- 1Department of Internal Medicine, Section of Medical Oncology and Hematology, Spital Limmattal, Schlieren, Switzerland
- 2Department of Radiology, Spital Limmattal, Schlieren, Switzerland
- 3Department of Pathology and Molecular Pathology, University Hospital Zurich (USZ) and University of Zurich (UZH), Zurich, Switzerland
- 4Department of Medical Oncology and Hematology, University Hospital Zurich (USZ) and University of Zurich (UZH), Zurich, Switzerland
Background: Colorectal cancer rarely harbors rearrangements in the ALK gene, and the therapeutic significance of non-canonical or functionally unclear ALK fusions remains poorly defined. We report a case of metastatic CRC with an ALK–CEP44 fusion not previously described in this tumor type, associated ALK overexpression, a notable clinical response to the ALK inhibitor alectinib, and rapid development of multiple ALK resistance mutations.
Case Summary: A 60-year-old male patient was diagnosed with stage IIIA right-sided CRC. Six months after adjuvant chemotherapy, he developed liver metastases. Comprehensive molecular profiling revealed strong ALK expression, a novel ALK–CEP44 fusion predicted to lack a functional kinase domain, and additional ALK alterations. Palliative chemotherapy induced a temporary response. Upon progression, treatment with alectinib led to rapid clinical, radiological and biochemical improvement. However, disease progression recurred shortly thereafter, and next-generation sequencing revealed four resistance-associated ALK mutations. The patient ultimately died due to progressive liver failure.
Conclusion: ALK-targeted therapy may provide benefit in selected CRC cases with atypical ALK alterations, even when the oncogenic role is uncertain. Comprehensive molecular profiling and timely therapeutic decisions are essential in managing such rare and complex cases.
Introduction
The treatment of metastatic colorectal cancer has seen some limited advances with targeted agents (1). Most druggable driver mutations are rare in colorectal cancer, with exception of KRAS mutations, occurring in around 50% of mCRC. However, the benefit of KRAS targeted agents has remained marginal so far (1, 2). Therefore, it is essential to search for and address the occasional druggable driver mutation which may provide a relevant benefit to selected patients. Certain molecular subtypes, including rare anaplastic lymphoma kinase (ALK) gene rearrangements, remain poorly characterized and require individualized treatment approaches. ALK fusions are most frequently associated with non-small cell lung cancer (NSCLC) but have also been identified in various other tumor types, including gastrointestinal cancers (3). The incidence of ALK gene alterations in CRC is estimated at less than 1% (4). They are typically detected only when an extended next-generation-sequencing (NGS) panel is performed, as routine screening for these alterations remains uncommon.
ALK-positive CRC is frequently associated with right-sided tumors, high microsatellite instability (MSI-H), and KRAS/BRAF wild-type status (3, 5). Notably, ALK-positive CRC may exhibit distinct clinical behaviors, including a more aggressive course and variable responses to standard chemotherapy (6). Case reports and small case series suggest that patients with ALK-positive mCRC may benefit significantly from ALK-targeted therapies, with partial responses observed in a few cases (5–9). These findings highlight the importance of comprehensive molecular profiling to guide therapeutic decisions in this rare subset of CRC.
This report presents a unique case of mCRC with ALK expression by immunohistochemistry (IHC), multiple ALK mutations and a novel fusion involving ALK and the centrosomal protein 44 (CEP44), which structural analysis suggests being non-functional. Despite this complex and ultimately difficult to interpret genotype, the patient exhibited a significant response to alectinib, highlighting the complexity of ALK-driven tumor biology and the difficulty of predicting treatment response in CRC. The case underscores the challenges of managing rare ALK-positive CRC, including the rapid development of resistance mutations following targeted therapy, and emphasizes the need for further research on response prediction and therapeutic strategies addressing ALK alterations.
Case presentation
A 60-year-old male patient presented in March 2023 for a screening colonoscopy. During the procedure, an adenocarcinoma of the distal ascending colon was identified. The patient was in good health and asymptomatic at the time. In April 2023, a laparoscopic right hemicolectomy was performed, with an uneventful recovery. Histopathological evaluation confirmed a poorly differentiated adenocarcinoma, classified as pT2 pN1b (stage IIIA) according to UICC 8th Edition, with preserved mismatch repair (pMMR) proteins. Due to the family history of colorectal and other cancers, the patient underwent genetic counseling and germline testing for hereditary cancer syndromes. No pathogenic mutations were detected. The initial carcinoembryonic antigen (CEA) level was 2.5 µg/L. Following surgery, adjuvant chemotherapy with capecitabine and oxaliplatin began in May 2023 and was completed in August 2023.
In September 2023, the patient began experiencing fever and complained of upper right abdominal pain. A computed tomography (CT) confirmed multiple liver metastases, which were subsequently confirmed through a liver biopsy. Molecular testing using the FoundationOne® CDx test (FOUNDATION MEDICINE, INC. CAMBRIDGE, MA, USA) revealed a number of ALK mutations, along with an ALK-CEP44 fusion. Alongside IHC showed ALK expression, correlating with a low-level amplification of the p-arm of chromosome 2, locus of ALK gene (see Figure 1).
Despite the complex genotype which raised the question whether ALK was activated, first-line palliative therapy with FOLFIRI and panitumumab (as bevacizumab was contraindicated after a cholecystectomy which was performed few days ago) was initiated as this is one of the approved therapeutic options in this setting. However, given the circumstances and in anticipation of potential disease progression, a cost coverage request for alectinib was already submitted. The initial therapy led to a partial remission by December 2023, with symptom improvement and decreased tumor burden evidenced by laboratory and imaging findings.
Soon after, in January 2024, however, the patient experienced worsening fatigue, fever, jaundice and right upper quadrant pain. Blood tests indicated a significant increase in liver enzymes and the CEA rose sharply to 57.3 µg/L (see Figure 2). Imaging showed disease progression, which was the reason to switch therapy to the ALK inhibitor alectinib. The patient responded well to alectinib. Within a few days jaundice and fever subsided, abdominal pain disappeared, and CEA dropped to a normal value after one month. ECOG Performance state was 0. In March 2024, a CT scan showed a partial remission according to RECIST v1.1. Alectinib was continued.
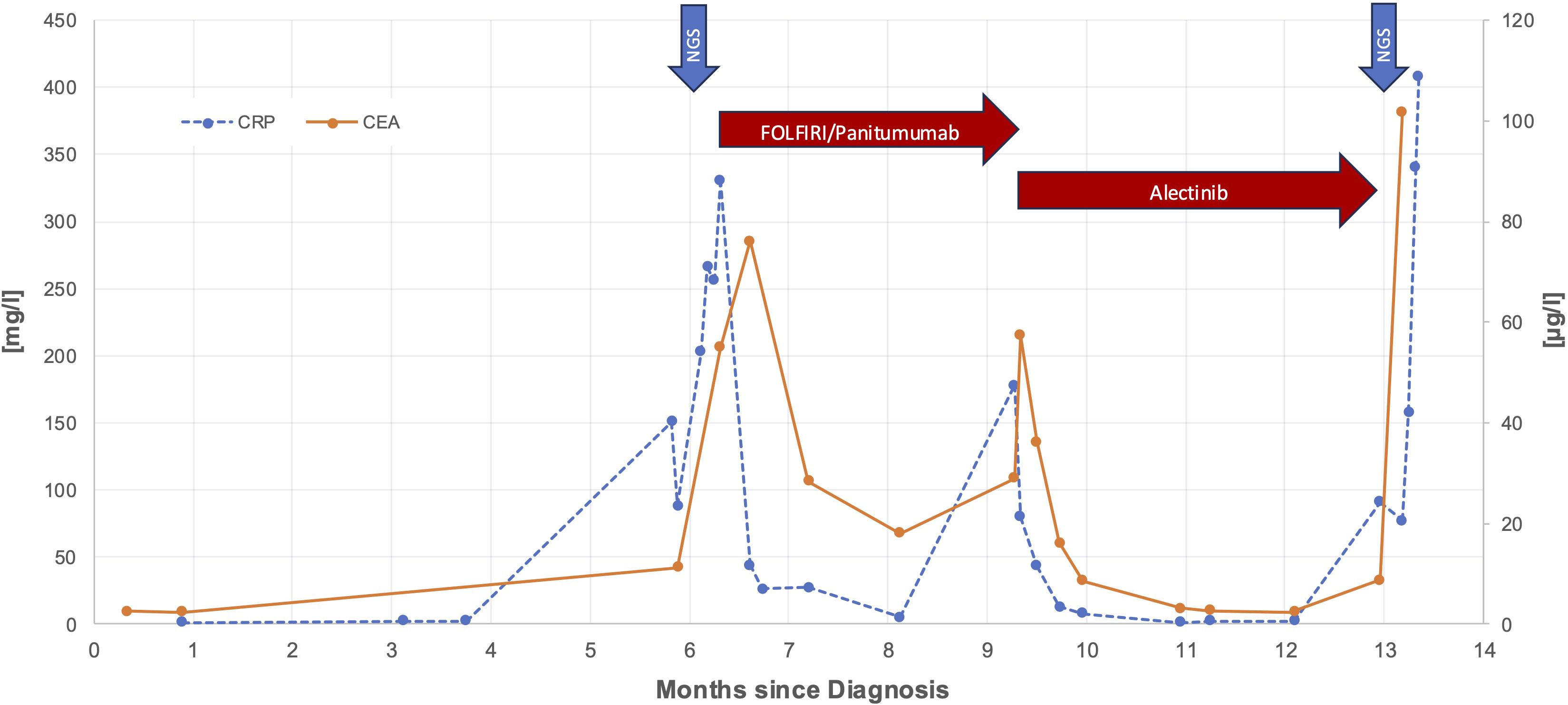
Figure 2. Time course of CEA and CRP levels during treatment period. Red arrows are highlighting responses to FOLFIRI/panitumumab and alectinib and blue arrows mark the time points of the first molecular analysis from liver biopsy and the second molecular analysis from peripheral blood, respectively.
However, only a few weeks later, in April 2024, again the patient’s condition swiftly deteriorated, with fever, severe fatigue, abdominal distention and now ascites. CEA and CRP increased again, and liver enzymes were markedly elevated. A CT scan confirmed extensive progression of liver metastases. A second NGS from liquid biopsy showed several new ALK mutations (see Figure 3). Considering the worsening liver function and aggressive disease spread, an emergency switch to FOLFIRI and bevacizumab was made as a bridging therapy while awaiting initiation of lorlatinib, a third generation ALK-inhibitor.
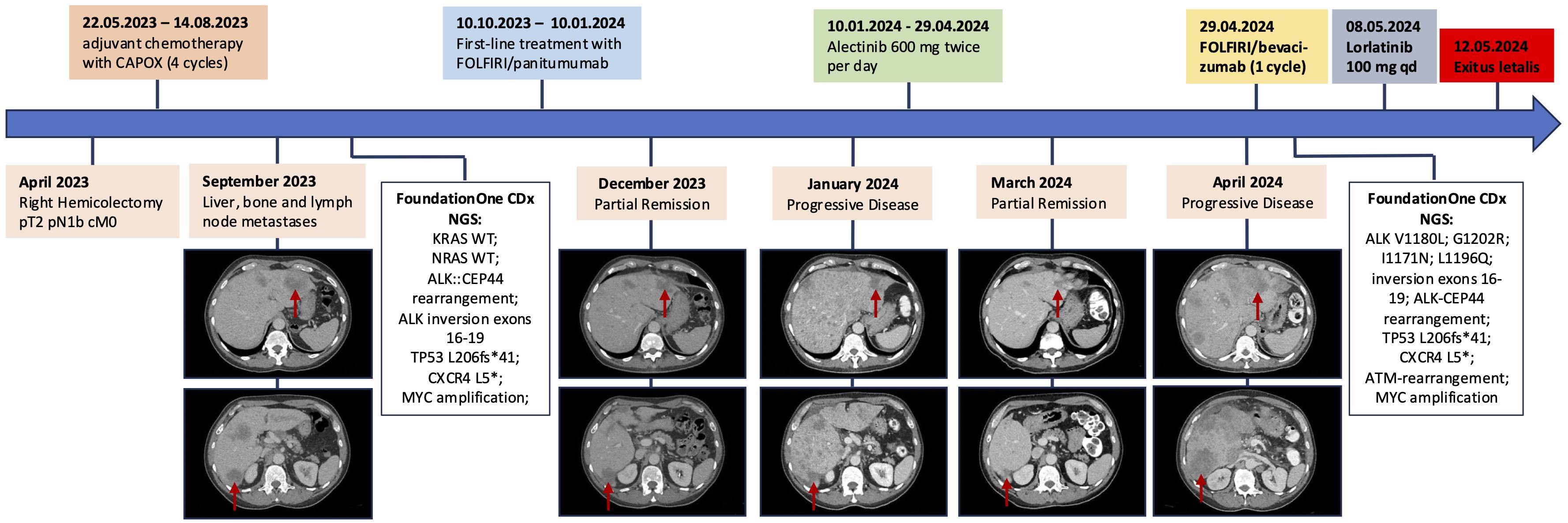
Figure 3. Timeline of the patient’s clinical history, summarizing therapeutic interventions, computed tomography findings with focus on liver metastases, and molecular profiling results at two time points. The red arrows each point to the liver metastases in segment II and segment VI, respectively.
Treatment with lorlatinib was initiated shortly thereafter. Nevertheless, the patient’s liver function continued to decline, and his general condition worsened. Ultimately, the patient died in May 2024.
Discussion
This case highlights a unique clinical scenario involving a mCRC with ALK overexpression by IHC and harboring multiple ALK mutations as well as an ALK-CEP44 fusion. The patient responded quickly and well to the ALK inhibitor alectinib, but a swift emergence of multiple resistance-associated mutations and ultimately clinical resistance were observed. To our knowledge, the ALK-CEP44 fusion has only been described once in a patient with NET and is unprecedented in colorectal cancer (10). ALK fusions in CRC are rare and most commonly involving fusion partners such as EML4, STRN and CAD (6, 7, 11). Typically, oncogenic ALK fusions result in constitutive kinase activation due to the loss of regulatory N-terminal domains, leading to sustained ALK phosphorylation and downstream oncogenic signaling (12). However, in this case, structural analysis suggested that the ALK-CEP44 fusion lacked a fully intact kinase domain, likely preventing constitutive phosphorylation and downstream oncogenic signaling. Despite presumed absence of ALK-driven oncogenic activity, IHC demonstrated strong ALK expression. This correlated with a low-level amplification of the p-arm of chromosome 2, locus of ALK gene. Targeted therapy with alectinib led to a significant clinical, biochemical, and radiological response raising questions about the mechanism of action, potential off-target effects or alternative sensitivity mechanisms. However, the response was transient, underlining the potential limitations of monotherapy in cases with complex or incompletely understood molecular drivers.
The aggressive progression and drug resistance observed during the course of the disease are to some extent consistent with findings from other ALK-positive CRC cases. Studies on ALK-positive CRCs with fusion partners such as STRN and EML4 report similar rapid disease progression and limited efficacy of standard ALK inhibitors, often due to secondary mutations or activation of bypass signaling pathways (4, 7, 13). However, in this case, the aggressive progression and relatively short duration of response to alectinib may be attributed, at least in part, to the likely modest ALK-oncogenic activity of ALK expression.
CEP44 plays a critical role in centrosome cohesion, which is essential for maintaining chromosomal stability during cell division. By stabilizing rootletin, a key component of the centrosome linker, CEP44 ensures the structural integrity necessary for centriole-to-centrosome conversion (14, 15). Therefore, disruptions in CEP44 can lead to chromosomal instability, which may have further contributed to the aggressive progression observed in this case (15, 16).
After alectinib therapy four concurrent mutations in the ALK gene - V1180L, I1171N, G1202R and L1196Q - were identified. All of them have been associated with resistance to alectinib and other second-line ALK-inhibitors (17–19). The emergence of four distinct ALK resistance mutations following a short course of alectinib therapy is striking. In other studies, the presence of multiple ALK resistance mutations has typically been associated with prior exposure to two or more ALK inhibitors, suggesting a cumulative selective pressure over time (13). Additionally, it has been proposed that highly potent ALK inhibitors, such as lorlatinib, may selectively drive the development of multiple concurrent resistance mutations (20). In this case, the rapid acquisition of such mutations under the selective pressure of alectinib alone points to a high degree of tumor adaptability, likely contributing to the aggressive disease progression observed.
Among the identified mutations, L1196Q stands out due to its rarity and limited characterization in the literature. Unlike other ALK resistance mutations, L1196Q has been reported in only a few studies (18, 21). It has been shown to reduce the efficacy of lorlatinib by altering binding interactions, though its impact may be less pronounced compared to the structurally similar L1196M mutation, which is a known driver of lorlatinib resistance, especially in compound with G1202R (21, 22). Despite uncertainties about its efficacy in the context of the given mutations, lorlatinib was selected as the next therapeutic line. Unfortunately, the rapid progression of the disease precluded a meaningful assessment of therapeutic response. This highlights the limited targeted options after resistance and the urgent need for earlier access to next-generation inhibitors in this setting.
Conclusion
This case underscores the importance of early testing for actionable driver mutations and timely planning of targeted therapies in aggressive ALK-positive colon cancers. Early access to such treatments could reduce delays and potentially prevent rapid progression, as observed here. Evidence from ALK-positive CRC cases suggests that initiating targeted therapy earlier, rather than adhering to standard second-line regimens, may benefit patients with high-risk ALK fusions and merits further investigation in clinical studies (7, 9). However, this case foremost highlights the challenges of predicting therapy response from molecular testing results. While some genetic aberrations confer a clear sensitivity to targeted therapy, most others do not. In particular, the interplay of complex genetic aberrations and the predictive potential of novel testing modalities, for example spatial transcriptomics and spatial proteomics, remain to be explored.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Genetic testing was conducted via a commercial platform (FoundationOne® CDx), and raw data are not publicly available. Further inquiries can be directed to the corresponding author.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
SH: Visualization, Conceptualization, Investigation, Writing – review & editing, Writing – original draft. CE: Investigation, Writing – review & editing, Resources, Supervision. SP: Visualization, Writing – review & editing, Validation. MZ: Methodology, Writing – review & editing, Investigation. AW: Writing – review & editing. TM: Writing – review & editing, Supervision.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2025.1613235/full#supplementary-material
References
1. Manzi J, Hoff CO, Ferreira R, Pimentel A, Datta J, Livingstone AS, et al. Targeted therapies in colorectal cancer: recent advances in biomarkers, landmark trials, and future perspectives. Cancers. (2023) 15:3023. doi: 10.3390/cancers15113023
2. Meng M, Zhong K, Jiang T, Liu Z, Kwan HY, Su T, et al. The current understanding on the impact of KRAS on colorectal cancer. BioMed Pharmacother. (2021) 140:111717. doi: 10.1016/j.biopha.2021.111717
3. Pagani F, Randon G, Guarini V, Raimondi A, Prisciandaro M, Lobefaro R, et al. The landscape of actionable gene fusions in colorectal cancer. Int J Mol Sci. (2019) 20:5319. doi: 10.3390/ijms20215319
4. Lai AZ, Schrock AB, Erlich RL, Ross JS, Miller VA, Yakirevich E, et al. Detection of an ALK fusion in colorectal carcinoma by hybrid capture-based assay of circulating tumor DNA. Oncol. (2017) 22:774–9. doi: 10.1634/theoncologist.2016-0376
5. Li ZJ, Pat Fong W, Zhang DS, Luo HY, Chen DL, Cai YY, et al. Exploring ALK fusion in colorectal cancer: a case series and comprehensive analysis. NPJ Precis Oncol. (2024) 8:100. doi: 10.1038/s41698-024-00598-7
6. Yakirevich E, Resnick MB, Mangray S, Wheeler M, Jackson CL, Lombardo KA, et al. Oncogenic ALK fusion in rare and aggressive subtype of colorectal adenocarcinoma as a potential therapeutic target. Clin Cancer Res. (2016) 22:3831–40. doi: 10.1158/1078-0432.CCR-15-3000
7. Ambrosini M, Del Re M, Manca P, Hendifar A, Drilon A, Harada G, et al. ALK inhibitors in patients with ALK fusion–positive GI cancers: an international data set and a molecular case series. JCO Precis Oncol. (2022) 6):e2200015. doi: 10.1200/PO.22.00015
8. Hsiao SY, He HL, Weng TS, Lin CY, Chao CM, Huang WT, et al. Colorectal cancer with EML4-ALK fusion gene response to alectinib: A case report and review of the literature. Case Rep Oncol. (2021) 14:232–8. doi: 10.1159/000511069
9. Hu T, Zhan J, Li L, He Y, Lin Y, Wang J, et al. Anaplastic lymphoma kinase (ALK) inhibitors show activity in colorectal cancer with ALK rearrangements: case series and literature review. Oncol. (2024) 30(1):oyae020. doi: 10.1093/oncolo/oyae020
10. Chen H, Wu Y, Wu X, Wang K, Xia Q, Wang Q, et al. Dramatic response to ensartinib in metastatic neuroendocrine tumors with a novel CEP44-ALK fusion: A case report and literature review. Clin Respir J. (2024) 18:e70040. doi: 10.1111/crj.70040
11. Medico E, Russo M, Picco G, Cancelliere C, Valtorta E, Corti G, et al. The molecular landscape of colorectal cancer cell lines unveils clinically actionable kinase targets. Nat Commun. (2015) 6:7002. doi: 10.1038/ncomms8002
12. Hallberg B and Palmer RH. Mechanistic insight into ALK receptor tyrosine kinase in human cancer biology. Nat Rev Cancer. (2013) 13:685–700. doi: 10.1038/nrc3580
13. Gainor JF, Dardaei L, Yoda S, Friboulet L, Leshchiner I, Katayama R, et al. Molecular mechanisms of resistance to first- and second-generation ALK inhibitors in ALK -rearranged lung cancer. Cancer Discov. (2016) 6:1118–33. doi: 10.1158/2159-8290.CD-16-0596
14. Hossain D, Shih SYP, Xiao X, White J, and Tsang WY. Cep44 functions in centrosome cohesion by stabilizing rootletin. J Cell Sci. (2020) 133:jcs239616. doi: 10.1242/jcs.239616
15. Atorino ES, Hata S, Funaya C, Neuner A, and Schiebel E. CEP44 ensures the formation of bona fide centriole wall, a requirement for the centriole-to-centrosome conversion. Nat Commun. (2020) 11:903. doi: 10.1038/s41467-020-14767-2
16. Hanahan D and Weinberg RA. Hallmarks of cancer: the next generation. Cell. (2011) 144:646–74. doi: 10.1016/j.cell.2011.02.013
17. Lin JJ, Riely GJ, and Shaw AT. Targeting ALK: precision medicine takes on drug resistance. Cancer Discov. (2017) 7:137–55. doi: 10.1158/2159-8290.CD-16-1123
18. Latif H and Liu SV. Novel ALK mutation with durable response to brigatinib—a case report. Transl Lung Cancer Res. (2020) 9:2145–8. doi: 10.21037/tlcr-20-145
19. Pan Y, Deng C, Qiu Z, Cao C, and Wu F. The resistance mechanisms and treatment strategies for ALK-rearranged non-small cell lung cancer. Front Oncol. (2021) 11:713530. doi: 10.3389/fonc.2021.713530
20. Dagogo-Jack I, Rooney M, Lin JJ, Nagy RJ, Yeap BY, Hubbeling H, et al. Treatment with next-generation ALK inhibitors fuels plasma ALK mutation diversity. Clin Cancer Res. (2019) 25:6662–70. doi: 10.1158/1078-0432.CCR-19-1436
21. Furuta H, Araki M, Masago K, Sagae Y, Fujita S, Seto K, et al. Novel resistance mechanisms including L1196Q, P1094H, and R1248_D1249 insertion in three patients with NSCLC after ALK tyrosine kinase inhibitor treatment. J Thorac Oncol. (2021) 16:477–82. doi: 10.1016/j.jtho.2020.09.023
Keywords: colorectal cancer, ALK gene mutation, targeted therapy, drug resistance, case report
Citation: Hofmann S, Egger C, Potthast S, Zoche M, Wicki A and Mehra T (2025) Case Report: Metastatic colorectal cancer with ALK–CEP44 fusion and rapid resistance development. Front. Oncol. 15:1613235. doi: 10.3389/fonc.2025.1613235
Received: 16 April 2025; Accepted: 29 May 2025;
Published: 18 June 2025.
Edited by:
Tamer Saad Kaoud, The University of Texas at Austin, United StatesReviewed by:
Eswari Dodagatta-Marri, University of California, San Francisco, United StatesChien-Ming Chao, Chi Mei Medical Center, Taiwan
Copyright © 2025 Hofmann, Egger, Potthast, Zoche, Wicki and Mehra. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Silvan Hofmann, c2V2aS5ob2ZtYW5uQGhvdG1haWwuY29t
 Silvan Hofmann
Silvan Hofmann Claudine Egger1
Claudine Egger1 Martin Zoche
Martin Zoche Andreas Wicki
Andreas Wicki Tarun Mehra
Tarun Mehra