- 1Division of Thoracic Tumor Multimodality Treatment, Cancer Center, West China Hospital, Sichuan University, Chengdu, China
- 2West China Hospital Sichuan University, Meishan Hospital, Meishan, China
- 3Department of Oncology, Meishan People’s Hospital, Meishan, China
Background: We aimed to evaluate the efficacy of thoracic radiotherapy (TRT) combined with immune checkpoint inhibitors (ICIs) in patients with advanced non-small-cell lung cancer (NSCLC) in real-world clinical settings and identify predictive subgroups that may benefit most from this approach.
Methods: We retrospectively reviewed the medical records of patients with advanced NSCLC who were treated with ICIs at West China Hospital from January 2015 to May 2022.
Results: A total of 302 patients with advanced NSCLC were included in this study. Among them, 54.3% (164/302) received ICIs in combination with TRT and were assigned to the TRT+ICIs group, while 45.7% (138/302) received ICIs alone and were assigned to the ICIs-only group. The median overall survival (OS) was significantly longer in the TRT+ICIs group (34.7 months) than in the ICIs-only group (27.1 months; P = 0.016). Additionally, the 24-month and 36-month OS rates were notably higher in the TRT+ICIs group (63.7% and 49.0%, respectively) than in the ICIs-only group (55.1% and 16.2%). Subgroup analysis of OS between the TRT+ICIs and ICIs-only groups identified factors associated with improved survival, including male sex, former smoking, Eastern Cooperative Oncology Group (ECOG) performance status 0–1, stage IIIb–c, high albumin level, and low neutrophil-to-lymphocyte (NLR) level. Multivariate analysis identified receipt of TRT, programmed death-ligand 1 (PD-L1) expression < 1%, PD-L1 ≥ 50%, and NLR as statistically significant independent prognostic factors for OS (P < 0.05). The combination treatment was well-tolerated, with an acceptable safety profile.
Conclusion: Our findings suggest that adding TRT to immunotherapy improves survival outcomes in patients with advanced NSCLC.
1 Introduction
Immune checkpoint inhibitors (ICIs) have revolutionized the standard treatment strategies for patients with advanced and metastatic non-small cell lung cancer (NSCLC). ICIs, such as anti-programmed cell death protein 1 (PD-1) and programmed cell death ligand 1 (PD-L1), have been demonstrated to prolong overall survival (OS) in patients with metastatic NSCLC and have been approved as first-line systemic therapy for advanced NSCLC (1–3). However, the efficacy of ICIs is limited by both primary and acquired resistance (4). Preclinical studies have demonstrated that combining radiotherapy (RT) with ICIs offers synergistic benefits, including RT-induced tumor debulking, triggering immunogenic cell death, releasing tumor-associated antigens, activating tumor-associated dendritic cells, remodeling immunosuppressive tumor microenvironments, and modulating immune checkpoint molecule upregulation (5–8). Recently, an increasing number of clinical trials have explored the efficacy of combining ICIs with RT for NSCLC. Notably, the phase III PACIFIC (9)and GEMSTOM 301 (10) trials established the combination of RT and ICIs as a treatment modality for unresectable, stage III NSCLC. A phase I/II trial demonstrated that pembrolizumab, with or without concurrent RT, did not significantly affect the objective response rates (ORR) or progression-free survival (PFS) in patients with metastatic NSCLC (11). Numerous studies have suggested that combining RT and ICIs may benefit NSCLC treatment, though some have reported conflicting results (12, 13). These discrepancies are likely attributable to patient heterogeneity and variations in the modalities of combination therapy. Therefore, we aimed to evaluate the clinical outcomes of combining thoracic radiotherapy (TRT) and ICIs in patients with advanced NSCLC in real-world clinical practice and identify subgroups that may benefit most from this approach.
2 Materials and methods
2.1 Patients
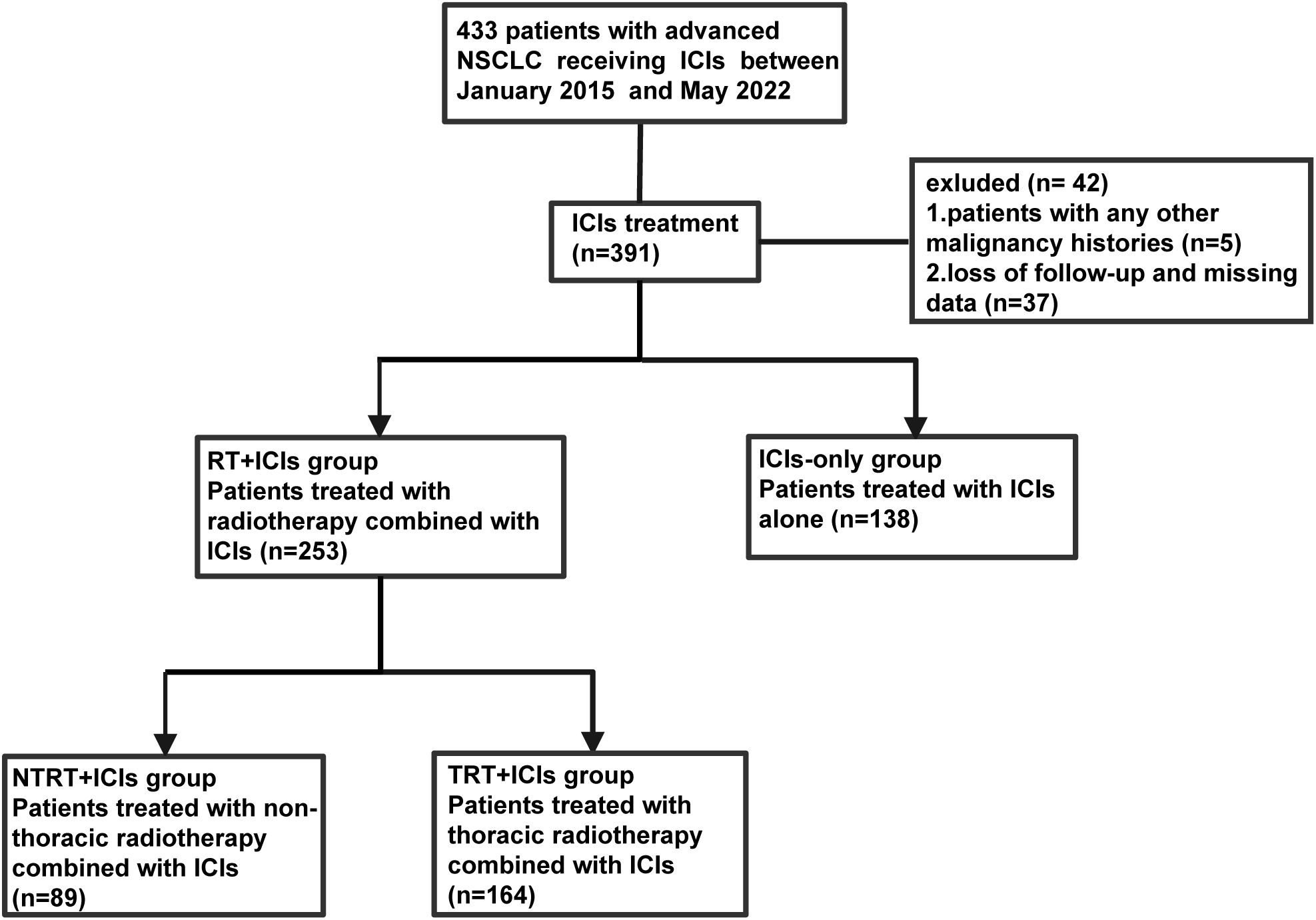
The medical records of patients diagnosed with advanced NSCLC and treated with ICIs, with or without TRT, at West China Hospital from January 2015 to May 2022 were included in this retrospective study. The inclusion criteria were as follows (1): patients were pathologically or cytologically confirmed to have stage IIIb–IV advanced NSCLC [according to the American Joint Committee on Cancer’s Cancer Staging Manual, 8th edition (14)] (2); patients received at least one dose of ICIs, with or without TRT, including definitive, palliative radiotherapy, and recurrence after definitive radiotherapy. The exclusion criteria were as follows (1): patients with a history of other malignancies (2); loss of follow-up or missing data; and (3) patients who received only RT from sites other than TRT (Figure 1).
The enrolled patients were categorized into two groups based on whether they received TRT. Patients who received ICIs combined with TRT were assigned to the TRT+ICIs group, while those who received only ICIs without RT were assigned to the ICIs-only group. The collected data included baseline patient demographics, pathology, stage at the initiation of ICI treatment, Eastern Cooperative Oncology Group (ECOG) performance status, PD-L1 expression, epidermal growth factor receptor (EGFR), anaplastic lymphoma kinase (ALK), proto-oncogene receptor tyrosine kinase (ROS1) aberration status, prior lines of systemic treatment, number of involved organs, albumin level at the initiation of ICIs, neutrophil-to-lymphocyte ratio (NLR) at initiation of ICIs, immunotherapy regimens, RT details, and follow-up data. This study was approved by the Ethics Committee of West China Hospital.
2.2 Treatments
The systemic immunotherapies used included pembrolizumab, nivolumab, durvalumab, atezolizumab, sintilimab, camrelizumab, tislelizumab, and penpulimab. The ICI regimens comprised monotherapy, combination with chemotherapy, or co-administration with a vascular endothelial growth factor receptor inhibitor. Patients in the TRT+ICIs group received a median of six cycles of immunotherapy (range: 1–39), whereas those in the ICIs-only group received a median of five cycles (range: 1–32).
Regarding the sequence of ICIs and RT, our study included both synchronous and sequential treatment approaches. For patients who received multiple courses of TRT, the course closest to the initiation of ICI therapy was selected for subsequent analysis. Patients received daily fractions ranging from 1.8 to 3 Gy, undergoing 10 to 30 fractions of conventional fractionated radiotherapy (CFRT) or 3 to 10 fractions of stereotactic body radiotherapy (SBRT), with a dose of 5.0 to 10.0 Gy per fraction for target organs. The biologically effective dose (BED) was calculated using the formula: BED = n * d * (1+ d ÷ [α/β]), where n = number of fractions, d = dose per fraction, and 10 is the assumed alpha/beta ratio for NSCLC tumors (15, 16). All patients received intensity-modulated RT or volumetric-modulated arc therapy, with a median BED dose of 60.0 Gy10 (range: 27.3 Gy10–180 Gy10). Adverse effects (AEs) were evaluated according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.03 (NCI CTCAE v4.03).
2.3 Statistical analysis
Ordinal variables were analyzed using the Mann–Whitney U test, while categorical variables were analyzed using the chi-square test, with a predetermined alpha level of 0.05 for statistical significance. All continuous parameters were examined for homogeneity of variance using Levene’s test, and comparisons were made using Student’s t-test. If the distribution significantly deviated from the normal, the Mann–Whitney U test was used. Albumin levels and NLR were categorized into two groups based on their median values. The primary endpoint of this study was the effect of TRT combined with ICI therapy on OS, which was measured from the start of ICI treatment to the time of death or censoring at the last follow-up. The Kaplan–Meier method was used to plot survival curves and estimate median OS. Differences in OS were compared using the log-rank test. In the subgroup analyses, the effect of adding TRT to ICIs on OS was assessed among the pre-set subgroups using Cox proportional hazards models, with results presented in a forest map. Bonferroni correction was adopted to enhance the credibility of the results. Univariate and multivariate analyses were performed using Cox proportional hazards regression models. Statistical significance was set at P < 0.05. All statistical analyses were performed using IBM SPSS Statistics (version 27.0; Armonk, NY, USA) and R (version 4.1.1; Vienna, Austria).
3 Results
3.1 Baseline characteristics
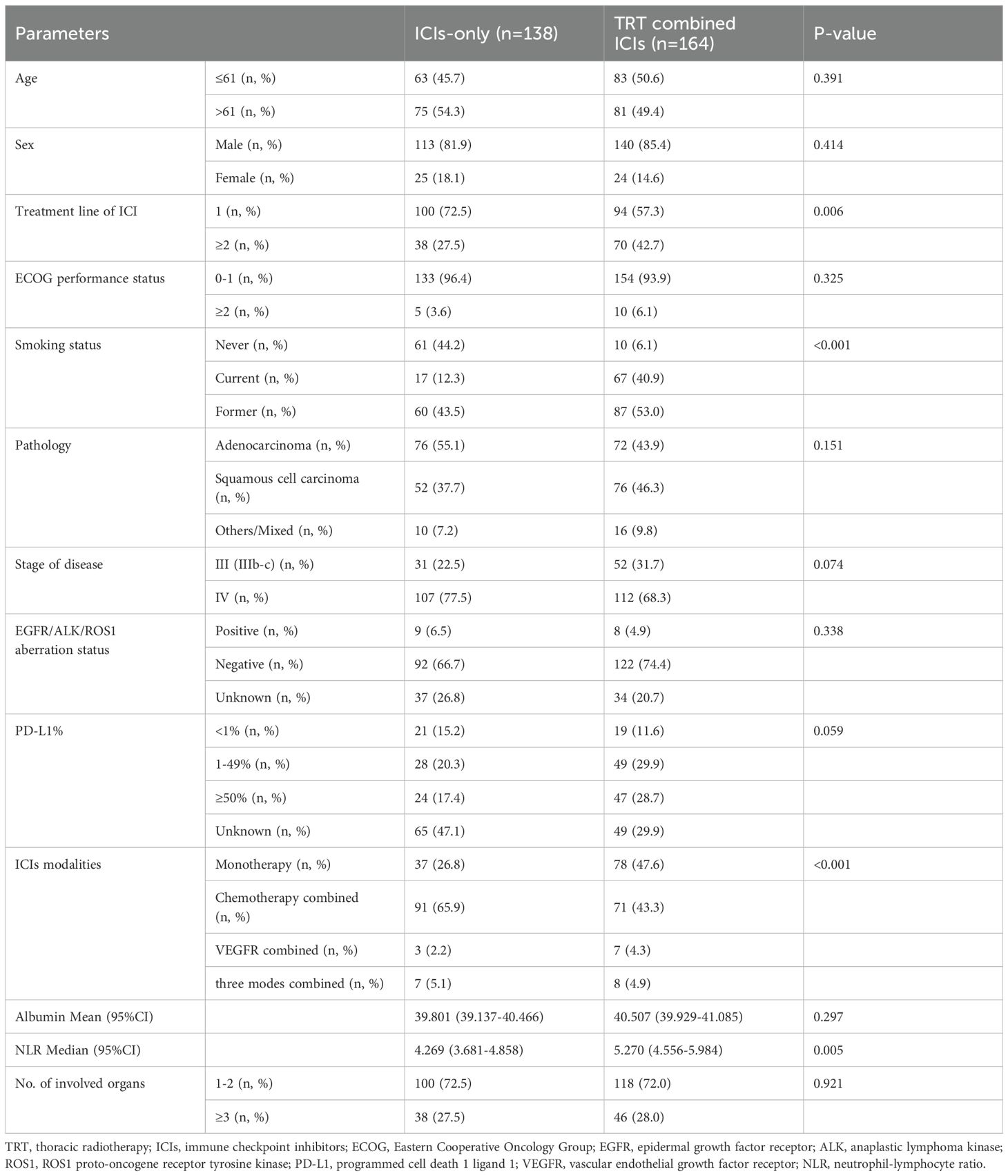
A total of 302 patients with advanced NSCLC who met the inclusion criteria were included in this study. As of the cut-off date, November 10, 2022, the median follow-up time was 26.6 months (95% confidence interval [CI]: 24.6–28.6). The patients were categorized into two groups based on whether they received TRT. Ultimately, 54.3% (164/302) of the patients were enrolled in the TRT+ICIs group, while 45.7% (138/302) were in the ICIs-only group. Baseline patient demographics and treatment characteristics of the two groups are summarized in Table 1. Although the baseline characteristics were generally well-balanced between the two groups, several notable differences were observed. Specifically, a greater proportion of patients in the TRT+ICIs group than in the ICIs-only group received ICIs as second-line or later-line therapy, were treated with ICIs monotherapy, and showed a higher NLR at the start of treatment. In contrast, the ICIs-only group had a higher proportion of never-smokers. Furthermore, more patients in the ICIs-only group received chemotherapy in combination with ICIs.
3.2 Efficacy
In the entire cohort, 45.7% (138/302) of patients died, with a median OS of 28.6 months (95% CI: 25.9–34.7). In the TRT+ICIs group, 44.5% (73/164) of patients reached the endpoint, while 47.1% (65/138) of patients in the ICIs-only group died. The median OS was significantly longer in the TRT+ICIs group (34.7 months) (95% CI: 25.9–41.4) than in the ICIs-only group (27.1 months) (P = 0.016) (95% CI: 23.0–31.3; Figure 2). Notably, the 24-month and 36-month OS rates were higher in the TRT+ICIs group at 63.7% (95% CI: 56.1%–72.4%) and 49.0% (95% CI: 40.3%–59.6%) than in the ICIs-only group, which had rates of 55.1% (95% CI: 45.8%–66.2%) and 16.2% (95% CI: 7.5%–34.8%), respectively.
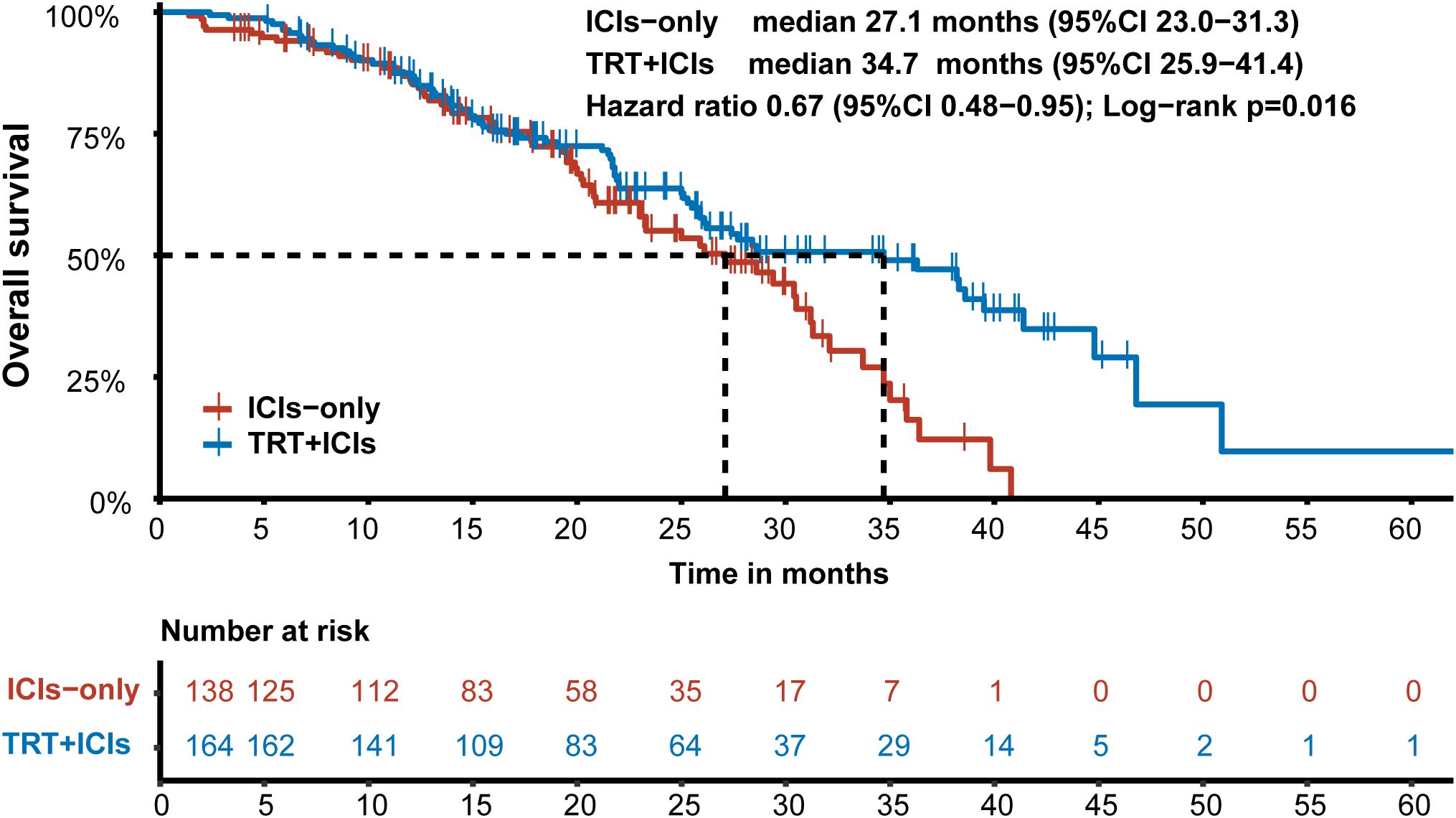
Figure 2. Kaplan-Meier survival curve of overall survival in the TRT+ICIs group and the ICIs-only group.
We then conducted an exploratory subgroup analysis of OS between the two groups (Figure 3). The TRT+ICIs group demonstrated superior outcomes in men (hazard ratio [HR], 0.621; 95% CI, 0.422–0.915; P = 0.016), patients with an ECOG performance status of 0–1 (HR, 0.68; 95% CI, 0.478–0.969; P = 0.033), former smokers (HR, 0.568; 95% CI, 0.342–0.941; P = 0.028), patients with stage IIIb-c diseases (HR, 0.508; 95% CI, 0.267–0.968; P = 0.039), patients with high albumin levels (HR, 0.471; 95% CI, 0.273–0.813; P = 0.007), and patients with low NLR (HR, 0.558; 95% CI, 0.315–0.991; P = 0.047). Then, we applied Bonferroni correction (α = 0.05/13 subgroups = 0.0038) to all subgroup comparisons. As noted, this stringent adjustment rendered all comparisons non-significant.
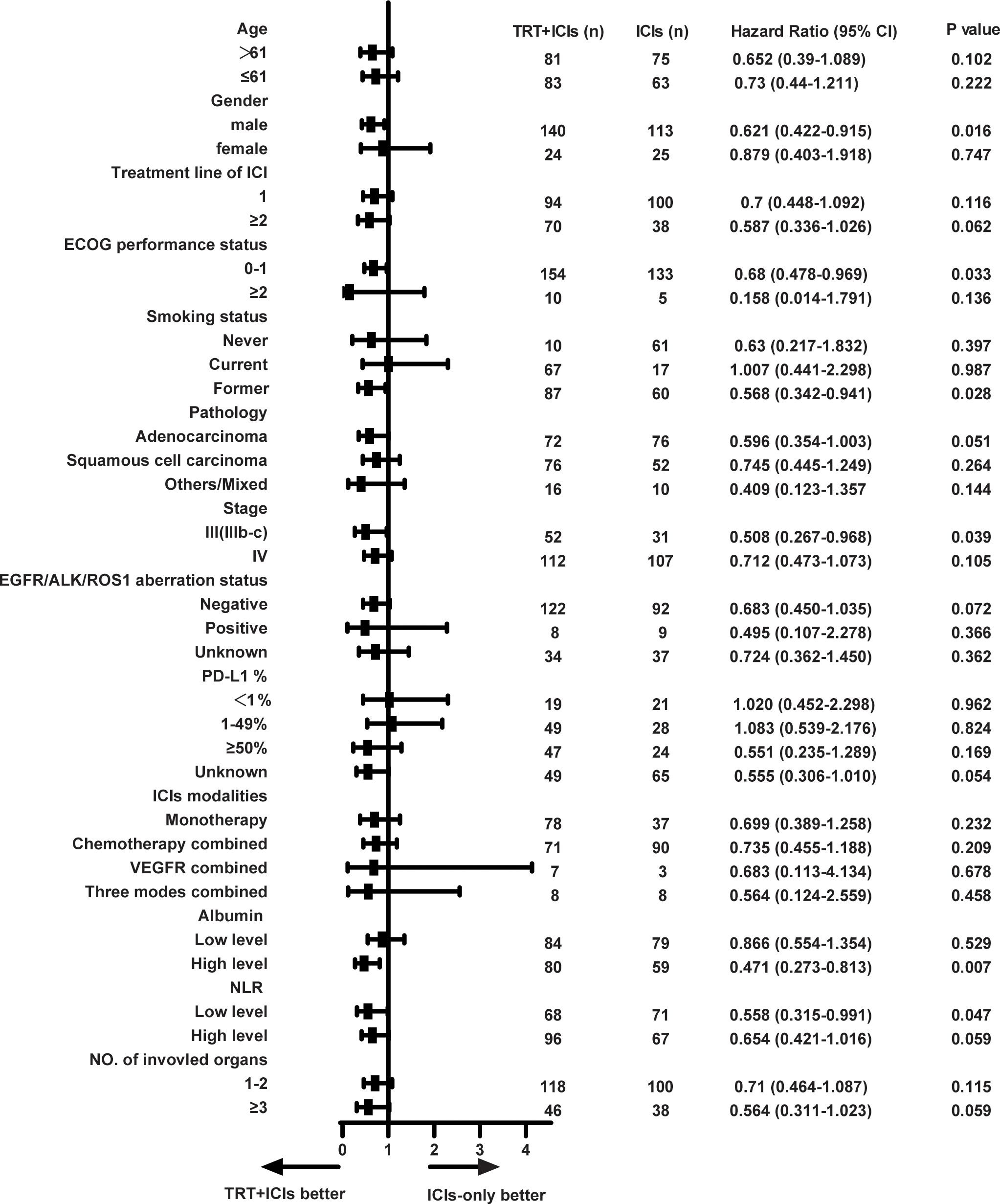
Figure 3. Forest plot of subgroup analysis on overall survival. TRT, thoracic radiotherapy; ICIs, immune checkpoint inhibitors; ECOG, Eastern Cooperative Oncology Group; EGFR, epidermal growth factor receptor; ALK, anaplastic lymphoma kinase; ROS1, ROS1 proto-oncogene receptor tyrosine kinase; PD-L1, programmed cell death 1 ligand 1; VEGFR, vascular endothelial growth factor receptor; NLR, neutrophil-lymphocyte ratio. * Exploratory subgroup analyses shown without multiplicity adjustment. All subgroup analyses became non-significant following Bonferroni correction (adjusted α = 0.0038).
Univariate survival analysis was performed to assess the association between OS and clinical characteristics, including age, sex, smoking status, prior lines of systemic treatment, ECOG performance status, pathology, stage, EGFR/ALK/ROS1 aberration status, PD-L1 expression, TRT, ICI modalities, number of involved organs, albumin level, and NLR. The results indicated that receiving TRT (HR, 0.655; 95% CI, 0.463–0.926; P = 0.017), PD-L1 < 1% (P = 0.003), PD-L1 ≥ 50% (HR, 0.348; 95% CI, 0.200–0.606; P < 0.001), albumin level (HR, 0.663; 95% CI, 0.471–0.934; P = 0.019), and NLR (HR, 1.600; 95% CI, 1.132–2.260; P = 0.008) were significant prognostic factors for OS (Table 2). Factors with P < 0.05 in the univariate analysis were included in the multivariate analysis. Multivariate analysis revealed that only receiving TRT (HR, 0.694; 95% CI, 0.485–0.994; P = 0.046), PD-L1 < 1% (P = 0.021), PD-L1 ≥ 50% (HR, 0.413; 95% CI, 0.235–0.727; P = 0.002), and NLR (HR, 1.528; 95% CI, 1.070–2.183; P = 0.020) remained statistically significant independent prognostic factors for OS (P < 0.05), as detailed in Table 2.
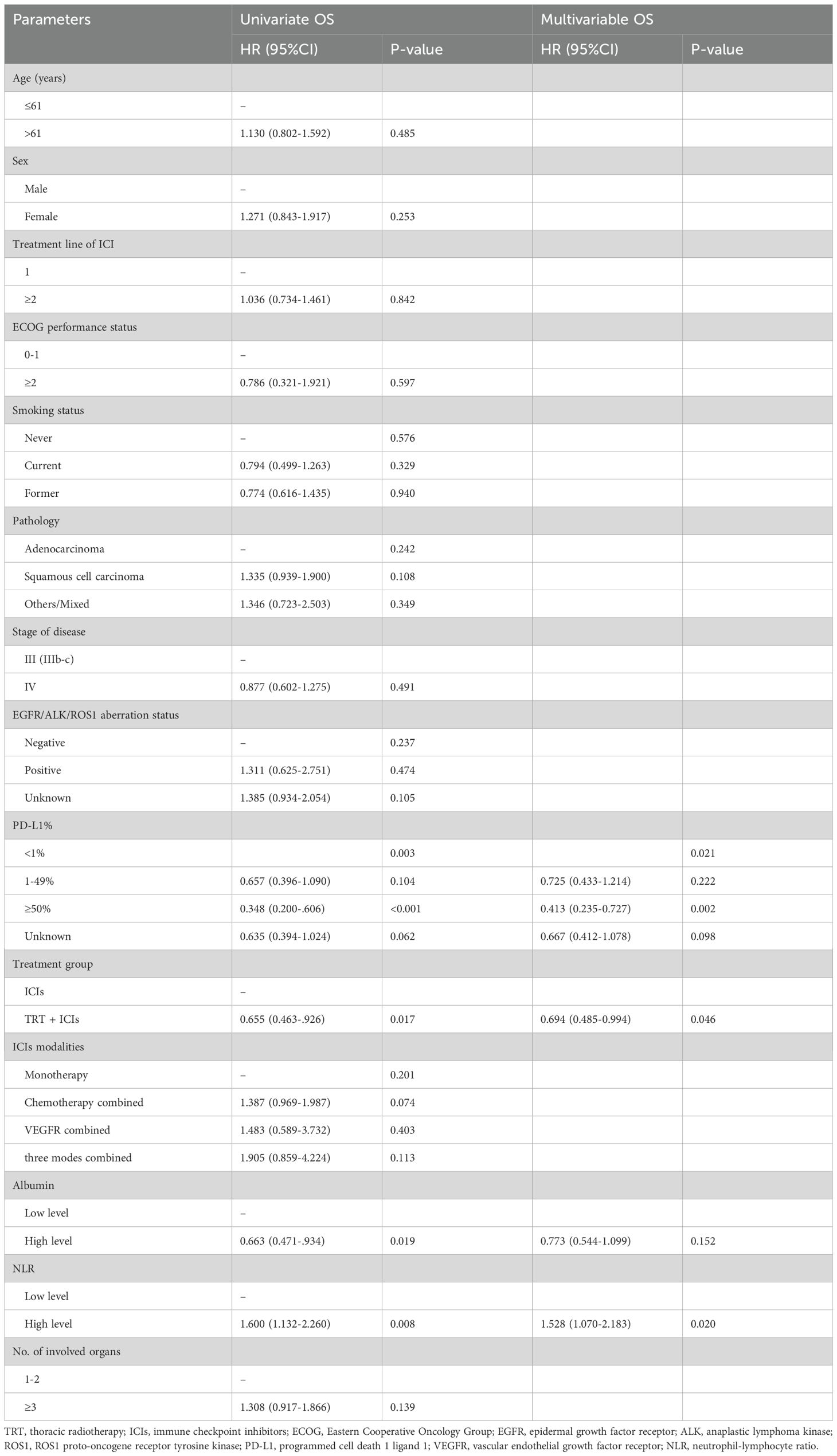
Table 2. Univariate and multivariable analysis of overall survival in the TRT+ICIs group and the ICIs-only group.
To investigate the optimal treatment combining TRT with ICIs, we conducted an exploratory subgroup analysis of OS in the TRT+ICIs group. Various TRT parameters were assessed. As illustrated in Figure 4A, patients who received SBRT had a higher median OS (38.2 months) (95% CI: 25.9–NA) than that of patients receiving CFRT (27.4 months) (95% CI: 25.3–NA). However, this difference was not statistically significant (P = 0.823). Additionally, we evaluated the impact of the TRT dose on OS by grouping patients based on BED equivalent doses of 60 Gy10. To examine the influence of the sequence between TRT and ICIs on prognosis, patients were stratified into two groups: those who received TRT before ICIs and those who received ICIs before TRT. Kaplan–Meier survival curves for the TRT dose and treatment sequence are presented in Figures 4B, C. However, no statistically significant differences were observed between these subgroups.
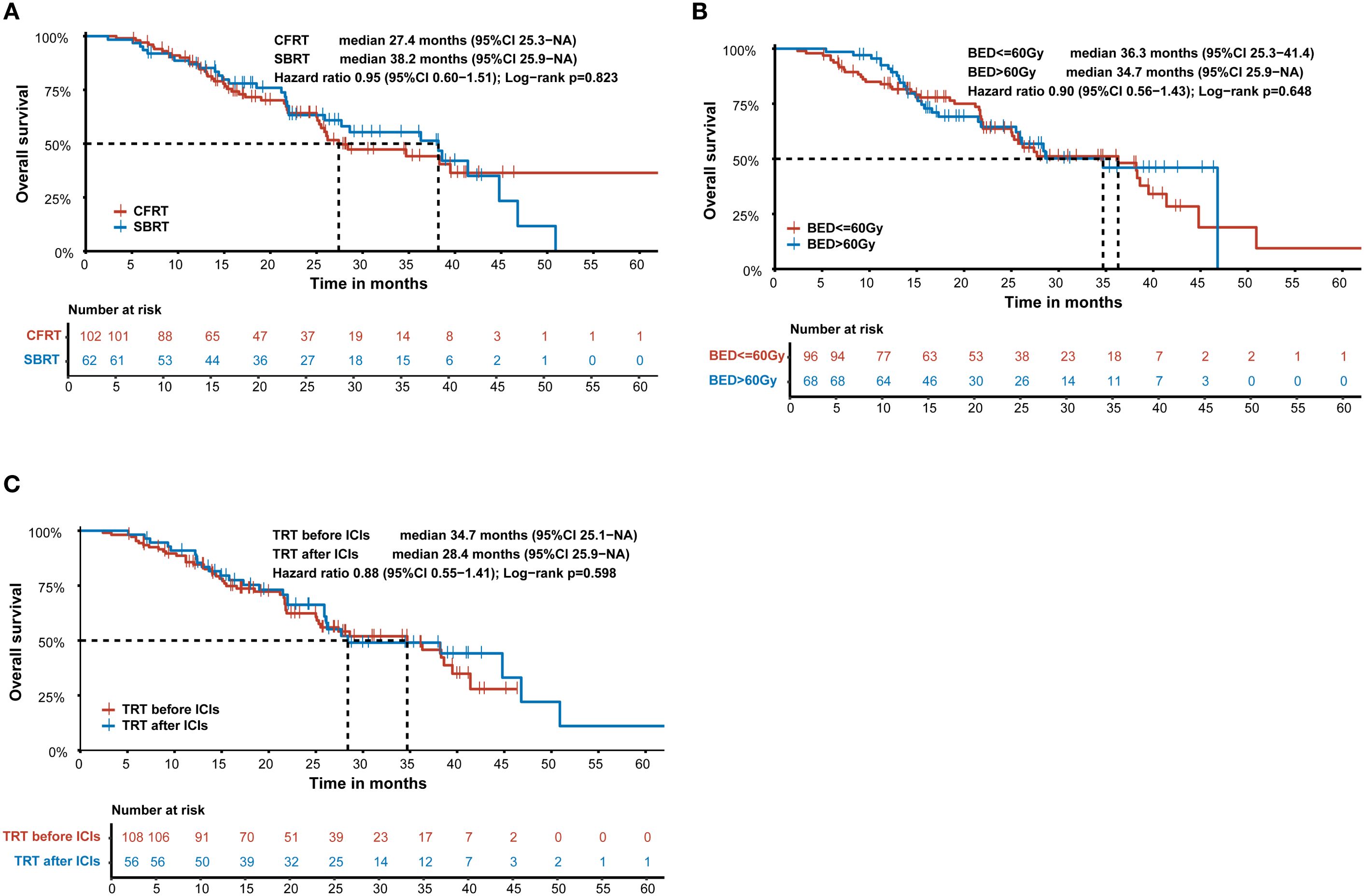
Figure 4. Kaplan-Meier survival curve of overall survival according to (A) SBRT and CFRT in the TRT+ICIs group. (B) the dose of TRT in the TRT+ICIs group. (C) the sequence between TRT and ICIs in the TRT+ICIs group. SBRT, stereotactic body radiotherapy; CFRT, conventional fractionated radiotherapy; BED, biologically effective dose; TRT, thoracic radiotherapy; ICIs, immune checkpoint inhibitors.
3.3 Treatment-related adverse events
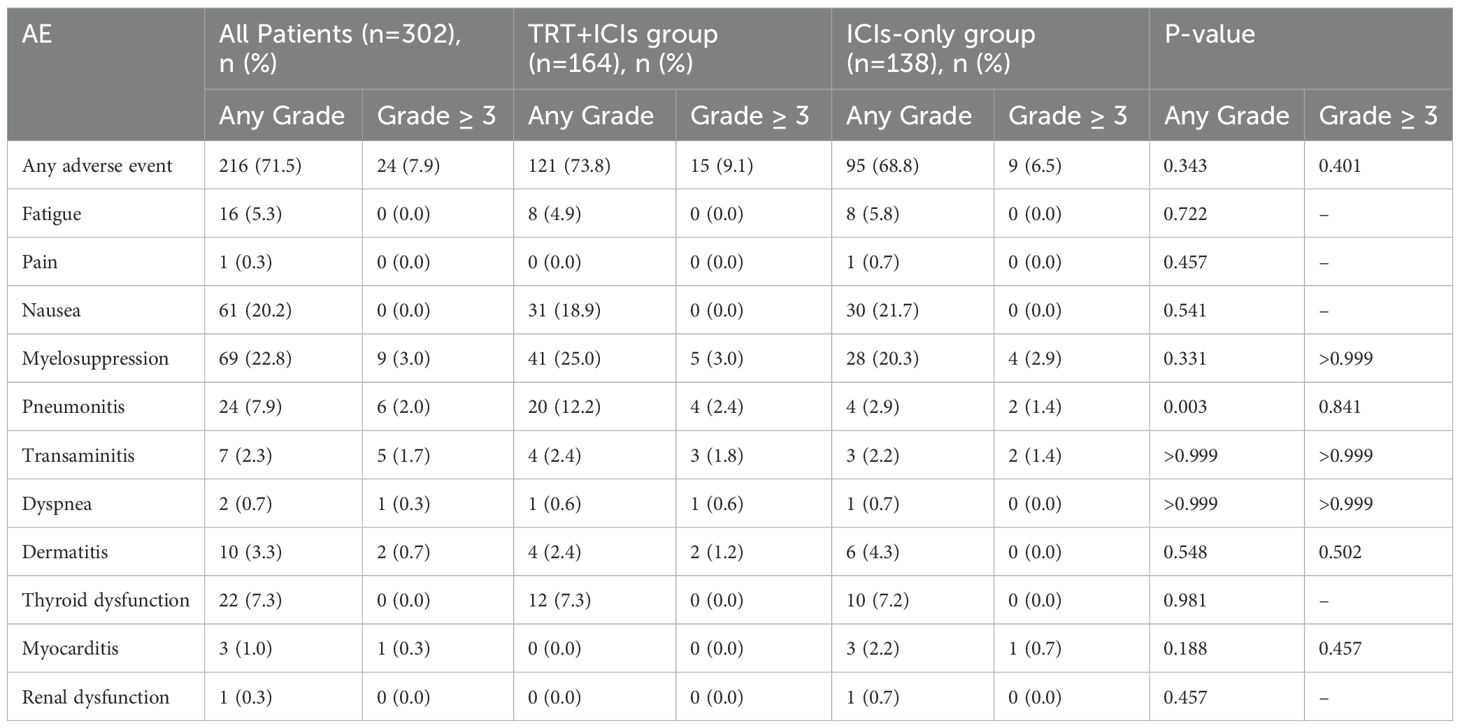
The treatment-related AEs are summarized in Table 3. Among the 302 patients, 216 (71.5%) experienced at least one AE potentially related to therapy—121 (73.8%) in the TRT+ICIs group and 95 (68.8%) in the ICIs-only group (P = 0.343). The most common AEs were myelosuppression (41 [25.0%] in the TRT+ICIs group vs. 28 [20.3%] in the ICIs-only group; P = 0.331) and nausea (31 [18.9%] in the TRT+ICIs group vs. 30 [21.7%] in the ICIs-only group; P = 0.541). Overall, 24 (7.9%) patients experienced grade 3 or higher AEs (15 [9.1%] in the TRT+ICIs group and 9 [6.5%] in the ICIs-only group; P =0.401). The most frequent grade 3 or higher AEs included myelosuppression (5 [3%] in the TRT+ICIs group vs. 4 [2.9%] in the ICIs-only group; P > 0.999), pneumonitis (4 [2.4%] in the TRT+ICIs group vs. 2 [1.4%] in the ICIs-only group; P = 0.841), and transaminitis (3 [1.8%] in the TRT+ICIs group vs. 2 [1.4%] in the ICIs-only group; P > 0.999).
The median onset time of pneumonitis in the entire cohort was 82 days (95% CI: 29.408–134.592) after the initiation of ICIs, with a median duration of 32 days (95% CI: 24.645–39.355). In the TRT+ICIs group, pneumonitis occurred at a median of 82 days (95% CI: 13.400–150.600) after ICIs initiation and 88 days (95% CI: 55.130–120.870) after TRT initiation, with a median duration of 36 days (95% CI: 29.070–42.930). Given the limited number of patients with pneumonitis in the ICIs-only group (n = 4), separate calculations were not performed.
4 Discussion
In our study, the combination of TRT and ICIs significantly prolonged OS and enhanced the 24-month and 36-month OS rates in patients with advanced NSCLC compared with ICIs alone. This highlights the efficacy of this combination strategy for treating advanced NSCLC. A secondary analysis of the KEYNOTE-001 trial showed that patients who had previously received radiotherapy exhibited longer PFS (4.4 months vs. 2.1 months, P = 0.019) and OS (10.7 months vs. 5.3 months, P = 0.026) than did those who did not, with an acceptable safety profile (17). Theelen et al. performed a secondary analysis of the MD Anderson Cancer Center (phase 1/2) and Pembrolizumab and Radiotherapy (phase 2) trials, where the thorax was the most common site targeted by radiotherapy (18). The combination of pembrolizumab and radiotherapy demonstrated a higher OS rate than that of pembrolizumab alone (19.2 months vs. 8.7 months, P = 0.0004), and PFS was significantly improved in the combination group (9.0 months vs. 4.4 months, P = 0.045). Additional radiotherapy significantly enhanced the out-of-field response rates (abscopal response rate: 41.7% vs. 19.7%, P = 0.0039; abscopal disease control rate: 65.3% vs. 43.4%, P = 0.0071). Moreover, neither of the two aforementioned trials achieved the predefined primary endpoints (11, 19). The phase III PACIFIC trial (9) demonstrated that the consolidation therapy with durvalumab following platinum-based concurrent chemoradiotherapy significantly improved PFS (P < 0.0001) and OS (P = 0.00251) in patients with unresectable stage III NSCLC. In conclusion, our findings are consistent with the current research, suggesting that the combination of RT with ICIs has considerable potential for the treatment of advanced NSCLC.
Although the clinical characteristics were not perfectly balanced between the two groups, a greater proportion of patients in the TRT+ICIs group received ICIs as second-line or later-line therapy, were treated with ICIs monotherapy, and showed a higher NLR at the start of treatment. According to the KEYNOTE-001 trial, enrolled patients with confirmed locally advanced or metastatic NSCLC received pembrolizumab (20). Treatment-naive patients demonstrated superior outcomes, with a median OS of 22.3 months (95% CI 17.1-32.3) compared to 10.5 months (95% CI 8.6-13.2) in previously treated patients. The 5-year OS rates were 23.2% and 15.5% for treatment-naive and previously treated cohorts, respectively. A retrospective cohort study by Heyward et al. analyzing SEER-Medicare data (2013–2019) from 17,681 individuals found that ICI plus chemotherapy showed significantly reduced mortality risk compared to ICI monotherapy in the first-line treatment setting (21). A Meta-analysis included data from 17 Phase III clinical trials involving 10,283 patients and demonstrated that immunotherapy significantly improves survival in lung cancer patients regardless of smoking status (never-smokers, former, or current smokers), with no significant interaction effects observed between treatment outcomes and smoking history (22). Ksienski et al. found that higher NLR significantly correlated with shorter OS in stage III NSCLC patients treated with durvalumab (23). Similarly, Lin et al. observed that increased NLR predicted worse OS in NSCLC patients receiving neoadjuvant therapy followed by surgical resection (24). All the aforementioned studies consistently demonstrated that the TRT+ICI group had poorer predicted survival outcomes. However, contrary to these expectations, the OS in the TRT+ICIs group was significantly longer than that in the ICIs-only group, providing further evidence for the effectiveness of this combined treatment strategy.
Preclinical evidence indicates that the combination of RT and ICIs produces synergistic effects through multiple mechanisms involving radiation-induced tumor cell killing that generates an in situ vaccine effect, enhances antigen presentation and dendritic cell activation, reprograms the immunosuppressive tumor microenvironment, and modulates immune checkpoint molecule expression (5, 6, 8, 25, 26). This study further confirms the therapeutic efficacy of the combined treatment.
The subgroup analysis of OS between the TRT+ICIs and ICIs-only groups identified factors associated with improved survival, including male sex (27), former smoking, ECOG performance status 0–1 (23, 28), stage IIIb-c (9, 10), high albumin level (29), and low NLR level (23, 28, 30). These findings almost align with those of previous studies, supporting the validity of our results. Only former smoking demonstrated a survival benefit inconsistent with the aforementioned meta-analysis results (22). This discrepancy may relate to the limited sample size in this study. In addition, the subgroup findings should be interpreted cautiously rather than definitive conclusions, particularly given non-significance after multiplicity correction. These results require prospective validation in adequately powered studies.
In the univariate survival analysis, TRT, PD-L1 expression, albumin level, and NLR were statistically significant. However, in the multivariate analysis, only TRT, PD-L1 expression, and NLR were independent predictors of prognosis. A significant association with OS was observed only in patients with PD-L1 < 1% and ≥ 50%. At the baseline level, the absolute proportion of PD-L1 ≥ 50% in the TRT + ICI group was indeed higher than that in the ICIs-only group (28.7% vs. 17.4%). However, there was no statistically significant difference between the two groups (P = 0.059). Moreover, in the multivariate analysis, the regression model was constructed to adjust for other confounding factors. This adjustment strategy ensures the reported protective effect of PD-L1 ≥50% is independent of baseline group differences. Patients in the PD-L1 ≥ 50% group were more likely than those in the PD-L1 < 1% group to benefit from treatment with ICIs, which is consistent with previous findings (3). No significant association was observed for the PD-L1 1-49% group, potentially due to the limited sample size. Ksienski et al. reported that elevated NLR was significantly associated with shorter OS in patients with stage III NSCLC treated with durvalumab (23).
Few studies have evaluated the optimal parameters for RT. In our study, we categorized RT techniques primarily into CFRT and SBRT. Our findings suggest that both CFRT and SBRT may enhance immunological efficacy. In the PACIFIC trial (9), patients received consolidation therapy with durvalumab following platinum-based concurrent chemoradiotherapy, with a typical CFRT dose of 60–66 Gy delivered in 30–33 fractions. A previous retrospective study concluded that SBRT (P = 0.013) and concurrent RT combined with ICIs (P = 0.002) were significantly associated with improved outcomes (31). Li et al. demonstrated that both CFRT (n = 75) and SBRT (n = 42), as well as intrathoracic RT (n = 76) and extrathoracic RT (n = 43), when combined with immunotherapy, could enhance survival (27). However, their study was limited by its relatively small sample size. Verma et al. reported that TRT in combination with ICIs is safe for patients, regardless of the different RT techniques and fractionation schedules (32).
In our study, no statistically significant difference was observed in the overall rate of treatment-related AEs between the TRT+ICIs and ICIs-only groups. Similarly, the incidences of grade 3 or higher AEs were comparable between the two groups. Although the overall incidence of pneumonitis was significantly higher in the TRT+ICIs group (12.2% vs. 2.9%, P = 0.003) than in the ICIs-only group, the majority of cases were mild to moderate (grade 1 or 2). Importantly, the incidence of clinically significant grade 3 or higher pneumonitis did not differ substantially between the two groups (2.4% vs. 1.4%; P = 0.841). Collectively, these findings suggest that the safety profile of thoracic radiotherapy combined with immunotherapy is well-tolerated and acceptable.
However, this study has some limitations. First, it was a retrospective, single-center study, which may have introduced some selection bias. Second, the PD-L1 expression status was not available for some patients. Third, in clinical practice, differentiating between radiation pneumonitis and immune-related pneumonitis remains a significant diagnostic challenge due to overlapping radiographic features, frequent concurrent administration of corticosteroids and limitations in retrospective study. Consequently, this study was virtually impossible to accurately distinguish between these two types of pneumonitis. Further well-designed randomized controlled trials are necessary to investigate the synergistic effects of RT and ICIs.
5 Conclusion
Our study supports the efficacy and safety of combining thoracic radiotherapy with immunotherapy in patients with advanced NSCLC. Additionally, this study delineates specific patient characteristics that may predict a favorable response to the proposed combination therapy, thereby providing a foundation for future research.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the Ethics Committee of West China Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because the retrospective design, and analyses were conducted following the Declaration of Helsinki.
Author contributions
YZ: Conceptualization, Data curation, Formal analysis, Funding acquisition, Validation, Visualization, Writing – original draft, Writing – review & editing. YC: Conceptualization, Data curation, Formal analysis, Validation, Visualization, Writing – original draft, Writing – review & editing. XZ: Investigation, Resources, Writing – review & editing. YG: Investigation, Resources, Writing – review & editing. YX: Investigation, Resources, Writing – review & editing. BZ: Investigation, Resources, Writing – review & editing. FP: Investigation, Resources, Writing – review & editing. MH: Investigation, Resources, Writing – review & editing. YL: Investigation, Resources, Writing – review & editing. YML: Conceptualization, Funding acquisition, Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the 1·3·5 project for disciplines of excellence–Clinical Research Incubation Project, West China Hospital, Sichuan University (No. 21HXFH054) and the Sichuan Medical and Health Care Promotion Institute Scientific Research Project (No. KY2024QN0167).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
TRT, Thoracic radiotherapy; ICIs, Immune checkpoint inhibitors; NSCLC, Non-small cell lung cancer; OS, Overall survival; ECOG, Eastern Cooperative Oncology Group; PD-L1, Programmed death-ligand 1; RT, Radiotherapy; ORR, Objective response rate; PFS, Progression-free survival.
References
1. Novello S, Kowalski DM, Luft A, Gümüş M, Vicente D, Mazières J, et al. Pembrolizumab plus chemotherapy in squamous non-small-cell lung cancer: 5-year update of the phase III KEYNOTE-407 study. J Clin Oncol. (2023) 41:1999–2006. doi: 10.1200/jco.22.01990
2. de Castro G, Kudaba I, Wu Y-L, Lopes G, Kowalski DM, Turna HZ, et al. Five-year outcomes with pembrolizumab versus chemotherapy as first-line therapy in patients with non–small-cell lung cancer and programmed death ligand-1 tumor proportion score ≥ 1% in the KEYNOTE-042 study. J Clin Oncol. (2023) 41:1986–91. doi: 10.1200/jco.21.02885
3. Garassino MC, Gadgeel S, Speranza G, Felip E, Esteban E, Dómine M, et al. Pembrolizumab plus pemetrexed and platinum in nonsquamous non-small-cell lung cancer: 5-year outcomes from the phase 3 KEYNOTE-189 study. J Clin Oncol. (2023) 41:1992–8. doi: 10.1200/jco.22.01989
4. Fares CM, Van Allen EM, Drake CG, and Allison JP. and hu-lieskovan S mechanisms of resistance to immune checkpoint blockade: why does checkpoint inhibitor immunotherapy not work for all patients? Am Soc Clin Oncol Educ Book. (2019) 39:147–64. doi: 10.1200/edbk_240837
5. Xia WY, Feng W, Zhang CC, Shen YJ, Zhang Q, Yu W, et al. Radiotherapy for non-small cell lung cancer in the immunotherapy era: the opportunity and challenge-a narrative review. Transl Lung Cancer Res. (2020) 9:2120–36. doi: 10.21037/tlcr-20-827
6. Diamond JM, Vanpouille-Box C, Spada S, Rudqvist NP, Chapman JR, Ueberheide BM, et al. Exosomes shuttle TREX1-sensitive IFN-stimulatory dsDNA from irradiated cancer cells to DCs. Cancer Immunol Res. (2018) 6:910–20. doi: 10.1158/2326-6066.Cir-17-0581
7. Jarosz-Biej M, Smolarczyk R, Cichoń T, and Kułach N. Tumor microenvironment as A “Game changer” in cancer radiotherapy. Int J Mol Sci. (2019) 20. doi: 10.3390/ijms20133212
8. Wang NH, Lei Z, Yang HN, Tang Z, Yang MQ, Wang Y, et al. Radiation-induced PD-L1 expression in tumor and its microenvironment facilitates cancer-immune escape: a narrative review. Ann Transl Med. (2022) 10:1406. doi: 10.21037/atm-22-6049
9. Spigel DR, Faivre-Finn C, Gray JE, Vicente D, Planchard D, Paz-Ares L, et al. Five-year survival outcomes from the PACIFIC trial: durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. J Clin Oncol. (2022) 40:1301–11. doi: 10.1200/jco.21.01308
10. Zhou Q, Chen M, Jiang O, Pan Y, Hu D, Lin Q, et al. Sugemalimab versus placebo after concurrent or sequential chemoradiotherapy in patients with locally advanced, unresectable, stage III non-small-cell lung cancer in China (GEMSTONE-301): interim results of a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol. (2022) 23:209–19. doi: 10.1016/s1470-2045(21)00630-6
11. Welsh J, Menon H, Chen D, Verma V, Tang C, Altan M, et al. Pembrolizumab with or without radiation therapy for metastatic non-small cell lung cancer: a randomized phase I/II trial. J Immunother Cancer. (2020) 8. doi: 10.1136/jitc-2020-001001
12. Iyengar P, Hu C, Gomez DR, Timmerman RD, Simone CB, Robinson CG, et al. NRG-LU002: Randomized phase II/III trial of maintenance systemic therapy versus local consolidative therapy (LCT) plus maintenance systemic therapy for limited metastatic non-small cell lung cancer (NSCLC). J Clin Oncol. (2024) 42:8506–6. doi: 10.1200/JCO.2024.42.16_suppl.8506
13. Wang P, Yin T, Zhao K, Yu J, and Teng F. Efficacy of single-site radiotherapy plus PD-1 inhibitors vs PD-1 inhibitors for oligometastatic non-small cell lung cancer. J Cancer Res Clin Oncol. (2021) 148:1253–61. doi: 10.1007/s00432-021-03849-3
14. Detterbeck FC, Boffa DJ, Kim AW, and Tanoue LT. The eighth edition lung cancer stage classification. Chest. (2017) 151:193–203. doi: 10.1016/j.chest.2016.10.010
15. Fowler JF. 21 years of biologically effective dose. Br J Radiol. (2010) 83:554–68. doi: 10.1259/bjr/31372149
16. Chi A, Wen S, Liao Z, Fowler J, Xu J, Nguyen NP, et al. What would be the most appropriateα/βRatio in the setting of stereotactic body radiation therapy for early stage non-small cell lung cancer. BioMed Res Int. (2013) 2013:1–8. doi: 10.1155/2013/391021
17. Shaverdian N, Lisberg AE, Bornazyan K, Veruttipong D, Goldman JW, Formenti SC, et al. Previous radiotherapy and the clinical activity and toxicity of pembrolizumab in the treatment of non-small-cell lung cancer: a secondary analysis of the KEYNOTE-001 phase 1 trial. Lancet Oncol. (2017) 18:895–903. doi: 10.1016/s1470-2045(17)30380-7
18. Theelen WSME, Chen D, Verma V, Hobbs BP, Peulen HMU, Aerts JGJV, et al. Pembrolizumab with or without radiotherapy for metastatic non-small-cell lung cancer: a pooled analysis of two randomised trials. Lancet Respir Med. (2021) 9:467–75. doi: 10.1016/s2213-2600(20)30391-x
19. Theelen WSME, Peulen HMU, Lalezari F, van der Noort V, de Vries JF, Aerts JGJV, et al. Effect of pembrolizumab after stereotactic body radiotherapy vs pembrolizumab alone on tumor response in patients with advanced non–small cell lung cancer. JAMA Oncol. (2019) 5:1276–82. doi: 10.1001/jamaoncol.2019.1478
20. Garon EB, Hellmann MD, Rizvi NA, Carcereny E, Leighl NB, Ahn MJ, et al. Five-year overall survival for patients with advanced non–Small-cell lung cancer treated with pembrolizumab: results from the phase I KEYNOTE-001 study. J Clin Oncol. (2019) 37:2518–27. doi: 10.1200/jco.19.00934
21. Heyward J, Lesko CR, Murray JC, Mehta HB, and Segal JB. Harm-benefit balance of immune checkpoint inhibitors in non-small cell lung cancer. JAMA Oncol. (2025) 11:707–16. doi: 10.1001/jamaoncol.2025.0985
22. Luo D, Yang D, Cao D, Gong Z, He F, Hou Y, et al. Effect of smoking status on immunotherapy for lung cancer: a systematic review and meta-analysis. Front Oncol. (2024) 14:1422160. doi: 10.3389/fonc.2024.1422160
23. Ksienski D, Truong PT, Bone JN, Egli S, Clarkson M, Patterson T, et al. Durvalumab following chemoradiotherapy for stage III non-small cell lung cancer: differences in survival based on age and post-progression systemic therapy. Target Oncol. (2025) 20:149–60. doi: 10.1007/s11523-024-01111-7
24. Lin Y, Huang R, Liu Q, Yan X, Liao G, Pan M, et al. Prognostic factors for non-small cell lung cancer after neoadjuvant therapy and surgery: a retrospective observational study. J Thorac Dis. (2025) 17:2841–55. doi: 10.21037/jtd-2024-1651
25. Zhang Z, Liu X, Chen D, and Yu J. Radiotherapy combined with immunotherapy: the dawn of cancer treatment. Signal Transduct Target Ther. (2022) 7:258. doi: 10.1038/s41392-022-01102-y
26. Hu Y, Paris S, Sahoo N, Wang Q, Wang Q, Barsoumian HB, et al. Superior antitumor immune response achieved with proton over photon immunoradiotherapy is amplified by the nanoradioenhancer NBTXR3. J Nanobiotechnology. (2024) 22:597. doi: 10.1186/s12951-024-02855-0
27. Li S, Chen K, Yang M, Hlaing SS, Chen M, Gu P, et al. Radiotherapy improves the outcomes of immunotherapy with Sintilimab in non-small-cell lung cancer: A real-world analysis. Front Immunol. (2022) 13:991431. doi: 10.3389/fimmu.2022.991431
28. Musaelyan AA, Odintsova SV, Musaelyan KA, Urtenova MA, Solovyova EP, Menshikova LI, et al. Predictive markers of response to immune checkpoint inhibitor rechallenge in metastatic non-small cell lung cancer. Explor Target Antitumor Ther. (2024) 5:1271–88. doi: 10.37349/etat.2024.00275
29. MacDonald M, Poei D, Leyba A, Diep R, Chennapan K, Leon C, et al. Real world prognostic utility of platelet lymphocyte ratio and nutritional status in first-line immunotherapy response in stage IV non-small cell lung cancer. Cancer Treat Res Commun. (2023) 36:100752. doi: 10.1016/j.ctarc.2023.100752
30. Yu C, Jiang H, Wang L, Jiang Z, and Jin C. Baseline (derived) neutrophil-lymphocyte ratio associated with survival in gastroesophageal junction or gastric cancer treated with ICIs. Front Oncol. (2025) 15:1404695. doi: 10.3389/fonc.2025.1404695
31. Zhou ZC, Chen KY, Li N, Xie MY, Sheng JM, Fan Y, et al. Real-world utilization of PD-1/PD-L1 inhibitors with palliative radiotherapy in patients with metastatic non-small cell lung cancer. Thorac Cancer. (2022) 13:2291–300. doi: 10.1111/1759-7714.14553
Keywords: immune checkpoint inhibitors, thoracic radiotherapy, non-small cell lung cancer, real-world study, survival outcomes
Citation: Zou Y, Chen Y, Zhou X, Gong Y, Xu Y, Zou B, Peng F, Huang M, Lu Y and Liu Y (2025) Combining immune checkpoint inhibitors with thoracic radiotherapy enhances outcomes in advanced non-small-cell lung cancer: a real-world study. Front. Oncol. 15:1611528. doi: 10.3389/fonc.2025.1611528
Received: 14 April 2025; Accepted: 11 July 2025;
Published: 06 August 2025.
Edited by:
Manash K. Paul, Manipal Academy of Higher Education, IndiaCopyright © 2025 Zou, Chen, Zhou, Gong, Xu, Zou, Peng, Huang, Lu and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yongmei Liu, bHltaTc1QDE2My5jb20=
†These authors share first authorship
 Yao Zou1,2,3†
Yao Zou1,2,3† Xiaojuan Zhou
Xiaojuan Zhou Youling Gong
Youling Gong Bingwen Zou
Bingwen Zou Feng Peng
Feng Peng Meijuan Huang
Meijuan Huang You Lu
You Lu Yongmei Liu
Yongmei Liu