- 1Department of Laboratory, The First People’s Hospital of Yibin, Yibin, China
- 2Department of Oncology, The First People’s Hospital of Yibin, Yibin, China
Background: Circulating tumor DNA (ctDNA), as a liquid biopsy biomarker, is undergoing extensive evaluation for its clinical utility across multiple tumor management scenarios. This study aimed to assess the prognostic value of ctDNA in esophageal cancer (EC) patients at different treatment time points through a systematic review and meta-analysis.
Materials and methods: A comprehensive search of the PubMed, Embase, and Cochrane Library databases from construction to October 2024 was conducted, and studies investigating the association between ctDNA and progression-free survival (PFS) and overall survival (OS) in EC patients were screened for inclusion. Primary outcomes included PFS/OS by ctDNA status at different time points (baseline, after neoadjuvant therapy, and during follow-up). Risk ratios (HRs) for PFS/OS with positive ctDNA tests at various time points were combined, and subgroup analyses were conducted for tumor-informed and non-tumor-informed testing of ctDNA.
Results: A total of 22 studies involving 1519 patients were finally included in this meta-analysis. In univariate analyses, detection of ctDNA was associated with poorer PFS at baseline (HR = 1.64, 95% CI:1.30-2.07), after neoadjuvant therapy (HR = 3.97, 95% CI: 2.68-5.88) and during follow-up (HR = 5.42, 95% CI:3.97-7.38). Similarly, detection of ctDNA at all time points was associated with poorer OS (at baseline: HR = 2.02, 95% CI:1.36-2.99; after neoadjuvant therapy: HR = 3.41, 95% CI: 2.08-5.59; and during follow-up: HR = 4.93, 95% CI:3.31-7.34). Similar PFS and OS outcomes were observed in multivariate analyses. The uni- and multivariate combined HRs for PFS/OS with ctDNA detected at different time points were numerically related as: baseline < after neoadjuvant therapy < during follow-up(PFS:1.90→4.07→5.22; OS:2.39→3.15→5.37). When ctDNA was detected in combined tumor-informed assays at baseline and after neoadjuvant therapy, most HRs for recurrence and mortality risk showed a trend toward higher values compared with non-tumor-informed assays. ctDNA test positivity predicted clinical recurrence an average of 4.53 months earlier (range: 0.98-11.6 months) than conventional radiological imaging techniques.
Conclusions: Positive ctDNA testing was associated with poorer prognosis throughout the treatment period of EC patients, and the prognostic value of monitoring ctDNA status increased with time from baseline to follow-up.
Systematic Review Registration: https://www.crd.york.ac.uk/PROSPERO/view/CRD42024612909, identifier CRD42024612909.
1 Introduction
According to the Global Cancer Observatory 2022, esophageal cancer (EC) is the 11th most common cancer and the 7th leading cause of cancer deaths globally, resulting in about 510,000 new cases and about 450,000 deaths (1).EC mainly consists of esophageal squamous cell carcinoma (ESCC) and esophageal adenocarcinoma (EAC). EC patients are asymptomatic or atypical in the early stages. Because of their very aggressive biology it often leads to a poor prognosis due to significant progression of the disease by the time of diagnosis. In most countries and regions, the 5-year survival rate after EC diagnosis is between 10% and 30% (2). Currently, the treatment of EC is based on a staged combination model, in which patients with locally advanced disease are usually treated radically with a multimodal approach including surgery, e.g., neoadjuvant therapy in combination with surgery, and palliatively with advanced (metastatic or disseminated) and recurrent disease (3). Although the treatment modalities of EC have improved significantly, the clinical management of EC is still unable to accurately and timely capture the recurrence risk of patients under the current clinical technology, resulting in no significant improvement in their prognosis. There is an urgent need to develop a new biomarker to predict the risk of EC patients at different stages of treatment and to implement individualized treatment so that timely intervention can be made to improve overall survival outcomes.
In recent years, more and more researchers have begun to focus on applying liquid biopsy in oncology, among which circulating tumor DNA (ctDNA) has attracted much attention (4). ctDNA is released into the bloodstream during apoptosis or necrosis of tumors and through circulating tumor cells (5–8). Compared with clinically available serum tumor biomarkers (e.g., CEA, SCC), ctDNA has higher sensitivity and specificity (8, 9). ctDNA has a very short half-life of approximately 2 hours, which allows it to reflect the real-time tumor load of a tumor patient more accurately and to be used as a dynamic biomarker for tracking the presence of tumors (10, 11). Some studies have reported the application of ctDNA in the early diagnosis of tumors, identification of tiny residual lesions, response to treatment, and survival prognosis, respectively (12–15). Most studies focused on the relationship between ctDNA and survival prognosis in EC. There are three systematic reviews and meta-analyses on the relationship between ctDNA and EC prognosis (16–18), the most comprehensive of which is from Zhang and Jin et al. (18). They conducted a complete subgroup analysis of the prognosis of ctDNA in patients with EC in terms of different ethnicities, histological subtypes, anatomical locations, ctDNA testing methods, testing times, and different treatment modalities. Although previous studies have confirmed the prognostic value of ctDNA in EC, a core issue that has been overlooked is that the vast majority of research has pooled ctDNA data collected at different time points for analysis, failing to adequately account for the inherent dynamic evolution of ctDNA levels over time and in response to treatment. This ‘static’ analytical approach fails to capture the full value of ctDNA as a biomarker that reflects tumor burden and treatment response in real time. This study aims to systematically evaluate the prognostic value of ctDNA at three critical clinical time points (baseline, after neoadjuvant therapy, and follow-up) using a “dynamic” framework. It further compares the differences in clinical recurrence detection timing between ctDNA and traditional imaging techniques, seeking to provide precise evidence for the application of ctDNA at specific clinical decision points.
2 Materials and methods
The present systematic review and meta-analysis were conducted by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (Supplementary Table S1) and the Assessing Methodological Quality in Systematic Reviews (AMSTAR) guidelines (19, 20). The study protocol was registered on the International Prospective Register of Systematic Reviews (PROSPERO) (ID: CRD42024612909).
2.1 Literature retrieval
We searched PubMed, Embase, and the Cochrane Library for studies published from inception to 23 October 2024, with no language restriction. The search strategy was for studies related to “esophageal neoplasms” and “circulating tumor DNA,” and the search formula took the form of a combination of subject terms plus free words/synonyms, with the complete search string shown in the Supplementary Table S2. All abstracts identified by the search strategy were screened first, followed by a review of potentially eligible full texts, and eligible conference abstracts were also included in the study. Two authors(MW and CX) independently performed study screening, data extraction, and quality assessment. Any disagreements were resolved through discussion with the other author(SW).
2.2 Study selection
Inclusion criteria: (a) clinical studies (prospective or retrospective); (b) patients with pathologically confirmed primary EC, regardless of the specific treatment modality; we classified adenocarcinoma of the esophagogastric junction as EAC due to its similarity to EAC in terms of clinical features and treatment strategy; (c) plasma samples with measurable ctDNA; there were no limitations on the methods of detection and analysis of ctDNA, considering the lack of a gold standard and the lack of direct comparisons between the different methods; and (d) provide data on the association between ctDNA and outcome [progression-free survival (PFS) and overall survival (OS)].
Exclusion criteria: (a) case reports, comments, letters, reviews, and systematic reviews and meta-analyses; (b) non-human studies; (c) replicated studies; (d) no relevant data.
2.3 Data extraction
Data to be extracted include (a) general information (title, authors, date of publication and type of study);(b) Patient population characteristics (sample size, tissue subtype, clinical stage and treatment modality); (c) The timing of ctDNA testing of plasma samples, the method of testing, and whether the test is tumor-informed and significantly mutated genes;(d) Duration of follow-up and advance time to ctDNA detection of disease recurrence;(e) Hazard ratios (HRs) and 95% confidence intervals (CIs) for PFS and OS. If survival data were not provided directly in the text, Engauge Digitizer version 11.1 could be used to extract non-numerical survival data from Kaplan-Meier curves if necessary, and HRs and 95% CIs could be calculated using Tierney’s method (21).
2.4 Quality assessment
The risk of bias for included studies was assessed using the Newcastle-Ottawa Scale (NOS) instrument. This instrument consists of three components: selection, comparability, and outcome, with studies scored from 0 to 9 (22). Scores of 0-3, 4-6, and 7–9 were defined as low, moderate, and high-quality studies, respectively.
2.5 Objectives and outcomes
The different time points for ctDNA assessment are classified as baseline (after diagnosis and before any treatment), after neoadjuvant therapy (after neoadjuvant therapy and before surgery), and during follow-up (during adjuvant therapy or follow-up). ctDNA testing can be either tumor-informed or non-tumor-informed, with tumor-informed assays defined as ctDNA assessments based on initial genomic analysis of primary tumor tissue samples to identify tumor-derived alterations. In contrast, non-tumor-informed assays do not require concomitant tumor tissue sequencing (9, 23).
The main objective was to assess whether patients with positive ctDNA tests in plasma samples at different time points had a worse prognosis (PFS and OS) than patients with negative ctDNA tests. The definitions of PFS and OS were based on the studies. PFS includes any recurrence events, such as disease-free survival (DFS), recurrence-free survival (RFS), freedom from progression (FFP), and time to progression (TTP); OS includes any type of mortality event, such as disease-specific survival (DSS) and cancer-specific death (CSD). The secondary objective was to perform subgroup analyses of ctDNA tumor-informed or non-tumor-informed testing at different time points. Finally, the time to detection of clinical recurrence by ctDNA was compared with conventional imaging techniques.
2.6 Statistical analysis
ctDNA was considered a binary (positive/negative) variable for the analysis. Univariate and multivariate HRs and 95% CIs were extracted, if available, and then combined separately. Multivariate HRs were extracted irrespective of the variables included in the model for each study. HRs greater than 1 indicated that patients with EC who tested positive for ctDNA had a poor prognosis. The I2 statistic was used to assess statistical heterogeneity between studies. If statistical homogeneity was observed, a fixed-effects model was used to combine the results (P > 0.05 or I2 < 50%), whereas a random-effects model was used if statistical heterogeneity existed (P < 0.05 or I2 > 50%). If a high degree of heterogeneity existed, sensitivity analyses were performed by systematically excluding individual studies to assess the stability of the results. In addition, publication bias was examined for results containing 10 or more studies using visual assessment of funnel plots. All analyses were performed using RevMan 5.3 software (Cochrane, London, UK).
3 Results
3.1 Search results and essential characteristics
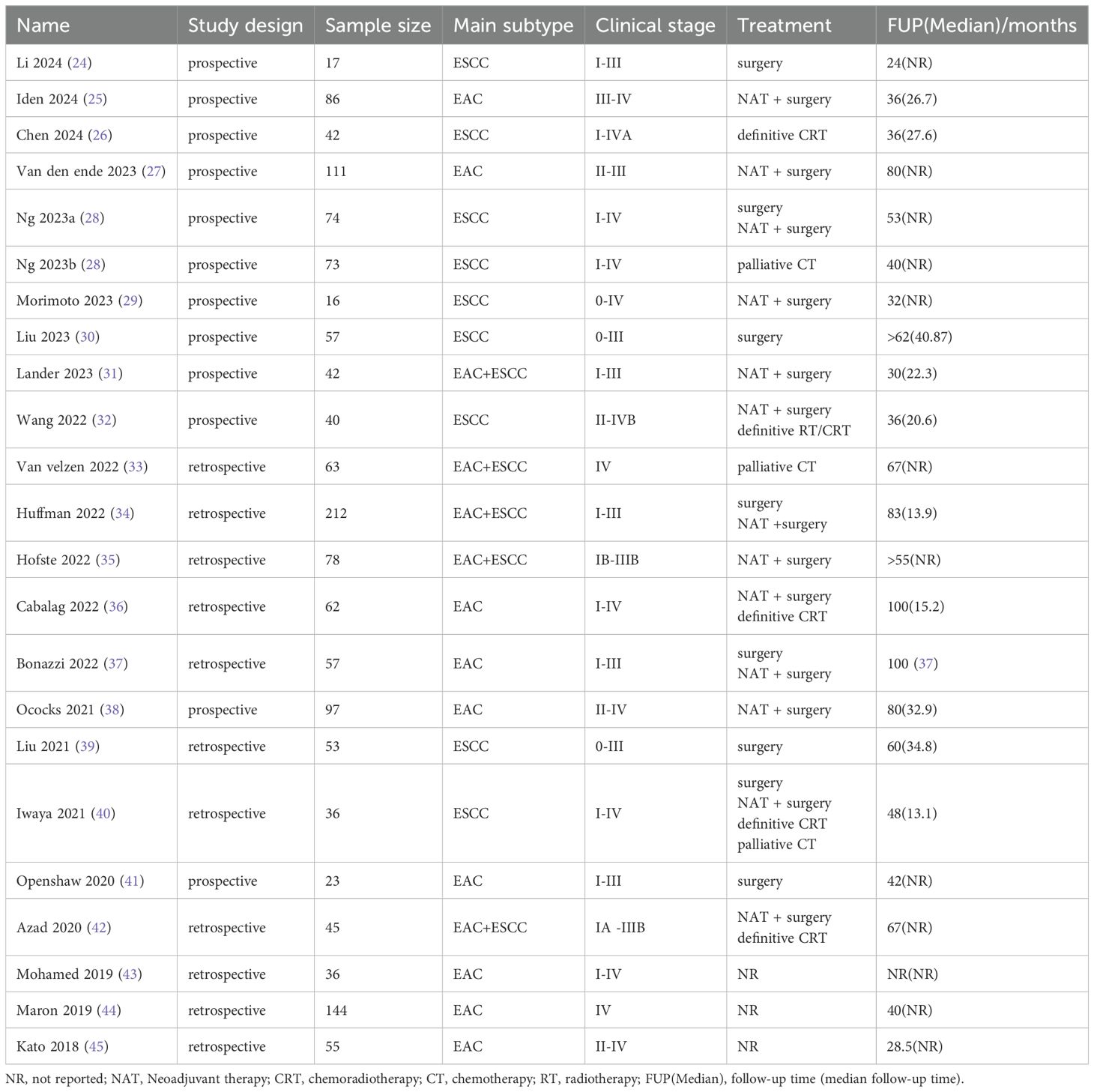
From the 765 records identified, this systematic review and meta-analysis finally screened for the inclusion of 22 studies (involving 1519 patients) (24–45), including 20 full-length articles and two conference abstracts (31, 43). Supplementary Figure S1 shows a flowchart of the study selection process according to PRISMA. The primary clinical characteristics included in the studies are shown in Table 1. Of the included studies, 11 were prospective, and 11 were retrospective. 9 studies focused on EAC, eight on ESCC, and the remaining five on EAC+ESCC. Based on TNM staging, it was estimated that approximately 74.0% of the study population were in stage 0-III, and 26.0% were in stage IV. These studies included the following treatment modalities: (1) curative surgery (24, 28, 30, 34, 37, 39–41); (2) neoadjuvant therapy followed by surgery (25, 27–29, 31, 32, 34–38, 40, 42); (3) definitive radiotherapy/radiochemotherapy (26, 32, 36, 40, 42); and (4) palliative chemotherapy (28, 33, 40). Follow-up time ranges from 24 to 100 months.
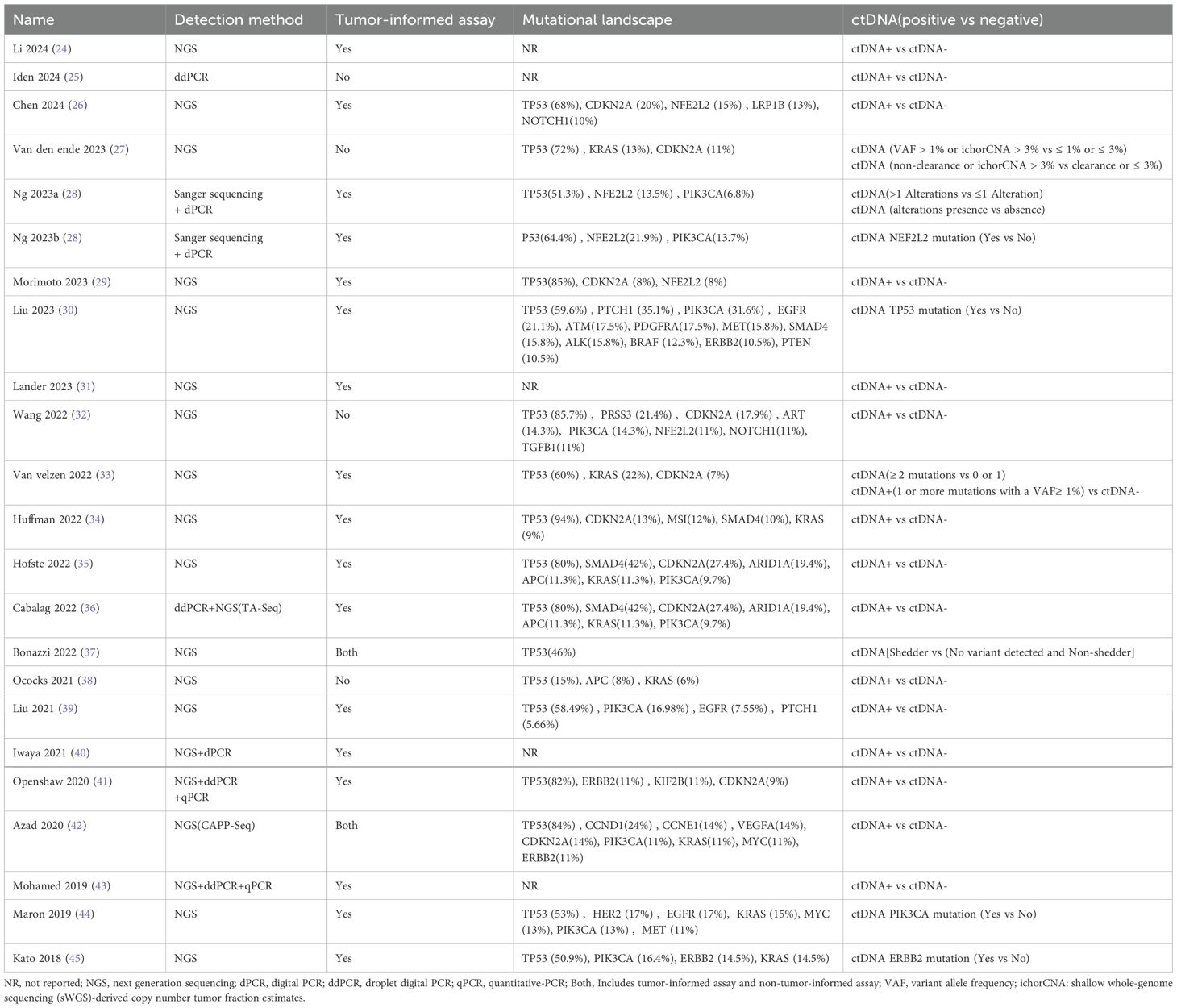
Specific information related to ctDNA in the included studies is presented in Table 2. Detection of ctDNA Two main methods were applied: (1) next-generation sequencing (NGS)-based assays, including TA-Seq and CAPP-Seq. (2) digital PCR (dPCR)–based assays, including droplet digital PCR (ddPCR). Most studies (18/22) had tumor-informed ctDNA assays (24, 26, 28–31, 33–37, 39–45). These 18 studies reported the genetic mutations detected in tumor tissue and/or plasma ctDNA.TP53 was the most commonly detected mutation, either in ESCC or EAC. Most TP53 variants are either missense or nonsense mutations. Other frequently mutated genes include NFE2L2, CDKN2A, PIK3CA, NOTCH1, ERBB2, ARID1A and SMAD4. Notably, most of the highly prevalent mutated genes are tumor suppressor genes. These gene mutations have been partially shown to be associated with poor prognosis in EC patients. The specific definitions of ctDNA-positive/negative status vary across studies. Most studies directly categorize patients into ctDNA-detectable and ctDNA-undetectable groups. At the same time, a minority employ classification methods that include variant allele frequency (VAF) thresholds combined with tumor fraction levels, mutation burden levels, or the presence/absence of specific genetic alterations.
3.2 ctDNA detection at baseline
11 studies (N = 647 patients) and 11 studies (N = 696 patients) reported associations between ctDNA testing at baseline with PFS and OS, respectively. In univariate and multivariate analyses, a positive ctDNA test at baseline was associated with poorer PFS (univariate: HR = 1.64, 95% CI:1.30-2.07, multivariate: HR = 2.88, 95% CI:1.95-4.25) and OS (univariate: HR = 2.02, 95% CI:1.36-2.99, multivariate: HR = 3.79, 95% CI:1.48-9.65) (Figure 1A, 2A, Supplementary Table S3). Similar results were obtained when further subgroup analyses of tumor-informed and non-tumor-informed testing were performed in univariate and multivariate analyses (Figures 1B, C, 2B, C, Supplementary Table S4).In addition, the combined HR of studies using tumor-informed testing in univariate and multivariate analyses was numerically higher or equal to that of studies using non-tumor-informed testing (Figures 1B, C, 2B, C, Supplementary Table S4). A high degree of statistical heterogeneity could be observed across the full range of analyses in which ctDNA was associated with OS, and we used a random-effects model followed by sensitivity analyses by excluding studies one by one, and found that none of the studies significantly altered the primary outcome. Thus, our results are reliable and stable. Supplementary Tables S3 and S4 summarize the associations between ctDNA testing and survival outcomes at different time points.
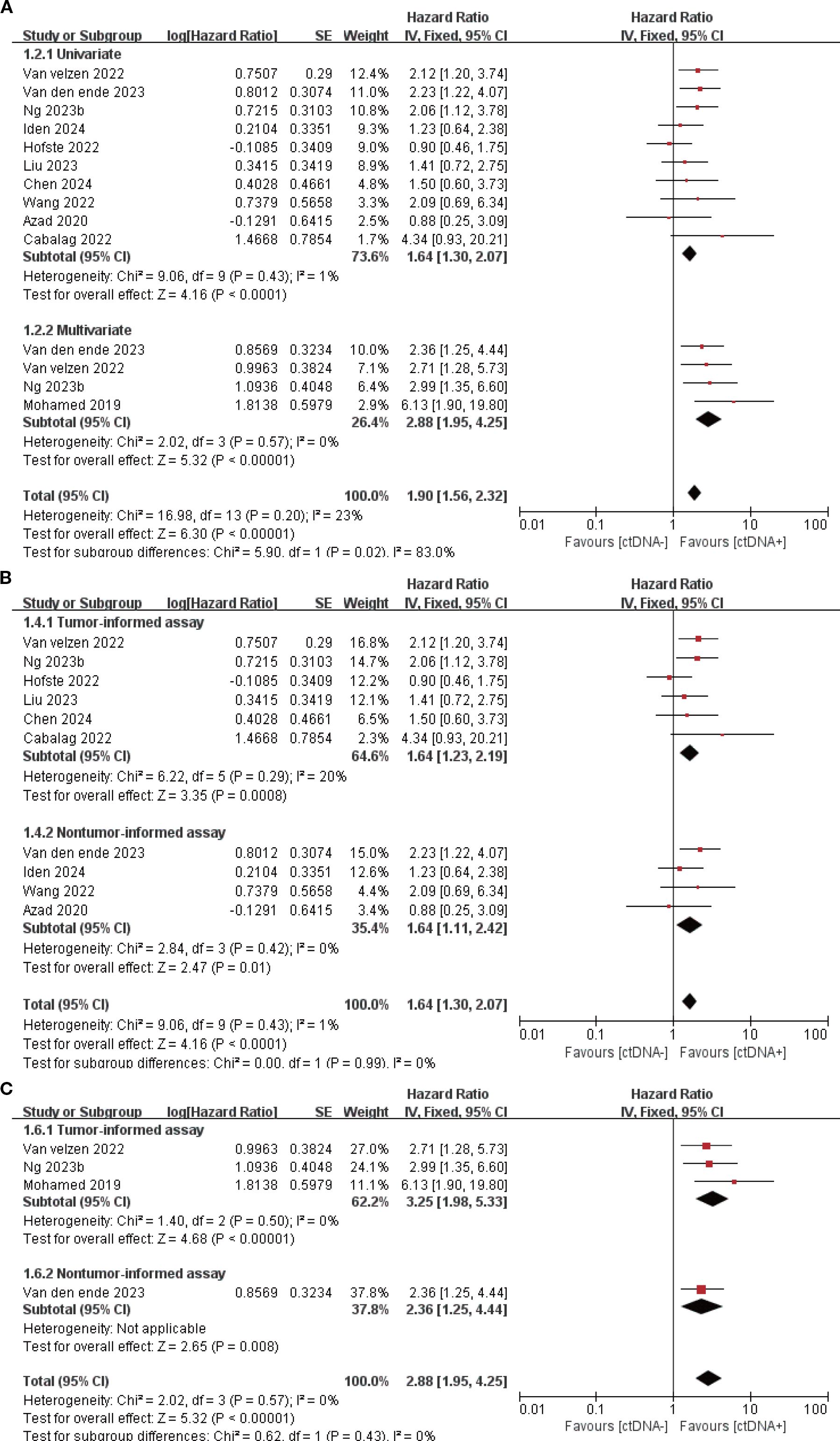
Figure 1. Association of ctDNA testing at baseline with PFS (fixed-effects model). (A) Univariate and multivariate analyses. Subgroup analyses of tumor-informed and non-tumor-informed assays in univariate (B) and multivariate (C) analyses.
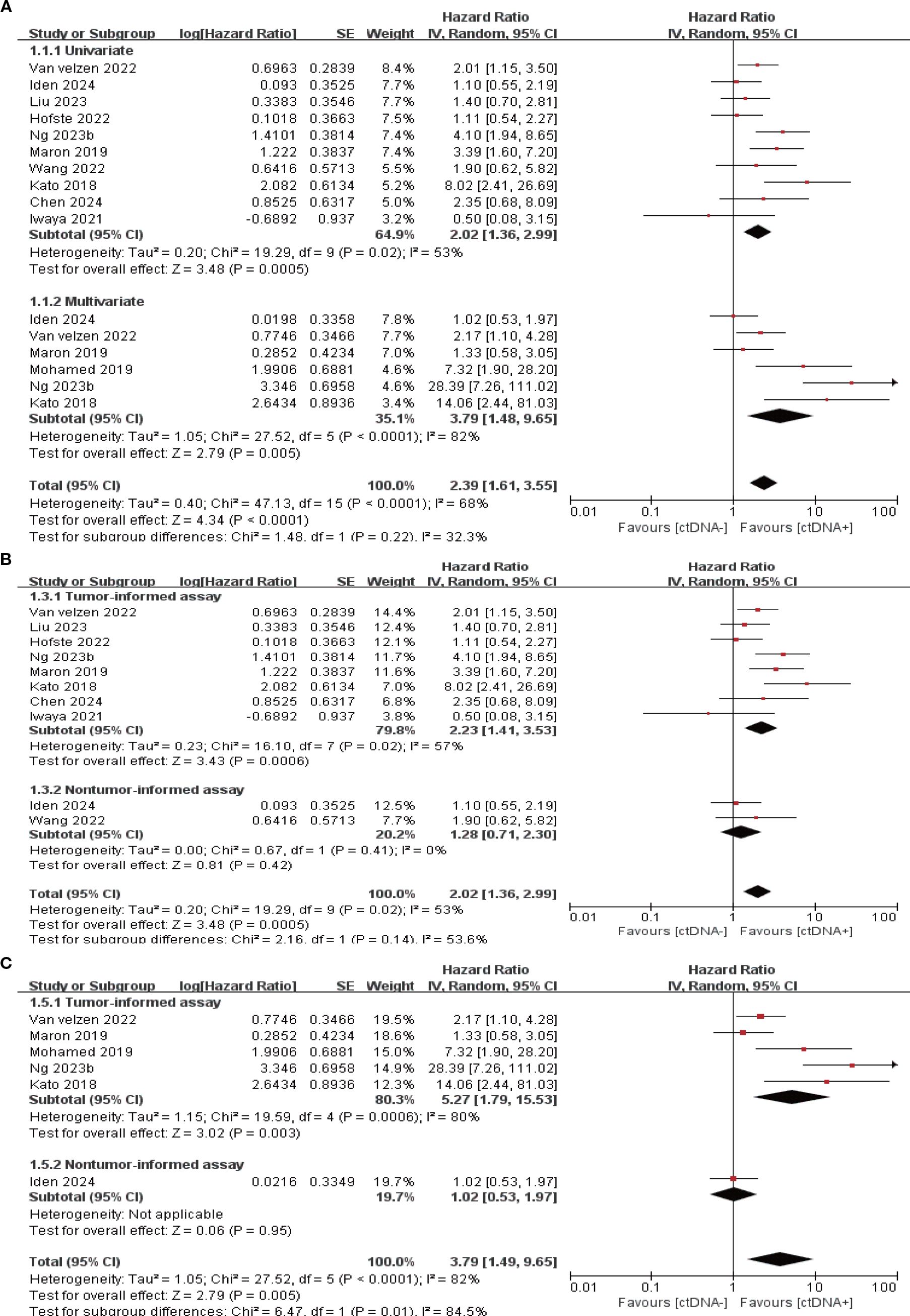
Figure 2. Association of ctDNA testing at baseline with OS (random-effects model). (A) Univariate and multivariate analyses. Subgroup analyses of tumor-informed and non-tumor-informed assays in univariate (B) and multivariate (C) analyses.
3.3 ctDNA detection after neoadjuvant therapy
7 studies (N = 301 patients) and 6 studies (N = 225 patients) reported the association between ctDNA testing after neoadjuvant therapy with PFS and OS, respectively. In univariate and multivariate analyses, a positive ctDNA test after neoadjuvant therapy was associated with poorer PFS (univariate: HR = 3.97, 95% CI:2.68-5.88, multivariate: HR = 4.21, 95% CI:2.67-6.64) and OS (univariate: HR = 3.41, 95% CI:2.08-5.59, multivariate: HR = 2.70, 95% CI:1.34-5.41) (Figures 3A, 4A, Supplementary Table S3). Similar results were obtained when further subgroup analyses of tumor-informed and non-tumor-informed testing were performed in univariate and multivariate analyses (Figures 3B, C, 4B, C, Supplementary Table S4). In addition, the combined HR was numerically higher for most of the studies using tumor-informed testing in univariate and multivariate analyses than for non-tumor-informed testing (Figures 3B, 4B, C, Supplementary Table S4). In addition, we found that the combined univariate and multivariate HRs for PFS/OS were numerically higher when ctDNA was detected after neoadjuvant therapy(PFS: HR = 4.07, OS: HR = 3.15) than at baseline (PFS: HR = 1.90, OS: HR = 2.39) (Supplementary Table S3).
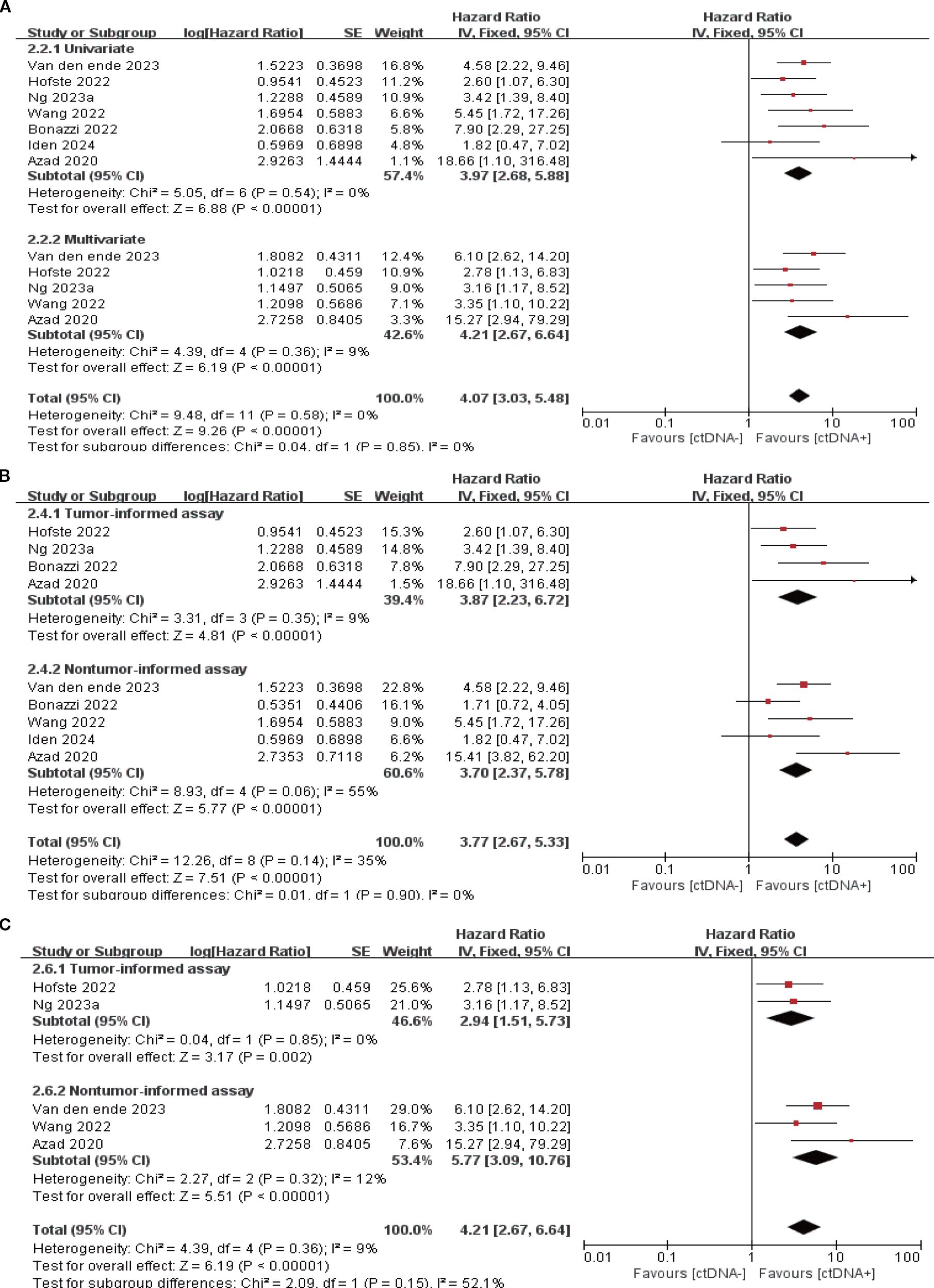
Figure 3. Association of ctDNA testing after neoadjuvant therapy with PFS (fixed-effects model). (A) Univariate and multivariate analyses. Subgroup analyses of tumor-informed and non-tumor-informed assays in univariate (B) and multivariate (C) analyses.
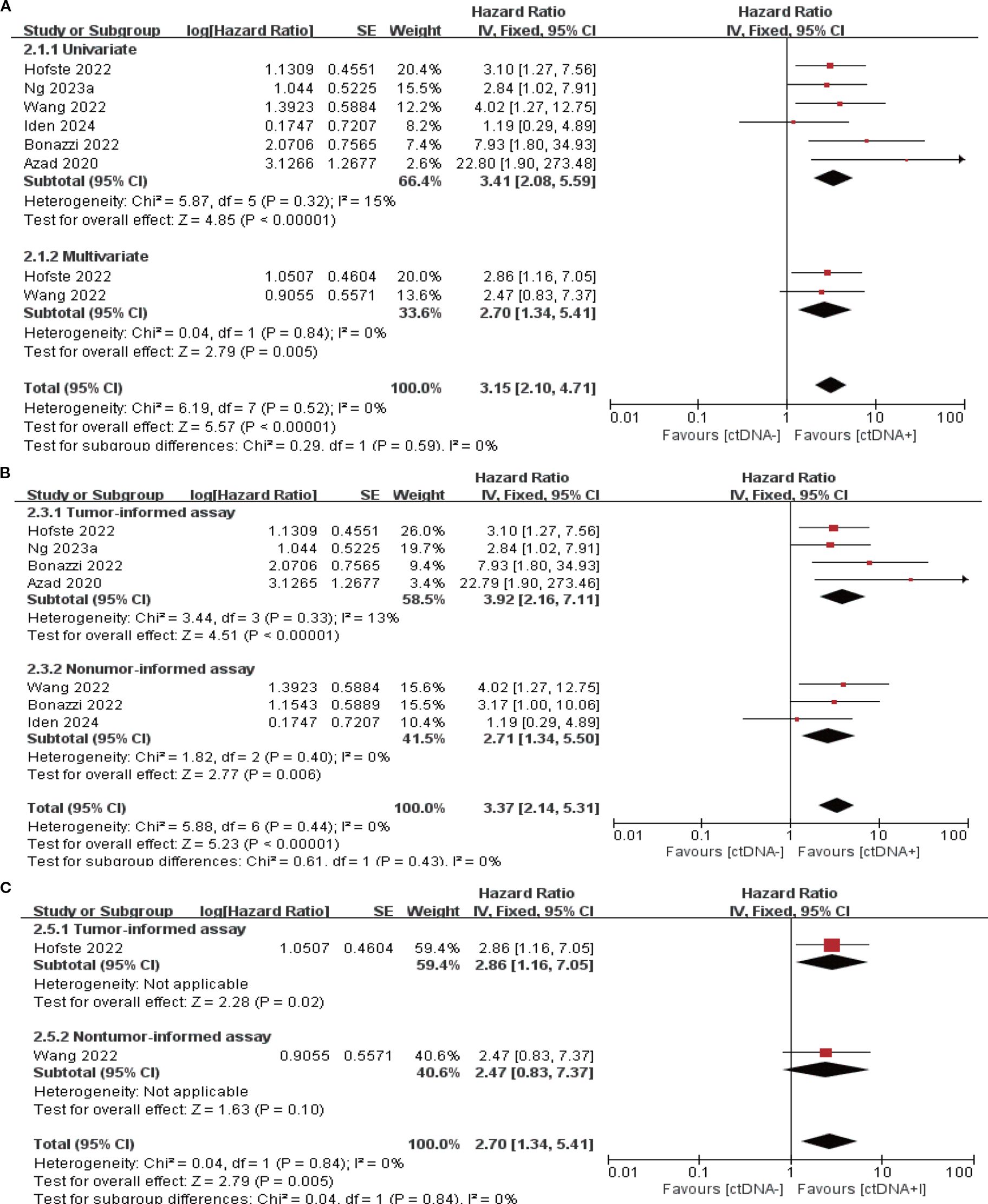
Figure 4. Association of ctDNA testing after neoadjuvant therapy with OS (fixed-effects model). (A) Univariate and multivariate analyses. Subgroup analyses of tumor-informed and non-tumor-informed assays in univariate (B) and multivariate (C) analyses.
3.4 ctDNA detection during the follow-up period
14 studies (N = 470 patients) and 8 studies (N = 274 patients) reported the association between ctDNA testing at follow-up with PFS and OS, respectively. In univariate and multivariate analyses, a positive ctDNA test at follow-up was associated with poorer PFS (univariate: HR = 5.42, 95% CI:3.97-7.38, multivariate: HR = 4.11, 95% CI:1.86-9.08) and OS (univariate: HR = 4.93, 95% CI:3.31-7.34, multivariate: HR = 6.69, 95% CI:3.54-12.62) (Figure 5A, 6A, Supplementary Table S3). Similar results were obtained when further subgroup analyses of tumor-informed and non-tumor-informed testing were performed in univariate and multivariate analyses (Figures 5B, C, 6B, C, Supplementary Table S4). In addition, we found that the combined univariate and multivariate HRs for PFS/OS were numerically higher when ctDNA was detected during follow-up (PFS: HR = 5.22, OS: HR = 5.37) than after neoadjuvant therapy (PFS: HR = 4.07, OS: HR = 3.15) and at baseline (PFS: HR = 1.90, OS: HR = 2.39) (Supplementary Table S3).
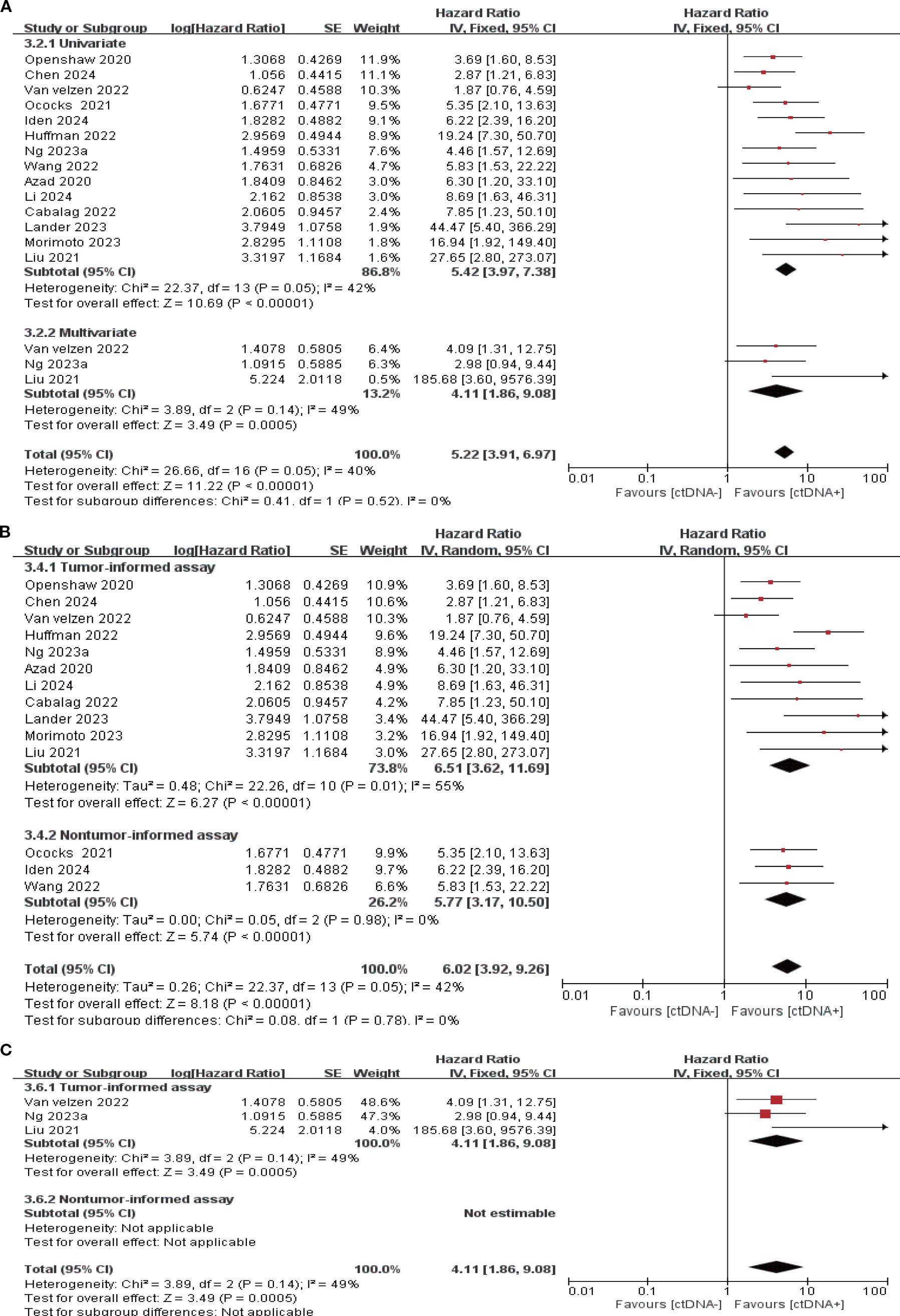
Figure 5. Association of ctDNA testing during follow-up with PFS (fixed/random-effects model). (A) Univariate and multivariate analyses. Subgroup analyses of tumor-informed and non-tumor-informed assays in univariate (B) and multivariate (C) analyses.
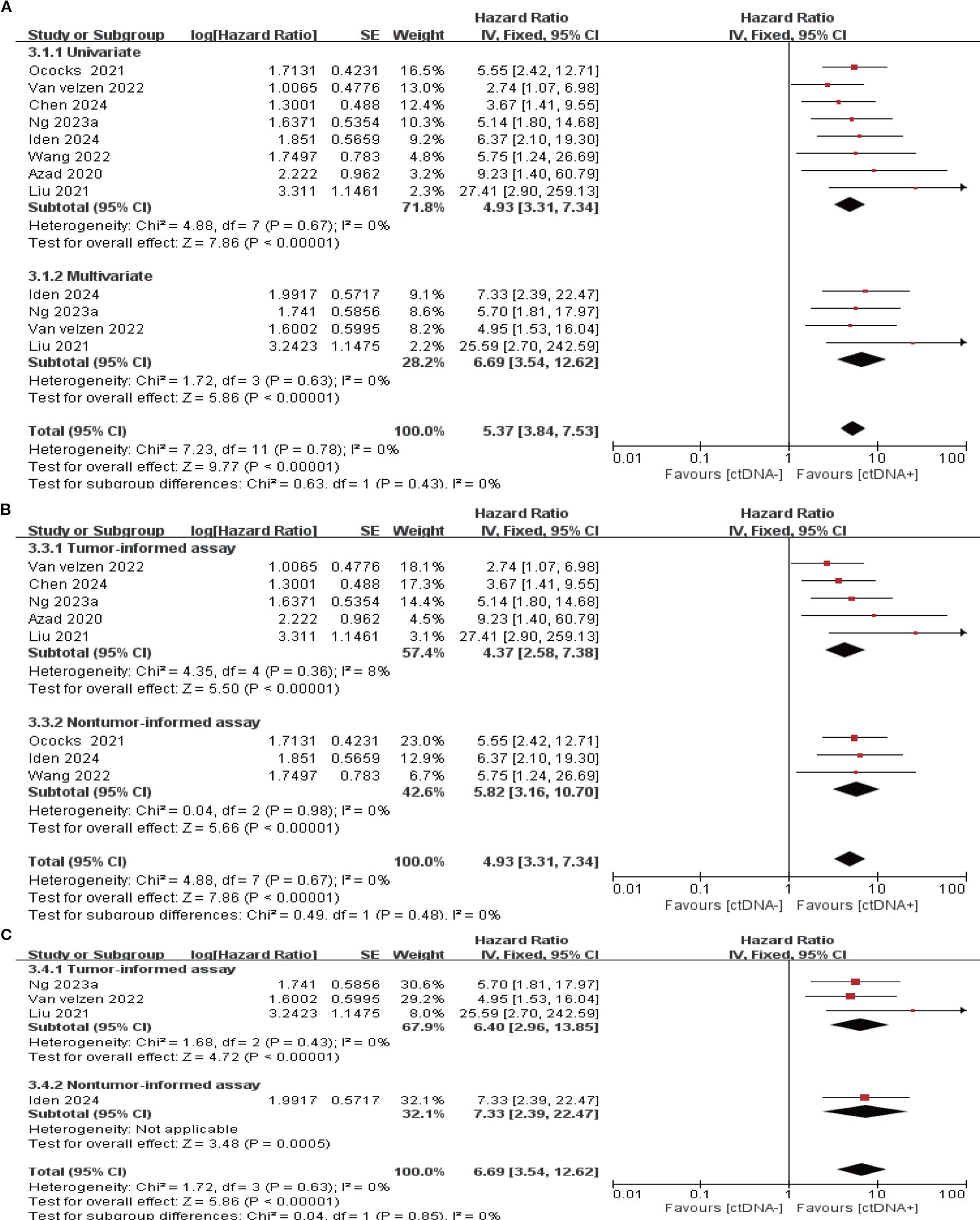
Figure 6. Association of ctDNA testing during follow-up with OS (fixed-effects model). (A) Univariate and multivariate analyses. Subgroup analyses of tumor-informed and non-tumor-informed assays in univariate (B) and multivariate (C) analyses.
3.5 Lead time
There are 9 studies (26, 28, 29, 32, 36, 40-43) comparing the mean time to the prediction of clinical recurrence by ctDNA testing with conventional radiological imaging techniques (e.g., CT, PET-CT). The results showed that ctDNA test positivity predicted clinical recurrence on average 4.53 months earlier (range: 0.98-11.6 months) than imaging techniques.
3.6 Risk of bias assessment and publication bias
We used the Newcastle-Ottawa scale to assess the risk of bias for the included studies. The results showed that all 22 included studies scored≥6. Supplementary Table S5 comprehensively describes the results of the quality assessment of the included literature. Publication bias was assessed using a funnel plot for the univariate analysis of baseline PFS, baseline OS, and follow-up PFS (Supplementary Figure S2). The results suggest the possibility of publication bias in the univariate analysis of follow-up PFS.
4 Discussion
This study differs from previous systematic reviews and meta-analyses in that it systematically elucidates the prognostic value of ctDNA in EC from a dynamic temporal perspective. By employing univariate and multivariate statistical methods combined with tumor-informed and non-informed analysis strategies, we provide a more comprehensive, time-specific basis for risk stratification and efficacy assessment.
Genomic alterations are key drivers of EC development and metastasis (46). Studies indicate that the most frequently mutated genes in ESCC include TP53, NFE2L2, and MLL2, while EAC predominantly involves TP53, CDKN2A, and ARID1A (47). Among these, TP53 exhibits the highest mutation rate in both subtypes (47–49) and correlates with poorer survival outcomes (50). Since Hsieh et al. (51) first reported the association between plasma cell-free DNA and EC recurrence and survival, numerous prospective and retrospective studies have focused on the prognostic value of ctDNA in EC. This systematic review and meta-analysis primarily assessed the clinical significance of ctDNA monitoring at three time points: baseline, after neoadjuvant therapy, and during follow-up.
Baseline ctDNA positivity is often associated with greater tumor burden and more advanced clinical staging (25–27, 33, 35, 43), suggesting its potential as a biomarker reflecting EC tumor burden and assessing prognosis. Although some included studies did not find a significant association with prognosis (25, 26, 32, 35, 40), others reported a clear correlation (27, 28, 33, 36, 43). The pooled results of this meta-analysis indicate that baseline ctDNA positivity is a significant risk factor for recurrence and mortality in EC patients, consistent with reports in other cancer types such as breast and lung cancer (15, 52, 53). Further analysis revealed that ctDNA positivity detected after neoadjuvant therapy and during follow-up was also significantly associated with higher risks of recurrence and mortality. Notably, the combined hazard ratio (HR) for recurrence or death following ctDNA positivity after neoadjuvant therapy was approximately 1–2 times that of baseline status, suggesting superior predictive efficacy. The HR for ctDNA positivity during follow-up further increased to 1–2 times that after neoadjuvant therapy, indicating that its prognostic value progressively escalates over time, peaking during follow-up. This precisely quantified pattern of risk evolution, systematically elucidated for the first time in the EC field through meta-analysis, highlights the significant advantage of dynamic monitoring over single-time-point detection. Notably, this pattern of risk change highly correlates with the dynamic clearance pattern of ctDNA. Multiple studies consistently demonstrate that a decrease or clearance of ctDNA levels during or after treatment is significantly associated with improved patient survival, with those maintaining persistent negativity or achieving clearance exhibiting better prognosis (25, 26, 32). Therefore, our discovery of this dynamic pattern represents a significant addition to previous review conclusions and provides a solid foundation for the clinical translation of ctDNA.
More importantly, viewed from the perspective of tumor ecology and evolution (54), these dynamic patterns reflect the adaptive evolution of tumor clonal populations under therapeutic pressure. Persistent ctDNA positivity after neoadjuvant therapy may indicate the selection and amplification of adaptive resistance subclones, while its clearance may reflect effective suppression of the dominant clone by treatment. This understanding extends beyond the static interpretation framework of traditional molecular biomarkers, situating ctDNA dynamics within the ecological system of tumor-microenvironment-therapy interactions. It provides a novel theoretical perspective for deepening our understanding of treatment response and resistance mechanisms.
Based on these time-specific risk quantification evidence and theoretical understanding, persistent ctDNA positivity after neoadjuvant therapy should be regarded as a strong indicator of tumor resistance to treatment and extremely high recurrence risk. Regardless of imaging findings, these patients should be considered for intensified adjuvant therapy (e.g., immunotherapy, extended chemotherapy, or targeted therapy) to eradicate residual disease. For ctDNA positivity emerging during follow-up, immediate initiation of more frequent imaging (e.g., PET-CT) is warranted, along with exploring preemptive treatment before clinical recurrence. This study provides evidence-based support for incorporating ctDNA-guided temporal stratification into the comprehensive management of EC patients, advancing from prognostic assessment to personalized intervention. However, the primary challenge to clinical translation remains the absence of standardized detection thresholds and unified reporting standards. Future research should focus on establishing clinically actionable criteria for ctDNA positivity at various time points. Prospective intervention trials—such as randomized controlled trials evaluating intensified treatment strategies for ctDNA-positive patients—are needed to validate the actual survival benefit. Ongoing clinical trials (e.g., NCT06914011, NCT05965479) provide an initial framework for ctDNA-guided therapy.
In colorectal and breast cancers, tumor-informed ctDNA testing demonstrated superior performance compared to non-tumor-informed assay, exhibiting higher sensitivity (52, 55, 56). This study found that the prognostic value of ctDNA derived from both testing methods was generally consistent across three time points: baseline, after neoadjuvant therapy, and follow-up. Notably, at baseline and after neoadjuvant therapy stages, the hazard ratios (HRs) for recurrence and mortality risk derived from the tumor-informed assay tended to be numerically higher than those from the non-tumor-informed assay, suggesting potential advantages during periods of high tumor burden due to greater sensitivity. However, given the limited research data and high uncertainty surrounding the non-tumor-informed assay, the actual effect of the tumor-informed assay may be overestimated. The conclusion presented here is only a preliminary hypothesis: a tumor-informed assay may be more sensitive in identifying high-risk recurrence and mortality in EC patients. Currently, systematic comparative studies in the EC field are lacking, and further research is needed to clarify the differences and clinical applicability of these two strategies.
During the tumor-free interval following treatment, when imaging fails to detect lesions, serial liquid biopsies can be used for early prediction of recurrence risk. This study found that in EC, the emergence of ctDNA positivity occurred on average 4.53 months earlier than imaging detection. This trend has been consistently reported across multiple tumor types, including breast cancer and colorectal cancer (52, 57, 58). Current clinical guidelines still regard imaging as the gold standard for recurrence diagnosis and treatment decisions. It is important to note that intervention based solely on ctDNA positivity carries risks, such as false positives due to clonal hematopoiesis or technical errors, which may lead to overtreatment. Therefore, after detecting ctDNA positivity, it is recommended to promptly combine imaging examinations for careful evaluation to ensure robust clinical decision-making and patient safety. Furthermore, the current early warning value of ctDNA remains based on limited studies and requires further validation across diverse populations and healthcare settings. Future large-scale, rigorously designed multicenter prospective studies are necessary to confirm these findings.
This study has several limitations. First, outcome measures were grouped into two broad categories—”recurrence-related (including PFS, DFS, RFS, and FFP)” and “mortality-related (including OS, DSS, and CSD)”—resulting in a certain lack of outcome consistency. Second, criteria for defining ctDNA positivity varied across studies, and the absence of standardized testing protocols reduced comparability of results. Third, the study did not perform stratified analyses by histological subtype (EAC vs. ESCC) or geographic region (Asia vs. Western countries). Given the significant molecular and clinical differences between these subtypes, along with regional heterogeneity in clinical practice and epidemiological backgrounds, this limitation restricts the generalizability of findings and the interpretability of subgroup analyses. Additionally, substantial heterogeneity existed in baseline OS analyses. Although random-effects models and sensitivity analyses were employed to ensure robustness, interpretation of results may still be affected. At the same time, the studies included primarily utilized univariate HRs, which may introduce residual confounding bias. This heterogeneity and bias primarily stem from clinical and methodological variations. Moreover, the included studies were evenly split between prospective and retrospective designs. Although quality assessments controlled for bias, further high-quality prospective studies are needed for validation. Finally, despite comprehensive search strategies, publication bias may still exist in some analyses. Given these limitations, future research should focus on: ① Establishing unified standards for ctDNA detection and reporting to enhance comparability of results; ② Conducting large-scale, multicenter, prospective studies targeting specific histological subtypes and regional populations, and integrating multidimensional data for individual patient data (IPD)-based meta-analyses to elucidate interactions among factors and more accurately assess the prognostic value of ctDNA; ③ Collectively enhancing the reliability and clinical applicability of relevant evidence through the aforementioned measures.
5 Conclusion
Throughout the entire treatment course, positive ctDNA detection consistently correlated with poor prognosis in EC patients. Notably, its prognostic value increased over time, continuously strengthening from baseline to follow-up stages. This study supports ctDNA as an effective dynamic prognostic biomarker and preliminarily explores its potential feasibility for integration into the treatment management workflow for EC patients.measures
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author/s.
Author contributions
MW: Writing – original draft, Investigation, Data curation, Conceptualization, Formal Analysis, Visualization, Validation, Methodology. CX: Writing – original draft, Methodology, Formal Analysis, Investigation. SW: Formal Analysis, Validation, Writing – review & editing, Investigation. YQ: Writing – review & editing, Data curation, Visualization. ZH: Visualization, Writing – review & editing. PG: Visualization, Conceptualization, Writing – review & editing, Supervision, Project administration.
Funding
The author(s) declare that no financial support was received for the research, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2025.1608872/full#supplementary-material
References
1. Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2024) 74:229–63. doi: 10.3322/caac.21834
2. Allemani C, Matsuda T, Di Carlo V, Harewood R, Matz M, Nikšić M, et al. Global surveillance of trends in cancer survival 2000-14 (Concord-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. (2018) 391:1023–75. doi: 10.1016/s0140-6736(17)33326-3
3. Pennathur A, Gibson MK, Jobe BA, and Luketich JD. Oesophageal carcinoma. Lancet. (2013) 381:400–12. doi: 10.1016/s0140-6736(12)60643-6
4. Cescon DW, Bratman SV, Chan SM, and Siu LL. Circulating tumor DNA and liquid biopsy in oncology. Nat Cancer. (2020) 1:276–90. doi: 10.1038/s43018-020-0043-5
5. Jahr S, Hentze H, Englisch S, Hardt D, Fackelmayer FO, Hesch RD, et al. DNA fragments in the blood plasma of cancer patients: quantitations and evidence for their origin from apoptotic and necrotic cells. Cancer Res. (2001) 61:1659–65. doi: 10.1002/1097-0142(20010215)91:4<874::AID-CNCR1076>3.0.CO;2-O
6. Thierry AR, Mouliere F, Gongora C, Ollier J, Robert B, Ychou M, et al. Origin and quantification of circulating DNA in mice with human colorectal cancer xenografts. Nucleic Acids Res. (2010) 38:6159–75. doi: 10.1093/nar/gkq421
7. Rostami A, Lambie M, Yu CW, Stambolic V, Waldron JN, and Bratman SV. Senescence, necrosis, and apoptosis govern circulating cell-free DNA release kinetics. Cell Rep. (2020) 31:107830. doi: 10.1016/j.celrep.2020.107830
8. Cheng F, Su L, and Qian C. Circulating tumor DNA: A promising biomarker in the liquid biopsy of cancer. Oncotarget. (2016) 7:48832–41. doi: 10.18632/oncotarget.9453
9. Lee MS, Kaseb AO, and Pant S. The emerging role of circulating tumor DNA in non-colorectal gastrointestinal cancers. Clin Cancer Res. (2023) 29:3267–74. doi: 10.1158/1078-0432.Ccr-22-3626
10. Dawson SJ, Tsui DW, Murtaza M, Biggs H, Rueda OM, Chin SF, et al. Analysis of circulating tumor DNA to monitor metastatic breast cancer. N Engl J Med. (2013) 368:1199–209. doi: 10.1056/NEJMoa1213261
11. Bettegowda C, Sausen M, Leary RJ, Kinde I, Wang Y, Agrawal N, et al. Detection of circulating tumor DNA in early- and late-stage human Malignancies. Sci Transl Med. (2014) 6:224ra24. doi: 10.1126/scitranslmed.3007094
12. Cohen JD, Li L, Wang Y, Thoburn C, Afsari B, Danilova L, et al. Detection and localization of surgically resectable cancers with a multi-analyte blood test. Science. (2018) 359:926–30. doi: 10.1126/science.aar3247
13. McDonald BR, Contente-Cuomo T, Sammut SJ, Odenheimer-Bergman A, Ernst B, Perdigones N, et al. Personalized circulating tumor DNA analysis to detect residual disease after neoadjuvant therapy in breast cancer. Sci Transl Med. (2019) 11:eaax7392. doi: 10.1126/scitranslmed.aax7392
14. Al-Showbaki L, Wilson B, Tamimi F, Molto C, Mittal A, Cescon DW, et al. Changes in circulating tumor DNA and outcomes in solid tumors treated with immune checkpoint inhibitors: A systematic review. J Immunother Cancer. (2023) 11:e005854. doi: 10.1136/jitc-2022-005854
15. Chen D, Guo J, Huang H, Tian L, Xie Y, and Wu Q. Prognostic value of circulating tumor DNA in operable non-small cell lung cancer: A systematic review and reconstructed individual patient-data based meta-analysis. BMC Med. (2023) 21:467. doi: 10.1186/s12916-023-03181-2
16. Chidambaram S and Markar SR. Clinical utility and applicability of circulating tumor DNA testing in esophageal cancer: A systematic review and meta-analysis. Dis Esophagus. (2022) 35:doab046. doi: 10.1093/dote/doab046
17. Zhang Y, Du H, Wang N, Wang L, and Huang Y. An update of clinical value of circulating tumor DNA in esophageal cancer: A systematic review and meta-analysis. BMC Cancer. (2024) 24:129. doi: 10.1186/s12885-024-11879-6
18. Zhang H, Jin T, Peng Y, Luan S, Li X, Xiao X, et al. Association between plasma circulating tumor DNA and the prognosis of esophageal cancer patients: A meta-analysis. Int J Surg. (2024) 110:4370–81. doi: 10.1097/js9.0000000000001373
19. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The prisma 2020 statement: an updated guideline for reporting systematic reviews. Bmj. (2021) 372:n71. doi: 10.1136/bmj.n71
20. Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, et al. Amstar 2: A critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. Bmj. (2017) 358:j4008. doi: 10.1136/bmj.j4008
21. Tierney JF, Stewart LA, Ghersi D, Burdett S, and Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. (2007) 8:16. doi: 10.1186/1745-6215-8-16
22. Stang A. Critical evaluation of the newcastle-ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. (2010) 25:603–5. doi: 10.1007/s10654-010-9491-z
23. Campos-Carrillo A, Weitzel JN, Sahoo P, Rockne R, Mokhnatkin JV, Murtaza M, et al. Circulating tumor DNA as an early cancer detection tool. Pharmacol Ther. (2020) 207:107458. doi: 10.1016/j.pharmthera.2019.107458
24. Li X, Liu T, Bacchiocchi A, Li M, Cheng W, Wittkop T, et al. Ultra-sensitive molecular residual disease detection through whole genome sequencing with single-read error correction. EMBO Mol Med. (2024) 16:2188–209. doi: 10.1038/s44321-024-00115-0
25. Iden CR, Mustafa SM, Øgaard N, Henriksen T, Jensen S, Ahlborn LB, et al. Circulating tumor DNA predicts recurrence and survival in patients with resectable gastric and gastroesophageal junction cancer. Gastric Cancer. (2024) 28:83–95. doi: 10.1007/s10120-024-01556-9
26. Chen B, Liu S, Zhu Y, Wang R, Cheng X, Chen B, et al. Predictive role of ctdna in esophageal squamous cell carcinoma receiving definitive chemoradiotherapy combined with toripalimab. Nat Commun. (2024) 15:1919. doi: 10.1038/s41467-024-46307-7
27. van den Ende T, van der Pol Y, Creemers A, Moldovan N, Boers D, van Berge Henegouwen MI, et al. Genome-wide and panel-based cell-free DNA characterization of patients with resectable esophageal adenocarcinoma. J Pathol. (2023) 261:286–97. doi: 10.1002/path.6175
28. Ng HY, Ko JMY, Lam KO, Kwong DLW, Lo AWI, Wong IYH, et al. Circulating tumor DNA dynamics as prognostic markers in locally advanced and metastatic esophageal squamous cell carcinoma. JAMA Surg. (2023) 158:1141–50. doi: 10.1001/jamasurg.2023.4395
29. Morimoto Y, Matsuda S, Kawakubo H, Nakamura K, Kobayashi R, Hisaoka K, et al. Tumor burden monitoring with circulating tumor DNA during treatment in patients with esophageal squamous cell carcinoma. Ann Surg Oncol. (2023) 30:3747–56. doi: 10.1245/s10434-023-13194-z
30. Liu T, Li M, Cheng W, Yao Q, Xue Y, Wang X, et al. A clinical prognostic model for patients with esophageal squamous cell carcinoma based on circulating tumor DNA mutation features. Front Oncol. (2023) 12:1025284. doi: 10.3389/fonc.2022.1025284measures
31. Lander EM, Huffman B, Klempner SJ, Aushev VN, Carbonell JI, Ferguson J, et al. Circulating tumor DNA as a marker of recurrence risk in locoregional esophagogastric cancers with pathologic complete response. J Clin Oncol. (2023) 41:452. doi: 10.1200/jco.2023.41.4_suppl.452
32. Wang X, Yu N, Cheng G, Zhang T, Wang J, Deng L, et al. Prognostic value of circulating tumour DNA during post-radiotherapy surveillance in locally advanced esophageal squamous cell carcinoma. Clin Transl Med. (2022) 12:e1116. doi: 10.1002/ctm2.1116
33. van Velzen MJM, Creemers A, van den Ende T, Schokker S, Krausz S, Reinten RJ, et al. Circulating tumor DNA predicts outcome in metastatic gastroesophageal cancer. Gastric Cancer. (2022) 25:906–15. doi: 10.1007/s10120-022-01313-w
34. Huffman BM, Aushev VN, Budde GL, Chao J, Dayyani F, Hanna D, et al. Analysis of circulating tumor DNA to predict risk of recurrence in patients with esophageal and gastric cancers. JCO Precis Oncol. (2022) 6:e2200420. doi: 10.1200/po.22.00420
35. Hofste LSM, Geerlings MJ, von Rhein D, Tolmeijer SH, Weiss MM, Gilissen C, et al. Circulating tumor DNA-based disease monitoring of patients with locally advanced esophageal cancer. Cancers (Basel). (2022) 14:4417. doi: 10.3390/cancers14184417
36. Cabalag CS, Yates M, Corrales MB, Yeh P, Wong SQ, Zhang BZ, et al. Potential clinical utility of a targeted circulating tumor DNA assay in esophageal adenocarcinoma. Ann Surg. (2022) 276:e120–e6. doi: 10.1097/sla.0000000000005177
37. Bonazzi VF, Aoude LG, Brosda S, Lonie JM, Patel K, Bradford JJ, et al. Ctdna as a biomarker of progression in oesophageal adenocarcinoma. ESMO Open. (2022) 7:100452. doi: 10.1016/j.esmoop.2022.100452
38. Ococks E, Frankell AM, Masque Soler N, Grehan N, Northrop A, Coles H, et al. Longitudinal tracking of 97 esophageal adenocarcinomas using liquid biopsy sampling. Ann Oncol. (2021) 32:522–32. doi: 10.1016/j.annonc.2020.12.010
39. Liu T, Yao Q, and Jin H. Plasma circulating tumor DNA sequencing predicts minimal residual disease in resectable esophageal squamous cell carcinoma. Front Oncol. (2021) 11:616209. doi: 10.3389/fonc.2021.616209
40. Iwaya T, Endo F, Takahashi F, Tokino T, Sasaki Y, and Nishizuka SS. Frequent tumor burden monitoring of esophageal squamous cell carcinoma with circulating tumor DNA using individually designed digital polymerase chain reaction. Gastroenterology. (2021) 160:463–5.e4. doi: 10.1053/j.gastro.2020.09.035
41. Openshaw MR, Suwaidan AA, Ottolini B, Fernandez-Garcia D, Richards CJ, Page K, et al. Longitudinal monitoring of circulating tumour DNA improves prognostication and relapse detection in gastroesophageal adenocarcinoma. Br J Cancer. (2020) 123:1271–9. doi: 10.1038/s41416-020-1002-8
42. Azad TD, Chaudhuri AA, Fang P, Qiao Y, Esfahani MS, Chabon JJ, et al. Circulating tumor DNA analysis for detection of minimal residual disease after chemoradiotherapy for localized esophageal cancer. Gastroenterology. (2020) 158:494–505.e6. doi: 10.1053/j.gastro.2019.10.039
43. Mohamed AA, Openshaw MR, Ottolini B, Guttery D, Garcia DF, Richards CJ, et al. The role of baseline and early dynamics of ctdna in predicting response and prognosis of early and advanced gastroesophageal adenocarcinomas. Cancer Res. (2019) 79. doi: 10.1158/1538-7445.SABCS18-2281
44. Maron SB, Chase LM, Lomnicki S, Kochanny S, Moore KL, Joshi SS, et al. Circulating tumor DNA sequencing analysis of gastroesophageal adenocarcinoma. Clin Cancer Res. (2019) 25:7098–112. doi: 10.1158/1078-0432.Ccr-19-1704
45. Kato S, Okamura R, Baumgartner JM, Patel H, Leichman L, Kelly K, et al. Analysis of circulating tumor DNA and clinical correlates in patients with esophageal, gastroesophageal junction, and gastric adenocarcinoma. Clin Cancer Res. (2018) 24:6248–56. doi: 10.1158/1078-0432.Ccr-18-1128
46. Yang H, Wang F, Hallemeier CL, Lerut T, and Fu J. Oesophageal cancer. Lancet. (2024) 404:1991–2005. doi: 10.1016/s0140-6736(24)02226-8
47. Agency; CGARNAWGAUBC. Integrated genomic characterization of oesophageal carcinoma. Nature. (2017) 541:169–75. doi: 10.1038/nature20805
48. Song Y, Li L, Ou Y, Gao Z, Li E, Li X, et al. Identification of genomic alterations in oesophageal squamous cell cancer. Nature. (2014) 509:91–5. doi: 10.1038/nature13176
49. Gao YB, Chen ZL, Li JG, Hu XD, Shi XJ, Sun ZM, et al. Genetic landscape of esophageal squamous cell carcinoma. Nat Genet. (2014) 46:1097–102. doi: 10.1038/ng.3076
50. Fisher OM, Lord SJ, Falkenback D, Clemons NJ, Eslick GD, and Lord RV. The prognostic value of tp53 mutations in oesophageal adenocarcinoma: A systematic review and meta-analysis. Gut. (2017) 66:399–410. doi: 10.1136/gutjnl-2015-310888
51. Hsieh CC, Hsu HS, Chang SC, and Chen YJ. Circulating cell-free DNA levels could predict oncological outcomes of patients undergoing esophagectomy for esophageal squamous cell carcinoma. Int J Mol Sci. (2016) 17:2131. doi: 10.3390/ijms17122131
52. Nader-Marta G, Monteforte M, Agostinetto E, Cinquini M, Martins-Branco D, Langouo M, et al. Circulating tumor DNA for predicting recurrence in patients with operable breast cancer: A systematic review and meta-analysis. ESMO Open. (2024) 9:102390. doi: 10.1016/j.esmoop.2024.102390
53. Liu H, Chen J, Huang Y, Zhang Y, Ni Y, Xu N, et al. Prognostic significance of circulating tumor DNA in urothelial carcinoma: A systematic review and meta-analysis. Int J Surg. (2024) 110:3923–36. doi: 10.1097/js9.0000000000001372
54. Luo WR. Rethinking cancer. Zhonghua Zhong Liu Za Zhi. (2025) 47:463–7. doi: 10.3760/cma.j.cn112152-20250401-00145
55. Chan HT, Nagayama S, Otaki M, Chin YM, Fukunaga Y, Ueno M, et al. Tumor-informed or tumor-agnostic circulating tumor DNA as a biomarker for risk of recurrence in resected colorectal cancer patients. Front Oncol. (2022) 12:1055968. doi: 10.3389/fonc.2022.1055968
56. Santonja A, Cooper WN, Eldridge MD, Edwards PAW, Morris JA, Edwards AR, et al. Comparison of tumor-informed and tumor-naïve sequencing assays for ctdna detection in breast cancer. EMBO Mol Med. (2023) 15:e16505. doi: 10.15252/emmm.202216505
57. Henriksen TV, Tarazona N, Frydendahl A, Reinert T, Gimeno-Valiente F, Carbonell-Asins JA, et al. Circulating tumor DNA in stage iii colorectal cancer, beyond minimal residual disease detection, toward assessment of adjuvant therapy efficacy and clinical behavior of recurrences. Clin Cancer Res. (2022) 28:507–17. doi: 10.1158/1078-0432.Ccr-21-2404
Keywords: esophageal cancer, liquid biopsy, circulating tumor DNA, prognostic biomarker, systematic review and meta-analysis, tumor-informed assay
Citation: Wang M, Xiong C, Wang S, Qiu Y, Hou Z and Gao P (2025) Circulating tumor DNA predicts prognosis at different time points in patients with esophageal cancer: a systematic review and meta-analysis. Front. Oncol. 15:1608872. doi: 10.3389/fonc.2025.1608872
Received: 09 April 2025; Accepted: 08 September 2025;
Published: 25 September 2025.
Edited by:
Weiren Luo, The Second Affiliated hospital of Southern University of Science and Technology, ChinaReviewed by:
Xiaobin Shang, Chinese Academy of Medical Sciences and Peking Union Medical College, ChinaShutao Zheng, Xinjiang Medical University, China
Copyright © 2025 Wang, Xiong, Wang, Qiu, Hou and Gao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Peng Gao, Z3VsaV95c0AxNjMuY29t
 Min Wang
Min Wang Chunbin Xiong1
Chunbin Xiong1 Yu Qiu
Yu Qiu Peng Gao
Peng Gao