- Department of Obstetrics and Gynecology, The First Affiliated Hospital of Henan University of Chinese Medicine, Zhengzhou, China
Choriocarcinoma belongs to a group of highly aggressive malignant gestational trophoblastic neoplasms. Choriocarcinoma is classified into two types based on the origin: a gestational type that arises following a normal or abnormal pregnancy and a non-gestational type resulting from trophoblastic differentiation of germ cells or somatic carcinomas. Primary cervical choriocarcinoma is rare; in women without a recent pregnancy, it is frequently misdiagnosed as other cervical carcinomas, which typically require surgery. Herein, we present a case of primary cervical choriocarcinoma in a 47-year-old woman who presented with irregular vaginal bleeding for five months, with her last pregnancy six years ago. She was initially misdiagnosed with cervical squamous cell carcinoma. Further evaluations, including serum human chorionic gonadotropin (hCG) and comprehensive pathological evaluation, confirmed the diagnosis of gestational choriocarcinoma. Immunohistochemistry showed positive hCG staining, negative p40 and p63 staining, and a high Ki-67 index (60%). Choriocarcinomas of the cervix are uncommon and share similar morphological characteristics with squamous cell carcinoma. Following systemic chemotherapy, hCG levels markedly declined, and the lesion resolved with good response. This case highlights the diagnostic pitfalls associated with primary cervical gestational choriocarcinoma and the importance of clinical correlation, particularly in patients without a recent pregnancy.
Introduction
Choriocarcinoma is an aggressive type of malignant gestational trophoblastic neoplasm (GTN) that originates from trophoblastic cells and is characterized by a mixture of neoplastic syncytiotrophoblasts, intermediate trophoblasts, and cytotrophoblasts. The incidence of choriocarcinoma varies considerably across regions. In China, choriocarcinoma occurs in 1 out of every 2,882 pregnancies (1); in contrast, in Europe and North America, choriocarcinoma occurs in approximately 1 in 40,000 pregnancies and 1 in 40 cases of hydatidiform moles (1, 2). There are two distinct types of choriocarcinoma: the gestational type, which arises from a normal or abnormal pregnancy (e.g., hydatidiform mole), and the non-gestational type, which arises from trophoblastic differentiation of germ cell tumors or somatic carcinoma (3–5). Abnormal uterine bleeding is the most typical presenting symptom as a result of the endomyometrial invasion by the tumor (6). Choriocarcinoma has a high propensity for distant metastasis, and hemoptysis and abnormal neurological symptoms are initial presentations from lung and cerebral metastases, respectively (1, 7).
Gestational choriocarcinomas most frequently occur in the uterine body, whereas ovary is the most common site in the extrauterine form (8). Primary cervical choriocarcinoma is well documented in the published literature, although rare, with fewer than 200 reported cases (8). When choriocarcinoma arises in the cervix, diagnostic challenges emerge, as cervical choriocarcinoma closely mimics cervical squamous cell carcinoma (SCC). Additionally, differentiating between gestational and non-gestational choriocarcinoma is crucial, as the non-gestational form responds poorly to chemotherapy (3). Here, we present a patient with primary cervical choriocarcinoma and describe the clinical correlation and pathology features, highlighting the diagnostic challenge associated with distinguishing cervical choriocarcinoma and cervical SCC.
Case presentation
A 47-year-old female with no significant past medical history who presented at a regional community hospital with irregular vaginal bleeding for 5 months. The initial bleeding was outside of her usual menstrual cycle, intermittent, and appears fresh red. The bleeding stopped simultaneously, then recurred after 4 months, and she was referred for further examination. A pelvic ultrasound revealed cervical hypertrophy with multiple cystic areas and a small ovarian cyst. ThinPrep cytology showed no evidence of dysplasia or carcinoma. Two months later, the patient experienced significantly heavier, continuous bleeding. The patient received amoxicillin and unspecified traditional Chinese medicine without resolution of the symptoms. Thus, a cervical biopsy was performed and revealed atypical squamous cells suggestive of SCC. Because of the limited resources at the community hospital, she was transferred to our tertiary medical center for further evaluation. The patient underwent detailed gynecological examination. The vagina was patent with abundant dark red, non-foul-smelling bloody discharge. The cervix appeared enlarged with necrotic and erosion-like changes, measuring approximately 4 cm in diameter, with positive contact bleeding, firm consistency, and a thickened cervical canal. Cervical motion tenderness was present. The uterus was anteverted, normal in size, of medium consistency, mobile, and without significant tenderness. Bimanual examination revealed normal size of the uterosacral or cardinal ligaments, and no obvious abnormalities were noted in the bilateral adnexal regions. Colposcopy revealed a 4 cm necrotic and erosive lesion at the external cervical os. A repeat pelvic ultrasound demonstrated a 4.3 × 3.3 cm heterogeneous lesion with irregular borders spanning the entire cervix. Pelvic non diffuse MRI with and without contrast detected abnormal signals extending from the external os into the endocervical canal (Figures 1A, B). Given the location of the lesion, the biopsy findings of atypical cells, and the abnormal imaging results, a diagnosis of cervical SCC was favored. Written informed consent was obtained from the patient, and the study was approved by the ethics committee of the The First Affiliated Hospital of Henan University of Chinese Medicine.
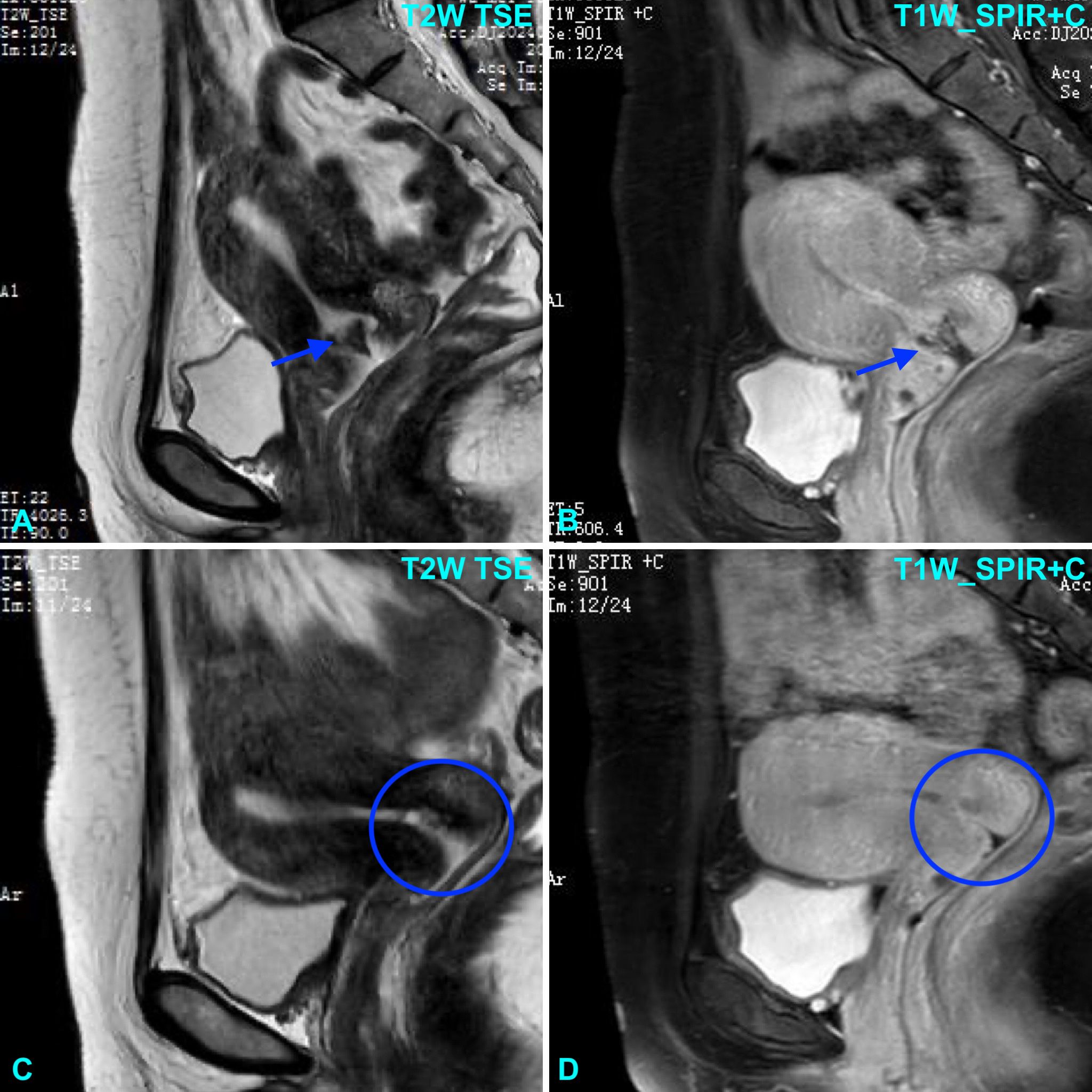
Figure 1. Pelvic MRI shows a lesion centered around the cervix on sagittal T2-weighted Turbo Spin Echo (TSE) image (A) and T1-weighted Spectral Presaturation with Inversion Recovery (SPIR) post-contrast image (B). Following chemotherapy, the lesion was no longer visible on sagittal T2-weighted TSE image (C) and T1-weighted SPIR post-contrast image (D).
As the patient was of childbearing age, a serum panel for HCG, progesterone, and estradiol was obtained to exclude ectopic pregnancy as a source of bleeding. The results showed a serum hCG level of 7,655 mIU/mL (with a repeat value of 7,346 mIU/mL), a progesterone level of 7.4 ng/mL, and an estradiol level of 107 pg/mL. These findings were perplexing because no radiological evidence of an ectopic pregnancy was observed, and the patient reported regular contraceptive use over the past 6 months with rare exceptions.
The cervical biopsy sample from the outside hospital was re-evaluated. Microscopically, the tumor exhibited an epithelioid appearance characterized by decidualization, marked nuclear pleomorphism, and a hemorrhagic background. Immunohistochemical analysis revealed the following results: hCG (+), p40 (–), CK5/6 (–), p16 (focal +), CEA (–), CK7 (focal +), MUC6 (–), Napsin A (–), p53 showing a wild-type pattern, and a Ki-67 index of approximately 5%. From the morphology and immunohistochemistry profile, a diagnosis of malignant GTN was favored. However, because the Ki-67 index is typically very high in choriocarcinoma, other differential diagnoses of gestational trophoblastic disease could not be excluded.
Immediate hysteroscopy revealed no endometrial abnormalities, and both endometrial biopsy and endocervical curettage were performed. The endocervical curettage specimen demonstrated a similar morphology of pleomorphic cells with squamoid appearance (Figures 2A–D). Immunohistochemical analysis showed CK (+), HPL (focal+), hCG (+), CD10 (–), CK5/6 (–), p40 (–), Inhibin A (focal, partial +), MUC4 (focal, partial +), p63 (–), and Ki-67 (60%). The presence of pleomorphic nuclei with abundant pink cytoplasm, hemorrhage, and positivity for hCG, with negative staining for p40 and p63 (Figures 3A–D), confirmed the diagnosis of choriocarcinoma. Whole-body CT scan revealed no abnormal lesions suggestive of metastasis.
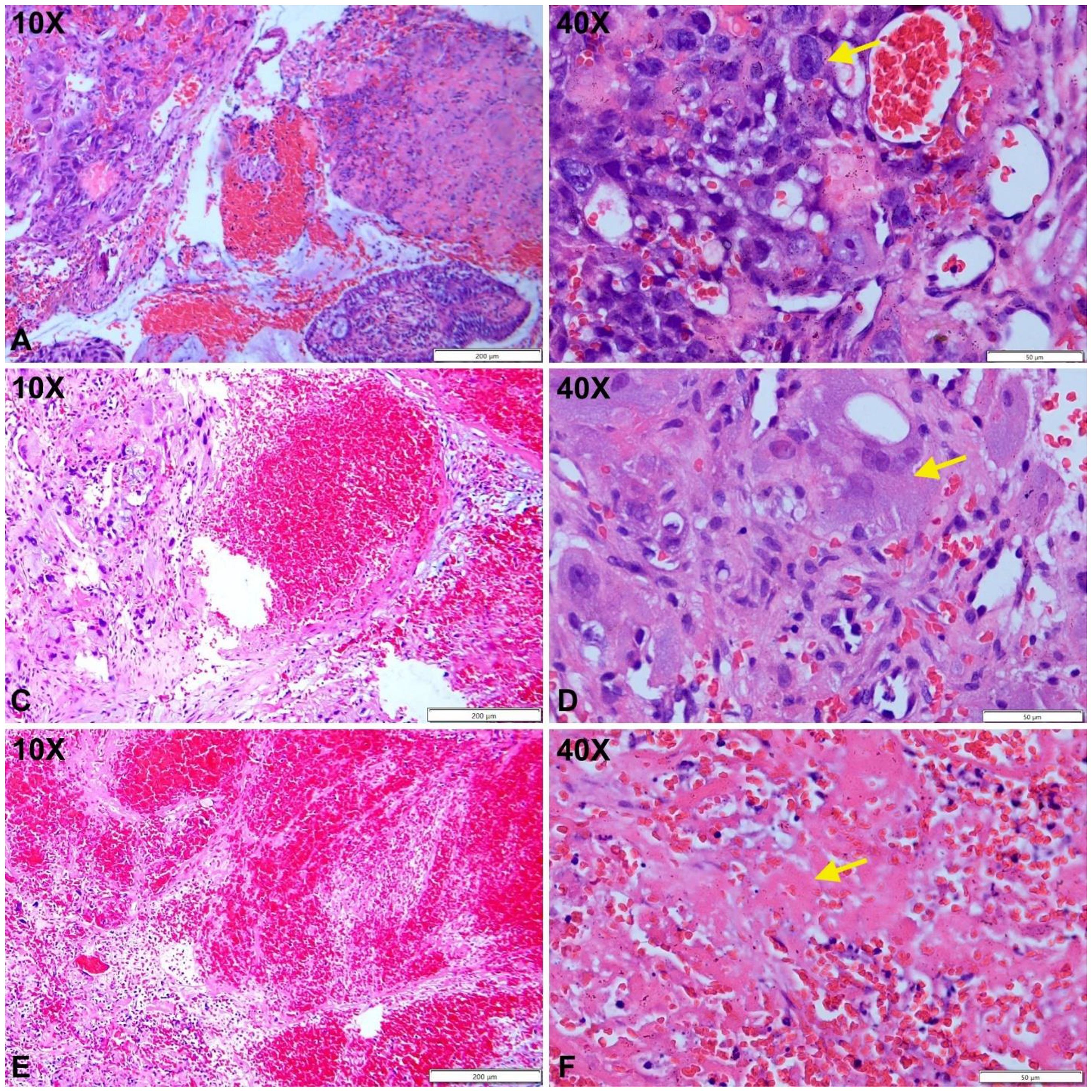
Figure 2. Cytotrophoblast proliferation with cytologic atypia (A, B, yellow arrow). Populations of atypical syncytiotrophoblasts are observed (C, D, yellow arrow). Areas of hemorrhage with fibrinoid necrosis are noted (E, F, yellow arrow).
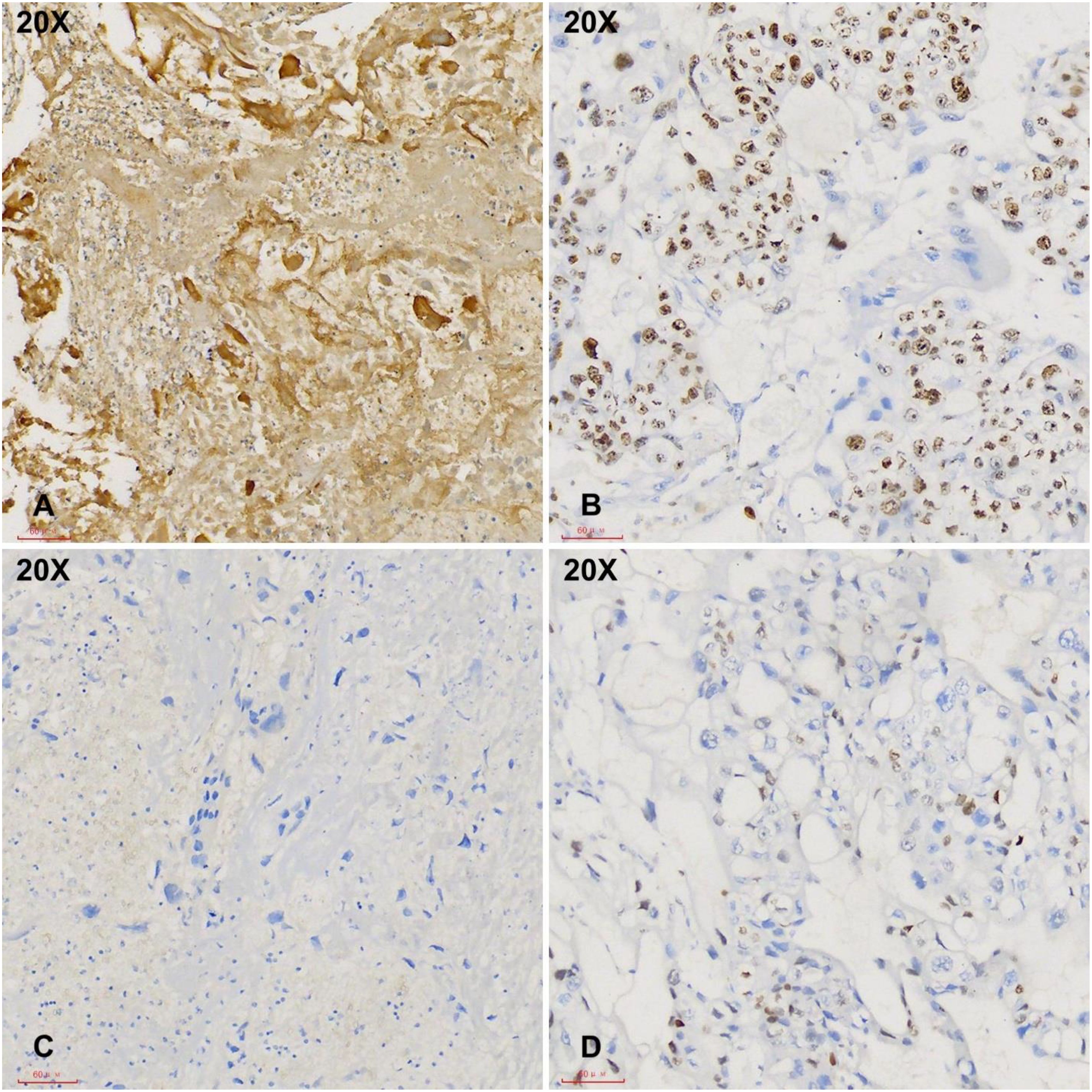
Figure 3. Immunohistochemical studies demonstrate strong hCG positivity in tumor cells with a high Ki-67 index (A, B, 20X). The tumor cells are negative for p40 and p63 (C, D, 20X).
The patient was over 40 years old with a full-term delivery 6 years prior; her serum HCG levels ranged between 103 and 104 mIU/mL, and the maximum diameter of the tumor was 3–5 cm. Following the 2000 GTN FIGO clinical staging and scoring system, she was diagnosed as stage I with a score of 9, indicating a high-risk subtype (9). The treatment of choriocarcinoma primarily involves systemic chemotherapy, with surgery playing an adjunctive role. The guidelines indicate high-risk GTN is managed with the etoposide, methotrexate, actinomycin D, cyclophosphamide, and vincristine (EMA/CO) chemotherapy regimen. After hysteroscopy and sampling, the patient’s serum hCG level dropped to 506 mIU/mL; following chemotherapy, it decreased to 150.9 mIU/mL, and after a second round of chemotherapy, it dropped to 3.6 mIU/mL (<5 mIU/mL). After completion of three rounds of consolidation chemotherapy, the cervical lesions disappeared (Figures 1C, D). The patient’s menstruation has returned to normal, and her hCG levels are monitored on a regular basis. Two additional cycles of EMA/CO chemotherapy regimen were further administered. The patient was followed up every three months and remained disease-free at six months.
Discussion
Choriocarcinoma is categorized as either gestational or non-gestational, and the difference is crucial in terms of optimal patient care. Gestational choriocarcinoma arises from the malignant transformation of trophoblastic cells following pregnancy. There are approximately 50% of cases associated with hydatidiform moles, 25% with miscarriages, 22.5% seen after a normal pregnancy, and 2.5% following an ectopic pregnancy in women of childbearing age (10–12). Approximately 2–3% of complete moles and less than 1% of partial moles progress to choriocarcinoma (2, 9, 13). The current patient developed gestational choriocarcinoma six years after a full-term delivery when she was 41 years old. The interval between antecedent pregnancy and disease onset is variable, with the longest reported interval of approximately 20 years (14). The pathogenesis of cervical GTN remains unclear. Cervical metastasis may occur despite a primary uterine tumor that spontaneously regresses; alternatively, it may be caused by the malignant transformation of a cervically implanted fetus or trophoblastic cells that migrate to the cervix and transform to malignancy (15, 16). Primary cervical choriocarcinoma may also develop after ectopic pregnancy in cervix and it can have an even normal level of serum hCG (17, 18). The current case represents a true primary cervical choriocarcinoma, as there was no evidence of tumors in the endometrial cavity.
Non-gestational choriocarcinoma results from abnormal differentiation of primordial germ cells during embryonic development. This type of choriocarcinoma is not related to pregnancy and can occur in both men and women, often in conjunction with germ cell tumors such as dysgerminomas and teratomas in the ovary (19, 20). The disease often metastasizes to organs like the lungs, liver, and brain, resulting in a poor prognosis (19). Additionally, a rare but well-established type of non-gestational choriocarcinoma is seen in post-menopausal women, which represents trophoblastic differentiation from somatic malignancy (21, 22).
In patients of reproductive age who present with cervical lesions associated with profuse bleeding, cervical choriocarcinoma is an important differential diagnosis. If serum hCG is not evaluated or unremarkable, pathology examination is the most reliable method to further confirm the diagnosis of choriocarcinoma. Microscopically, choriocarcinoma is characteristic but also shares many features similar to its mimickers. Choriocarcinoma shows marked cytologic atypia, high mitotic activity, and the Ki-67 index is often over 90% (23). The tumor typically appears as an infiltrative mass with extensive hemorrhaging, necrosis, and lymphovascular invasion (24). While the presence of chorionic villi usually rules out the diagnosis of choriocarcinoma, malignant trophoblasts may extend from the villi in term placentas forming intraplacental choriocarcinoma (24, 25). For diagnosis, immunohistochemistry stains such as hCG, HSD3B1, hPL, and inhibin A, are necessary to confirm malignant trophoblasts (5, 26).
In cervical biopsy, SCC is a major diagnostic pitfall, and a patient can be easily misdiagnosed given the morphological and immunohistochemical profile overlap. Both choriocarcinoma and SCC can show significant infiltrative growth, severe nuclear pleomorphism, and b-hCG positivity (27). It is important to note that true squamous differentiation would be positive for one of the two squamous markers, p63 or p40 (28), as long as most cervical SCCs are HPV-mediated and exhibit strong p16 positivity (29). Cervical choriocarcinoma is diagnosed on the basis of diffusely positive hCG, negative p63 and p40, and focal p16. The primary cervical gestational type is further evident by the absence of lesions in the uterine cavity and a very high serum hCG level. The patient has a long latent history (6 years) between the pregnancy and the development of choriocarcinoma. Two possible explanations include an undetected asymptomatic pregnancy with pregnancy loss during the long latent period, and another theory is that retained trophoblastic tissue residing in the cervix following the antecedent pregnancy could remain dormant for years before developing into invasive gestational trophoblastic neoplasms (30).
An important clinical implication of making an accurate diagnosis in this setting is that stage IA cervical squamous cell carcinoma is typically treated with cone biopsy or loop electrosurgical excision procedure, with or without sentinel lymph node mapping, radiation, or extensive surgery, depending on the presence of lymphovascular invasion (31). In contrast, cervical gestational trophoblastic neoplasms are managed primarily with chemotherapy, often combined with surgery (16). The clinical significance of choriocarcinoma developing after a long latent period following an antenatal pregnancy is unclear, particularly regarding whether there is a difference in prognosis or chemotherapy treatment response.
Somatic carcinoma with trophoblastic differentiation is an important differential diagnosis in the current patient, given the associated poor prognosis and different treatment regimens (32). The development of these tumors is not linked to gestational trophoblasts or germ cells (33). Razack et al. reported a case of HPV-associated SCC with trophoblastic differentiation in the cervix and an elevated serum hCG level of 78 mIU/mL (normal <5 mIU/mL). Histologically, the tumor was invasive, showed irregular nuclei membrane, and was devoid of squamous differentiation. The tumor was strongly positive for p16 and GATA3 with a very high Ki-67 index, which strongly mimicked malignant GTNs. In the current patient, the hCG level was over 7000 mIU/mL in two assessments; together with the strong positive hCG detected by immunohistochemical stain, a somatic carcinoma with trophoblastic differentiation can be ruled out. Generally, when immunostain and serum levels of hCG are equivocal, short tandem repeat genotyping is required to confirm the gestational nature with a unique paternal genome that is not present in somatic malignancies (33).
The FIGO staging system categorizes GTN into low and high-risk cases by prognostic scores (stages 7 and 8) determined using a scoring system that takes into account the patient’s age, previous pregnancy, months from pregnancy, hCG level, tumor size, and metastases (9). This patient was at stage I, with a score of 9, indicating a high-risk subtype. Patients with low-risk GTN are treated with single-agent chemotherapy (methotrexate or actinomycin D) (34). Chemotherapy is recommended for 2–3 courses after hCG levels have been normalized. Alternative regimens may be used if the response plateaus (12). GTN that presents as high risk should be treated with the EMA/CO regimen (35). Brain metastases may require additional treatments like cranial radiotherapy, and etoposide and cisplatin are used for induction chemotherapy for extensive metastasis (36, 37). Chemotherapy is the mainstay of GTN treatment, and hysterectomy is usually not required. However, it can reduce chemotherapy cycles in low-risk patients not seeking fertility preservation (38). Surgery is also essential for controlling bleeding or addressing chemotherapy resistance in patients with resistant lesions or severe hemorrhage causing anemia (38, 39).
The current case highlights the diagnostic pitfalls inherent in primary cervical gestational choriocarcinoma, particularly in patients without a recent pregnancy, in which histopathological features overlap with those of more common cervical malignancies. This convergence underscores the need for meticulous clinical correlation to guide appropriate treatment regimens and avoid unnecessary surgical intervention.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the ethics committee of the The First Affiliated Hospital of Henan University of Chinese Medicine. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
JS: Writing – original draft, Data curation, Validation, Investigation. PC: Methodology, Writing – review & editing, Project administration, Conceptualization.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Zhang W, Liu B, Wu J, and Sun B. Hemoptysis as primary manifestation in three women with choriocarcinoma with pulmonary metastasis: a case series. J Med Case Rep. (2017) 11:110. doi: 10.1186/s13256-017-1256-9
2. Lurain JR. Gestational trophoblastic disease I: epidemiology, pathology, clinical presentation and diagnosis of gestational trophoblastic disease, and management of hydatidiform mole. Am J Obstet Gynecol. (2010) 203:531–9. doi: 10.1016/j.ajog.2010.06.073
3. Wang L, Wan Y, Sun Y, Zhang X, Cheng X, Wu M, et al. Pure nongestational uterine choriocarcinoma in postmenopausal women: a case report with literature review. Cancer Biol Ther. (2019) 20:1176–82. doi: 10.1080/15384047.2019.1617564
4. Shih I-M, Mazur MT, and Kurman RJ. Gestational trophoblastic tumors and related tumor-like lesions. In: Kurman RJ, Ellenson LH, and Ronnett BM, editors. Blaustein’s Pathology of the Female Genital Tract. Springer US, Boston, MA (2011). p. 1075–135.
5. Olson MT, Gocke CD, Giuntoli RL 2nd, and Shih Ie M. Evolution of a trophoblastic tumor from an endometrioid carcinoma–a morphological and molecular analysis. Int J Gynecol Pathol. (2011) 30:117–20. doi: 10.1097/PGP.0b013e3181f5ce68
6. Salahaldin RR, Abubaker ME, Abdalqader GM, Tuqan AR, Zaben BA, and Barghouthi I. Postpartum choriocarcinoma complicated by uterine perforation: A case report and literature review. Case Rep Women’s Health. (2025) 45:e00693. doi: 10.1016/j.crwh.2025.e00693
7. Song L, Li Q, Yin R, and Wang D. Choriocarcinoma with brain metastasis after term pregnancy: A case report. Med (Baltimore). (2018) 97:e12904. doi: 10.1097/MD.0000000000012904
8. Pérez Montiel CA and Varela Aguirre GJ. Primary non-gestational choriocarcinoma of the uterine cervix. Int J Gynecol Cancer. (2022) 32:111–2. doi: 10.1136/ijgc-2021-003061
9. Ngan HYS, Seckl MJ, Berkowitz RS, Xiang Y, Golfier F, Sekharan PK, et al. Diagnosis and management of gestational trophoblastic disease: 2021 update. Int J Gynaecol Obstet. (2021) 155 Suppl 1:86–93. doi: 10.1002/ijgo.13877
10. Essel KG, Shafer A, Bruegl A, Gershenson DM, Drury LK, Ramondetta LM, et al. Complete resection is essential in the surgical treatment of gestational trophoblastic neoplasia. Int J Gynecol Cancer. (2018) 28:1453–60. doi: 10.1097/IGC.0000000000001348
11. Bagshawe KD, Golding PR, and Orr AH. Choriocarcinoma after hydatidiform mole. Studies related to effectiveness of follow-up practice after hydatidiform mole. Br Med J. (1969) 3:733–7. doi: 10.1136/bmj.3.5673.733
12. Soper JT. Gestational trophoblastic disease. Obstet Gynecol. (2006) 108:176–87. doi: 10.1097/01.AOG.0000224697.31138.a1
13. Berkowitz RS and Goldstein DP. Chorionic tumors. N Engl J Med. (1996) 335:1740–8. doi: 10.1056/NEJM199612053352306
14. Tomoda Y, Arii Y, Kaseki S, Asai Y, Gotoh S, Suzuki T, et al. Surgical indications for resection in pulmonary metastasis of choriocarcinoma. Cancer. (1980) 46:2723–30. doi: 10.1002/1097-0142(19801215)46:12<2723::AID-CNCR2820461231>3.0.CO;2-0
15. Martin BR 3rd, Orr JW Jr, and Austin JM Jr. Cervical choriocarcinoma associated with an intrauterine contraceptive device: a case report. Am J Obstet Gynecol. (1983) 147:343–4. doi: 10.1016/0002-9378(83)91127-4
16. Wang X, Yang J, Wan X, Feng F, Zhao J, Ren T, et al. Identification and treatment of primary cervical gestational trophoblastic neoplasia: a retrospective study of 13 patients and literature review. Orphanet J Rare Dis. (2021) 16:480. doi: 10.1186/s13023-021-02111-w
17. Mitrovic S, Arsenijevic PS, Kljakic D, Djuric JM, Milosavljevic MZ, Protrka ZM, et al. Gestational choriocarcinoma of the cervix. Arch Iran Med. (2014) 17:783–5. Available online at: https://pubmed.ncbi.nlm.nih.gov/25365622/.
18. Mehra R, Huria A, Gupta P, and Mohan H. Choriocarcinoma with negative urinary and serum beta human chorionic gonadotropin (betaHCG)–a case report. Indian J Med Sci. (2005) 59:538–41. doi: 10.4103/0019-5359.19197
19. Yang Y, Zhang X, Chen D, Liu L, and Hao L. Adolescent non-gestational ovarian choriocarcinoma: report of a case and review of literature. Int J Clin Exp Pathol. (2019) 12:1788–94. Available online at: https://pubmed.ncbi.nlm.nih.gov/31933999/.
20. Buza N and Hui P. Genotyping diagnosis of gestational trophoblastic disease: frontiers in precision medicine. Modern Pathol. (2021) 34:1658–72. doi: 10.1038/s41379-021-00831-9
21. Zhang Z, Zhao Z, Wang J, Cui R, and Feng F. Endometrial clear cell carcinoma with non-gestational choriocarcinoma differentiation: use of rapamycin maintenance. Int J Gynecol Cancer. (2023) 33:988–94. doi: 10.1136/ijgc-2023-004320
22. Buza N, Baine I, and Hui P. Precision genotyping diagnosis of lung tumors with trophoblastic morphology in young women. Modern Pathol. (2019) 32:1271–80. doi: 10.1038/s41379-019-0275-z
23. Hui P. Gestational trophoblastic tumors: A timely review of diagnostic pathology. Arch Pathol Lab Med. (2019) 143:65–74. doi: 10.5858/arpa.2018-0234-RA
24. Jiao L, Ghorani E, Sebire NJ, and Seckl MJ. Intraplacental choriocarcinoma: Systematic review and management guidance. Gynecol Oncol. (2016) 141:624–31. doi: 10.1016/j.ygyno.2016.03.026
25. Black JO, Rufforny-Doudenko I, and Shehata BM. Pathologic quiz case: third trimester placenta exhibiting infarction. Intraplacental Choriocarcinoma Arch Pathol Lab Med. (2003) 127:e340–2. doi: 10.5858/2003-127-e340-PQCTTP
26. Kalhor N, Ramirez PT, Deavers MT, Malpica A, and Silva EG. Immunohistochemical studies of trophoblastic tumors. Am J Surg Pathol. (2009) 33:633–8. doi: 10.1097/PAS.0b013e318191f2eb
27. Hameed A, Miller DS, Muller CY, Coleman RL, and Albores-Saavedra J. Frequent expression of beta-human chorionic gonadotropin (beta-hCG) in squamous cell carcinoma of the cervix. Int J Gynecol Pathol. (1999) 18:381–6. doi: 10.1097/00004347-199910000-00014
28. Bishop JA, Montgomery EA, and Westra WH. Use of p40 and p63 immunohistochemistry and human papillomavirus testing as ancillary tools for the recognition of head and neck sarcomatoid carcinoma and its distinction from benign and Malignant mesenchymal processes. Am J Surg Pathol. (2014) 38:257–64. doi: 10.1097/PAS.0000000000000119
29. Sabatini ME and Chiocca S. Human papillomavirus as a driver of head and neck cancers. Br J Cancer. (2020) 122:306–14. doi: 10.1038/s41416-019-0602-7
30. Zribi A, Mazroui RA, Sayani R, and Burney IA. An Unusual Presentation of Choriocarcinoma in a Postmenopausal Woman: A case report. Sultan Qaboos Univ Med J. (2024) 24:135–8. doi: 10.18295/squmj.5.2023.036
31. Guimarães YM, Godoy LR, Longatto-Filho A, and Reis RD. Management of early-stage cervical cancer: A literature review. Cancers (Basel). (2022) 14:575. doi: 10.3390/cancers14030575
32. Xie Y, Li Q, Yang K, Liu T, Liu J, and Li L. Endometrial carcinoma with significant choriocarcinomatous differentiation in a nulliparous woman: a case report and literature review. Gynecol Pelvic Med. (2022) 5:8. doi: 10.21037/gpm-21-59
33. Razack R, Addae H, and Hui P. High-risk HPV associated carcinoma with trophoblastic differentiation. A case report highlighting features of an aggressive somatic Malignancy with elevated BHCG levels. Hum Pathol Rep. (2023) 34:300722. doi: 10.1016/j.hpr.2023.300722
34. Alobaid A, Ahmeed S, Abuzaid M, Aldakhil L, and Abu-Zaid A. Low-risk gestational trophoblastic neoplasia: A single-center experience from Saudi Arabia. Avicenna J Med. (2019) 9:89–93. doi: 10.4103/ajm.AJM_188_18
35. Albright BB, Ellett T, Knochenhauer HE, Goins EC, Monuszko KA, Kaplan SJ, et al. Treatments and outcomes in high-risk gestational trophoblastic neoplasia: A systematic review and meta-analysis. Bjog. (2023) 130:443–53. doi: 10.1111/1471-0528.17374
36. Barcellini A, Fodor A, Charalampopoulou A, Cassani C, Locati LD, Cioffi R, et al. Radiation therapy for gestational trophoblastic neoplasia: forward-looking lessons learnt. Cancers (Basel). (2023) 15:4817. doi: 10.3390/cancers15194817
37. Nakamura B, Cowan M, Griffin BB, Victoria Fischer J, Lurain JR, and Strohl AE. Successful management of stage IV epithelioid trophoblastic tumor using multimodality treatment: A case report. Gynecol Oncol Rep. (2021) 37:100802. doi: 10.1016/j.gore.2021.100802
38. Ramesan CK, Thomas DS, Sebastian A, Thomas V, Thomas A, George R, et al. Role of hysterectomy in gestational trophoblastic neoplasia. Indian J Surg Oncol. (2021) 12:386–90. doi: 10.1007/s13193-021-01328-2
Keywords: cervix, choriocarcinoma, diagnosis, hCG, gestational trophoblastic disease
Citation: Shi J and Chen P (2025) Diagnostic pitfall in primary cervical gestational choriocarcinoma: a case report. Front. Oncol. 15:1608856. doi: 10.3389/fonc.2025.1608856
Received: 09 April 2025; Accepted: 08 August 2025;
Published: 27 August 2025.
Edited by:
Ottavia D’Oria, Sapienza University of Rome, ItalyReviewed by:
Ashraf Talaat Youssef, Fayoum University, EgyptPing Li, Jinan University, China
Neetha Vyas, K S Hegde Medical Academy, India
Copyright © 2025 Shi and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ping Chen, cGluZ3Bpbmc2NzY4QDEyNi5jb20=
 Jiayu Shi
Jiayu Shi Ping Chen*
Ping Chen*