- 1Department of Pharmacy, Hai’an People’s Hospital, Nantong, China
- 2Department of Oncology, The First Affiliated Hospital of Nanjing Medical University, Nanjing, China
- 3Department of Pharmacy, The First Affiliated Hospital of Nanjing Medical University, Nanjing, China
Studies have shown that the application of immune checkpoint inhibitors (ICIs) in solid tumors can easily affect the endocrine system, causing disorders of thyroid function, which are usually reversible after treatment cessation in most patients. Serplulimab is a PD-1 inhibitor and is used for treating small cell lung cancer (SCLC). Previous adverse reactions mention the impact of Serplulimab on the release and synthesis of thyroid hormones, but the occurrence of hyperthyroidism and hypothyroidism in the same patient has not ever been reported. We hereby report a 78-year-old patient with SCLC who developed severe hyperthyroidism after the second cycle of Serplulimab treatment. This condition lasted for one month and improved to the normal level, but it turned into hypothyroidism without any distinguishing clinical features after using Serplulimab again. The changes of relevant thyroid hormone levels in each cycle should be recorded and corresponding interventions should be implemented based on features and hormone levels. Moreover, it is necessary to combine the baseline characteristics to predict the possible adverse reactions of the patients in advance and conduct pharmacological monitoring as early as possible.
Introduction
Immune checkpoint inhibitors (ICIs) are currently widely used in the treatment of lung cancer. Serplulimab, a recombinant programmed death 1 (PD-1) inhibitor independently developed in China, has been approved in recent years for use in combination with chemotherapy (etoposide plus carboplatin) as a first-line treatment for extensive-stage small cell lung cancer (1). To further improve therapeutic efficacy and prognosis quality, we are particularly concerned about immune-related adverse events after Serplulimab treatment. The endocrine-related adverse reactions caused by Serplulimab require vigilance from clinical practitioners, mainly including hyperthyroidism or hypothyroidism, pituitary inflammation, adrenal insufficiency, and hyperglycemia (2). In Cheng’s clinical trial, the Serplulimab group experienced a total of 26.2% thyroid dysfunction and 6.2% hyperglycemia (3). Also, Ning et al. introduced a 55-year-old male patient with SCLC who developed type 1 diabetes 68th week of receiving Serplulimab (4). The main practice contradiction of immune-related thyroid injury is that most patients have no obvious symptoms in the early stage, which is easy to be missed (5). Patial patients may have most common manifestations, like fatigue, anorexia, bradycardia etc. While others experience a short period of thyrotoxicosis including sweating, tremor, emaciation, palpitation etc. (6). Here we report a case of asymptomatic hyperthyroidism induced by Serplulimab and followed by hypothyroidism, which is alleviated through the alternative treatment of levothyroxine. Thyroid function should be routinely monitored before ICIs’ treatment, since adverse effects are more likely to occur in the first 3 months. By reviewing relevant domestic and international case reports of adverse reaction and guiding principles, we analyze and discuss the clinical characteristics and intervention measures of immune-related thyroid dysfunction caused by PD-1 inhibitors. Through this case, we propose a comprehensive pharmaceutical intervention for patients with asymptomatic thyroid dysfunction, aiming to provide references for better clinical management of such adverse reactions.
Case presentation
A 78-year-old male patient, weighing 65 kg with a body surface area of 1.71 m², sought medical attention due to the presence of blood-streaked sputum upon waking without any obvious cause. On May 23, 2024, he was admitted to the Oncology Department of Jiangsu Provincial People’s Hospital following a confirmed diagnosis of SCLC (T4N2M1, Stage IV). From May 26, 2024, to November 6, 2024, the patient underwent six cycles of immunotherapy combined with chemotherapy: Serplulimab 200 mg on day 1, Etoposide 160 mg on days 1 to 2 or days 1 to 3, and Carboplatin 460 mg/400 mg on day 1. During this period, the patient experienced mild bone marrow suppression and severe thyroid dysfunction. The patient has a 27-year history of hypertension and has been regularly taking Levamlodipine Besylate, Rosuvastatin Calcium Tablets, and Clopidogrel Bisulfate Tablets. The patient worked as a carpenter with a history of occupational exposure to dust and radioactive materials and has not quit smoking or drinking. Discharge diagnosis including: Immunotherapy for malignant tumors, Maintenance chemotherapy for malignant tumors, Malignant neoplasm of the lung, Hypertension, Primary hypothyroidism.
On November 5, 2024, the patient was admitted for the 6th cycle of immunotherapy combined with chemotherapy: Serplulimab 200 mg on day 1, Etoposide 160mg on days 1–2 and 100mg on day 3, and Carboplatin 400 mg on day 1, supplemented with antiemetic and gastric-protective treatments. On July 25, 2024, the examination revealed hyperthyroidism after the 2nd cycle treatment. The level of free triiodothyronine (FT3) and free thyroxine (FT4) were respectively as high as 30.10 pmol/L and 93.90 pmol/L, while thyrotropin TSH) dropped to less than 0.005 mIU/L. Meanwhile, the thyroglobulin autoantibody (TGAb) at 223.0 IU/ml and anti-thyroid peroxidase (TPOAb) at 212.0 IU/ml (the standard value of TPOAb<34 IU/ml). After receiving the fourth cycle of treatment without any intervention of the treatment plan, the patient’s FT3 and FT4 levels dropped to <0.600 pmol/L and 0.62 pmol/L, while TSH rose to 93.640 mIU/L. This condition persisted until October 5, 2024, when a subsequent hospital examination diagnosed hypothyroidism (the changes of thyroid indicators in Figure 1).
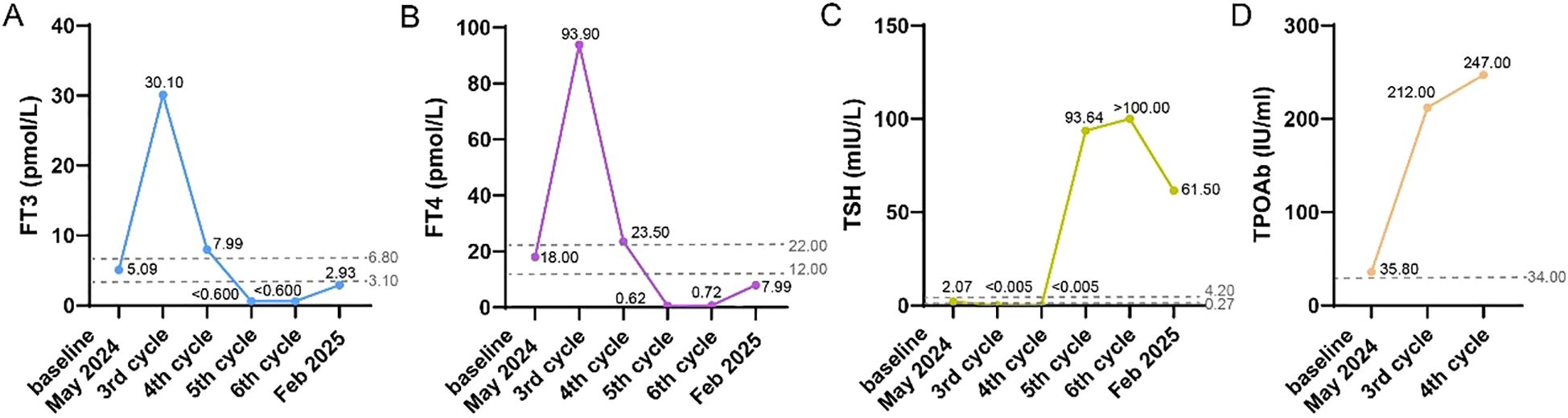
Figure 1. The changes of thyroid indicators from May 2024 (the baseline) to Feb 2025 (three months after the completion of 6 cycles of Serplulimab treatment), including FT3 (A), FT4 (B), TSH (C), TPOAb (D).
During the treatment, the patient remained in good condition without significant discomfort. However, persistent hypothyroidism led to abnormalities in liver and kidney function, myocardial function, and coagulation parameters after 6th cycle (as detailed in the auxiliary examination results in Table 1). To ensure the efficacy of antitumor therapy, Levothyroxine Sodium Tablets (Euthyrox) were administered to regulate thyroid function since 5th cycle. With continuous drug intervention, the laboratory indexes of each organ gradually recovered in February 2025.
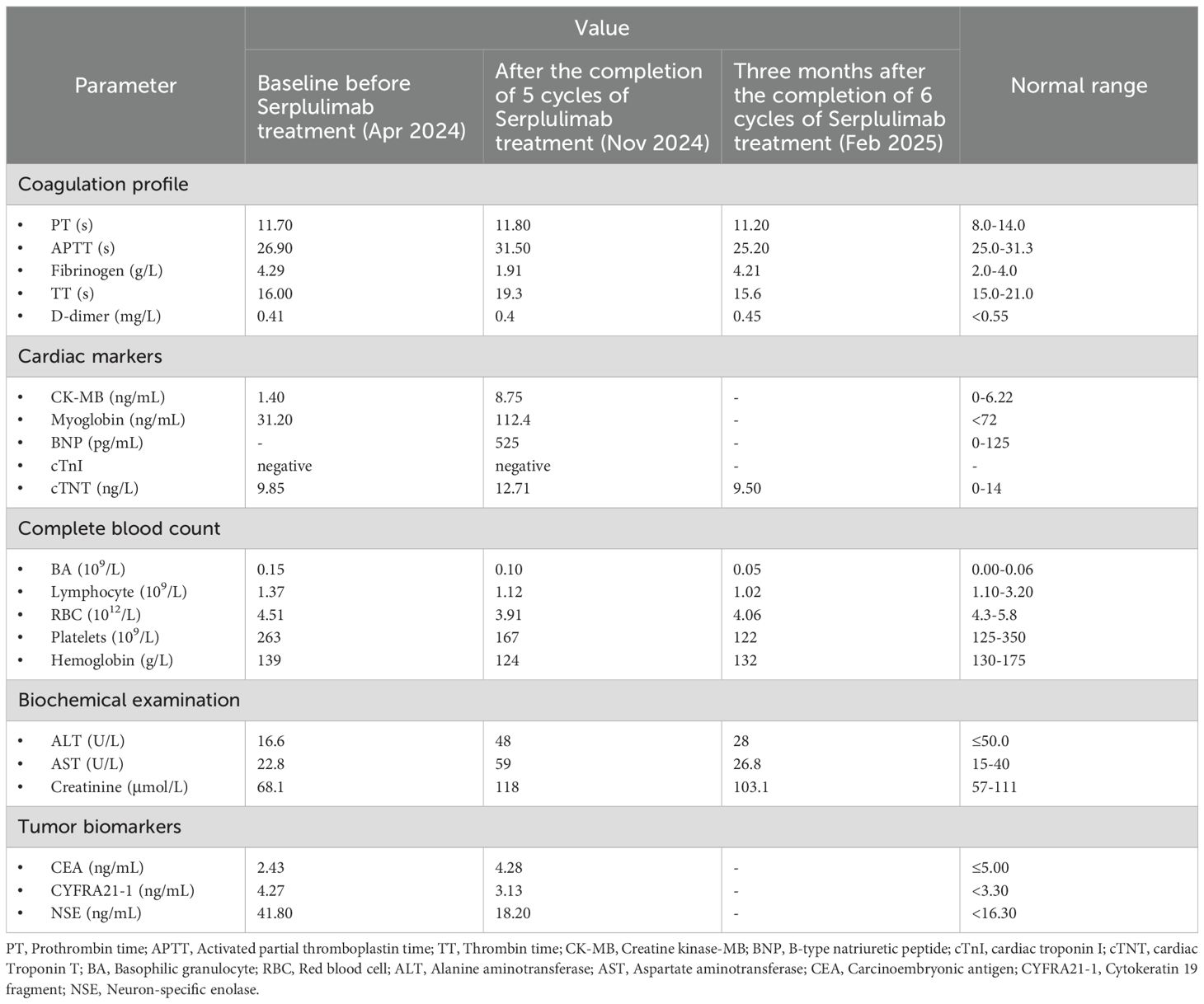
Table 1. The biochemical and relevant parameters of baseline and after cycles of Serplulimab treatment.
Discussion
The patient was treated with a regimen of Serplulimab combined with Etoposide and Carboplatin. After the second cycle of treatment, hyperthyroidism developed and persisted until August 29, 2024, during the fourth cycle of treatment. Thyroid function tests indicated that FT3, FT4, TGAb, and TPOAb levels had risen to several times the standard range, while TSH had decreased to <0.005 mIU/L. By October 2, 2024, during hospitalization, the patient transitioned to secondary hypothyroidism, which persisted until the current admission. Reviewing adverse reaction reports and multiple clinical trial results, there is no documented evidence of endocrine system disorders caused by Etoposide or Carboplatin in lung cancer patients. In contrast, Serplulimab, which mobilizes the body’s immune system to eliminate tumor cells, is more likely to cause changes in endocrine hormones, such as thyroid and adrenal function. Common endocrine-related adverse reactions associated with Serplulimab include: very common hypothyroidism; common hyperthyroidism and thyroiditis; and rare other thyroid disorders, goiter, hypophysitis, and adrenal-related diseases (7, 8). Chinese Expert Consensus on the Emergency Management of Major Endocrine Adverse Reactions Induced by ICIs states that patients who receive ICIs combination treatment may not show any sensible symptoms of thyroid dysfunction at low incidence, but instead exhibit a brief period of thyrotoxicosis (being a surge in the release of FT3 and FT4) (9). Two to twelve weeks later, hypothyroidism occurred, which was similar to the natural course of thyroiditis. The ASTRUM-505 randomized clinical trial compared the impact of Serplulimab versus placebo combined with chemotherapy on the survival of small cell lung cancer patients (3). The Serplulimab group demonstrated a longer median overall survival (15.4 months vs. 10.9 months). However, the Serplulimab group also had a significantly higher incidence of hypothyroidism (14.9% vs. 5%) and hyperthyroidism (11.3% vs. 3.1%) of any severity compared to the placebo group. Although Serplulimab demonstrated significant antitumor efficacy in the course of this patient’s treatment, the incidence of endocrine system dysfunction it caused was second only to that of hematologic toxicity. In this case, the patient received Serplulimab for six times. Using the WHO-UMC causality assessment method, the relationship was judged as “possible,” while the APS scoring method yielded a score of 7, indicating a causality level of “probable.”
In a retrospective data study by Iwamoto et al. (10), it was shown that patients who developed thyroid dysfunction following ICI treatment had significantly higher survival rates. The greater the degree of thyroid dysfunction, the higher the remission rate of ICI treatment. The potential pathophysiological reason for thyroid dysfunction induced by immunotherapy is believed to be immune-mediated acute inflammation, followed by destruction of the thyroid gland (11). Serplulimab treatment directly activates T cells, inducing autoimmune side effects, or alters the expression of human leukocyte antigen-isotype (HLA-DR), indirectly increasing T cell activation (12). Among patients receiving Serplulimab, hyperthyroidism typically occurs earlier, with a median onset time of approximately 1.77 months and a duration of 1.54 months. In contrast, the median time to the onset of hypothyroidism is 3.65 months (7, 8), which explains why the patient’s initial endocrine abnormality manifested as hyperthyroidism. The median half-life of Serplulimab after the first dose and at steady state is approximately 19.0 days and 24.4 days, respectively. After discontinuing the medication, the median time to the resolution of hypothyroidism is 1.87 months. When this patient developed endocrine system disorders, the clear duration far exceeded the 3- to 4-week cycle of antitumor treatment, resulting in continuous thyroid dysfunction. On the other hand, discontinuing immunotherapy due to non-grade 4 severe thyroid dysfunction would significantly impact the patient’s progression-free survival.
Prior to initiating antitumor therapy on May 25, 2024, the patient’s thyroid function indicators were within normal ranges, with no evidence of primary hyperthyroidism or hypothyroidism. However, it is noteworthy that the baseline level of TPOAb was elevated at 35.8 IU/mL, exceeding the normal range. A search on the PubMed database using the keywords “immunotherapy” and “hypothyroidism” revealed that immune checkpoint inhibitor (ICI)-induced hypothyroidism is, to some extent, predictable. A study involving 53 non-small cell lung cancer (NSCLC) patients treated with Nivolumab measured TPOAb and TGAb levels to predict the likelihood of hypothyroidism. The study found that among the 9 patients with baseline TPOAb positivity, 44% (4 out of 9) developed ICI-related hypothyroidism (13). In this case, the patient experienced the less common progression from hyperthyroidism to hypothyroidism without intervention. A possible contributing factor was the elevated baseline levels of TGAb and TPOAb, which became high-risk factors for thyroid dysfunction after the initiation of Serplulimab treatment (14, 15).
According to the Chinese Expert Consensus on the Diagnosis and Treatment of Thyroid Diseases in the Elderly (2021 Edition), drug-induced hyperthyroidism or abnormalities are relatively common. For endocrine diseases of grade 3 or lower severity caused by immunotherapy, treatment may be paused or symptom-relieving medications may be administered based on the safety and tolerance of individual patients. In our case, we followed the guideline of chronic-phase over-treatment, only clinical symptoms and hormones were monitored and no measures were required. If there have other organic changes or TSH>10 mIU/L, levothyroxine sodium is first-line recommended and the initial dose is from 12.5 to 50 μg/d. The initial dose should not be excessive, and the dosage should be adjusted gradually. For patients aged 70 or older, or those with arrhythmias or osteoporosis, serum TSH levels are generally controlled within 4–7 mIU/L (6). The patient takes levothyroxine sodium 50 μg/d continuously since Oct 2024 to reduce other complications caused by endocrine-related adverse reactions. In addition, TPOAb status should be considered when TSH is between 5 to 10 mIU/L. Long-term over-replacement therapy leading to iatrogenic hyperthyroidism can easily cause atrial fibrillation, osteoporosis, sarcopenia, and frailty, necessitating regular monitoring and evaluation (16).
Conclusion
Immune-related thyroid dysfunction is a relatively common adverse reaction following ICI treatment. However, for elderly patients, it can have secondary effects on organs such as the heart, liver, and kidneys, and severe conditions may hinder the progress of antitumor therapy. Clinicians and pharmacists should fully recognize the adverse reactions and their timing related to the endocrine system caused by ICI use. They should promptly monitor relevant signs in cancer patients and pay attention to potentially overlooked pathological manifestations, such as surface discomfort or fatigue, during the course of the disease. We focus on changes in thyroid hormones after receiving chemotherapy plus Serplulimab and changes in TPOAb level associated with autoimmune attack in this study. Unfortunately, relevant FT3, FT4 and TSH measures were not recorded in the course during 2nd cycle. The patient’s general condition had improved, and he was discharged with instructions to continue taking Euthyrox as prescribed. Oral medication adherence was ensured with regular phone follow-up and outpatient service. It suggests that the antitumor treatment plan should be adjusted individually based on the patient’s physical strength and constitution, when patients are highly sensitive to medications. Additionally, for patients with baseline positivity for thyroid-related autoantibodies, as in this case, may become potential risk factors for immune-related reactions in the future. Early intervention or preventive measures should be implemented before treatment to avoid further progression of adverse reactions.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article. Written informed consent was obtained from the participant/patient(s) for the publication of this case report.
Author contributions
LL: Data curation, Investigation, Writing – original draft. LXL: Writing – review & editing. WF: Writing – review & editing. XL: Methodology, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Cheng Y, Zhang S, Han L, Wu L, Chen J, Zhao P, et al. First-line serplulimab plus chemotherapy in extensive-stage small-cell lung cancer: Updated results and biomarker analysis from the ASTRUM-005 randomized clinical trial. Cancer Commun (Lond). (2025) 45:990–1009. doi: 10.1002/cac2.70032
2. Magee DE, Hird AE, Klaassen Z, Sridhar SS, Nam RK, Wallis CJD, et al. Adverse event profile for immunotherapy agents compared with chemotherapy in solid organ tumors: a systematic review and meta-analysis of randomized clinical trials. Ann Oncol. (2020) 31:50–60. doi: 10.1016/j.annonc.2019.10.008
3. Cheng Y, Han L, Wu L, Chen J, Sun H, Wen G, et al. Effect of first-line serplulimab vs placebo added to chemotherapy on survival in patients with extensive-stage small cell lung cancer: the ASTRUM-005 randomized clinical trial. JAMA. (2022) 328:1223–32. doi: 10.1001/jama.2022.16464
4. Ning P, Liu S, and Cao H. Rare, late onset of immune checkpoint inhibitor-induced type 1 diabetes mellitus in a patient with small-cell lung cancer treated with serplulimab: a case report and review of the literature. J Med Case Rep. (2024) 18:51. doi: 10.1186/s13256-023-04248-7
5. Brahmer JR, Abu-Sbeih H, Ascierto PA, Brufsky J, Cappelli LC, Cortazar FB, et al. Society for Immunotherapy of Cancer (SITC) clinical practice guideline on immune checkpoint inhibitor-related adverse events. J Immunother Cancer. (2021) 9:e002435. doi: 10.1136/jitc-2021-002435
6. Iwama S, Kobayashi T, Yasuda Y, and Arima H. Immune checkpoint inhibitor-related thyroid dysfunction. Best Pract Res Clin Endocrinol Metab. (2022) 36:101660. doi: 10.1016/j.beem.2022.101660
7. Zhou C, Hu Y, Arkania E, Kilickap S, Ying K, Xu F, et al. A global phase 3 study of serplulimab plus chemotherapy as first-line treatment for advanced squamous non-small-cell lung cancer (ASTRUM-004). Cancer Cell. (2024) 42:198–208.e3. doi: 10.1016/j.ccell.2023.12.004
8. Song Y, Zhang B, Xin D, Kou X, Tan Z, Zhang S, et al. First-line serplulimab or placebo plus chemotherapy in PD-L1-positive esophageal squamous cell carcinoma: a randomized, double-blind phase 3 trial. Nat Med. (2023) 29:473–82. doi: 10.1038/s41591-022-02179-2
9. Arima H, Iwama S, Inaba H, Ariyasu H, Makita N, Otsuki M, et al. Management of immune-related adverse events in endocrine organs induced by immune checkpoint inhibitors: clinical guidelines of the Japan Endocrine Society. Endocr J. (2019) 66:581–6. doi: 10.1507/endocrj.EJ19-0163
10. Iwamoto Y, Kimura T, Dan K, Ohnishi M, Takenouchi H, Iwamoto H, et al. Immune checkpoint inhibitor-induced hypothyroidism predicts treatment response in Japanese subjects. Front Endocrinol (Lausanne). (2023) 14:1221723. doi: 10.3389/fendo.2023.1221723
11. Muir CA, Tsang VHM, Menzies AM, and Clifton-Bligh RJ. Immune related adverse events of the thyroid - A narrative review. Front Endocrinol (Lausanne). (2021) 13:886930. doi: 10.3389/fendo.2022.886930
12. Muir CA, Clifton-Bligh RJ, Long GV, Scolyer RA, Lo SN, Carlino MS, et al. Thyroid immune-related adverse events following immune checkpoint inhibitor treatment. J Clin Endocrinol Metab. (2021) 106:e3704–13. doi: 10.1210/clinem/dgab263
13. Maekura T, Naito M, Tahara M, Ikegami N, Kimura Y, Sonobe S, et al. Predictive factors of nivolumab-induced hypothyroidism in patients with non-small cell lung cancer. In Vivo. (2017) 31:1035–9. doi: 10.21873/invivo.11166
14. Karaviti D, Kani ER, Karaviti E, Gerontiti E, Michalopoulou O, Stefanaki K, et al. Thyroid disorders induced by immune checkpoint inhibitors. Endocrine. (2024) 85:67–79. doi: 10.1007/s12020-024-03718-2
15. Luo J, Martucci VL, Quandt Z, Groha S, Murray MH, Lovly CM, et al. Immunotherapy-mediated thyroid dysfunction: genetic risk and impact on outcomes with PD-1 blockade in non-small cell lung cancer. Clin Cancer Res. (2021) 27:5131–40. doi: 10.1158/1078-0432.CCR-21-0921
Keywords: case report, immunotherapy, hyperthyroidism, hypothyroidism, adverse reaction
Citation: Lu L, Liu L, Fang W and Liu X (2025) Case Report: Serplulimab-induced thyroid dysfunction in a patient with advanced small cell lung cancer. Front. Oncol. 15:1611425. doi: 10.3389/fonc.2025.1611425
Received: 14 April 2025; Accepted: 21 October 2025;
Published: 04 November 2025.
Edited by:
Tao Zhang, West China Hospital, ChinaReviewed by:
Dmitry Aleksandrovich Zinovkin, Gomel State Medical University, BelarusAlexandra Chera, Carol Davila University of Medicine and Pharmacy, Romania
Copyright © 2025 Lu, Liu, Fang and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wentong Fang, Znd0ZnVAMTYzLmNvbQ==; Xiaojian Liu, MTA4NTk3NTA1NkBxcS5jb20=
 Lu Lu
Lu Lu Lingxiang Liu
Lingxiang Liu Wentong Fang3*
Wentong Fang3* Xiaojian Liu
Xiaojian Liu