment. Exclusion criteria include the presence of synchronous malignancies, severe cognitive impairment, and residence outside of Korea. Patients are recruited from both existing cohort studies and newly diagnosed cases beginning in June 2023. At the time of enrollment into the TRC, participants provide informed consent, including agreement to be contacted and considered for relevant clinical trials when suitable interventions become available. Data is collected across five domains: sociodemographic characteristics, health behaviors, disease and treatment information, nutritional status, and quality of life. Study visits are scheduled at diagnosis, prior to surgery, at 1, 3, 6, and 12 months post-surgery, and annually thereafter for up to 10 years. As of June 2025, a total of 448 participants have been enrolled, representing approximately 75% of the target. To achieve this, our TRC employs three key strategies. First, we leverage three existing prospective studies to efficiently identify and enroll long-term survivors. Second, we collect a wide range of data on HRQoL, health behaviors, and environmental factors to enable a multidimensional understanding of survivorship. Third, we collect multiple times within short-term interval points, allowing seamless linkage between the cohort and appropriate trials. This study effectively designs a trial-ready cohort of survivors of esophageal cancer using a unique strategy to overcome cohort construction challenges, aiming to generate valuable data on quality of life and serve as a platform for tailored interventions.
1 Introduction
Esophageal cancer is the eleventh most common cancer worldwide and seventh in terms of mortality (1). Over the past 20 years, the five-year survival rate of esophageal cancer increased from 21.7% (2001–2005) to 43.2% (2018–2022). Consequently, the number of esophageal cancer survivors has steadily increased (2). However, most previous studies have followed esophageal cancer survivors for only 1–2 years postoperatively (3–5) or were cross-sectional in design, limiting insights into the long-term impact of treatment (6–8). Only a few cohort studies have extended follow-up beyond five years, but they included fewer than 200 patients, with high rates of loss to follow-up (9, 10). Although some previous studies have tracked esophageal cancer survivors’ QoL for over 5 years postoperatively, they exhibit limitations. A study in China reported follow-up data up to 9 years after surgery for over 300 patients, however, it cannot be regarded as a true longitudinal assessment, as more than 60% of participants completed the QoL assessment only once (11). Similarly, another Chinese study tracked 232 postoperative patients for a median of 80 months, yet QoL data were collected only during the first two years, with no assessments beyond 24 months (12). The OSCAR study from Sweden followed over 400 patients and achieved repeated fatigue assessments up to 5 years with relatively high retention. However, its scope was limited to cancer-related fatigue, lacking a broader multidimensional evaluation of QoL (13).
Based on the previous studies, it is well established that esophageal cancer survivors frequently experience substantially impaired health-related quality of life (HRQoL), primarily due to slow postoperative recovery and persistent symptoms (14). More than 50% of patients who had undergone esophagectomy reported dysphagia, 60% experienced dumping syndrome, and 33% suffered from severe weight loss (>10%) (15). While cancer survivorship guidelines recommend comprehensive follow-up care (16), current practices remain largely limited to medical surveillance and tumor-specific monitoring. In particular, esophageal cancer survivors often face socioeconomic disadvantages and have limited access to recruitment platforms (17), making it difficult to identify eligible individuals for supportive care interventions and to ensure timely delivery of appropriate care (18).
Given these barriers to long-term follow-up and interventional research, trial-ready cohorts (TRCs) represent a promising strategy. TRCs are prospective, well-characterized cohorts that enable the continuous identification of high-risk subgroups and allow for the rapid initiation of targeted intervention trials (19). Although TRCs have been successfully adopted in various fields, including cardiology and rare cancers, their application to cancer survivorship remains limited. Therefore, the objective of this study is to describe the protocol for the development and implementation of a prospective, platform-based, trial-ready cohort.
2 Methods
2.1 Study design and study population
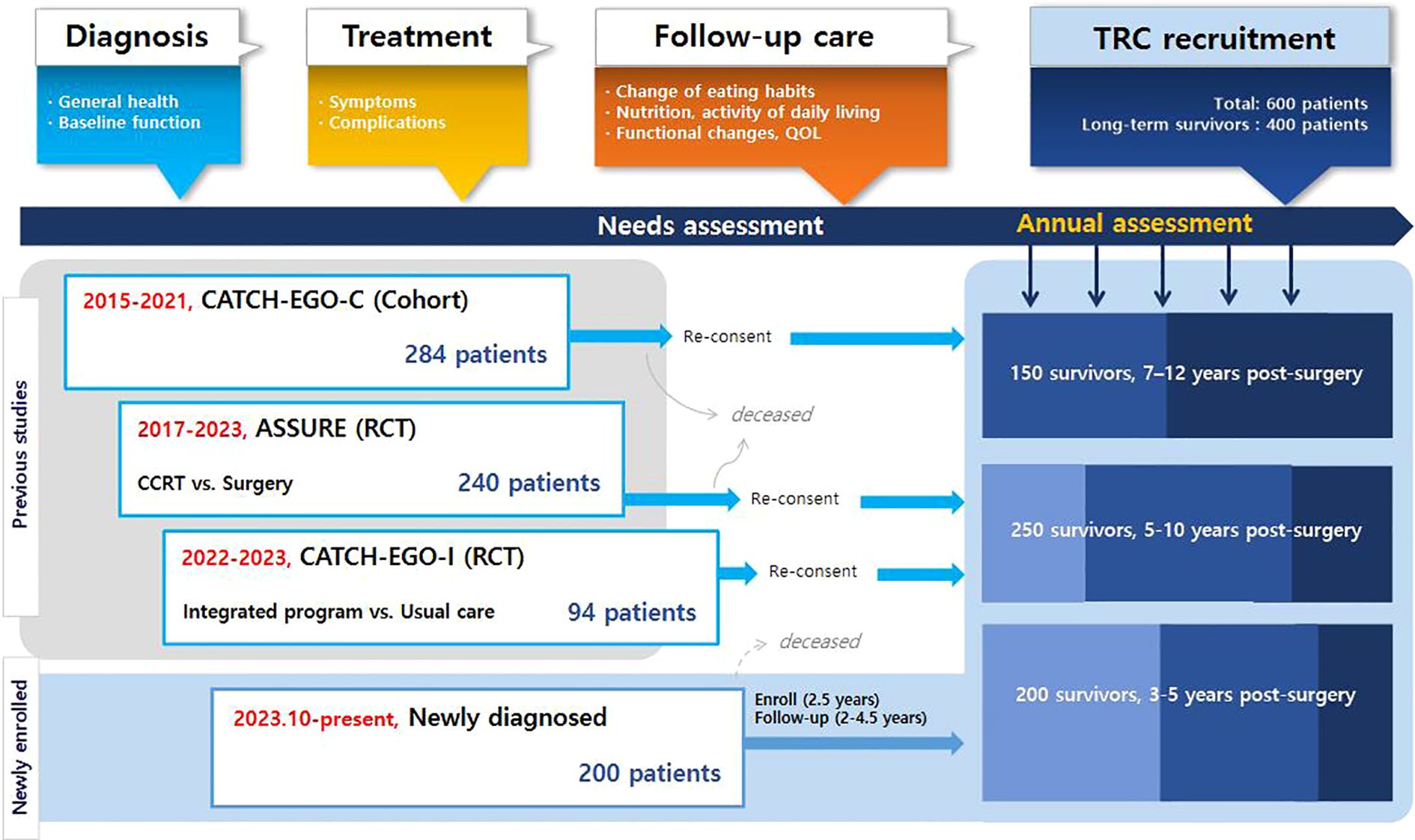
This is a prospective multicenter TRC study involving patients with esophageal cancer (Figure 1). The eligibility criteria are as follows: 1) patients diagnosed with primary esophageal cancer, including squamous cell carcinoma or adenocarcinoma, confirmed through endoscopic evaluation, imaging studies such as CT or PET-CT, and histopathological examination of biopsy specimens by board-certified pathologists, 2) patients who had or will have treatment for curative purposes, 3) patients who understand and write Korean at a native level, and 4) patients who provided informed consent. Patients are excluded if 1) they have a previous history at the time of esophageal cancer diagnosis including synchronous cancers, other cancers within 3 years, or esophagectomy, 2) they have severe cognitive impairment, including dementia and schizophrenia or 3) reside overseas that hinders regular participation. At the time of enrollment into the TRC, participants provide informed consent, including agreement to be contacted and considered for relevant clinical trials when suitable interventions become available.
Given the low incidence and relatively high mortality rates, we recruit patients from three existing cohort studies of esophageal cancer as well as from newly diagnosed cases. For newly diagnosed patients, no restrictions are applied regarding age, comorbidities, or treatment modality in order to capture a broader range of survivorship needs.
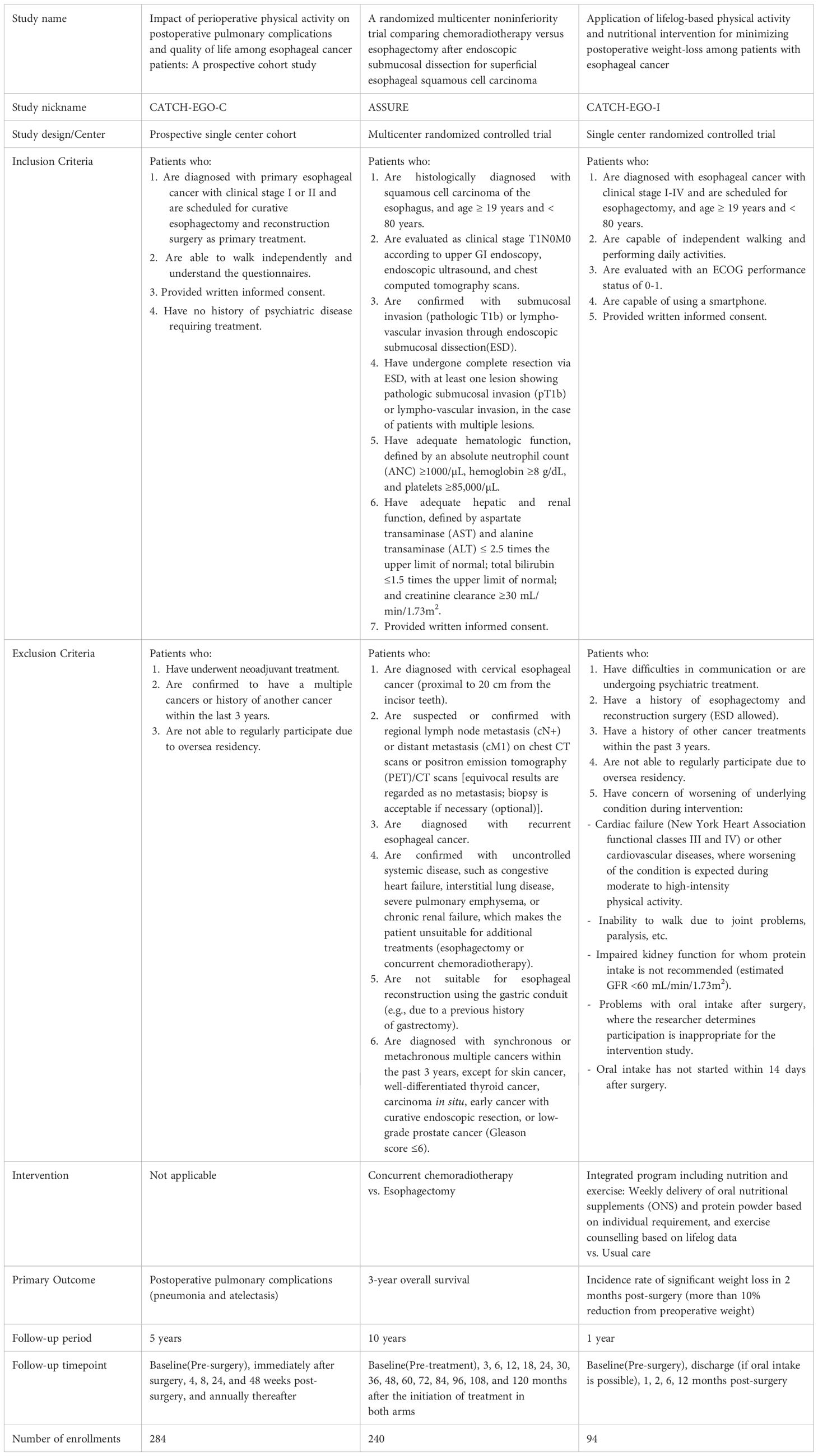
Starting in June 2023, patients participating in the existing cohorts are recruited during their surveillance visits to treatment hospitals, typically during annual visits to outpatient clinics Detailed information on existing cohorts are followings: The CATCH-EGO-C study (ClinicalTrials.gov ID: NCT03231462) is a prospective cohort study investigating how perioperative physical activity affects postoperative pulmonary complications and quality of life (QoL) in patients with esophageal cancer. The study recruited 284 patients with stage I-II esophageal cancer who underwent curative surgery at Samsung Medical Center since Mar 2015. The ASSURE study (ClinicalTrials.gov ID: NCT03306901) is a multicenter randomized controlled non-inferiority trial that compared the survival rates of chemoradiotherapy and esophagectomy after endoscopic submucosal dissection (ESD) in patients with superficial esophageal squamous cell carcinoma (clinical stage T1N0M0). The trial enrolled 240 patients (121 and 119 in the surgery and concomitant chemoradiotherapy groups, respectively) in stage I starting in Oct 2017, and is following up on clinical outcomes from seven hospitals across South Korea (Samsung Medical Center, Asan Medical Center, Seoul National University Hospital, Seoul National University Bundang Hospital, National Cancer Center, Gangnam Severance Hospital, and Pusan National University Hospital). Another study, CATCH-EGO-I (CRIS Registration Number: KCT0006446), is a randomized controlled trial that is comparing severe weight loss between patients with stage I-IV esophageal cancer receiving weekly integrated program for 2 months (n=48) and usual care (n=46) after surgery. The program consisted of delivery of oral nutritional supplements (ONS) and protein powder, and exercise counselling based on individual requirement and lifelog data, following up to 12 months (Table 1) At enrollment, the three cohorts differed in prior follow-up duration (CATCH-EGO-C: 8.3 years, ASSURE: 5.7 years, and CATCH-EGO-I: 1.2 years), resulting in participants being enrolled at different stages of follow-up depending on trial timelines. These variations were addressed during data harmonization by aligning common variables and timepoints.
Newly diagnosed patients are those with primary esophageal cancer planned for curative resection and reconstruction at clinical stages I-III, including those who receive neoadjuvant treatment. All patients are enrolled before the start of any treatment and recruited from the thoracic surgery outpatient clinics at the Samsung Medical Center, Asan Medical Center, Seoul National University Hospital, and Seoul National University Bundang Hospital. The physician explains the study to eligible patients and obtain their informed consent. To better understand the diverse unmet needs of patients, we have decided to expand the inclusion criteria to encompass patients with advanced stages of the disease, thereby capturing a broader range of experiences and challenges. Patients can be excluded from the study after enrollment under the following conditions: 1) enrolled in the study with the intention of receiving curative treatment (surgery or anticancer radiation therapy) for esophageal cancer but did not undergo treatment, or 2) primary treatment strategy shifted from curative to non-curative due to disease progression or other reasons. This study has been approved by the institutional review boards of all the participating hospitals.
2.2 Variables and measurement
Although the three source cohorts differ in study design, comprising observational and interventional studies, the data collection process has been standardized across all cohorts. This includes harmonization of variable definitions, measurement timepoints, and patient-reported outcome measures (PROMs), allowing for consistent and comparable data across participants regardless of cohort origin.
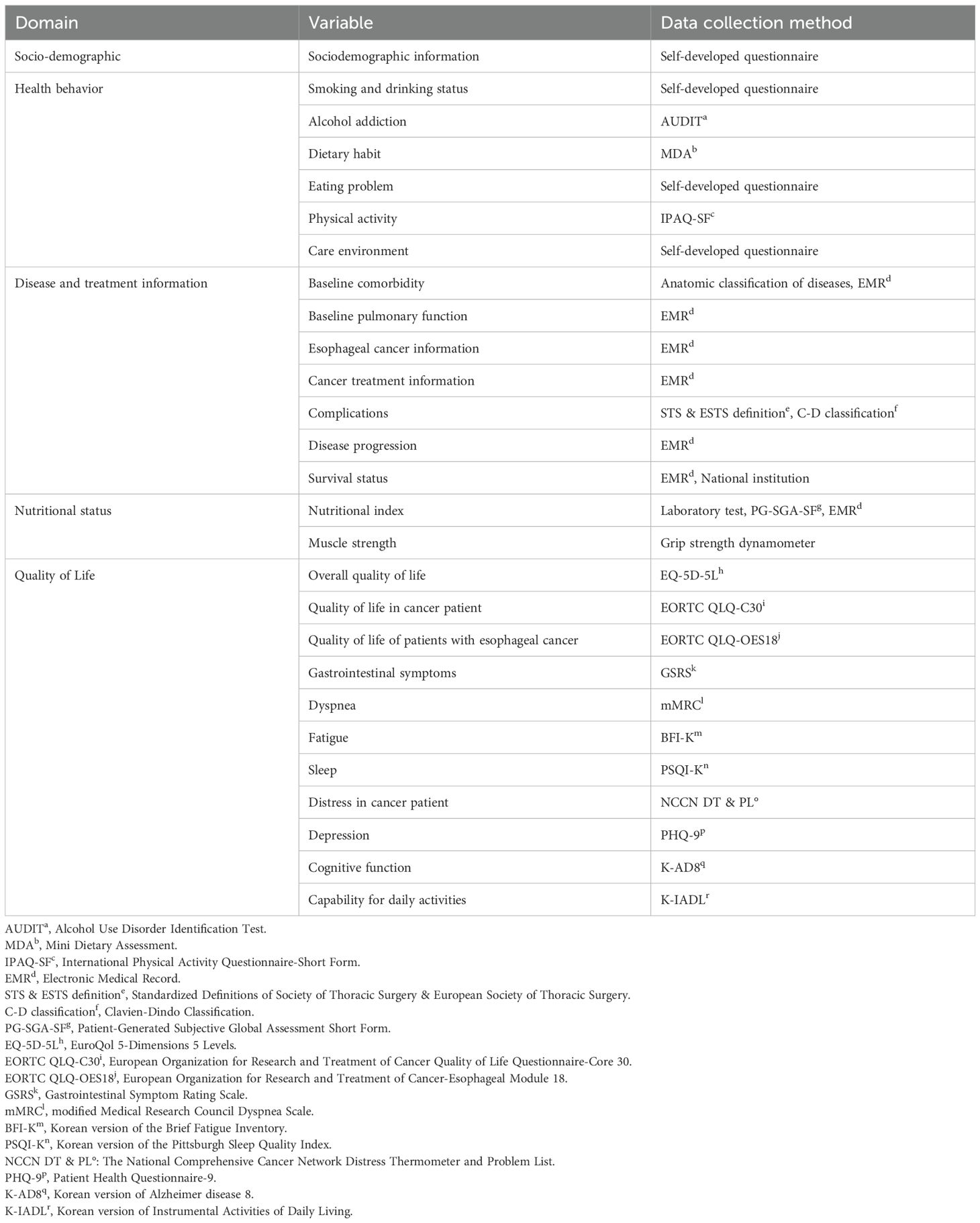
Information is collected on five domains: sociodemographics, health behavior, disease and treatment information, nutritional status, and QoL. Data are collected during regular clinic visits of the patients, as the visit schedules aligned as much as possible with the standard esophageal cancer treatment process. Visits are scheduled at the time of enrollment (baseline, T0); pre-surgery (T1); 1 (T2), 3 (T3), 6 (T4), and 12 months post-surgery (T5); and annually thereafter (T6–T19). T0 visit is planned at the time of diagnosis (pretreatment). The T1 visit applies only to patients who undergo neoadjuvant (radiation) therapy before surgery. The visit window for each timepoint varies: the T0 visit can be scheduled at any time between enrollment and treatment initiation, the T1 visit can be scheduled from 2 weeks after the completion of neoadjuvant treatment until before surgery, and the visit windows for T2, T3, T4, and T5 to T19 are ±14 days, -1 month, ± 1 month, and ±2 months, respectively.
The sociodemographic domain includes participants’ sex, date of birth, education level, marital status, occupation, and family income. Sociodemographic information is gathered using self-report questionnaires. Marital status, occupation, and family income which are expected to change over time, are collected at T0 and consecutively starting from T4, whereas all other data are collected only at T0.
The health behavior domain includes smoking and drinking status, alcohol addiction, eating habits, diet, physical activity, and care environment which provides information on the current state of the patient’s caregiver. This domain measures patients’ health-related behaviors and environmental factors that affect their behavioral choices. Data are collected at every study visit from T0, except for smoking and drinking status, alcohol addiction, physical activity, and care environment which are not evaluated at immediate post-surgery such as T2 and T3. Most of the variables use patient-reported outcome measurements (PROMs) for data collection (Table 2) except smoking and drinking status, eating problem, and care environment, which are assessed using self-developed questionnaires.
To investigate the relationship between disease characteristics and outcomes, the disease and treatment information domain comprises baseline comorbidities, baseline pulmonary function, esophageal cancer information, cancer treatment information, complications, disease progression, and survival status. Every information within this domain is collected by reviewing electronic medical records (EMR), with some comorbidity data gathered using self-developed questionnaires according to anatomic classification. Information on newly diagnosed disease following treatment is also collected during the patient’s study visits.
The nutritional status domain includes muscle strength and nutritional indices, such as body weight, body mass index (BMI), laboratory results, and overall nutritional evaluation. Muscle strength is measured by averaging the grip strength dynamometer values from both hands. Laboratory results consist of albumin, total protein, hemoglobin, white blood cell, absolute lymphocyte count, absolute neutrophil count, and platelet. Data on each variable is collected at every visit from T0 to the end of the study period. Body weight, BMI, and laboratory results are obtained through EMR review, whereas the nutritional assessment utilizes Patient-Generated Subjective Global Assessment Short Form.
The QoL domain includes overall QoL, QoL in patients with cancer, QoL in patients with esophageal cancer, gastrointestinal symptoms, dyspnea, fatigue, sleep, distress in cancer patient, depression, cognitive function, and capability for daily activities. These variables are used to assess the burden of symptoms and QoL of the patients from various perspectives. QoL is collected at baseline; T0, pre-treatment (T1, if applicable), 6 months post-treatment (T4), and annually from 12 months post-treatment (T5), using PROMs (Table 2). Cognitive function is assessed biannually from T5.
2.3 Statistical analysis
Based on the assumption that approximately 20% of esophageal cancer survivors experience significant issues related to impaired HRQoL, we estimated that at least 80 patients with substantial unmet needs would be required to develop predictive models and define intervention targets. Considering potential attrition and the need for subgroup analyses, we determined that a total sample size of 600 participants would be appropriate.
We will classify patients into a “risk group” (those experiencing a clinically significant decline in QoL by year 3 post-surgery) and a “non-risk group,” and compare baseline characteristics using t-tests and chi-square tests, as appropriate. Mixed-effects models will then be used to examine longitudinal differences in QoL trajectories between the two groups. To examine longitudinal differences in QoL trajectories between the two groups, we will employ linear mixed-effects models to account for repeated measurements within individuals. In these models, time will be treated as a fixed effect, and participant identifier will be included as a random intercept to model intra-individual correlation. To account for potential heterogeneity across cohorts, such as differences in recruitment period or baseline characteristics, the cohort source will also be modeled as a random effect. This hierarchical structure will allow us to account for clustering without inflating the variance of fixed-effect estimates. Baseline covariates and interaction terms will be included to explore effect modification over time. Variable selection will be informed by existing literature and preliminary univariable analyses, with the final model determined using likelihood ratio tests (significance level set at p < 0.05). Multivariable logistic regression will be conducted to identify predictors of risk group membership, including variables such as baseline nutritional status (BMI, PG-SGA-SF), symptom severity, and psychosocial factors.
Planned subgroup analyses will be stratified by age (<65 vs ≥65), sex, type of surgery (e.g., minimally invasive vs open), and treatment modality (surgery alone vs surgery + neoadjuvant or adjuvant therapy). These strata were chosen based on prior studies showing differential impacts on QoL and recovery patterns among esophageal cancer survivors. Sensitivity analyses stratified by cohort will be conducted to assess the robustness and consistency of findings.
3 Results
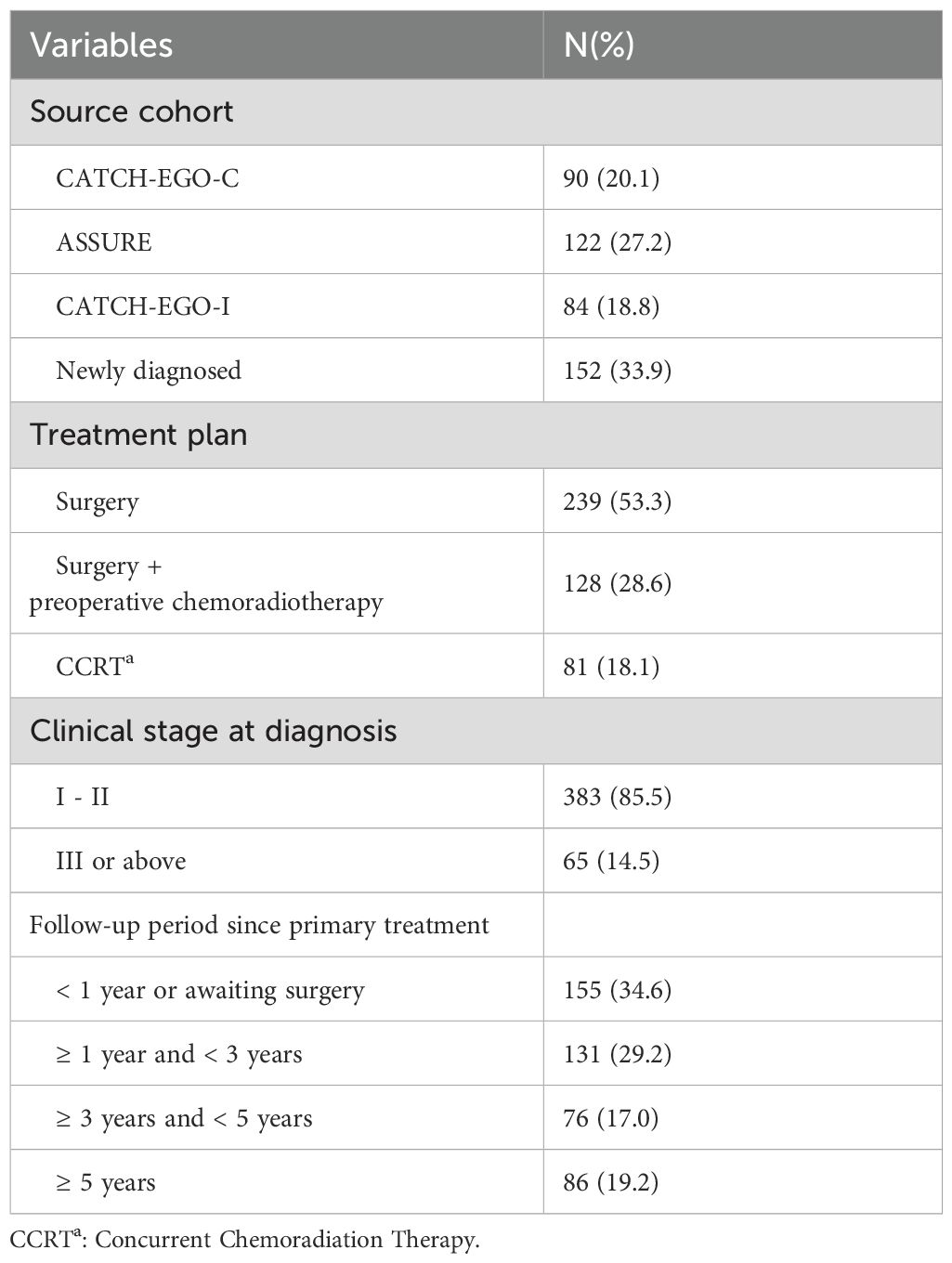
We expect that > 50% of long-term survivors will be enrolled from existing cohorts, and that newly diagnosed patients will also be recruited. As of June 2025, the median follow-up duration since their primary treatment was 72.8 months for the participants from CATCH-EGO-C (n = 90), 37.9 months for the participants from ASSURE (n = 122), and 22.7 months for the participants from CATCH-EGO-I (n = 84). As the cohorts are still under active follow-up and final data consolidation is pending, loss to follow-up rates have not yet been finalized and are not reported in this protocol paper. For newly enrolled patients (n = 152), follow-up is also ongoing, with a median follow-up duration of 5.2 months from surgery (Table 3).
4 Discussion
4.1 Key strategies
This study aims to establish a TRC of patients with esophageal cancer through prospective recruitment from multiple centers. The primary objective is to develop a comprehensive cohort capable of assessing the unmet needs and HRQoL of survivors, and to facilitate timely referral to appropriate interventions when patients are eligible and willing to participate. To achieve this, our TRC employs three key strategies. First, we leverage three existing prospective studies to efficiently identify and enroll long-term survivors. Second, we collect a wide range of data on HRQoL, health behaviors, and environmental factors to enable a multidimensional understanding of survivorship. Third, we collect multiple times within short-term interval points, allowing seamless linkage between the cohort and appropriate trials.
First, we use three existing prospective studies to efficiently identify and enroll long-term survivors. Although esophageal cancer highlights the critical need for long-term research, previous esophageal cancer cohorts have generally enrolled < 200 patients and have faced significant losses to follow-up (20). Given the low incidence and high mortality of the disease, enrolling and retaining patients over time is inherently difficult. To overcome these challenges, we adopted an innovative cohort development strategy to enhance recruitment efficiency and build a robust longitudinal dataset. Specifically, we implemented a dual enrolment approach that includes both newly diagnosed patients and long-term survivors from three existing prospective studies: CATCH-EGO-C, ASSURE, and CATCH-EGO-I. These studies were originally designed to collect baseline HRQoL data, which is often lacking in retrospective or real-world datasets. By leveraging these cohorts, we aim to efficiently recruit long-term survivors within a relatively short timeframe, reducing both time and resource burdens while maintaining high-quality data. Each cohort contributes distinct strengths—CATCH-EGO-C focused on physical activity, ASSURE collected detailed adverse event data, and CATCH-EGO-I incorporated lifelog-based nutritional and physical activity interventions. Despite these differences, all three studies utilized identical patient-reported outcome measures (PROMs) and aligned measurement timepoints, allowing for integration into a single, unified cohort with consistent and comparable QoL data.
Second, we collect a wide range of data to enable a multidimensional understanding of survivorship. Prior qualitative studies have shown that survivors of esophageal cancer report complex challenges in managing their symptoms, diet, psychosocial factors, and social support (21). A focus group study revealed that the HRQoL of patients is heavily influenced by various interconnected factors. For instance, physical symptoms such as diarrhea lead to psychological and social issues, including anxiety and isolation (22), highlighting the need for multidimensional research on QoL in survivors of esophageal cancer. However, many studies used limited measurements, capturing only a narrow scope of patient QoL. For instance, previous studies such as the Prospective Observational Cohort Study of Esophageal-gastric cancer and the Swedish National Registry for Esophageal and Gastric Cancer (NREV) have relied on the European Organization for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire Core 30 (QLQ-C30) and its esophageal cancer-specific module, QLQ-OG25, to assess QoL in patients with esophageal cancer (10, 23, 24). The EORTC QLQ-C30 is designed to measure cancer-related QoL across five functional domains and various symptom scales, while the QLQ-OG25 focuses on esophageal-specific symptoms such as dysphagia, reflux, and eating difficulties. Although these instruments are valuable, they do not comprehensively capture all aspects of QoL relevant to esophageal cancer survivors. Chronic issues such as altered gastrointestinal function, severe fatigue, dietary restrictions, sleep disturbances, and fear of cancer recurrence—common after esophagectomy—are often underrepresented. To address this gap, our study employs a broader set of QoL assessments that capture long-term survivorship issues in greater detail. In fact, a potential application currently under development involves a structured nutritional intervention program tailored to the unique dietary challenges faced by esophageal cancer survivors. This detailed understanding will facilitate the identification of specific unmet needs and enable the design of tailored interventions or clinical trials, contributing to a more comprehensive approach to survivorship care in esophageal cancer.
Third, our study collects multiple time points, enabling us to track the trajectory of HRQoL from the preoperative phase onwards. This approach also allows seamless linkage between the cohort and appropriate intervention trials. Curative surgery remains the standard primary treatment for esophageal cancer, but it leads to a permanent decline in gastric function, resulting in significant physiological changes. Therefore, substantial differences in patient conditions before and after surgery are well recognized (15, 25). Despite this, many previous studies such as Swedish cohort study have not included preoperative QoL assessments (10, 24, 26–29). Consequently, these studies such as NREV cohort could only report postoperative QoL trends or relied on alternative methods for comparison (24). Additionally, previous studies such as Sweden cohort study have assessed patient QoL at relatively long intervals. QoL assessments in prior research typically occurred at 6 months, 3 years, and 5 years post-surgery, with intervals of at least 5 years, depending on the study objectives (26, 28). To overcome these limitations, we collect data at shorter intervals during annual assessments. Also, by measuring QoL in its most fluctuant and vulnerable period; immediately post-surgery; we can gain valuable insights into short-term changes that may influence long-term outcomes. We believe that gathering comparable and periodic data provides a clearer understanding of the overall trends in the QoL of survivors and offers new insights into the relationship between the postoperative period and long-term QoL outcomes.
4.2 Limitations
Despite these strengths, our study has some limitations. First, the predominance of Korean participants may limit the generalizability of our findings. There are epidemiological differences in esophageal cancer between Asian and Western populations; while adenocarcinoma accounts for > 60% of esophageal cancer cases in the West, squamous cell carcinoma is more common in the East (30). This distinction influences treatment approaches, with squamous cell carcinoma often requiring total esophagectomy, leading to significant and permanent physical changes that profoundly affect QoL (31). Therefore, our findings may not be generalizable to populations with different epidemiological profiles. We aim to mitigate this limitation by conducting this study as a multicenter study to include patients from a broader range of clinical settings, which may help capture some variability in patient characteristics. However, future expansion of the TRC to multinational settings is currently under discussion. In particular, we are exploring collaborations across East Asia, where squamous cell carcinoma predominates, and considering adaptations of the PROM framework to accommodate Western cohorts primarily affected by adenocarcinoma. This would allow for cross-cultural validation and broader applicability of our findings.
4.3 Implications
This study presents a well-designed TRC of survivors of esophageal cancer. Although constructing a large longitudinal cohort of this population poses challenges, we have implemented a distinct strategy to overcome these limitations. We anticipate that our cohort will provide valuable data to generate new insights into the QoL of long-term survivors of esophageal cancer and serve as a platform for prompt connections toward proper interventions. While this manuscript describes the initial design and infrastructure of the TRC, the cohort is actively ongoing, with longitudinal follow-up proceeding according to protocol. Once complete, the cohort is likely to be widely cited as a methodological and data-rich reference point in the field of esophageal cancer survivorship.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Samsung Medical Centre (IRB No. SMC 2023-05-116), Asan Medical Centre (IRB No. S2023-2290-0003), Seoul National University Hospital (IRB No. H-2311-020-1480), Seoul National University Bundang Hospital (IRB No. B-2312-870-402), National Cancer Centre (IRB No. NCC2024-0002), Gangnam Severance Hospital (IRB No. 3-2024-0005), and Pusan National University Hospital (IRB No. 2401-005-135). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
JH: Data curation, Formal analysis, Methodology, Visualization, Writing – original draft, Writing – review & editing. GL: Conceptualization, Investigation, Supervision, Writing – original draft, Writing – review & editing. JuC: Conceptualization, Investigation, Methodology, Project administration, Supervision, Writing – review & editing. SY: Investigation, Methodology, Writing – review & editing, Data curation. YS: Data curation, Writing – review & editing. YC: Data curation, Writing – review & editing. JoC: Data curation, Writing – review & editing. SP: Data curation, Writing – review & editing. YJ: Data curation, Writing – review & editing. JL: Data curation, Writing – review & editing. DK: Data curation, Writing – review & editing, Conceptualization, Investigation, Methodology, Project administration, Supervision. HK: Conceptualization, Data curation, Investigation, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was supported by a grant from the Korean Cancer Survivors Healthcare R&D Project through the National Cancer Center, funded by the Ministry of Health & Welfare, Republic of Korea (RS-2023-CC139856).
Acknowledgments
The authors would like to thank the patients and healthcare professionals who participated in this study, as well as the staff of the National Cancer Center for their support and cooperation.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2024) 74:229–63. doi: 10.3322/caac.21834
2. Park EH, Jung KW, Park NJ, Kang MJ, Yun EH, Kim HJ, et al. Cancer statistics in korea: incidence, mortality, survival, and prevalence in 2022. Cancer Res Treat. (2025) 57:312–30. doi: 10.4143/crt.2025.264
3. Noordman BJ, Verdam MGE, Lagarde SM, Hulshof M, van Hagen P, van Berge Henegouwen MI, et al. Effect of neoadjuvant chemoradiotherapy on health-related quality of life in esophageal or junctional cancer: results from the randomized CROSS trial. J Clin Oncol. (2018) 36:268–75. doi: 10.1200/JCO.2017.73.7718
4. Schandl A, Johar A, Anandavadivelan P, Vikstrom K, Malberg K, and Lagergren P. Patient-reported outcomes 1 year after oesophageal cancer surgery. Acta Oncol. (2020) 59:613–9. doi: 10.1080/0284186X.2020.1741677
5. Liu YJ, Schandl A, Markar S, Johar A, and Lagergren P. Psychological distress and health-related quality of life up to 2 years after oesophageal cancer surgery: nationwide population-based study. BJS Open. (2021) 5:zraa038. doi: 10.1093/bjsopen/zraa038
6. Doherty MK, Leung Y, Su J, Naik H, Patel D, Eng L, et al. Health utility scores from EQ-5D and health-related quality of life in patients with esophageal cancer: a real-world cross-sectional study. Dis Esophagus. (2018) 31:doy058. doi: 10.1093/dote/doy058
7. Liu Q, Zeng H, Xia R, Chen G, Liu S, Zhang Z, et al. Health-related quality of life of esophageal cancer patients in daily life after treatment: A multicenter cross-sectional study in China. Cancer Med. (2018) 7:5803–11. doi: 10.1002/cam4.1817
8. Markar SR, Zaninotto G, Castoro C, Johar A, Lagergren P, Elliott JA, et al. Lasting symptoms after esophageal resection (LASER): european multicenter cross-sectional study. Ann Surg. (2022) 275:e392–400. doi: 10.1097/SLA.0000000000003917
9. Cheng Z, Johar A, Lagergren J, Schandl A, and Lagergren P. Disease-specific health-related quality of life trajectories up to 15 years after curative treatment for esophageal cancer-a prospective cohort study. Cancer Med. (2024) 13:e7466. doi: 10.1002/cam4.7466
10. Derogar M and Lagergren P. Health-related quality of life among 5-year survivors of esophageal cancer surgery: a prospective population-based study. J Clin Oncol. (2012) 30:413–8. doi: 10.1200/JCO.2011.38.9791
11. Chen L, Wang H, Qi Z, Liang L, Guo C, He Y, et al. Dynamics of long-term quality of life after treatment for esophageal cancer: A community-based patient study. JCO Glob Oncol. (2024) 10:e2400044. doi: 10.1200/GO.24.00044
12. Qiu LH, Liang SH, Wu L, Huang YY, Yang TZ, Li CZ, et al. Longitudinal assessment of quality of life indicators and prognosis in esophageal cancer patients with curative resection. J Thorac Dis. (2024) 16:6064–80. doi: 10.21037/jtd-24-311
13. Cheng Z, Johar A, Nilsson M, Schandl A, and Lagergren P. Cancer-related fatigue trajectories up to 5 years after curative treatment for oesophageal cancer. Br J Cancer. (2024) 130:628–37. doi: 10.1038/s41416-023-02551-0
14. Donohoe CL, McGillycuddy E, and Reynolds JV. Long-term health-related quality of life for disease-free esophageal cancer patients. World J Surg. (2011) 35:1853–60. doi: 10.1007/s00268-011-1123-6
15. Soriano TT, Eslick GD, and Vanniasinkam T. Long-term nutritional outcome and health related quality of life of patients following esophageal cancer surgery: A meta-analysis. Nutr Cancer. (2018) 70:192–203. doi: 10.1080/01635581.2018.1412471
16. Mollica MA, McWhirter G, Tonorezos E, Fenderson J, Freyer DR, Jefford M, et al. Developing national cancer survivorship standards to inform quality of care in the United States using a consensus approach. J Cancer Surviv. (2024) 18:1190–9. doi: 10.1007/s11764-024-01602-6
17. Xie SH and Lagergren J. Social group disparities in the incidence and prognosis of oesophageal cancer. U Eur Gastroenterol J. (2018) 6:343–8. doi: 10.1177/2050640617751254
18. Cheng C, Ho RTH, Guo Y, Zhu M, Yang W, Li Y, et al. Development and feasibility of a mobile health-supported comprehensive intervention model (CIMmH) for improving the quality of life of patients with esophageal cancer after esophagectomy: prospective, single-arm, nonrandomized pilot study. J Med Internet Res. (2020) 22:e18946. doi: 10.2196/18946
19. Aisen PS, Jimenez-Maggiora GA, Rafii MS, Walter S, and Raman R. Early-stage Alzheimer disease: getting trial-ready. Nat Rev Neurol. (2022) 18:389–99. doi: 10.1038/s41582-022-00645-6
20. Boshier PR, Klevebro F, Savva KV, Waller A, Hage L, Hanna GB, et al. Assessment of health related quality of life and digestive symptoms in long-term, disease free survivors after esophagectomy. Ann Surg. (2022) 275:e140–e7. doi: 10.1097/SLA.0000000000003829
21. Li ZY, Ren JY, Zhong JD, and Zhang JE. Understanding the supportive care needs among discharged patients with esophageal cancer after esophagectomy: A qualitative study. Eur J Oncol Nurs. (2023) 64:102337. doi: 10.1016/j.ejon.2023.102337
22. Malmström M, Ivarsson B, Johansson J, and Klefsgård R. Long-term experiences after oesophagectomy/gastrectomy for cancer—A focus group study. Int J Nurs Stud. (2013) 50:44–52. https://doi.org/10.1016/j.ijnurstu.2012.08.011
23. Sunde B, Lindblad M, Malmstrom M, Hedberg J, Lagergren P, and Nilsson M. Health-related quality of life one year after the diagnosis of oesophageal cancer: a population-based study from the Swedish National Registry for Oesophageal and Gastric Cancer. BMC Cancer. (2021) 21:1277. doi: 10.1186/s12885-021-09007-9
24. van Kleef JJ, Dijksterhuis WPM, van den Boorn HG, Prins M, Verhoeven RHA, Gisbertz SS, et al. Prognostic value of patient-reported quality of life for survival in oesophagogastric cancer: analysis from the population-based POCOP study. Gastric Cancer. (2021) 24:1203–12. doi: 10.1007/s10120-021-01209-1
25. Park DP, Welch CA, Harrison DA, Palser TR, Cromwell DA, Gao F, et al. Outcomes following oesophagectomy in patients with oesophageal cancer: a secondary analysis of the ICNARC Case Mix Programme Database. Crit Care. (2009) 13:S1. doi: 10.1186/cc7868
26. Cheng Z, Johar A, Lagergren J, Schandl A, and Lagergren P. Health-related quality of life trajectories up to 15 years after curative treatment for esophageal cancer: a prospective cohort study. Int J Surg. (2024) 110:1537–45. doi: 10.1097/JS9.0000000000001026
27. Klevebro F, Johar A, and Lagergren P. Impact of co-morbidities on health-related quality of life 10 years after surgical treatment of oesophageal cancer. BJS Open. (2020) 4:601–4. doi: 10.1002/bjs5.50303
28. Schandl A, Cheng Z, Johar A, and Lagergren P. Health-related quality of life 15 years after oesophageal cancer surgery: a prospective nationwide cohort study. J Cancer Surviv. (2023) 17:815–25. doi: 10.1007/s11764-022-01257-1
29. Schandl A, Lagergren J, Johar A, and Lagergren P. Health-related quality of life 10 years after oesophageal cancer surgery. Eur J Cancer. (2016) 69:43–50. doi: 10.1016/j.ejca.2016.09.032
30. Domper Arnal MJ, Ferrandez Arenas A, and Lanas Arbeloa A. Esophageal cancer: Risk factors, screening and endoscopic treatment in Western and Eastern countries. World J Gastroenterol. (2015) 21:7933–43. doi: 10.3748/wjg.v21.i26.7933
Keywords: esophageal cancer survivor, health-related quality of life, trial-ready cohort, cancer survivorship, long-term follow-up, patient-reported outcome
Citation: Han J, Lee G, Cho J, You S, Shim YM, Choi YS, Cho JH, Park SY, Jeon YJ, Lee J, Kang D and Kim HK (2025) A trial-ready cohort for finding unmet needs and improving quality of life among patients with esophageal cancer: a multicenter prospective cohort study. Front. Oncol. 15:1607741. doi: 10.3389/fonc.2025.1607741
Received: 08 April 2025; Accepted: 10 July 2025;
Published: 23 July 2025.
Edited by:
Sharon R. Pine, University of Colorado Anschutz Medical Campus, United StatesReviewed by:
Zhijian Hu, Fujian Medical University, ChinaKoshiro Ishiyama, National Cancer Centre, Japan
Copyright © 2025 Han, Lee, Cho, You, Shim, Choi, Cho, Park, Jeon, Lee, Kang and Kim. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Danbee Kang, ZGJlZS5rYW5nQHNra3UuZWR1; Hong Kwan Kim, aGtraW10c0BnbWFpbC5jb20=
†These authors have contributed equally to this work and share first authorship
 Jiyoon Han
Jiyoon Han Genehee Lee
Genehee Lee Juhee Cho1,2,4
Juhee Cho1,2,4 Danbee Kang
Danbee Kang Hong Kwan Kim
Hong Kwan Kim