- 1Department of Gastroenterology, Hepatology and Infectious Diseases, University Hospital Düsseldorf, Medical Faculty of Heinrich Heine University Düsseldorf, Düsseldorf, Germany
- 2Epidemiology, IQVIA, Frankfurt, Germany
Background: Pancreatic cancer is a highly lethal cancer with increasing incidence and poor prognosis due to late diagnosis. While several risk factors are known, evidence on a potential role of autoimmune diseases remains limited. Given the increasing prevalence of autoimmune diseases and their association with various malignancies, this study aims to investigate their potential association with pancreatic cancer.
Methods: 5,440 patients with a first diagnosis of pancreatic cancer and 27,200 propensity score matched individuals without cancer were identified from the Disease Analyzer database (IQVIA). The outcome of the study was the association between the diagnosis of pancreatic cancer and a patient´s history of autoimmune disease.
Results: Inflammatory bowel disease (OR: 1.69; 95% CI: 1.34-2.12) and rheumatoid arthritis (OR: 1.20; 95% CI: 1.03-1.41) were significantly associated with increased odds of pancreatic cancer. The OR was 1.25 for systemic lupus erythematosus and 1.26 for multiple sclerosis without reaching a statistical significance. In sex-stratified analyses, inflammatory bowel disease was strongly associated with pancreatic cancer in women (OR: 2.14; 95% CI: 1.59-2.89) but not in men (OR: 1.24; 95% CI: 0.86-1.78). A positive association between rheumatoid arthritis and pancreatic cancer was also observed in women (OR: 1.26; 95% CI: 1.03-1.53) but not in men (OR: 1.09; 95% CI: 1.03-1.53-1.44). In addition, the ORs for SLE (1.82) and MS (1.45) were increased in women to a clinically relevant extent that did not reach the significance level of <0.05. A similar increase was not observed in male patients.
Conclusion: Autoimmune disease may be associated with an increased risk of developing pancreatic cancer, particularly in women. This highlights the importance of addressing gender differences in medical practice, particularly in relation to disease screening and surveillance.
Introduction
Pancreatic cancer is one of the deadliest cancers and a persistent global health challenge. It is the seventh leading cause of cancer-related death worldwide, with an estimated 495,773 new cases and 466,003 deaths recorded in 2020 alone (1, 2). The incidence of pancreatic cancer has been steadily increasing over the past few decades, reflecting changes in lifestyle and dietary habits (3). The prognosis for pancreatic cancer remains poor, with an overall 5-year survival rate of approximately 12% (4). Treatment strategies typically include surgery for localized pancreatic cancer, often in combination with neoadjuvant or adjuvant chemotherapy and, in some cases, radiotherapy (5–7). For metastatic pancreatic cancer, the focus shifts to systemic treatments, including chemotherapy, targeted therapies, and immunotherapy (8–11). One of the main reasons for the poor prognosis is the late diagnosis. It is therefore particularly important to identify risk factors so that high-risk patients can be screened more effectively. Established risk factors for the development of pancreatic cancer include smoking (12), both active and passive, and obesity (13). In addition, metabolic disorders such as type 2 diabetes mellitus (14) and chronic inflammation of the pancreas increase the risk (15). Excessive alcohol consumption also contributes to an increased risk (16). However, there is still a lack of clearly defined risk factors for which the implementation of targeted screening has a proven benefit.
Similar to the rising incidence of pancreatic cancer, the prevalence of autoimmune diseases has also increased (17). Autoimmune diseases, such as chronic inflammatory bowel disease, increase the risk not only of colorectal cancer but also of extraintestinal malignancies, including cholangiocarcinoma, skin cancer, hematological malignancies, genitourinary cancers, cervical cancer, and prostate cancer (18). The presence of rheumatoid arthritis may also increase the risk of cancer. A study of approximately 1.3 million women demonstrated that rheumatoid arthritis was significantly associated with an increased risk of lung, cervical, and oropharyngeal cancers, as well as hematological malignancies (19). The presence of systemic lupus erythematosus or multiple sclerosis is also associated with an increased risk of both hematologic malignancies and solid tumors, such as lung cancer (20, 21).
An link between these autoimmune diseases and the incidence of pancreatic cancer remains unclear, and the aim of this study is to investigate this relationship.
Materials and methods
Database
This study used data from the Disease Analyzer database (IQVIA). Details of this database have been published previously (22). In brief, the Disease Analyzer database contains data on demographic variables, diagnoses and prescriptions of outpatients from general practices in Germany. The database covers approximately 1300 general practices in Germany. The panel of practices included in the Disease Analyzer database has previously been shown to be representative of office-based practices in Germany (22). The database has been widely used for epidemiological studies in recent years including cancer studies (23, 24).
Study population
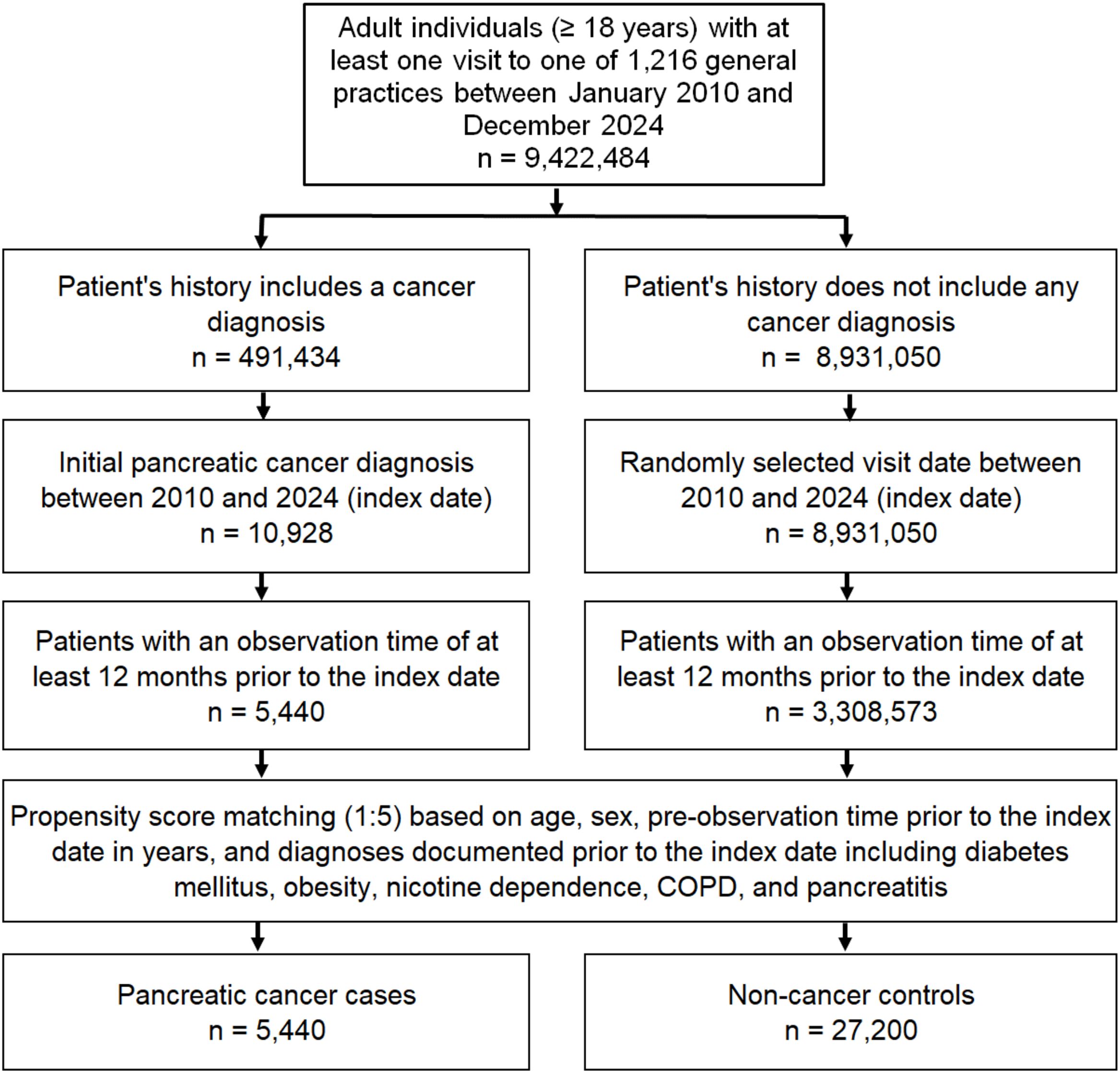
The study population included all patients aged ≥18 years with a first diagnosis of pancreatic cancer (ICD-10 codes: C25) between January 2010 and December 2024 (index date) who had at least one year of follow-up before the index date. Controls were individuals without a history of cancer who were matched (5:1) by nearest neighbor propensity scores based on age, sex, pre-observation time prior to the index date in years, and documented diagnoses prior to the index date, including diabetes mellitus (ICD-10: E10-E14), obesity (ICD-10: E66), nicotine dependence (ICD-10: F17), chronic obstructive pulmonary disease (COPD) (ICD-10: J44), and history of pancreatitis (ICD-10: K85, K86). Diabetes, obesity, pancreatitis, and tobacco use are considered risk factors for pancreatic cancer (25). As information on tobacco use is not available, we used diagnoses of nicotine dependence and COPD which may proxy for smoking behavior. For individuals without cancer (controls), the index date was a randomly selected visit date between January 2010 and December 2024. The flow diagram of study participants is shown in Figure 1. For propensity score matching, a standardized mean difference (SMD) of less than 0.1 was allowed, indicating that adequate covariate balance has been achieved.
Study outcome
The outcome of the study was the association between autoimmune diseases documented in the complete patient history before the index date and the diagnosis of pancreatic cancer. The diagnoses of interest were inflammatory bowel disease (IBD) (ICD-10: K50, K51), rheumatoid arthritis (ICD-10: M05, M06), psoriasis (ICD-10: L40), systemic lupus erythematosus (SLE) (ICD-10: M32), autoimmune thyroiditis (ICD-10: E06.3), and multiple sclerosis (ICD-10: G35).
Statistical analyses
To examine whether the diagnosis of pancreatic cancer was associated with the patient history of autoimmune disease, we used multivariable conditional logistic regression models and estimated adjusted odds ratios (AORs) with 95% confidence intervals (95%CI). ORs were adjusted for age, sex, and documented diagnoses prior to the index date which were included in the PS matching. This model was also calculated separately for female and male patients. A p-value <0.05 was considered statistically significant. All analyses were performed using SAS version 9.4 (SAS Institute, Cary, US).
Results
Baseline characteristics
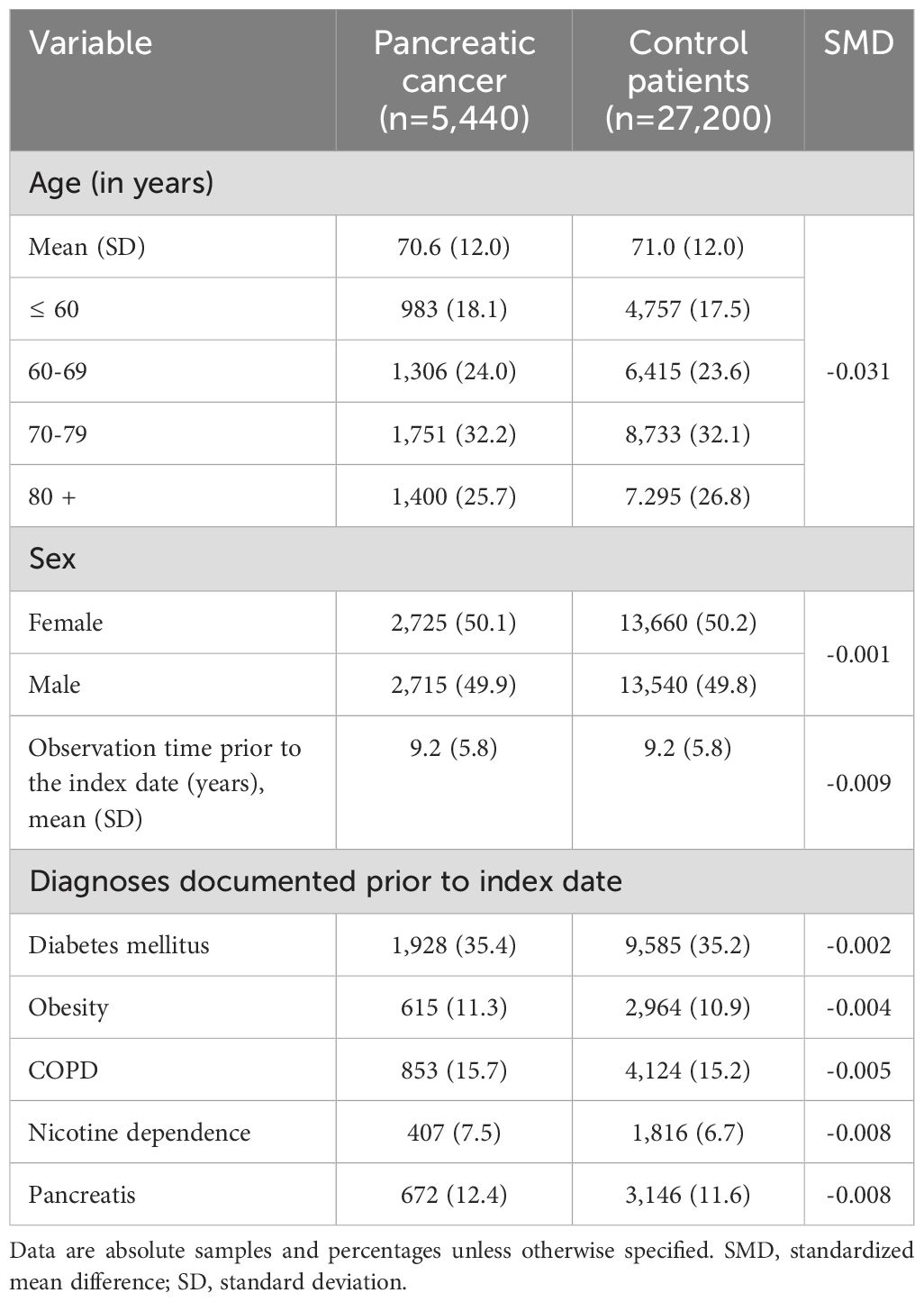
After 1:5 matching, 5,440 cases (patients with pancreatic cancer) and 27,200 controls (patients without cancer) were available for analyses. The mean age at the index date was 70.6 in patients with pancreatic cancer compared to 71.0 years in the control group. There was no significant differences between the two groups in terms of sex distribution. In both cohorts, 50% of the included patients were female. When examining the predefined co-diagnoses, there was no significant difference in the occurrence of diabetes mellitus between patients with pancreatic cancer and the control group. In contrast, patients with pancreatic cancer showed a slightly higher occurrence of overweight, COPD, nicotine dependence, and pancreatitis. On average, both cases and controls had 9.2 years of pre-observation time prior to index date. The prevalence of the most predefined co-diagnoses is shown in Table 1.
Association between the history of autoimmune diseases and pancreatic cancer diagnosis
Table 2 shows the proportion of case and controls diagnosed with each of autoimmune disease as well as results of logistic regression analysis. In the total population, inflammatory bowel diseases (OR: 1.69; 95% CI: 1.34-2.12) and rheumatoid arthritis (OR: 1.20; 95% CI: 1.03-1.41) were associated with increased odds of developing pancreatic cancer. The OR was 1.25 for Systemic lupus erythematosus (SLE) and 1.26 for multiple sclerosis (MS), however, due to small samples of patients with these diseases, the p-value of <0.05 was not reached. The analysis of psoriasis and autoimmune thyroiditis showed no association with pancreatic cancer (Table 2).
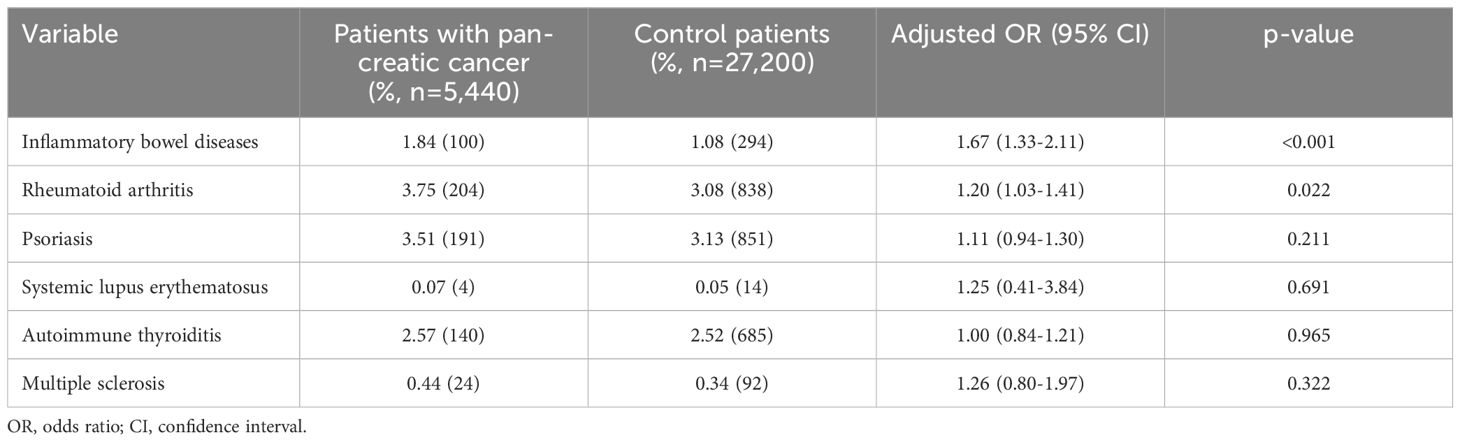
Table 2. Association between autoimmune disorders and pancreatic cancer diagnosis in patients followed in general practices in Germany.
A sex-depended association between autoimmune disease and pancreatic cancer
As autoimmune diseases are significantly more common in women, we subsequently performed a sex-stratified analysis (Table 3). Here, we found that inflammatory bowel disease was strongly associated with pancreatic cancer in women (AOR: 2.14; 95% CI: 1.59-2.89) but not in men (AOR: 1.24; 95% CI: 0.86-1.78). A positive association between rheumatoid arthritis and pancreatic cancer was also observed in women (AOR: 1.26; 95% CI: 1.03-1.53) but not in men (AOR: 1.09; 95% CI: 1.03-1.53-1.44). In addition, the ORs for SLE (1.82) and MS (1.45) were increased in women to a clinically relevant extent that did not reach the significance level of <0.05. A similar increase was not observed in male patients. The analysis of psoriasis and autoimmune thyroiditis revealed no significant differences between male and female patients.
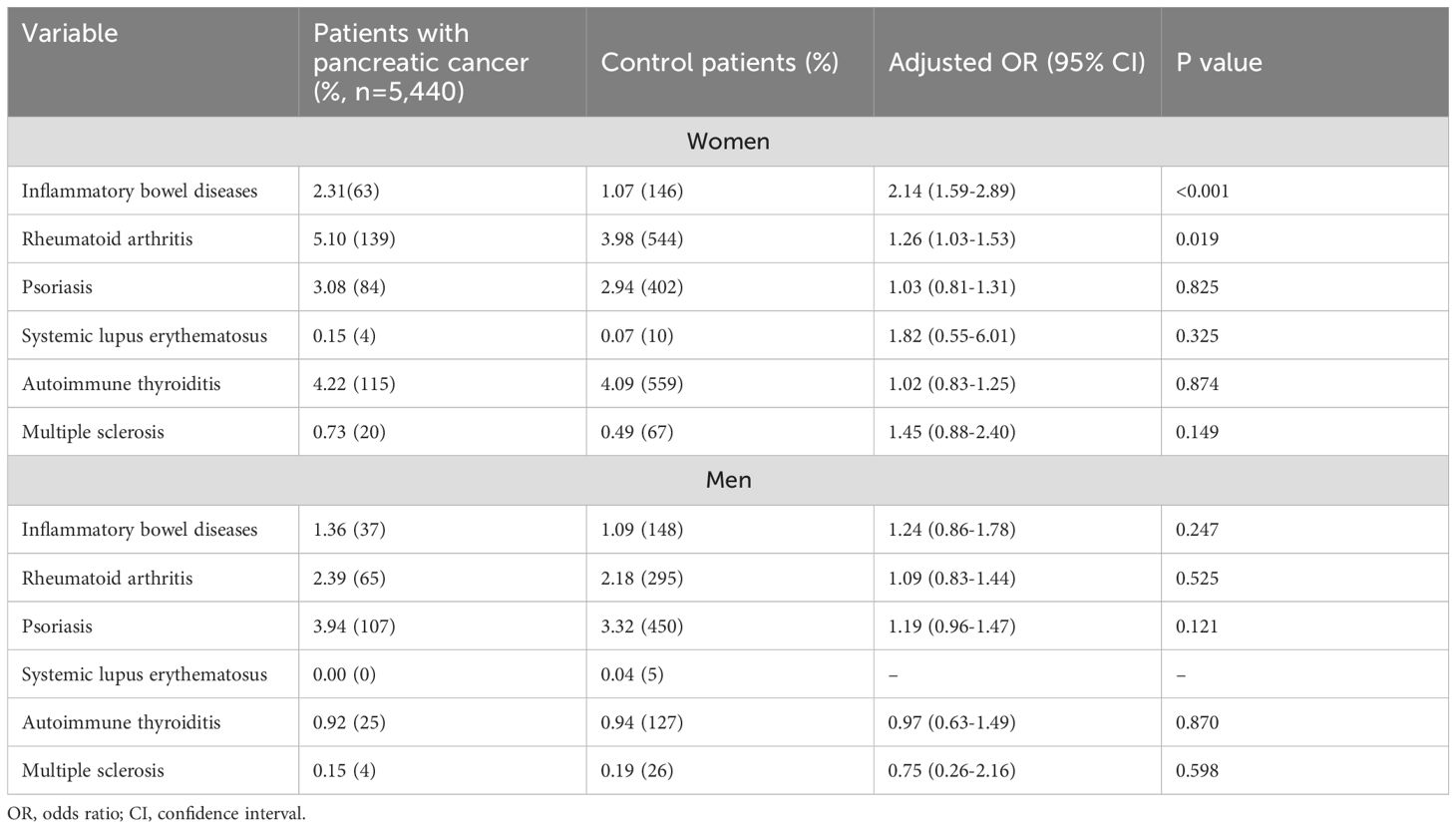
Table 3. Association between sex, autoimmune disorders, and pancreatic cancer diagnosis among patients followed in general practices in Germany.
Discussion
In summary, this study shows that the presence of IBD or RA was associated with increased odds of pancreatic cancer. Further analysis showed that this association was mainly observed in women and not in men.
Systemic inflammation plays a crucial role in the development and progression of pancreatic cancer. Chronic inflammatory states lead to a tumor-promoting microenvironment through the release of pro-inflammatory cytokines, chemokines, and growth factors. These mediators can induce DNA damage, promote malignant transformation, and facilitate tumor growth and immune evasion (26). Inflammatory pathways, such as NF-κB and STAT3, are often activated in pancreatic tissue under chronic inflammatory conditions, further promoting carcinogenesis. Persistent inflammation may also contribute to desmoplasia and fibrosis, both hallmarks of pancreatic ductal adenocarcinoma, creating a microenvironment conducive to tumor progression (27, 28). Unfortunately, the database does not contain markers of systemic inflammation, such as CRP or leukocyte counts. This is an important limitation of the present study.
In patients with inflammatory bowel disease systemic inflammation results primarily from chronic inflammation of the intestinal mucosa. In diseases such as Crohn’s disease and ulcerative colitis, the intestinal barrier is compromised, allowing microbial products such as lipopolysaccharides (LPS) to enter the bloodstream (29). This triggers an immune response beyond the gut, resulting in systemic inflammation. Key pro-inflammatory mediators involved include TNF-α, IL-6, IL-1β, and IFN-γ (30). These cytokines not only maintain local intestinal inflammation but also circulate systemically, activating immune cells and promoting a pro-tumorigenic environment in distant organs, including the pancreas. Chronic immune activation and cytokine signaling can lead to DNA damage, cellular stress, and changes in the tissue microenvironment that may contribute to carcinogenesis (31).
In rheumatoid arthritis, systemic inflammation results from an ongoing autoimmune response that primarily targets the synovial joints. This chronic immune activation leads to widespread inflammatory effects beyond the joints, driven by the sustained activation of immune cells such as macrophages, T cells, and B cells (32). As a result, pro-inflammatory cytokines, particularly TNF-α, IL-6, IL-1β, IL-23 and IL-17, are released into the systemic circulation (33). These mediators contribute to a chronic inflammatory state that can affect distant tissues and organs, promoting oxidative stress, endothelial dysfunction, and immune dysregulation, all of which can facilitate carcinogenesis in organs such as the pancreas. Another possible cause for the increased odd of pancreatic cancer could be the patients’ therapy. Most patients with RA receive disease-modifying antirheumatic drugs (DMARDs). It has already been shown that this immunosuppression can increase the risk of developing cancer (34); however, no study exists specifically addressing the development of pancreatic cancer. Unfortunately, our database does not contain information on whether and which patients received DMARDs. Therefore, follow-up studies are necessary to clarify this issue.
Systemic lupus erythematosus increases the risk of developing certain cancers, particularly non-Hodgkin lymphoma, due to chronic activation and dysregulation of the immune system (35). At the core of SLE is a breakdown in immune tolerance, driven primarily by overactive B cells. These cells, which normally produce antibodies to fight infection, become autoreactive in SLE, producing autoantibodies that mistakenly target the body’s own tissues (36). These cells show increased activation, resist normal cell death, and receive excessive survival signals such as BAFF. This persistent activation and expansion of B cells leads to systemic inflammation, persistent immune stimulation, and an increased risk of malignant transformation, particularly in lymphoid tissues (37). The role of B cells in the development of pancreatic cancer is not yet fully understood. However, there is evidence that B lymphocytes can secrete neoplastic growth factors and immunosuppressive cytokines, thereby promoting immune evasion and cancer progression (38). Furthermore, increased infiltration of B cells into the tumor has been associated with poorer patient prognosis (39). This supports the hypothesis that patients with SLE, which is characterized by overactive B cells, may also have an increased risk of developing pancreatic cancer. In our study, the number of patients with SLE was too small to detect a significant difference. Further studies are needed to clarify this possible association.
Women are disproportionately affected by autoimmune diseases such as inflammatory bowel disease and rheumatoid arthritis, a disparity that has been attributed to complex interactions between hormonal, genetic, and immunological factors. Estrogen and other sex hormones modulate immune responses by influencing cytokine production, T cell differentiation, and B cell activity (40). Studies have shown that estrogen enhances Th2-mediated immune responses and promotes the survival of autoreactive B cells, thereby increasing susceptibility to autoimmunity (41). In addition, many immune-related genes are located on the X chromosome, and women with two X chromosomes, are subject to incomplete X-chromosome inactivation, which may lead to overexpression of these genes (42, 43). Taken together, these findings suggest that the increased immune responsiveness in women, while beneficial in some contexts, predisposes them to a higher risk of developing autoimmune disease (43).
Our study results are consistent with the biological rationale that the presence of an autoimmune disease leads to increased systemic inflammation, thereby contributing to higher odds of developing pancreatic cancer. Interestingly, the overall incidence of pancreatic cancer is slightly higher in men than in women (44). This may be partly explained by the higher prevalence of risk factors such as smoking and alcohol consumption among men. However, autoimmune diseases also occur in men, although less frequently than in women. Interestingly, our sex-specific analysis showed that in men with a history of autoimmune disease, the odds of developing pancreatic cancer was not increased. The reason for this finding is unclear; it is possible that women exhibit a higher degree of systemic inflammation. This remains speculative and further underscores the need for sex-specific research.
Currently, there is no effective or widely recommended screening method for pancreatic cancer in the general population, primarily due to the disease’s typically late onset and lack of reliable early detection tools (7). However, screening may be considered for individuals at increased risk, including those with a strong family history of pancreatic cancer, known genetic syndromes (such as BRCA mutations or Lynch syndrome), or familial pancreatic cancer. Chronic pancreatitis is also recognized as a risk factor for pancreatic cancer, and patients with long-standing or hereditary pancreatitis may be considered for targeted surveillance. In high-risk individuals, imaging techniques such as endoscopic ultrasound (EUS) or magnetic resonance imaging (MRI) may be used, though their effectiveness in reducing mortality remains under investigation (45). However, further research is needed to determine whether women with a history of autoimmune disease should be screened for pancreatic cancer.
Our study has several limitations that are inherent to the database analysis and study design. First, we cannot exclude the possibility of misclassification of diagnoses or the absence of certain codes within the ICD‐10 coding system. Second, the database does not specify whether the pancreatic carcinoma patients have pancreatic ductal adenocarcinoma (PDAC) or neuroendocrine tumor (NET). This distinction would be important to know, as these entities differ significantly in their biological characteristics. In addition, the German Disease Analyzer Database does not include information on patients´ lifestyle, socioeconomic status or survival. It would be very interesting to know whether pancreatic cancer patients with a history of autoimmune disease have a different outcome compared to pancreatic cancer patients without a history of autoimmune disease. Furthermore, the case numbers for SLE and MS were too small to draw statistically significant results. In addition, not all major autoimmune diseases could be analyzed, because, for example, atopic dermatitis is rarely documented by general practitioners. As a result, it is difficult to make broad statements about autoimmune diseases in general.
However, the database provides a valuable perspective on general practitioner consultations in Germany, and the identification of inflammatory bowel disease and rheumatoid arthritis as factors associated with a pancreatic cancer in female patients may provide a basis for initiating further research on this topic.
In conclusion, we have shown that autoimmune diseases are associated with pancreatic cancer, particularly in women. This again highlights the need for greater attention to gender differences in medicine, especially in disease screening.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author contributions
SL: Formal Analysis, Visualization, Writing – original draft, Writing – review & editing. FH: Formal Analysis, Visualization, Writing – original draft, Writing – review & editing. TL: Writing – original draft, Writing – review & editing. CR: Conceptualization, Writing – original draft, Writing – review & editing. KK: Formal Analysis, Visualization, Writing – review & editing, Software, Writing – original draft, Methodology.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. There was no specific funding for this study. In general, work in group of TL was funded by the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation program through the ERC Consolidator Grant PhaseControl (Grant Agreement 771083). The laboratory of TL was further funded by the German Cancer Aid (Deutsche Krebshilfe -110043), the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) -403224013, 279874820, 461704932, 440603844, the German Ministry of Health (BMG -DEEP LIVER 2520DAT111) and support from the Medical Faculty of the Heinrich Heine University.
Conflict of interest
Author KK was employed by company IQVIA.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Permuth JB, Powers BD, Hodul PJ, and Florida Pancreas C. A path forward for understanding and addressing multifaceted pancreatic cancer disparities. Gastroenterology. (2022) 163:51–3. doi: 10.1053/j.gastro.2022.04.047
2. Ilic I and Ilic M. International patterns in incidence and mortality trends of pancreatic cancer in the last three decades: A joinpoint regression analysis. World J Gastroenterol. (2022) 28:4698–715. doi: 10.3748/wjg.v28.i32.4698
3. The Lancet Gastroenterology H. Cause for concern: the rising incidence of early-onset pancreatic cancer. Lancet Gastroenterol Hepatol. (2023) 8:287. doi: 10.1016/S2468-1253(23)00039-0
4. Orth M, Metzger P, Gerum S, Mayerle J, Schneider G, Belka C, et al. Pancreatic ductal adenocarcinoma: biological hallmarks, current status, and future perspectives of combined modality treatment approaches. Radiat Oncol. (2019) 14:141. doi: 10.1186/s13014-019-1345-6
5. Springfeld C, Ferrone CR, Katz MHG, Philip PA, Hong TS, Hackert T, et al. Neoadjuvant therapy for pancreatic cancer. Nat Rev Clin Oncol. (2023) 20:318–37. doi: 10.1038/s41571-023-00746-1
6. Neoptolemos JP, Kleeff J, Michl P, Costello E, Greenhalf W, and Palmer DH. Therapeutic developments in pancreatic cancer: current and future perspectives. Nat Rev Gastroenterol Hepatol. (2018) 15:333–48. doi: 10.1038/s41575-018-0005-x
7. Wood LD, Canto MI, Jaffee EM, and Simeone DM. Pancreatic cancer: pathogenesis, screening, diagnosis, and treatment. Gastroenterology. (2022) 163:386–402 e381. doi: 10.1053/j.gastro.2022.03.056
8. Rojas LA and Balachandran VP. Scaling the immune incline in PDAC. Nat Rev Gastroenterol Hepatol. (2021) 18:453–4. doi: 10.1038/s41575-021-00475-9
9. Dickson I. Improved CAR T therapy for PDAC. Nat Rev Gastroenterol Hepatol. (2021) 18:456. doi: 10.1038/s41575-021-00476-8
10. Park W, Chawla A, and O’Reilly EM. Pancreatic cancer: A review. JAMA. (2021) 326:851–62. doi: 10.1001/jama.2021.13027
11. Timmer FEF, Geboers B, Nieuwenhuizen S, Dijkstra M, Schouten EAC, Puijk RS, et al. Pancreatic cancer and immunotherapy: A clinical overview. Cancers (Basel). (2021) 13(16):4138. doi: 10.3390/cancers13164138
12. Gallaway MS, Henley SJ, Steele CB, Momin B, Thomas CC, Jamal A, et al. Surveillance for cancers associated with tobacco use - United States, 2010-2014. MMWR Surveill Summ. (2018) 67:1–42. doi: 10.15585/mmwr.ss6712a1
13. Collaborators GBDO, Afshin A, Forouzanfar MH, Reitsma MB, Sur P, Estep K, et al. Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med. (2017) 377:13–27. doi: 10.1056/NEJMoa1614362
15. Malka D, Hammel P, Maire F, Rufat P, Madeira I, Pessione F, et al. Risk of pancreatic adenocarcinoma in chronic pancreatitis. Gut. (2002) 51:849–52. doi: 10.1136/gut.51.6.849
16. Naudin S, Li K, Jaouen T, Assi N, Kyro C, Tjonneland A, et al. Lifetime and baseline alcohol intakes and risk of pancreatic cancer in the European Prospective Investigation into Cancer and Nutrition study. Int J Cancer. (2018) 143:801–12. doi: 10.1002/ijc.31367
17. Conrad N, Misra S, Verbakel JY, Verbeke G, Molenberghs G, Taylor PN, et al. Incidence, prevalence, and co-occurrence of autoimmune disorders over time and by age, sex, and socioeconomic status: a population-based cohort study of 22 million individuals in the UK. Lancet. (2023) 401:1878–90. doi: 10.1016/S0140-6736(23)00457-9
18. Faye AS, Holmer AK, and Axelrad JE. Cancer in inflammatory bowel disease. Gastroenterol Clin North Am. (2022) 51:649–66. doi: 10.1016/j.gtc.2022.05.003
19. Yang TO, Floud S, Reeves GK, and Million Women Study C. Rheumatoid arthritis and cancer risk in the Million Women Study. Int J Epidemiol. (2024) 53(2):dyae006. doi: 10.1093/ije/dyae006
20. Hardenbergh D, Naik R, Manno R, Azar A, Monroy Trujillo JM, Adler B, et al. The cancer risk profile of systemic lupus erythematosus patients. J Clin Rheumatol. (2022) 28:e257–62. doi: 10.1097/RHU.0000000000001729
21. Liu Z, Fan T, Mo X, Kan J, and Zhang B. Association between multiple sclerosis and cancer risk: A two-sample Mendelian randomization study. PloS One. (2024) 19:e0298271. doi: 10.1371/journal.pone.0298271
22. Rathmann W, Bongaerts B, Carius HJ, Kruppert S, and Kostev K. Basic characteristics and representativeness of the German Disease Analyzer database. Int J Clin Pharmacol Ther. (2018) 56:459–66. doi: 10.5414/CP203320
23. Krieg S, Loosen S, Krieg A, Luedde T, Roderburg C, and Kostev K. Association between iron deficiency anemia and subsequent stomach and colorectal cancer diagnosis in Germany. J Cancer Res Clin Oncol. (2024) 150:53. doi: 10.1007/s00432-023-05534-z
24. Grewe S, Jordens MS, Roderburg C, Leyh C, Labuhn S, Luedde T, et al. Elevated hbA1c levels are associated with a risk of pancreatic cancer: A case-control study. J Clin Med. (2024) 13(18):5584. doi: 10.3390/jcm13185584
25. Zhao Z and Liu W. Pancreatic cancer: A review of risk factors, diagnosis, and treatment. Technol Cancer Res Treat. (2020) 19:1533033820962117. doi: 10.1177/1533033820962117
26. Padoan A, Plebani M, and Basso D. Inflammation and pancreatic cancer: focus on metabolism, cytokines, and immunity. Int J Mol Sci. (2019) 20(3):676. doi: 10.3390/ijms20030676
27. Loncle C, Bonjoch L, Folch-Puy E, Lopez-Millan MB, Lac S, Molejon MI, et al. IL17 functions through the novel REG3beta-JAK2-STAT3 inflammatory pathway to promote the transition from chronic pancreatitis to pancreatic cancer. Cancer Res. (2015) 75:4852–62. doi: 10.1158/0008-5472.CAN-15-0896
28. Cani PD, Osto M, Geurts L, and Everard A. Involvement of gut microbiota in the development of low-grade inflammation and type 2 diabetes associated with obesity. Gut Microbes. (2012) 3:279–88. doi: 10.4161/gmic.19625
29. Stephens M and von der Weid PY. Lipopolysaccharides modulate intestinal epithelial permeability and inflammation in a species-specific manner. Gut Microbes. (2020) 11:421–32. doi: 10.1080/19490976.2019.1629235
30. Yang L, Wu G, Wu Q, Peng L, and Yuan L. METTL3 overexpression aggravates LPS-induced cellular inflammation in mouse intestinal epithelial cells and DSS-induced IBD in mice. Cell Death Discov. (2022) 8:62. doi: 10.1038/s41420-022-00849-1
31. Francescone R, Hou V, and Grivennikov SI. Cytokines, IBD, and colitis-associated cancer. Inflammation Bowel Dis. (2015) 21:409–18. doi: 10.1097/MIB.0000000000000236
32. Weyand CM and Goronzy JJ. The immunology of rheumatoid arthritis. Nat Immunol. (2021) 22:10–8. doi: 10.1038/s41590-020-00816-x
33. Kondo N, Kuroda T, and Kobayashi D. Cytokine networks in the pathogenesis of rheumatoid arthritis. Int J Mol Sci. (2021) 22(20):10922. doi: 10.3390/ijms222010922
34. Sendaydiego X, Gold LS, Dubreuil M, Andrews JS, Reid P, Liew DFL, et al. Use of biologic or targeted synthetic disease-Modifying antirheumatic drugs and cancer risk. JAMA Netw Open. (2024) 7:e2446336. doi: 10.1001/jamanetworkopen.2024.46336
35. Song L, Wang Y, Zhang J, Song N, Xu X, and Lu Y. The risks of cancer development in systemic lupus erythematosus (SLE) patients: a systematic review and meta-analysis. Arthritis Res Ther. (2018) 20:270. doi: 10.1186/s13075-018-1760-3
36. Weinstein A, Alexander RV, and Zack DJ. A review of complement activation in SLE. Curr Rheumatol Rep. (2021) 23:16. doi: 10.1007/s11926-021-00984-1
37. Mockel T, Basta F, Weinmann-Menke J, and Schwarting A. B cell activating factor (BAFF): Structure, functions, autoimmunity and clinical implications in Systemic Lupus Erythematosus (SLE). Autoimmun Rev. (2021) 20:102736. doi: 10.1016/j.autrev.2020.102736
38. Minici C, Testoni S, and Della-Torre E. B-lymphocytes in the pathophysiology of pancreatic adenocarcinoma. Front Immunol. (2022) 13:867902. doi: 10.3389/fimmu.2022.867902
39. Zhu H, Xu J, Wang W, Zhang B, Liu J, Liang C, et al. Intratumoral CD38(+)CD19(+)B cells associate with poor clinical outcomes and immunosuppression in patients with pancreatic ductal adenocarcinoma. EBioMedicine. (2024) 103:105098. doi: 10.1016/j.ebiom.2024.105098
40. Kovats S. Estrogen receptors regulate innate immune cells and signaling pathways. Cell Immunol. (2015) 294:63–9. doi: 10.1016/j.cellimm.2015.01.018
41. Salem ML. Estrogen, a double-edged sword: modulation of TH1- and TH2-mediated inflammations by differential regulation of TH1/TH2 cytokine production. Curr Drug Targets Inflammation Allergy. (2004) 3:97–104. doi: 10.2174/1568010043483944
42. Bottema RW, Kerkhof M, Reijmerink NE, Koppelman GH, Thijs C, Stelma FF, et al. X-chromosome Forkhead Box P3 polymorphisms associate with atopy in girls in three Dutch birth cohorts. Allergy. (2010) 65:865–74. doi: 10.1111/j.1398-9995.2009.02291.x
43. Huret C, Ferraye L, David A, Mohamed M, Valentin N, Charlotte F, et al. Altered X-chromosome inactivation predisposes to autoimmunity. Sci Adv. (2024) 10:eadn6537. doi: 10.1126/sciadv.adn6537
44. Cronin KA, Scott S, Firth AU, Sung H, Henley SJ, Sherman RL, et al. Annual report to the nation on the status of cancer, part 1: National cancer statistics. Cancer. (2022) 128:4251–84. doi: 10.1002/cncr.34479
45. Goggins M, Overbeek KA, Brand R, Syngal S, Del Chiaro M, Bartsch DK, et al. Management of patients with increased risk for familial pancreatic cancer: updated recommendations from the International Cancer of the Pancreas Screening (CAPS) Consortium. Gut. (2020) 69:7–17. doi: 10.1136/gutjnl-2019-319352
Keywords: pancreatic cancer, PDAC, risk factor, autoimmune disease, inflammatory bowel disease, rheumatoid arthritis, systemic lupus erythematosus, multiple sclerosis
Citation: Loosen SH, Hansen FJ, Luedde T, Roderburg C and Kostev K (2025) A sex dependent association between the history of autoimmune disease and the development of pancreatic cancer: a case-control study of 32,640 patients. Front. Oncol. 15:1613787. doi: 10.3389/fonc.2025.1613787
Received: 17 April 2025; Accepted: 23 June 2025;
Published: 08 July 2025.
Edited by:
Julius Hollnberger, Klinik für Gastroenterologie und Hepatologie, GermanyReviewed by:
Saber A. Amin, University of Nebraska Medical Center, United StatesPascal Kohmann, German Cancer Research Center (DKFZ), Germany
Copyright © 2025 Loosen, Hansen, Luedde, Roderburg and Kostev. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sven H. Loosen, U3Zlbi5Mb29zZW5AbWVkLnVuaS1kdWVzc2VsZG9yZi5kZQ==
†These authors share first authorship
‡These authors share senior authorship
 Sven H. Loosen
Sven H. Loosen Frederik J. Hansen
Frederik J. Hansen Tom Luedde1
Tom Luedde1 Karel Kostev
Karel Kostev