- West China Hospital, Sichuan University, Chengdu, China
Pancreatoblastoma (PB) is a rare malignant neoplasm of the pancreas, primarily affecting children. While some reports have described the imaging characteristics of PB, detailed descriptions of its ultrasound (US) and contrast-enhanced ultrasound (CEUS) features in children are limited. We reported two cases of PB admitted to our hospital with detailed ultrasonographic features. The first case involved a 14-year-old girl who presented with intermittent, unexplained epigastric pain. CEUS revealed a hypoechoic mass with heterogeneous hyperenhancement in the pancreatic head. She underwent pancreaticoduodenectomy and remains disease-free to date. The second case was a 4-year-old boy with a palpable, unexplained mass in the right upper abdomen. US identified a well-defined, heterogeneous mass in the epigastric region with internal point-like hyperechoic areas. The intraoperative US showed portal vein cancer thrombus. He underwent tumor resection along with reconstruction of the portal and superior mesenteric veins. He subsequently received chemotherapy and remained disease-free to date.
Introduction
Pancreatoblastoma (PB) is one of the most common malignant pancreatic neoplasms in children (1, 2). It is typically a slow-growing tumor with non-specific clinical presentations, often resulting in delayed diagnosis until the disease has reached an advanced stage (3). Since its first description by Frable et al. (4), several reports have discussed the imaging features of PB; however, detailed accounts of its ultrasonic characteristics remain rare. In this report, we present two cases of pediatric PB, providing a comprehensive analysis of their ultrasonographic features and emphasized the role of CEUS and intraoperative ultrasound.
Cases presentation
Case 1
A 14-year-old girl presented with unprovoked intermittent epigastric pain for the past 6 months. She also reported occasional episodes of nausea and vomiting. Abdominal palpation revealed no tenderness, rebound tenderness, or muscle tension. No palpable abdominal masses were detected. Tumor markers, including carcinoembryonic antigen (CEA), carbohydrate antigen 125 (CA125), and carbohydrate antigen 19-9 (CA19-9), were not significantly elevated. However, alpha-fetoprotein (AFP) was elevated at 395 ng/mL.
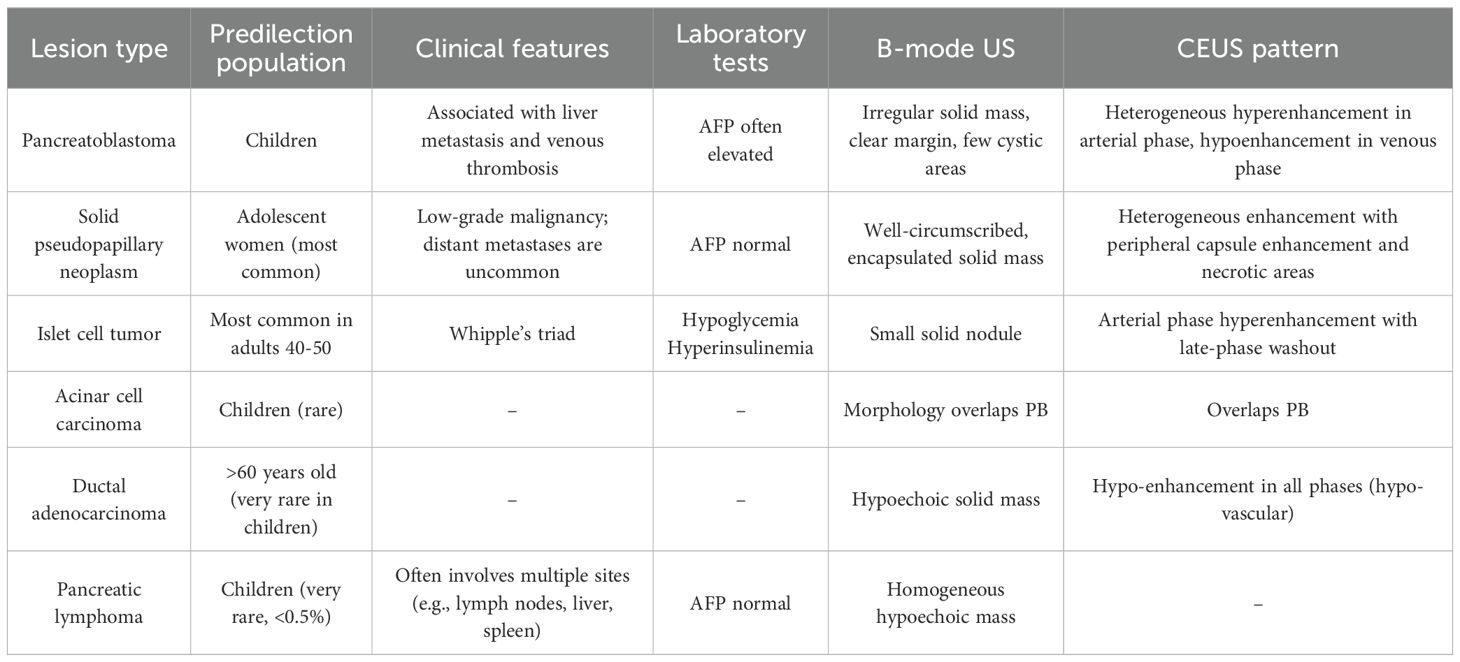
Abdominal US revealed an irregular, hypoechoic mass in the pancreatic head, measuring 5.6×4.8×6.3 cm, with indistinct margins and no obvious blood flow signals (Figure 1). The patient underwent CEUS with the patient’s consent for further diagnosis. A 1.2-mL US contrast agent SonoVue (Bracco, Milan, Italy) suspension was injected as a bolus (injection time <3 s) through the left cubital vein followed by a flush with 5 mL saline. The mass exhibits arterial-phase hyperenhancement of its solid components, demonstrating greater conspicuity than surrounding pancreatic parenchyma, with persistent non-enhancing necrotic regions (Figure 2A). Venous-phase imaging reveals rapid contrast washout in the solid components, resulting in hypoenhancement relative to pancreatic tissue, whereas necrotic areas maintain their avascular characteristics (Figure 2B). Contrast-enhanced computed tomography (CECT) showed a 6.0×4.5 cm soft tissue density tumor with indistinct margins in the head of the pancreas, exhibiting heterogeneous hyperenhancement and a hypodense necrotic area.
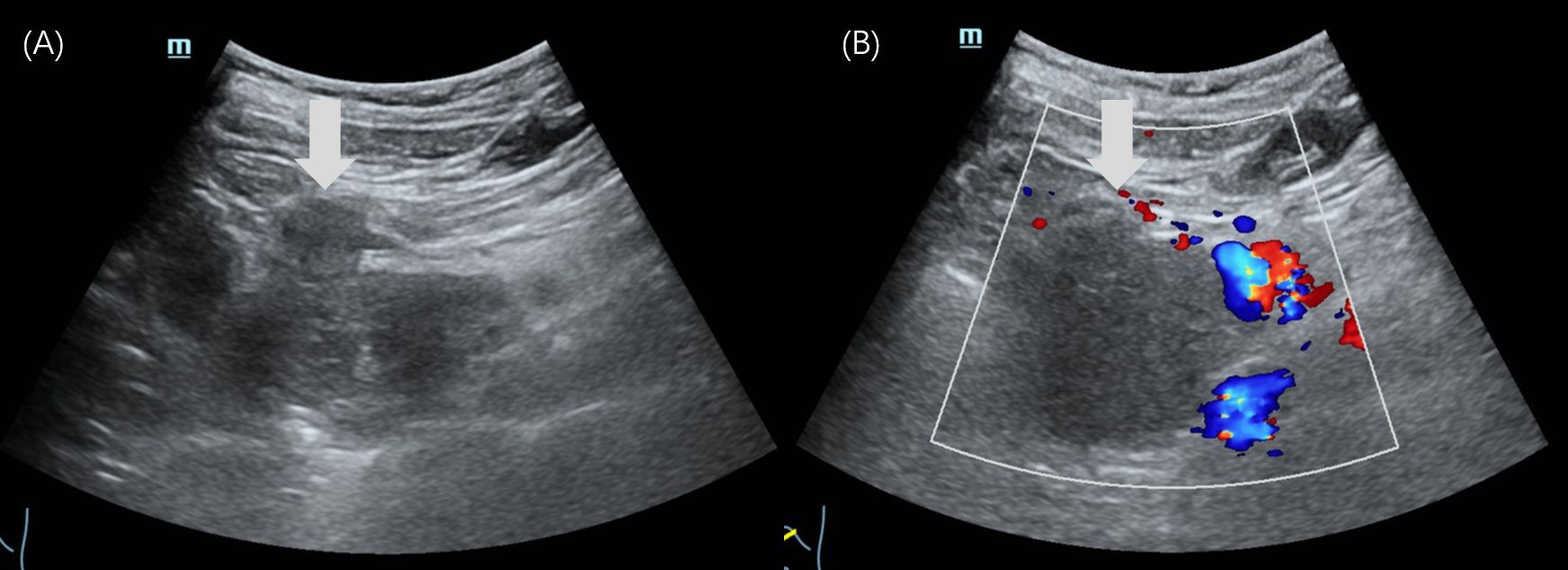
Figure 1. Ultrasound images of case 1. (A) The gray-scale US showed a 5.6×4.8×6.3cm irregular-shaped, indistinct-bounded, hypoechoic mass (arrow) in the pancreatic head. (B) The color Doppler imaging showed no obvious signal of blood flow within the mass (arrow).
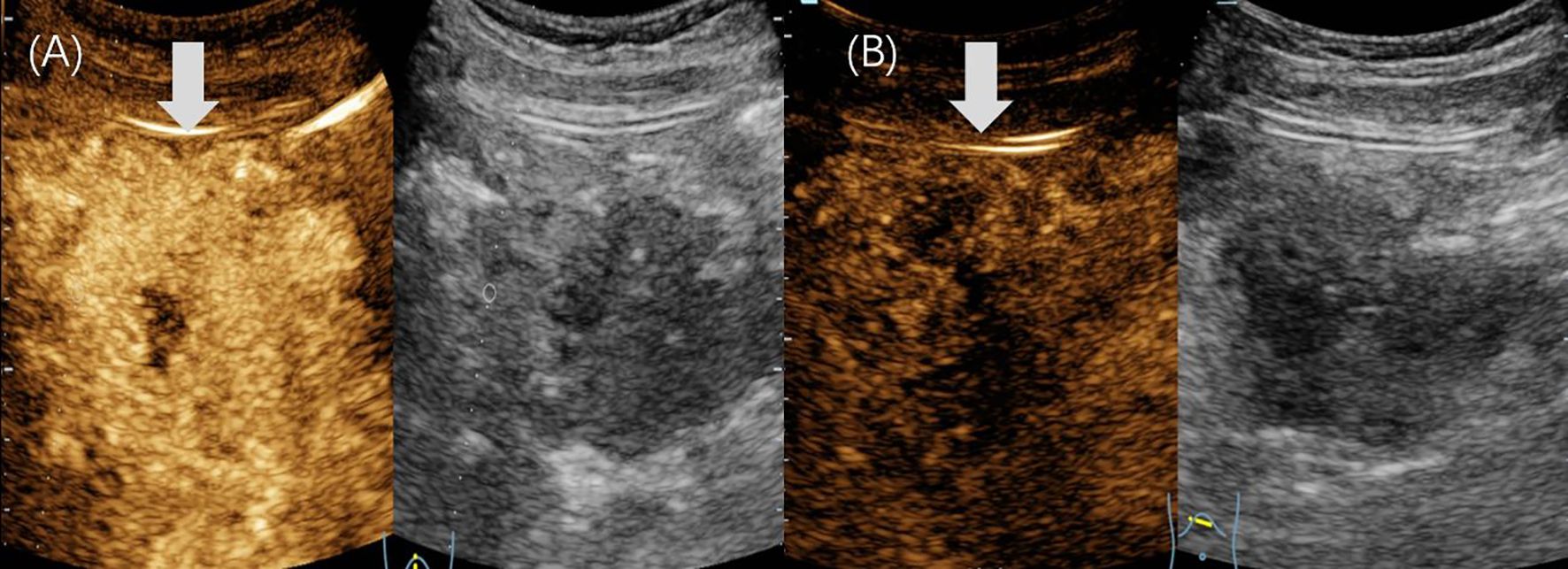
Figure 2. Contrast-enhanced ultrasound images of case 1. (A) In the arterial phase, the mass showed heterogeneous hyperenhancement (arrow). (B) The mass showed hypoenhancement (arrow) in the venous phase.
Based on the imaging findings, laboratory results, and the incidence of pancreatic tumors, the clinical diagnosis was pancreatoblastoma. She underwent pancreaticoduodenectomy, cholecystectomy, and resection and reconstruction of the portal and superior mesenteric veins. Intraoperative examination revealed a firm pancreas with marked inflammatory edema of the gland and surrounding tissues, whereas a hard, multinodular mass was identified in the pancreatic head and the uncinate process exhibited cystic-necrotic changes. Intraoperative US findings were consistent with the preoperative observations, with no new positive findings identified. Moreover, the clinical diagnosis was later confirmed by postoperative pathology. Immunohistochemical staining results were as follows: β-catenin (−), CK 8/18 (+), CgA (+), E-C 99 (+), GPC-3 (+). At the 23-month follow-up, there was no evidence of disease recurrence.
Case 2
A 4-year-old boy presented to our hospital with an unexplained mass in the right upper abdomen for 1 week. Since the onset of the condition, he did not report any abdominal pain, nausea, vomiting, fever, or jaundice. A computed tomography (CT) scan conducted at a local hospital revealed a mass in the upper abdomen, initially suspected to be a right adrenal neuroblastoma.
On examination, a firm, non-tender mass measuring approximately 8 × 10 cm was palpable in the right upper abdomen, with limited mobility. Tumor markers, including CEA and CA19-9, were not significantly elevated. However, both AFP and neuron-specific enolase (NSE) levels were elevated (AFP: 1210 ng/mL, NSE: 51.2 ng/mL). Abdominal US revealed a well-defined, heterogeneous mass in the epigastrium, measuring 10.6 × 7.1 cm, with internal point-like hyperechoic regions. Dotted linear blood flow was detected within the mass (Figure 3). No enlarged lymph nodes were observed in the abdominal cavity. Additionally, CECT revealed a mixed-density mass in the hepatogastric space, measuring approximately 10.5 × 6.5 cm, with low-density areas and scattered calcifications. In the arterial phase, the mass exhibited heterogeneous hyperenhancement.
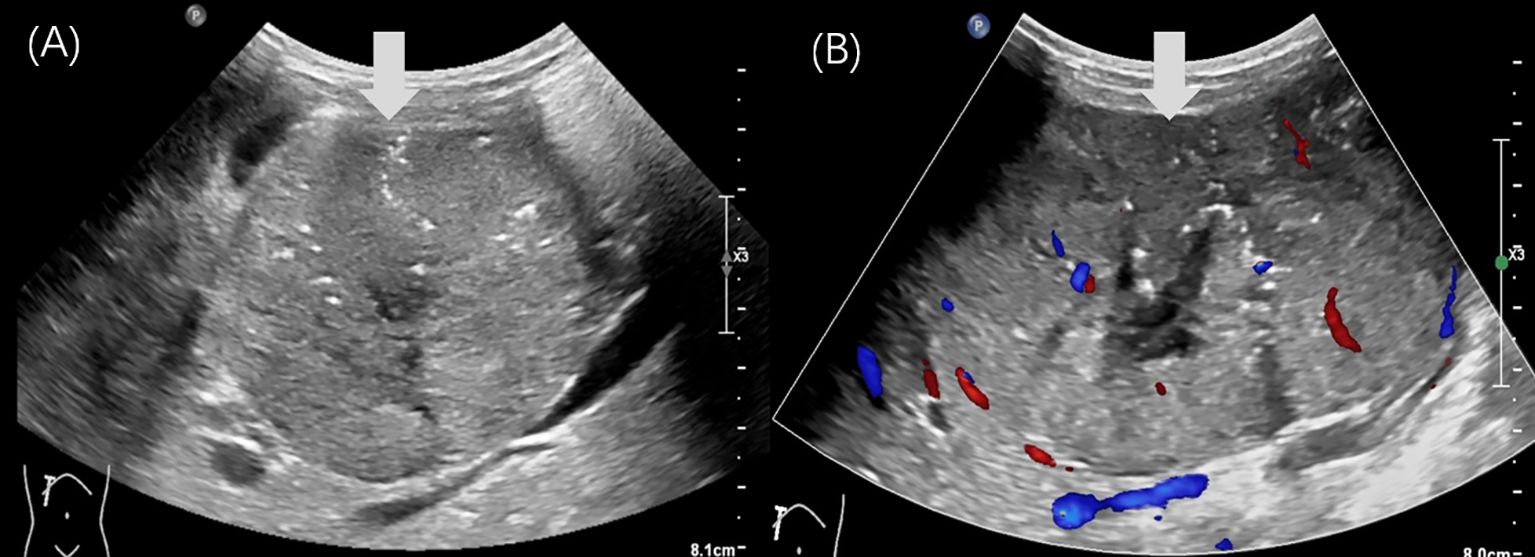
Figure 3. Ultrasound images of case 2. (A, B) The gray-scale US revealed a regular-shaped, heterogeneous, epigastric mass (arrow) with internal point-like hyperechoic, measuring 10.6×7.1cm in diameter. (B) The color Doppler imaging showed that a dotted linear blood flow (arrow) was visible within it.
Based on the imaging findings and laboratory results, the patient was clinically diagnosed with a retroperitoneal malignant tumor. Laparotomy revealed a large, cystic, and poorly mobile cystic-solid tumor in the retroperitoneal space. The mass demonstrated a vascular-rich surface, displaced the duodenum and hepatoduodenal ligament anteriorly, and invaded surrounding structures. It also encased the celiac trunk, portal vein, and its tributaries, compressing adjacent organs. The intraoperative US detected cancer thrombus in the portal vein (Figure 4), whereas no thrombus was detected in the inferior vena cava. Tumor resection was performed along with reconstruction of the portal and superior mesenteric veins. Postoperative histopathology revealed a round cell tumor with rosette formations under microscopic examination. Immunohistochemical staining showed the following results: β-catenin+ (nuclear staining of some cells), cytokeratin+, CK 19+, CD10+, CD99+, and Ki-67+ at 70%. These findings confirmed the diagnosis of pancreatoblastoma. The patient subsequently received chemotherapy. At the 21-month follow-up, there were no signs of tumor recurrence.
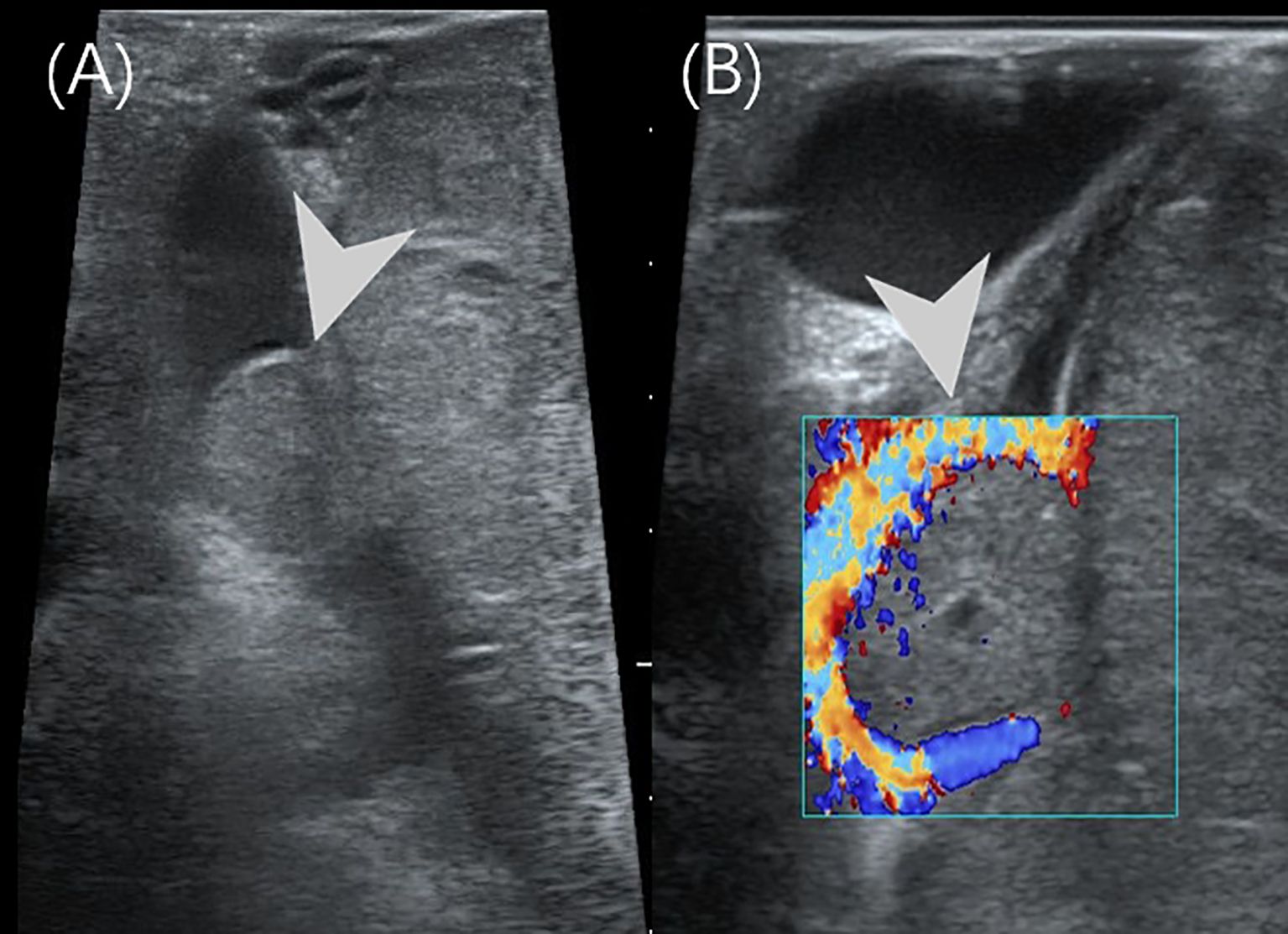
Figure 4. Intraoperative US of case 2. (A) The intraoperative US showed a 1.5×1.0cm hypoechoic nodules (arrowhead) in the main trunk of the portal vein. (B) The color Doppler imaging showed a filling defect in the main trunk of the portal vein.
Discussion
PB, also known as infantile pancreatic carcinoma, is an extremely rare pancreatic tumor in childhood, accounting for approximately 0.5% of pancreatic non-endocrine tumors (5). The term “pancreatoblastoma” was first introduced by Horie et al. in 1977 (6). PB predominantly affects young children, with a median age of 5 years (7). Boys are affected approximately twice as often as girls (8). Its incidence appears to be relatively higher in East Asia (9). PB is thought to arise from multipotent stem cells, as evidenced by the presence of endocrine components, acinar cells with zymogen granules, and elevated AFP levels (10). Approximately 25%–55% of PB patients exhibit elevated AFP levels (11). The primary symptoms reported were abdominal pain and a palpable abdominal mass, occasionally accompanied by anorexia, diarrhea, and fever. In cases where the tumor compresses the biliary system, obstructive jaundice may occur.
Imaging studies play a crucial role in the initial diagnosis and evaluation of PB, particularly in assessing tumor characteristics and the extent of invasion. Previous reports indicate that PB typically appears as a heterogeneous or predominantly hypoechoic mass on US, sometimes containing small fluid-filled areas (9). In our two cases, one patient presented with a hypoechoic mass, whereas the other had a heterogeneous mass. Remarkably, one of our patients underwent CEUS, which showed arterial-phase hyperenhancement of solid components with persistent non-enhancing necrotic regions, followed by rapid venous-phase washout. There have been no reports of CEUS findings in pediatric PB except one case with adult PB who showed equal enhancement during the arterial phase and slightly faster washout in the delayed phase (12), which was consistent with our finding. It is recommended that CEUS be performed immediately after the detection of a pancreatic lesion provided superior lesion perfusion, contrast, and spatial resolution, in order to significantly enhance the accuracy of initial diagnostic imaging. The particular advantage of CEUS in the pediatric population lies in its dynamic assessment of microvascular perfusion in real time, without exposing the child to ionizing radiation. Unlike the fixed phases of CT and magnetic resonance imaging (MRI), CEUS allows continuous observation of contrast uptake and washout patterns, which may provide unique hemodynamic information. In these presented cases, the rapid washout pattern observed in CEUS contributed key diagnostic information. Therefore, rather than replacing these modalities, CEUS serves as a valuable complementary tool—particularly in specific clinical scenarios such as initial evaluation, follow-up of known lesions, or when other imaging findings are equivocal. Future studies directly comparing the diagnostic performance of these modalities in pediatric pancreatic tumors would be of significant clinical value.
The incidence of PB is very low, it often needs to be differentiated from other four main types of pancreatic tumors in children: solid pseudopapillary neoplasm (SPN), islet cell tumor, acinar cell carcinoma (ACC), and ductal adenocarcinoma (DAC) (1). SPN is currently regarded as the most common pancreatic tumor in children (2). It predominantly occurs in older children, particularly among adolescent women (13). On grayscale US, SPN typically presents as a well-defined, regular-shaped solid mass with a capsule, whereas on CEUS, it characteristically demonstrates heterogeneous enhancement with peripheral capsule enhancement and internal necrotic non-enhancement areas (14). Although SPN of the pancreas is classified as a low-grade malignancy, distant metastases and venous thrombosis are uncommon. A key differentiating feature is the serum AFP level: While SPN typically presents with normal AFP, elevated AFP is frequently observed in PB and serves as an important diagnostic clue (13). US and other imaging findings of PB include irregular morphology, clear boundaries, solid composition, and minimal evidence of large cystic areas or necrosis with heterogeneous hyperenhancement in the arterial phase and hypoenhancement in the venous phase. Additionally, PB may present secondary complications such as liver metastasis and venous thrombosis, one of two cases admitted to our hospital exhibited related secondary changes identified through intraoperative US.
Islet cell tumors are a type of functioning neuroendocrine tumor that typically occur in adults aged 40 to 50, often presenting with refractory hypoglycemia in children, so they are detected early. The size of mass is usually small, and it is easy to diagnose correctly depending on hypoglycemia in laboratory examinations, a conclusion further supported by its characteristic hypervascular pattern on CEUS, typically demonstrating rapid hyperenhancement in the arterial phase followed by washout (15). Approximately 15% of pediatric pancreatic tumors are ACCs (16), although cases of pancreatic ACCs in children are rare (17). Differentiating ACC from PB can be challenging due to their overlapping morphological and immunohistochemical features (18). The ability of radiomics to capture subtle tissue heterogeneity through texture and higher-order features offers a powerful tool for distinguishing phenotypically similar pancreatic tumors (19, 20). For instance, CT-based radiomic models can differentiate pancreatic neuroendocrine tumors from DAC with high accuracy (AUC 0.86–0.99) (20). This principle could potentially aid in refining SPN and PB differentiation by quantifying texture or enhancement differences. DACs are more common in individuals over 60 years of age and are exceedingly rare in children (21). On CEUS, DAC typically exhibits hypoenhancement in all phases due to its low vascular density (15). In addition to the above differential diagnosis, pediatric pancreatic lymphoma also needs to be differentiated with PB, consisting of less than 0.5% of pancreatic cancers (22). AFP levels in pancreatic lymphoma generally remain within the normal limits. On ultrasound, pancreatic lymphoma typically appears as a homogeneous hypoechoic mass confined to the pancreas (22). Lymphoma frequently involves multiple sites. Further examinations of retroperitoneal and superficial lymph enlargement, intestinal wall, liver, and spleen lesions suggest the possibility of lymphoma, but the final diagnosis still needs pathological examination. In summary, the differentiation of pediatric pancreatic lesions—with PB as the central focus—should be approached comprehensively, taking into account incidence, clinical presentation, laboratory markers, and ultrasound imaging features. To facilitate a clearer and more concise comparison, the key information is summarized in the table (Table 1) below.
The final definitive diagnosis of PB relies on histopathology. Histological findings include the presence of characteristic squamous corpuscles and tumor cells with acinar, glandular, or undifferentiated features (3). Complete tumor resection is a key factor influencing prognosis in children with PB. For patients in whom surgical resection is not feasible, preoperative chemotherapy is recommended to reduce tumor size and create conditions favorable for surgery. Additionally, appropriate postoperative chemotherapy can help prevent tumor recurrence and prolong survival in cases of incomplete tumor resection (1). In our two cases, intraoperative ultrasound revealed a portal vein cancer thrombus, directly influencing the surgical approach. As a simple bedside imaging modality, intraoperative US provides surgeons with real-time information about lesions, as well as the extent of malignant tumor invasion into surrounding tissues and blood vessels. Therefore, intraoperative US should be performed as much as possible during surgery in PB children as an important addition to routine imaging examinations in order to ensure the surgery effectiveness and the best prognosis. This aligns with the broader role of intraoperative imaging guidance in minimally invasive pancreatic surgery (MIPS), where real-time anatomical visualization is critical for assessing resectability and preventing vascular injury (23). While PB surgery is frequently performed via open approaches given its rarity in children and potential for vascular involvement, the principles of intraoperative imaging, such as confirming tumor margins or vascular invasion, remain equally relevant (19).
In summary, accurately diagnosing PB prior to surgery remains a significant challenge. While PB lacks specific imaging characteristics, imaging can still provide clinicians with critical information regarding the tumor’s location, size, nature, extent of invasion, and blood supply, which aids in early and accurate diagnosis and treatment. Compared with 2D gray-scale US, CEUS offers more comprehensive and detailed information of lesions, enhancing the likelihood and accuracy of early diagnosis. Intraoperative US can provide real-time insights about the extent of malignant tumor invasion, assisting surgeons in making intraoperative decisions to ultimately achieving a better prognosis. It is important to note that the present report is limited to two cases, which curtails the generalizability of our sonographic observations. Future efforts should focus on large-scale, multicenter collaborations to corroborate the described imaging features of pediatric PB and to definitively establish the value of CEUS in standard diagnostic protocols.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Written informed consent was obtained from the individual(s), and minor(s)’ legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article. Written informed consent was obtained from the participant/patient(s) for the publication of this case report.
Author contributions
XZ: Writing – original draft. HY: Writing – review & editing. JY: Writing – review & editing. BX: Writing – review & editing. JL: Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Lee YJ and Hah JO. Long-term survival of pancreatoblastoma in children. J Pediatr hematology/oncology. (2007) 29:845–7. doi: 10.1097/MPH.0b013e3181581576
2. Ahmed TS, Chavhan GB, Navarro OM, and Traubici J. Imaging features of pancreatic tumors in children: 13-year experience at a pediatric tertiary hospital. Pediatr radiology. (2013) 43:1435–43. doi: 10.1007/s00247-013-2721-2
3. Huang Y, Yang W, Hu J, Zhu Z, Qin H, Han W, et al. Diagnosis and treatment of pancreatoblastoma in children: a retrospective study in a single pediatric center. Pediatr Surg Int. (2019) 35:1231–8. doi: 10.1007/s00383-019-04524-y
4. Frable WJ, Still WJ, and Kay S. Carcinoma of the pancreas, infantile type. A light and electron microscopic study. Cancer. (1971) 27:667–73. doi: 10.1002/1097-0142(197103)27:3<667::AID-CNCR2820270323>3.0.CO;2-N
6. Horie A, Yano Y, Kotoo Y, and Miwa A. Morphogenesis of pancreatoblastoma, infantile carcinoma of the pancreas: report of two cases. Cancer. (1977) 39:247–54. doi: 10.1002/1097-0142(197701)39:1<247::AID-CNCR2820390138>3.0.CO;2-F
7. Mercadal-Hally M, Vaidya S, Vilca-Melendez H, Heaton N, Dhawan A, and Grammatikopoulos T. Pancreatoblastoma: A rare indication for liver transplantation in children. Hepatobiliary pancreatic Dis international: HBPD Int. (2020) 19:499–501. doi: 10.1016/j.hbpd.2020.07.003
8. Cao L and Liu D. Diagnosis and treatment of pancreatoblastoma in China. Pancreas. (2007) 34:92–5. doi: 10.1097/01.mpa.0000240610.33936.cb
9. Roebuck DJ, Yuen MK, Wong YC, Shing MK, Lee CW, and Li CK. Imaging features of pancreatoblastoma. Pediatr radiology. (2001) 31:501–6. doi: 10.1007/s002470100448
10. Gu WZ, Zou CC, Zhao ZY, Liang L, and Tang HF. Childhood pancreatoblastoma: clinical features and immunohistochemistry analysis. Cancer letters. (2008) 264:119–26. doi: 10.1016/j.canlet.2008.01.026
11. Nijs E, Callahan MJ, and Taylor GA. Disorders of the pediatric pancreas: imaging features. Pediatr radiology. (2005) 35:358–73. doi: 10.1007/s00247-004-1326-1
12. Li J, Peng C, Fan X, Wang L, and Wang J. Adult pancreatoblastoma: a case report. J Int Med Res. (2021) 49:3000605211039565. doi: 10.1177/03000605211039565
13. Yang Z, Gong Y, Ji M, Yang B, and Qiao Z. Differential diagnosis of pancreatoblastoma (PB) and solid pseudopapillary neoplasms (SPNs) in children by CT and MR imaging. Eur radiology. (2021) 31:2209–17. doi: 10.1007/s00330-020-07309-3
14. De Robertis R, D’Onofrio M, Crosara S, Dal Corso F, Barbi E, Canestrini S, et al. Contrast-enhanced ultrasound of pancreatic tumours. Australas J Ultrasound Med. (2014) 17:96–109. doi: 10.1002/j.2205-0140.2014.tb00032.x
15. Bartolotta TV, Randazzo A, Bruno E, Alongi P, and Taibbi A. Focal pancreatic lesions: role of contrast-enhanced ultrasonography. Diagnostics (Basel Switzerland). (2021) 11:957–971. doi: 10.3390/diagnostics11060957
16. Klimstra DS. Nonductal neoplasms of the pancreas. Modern pathology: an Off J United States Can Acad Pathology Inc. (2007) 20 Suppl 1:S94–112. doi: 10.1038/modpathol.3800686
17. Huang Y, Cao YF, Lin JL, Gao F, and Li F. Acinar cell cystadenocarcinoma of the pancreas in a 4-year-old child. Pancreas. (2006) 33:311–2. doi: 10.1097/01.mpa.0000229011.70161.d9
18. Illyés G, Luczay A, Benyó G, Kálmán A, Borka K, Köves K, et al. Cushing’s syndrome in a child with pancreatic acinar cell carcinoma. Endocrine pathology. (2007) 18:95–102. doi: 10.1007/s12022-007-0018-4
19. De Robertis R, Todesco M, Autelitano D, Spoto F, and D’Onofrio M. The role of radiomics in hepato-bilio-pancreatic surgery: a literature review. Artif Intell Surgery. (2023) 3:166–79. doi: 10.20517/ais.2023.18
20. Grewal M, Ahmed T, and Javed AA. Current state of radiomics in hepatobiliary and pancreatic Malignancies. Artif Intell Surgery. (2023) 3:217–32. doi: 10.20517/ais.2023.28
21. Lüttges J, Stigge C, Pacena M, and Klöppel G. Rare ductal adenocarcinoma of the pancreas in patients younger than age 40 years. Cancer. (2004) 100:173–82. doi: 10.1002/cncr.11860
22. Anand D, Lall C, Bhosale P, Ganeshan D, and Qayyum A. Current update on primary pancreatic lymphoma. Abdominal Radiol (New York). (2016) 41:347–55. doi: 10.1007/s00261-015-0620-8
Keywords: pancreatoblastoma, ultrasound, contrast-enhanced ultrasound, intraoperative ultrasound, pediatrics, case report
Citation: Zeng X, Yan H, Yang J, Xiang B and Liu J (2025) Case Report: The crucial role of contrast-enhanced ultrasound and intraoperative ultrasound in diagnosing pediatric pancreatoblastoma. Front. Oncol. 15:1610618. doi: 10.3389/fonc.2025.1610618
Received: 23 May 2025; Accepted: 31 October 2025;
Published: 19 November 2025.
Edited by:
Shihori Tanabe, National Institute of Health Sciences (NIHS), JapanReviewed by:
Heba Taher, Cairo University, EgyptAntonio Galluzzo, Careggi University Hospital, Italy
Copyright © 2025 Zeng, Yan, Yang, Xiang and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Juxian Liu, bGl1anV4aWFuQHdjaHNjdS5jbg==
 Xiangfeng Zeng
Xiangfeng Zeng Hualin Yan
Hualin Yan Jiali Yang
Jiali Yang Juxian Liu
Juxian Liu