- 1Cancer Institute, Xuzhou Medical University, Xuzhou, Jiangsu, China
- 2Center of Clinical Oncology, The Affiliated Hospital of Xuzhou Medical University, Xuzhou, Jiangsu, China
- 3Jiangsu Center for the Collaboration and Innovation of Cancer Biotherapy, Cancer Institute, Xuzhou Medical University, Xuzhou, Jiangsu, China
Cancer immunotherapy represents a paradigm shift in oncology by leveraging the immune system to target tumors. The therapeutic efficacy of these approaches depends critically on identifying molecular targets that enhance treatment responses while limiting toxicity. CD70, a TNF family member, has emerged as a promising target due to its overexpression in hematologic malignancies and solid tumors contrasted with restricted expression in healthy tissues. This differential expression profile implies that CD70-directed therapies could achieve tumor-specific cytotoxicity with reduced off-target effects. Nevertheless, key challenges persist, including optimizing delivery systems and elucidating the immunological consequences of CD70 modulation. This review synthesizes recent progress in CD70-targeted immunotherapy, evaluating both its therapeutic potential and current constraints to guide future clinical translation.
1 Introduction
Cancer immunotherapy has redefined oncology by mobilizing the immune system against malignancies (1). Its clinical successes across diverse cancers highlight the need to identify molecular targets that maximize therapeutic benefit while mitigating adverse events. CD70, a TNF family protein, exemplifies such a target with emerging therapeutic relevance.
As a type II transmembrane protein, CD70 activates T and B cell proliferation and differentiation through CD27 engagement, thereby modulating immune responses (2). While constitutively suppressed in normal tissues, CD70 becomes aberrantly expressed in hematologic and solid tumors (2), positioning it as a compelling target for immunotherapy. Anti-CD70 monoclonal antibodies suppress tumor progression by augmenting immune-mediated cytotoxicity (3–5). Combinatorial regimens incorporating CD70-targeted agents exhibit synergistic effects with conventional therapies. CD70-directed CAR T cells display precise antitumor activity, while bispecific CAR T cells co-targeting CD70 and immune effectors amplify tumoricidal responses (6, 7).
This review critically assesses CD70’s role in cancer immunotherapy by analyzing its mechanistic contributions to tumorigenesis and surveying current targeting strategies. We evaluate the clinical promise and limitations of these approaches, underscoring CD70’s significance in advancing therapeutic innovation. A deeper understanding of CD70 biology may catalyze the development of precision immunotherapies to improve cancer outcomes.
2 Expression of CD70 and CD27
CD70, a TNF family member, functions as a type II transmembrane glycoprotein with a 50 kDa molecular weight that forms trimers. Under physiological conditions, CD70 expression occurs transiently in antigen-activated B and T cells, natural killer (NK) cells, and mature dendritic cells (DCs) (2). Hematological malignancies demonstrate co-expression of CD70 and CD27 in leukemia, B-cell lymphoma, multiple myeloma, and T-cell lymphoma (2). CD70 also appears in solid tumors such as renal cell carcinoma, nasopharyngeal carcinoma, glioblastoma, melanoma, and carcinomas of the lung, cervix, breast, ovary, and mesothelium (8–13).
CD27, a TNFR family member, is a 55 kDa type I transmembrane protein that forms dimers. Physiologically, CD27 localizes to naive T cells, memory B and T cells, and certain NK cell subsets (2). In hematological malignancies, tumor cells co-expressing CD27 and CD70 evade immune surveillance within the tumor microenvironment, thereby promoting disease progression (14, 15).
3 The CD70/CD27 axis
The CD70-CD27 axis functions through (CD272)3-(CD703)2 complex formation (16). Axis activation triggers extracellular CD27 cleavage, releasing soluble sCD27 fragments in both physiological and pathological contexts (14).
CD27 signaling engages TRAF2/5 to activate NF-κB and JNK pathways, driving T-cell proliferation, survival and differentiation. While promoting effector and memory T-cell development, this axis suppresses Th17 differentiation. CD70 signaling induces cytokine production in CD4+ and CD8+ T cells. Coquet et al. showed thymic CD27-CD70 interaction prevents regulatory T-cell (Treg) apoptosis, expanding the Treg pool (17). Paradoxically, the axis also triggers apoptosis via Siva and caspase proteins (18)(Figure 1).
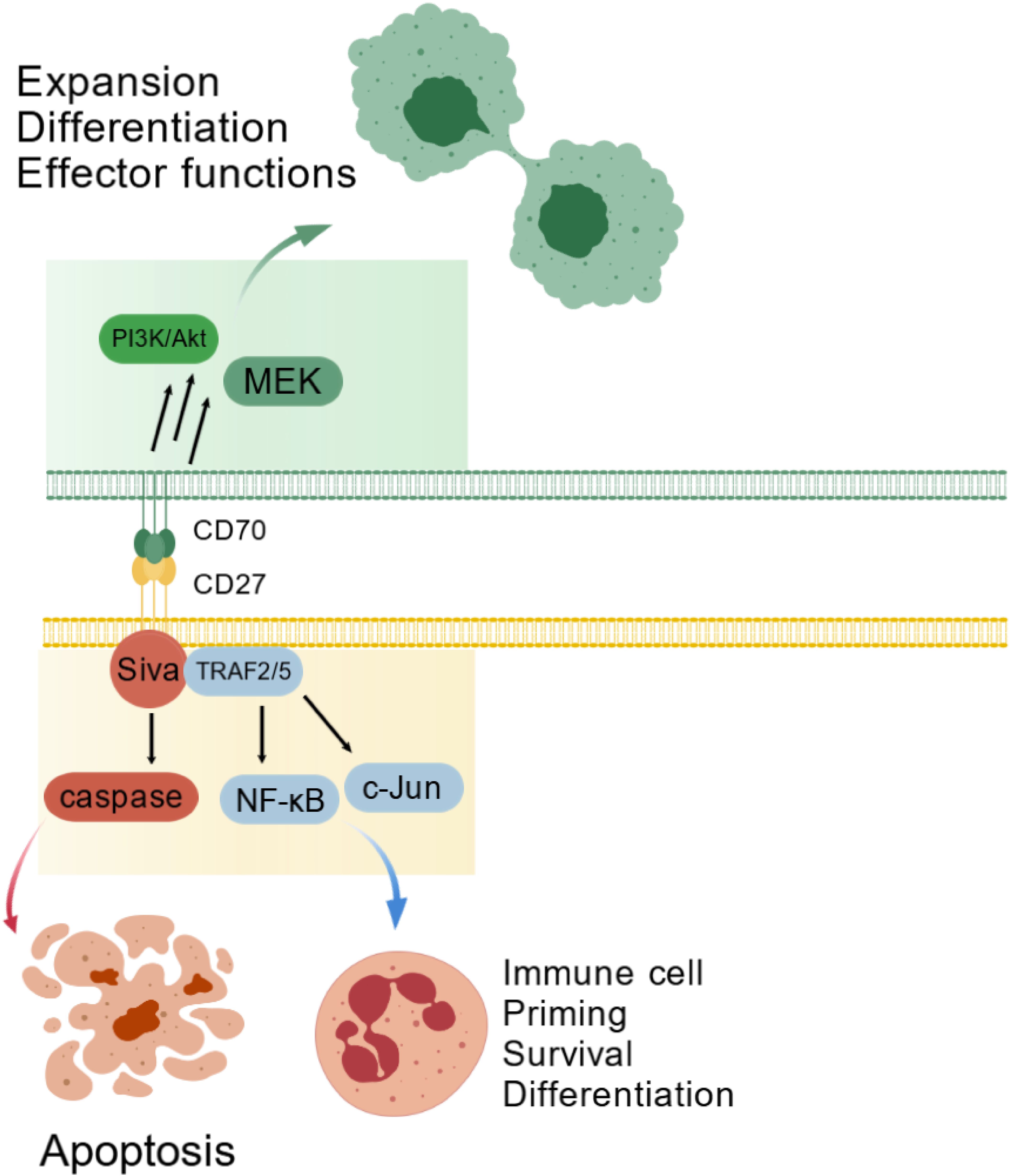
Figure 1. The CD70-CD27 axis in physiological immune regulation. The CD70-CD27 interaction plays a critical role in regulating immune cell proliferation, survival, and differentiation through TRAF2/5-mediated activation of the NF-κB and c-Jun signaling pathways. Conversely, this interaction may also trigger apoptosis via Siva-dependent caspase pathway activation. CD70 reverse signaling additionally stimulates the PI3K/Akt pathway, influencing cell survival, differentiation, and effector functions.
In solid tumors, CD70 signaling associates with cancer stemness and epithelial-mesenchymal transition (EMT) by upregulating EMT-related genes (SOX2, CD44, vimentin, Snail, Slug, β-catenin) while activating MAPK and overexpressing RhoE (2). This signal impairs T-cell function through three mechanisms: expanding suppressive Tregs, inducing T-cell exhaustion (evidenced by PD-1/TIM-3 expression in follicular B-cell lymphoma patients), and promoting apoptosis (2). Felix M Wensveen demonstrated that strong antigen stimulation activates CD95/CD95L through this axis, inducing T-cell apoptosis (19) (Figure 2).
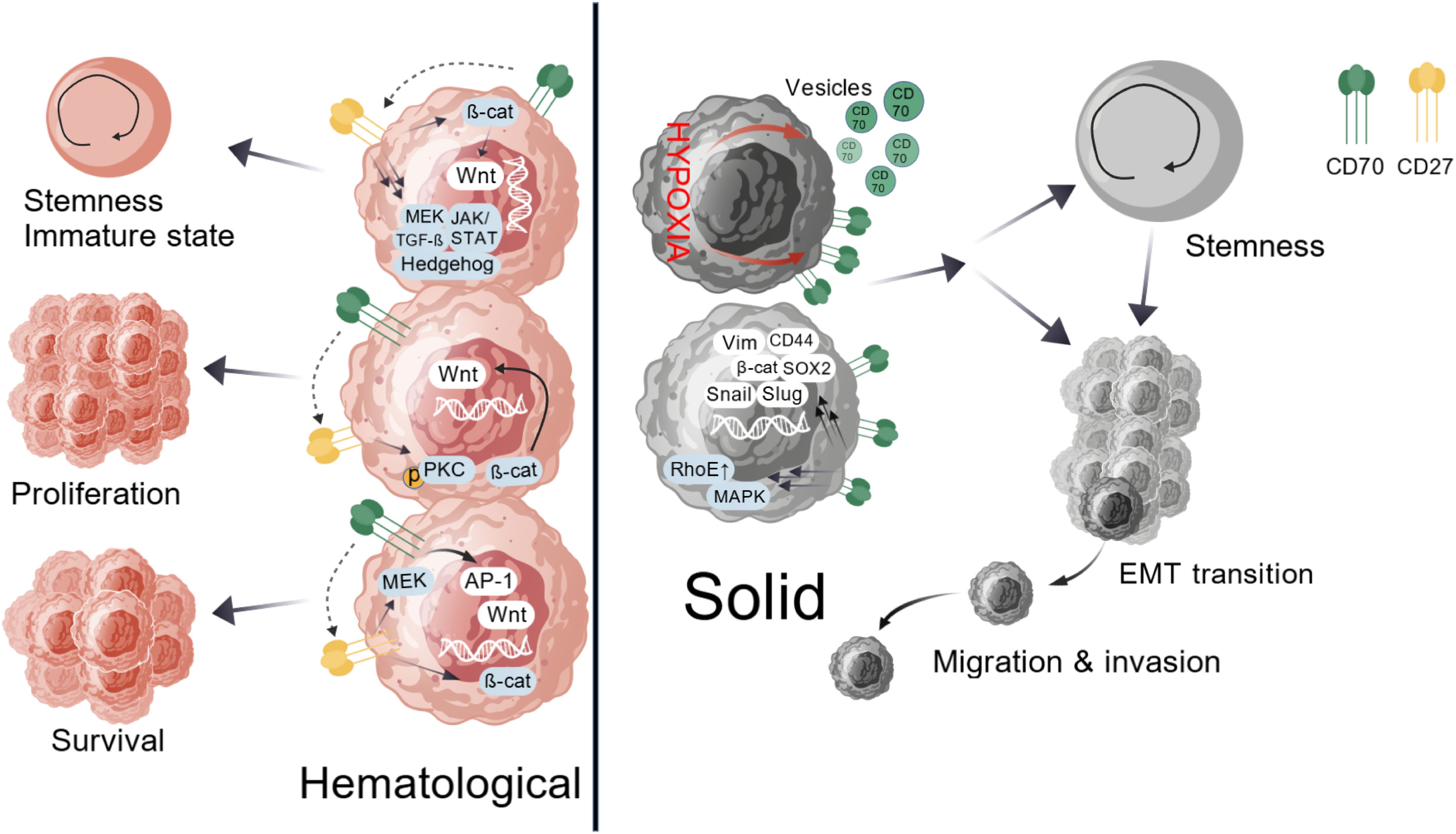
Figure 2. Pathogenic CD70-CD27 signaling in cancer. In hematological malignancies, CD70-CD27 signaling activates canonical Wnt, JAK/STAT, Hedgehog, and TGF-β pathways, which maintain cellular stemness and an immature phenotype. CD27 crosslinking via protein kinase C or β-catenin-dependent mechanisms, along with CD70 reverse signaling, drives malignant cell proliferation. This axis sustains survival through synergistic regulation of MEK pathway kinases and transcription factor AP-1, coupled with β-catenin-dependent Wnt pathway activation. Within solid tumors, CD70 signaling fosters cancer stem cell characteristics and epithelial-mesenchymal transition by upregulating EMT-associated transcriptional regulators such as SOX2, CD44, Vimentin, Snail, Slug, and β-catenin while concurrently activating MAPK signaling and inducing RhoE overexpression. Hypoxia emerges as a critical modulator of CD70 expression, directly influencing tumor stemness, migration, and invasive potential.
The CD70-CD27 axis exhibits dual roles in hematopoiesis and oncogenesis. During normal development, tightly controlled CD70 expression supports immune cell priming and differentiation via TRAF2/5-dependent NF-κB and c-Jun activation (2), while Siva-mediated caspase activation can induce apoptosis (14). CD70 reverse signaling activates PI3K/Akt and MEK pathways, modulating cell expansion and effector functions (20) (Figure 1). In cancer, malignant cell co-expression creates autocrine proliferation loops. Hematologic malignancies show activated Wnt, JAK/STAT, Hedgehog, and TGF-β pathways that maintain stemness (21), with CD27 crosslinking and CD70 reverse signaling further driving proliferation (21). Survival pathways include MEK kinases, AP-1 activation, and Wnt/β-catenin signaling (22, 23). Solid tumors link CD70 to cancer stem cells and EMT (24) through EMT gene induction and MAPK/RhoE activation (2). Hypoxia potently regulates CD70 expression, enhancing stemness and invasiveness (25) (Figure 2).
This axis critically regulates immunity: NK cell CD70 activates CD27+ T cells to boost CTL responses and IFN-γ production, while DC CD70 sustains CD8+ T cell immunity (26). Germinal center B-cell interactions with CD27 modulate B-cell activation, plasma cell differentiation, and humoral responses (27).
4 Anti-tumor drugs targeting CD70
4.1 mAbs and ADCs
Monoclonal antibodies (mAbs) originate from a single B cell clone, producing a homogeneous population that binds to a specific antigenic epitope. Their mechanisms include antibody-dependent cellular cytotoxicity (ADCC), antibody-dependent cellular phagocytosis (ADCP), and complement-dependent cytotoxicity (CDC). By blocking CD70-CD27 interactions, mAbs may disrupt signaling pathways promoting blast proliferation and survival, potentially limiting immune evasion and augmenting antigen-specific T cell responses. Recent developments include anti-CD70 mAbs such as SEA-CD70.
Antibody-drug conjugates (ADCs) combine a monoclonal antibody with a cytotoxic payload via a linker, enabling targeted drug delivery. CD70-targeted ADCs like SGN-75 and MDX-1203 are under investigation for hematologic and solid tumors.
4.2 Anti-CD70 CAR-T
Unlike mAbs and ADCs, chimeric antigen receptor T-cell (CAR-T) immunotherapy functions as a dynamic immunotherapy, with engineered T cells capable of in vivo expansion and differentiation. This reduces dosing frequency while enhancing antigen recognition and tumor specificity. CAR-T cells also recruit immune cells through cytokine secretion, amplifying antitumor responses.
CAR-T technology merges antibody-like antigen targeting with T cell cytotoxicity by engineering chimeric antigen receptors. These receptors typically comprise an antigen-binding domain, hinge region, transmembrane domain, costimulatory domain, and signaling region (28). CD70’s tumor-selective expression makes it an attractive target. CD70-directed CAR-T has shown efficacy in acute myeloid leukemia, renal cancer, and glioblastoma.
4.2.1 Anti-CD70 CAR-T and AML
Acute myeloid leukemia (AML) remains a therapeutic challenge, but CD70’s expression on leukemic blasts, not hematopoietic stem cells, positions it as a viable CAR-T target (29). Current CD70-targeted CAR-T approaches fall into two categories.
Tim Saucer et al. (29) engineered structurally diverse CD70 single-chain variable fragment CAR-T (CD70scFv CAR-T), demonstrating variable expression and antitumor activity. Wu et al. confirmed its antileukemic effects in preclinical models, though complete eradication proved unattainable (30). Despite their high binding affinity and specificity, scFvs present several limitations: Certain scFv constructs exhibit self-aggregation tendencies, which promote CAR clustering and subsequent tonic signaling that ultimately induces T-cell exhaustion. Additionally, murine-derived scFvs may trigger immunogenic responses.
The CD70-specific CAR incorporating truncated CD27 (tCD27) demonstrated superior functionality compared to conventional scFv-based CAR constructs. After Donald R. Shaffer et al. (31) pioneered full-length CD27-CD3 fusion constructs for B cell malignancies, Wang et al. (32) enhanced potency by truncating CD27’s intracellular domain and incorporating 4-1BB, though metalloproteinase-mediated CD27 cleavage impaired CD70 binding (33–36). However, CD27 engagement with CD70 triggers matrix metalloproteinase-mediated cleavage, which reduces functional CAR expression on T-cells and significantly impairs the in vivo efficacy of tCD27-based CAR-T therapy. Leick et al. (37) improved synapse affinity by pretreating with azacitidine to increase antigen density, while structural modifications to CAR domains mitigated metalloproteinase susceptibility.
To address this challenge, Cheng et al. (38) developed nanobody-based anti-CD70-CAR T-cells (nb70CAR-T) incorporating two distinct heavy-chain antibody variable domains (VHHs) for enhanced CD70 recognition. This design minimizes self-aggregation and immunogenicity compared to murine-derived scFv constructs. The researchers further employed CRISPR-Cas9 to disrupt the CD70 gene in T-cells, preventing fratricide. They also examined epigenetic modulators to control CD70 expression on AML cells, thereby optimizing nb70CAR-T efficacy.
4.2.2 Anti-CD70 CAR-T and RCC
Renal cell carcinoma (RCC), a highly malignant tumor originating from the renal parenchyma and tubule epithelium, frequently overexpresses CD70 despite its role as a costimulatory immune molecule (39, 40). The rapid internalization of CD70 by antibodies enhances its therapeutic appeal in RCC (41, 42). ALLO-316, an allogeneic anti-CD70 CAR-T therapy, employs TALENs to disrupt TRAC and CD52 while incorporating a Rituximab recognition site to modulate T-cell overactivation via CD52 antibody and Fludarabine/Cyclophosphamide synergy. These allogeneic CAR-T designs circumvent GVHD risks while maintaining broad applicability. CD70+ T cells further correlate with a GvHD progression post-transplant, suggesting their suppression could mitigate this complication (43).
4.2.3 Anti-CD70 CAR-T and glioma
Gliomas, the predominant primary brain tumors, originate from malignant glial cells. CD70 overexpression independently predicts poor survival in low-grade glioma (LGG) and glioblastoma (GBM) multiforme (44) and promotes macrophage infiltration and CD8+ T-cell apoptosis (10, 44), making it a compelling CAR-T target (10, 31, 32). Jin et al. linked CD70 to immunosuppression and augmented anti-tumor responses by engineering IL-8 receptor (CXCR1/CXCR2)-modified anti-CD70 CAR-T, leveraging radiation-induced IL-8 release to enhance tumor trafficking (45). Ji et al. enabled blood-brain barrier penetration by incorporating rabies virus glycoprotein 29 into anti-CD70 CAR-T (46), while Zhu et al. combined lysosomal virus infection with CAR-T to shift the microenvironment toward pro-inflammatory effector cell dominance (47).
4.3 CD70-CAR-NK
CD70 chimeric antigen receptor natural killer cells (CD70-CAR-NK), engineered with IL-15, hnCD16, and CD70 knockout, target tumor cells and cancer-associated fibroblasts with minimal off-tumor effects, exhibiting potent cytotoxicity and prolonged persistence (48). IL-15 stimulation amplifies CAR expression and cytokine secretion, improving tumor control in xenografts, particularly with repeated dosing (49). These cells also deplete alloreactive T cells, enhancing engraftment (50). Their off-the-shelf feasibility and dual targeting of tumor and stromal compartments address key CAR-T limitations, positioning them as versatile candidates for clinical translation.
4.4 Comparison of CD70-targeted therapeutic approaches
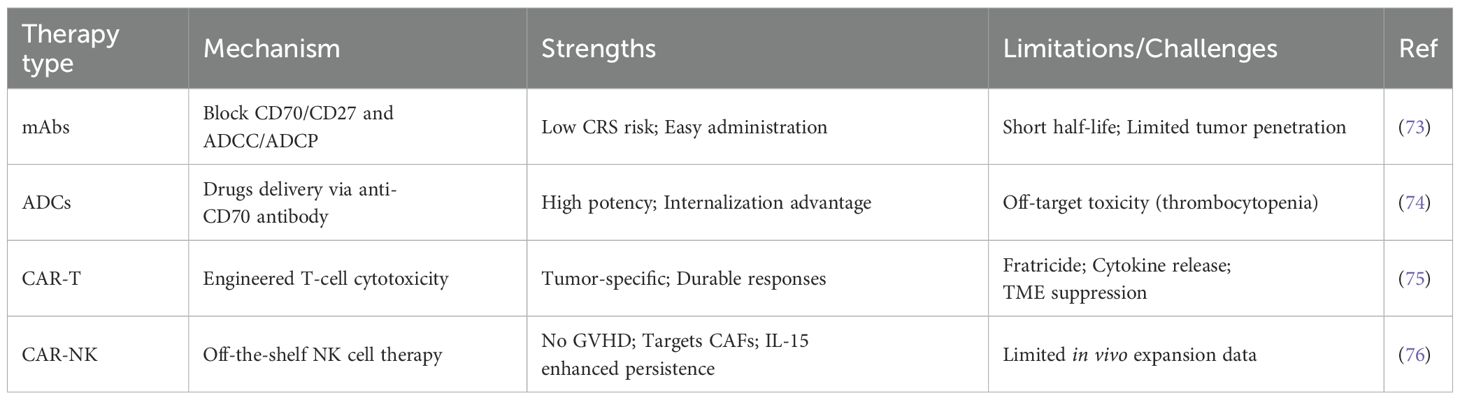
CD70-directed modalities exhibit distinct profiles: mAbs block CD70/CD27 interactions with low cytokine release syndrome risk but poor tumor penetration; ADCs deliver potent toxins yet face thrombocytopenia; CAR-Ts achieve durable responses despite fratricide and tumor microenvironment (TME) suppression; CAR-NKs offer GVHD-free, off-the-shelf therapy with stromal targeting but limited expansion. These trade-offs underscore the need for context-specific optimization (Table 1).
The inherent heterogeneity of these therapies also manifests in their safety profiles, particularly the contrasting efficacy-toxicity trade-offs between CAR-T cells and ADCs. CAR-T therapy frequently induces CRS, while ADCs predominantly cause hematologic toxicities. CRS results from on-target immune activation, which often correlates with therapeutic efficacy in lymphomas but requires careful immunomodulation to control severe inflammation without eliminating CAR-T cells (51). In contrast, ADC-induced myelosuppression arises from off-target payload effects on bone marrow, lacking any therapeutic benefit and often forcing dose reductions that diminish antitumor activity (52). These distinct mechanisms necessitate different management strategies: CRS benefits from early cytokine blockade with agents like anti-IL-6, whereas ADC toxicities require supportive care and dose adjustments. Clinicians must balance CAR-T’s acute, potentially efficacy-associated toxicities against ADCs’ cumulative hematologic risks when selecting treatments.
4.5 Clinical landscape of CD70 therapies
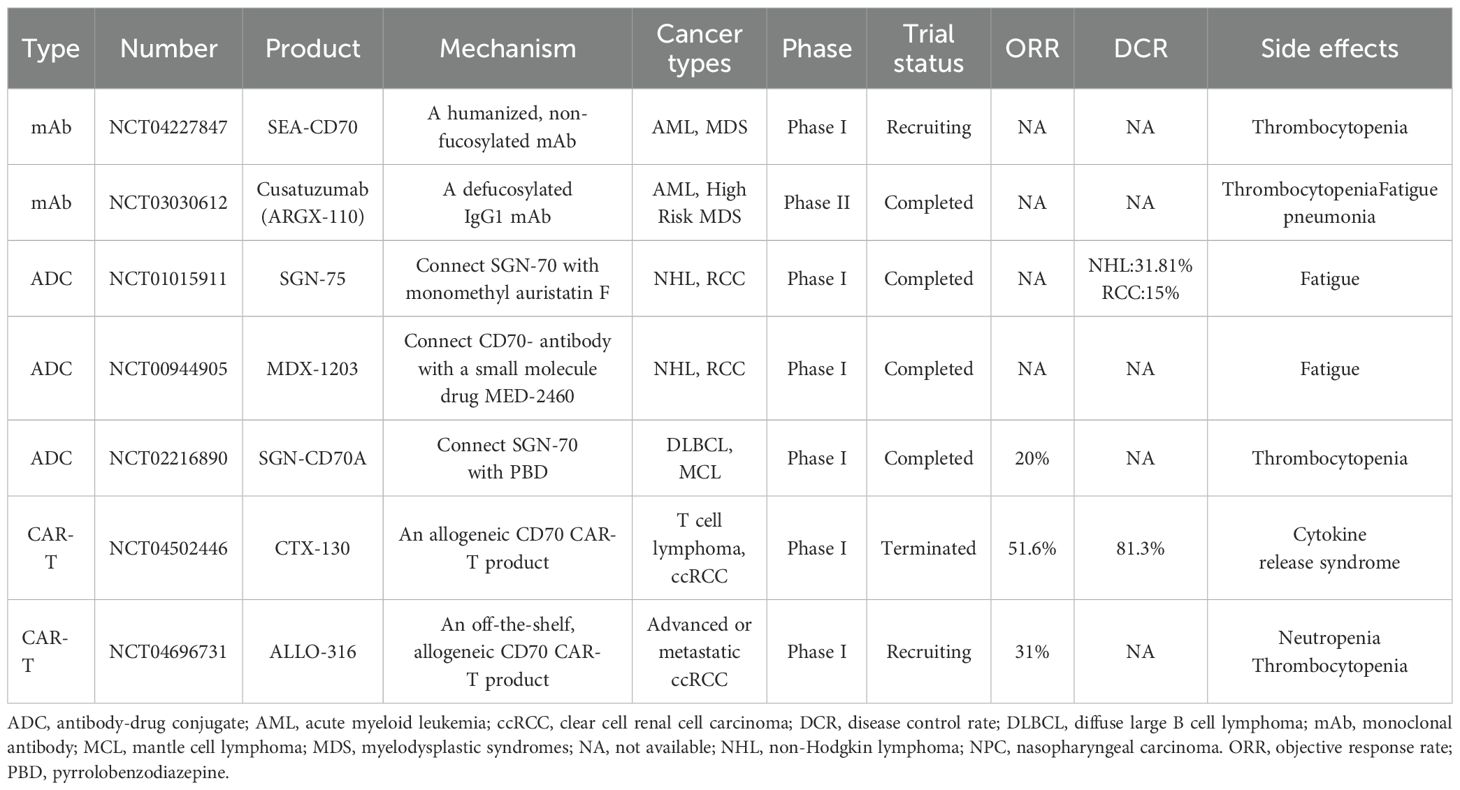
Targeted therapies against CD70 have advanced considerably, though each modality presents unique clinical outcomes and limitations. Table 2 summarizes ongoing and completed trials across mAb, ADC, and CAR-T modalities (Table 2). CD70-targeted therapies exhibit divergent clinical outcomes depending on therapeutic modality. The anti-CD70 monoclonal antibody cusatuzumab induced complete remission in 37% of acute myeloid leukemia patients when combined with azacitidine, with median overall survival reaching 11.5 months, but showed only stable disease in nasopharyngeal carcinoma before trial termination due to limited efficacy and adverse events including fatigue and pneumonia (NCT03030612). While cusatuzumab blocks CD70-CD27 interaction and mediates antibody-dependent cellular cytotoxicity, its efficacy appears restricted primarily to hematologic malignancies rather than solid tumors. Early-phase trials of SEA-CD70 suggest acceptable safety and preliminary antitumor activity, though comprehensive efficacy data remain unavailable.
Among antibody-drug conjugates, SGN-75 demonstrated limited activity in RCC and non-Hodgkin lymphoma before development ceased following cases of immune-mediated thrombocytopenic purpura. The pyrrolobenzodiazepine-conjugated SGN-CD70A produced modest responses in diffuse large B-cell and mantle cell lymphomas but was constrained by dose-limiting thrombocytopenia occurring in 75% of patients. Although SGN-CD70A demonstrated a 78% disease control rate in metastatic RCC, its clinical development was ultimately constrained by marginal efficacy. MDX-1203 stabilized disease in 69% of patients but induced severe hypersensitivity reactions and delayed toxicities such as pleural effusions, prompting discontinuation (NCT00944905). These ADC toxicities predominantly involve hematologic complications, suggesting payload-related mechanisms despite CD70’s tumor-selective expression.
CAR-T cell therapies demonstrate comparatively robust clinical activity. The allogeneic CTX130 induced durable complete remissions in cRCC with an 81.3% disease control rate and manageable toxicity, leveraging direct T-cell cytotoxicity without ADC-associated payload effects. In T-cell malignancies, CTX130 yielded objective responses in 51.6% of patients at elevated doses. ALLO-316, another investigational CAR-T product, shows early promise in RCC. While CAR-T therapies enable sustained immune-mediated tumor clearance, they face challenges including cytokine release syndrome and complex manufacturing requirements.
Recent late-phase trials reveal both progress and limitations in emerging therapies. SGN-CD70A showed response rates of 20-30% but was hampered by severe thrombocytopenia (Grade ≥3: 40-50%), which constrained its clinical utility (NCT02216890). ALLO-316 produced durable responses (30-40% ORR) in CD70-high ccRCC patients, with certain remissions persisting beyond 12 months and a favorable safety profile characterized by mild CRS and negligible neurotoxicity (NCT04696731). The dose-limiting toxicity of SGN-CD70A contrasts with ALLO-316’s sustained activity, reinforcing CD70 as a viable target in solid tumors. Refining biomarker selection and CAR-T production protocols may improve therapeutic outcomes, suggesting allogeneic approaches could complement ADCs for CD70-positive malignancies.
Monoclonal antibodies and antibody-drug conjugates confront efficacy and toxicity barriers, whereas CAR-T therapies produce durable responses but require optimization to reduce immune-related adverse events and enhance production scalability. Future development should focus on engineered antibody-drug conjugates with refined linker-payload systems, combination strategies with immune checkpoint inhibitors, and improved allogeneic CAR-T platforms to broaden clinical applicability. These collective efforts affirm CD70’s therapeutic potential while emphasizing the need for modality-specific optimization.
5 CD70 and cancer diagnosis
Beyond its therapeutic potential, CD70 expression patterns also facilitate novel diagnostic applications. 18F-FDG PET/CT has been widely used in clinical diagnostics, but its effectiveness in ccRCC remains limited due to inconsistent glucose metabolism and reduced tracer uptake. The incorporation of CD70-targeted PET/CT imaging has improved diagnostic accuracy for ccRCC. A recent pilot clinical trial assessed two novel CD70-specific single-domain antibody tracers, (68Ga)Ga-NOTA-RCCB3 and (68Ga)Ga-NOTA-RCCB6, revealing that (68Ga)Ga-NOTA-RCCB6 immuno-PET/CT effectively identifies metastasis and postoperative recurrence in ccRCC (53). Further development of the CD70-targeted tracer (18F)RCCB6 has demonstrated its diagnostic precision in detecting ccRCC metastasis across preclinical models and human studies (54). In a ccRCC patient-derived xenograft model, (18F)RCCB6 exhibited substantial tumor-specific uptake (10.63% ± 1.21% ID/g), with CD70 blockade significantly reducing this signal (0.53% ± 0.04% ID/g; p = 0.002), confirming target engagement. Initial clinical assessment (NCT06148220) showed robust (18F)RCCB6 uptake in metastatic ccRCC lesions involving lung, pancreas, muscle, bone, and intracranial sites (54). Subsequent analysis of imaging data from 15 patients further validated the clinical utility of [18F]RCCB6 for postoperative surveillance and treatment response assessment (55). A current multicenter trial (NCT06680089) is systematically investigating the diagnostic and prognostic potential of (18F)RCCB6 immuno-PET/CT in metastatic ccRCC.
6 Discussion
CAR-T therapy has demonstrated remarkable success in treating hematologic malignancies, but its efficacy against solid tumors remains limited. The TME impedes CAR-T cell infiltration, promoting exhaustion and dysfunction (56), while tumor antigens often exhibit low specificity, high heterogeneity, and sparse expression (57). Overcoming these barriers requires innovative CAR-T designs tailored to the unique challenges of solid tumors.
6.1 The challenge of TME
The TME drives cancer progression and treatment resistance by harboring immunosuppressive cells such as MDSCs and Tregs, alongside inhibitory signals like PD-L1 and TGF-β (58). These factors collectively suppress CAR-T cell activity. Strategies to enhance efficacy include chemotherapy to deplete immunosuppressive populations and checkpoint blockade to reactivate T cell function. Such interventions may remodel the TME, improving CAR-T cell persistence and anti-tumor responses. Key strategies involve targeting the CXCR4/CXCL12 axis to enhance T-cell infiltration in immunologically cold tumors (59, 60); while JAK/STAT or PI3K inhibitors can suppress myeloid-derived immunosuppression by blocking TAM/MDSC function and their secretion of IL-10 and TGF-β (61); Local Treg depletion via anti-CD25 antibodies or immunotoxins reduces suppression of effector T cells (62–64); whereas IL-15 fusion proteins directly stimulate CD8 T cells and NK cells (65, 66); DNA-damaging agents or PARP inhibitors may further augment immunogenicity by increasing mutational burden and neoantigen presentation (67). These approaches require customization based on tumor-specific TME features—such as PDAC’s CAF barrier or GBM’s low mutational load—and should be combined strategically to maximize immune activation while minimizing autoimmune toxicity.
6.2 The challenge of tumor antigens
CD70 offers a promising target due to its tumor-restricted expression and minimal off-target effects. Disrupting the CD70-CD27 axis further curbs tumor growth. However, broader challenges persist: tumor antigens often lack specificity, exhibit heterogeneity, or are expressed at low densities (57).
6.2.1 The challenge of low specificity
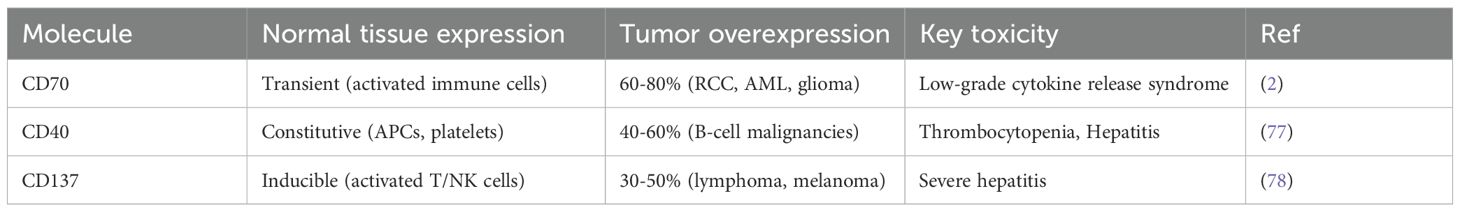
While tumor-specific antigens (TSAs) are ideal targets, their rarity necessitates reliance on tumor-associated antigens (TAAs) like CD70, which is overexpressed in malignancies but transiently in activated lymphocytes. This selectivity reduces off-target toxicity, yet CD70 expression on CAR-T cells risks fratricide (68). Gene editing to eliminate CD70, TCR, and MHC genes can prevent GVHD, while bispecific CAR-T cells or synNotch receptors may further mitigate fratricide. Compared with other TNF family targets, CD70 has highly heterogeneous expression, low off-target toxicity and high efficacy (Table 3).
6.2.2 The challenge of high heterogeneity
Antigen heterogeneity limits monospecific CAR-T therapies. Bispecific designs, such as B7-H3/CD70-targeting tanCAR-T cells (6) or CD19/CD70 dualCAR-T cells (7), broaden target recognition. Alternatively, BiTE-secreting CAR-T cells (69) or synNotch receptors (59) enhance efficacy against heterogeneous tumors without off-target effects.
6.2.3 The challenge of low density
Low antigen density compromises CAR-T cell recognition. Epigenetic modulators like decitabine, chemotherapy, or radiotherapy can upregulate target expression. For instance, cisplatin elevates CD70 in NSCLC (8), while irradiation boosts CD70 in prostate cancer models (70). These approaches may sensitize tumors to CAR-T therapy.
6.3 Optimizing CAR-T design
Structural modifications to CAR-T have enhanced its efficacy and specificity through targeted engineering of key domains. Hinge, transmembrane, co-stimulatory, and signaling domains critically influence CAR-T functionality.
CD70-targeted CAR-T therapies have advanced through iterative generations. First-generation constructs, comprising anti-CD70 scFv linked to CD3ζ, induce T-cell activation but exhibit poor persistence due to lacking co-stimulation. Second-generation CARs incorporate CD28 or 4-1BB domains, with CD28 enhancing cytotoxicity and 4-1BB improving metabolic fitness and durability—key for mitigating fratricide from CD70’s expression on activated T-cells. Third-generation designs combine multiple co-stimulatory domains (e.g., CD28-4-1BB-CD3ζ) but risk excessive tonic signaling without clear preclinical superiority. Next-generation approaches integrate safety modules: (i) CRISPR-mediated CD70 knockout to prevent fratricide, (ii) cytokine-secreting armored CARs (e.g., IL-15) to counter immunosuppression, and (iii) logic-gated systems to reduce off-tumor toxicity. Persistent challenges include exhaustion from tonic signaling, antigen heterogeneity in solid tumors, and B-cell aplasia due to CD70 expression on plasma cells. Clinical trials are evaluating optimized second- and next-generation constructs.
6.4 CD70-targeted CAR-T therapy faces a critical challenge: fratricide
Fratricide occurs when CAR-T cells self-destruct through mutual recognition of surface-expressed CD70 (71). Mechanistically, this process involves scFv-mediated binding of CARs to CD70 on neighboring T cells (trans-interaction), which triggers cytotoxic activation (68). Beyond T-cell self-killing, CD70 sharing also drives off-target toxicity in vital tissues, particularly renal proximal tubular epithelial cells in RCC. Therapeutic targeting of these constitutively CD70-expressing cells—whether by ADCs or CAR-T-derived cytokines—induces direct cellular damage or cytotoxic payload internalization. Preclinical models and clinical ADC data demonstrate that RCC patients consequently develop dose-limiting acute kidney injury, characterized by elevated serum creatinine and proteinuria.
Two principal strategies have emerged to address fratricide and its downstream effects: (1) For scFv-based CARs, cis-masking prevents self-recognition while preserving tumor targeting, thereby eliminating trans-interaction-mediated fratricide and facilitating clinical translation (68). (2) In VHH-based nanoCAR platforms, CRISPR/Cas9-mediated CD70 knockout resolves both trans (fratricide) and cis interactions, the latter of which induces severe T-cell exhaustion through nanoCAR binding to CD70 on the same cell (72). Single-cell transcriptomics confirms this approach prevents exhaustion and restores antitumor efficacy in lymphoma PDX models, while simultaneously reducing renal toxicity by eliminating CD70 from T cells that could exacerbate off-target damage (72).
6.5 Evolution of CD70-targeted therapies
Advances in gene editing, AI, and delivery systems are addressing current limitations. CRISPR enables multiplexed edits (e.g., CD70/PD-1 knockout) and TME-responsive elements, while AI models predict resistance patterns from single-cell data. Delivery innovations like conditionally activated vectors and LNPs, combined with epigenetic modulators (e.g., azacitidine) or adenosine axis inhibitors, may overcome resistance. Key priorities include: (1) A global CD70 expression atlas, (2) Trials of allogeneic CD70-CAR-NK for solid tumors, (3) Companion diagnostics via PET tracers and computational modeling.
7 Conclusion
CD70, minimally expressed in normal tissues but upregulated in malignancies, regulates immune cell function physiologically yet promotes tumor progression. Current immunotherapies—mAbs, ADCs, and CAR-T—show promise, particularly in hematologic cancers. Challenges remain in overcoming TME suppression and antigen heterogeneity. Optimizing CAR-T design, enhancing tumor infiltration, and modulating the TME are critical to realizing CD70’s therapeutic potential.
Author contributions
RW: Funding acquisition, Writing – original draft. JC: Software, Writing – original draft. GW: Supervision, Writing – review & editing. LH: Visualization, Supervision, Funding acquisition, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was supported by the National Natural Science Foundation of China (Grant No. 82203172), Innovation and entrepreneurship training program for college students (No. 202310313050Z).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Riley RS, June CH, Langer R, and Mitchell MJ. Delivery technologies for cancer immunotherapy. Nat Rev Drug Discov. (2019) 18:175–96. doi: 10.1038/s41573-018-0006-z
2. Flieswasser T, Van den Eynde A, Van Audenaerde J, De Waele J, Lardon F, Riether C, et al. The CD70-CD27 axis in oncology: the new kids on the block. J Exp Clin Cancer Res. (2022) 41:12. doi: 10.1186/s13046-021-02215-y
3. Abaza Y and Fathi AT. Monoclonal antibodies in acute myeloid leukemia-are we there yet? Cancer J. (2022) 28:37–42. doi: 10.1097/PPO.0000000000000577
4. Gallazzi M, Ucciero MAM, Faraci DG, Mahmoud AM, Al Essa W, Gaidano G, et al. New frontiers in monoclonal antibodies for the targeted therapy of acute myeloid leukemia and myelodysplastic syndromes. Int J Mol Sci. (2022) 23:7542. doi: 10.3390/ijms23147542
5. Wang H, Kaur G, Sankin AI, Chen F, Guan F, and Zang X. Immune checkpoint blockade and CAR-T cell therapy in hematologic Malignancies. J Hematol Oncol. (2019) 12:59. doi: 10.1186/s13045-019-0746-1
6. Yang M, Tang X, Zhang Z, Gu L, Wei H, Zhao S, et al. Tandem CAR-T cells targeting CD70 and B7-H3 exhibit potent preclinical activity against multiple solid tumors. Theranostics. (2020) 10:7622–34. doi: 10.7150/thno.43991
7. Tu S, Zhou X, Guo Z, Huang R, Yue C, He Y, et al. CD19 and CD70 dual-target chimeric antigen receptor T-cell therapy for the treatment of relapsed and refractory primary central nervous system diffuse large B-cell lymphoma. Front Oncol. (2019) 9:1350. doi: 10.3389/fonc.2019.01350
8. Jacobs J, Zwaenepoel K, Rolfo C, Van den Bossche J, Deben C, Silence K, et al. Unlocking the potential of CD70 as a novel immunotherapeutic target for non-small cell lung cancer. Oncotarget. (2015) 6:13462–75. doi: 10.18632/oncotarget.3880
9. Petrau C, Cornic M, Bertrand P, Maingonnat C, Marchand V, Picquenot JM, et al. CD70: A potential target in breast cancer? J Cancer. (2014) 5:761–4. doi: 10.7150/jca.10360
10. Jin L, Ge H, Long Y, Yang C, Chang YE, Mu L, et al. CD70, a novel target of CAR T-cell therapy for gliomas. Neuro Oncol. (2018) 20:55–65. doi: 10.1093/neuonc/nox116
11. Inaguma S, Lasota J, Czapiewski P, Langfort R, Rys J, Szpor J, et al. CD70 expression correlates with a worse prognosis in Malignant pleural mesothelioma patients via immune evasion and enhanced invasiveness. J Pathol. (2020) 250:205–16. doi: 10.1002/path.5361
12. Jilaveanu LB, Sznol J, Aziz SA, Duchen D, Kluger HM, and Camp RL. CD70 expression patterns in renal cell carcinoma. Hum Pathol. (2012) 43:1394–9. doi: 10.1016/j.humpath.2011.10.014
13. Liu N, Sheng X, Liu Y, Zhang X, and Yu J. Increased CD70 expression is associated with clinical resistance to cisplatin-based chemotherapy and poor survival in advanced ovarian carcinomas. Onco Targets Ther. (2013) 6:615–9. doi: 10.2147/OTT.S44445
14. Jacobs J, Deschoolmeester V, Zwaenepoel K, Rolfo C, Silence K, Rottey S, et al. CD70: An emerging target in cancer immunotherapy. Pharmacol Ther. (2015) 155:1–10. doi: 10.1016/j.pharmthera.2015.07.007
15. Flieswasser T, Camara-Clayette V, Danu A, Bosq J, Ribrag V, Zabrocki P, et al. Screening a broad range of solid and haematological tumour types for CD70 expression using a uniform IHC methodology as potential patient stratification method. Cancers (Basel). (2019) 11:1611. doi: 10.3390/cancers11101611
16. Wajant H. Therapeutic targeting of CD70 and CD27. Expert Opin Ther Targets. (2016) 20:959–73. doi: 10.1517/14728222.2016.1158812
17. Coquet JM, Ribot JC, Babala N, Middendorp S, van der Horst G, Xiao Y, et al. Epithelial and dendritic cells in the thymic medulla promote CD4+Foxp3+ regulatory T cell development via the CD27-CD70 pathway. J Exp Med. (2013) 210:715–28. doi: 10.1084/jem.20112061
18. Prasad KV, Ao Z, Yoon Y, Wu MX, Rizk M, Jacquot S, et al. CD27, a member of the tumor necrosis factor receptor family, induces apoptosis and binds to Siva, a proapoptotic protein. Proc Natl Acad Sci U.S.A. (1997) 94:6346–51. doi: 10.1073/pnas.94.12.6346
19. Wensveen FM, Unger PP, Kragten NA, Derks IA, ten Brinke A, Arens R, et al. CD70-driven costimulation induces survival or Fas-mediated apoptosis of T cells depending on antigenic load. J Immunol. (2012) 188:4256–67. doi: 10.4049/jimmunol.1102889
20. Arens R, Nolte MA, Tesselaar K, Heemskerk B, Reedquist KA, van Lier RA, et al. Signaling through CD70 regulates B cell activation and IgG production. J Immunol. (2004) 173:3901–8. doi: 10.4049/jimmunol.173.6.3901
21. Riether C, Schurch CM, Buhrer ED, Hinterbrandner M, Huguenin AL, Hoepner S, et al. CD70/CD27 signaling promotes blast stemness and is a viable therapeutic target in acute myeloid leukemia. J Exp Med. (2017) 214:359–80. doi: 10.1084/jem.20152008
22. Riether C, Schurch CM, Flury C, Hinterbrandner M, Druck L, Huguenin AL, et al. Tyrosine kinase inhibitor-induced CD70 expression mediates drug resistance in leukemia stem cells by activating Wnt signaling. Sci Transl Med. (2015) 7:298ra119. doi: 10.1126/scitranslmed.aab1740
23. Bertrand P, Maingonnat C, Penther D, Guney S, Ruminy P, Picquenot JM, et al. The costimulatory molecule CD70 is regulated by distinct molecular mechanisms and is associated with overall survival in diffuse large B-cell lymphoma. Genes Chromosomes Cancer. (2013) 52:764–74. doi: 10.1002/gcc.22072
24. Pich C, Sarrabayrouse G, Teiti I, Mariame B, Rochaix P, Lamant L, et al. Melanoma-expressed CD70 is involved in invasion and metastasis. Br J Cancer. (2016) 114:63–70. doi: 10.1038/bjc.2015.412
25. Tse SW, Tan CF, Park JE, Gnanasekaran J, Gupta N, Low JK, et al. Microenvironmental hypoxia induces dynamic changes in lung cancer synthesis and secretion of extracellular vesicles. Cancers (Basel). (2020) 12:2917. doi: 10.3390/cancers12102917
26. Al Sayed MF, Ruckstuhl CA, Hilmenyuk T, Claus C, Bourquin JP, Bornhauser BC, et al. CD70 reverse signaling enhances NK cell function and immunosurveillance in CD27-expressing B-cell Malignancies. Blood. (2017) 130:297–309. doi: 10.1182/blood-2016-12-756585
27. Achour A, Simon Q, Mohr A, Seite JF, Youinou P, Bendaoud B, et al. Human regulatory B cells control the T(FH) cell response. J Allergy Clin Immunol. (2017) 140:215–22. doi: 10.1016/j.jaci.2016.09.042
28. Deng W, Chen P, Lei W, Xu Y, Xu N, Pu JJ, et al. CD70-targeting CAR-T cells have potential activity against CD19-negative B-cell Lymphoma. Cancer Commun (Lond). (2021) 41:925–9. doi: 10.1002/cac2.12201
29. Sauer T, Parikh K, Sharma S, Omer B, Sedloev D, Chen Q, et al. CD70-specific CAR T cells have potent activity against acute myeloid leukemia without HSC toxicity. Blood. (2021) 138:318–30. doi: 10.1182/blood.2020008221
30. Wu G, Guo S, Luo Q, Wang X, Deng W, Ouyang G, et al. Preclinical evaluation of CD70-specific CAR T cells targeting acute myeloid leukemia. Front Immunol. (2023) 14:1093750. doi: 10.3389/fimmu.2023.1093750
31. Shaffer DR, Savoldo B, Yi Z, Chow KK, Kakarla S, Spencer DM, et al. T cells redirected against CD70 for the immunotherapy of CD70-positive Malignancies. Blood. (2011) 117:4304–14. doi: 10.1182/blood-2010-04-278218
32. Wang QJ, Yu Z, Hanada KI, Patel K, Kleiner D, Restifo NP, et al. Preclinical evaluation of chimeric antigen receptors targeting CD70-expressing cancers. Clin Cancer Res. (2017) 23:2267–76. doi: 10.1158/1078-0432.CCR-16-1421
33. Burchill MA, Tamburini BA, and Kedl RM. T cells compete by cleaving cell surface CD27 and blocking access to CD70-bearing APCs. Eur J Immunol. (2015) 45:3140–9. doi: 10.1002/eji.201545749
34. Kato K, Chu P, Takahashi S, Hamada H, and Kipps TJ. Metalloprotease inhibitors block release of soluble CD27 and enhance the immune stimulatory activity of chronic lymphocytic leukemia cells. Exp Hematol. (2007) 35:434–42. doi: 10.1016/j.exphem.2006.10.018
35. Pirillo C, Birch F, Tissot FS, Anton SG, Haltalli M, Tini V, et al. Metalloproteinase inhibition reduces AML growth, prevents stem cell loss, and improves chemotherapy effectiveness. Blood Adv. (2022) 6:3126–41. doi: 10.1182/bloodadvances.2021004321
36. Zhou Y, Liu X, Xu L, Tseng H, Cao Y, Jiang J, et al. Matrix metalloproteinase-8 is overexpressed in Waldenstrom’s macroglobulinemia cells, and specific inhibition of this metalloproteinase blocks release of soluble CD27. Clin Lymphoma Myeloma Leuk. (2011) 11:172–5. doi: 10.3816/CLML.2011.n.041
37. Leick MB, Silva H, Scarfo I, Larson R, Choi BD, Bouffard AA, et al. Non-cleavable hinge enhances avidity and expansion of CAR-T cells for acute myeloid leukemia. Cancer Cell. (2022) 40:494–508 e5. doi: 10.1016/j.ccell.2022.04.001
38. Cheng J, Ge T, Zhu X, Wang J, Zeng Y, Mu W, et al. Preclinical development and evaluation of nanobody-based CD70-specific CAR T cells for the treatment of acute myeloid leukemia. Cancer Immunol Immunother. (2023) 72:2331–46. doi: 10.1007/s00262-023-03422-6
39. Hintzen RQ, Lens SM, Beckmann MP, Goodwin RG, Lynch D, and van Lier RA. Characterization of the human CD27 ligand, a novel member of the TNF gene family. J Immunol. (1994) 152:1762–73. doi: 10.4049/jimmunol.152.4.1762
40. Junker K, Hindermann W, von Eggeling F, Diegmann J, Haessler K, and Schubert J. CD70: a new tumor specific biomarker for renal cell carcinoma. J Urol. (2005) 173:2150–3. doi: 10.1097/01.ju.0000158121.49085.ba
41. Law CL, Gordon KA, Toki BE, Yamane AK, Hering MA, Cerveny CG, et al. Lymphocyte activation antigen CD70 expressed by renal cell carcinoma is a potential therapeutic target for anti-CD70 antibody-drug conjugates. Cancer Res. (2006) 66:2328–37. doi: 10.1158/0008-5472.CAN-05-2883
42. Adam PJ, Terrett JA, Steers G, Stockwin L, Loader JA, Fletcher GC, et al. CD70 (TNFSF7) is expressed at high prevalence in renal cell carcinomas and is rapidly internalised on antibody binding. Br J Cancer. (2006) 95:298–306. doi: 10.1038/sj.bjc.6603222
43. Verma K, Croft W, Margielewska-Davies S, Pearce H, Stephens C, Diaconescu D, et al. CD70 identifies alloreactive T cells and represents a potential target for prevention and treatment of acute GVHD. Blood Adv. (2024) 8:4900–12. doi: 10.1182/bloodadvances.2024012909
44. Ge H, Mu L, Jin L, Yang C, Chang YE, Long Y, et al. Tumor associated CD70 expression is involved in promoting tumor migration and macrophage infiltration in GBM. Int J Cancer. (2017) 141:1434–44. doi: 10.1002/ijc.30830
45. Jin L, Tao H, Karachi A, Long Y, Hou AY, Na M, et al. CXCR1- or CXCR2-modified CAR T cells co-opt IL-8 for maximal antitumor efficacy in solid tumors. Nat Commun. (2019) 10:4016. doi: 10.1038/s41467-019-11869-4
46. Ji F, Xu L, Long K, Zhang F, Zhang M, Lu X, et al. Rabies virus glycoprotein 29 (RVG29) promotes CAR-T immunotherapy for glioma. Transl Res. (2023) 259:1–12. doi: 10.1016/j.trsl.2023.03.003
47. Zhu G, Zhang J, Zhang Q, Jin G, Su X, Liu S, et al. Enhancement of CD70-specific CAR T treatment by IFN-gamma released from oHSV-1-infected glioblastoma. Cancer Immunol Immunother. (2022) 71:2433–48. doi: 10.1007/s00262-022-03172-x
48. Van den Eynde A, Gehrcken L, Verhezen T, Lau HW, Hermans C, Lambrechts H, et al. IL-15-secreting CAR natural killer cells directed toward the pan-cancer target CD70 eliminate both cancer cells and cancer-associated fibroblasts. J Hematol Oncol. (2024) 17:8. doi: 10.1186/s13045-024-01525-w
49. Guo S, Lei W, Jin X, Liu H, Wang JQ, Deng W, et al. CD70-specific CAR NK cells expressing IL-15 for the treatment of CD19-negative B-cell Malignancy. Blood Adv. (2024) 8:2635–45. doi: 10.1182/bloodadvances.2023012202
50. Wang L, Wang Y, He X, Mo Z, Zhao M, Liang X, et al. CD70-targeted iPSC-derived CAR-NK cells display potent function against tumors and alloreactive T cells. Cell Rep Med. (2025) 6:101889. doi: 10.1016/j.xcrm.2024.101889
51. Jain MD, Smith M, and Shah NN. How I treat refractory CRS and ICANS after CAR T-cell therapy. Blood. (2023) 141:2430–42. doi: 10.1182/blood.2022017414
52. Conilh L, Sadilkova L, Viricel W, and Dumontet C. Payload diversification: a key step in the development of antibody-drug conjugates. J Hematol Oncol. (2023) 16:3. doi: 10.1186/s13045-022-01397-y
53. Zhou X, Wu Q, Zhai W, Zhang Y, Wu Y, Cao M, et al. CD70-targeted immuno-PET/CT imaging of clear cell renal cell carcinoma: A translational study. J Nucl Med. (2024) 65:1891–8. doi: 10.2967/jnumed.124.268509
54. Wu Q, Wu Y, Zhang Y, Guan Y, Huang G, Xie F, et al. ImmunoPET/CT imaging of clear cell renal cell carcinoma with [(18)F]RCCB6: a first-in-human study. Eur J Nucl Med Mol Imaging. (2024) 51:2444–57. doi: 10.1007/s00259-024-06672-3
55. Wu Q, Wu Y, Zhang Y, Guan Y, Huang G, Zheng J, et al. (18)F]RCCB6 immuno-positron emission tomography/computed tomography for postoperative surveillance in clear cell renal cell carcinoma: A pilot clinical study. Eur Urol. (2024) 86:372–4. doi: 10.1016/j.eururo.2024.06.020
56. Hou AJ, Chen LC, and Chen YY. Navigating CAR-T cells through the solid-tumour microenvironment. Nat Rev Drug Discov. (2021) 20:531–50. doi: 10.1038/s41573-021-00189-2
57. Chen N, Li X, Chintala NK, Tano ZE, and Adusumilli PS. Driving CARs on the uneven road of antigen heterogeneity in solid tumors. Curr Opin Immunol. (2018) 51:103–10. doi: 10.1016/j.coi.2018.03.002
58. Beatty GL and Moon EK. Chimeric antigen receptor T cells are vulnerable to immunosuppressive mechanisms present within the tumor microenvironment. Oncoimmunology. (2014) 3:e970027. doi: 10.4161/21624011.2014.970027
59. Biasci D, Smoragiewicz M, Connell CM, Wang Z, Gao Y, Thaventhiran JED, et al. CXCR4 inhibition in human pancreatic and colorectal cancers induces an integrated immune response. Proc Natl Acad Sci U.S.A. (2020) 117:28960–70. doi: 10.1073/pnas.2013644117
60. Wang Z, Moresco P, Yan R, Li J, Gao Y, Biasci D, et al. Carcinomas assemble a filamentous CXCL12-keratin-19 coating that suppresses T cell-mediated immune attack. Proc Natl Acad Sci U.S.A. (2022) 119. doi: 10.1073/pnas.2119463119
61. Bilotta MT, Antignani A, and Fitzgerald DJ. Managing the TME to improve the efficacy of cancer therapy. Front Immunol. (2022) 13:954992. doi: 10.3389/fimmu.2022.954992
62. Powell DJ Jr., Felipe-Silva A, Merino MJ, Ahmadzadeh M, Allen T, Levy C, et al. Administration of a CD25-directed immunotoxin, LMB-2, to patients with metastatic melanoma induces a selective partial reduction in regulatory T cells. vivo J Immunol. (2007) 179:4919–28. doi: 10.4049/jimmunol.179.7.4919
63. Rech AJ, Mick R, Martin S, Recio A, Aqui NA, Powell DJ Jr., et al. CD25 blockade depletes and selectively reprograms regulatory T cells in concert with immunotherapy in cancer patients. Sci Transl Med. (2012) 4:134ra62. doi: 10.1126/scitranslmed.3003330
64. Onda M, Kobayashi K, and Pastan I. Depletion of regulatory T cells in tumors with an anti-CD25 immunotoxin induces CD8 T cell-mediated systemic antitumor immunity. Proc Natl Acad Sci U.S.A. (2019) 116:4575–82. doi: 10.1073/pnas.1820388116
65. Ng S, Deng J, Chinnadurai R, Yuan S, Pennati A, and Galipeau J. Stimulation of natural killer cell-mediated tumor immunity by an IL15/TGFbeta-neutralizing fusion protein. Cancer Res. (2016) 76:5683–95. doi: 10.1158/0008-5472.CAN-16-0386
66. Knudson KM, Hicks KC, Ozawa Y, Schlom J, and Gameiro SR. Functional and mechanistic advantage of the use of a bifunctional anti-PD-L1/IL-15 superagonist. J Immunother Cancer. (2020) 8:e000493. doi: 10.1136/jitc-2019-000493
67. Staniszewska AD, Armenia J, King M, Michaloglou C, Reddy A, Singh M, et al. PARP inhibition is a modulator of anti-tumor immune response in BRCA-deficient tumors. Oncoimmunology. (2022) 11:2083755. doi: 10.1080/2162402X.2022.2083755
68. Panowski SH, Srinivasan S, Tan N, Tacheva-Grigorova SK, Smith B, Mak YSL, et al. Preclinical development and evaluation of allogeneic CAR T cells targeting CD70 for the treatment of renal cell carcinoma. Cancer Res. (2022) 82:2610–24. doi: 10.1158/0008-5472.CAN-21-2931
69. Choe JH, Watchmaker PB, Simic MS, Gilbert RD, Li AW, Krasnow NA, et al. SynNotch-CAR T cells overcome challenges of specificity, heterogeneity, and persistence in treating glioblastoma. Sci Transl Med. (2021) 13:eabe7378. doi: 10.1126/scitranslmed.abe7378
70. Bernstein MB, Garnett CT, Zhang H, Velcich A, Wattenberg MM, Gameiro SR, et al. Radiation-induced modulation of costimulatory and coinhibitory T-cell signaling molecules on human prostate carcinoma cells promotes productive antitumor immune interactions. Cancer Biother Radiopharm. (2014) 29:153–61. doi: 10.1089/cbr.2013.1578
71. Safarzadeh Kozani P, Safarzadeh Kozani P, and Rahbarizadeh F. CAR-T cell therapy in T-cell Malignancies: Is success a low-hanging fruit? Stem Cell Res Ther. (2021) 12:527. doi: 10.1186/s13287-021-02595-0
72. De Munter S, Buhl JL, De Cock L, Van Parys A, Daneels W, Pascal E, et al. Knocking out CD70 rescues CD70-specific nanoCAR T cells from antigen-induced exhaustion. Cancer Immunol Res. (2024) 12:1236–51. doi: 10.1158/2326-6066.CIR-23-0677
73. Jin BK, Odongo S, Radwanska M, and Magez S. NANOBODIES(R): A review of diagnostic and therapeutic applications. Int J Mol Sci. (2023) 24:5994. doi: 10.3390/ijms24065994
74. Khongorzul P, Ling CJ, Khan FU, Ihsan AU, and Zhang J. Antibody-drug conjugates: A comprehensive review. Mol Cancer Res. (2020) 18:3–19. doi: 10.1158/1541-7786.MCR-19-0582
75. Sterner RC and Sterner RM. CAR-T cell therapy: current limitations and potential strategies. Blood Cancer J. (2021) 11:69. doi: 10.1038/s41408-021-00459-7
76. Xie G, Dong H, Liang Y, Ham JD, Rizwan R, and Chen J. CAR-NK cells: A promising cellular immunotherapy for cancer. EBioMedicine. (2020) 59:102975. doi: 10.1016/j.ebiom.2020.102975
77. Tang T, Cheng X, Truong B, Sun L, Yang X, and Wang H. Molecular basis and therapeutic implications of CD40/CD40L immune checkpoint. Pharmacol Ther. (2021) 219:107709. doi: 10.1016/j.pharmthera.2020.107709
Keywords: CD70, cancer immunotherapy, CAR-T therapy, monoclonal antibodies, tumor microenvironment
Citation: Wu R, Chen J, Wang G and Han L (2025) CD70 as a target in cancer immunotherapy: advances, challenges, and future directions. Front. Oncol. 15:1609840. doi: 10.3389/fonc.2025.1609840
Received: 11 April 2025; Accepted: 28 July 2025;
Published: 15 August 2025.
Edited by:
Tao Zhang, Memorial Sloan Kettering Cancer Center, United StatesReviewed by:
Yuxiang Wang, Case Western Reserve University, United StatesXiaoyi Zhang, Jacobi Medical Center, United States
Shukun Yang, Johnson & Johnson, United States
Copyright © 2025 Wu, Chen, Wang and Han. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gang Wang, d2FuZ2dAeHpobXUuZWR1LmNu; Lulu Han, TExIYW5AeHpobXUuZWR1LmNu
†These authors have contributed equally to this work and share first authorship
‡These authors have contributed equally to this work and share last authorship
 Ruchen Wu1†
Ruchen Wu1† Gang Wang
Gang Wang Lulu Han
Lulu Han