- 1Department of Oncology, Tianjin Union Medical Center, The First Affiliated Hospital of Nankai University, Tianjin, China
- 2Tianjin Cancer Institute of Integrative Traditional Chinese and Western Medicine, Tianjin Union Medical Center, The First Affiliated Hospital of Nankai University, Tianjin, China
- 3Department of Radiation Oncology, National Cancer Center/National Clinical Research Center/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
Background: In volumetric modulated arc therapy (VMAT), collimator angle selection impacts dose distribution and plan quality. Conventional VMAT plans use dual arcs with collimators set at 0˚. This study explores the dosimetric effects of using orthogonal collimator angles (0˚ and 90˚) in dual-arc VMAT.
Methods: Sixty patients with head and neck, thoracic, and abdominal tumors were analyzed. Two VMAT plans were generated: Plan A (0˚ collimator angle) and Plan B (0˚ and 90˚ collimator angles). Dosimetric endpoints included conformity index (CI), gradient measure (GM), homogeneity index (HI), dose to organs at risk (OARs), mean dose to normal tissues (Dmean, NT), monitor units (MU), and gamma pass rate (GPR).
Results: Plan B improved dosimetric outcomes over Plan A. HI decreased by 0.03 in the rectum, 0.01 in the breast, and 0.01 in the larynx. GM decreased by 0.15 cm in the rectum, 0.05 cm in the breast, and 0.01 cm in the larynx. OAR doses were reduced across sites, with notable decreases in the bladder (−4.62 Gy), left anterior descending artery (−3.99 Gy), and spinal cord (−1.79 Gy). Dmean,NT was slightly reduced in the rectum, breast and larynx. MU increased in rectum plans (+41 MU), but decreased in the breast and laryngeal plans by 38 MU and approximately 73 MU, respectively. All plans achieved GPR > 95%.
Conclusion: Incorporating orthogonal collimator angles (0˚ and 90˚) in dual-arc VMAT enhances dose conformity and spares OARs without compromising target coverage or delivery accuracy. This approach is clinically applicable with minimal workflow changes.
1 Background
In the field of radiation therapy, volumetric modulated arc therapy (VMAT) achieves high-precision irradiation of planning target volume through continuous gantry rotation and dynamic adjustment of the multileaf collimator (MLC) while maximizing the protection of the surrounding normal tissues (1–3). The selection of collimator angles has a significant effect on the dose distribution and plan quality of VMAT (4–6). Currently, patients undergoing radiation therapy typically receive double-arc VMAT plans with a collimator angle of 0˚ (5, 7–10).
Under the conventional double-arc field with a collimator angle of 0˚, the beam primarily irradiates the planning target along the anteroposterior direction (i.e., from the front to the back of the patient or vice versa) (11). This arrangement may lead to several limitations. The movement direction of the MLC leaves is parallel to the gantry rotation plane, restricting the flexibility of the leaves in the direction perpendicular to the gantry rotation (3, 12, 13). Additionally, the beam shape of the 0˚ collimator is relatively fixed and may not adapt well to complex planning target shapes and the protection needs of the surrounding normal tissues (7, 14, 15). In particular, when the planning target has a pronounced concave shape or a large curvature, the 0˚ collimator may fail to effectively avoid critical organs, leading to increased dose exposure and potential radiation-induced side effects (16). For organs with relatively flexible positioning, such as the small intestine in the pelvic cavity, the 0˚ collimator arrangement may lead to high doses in certain regions, increasing the risk of small intestine damage such as enteritis or intestinal obstruction (17, 18).
To reduce the dose exposure of normal tissues, this study introduced a 90˚ collimator angle into a conventional double-arc VMAT plan. This setting leverages the modulating capability of the MLC in the orthogonal direction, which is expected to improve the dose distribution in the planning target and protect critical normal organs. This study aimed to explore a simple and effective optimization method to enhance the quality and therapeutic effects of VMAT plans without significantly increasing the plan complexity. These findings aim to provide a new perspective for the design of radiation therapy plans, particularly in improving treatment efficiency and protecting normal tissues.
2 Methods
2.1 Case selection
This retrospective study randomly selected 60 patients who underwent VMAT at the Tianjin People’s Hospital in December 2024. The patient cohort included 20 patients each with laryngeal cancer, 20 with left-sided breast cancer, and 20 with rectal cancer. The prescribed doses for the target volumes were set at 60.06 Gy/33 fractions for laryngeal cancer, 50 Gy/25 fractions for breast cancer, and 48.6 Gy/27 fractions for rectal cancer. The study adhered to the guidelines of the medical ethics committee, and all patient data were anonymized and analyzed. All patients underwent computed tomography (CT) simulation using a 16-slice large-bore CT scanner (General Electric Company. (2015). Discovery RT 590. GE Healthcare).
2.2 Plan design
For each patient, the planning target volume (PTV) and organs at risk (OARs) were contoured using Eclipse Treatment Planning System based on patient imaging data. Two VMAT plans were designed and labeled Plan A and Plan B. Plan A used a conventional double-arc plan with a collimator angle of 0˚, whereas Plan B employed a double-arc plan with orthogonal collimator angles of 0˚ and 90˚. All other parameters, such as the beam energy, field size, and optimization objectives, were kept consistent during the planning process, with only the collimator angles being altered. All plans were designed and optimized by the same medical physicist to ensure the reliability and accuracy of the results. In this study, all treatment plans for patients with rectal cancer were standardized to ensure that 100% of the target volume received at least 95% of the prescribed dose.
2.3 Target evaluation
The dose distributions of the target volumes for both plans were meticulously assessed using the conformity index (CI) (19, 20), gradient measure (GM) (21), and homogeneity index (HI) (22).
The CI is a crucial quantitative metric in radiation therapy used to evaluate the conformity of the high-dose region to the PTV. A CI value closer to 1 indicated better conformity between the dose distribution and target, indicating that the shape and size of the high-dose region more closely matched the target. CI was calculated using the following formula:
where, is the target volume at the prescribed dose, is the PTV volume, and is the total volume covered by the prescribed dose.
The GM assesses the dose fall-off at the target margins, serving as an important parameter for evaluating the dose transition between the target and surrounding normal tissues. The GM is typically measured in centimeters, representing the distance from the target edge to the 50% prescription isodose line. A higher GM value indicates faster dose fall-off at the target edge, resulting in better protection of the surrounding normal tissues. GM was calculated as follows:
where V50% is the volume enclosed by the 50% prescription isodose line, and is the PTV volume.
The HI is a vital metric for evaluating the uniformity of the dose distribution within a target during radiation therapy. An HI value closer to 0 indicates a more uniform dose distribution within the target. HI was calculated using the following formula:
where D2 is the dose covering 2% of the target volume, D98 is the dose covering 98% of the target volume, and D50 is the dose covering 50% of the target volume, respectively.
2.4 OARs evaluation
In radiation therapy, dosimetric evaluation of OARs is crucial for ensuring treatment safety. The OARs of interest are listed in Table 1. The mean dose (Dmean, OAR) to OARs was used to assess the overall dose exposure, whereas the maximum dose (Dmax) was used to evaluate potential high-dose risks. Additionally, the ratio of total body volume receiving a specific percentage of the prescribed dose (20%, 40%, 60%, and 80%) was assessed and denoted as V20%, V40%, V60%, and V80%, respectively.
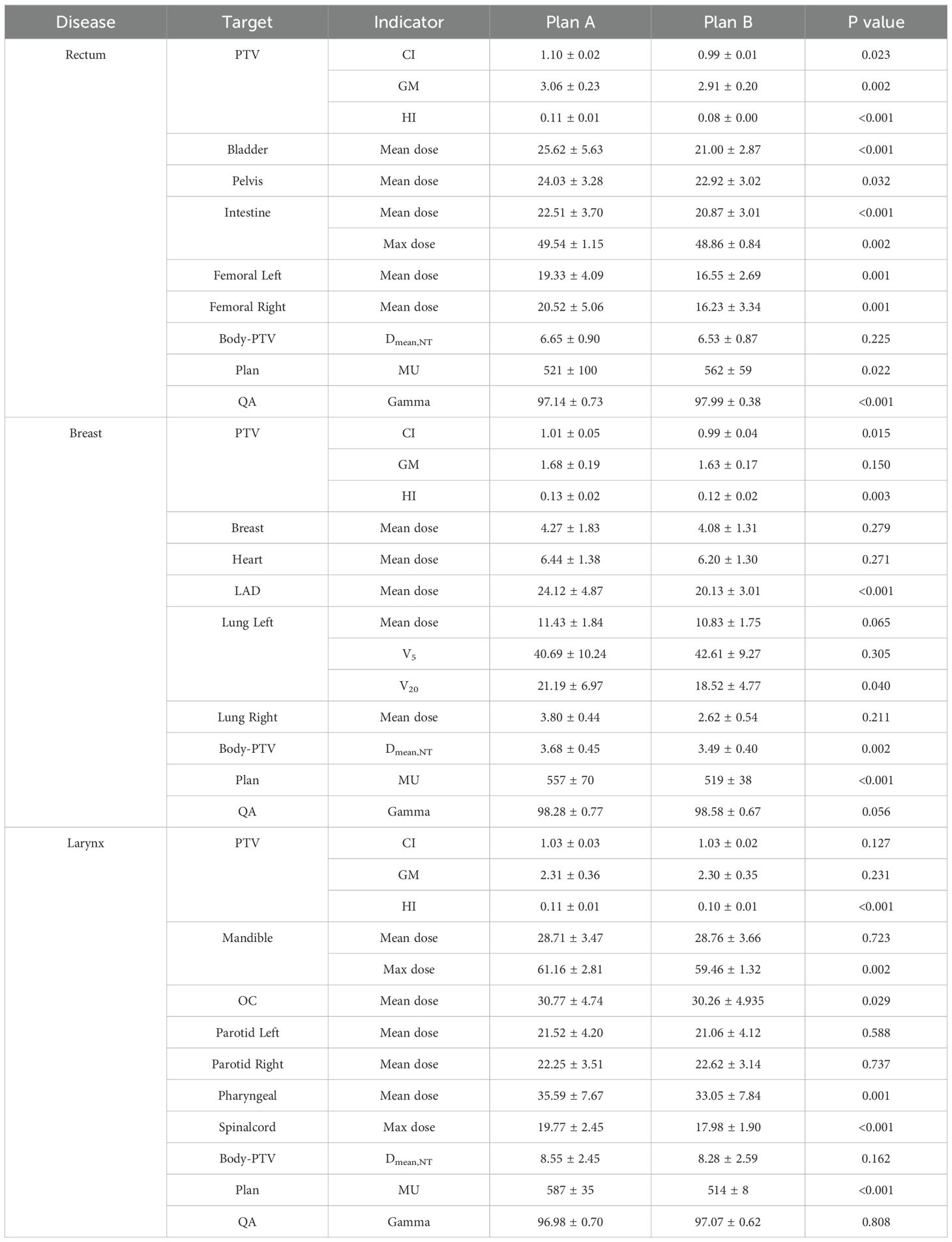
Table 1. The mean and variance of evaluation indicators for Planning Target Volumes(PTV), Organs at Risk (OARs), Monitor units (MU) and the gamma pass rate in two plans for rectal cancer, breast cancer, and laryngeal cancer.
The mean radiation dose to normal tissues (Dmean,NT) refers to the average radiation dose from normal tissues outside PTV in the radiotherapy plan. This is an important indicator for assessing the safety of a radiotherapy plan and reflects the extent of radiation exposure to normal tissues. The equation for calculating Dmean,NT is as follows:
where Vbody is the total irradiated volume (e.g., body volume), Dmean,body is the mean dose to the total irradiated volume, VPTV is the PTV volume, and Dmean,PTV is the mean dose to the PTV volume (with volume units in cm³ and dose units in Gy).
2.5 Plan evaluation
Monitor units (MU) are radiation therapy parameters that describe the total beam-on time required for a treatment plan. They reflect the irradiation intensity of the linear accelerator when executing the treatment plan. Under the same dose distribution, a lower total MU generally indicates a higher plan efficiency. In a treatment plan, the total MU is the sum of the MUs of all the beams.
Plan verification refers to the process of validating the dose distribution of a VMAT treatment plan during radiation therapy to ensure the accuracy and safety of the treatment plan. Since VMAT technology modulates volume by adjusting multiple parameters, such as the shape of the MLC opening, dose rate, and gantry rotation speed, its dose distribution is complex and requires individualized quality assurance (QA). In this study, an electronic portal imaging device (EPID) was used to compare the dose calculated using the planning system with the actual measured dose.
The gamma pass rate (GPR) is the percentage of points that meet specific dose difference and distance-to-agreement (DTA) criteria in gamma analysis. Gamma analysis was used to compare the planned dose distributions with measured dose distributions by calculating the gamma index for each point to assess the consistency between the two. Points with a gamma index < 1 are considered “passed” and the GPR is the percentage of these “passed” points out of the total evaluated points. In clinical practice, a commonly used gamma analysis criterion is a 3% dose difference and 3 mm DTA (3%/3 mm), with a pass rate of 90% being the acceptable standard. A higher GPR indicates better consistency between the planned and measured dose distributions, leading to higher treatment accuracy and safety.
2.6 Statistical analysis
A rigorous statistical treatment and analysis were conducted on the collected data. Comprehensive descriptive statistical analyses, including mean and standard deviation, were performed for all evaluation indicators encompassed by Plan A and Plan B. Given that the sample size for all analyses was only 20, the Kolmogorov–Smirnov (K–S) test (23) was employed to assess the normality of the data. The results of the normality test indicated that the majority of the data did not conform to a Gaussian distribution (p<0.05). Consequently, non-parametric methods were utilized for statistical comparisons. Specifically, the Wilcoxon signed-rank test (24) was applied for comparisons between the two groups. All statistical analyses were carried out using OriginPro 2024 software (OriginLab Corporation, Northampton, MA, USA). The significance threshold for this study was set at p<0.05.
3 Results
3.1 Target volume
In terms of target conformity, the CI for rectal, breast, and laryngeal cancers showed no significant differences between Plan A and Plan B, indicating consistent conformity between the two plans. The results can be seen in Figure 1a and Table 1. However, the average GM of Plan B was lower than that of Plan A across all three cancers, indicating a faster dose fall-off at the target margins and improved protection of surrounding normal tissues. Notably, the GM reduction in breast cancer was minimal (0.05 cm, p=0.150), and some individual cases exhibited higher GM in Plan B. The largest GM reduction was observed in rectal cancer (0.15 cm, p=0.002), followed by laryngeal cancer (0.01 cm). Notably, the reduction in breast cancer was minimal, suggesting a limited clinical impact and highlighting anatomical factors that may reduce the modulation advantage of orthogonal collimation in this site. The results are shown in Figure 1b and Table 1. Similarly, the HI of Plan B was lower than that of Plan A, indicating a more uniform dose distribution within the planning target. The HI reductions were 0.03 for rectal cancer (p<0.001), 0.01 for breast cancer (p=0.003), and 0.01 for laryngeal cancer (p<0.001). The results are presented in Figure 1c and Table 1. For clarity, P values are reported only when the comparison between plans resulted in notable or statistically significant differences.
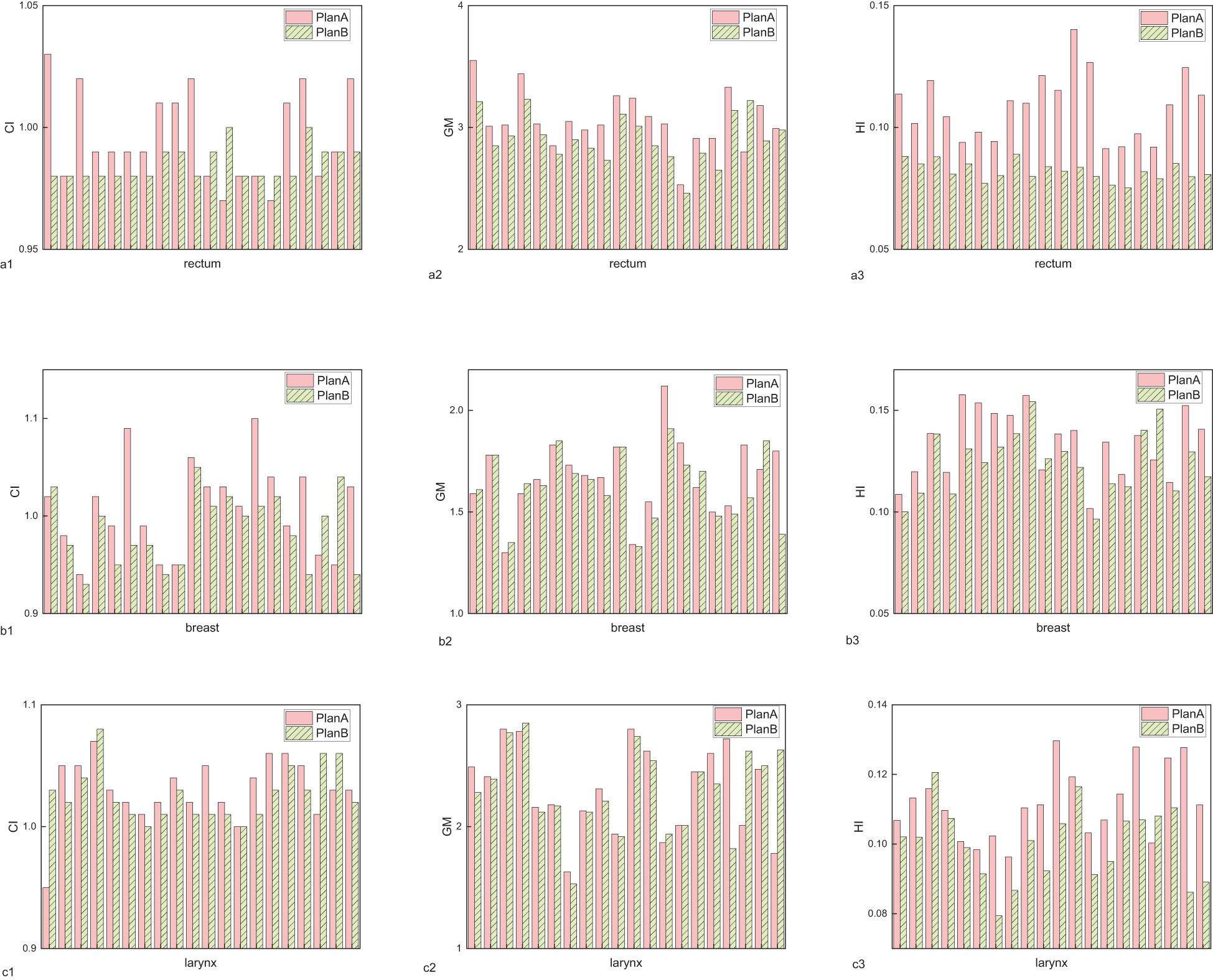
Figure 1. The assessment indicators for planning target volumes of rectal cancer, breast cancer, and laryngeal cancer. (a) conformity index (CI), (b) gradient measure (GM), (c) homogeneity index (HI).
3.2 OARs
For rectal cancer, Plan B demonstrated a reduction in the Dmean to the bladder, pelvis bone, intestine, and both femoral heads by 4.62 (p<0.001), 1.11 (p<0.001), 1.64 (p<0.001), left 2.78 (p=0.001) and right 4.29 Gy (p=0.001), respectively. The Dmax to the intestine decreased by 0.68 Gy (p=0.002). Dmean,NT decreased from 6.65 ± 0.90 Gy in Plan A to 6.53 ± 0.87 Gy in Plan B. The results are presented in Figure 2a and Table 1.
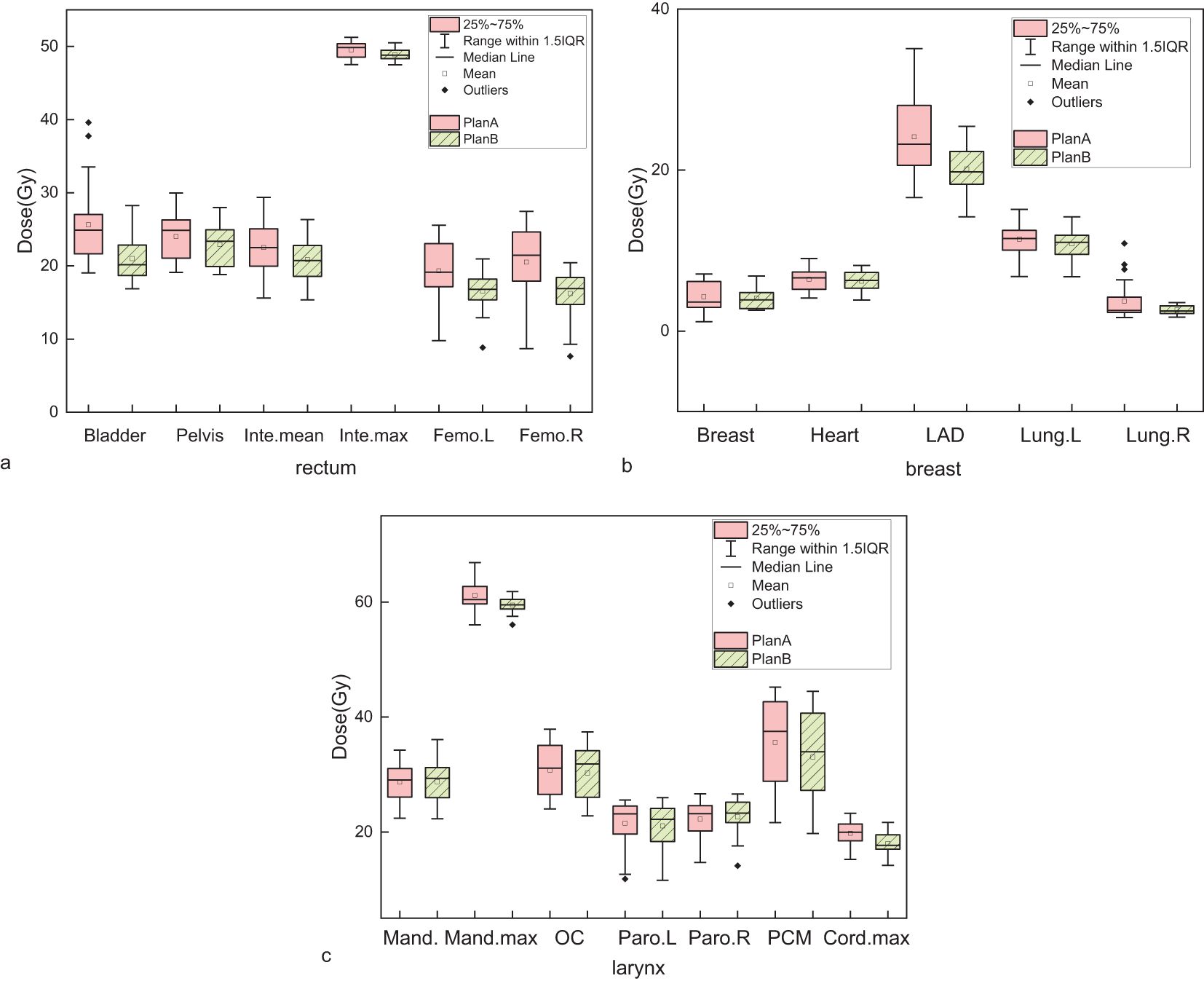
Figure 2. Comparison of mean or maximum dose to organs at risk (OARs) between Plan A (dual-arc VMAT with identical collimator angles) and Plan B (dual-arc VMAT with orthogonal collimator angles: 0˚ and 90˚). (a) Rectal cancer: bladder, pelvis, small intestine (Inte), left femoral head (Femo.L), and right femoral head (Femo.R); (b) Breast cancer: contralateral breast, heart, left anterior descending artery (LAD), ipsilateral lung (lung.L), and contralateral lung (lung.R); (c) Laryngeal cancer: mandible (Mand.), oral cavity (OC), left and right parotid glands (Paro.L and Paro.R), pharyngeal constrictor (PCM), and spinal cord. Each group contains 10 patients. Boxplots illustrate the distribution of dose values: boxes represent the interquartile range (IQR), horizontal lines indicate medians, whiskers extend to 1.5×IQR, and open circles denote statistical outliers. Slight dose reductions in Plan B are observed in several OARs, though absolute differences remain small in most cases.
For breast cancer, Plan B resulted in a decrease in the mean dose to the contralateral breast, heart, left anterior descending artery (LAD), ipsilateral lung and contralateral lung by 0.19, 0.24, 3.99 (p<0.001), 0.60, and 1.18 Gy, respectively. Dmean,NT decreased by 0.19 Gy (p=0.002). However, the volume of the affected lung receiving 5 Gy (V5:+1.92 Gy) increased, whereas the volume receiving 20 Gy (V20: -2.67 Gy) decreased. The results are shown in Figure 2b and Table 1.
For laryngeal cancer, the mean dose to mandible increased by 0.06 Gy, while the maximum dose decreased by 1.7 Gy (p=0.002). The mean doses to the oral cavity, left parotid gland, and pharyngeal constrictor decreased by 0.51 (p=0.029), 0.46, and 2.54 Gy (p=0.001), respectively. The mean dose to the right parotid gland increased by 0.37 Gy. The maximum dose to the spinal cord decreased by 1.79 Gy (p<0.001). Dmean,NT decreased by 0.27 Gy. The results can be seen in Figure 2c and Table 1.
3.3 Dose lines
In the analysis of isodose line volumes for rectal cancer, the ratios of total body volume receiving 20%, 40%, 60%, and 80% of the prescription dose (V20%, V40%, V60%, and V80%) were numerically lower in Plan B. However, these changes were small (typically <1%) and may reflect variability inherent to small-sample pilot data. The results are shown in Figure 3a and Table 2. For breast cancer, V20% and increased by 0.43%. While V40%, V60% and V80% decreased by 0.37%, 0.36% and 0.15%, respectively. The results are displayed in Figure 3b and Table 2. For laryngeal cancer, V40% and V80% slightly increased by 0.03%, and 0.58%, respectively. While V20% and V60% decreased by 0.61%, and 0.09%, respectively. The results are displayed in Figure 3c and Table 2.
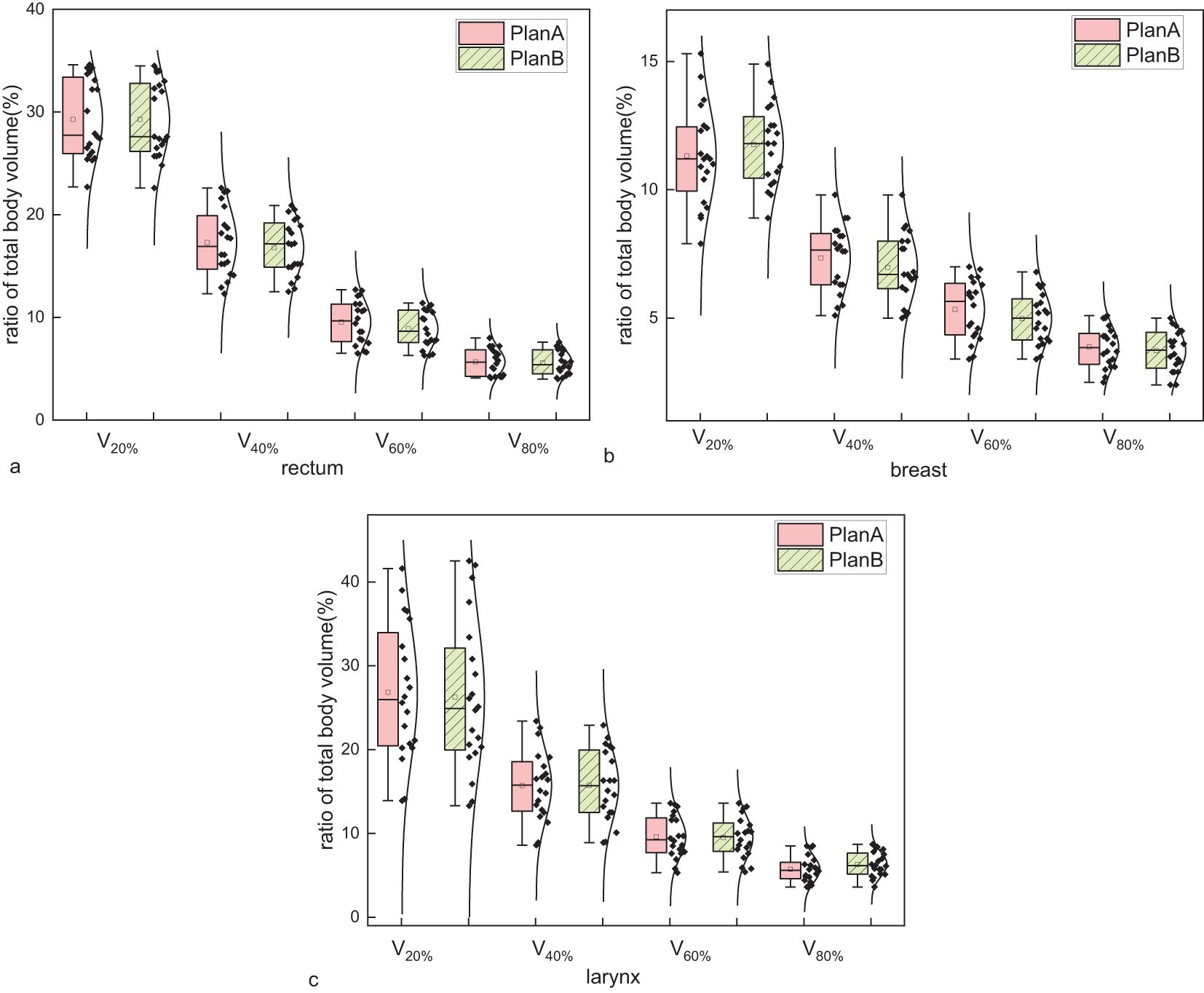
Figure 3. Comparison of the ratio of total body volume receiving 20%, 40%, 60%, and 80% of the prescription dose (V20%, V40%, V60%, and V80%) between Plan A and Plan B across three disease sites: (a) rectal cancer, (b) breast cancer, and (c) laryngeal cancer. Each metric reflects the percentage of the entire patient body exposed to respective isodose levels. Each group includes 20 patients. Boxplots represent distribution: the boxes show the interquartile range (IQR), horizontal lines indicate medians, whiskers extend to 1.5×IQR, and open circles represent outliers. Although Plan B showed numerically lower V-values in most cases, the differences were marginal, suggesting limited dosimetric impact on total body exposure.
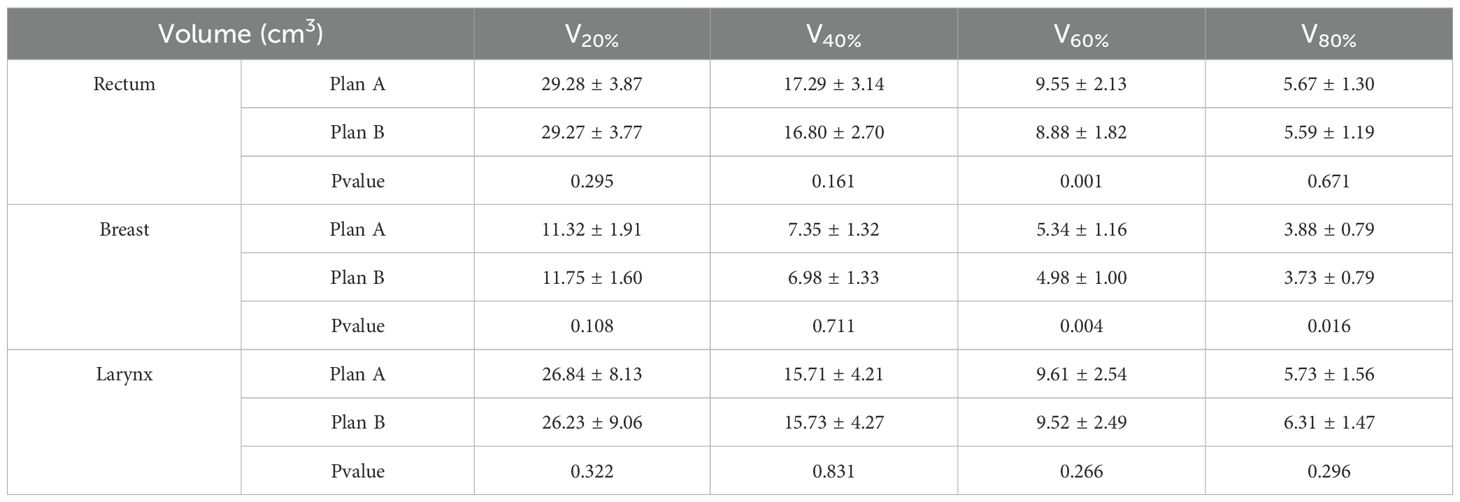
Table 2. Mean and variance of the ratio of total body volume corresponding to the prescribed dose percentages (20%, 40%, 60%, 80%).
3.4 Dose-volume histogram
In the analysis of DVH for OARs, Plan B showed significant changes compared to Plan A. For rectal cancer, the DVH for the bladder, pelvis bone, and intestine showed a notable reduction. Specifically, the bladder DVH showed a maximum relative volume reduction of 19.57% within a dose range of 11.1–27.4 Gy. The pelvis bone DVH showed a maximum reduction of 9.23% within the dose range of 22.3–36.6 Gy. The intestine DVH showed a maximum reduction of 4.87% within the dose range of 11.2–40.4 Gy. The left femoral head DVH showed a maximum reduction of 21.51% within the dose range of 14.8–26.1 Gy, while the right femoral head DVH showed a maximum reduction of 31.86% within the dose range of 15.1–26.6 Gy. The results are shown in Figure 4a.
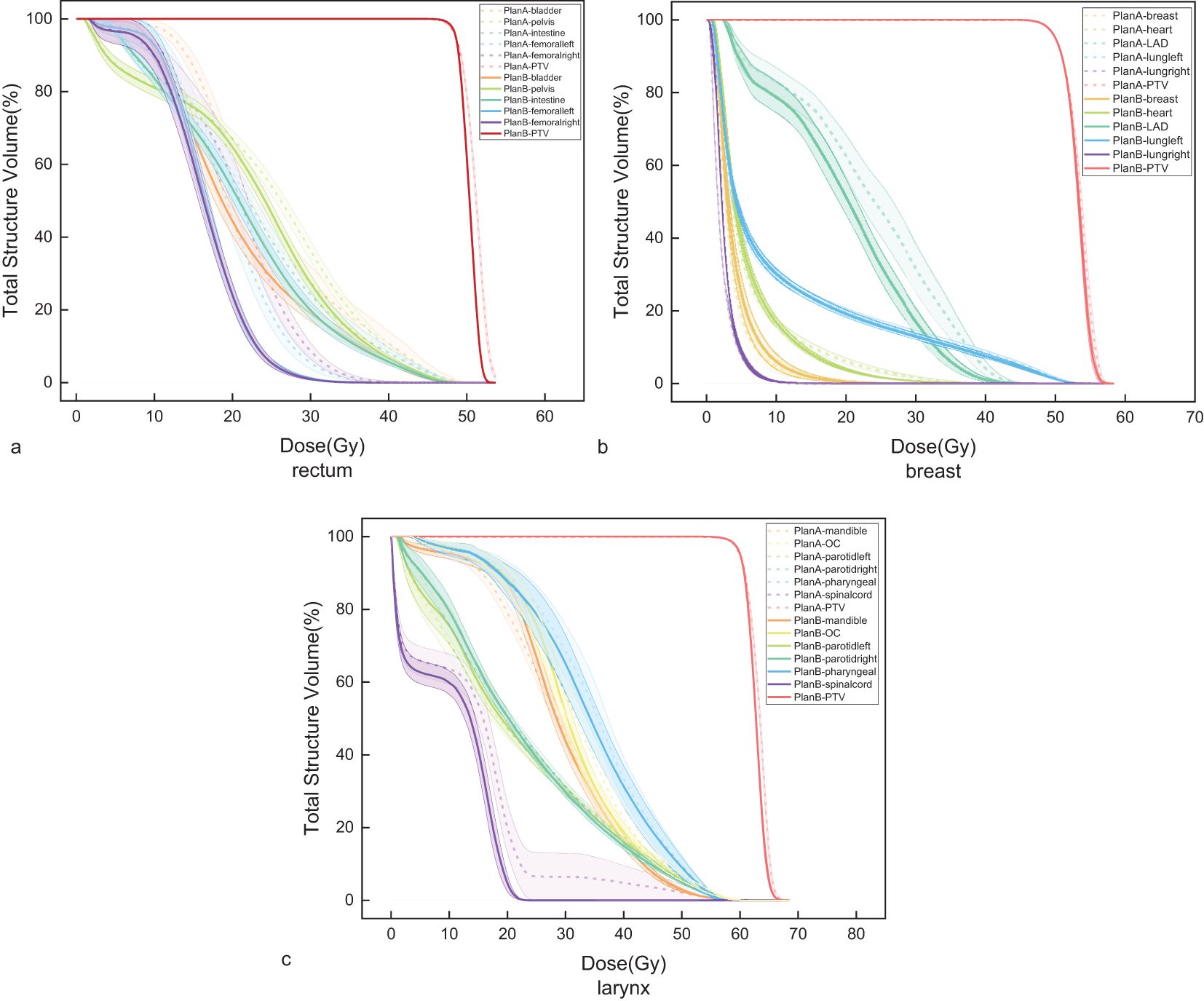
Figure 4. Dose-Volume Histogram (DVH) of the Planning Target Volumes(PTV) and Organs at Risk (OARs). (a) rectal cancer, (b) breast cancer, and (c) laryngeal cancer.
For breast cancer, Plan B showed an increase in the contralateral lung DVH within a dose range of 16.7–27.4 Gy, with a maximum increase of 0.15%. The contralateral breast DVH showed a maximum reduction of 0.15% within a dose range of 16.7–27.4 Gy. The heart DVH showed a maximum reduction of 2.35% within the dose range of 12.6–29.1 Gy. The LAD DVH showed the most significant reduction within the dose range of 15.1–37.0 Gy, with a maximum reduction of 15.76%. The DVH of the affected lung showed a maximum reduction of 0.87% within the dose range of 20.3–49.1 Gy. The results are shown in Figure 4b.
For laryngeal cancer, the mandibular DVH showed a maximum reduction of 2.03% within a dose range of 13.9–34.9 Gy. The oral cavity DVH showed a maximum reduction of 7.44% within a dose range of 29.2–39.9 Gy. The left parotid gland DVH showed a maximum reduction of 1.80% within the dose range of 34.9–56.1 Gy, while the right parotid gland DVH showed a maximum reduction of 1.62% within the dose range of 22.3–50.8 Gy. The pharyngeal constrictor DVH showed a maximum reduction of 5.23% within a dose range of 25.7–39.1 Gy. The spinal cord DVH showed a maximum reduction of 18.42% within a dose range of 13.8–22.4 Gy. The results are shown in Figure 4c.
3.5 Plan results
The mean value of MU for rectal (p=0.022), breast (p<0.001), and laryngeal cancers (p<0.001) were 521, 557, and 587 for Plan A, and 562, 519, and 514 for Plan B, respectively. The results are shown in Figure 5a. The gamma pass rate (GPR) for all plans exceeded 95%. The results are seen in Figure 5b.
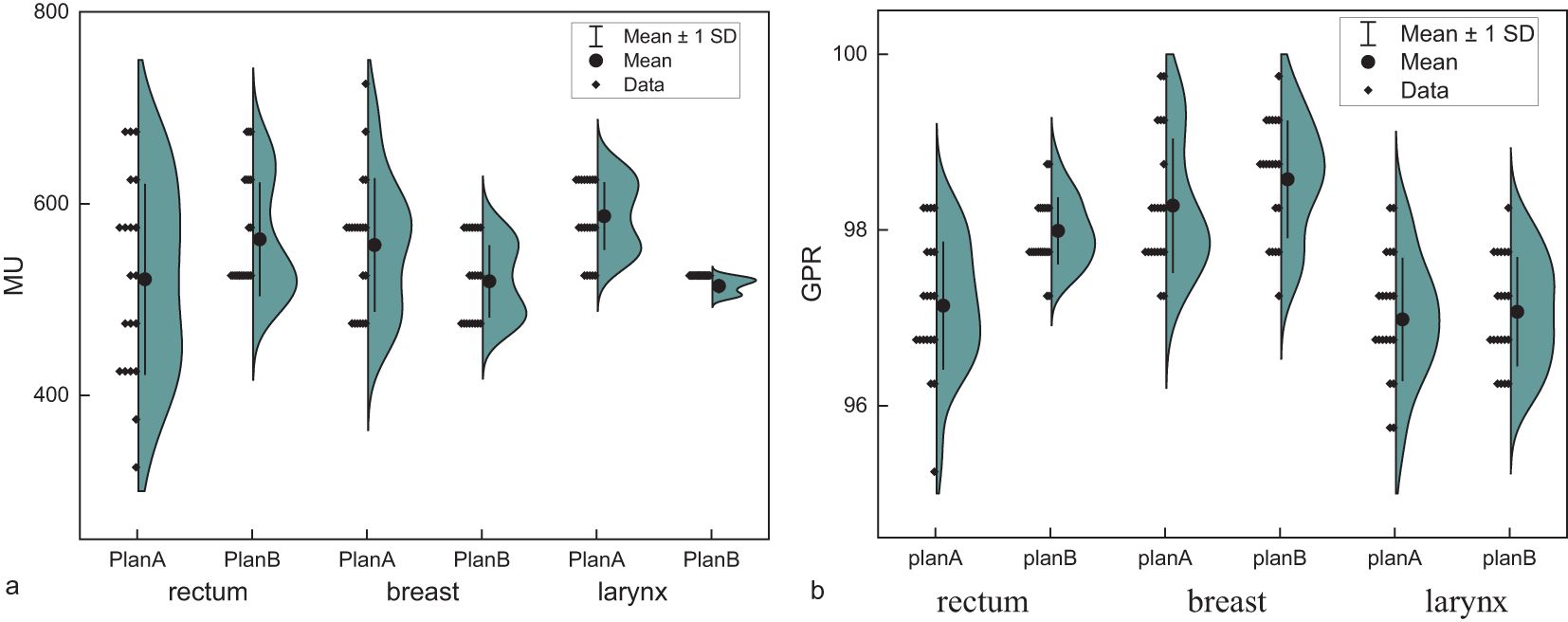
Figure 5. (a) Monitor units (MU) of Plan A and Plan B in rectal cancer, breast cancer, and laryngeal cancer. (b) The gamma pass rate (GPR) of Plan A and Plan B in rectal cancer, breast cancer, and laryngeal cancer.
4 Discussion
This study compared VMAT plans using a 90˚ collimator angle (Plan B) with conventional collimator angle plan (Plan A) to evaluate its potential benefits in optimizing planning target dose distribution and protecting OARs. The results revealed the significant advantages of Plan B in key dosimetric indicators, providing innovative ideas for radiotherapy plan design and holding significant clinical application value.
The results indicated that Plan B was comparable to Plan A regarding target dose coverage, achieving 95% coverage of the prescription dose. This suggests no significant difference in the overall dose coverage capability between the two plans. However, Plan B was significantly superior to Plan A regarding GM and HI. The decrease in the GM of Plan B indicates a faster dose fall-off at the target margins, which could protect the surrounding normal tissues more effectively. Moreover, the reduction in the HI in Plan B suggests a more uniform dose distribution within the target. This may be attributed to the introduction of a 90˚ collimator angle, which fully uses the modulating capability of the MLC in the orthogonal direction, thereby improving dose distribution.
Regarding OAR protection, Plan B demonstrated a significant dose reduction trend compared with Plan A. In particular, for the intestine, which is located flexibly within the pelvic cavity and close to the target, Plan B significantly reduced the dose exposure to the intestine through the optimized collimator angles. This reduces the risk of complications caused by high-dose irradiation, such as enteritis and intestinal obstruction. Additionally, the decrease in the Dmean to the bladder indicates that Plan B can avoid increased dose exposure to the bladder, thereby reducing the incidence of bladder irritation symptoms. For breast cancer, Plan B reduces the dose to important organs such as the contralateral breast, heart, and left anterior descending artery (LAD). Although the V5 volume of the affected lung increased, the V20 decreased, which is favorable for protecting normal tissues overall. This optimization strategy helps reduce the long-term effect of radiotherapy on the heart and coronary arteries while minimizing damage to the affected lung. Notably, V5 and V20 for the left lung (LungL) exhibited considerable inter-patient variability, with standard deviations exceeding 20% of the mean in some cases. This variability may arise from differences in chest wall curvature, lung expansion, and beam entry geometry, all of which can affect low-dose bath distribution. As such, these parameters should be interpreted with caution, especially in small pilot cohorts. For laryngeal cancer, Plan B exhibited more precise dose control in areas such as the mandible, oral cavity, parotid glands, and pharyngeal constrictors, with a significant reduction in the maximum dose to the spinal cord. This indicates that Plan B offers greater advantage in protecting important organs and tissues in patients with laryngeal cancer, thereby effectively reducing the risk of radiotherapy-related complications.
In rectal cancers, the ratio of total body volumes receiving 20%, 40%, 60%, and 80% of the prescribed dose (V20%, V40%, V60%, and V80%, respectively) decreased in Plan B. This indicates that Plan B can better protect the surrounding normal tissues during rectal cancer radiotherapy while maintaining adequate tumor irradiation. However, for breast cancer, the changes in Plan B dose lines are more complex. The volumes receiving 20% of the prescribed dose increased, whereas those receiving 40%, 60% and 80% decreased significantly. These changes may be related to the specific shape of the breast cancer target and surrounding tissues. Overall, Plan B effectively controlled the high-dose region volumes in optimizing isodose lines, particularly in radiotherapy plans for rectal and breast cancers. This optimization strategy helps reduce the potential damage to the surrounding normal tissues while ensuring adequate planning target irradiation. For laryngeal cancer, optimizing dose lines in Plan B must consider the balance between the low- and high-dose regions to achieve the best therapeutic effect.
Through a comparative analysis of radiotherapy plans for the three types of cancer, we found significant differences in the mean number of MUs between Plan A and Plan B across different cancer types, with inconsistent trends. These differences may be closely related to factors such as tumor location, radiotherapy techniques, dose distribution optimization strategies, and individual patient anatomy. For example, Plan B may focus on protecting normal tissues within the pelvic cavity in optimizing rectal cancer. By increasing the number of MUs, a more complex dose distribution was achieved to reduce the dose exposure to normal tissues. In the breast cancer optimization process, Plan B may prioritize the uniformity and conformity of the dose distribution. Adjusting the arc angles and MLC movements reduces the demand for the MUs. For laryngeal cancer, Plan B may focus more on precise irradiation of the laryngeal tumor and the cervical lymphatic drainage area. A more precise dose distribution was achieved by reducing the number of MUs.
Adjusting the collimator angle alters the propagation path and dose distribution of the radiation beam within a patient’s body. Introduction a 90˚ collimator angle allows the radiation beam to cover the target area more comprehensively, reducing the dose of cold and hot spots and achieving a more uniform and conformal dose distribution. Moreover, the complementary use of collimator angles at different angles can significantly reduce the dose exposure to OARs, particularly when the target has large curvature changes adjacent to or interwoven with OARs, and the advantages of the 90˚ collimator angle are more prominent. Target areas with large curvature changes have a concave shape, similar to the two separate targets. Such target structures are prone to problems similar to those of isolated island blockages when designed. Figure 6 illustrates the dose distribution and beam eye view (BEV) for 0˚ and 90˚ collimator angles.
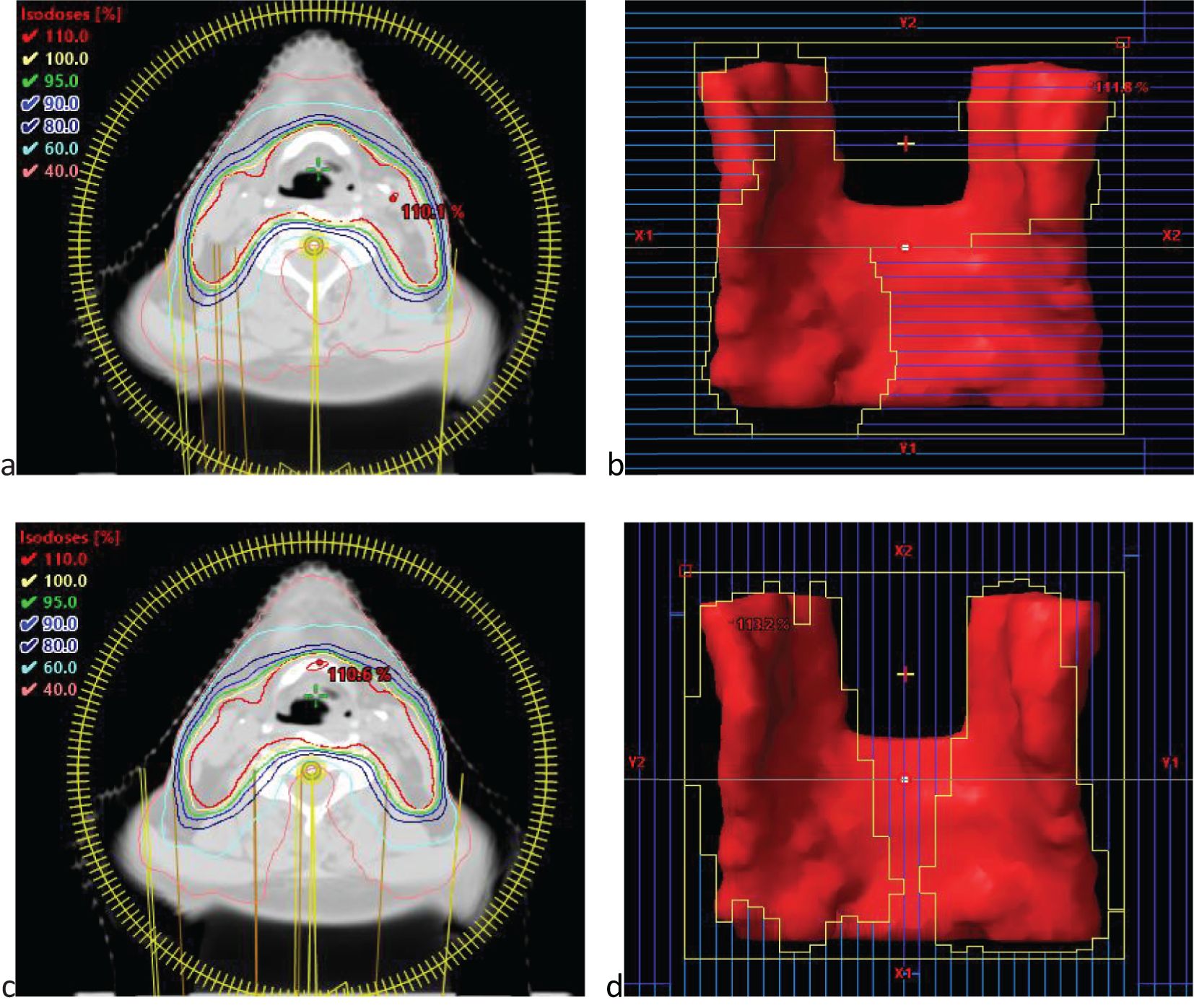
Figure 6. (a) The illustration of dose distribution under 0˚ collimator angles. (b) the beam eye view (BEV) under 0˚ collimator angles. (c) The illustration of dose distribution under 90˚ collimator angles. (d) the beam eye view (BEV) under 90˚ collimator angles.
The HyperArc orthogonal collimator technique has garnered widespread attention owing to its ability to achieve high-precision dose irradiation among modern radiotherapy techniques. However, applying this technique requires complex equipment and optimization algorithms. By simplifying the technical approach, this study explores whether combining a 90˚ collimator arc with a 0˚ arc can achieve effects similar to those of orthogonal collimators while avoiding dose inhomogeneity issues. This study investigates the effects of applying the 90˚ collimator arc in radiotherapy and the rationality for its combination with the 0˚ arc. This approach achieves a high dose conformity similar to the Halcyon orthogonal collimator and effectively improves dose uniformity within the target. This combination optimizes the direction and intensity distribution of dose irradiation through the synergistic action of multiple angles, thereby reducing the dose inhomogeneity caused by a single arc angle.
Although personalized collimator angle settings can potentially achieve improved dosimetric results, they are often associated with increased planning complexity and require substantial time investment, experience, and technical expertise from physicists. In contrast, our study employed a fixed orthogonal configuration with collimator angles of 0˚ and 90˚ for the two arcs in Plan B, without additional modulation. This simplified approach not only enhances reproducibility and planning efficiency but is also feasible for implementation on most modern treatment planning systems and linear accelerators, which support assigning different collimator angles for each arc. Therefore, the proposed method offers a practical balance between dosimetric benefit and clinical applicability. They require extensive clinical experience and advanced planning and design skills. In comparison, adding a 90˚ collimator angle does not require complex adjustments for each case and has strong operability and universality. This can simplify the plan design process and improve work efficiency while ensuring therapeutic effects. In actual radiotherapy, when managing many patients with different types of tumors, there is a need for a method that can ensure therapeutic effects while simplifying the plan design process and improving work efficiency. The VMAT plan optimization method of adding a 90˚ collimator angle meets this demand and has significant clinical application value.
Despite the advantages of the method design and result analysis, this study has some limitations. First, the sample size was small, with only 60 patients, which may have limited the statistical analysis. This was a proof-of-concept investigation. Future research should validate the statistical effects and clinical applicability by expanding the sample size. Second, future studies should further explore the application effects of the 90˚ collimator angle at other tumor sites and its comprehensive application value in combination with other optimization techniques, such as adaptive radiotherapy. In addition, this study focused solely on improvement in dosimetric indicators and did not involve long-term follow-up of clinical treatment effects. Future research should combine patient clinical prognostic data to evaluate the efficacy and safety of the optimized plan for actual treatment.
5 Conclusion
In summary, by comparing the dosimetric indicators of conventional 0˚ collimator angle (Plan A) and 0˚ and 90˚ orthogonal collimator angles (Plan B) in VMAT plans, this study explored the effect of the 90˚ collimator on target dose distribution and OAR protection. The results demonstrated that Plan B significantly outperformed Plan A in key dosimetric metrics, including improved homogeneity index (HI) and gradient measure (GM), as well as reduced dose exposure to OARs, while maintaining complete target coverage and high-precision plan delivery. Moreover, the implementation of Plan B does not require complex individualized adjustments, offering high clinical feasibility and generalizability. This study provides a simple and effective new concept for designing radiotherapy plans with significant clinical value. Future research should expand the sample size to further validate its statistical effects and clinical applicability, and evaluate the actual efficacy and safety of the optimized plan.
Data availability statement
The raw data generated in this study contains clinical information that cannot be disclosed to the journal. Requests to access the datasets should be directed to XH, bWVkaWNhbHBoeXNpY2lzdHNAMTYzLmNvbQ==.
Author contributions
XH: Writing – original draft, Formal analysis. CG: Writing – review & editing, Methodology, Formal analysis. SL: Writing – review & editing, Data curation. KM: Writing – review & editing, Project administration. HW: Writing – review & editing, Funding acquisition, Resources.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was supported by the Fundamental Research Funds for the Tianjin People’s Hospital (No.2024YJ021), the Tianjin Key Medical Discipline (Specialty) Construction Project (No.TJYXZDXK-053B), the Tianjin Key Specialty Construction Project for combining traditional Chinese and Western medicine (No.ZDZKKF02), National High Level Hospital Clinical Research Funding (2022-CICAMS-80102022203), and Tianjin Health Research Project (Grant No.TJWJ2025QN051).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that Generative AI was used in the creation of this manuscript. Generative AI was used in this article for grammatical and linguistic standardization.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Otto K. Volumetric modulated arc therapy: IMRT in a single gantry arc. Med Phys. (2008) 35:310–7. doi: 10.1118/1.2818738
2. Macchia G, Deodato F, Cilla S, Cammelli S, Guido A, Ferioli M, et al. Volumetric modulated arc therapy for treatment of solid tumors: current insights. Onco Targets Ther. (2017) 10:3755–72. doi: 10.2147/OTT.S113119
3. Peiris GS, Whelan B, Hardcastle N, and Sheehy SL. The effect of multi-leaf collimator leaf width on VMAT treatment plan quality. J Appl Clin Med Phys. (2025) 26:e70018. doi: 10.1002/acm2.70018
4. Isa M, Rehman J, Afzal M, and Chow JC. Dosimetric dependence on the collimator angle in prostate volumetric modulated arc therapy. Int J Cancer Ther Oncol. (2014) 2:20419. doi: 10.14319/ijcto.0204.19
5. Kim M, Kim B, Choi E, Shin YS, Park SG, Oh YK, et al. Analyzing collimator rotation angle influence on half-beam VMAT outcomes for prostate cancer: A comparative approach using statistical and machine learning methods. (2024). doi: 10.21203/rs.3.rs-4225871/v1
6. Saeed H, Sadiqullah HM, Ahmad HK, Shafiq S, Ahmed Q, Basit A, et al. The effect of varying collimator angles on VMAT planning of prostate cancer. Pakistan J Med Health Sci. (2023) 17:145–8. doi: 10.53350/pjmhs2023174145
7. Sun W, Chen K, Li Y, Xia W, Dong L, Shi Y, et al. Optimization of collimator angles in dual-arc volumetric modulated arc therapy planning for whole-brain radiotherapy with hippocampus and inner ear sparing. Sci Rep. (2021) 11:19035. doi: 10.1038/s41598-021-98530-7
8. Li X, Wu J, Palta M, Zhang Y, Sheng Y, Zhang J, et al. A collimator setting optimization algorithm for dual-arc volumetric modulated arc therapy in pancreas stereotactic body radiation therapy. Technol Cancer Res Treat. (2019) 18:1533033819870767. doi: 10.1177/1533033819870767
9. Sze HC, Lee MC, Hung WM, Yau TK, and Lee AW. RapidArc radiotherapy planning for prostate cancer: single-arc and double-arc techniques vs. intensity-modulated radiotherapy. Med Dosim. (2012) 37:87–91. doi: 10.1016/j.meddos.2011.01.005
10. Treutwein M, Hipp M, Koelbl O, and Dobler B. Searching standard parameters for volumetric modulated arc therapy (VMAT) of prostate cancer. Radiat Oncol. (2012) 7:108. doi: 10.1186/1748-717X-7-108
11. Li MH, Huang SF, Chang CC, Lin JC, and Tsai JT. Variations in dosimetric distribution and plan complexity with collimator angles in hypofractionated volumetric arc radiotherapy for treating prostate cancer. J Appl Clin Med Phys. (2018) 19:93–102. doi: 10.1002/acm2.12249
12. Ahn BS, Park SY, Park JM, Choi CH, Chun M, and Kim JI. Dosimetric effects of sectional adjustments of collimator angles on volumetric modulated arc therapy for irregularly-shaped targets. PloS One. (2017) 12:e0174924. doi: 10.1371/journal.pone.0174924
13. Huang SX, Yang SH, Zeng B, and Li XH. Personalized selection of unequal sub-arc collimator angles in VMAT for multiple brain metastases. Appl Radiat Isot. (2024) 214:111513. doi: 10.1016/j.apradiso.2024.111513
14. Serarslan A, Daştan Y, Aksu T, Yıldız RE, Gürsel B, Meydan D, et al. Ninety-degree angled collimator: a dosimetric study related to dynamic intensity-modulated radiotherapy in patients with endometrial carcinoma. BMC Cancer. (2023) 23:515. doi: 10.1186/s12885-023-11033-8
15. Abdulameer MS, Pallathadka H, Menon SV, Rab SO, Hjazi A, Kaur M, et al. Dosimetric effect of collimator rotation on intensity modulated radiotherapy and volumetric modulated arc therapy for rectal cancer radiotherapy. J Xray Sci Technol. (2024) 32:1331–48. doi: 10.3233/XST-240172
16. Durmuş IF, Esitmez D, Arslan GI, and Okumuş A. Dosimetric comparison of three-dimensional conformal, intensity-modulated radiotherapy, volumetric modulated arc therapy, and dynamic conformal arc therapy techniques in prophylactic cranial irradiation. Prog Med Phys. (2023) 34:41–7. doi: 10.14316/pmp.2023.34.4.41
17. Barillot I, Tavernier E, Peignaux K, Williaume D, Nickers P, Leblanc-Onfroy M, et al. Impact of post operative intensity modulated radiotherapy on acute gastro-intestinal toxicity for patients with endometrial cancer: results of the phase II RTCMIENDOMETRE French multicentre trial. Radiother Oncol. (2014) 111:138–43. doi: 10.1016/j.radonc.2014.01.018
18. Liao X, Wu F, Wu J, Peng Q, Yao X, Kang S, et al. Impact of positioning errors in the dosimetry of VMAT left-sided post mastectomy irradiation. Radiat Oncol. (2020) 15:1–7. doi: 10.1186/s13014-020-01556-w
19. Feuvret L, Noël G, Mazeron J. J., and Bey P. Conformity index: A review. Int J Radiat Oncol Biol Phys. (2006) 64:333–42. doi: 10.1016/j.ijrobp.2005.09.028
20. Hodapp N. The ICRU Report 83: prescribing, recording and reporting photon-beam intensity-modulated radiation therapy (IMRT). Strahlenther Onkol. (2012) 188:97–9. doi: 10.1007/s00066-011-0015-x
21. Duan Y, Lin Y, Wang H, Kang B, Feng A, Ma K, et al. How does the gradient measure of the lung SBRT treatment plan depend on the tumor volume and shape? Front Oncol. (2021) 11:781302.
22. Kataria T, Sharma K, Subramani V, Karrthick K. P., and Bisht S. S.. Homogeneity Index: An objective tool for assessment of conformal radiation treatments. J Med Phys. (2012) 37:207–13. doi: 10.4103/0971-6203.103606
23. Vrbik JSmall-sample corrections to Kolmogorov–Smirnov test statistic. Pioneer J Theor Appl Stat. (2018) 15:15–23.
24. McDonald JH. Wilcoxon signed-rank test - Handbook of Biological Statistics . Available online at: www.biostathandbook.com (Retrieved September 02, 2021).
Keywords: radiotherapy, radiotherapy treatment planning, volumetric modulated arc therapy, orthogonal collimator angles, dose conformity and homogeneity
Citation: Huang X, Guo C, Liu S, Men K and Wang H (2025) Dosimetric analysis of orthogonal collimator configuration in volumetric modulated arc therapy planning: a comparative study. Front. Oncol. 15:1612643. doi: 10.3389/fonc.2025.1612643
Received: 16 April 2025; Accepted: 30 June 2025;
Published: 29 July 2025.
Edited by:
Abdul K. Parchur, Medical College of Wisconsin, United StatesReviewed by:
Prarthana Pasricha, Carleton University, CanadaPraveenbalaji Rajendran, Harvard University, United States
Copyright © 2025 Huang, Guo, Liu, Men and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kuo Men, bWVua3VvMTI2QDEyNi5jb20=; Hui Wang, d2FuZ2h1aTJAdW1jLm5ldC5jbg==
†These authors have contributed equally to this work and share first authorship
‡These authors have contributed equally to this work
 Xin Huang
Xin Huang Chenlei Guo
Chenlei Guo Shuangtong Liu1,2†
Shuangtong Liu1,2† Kuo Men
Kuo Men