- 1Division of Head & Neck Tumor Multimodality Treatment, Cancer Center, West China Hospital, Sichuan University, Chengdu, China
- 2Department of Targeting Therapy & Immunology, Cancer Center, West China Hospital, Sichuan University, Chengdu, China
- 3Sichuan Kelun-Biotech Biopharmaceutical Co. Ltd., Chengdu, China
- 4Division of Abdominal Tumor Multimodality Treatment, Department of Radiation Oncology, Cancer Center, West China Hospital, Sichuan University, Chengdu, China
by Liu M, Liu Z, He S, Pei Y, Xu S, Ge J, Qing Y, Wei Y, Chen Y, Ai P and Peng X (2025) Front. Oncol. 15:1539514. doi: 10.3389/fonc.2025.1539514
In the published article “Shi Y, Qin X, Peng X, Zeng A, Li J, Chen C, et al. Efficacy and safety of KL-A167 in previously treated recurrent or metastatic nasopharyngeal carcinoma: a multicenter, single-arm, phase 2 study. Lancet Reg Health West Pac, 2023, 31: 100617” was not cited in the article. The citation has now been inserted in Methods, Data source, Paragraph 1 and should read:
“This study used data from an open-label, multicenter phase 2 clinical trial conducted between 2017 and 2019 at 42 hospitals in China involving 153 patients with NPC (Supplementary Figure 1) (6)”.
In the published article, there was an error in Table 1 as published. In the ECOG performance status row, the current version displays the numerical coding used for statistical modeling (i.e., 1 and 2). This should be revised to reflect the actual clinical values (ECOG = 0 and ECOG = 1), as presented to clinicians. The corrected Table 1 and its caption “Baseline characteristics of patients with and without immune-related adverse events (irAEs)” appear below.
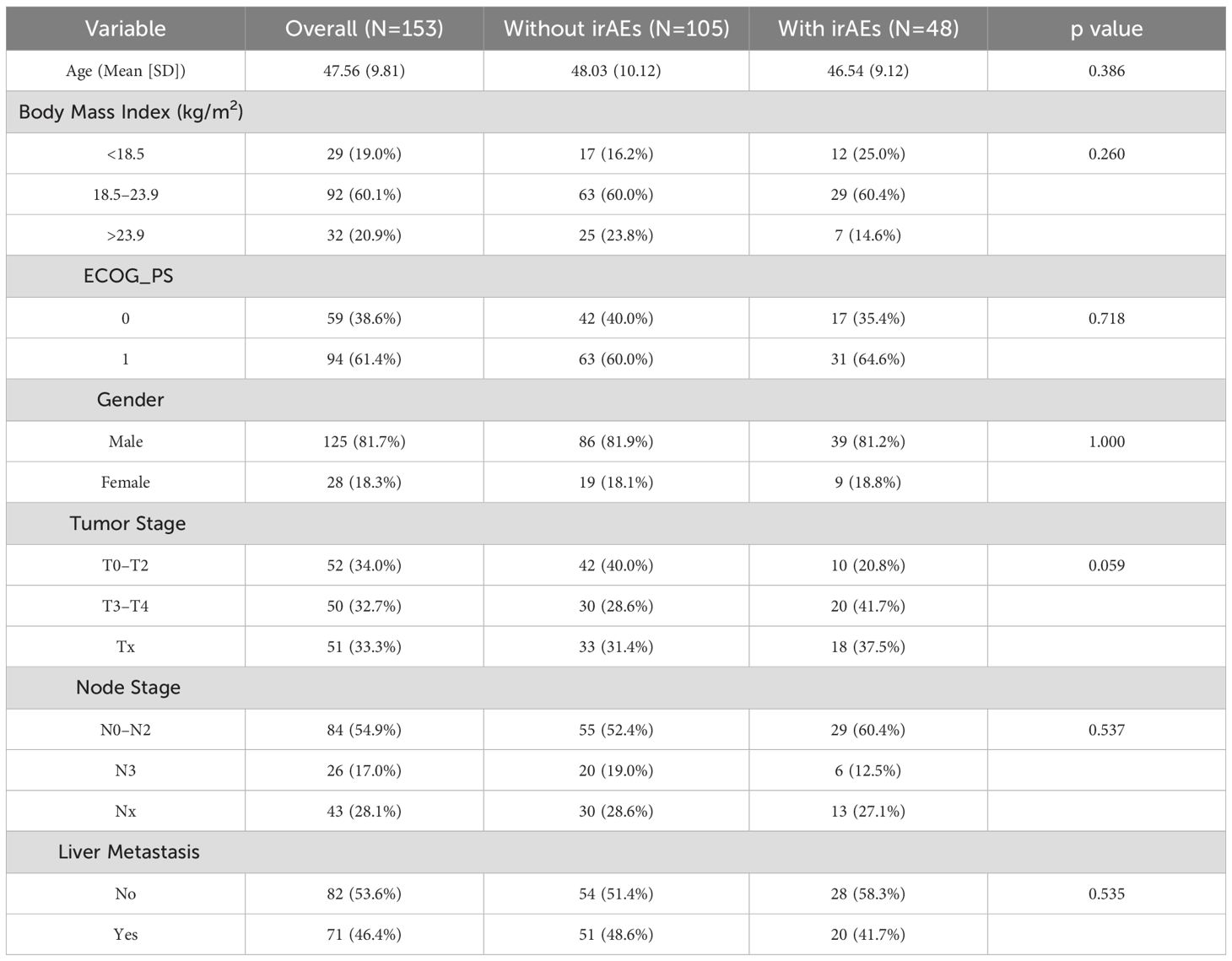
Table 1. Baseline characteristics of patients with and without immune-related adverse events (irAEs).
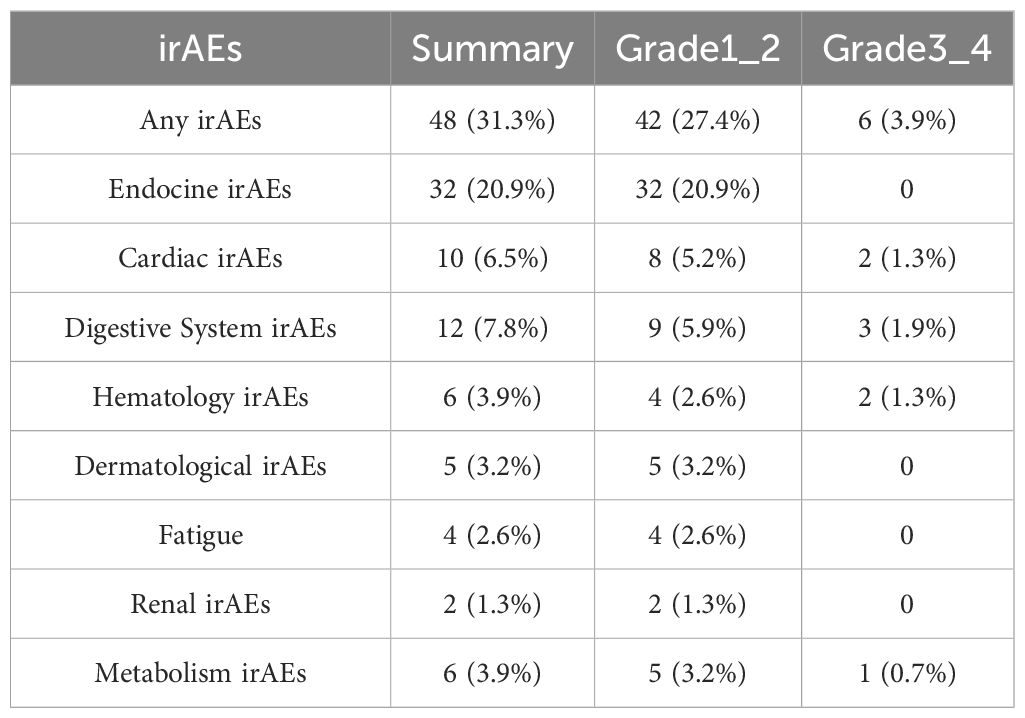
In the published article, there was an error in Table 2 as published. A portion of the fatigue-related data was inadvertently omitted during table compilation. Upon verification, the total number of fatigue events, including Grade 1–2, should be 4. The corrected Table 2 and its caption “Incidence and severity of immune-related adverse events (irAEs) among patients” appear below.
In the published article, there was an error in Figure 4 as published. After publication, we realized that the survival curve presented in Figure 4 overlaps with data previously published by Dr. Shihong Xu in Oral Oncology (2025; https://doi.org/10.1016/j.oraloncology.2024.107161). Dr. Xu is one of our research collaborators. Although using the same database, we analysed different markers. We focused on the predictive modeling. We acknowledge that including this figure may result in unintended duplication. To maintain clarity and proper attribution, we respectfully request the removal of Figure 4 from the published article.
In the published article, there was an error in Supplementary Figure 2. Upon verification, the total number of fatigue events, including Grade 1–2, should be 4.
In the published article, there was an error. As in the case of Figure 4, we removed the sentence discussing survival analysis, which overlapped with results already reported by Dr. Shihong Xu in Oral Oncology (2025). Therefore, we do not elaborate on this point further in the current article.
A correction has been made to Discussion, Paragraph 3. This sentence previously stated:
“Further KM survival curve analysis revealed that patients who experienced irAEs had better PFS, suggesting that the occurrence of irAEs may be associated with a better treatment response (Figure 4).”
This sentence has been removed.
The authors apologize for this error and state that this does not change the scientific conclusions of the article in any way. The original article has been updated.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Keywords: NPC, irAEs, PD-L1 inhibitors, biomarkers, nomogram
Citation: Liu M, Liu Z, He S, Pei Y, Xu S, Ge J, Qing Y, Wei Y, Chen Y, Ai P and Peng X (2025) Corrigendum: Development and validation of nomogram models for predicting immune-related adverse events in recurrent and metastatic nasopharyngeal carcinoma patients treated with PD-L1 inhibitors. Front. Oncol. 15:1610079. doi: 10.3389/fonc.2025.1610079
Received: 11 April 2025; Accepted: 28 April 2025;
Published: 15 May 2025.
Edited and Reviewed by:
Guopei Zhu, Shanghai Jiao Tong University, ChinaCopyright © 2025 Liu, Liu, He, Pei, Xu, Ge, Qing, Wei, Chen, Ai and Peng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ye Chen, NDYzNTI3NjE3QHFxLmNvbQ==; Ping Ai, YWlwMjAyM0AxNjMuY29t; Xingchen Peng, cHh4MjAxNEAxNjMuY29t
 Mengyuan Liu
Mengyuan Liu Zheran Liu
Zheran Liu Shuangshuang He
Shuangshuang He Yiyan Pei
Yiyan Pei Shihong Xu
Shihong Xu Junyou Ge3
Junyou Ge3 Ye Chen
Ye Chen Xingchen Peng
Xingchen Peng