- 1Department of Obstetrics and Gynecology in Sir Run Shaw Hospital, School of Medicine, Zhejiang University, Key Laboratory of Reproductive Dysfunction Management of Zhejiang Province, Hangzhou, China
- 2Polytechnic Institute of Zhejiang University, Hangzhou, Zhejiang, China
- 3Center for Clinical Genetics and Genomics, DIAN Diagnostics, Hangzhou, Zhejiang, China
- 4Assisted Reproduction Unit, Department of Obstetrics and Gynecology, Department of Laboratory Medicine, Sir Run Shaw Hospital, Zhejiang University School of Medicine, Key Laboratory of Reproductive Dysfunction Management of Zhejiang Province, Hangzhou, China
- 5DIAN Diagnostics, Hangzhou, Zhejiang, China
- 6Center for Precision Medicine, Zhejiang-California International NanoSystems Institute, Hangzhou, China
- 7Department of Pathology and Laboratory of Medicine, University of Rochester Medical Centre, Rochester, NY, United States
Ovarian cancer (OC) is a highly heterogeneous malignancy influenced by germline genetic factors, with BRCA1/2 mutations being well-established risk factors. Germline double heterozygosity (GDH), particularly involving rare combinations, remains poorly understood. This study presents the first report of BRCA1/BRIP1 GDH in a case of Chinese OC patients and compares their clinical characteristics and treatment responses to a patient with BRCA1/BRCA2 GDH. The BRCA1/BRIP1 GDH patient is a 46-year-old female diagnosed with advanced ovarian adenocarcinoma at clinical stage FIGO IVB, exhibited severe chemotherapy-induced toxicity and postoperative complications, including chylous leakage. In contrast, the BRCA1/BRCA2 GDH patient is a 44-year-old female with high-grade serous ovarian cancer at clinical stage FIGO IIIC, tolerated chemotherapy well. Both patients experienced clinical benefit from Olaparib maintenance therapy. Genetic testing confirmed pathogenic variants in both cases, revealing distinct clinical trajectories influenced by different GDH profiles. Our findings suggest that different GDH combinations may influence chemotherapy tolerance and therapeutic effectiveness in OC. BRCA1/BRIP1 GDH patients may require personalized dose adjustments to mitigate toxicity and optimize efficacy. This study underscores the clinical significance of GDH heterogeneity and the importance of comprehensive genetic testing for guiding individualized treatment strategies. Future research should focus on expanding sample sizes and conducting in-depth functional analyses to further clarify the clinical implications of different GDH types, ultimately refining treatment approaches for GDH-associated OC.
1 Introduction
Ovarian cancer (OC) is a heterogeneous group of malignancies originating in the ovaries, fallopian tubes, or peritoneum. It is the eighth leading cause of cancer-related mortality in women worldwide, with over 310,000 new cases and approximately 200,000 deaths reported in 2020 (1). Germline genetic factors play a significant role in the onset of breast cancer (BC) and OC. Carriers of pathogenic BRCA1 and BRCA2 variants are known to have an elevated risk of developing OC. The lifetime risk of OC in the general population is approximately 1.3% (2). Notably, the Homologous Recombination Repair (HRR) gene family is essential for genomic stability and DNA damage repair. According to the National Comprehensive Cancer Network (NCCN) (https://www.nccn.org/guidelines/category_2), different genetic variants are associated with varying risks of epithelial ovarian cancer (EOC), with BRCA1 variants conferring an absolute risk of 39–58%, BRCA2 variants 13–29%, and BRIP1 variants 5–15%.
BRCA1 and BRCA2 are critical regulators of multiple cellular processes, including transcriptional regulation, cell cycle control, and the DNA damage response. They play a pivotal role in DNA repair via homologous recombination. BRIP1, which interacts with BRCA1, is essential for maintaining genomic integrity and functions as a tumor suppressor (3). Variants in BRCA1 and BRCA2 disrupt the HRR pathway, compromising the ability of cancer cells to repair platinum-induced DNA damage and thereby increasing their sensitivity to platinum-based chemotherapy. Previous studies, based on both preclinical models and clinical data, have demonstrated that carriers of BRCA or BRIP1 gene variants exhibit significantly enhanced sensitivity to platinum-based chemotherapy, with notable improvements in overall survival (OS) (4, 5).
Most individuals carrying cancer susceptibility gene variants, such as BRCA1, BRCA2, and ATM, are heterozygous for a single pathogenic variant. Germline double heterozygosity (GDH) in OC and BC is rare. The advancement of sequencing technologies, particularly Next-Generation Sequencing (NGS), has facilitated the widespread adoption of multi-gene panel testing, leading to an increased number of reported GDH cases in recent years. GDH cases most commonly involve BRCA1 and BRCA2, with BC being the predominant associated malignancy (6–8). In terms of racial distribution, most GDH reports involve individuals of European descent (7, 9–11), while among Asian populations, South Korea has the highest reported incidence (8, 12, 13). GDH cases in China have been reported infrequently (14–16).
In this study, we describe two Chinese OC patients carrying different GDH variants (BRCA1/BRIP1 and BRCA1/BRCA2) and evaluate their chemotherapy tolerance, postoperative recovery, and responses to targeted therapy. The results show that the patient with the BRCA1/BRIP1 variants experienced greater chemotherapy toxicity and developed postoperative chylous leakage. In contrast, the patient with the BRCA1/BRCA2 mutation exhibited better chemotherapy tolerance. Notably, this study represents the first report of a BRCA1/BRIP1 GDH variant in a Chinese OC patient.
2 Manuscript formatting
2.1 The result of patient 1
In December 2021, a 46-year-old woman presented to a local hospital with abdominal distension. She had no significant past medical history. Cytological analysis of ascites confirmed adenocarcinoma, and PET-CT revealed a right adnexal mass with metastases involving the mesentery, left supraclavicular region, mediastinum, bilateral internal mammary regions, multiple subcapsular hepatic lymph nodes, and possible sacral bone involvement. The clinical stage was FIGO IVB. Due to limited medical resources during the COVID-19 pandemic, the patient was not a candidate for primary surgery and instead received individualized palliative chemotherapy at the local hospital (paclitaxel 260 mg, cisplatin 60 mg, and bevacizumab 900 mg) for five cycles. Although no imaging assessment was performed after these cycles. According to the patient’s self-report, there was significant symptomatic improvement, suggesting a partial response. Chemotherapy was discontinued due to severe gastrointestinal side effects, and the patient subsequently switched to oral traditional Chinese medicine.
In March 2023, the patient was referred to our hospital for further management. Repeat PET-CT demonstrated persistent disease with a similar extent of metastasis. She then received three cycles of neoadjuvant chemotherapy (paclitaxel and carboplatin). Follow-up imaging revealed more than a 30% reduction in tumor size, consistent with a partial response according to RECIST criteria. On May 17, 2023, the patient underwent open abdominal cytoreductive surgery for OC, including total hysterectomy, bilateral salpingo-oophorectomy, omentectomy, pelvic lymphadenectomy, presacral lymphadenectomy, and para-aortic lymphadenectomy. Residual lymph nodes (<1 cm) remained in the left supraclavicular fossa, right internal mammary region, and hepatic hilum, achieving R1 resection. Postoperatively, the patient received three additional cycles of paclitaxel, carboplatin, and bevacizumab, with the final cycle administered on August 2, 2023. Multiple dose adjustments were necessary due to significant hematologic toxicities ranging from grade 1 to 4. The postoperative period was complicated by chylous leakage, requiring specialized management. The patient is currently undergoing maintenance therapy with Olaparib. Over 17 months of outpatient follow-up, no new lesions have been detected, and the residual lymph nodes have remained stable. The disease remains stable.
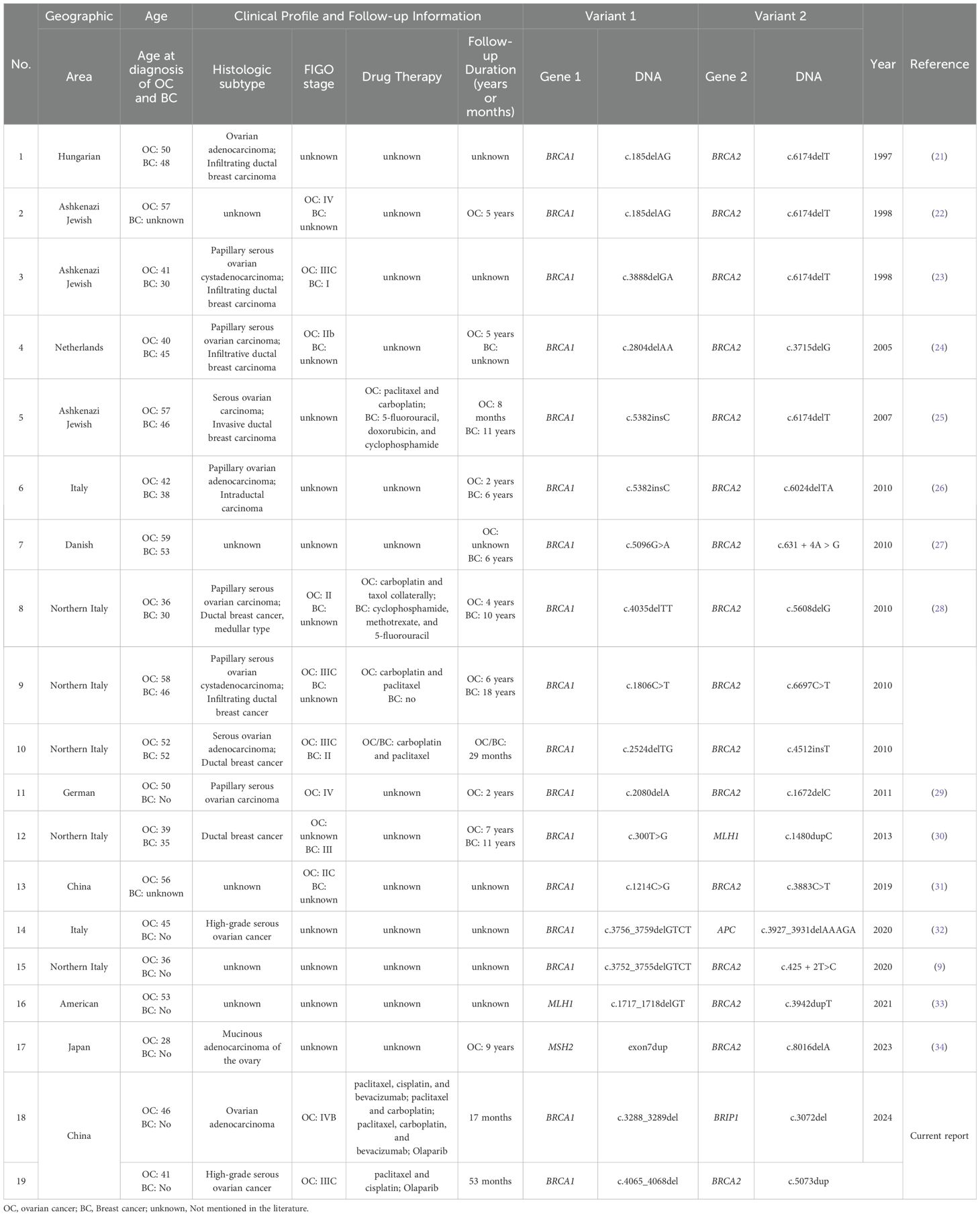
The Whole exome sequencing (WES) testing identified GDH variants in the patient, including (NM_007294.4) c.3288_3289del (p.Leu1098SerfsTer4) in exon 17 of BRCA1 and (NM_032043.3) c.3072del (p.Ser1025HisfsTer34) in exon 17 of BRIP1 (Figures 1A-D). According to the guidelines of the American College of Medical Genetics and Genomics (ACMG) (17), the BRCA1 variant was classified as pathogenic (PVS1+PS4+PM2_P), while the BRIP1 variant was classified as likely pathogenic (PVS1+ PM2_P). To further illustrate the potential structural impact of these variants, three-dimensional protein structure models were generated and are shown in Figures 2A, B.
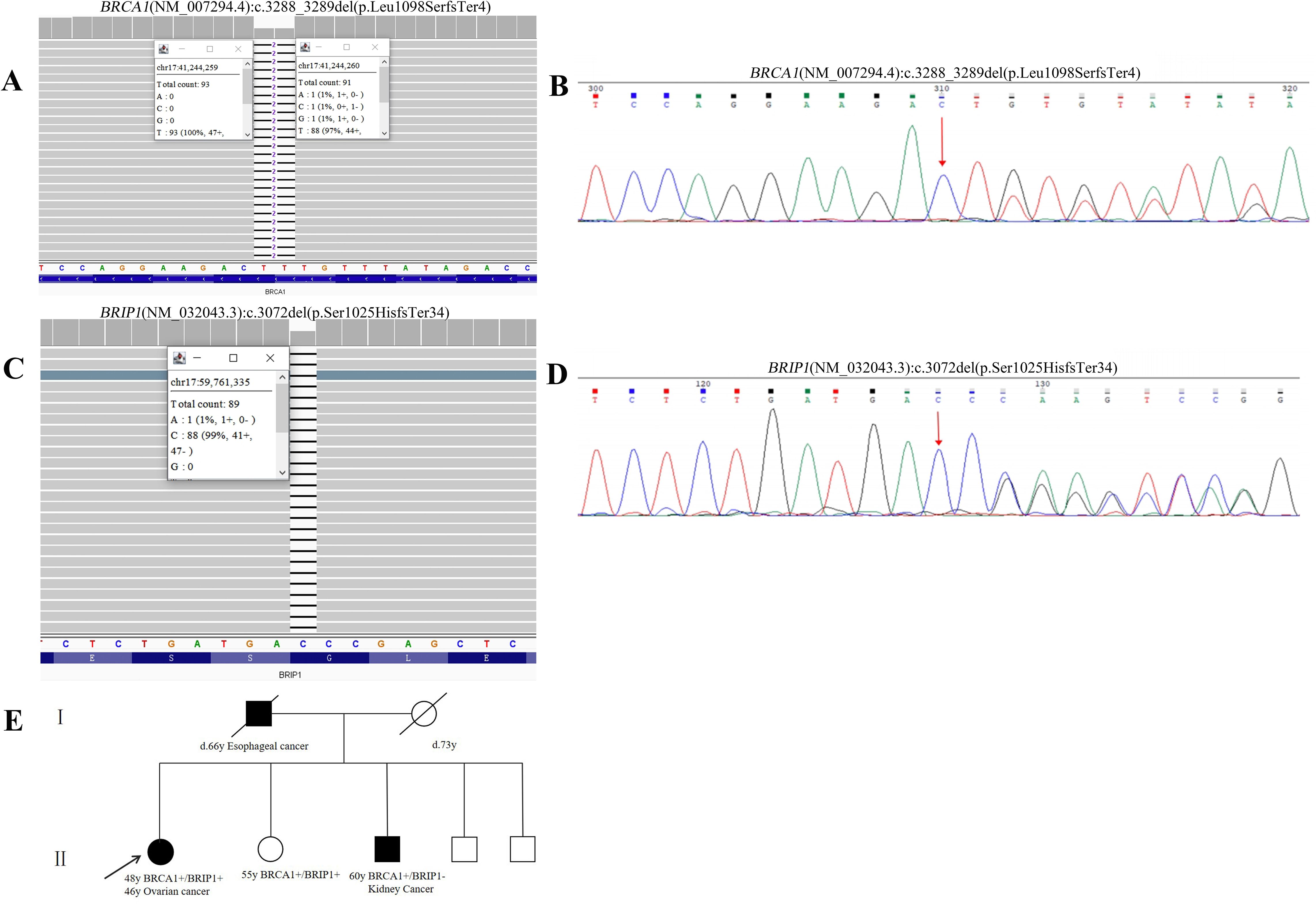
Figure 1. Pedigree and molecular test results of Patient 1. (A) WES data of BRCA1 (NM_007294.4):c.3288_3289del (p.Leu1098SerfsTer4) pathogenic variant. (B) Sanger sequencing result of the BRCA1 pathogenic variant. (C) WES data of BRIP1 (NM_032043.3):c.3072del (p.Ser1025HisfsTer34) likely pathogenic variant. (D) Sanger sequencing result of the BRIP1 likely pathogenic variant. Red arrows indicate the identified variants. (E) Pedigree of individual 1. Black indicates individuals with cancer. Cancer type and age at diagnosis are reported. The age for genetic testing and age of death are also reported. Family members carrying the pathogenic/likely pathogenic variant are marked with BRCA1+, BRIP1+.
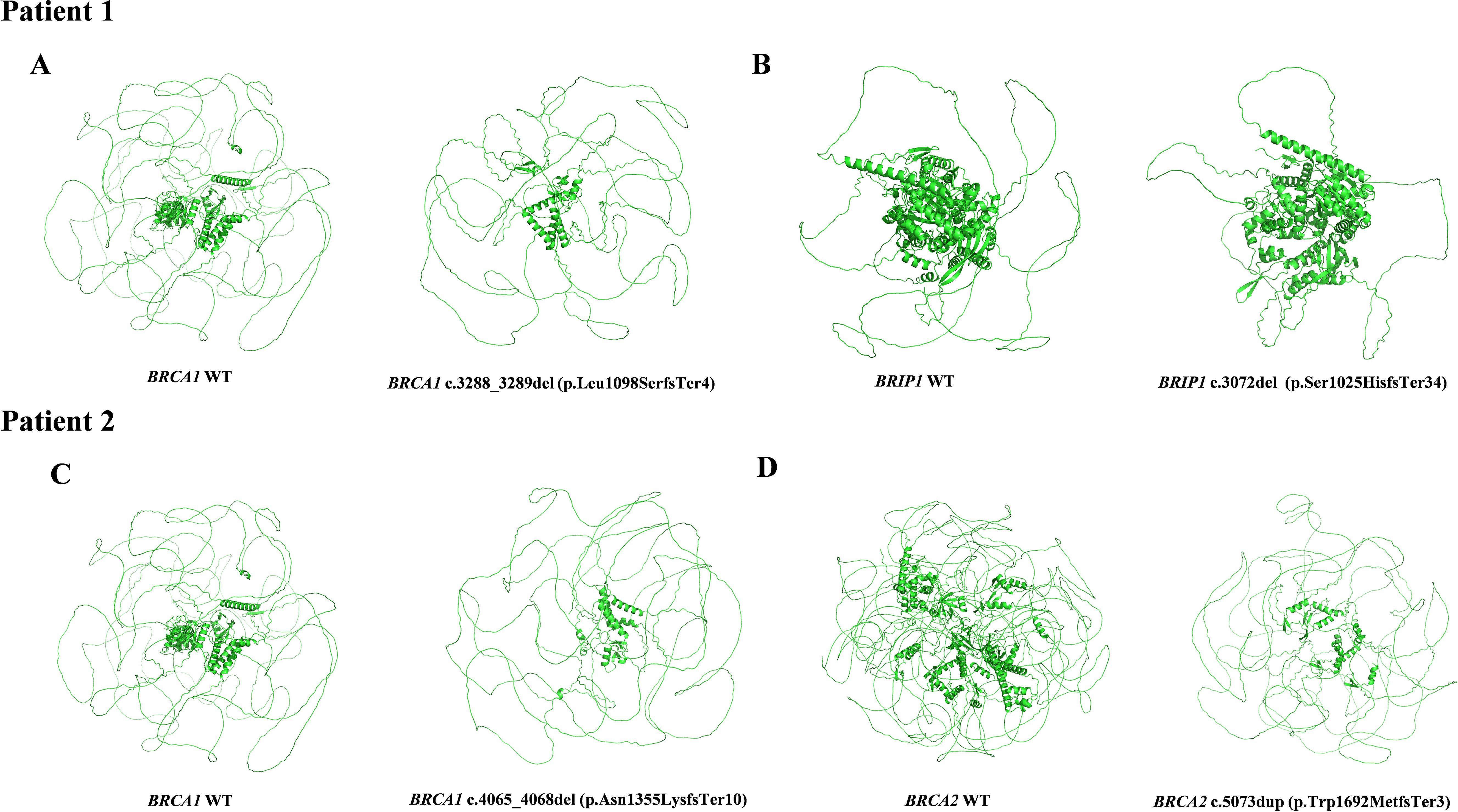
Figure 2. 3D structures of wild-type and variant proteins identified in Patient 1 and Patient 2. (A) BRCA1 WT vs. BRCA1 (NM_007294.4):c.3288_3289del (p.Leu1098SerfsTer4) 3D Structures in Patient 1. (B) BRIP1 WT vs. BRIP1 (NM_032043.3):c.3072del (p.Ser1025HisfsTer34) 3D Structures in Patient 1. (C) BRCA1 WT vs. BRCA1 (NM_007294.4):c.4065_4068del (p.Asn1355LysfsTer10) 3D Structures in Patient 2. (D) BRCA2 WT vs. BRCA2 (NM_000059.3):c.5073dup (p.Trp1692MetfsTer3) 3D Structures in Patient 2.
The proband’s father passed away from esophageal cancer at the age of 66, while her mother died of cerebral infarction at the age of 73. Among her siblings, one brother has kidney cancer, and her unaffected sister shares the same GDH as the proband. The brother with kidney cancer carries a single BRCA1 (NM_007294.4) c.3288_3289del (p.Leu1098SerfsTer4) variant. Based on familial genetic testing, the two germline variants were determined to be of paternal and maternal origin. Figure 1E shows the genealogic tree of this family.
2.2 The result of patient 2
The female proband was diagnosed with high-grade serous ovarian cancer (HGSOC) at the age of 44. In February 2020, she presented with abdominal distension, dull lower abdominal pain, and constipation. The patient had no significant medical comorbidities, no history of long-term medication use, infectious diseases, or allergies. Imaging revealed bilateral ovarian masses, pelvic peritoneal thickening, and ascites consistent with malignant ovarian tumors and metastasis. The patient underwent open abdominal cytoreductive surgery for OC, including total hysterectomy, bilateral salpingo-oophorectomy, appendectomy, omentectomy, resection of peritoneal lesions in the pelvic and abdominal cavities, and pelvic and para-aortic lymphadenectomy. Small residual miliary lesions remained on the diaphragm and mesentery, achieving R1 resection. According to the operative and pathological findings, the final pathological FIGO stage was determined to be IIIC. The patient underwent six cycles of TP regimen chemotherapy (paclitaxel combined with cisplatin) following surgery. Genetic testing identified two pathogenic variants: BRCA1 (NM_007294.4) c.4065_4068del (p.Asn1355LysfsTer10) and BRCA2 (NM_000059.4) c.5073dup (p.Trp1692MetfsTer3) (Figure 3). Based on ACMG guidelines, both variants were classified as pathogenic. Targeted therapy with Olaparib was initiated in August 2020, with dosing adjusted for hematologic parameters. Three-dimensional models of the protein structures were generated to provide additional insight into the structural consequences of these variants, as depicted in Figures 2C, D. The patient completed her last cycle of chemotherapy in July 2020 and has been followed up for 53 months, with no evidence of disease recurrence as of December 2024.
Figure 3. Pedigree and molecular test results of Patient 2. (A) WES data of the detected BRCA1 (NM_007294.4):c.4065_4068del (p.Asn1355LysfsTer10) pathogenic variant. (B) Sanger sequencing result of the BRCA1 pathogenic variant. (C) WES data of the detected BRCA2 (NM_000059.3):c.5073dup (p.Trp1692MetfsTer3) pathogenic variant. (D) Sanger sequencing result of the BRCA2 pathogenic variant. Red arrows indicate the identified variants. (E) Pedigree of the individual 2. Proband is indicated with an arrow. Black indicates individuals with cancer. Cancer type and age at diagnosis are reported. The age for genetic testing and age of death are also reported. Family members carrying the pathogenic/likely pathogenic variant are marked with BRCA1+, BRCA2+.
The proband underwent genetic counseling, during which a family pedigree was constructed. Sanger sequencing confirmed that her father carried the same genetic variant, while her brother tested negative. The family history revealed the mother had cervical cancer at the age of 63, and the father was diagnosed with throat cancer at the age of 60 and lung cancer at the age of 76. Another brother remains unaffected. Details of the familial cancer history are depicted in Figure 3E.
2.3 Discussion
This study is the first to report BRCA1/BRIP1 GDH in OC and evaluates its clinical impact and treatment response. Additionally, a BRCA1/BRCA2 GDH case is included for comparative analysis. Marked differences in treatment response and toxicity profiles were observed between the two patients. The patient with BRCA1/BRIP1 GDH demonstrated substantial chemotherapy-induced toxicity and postoperative complications, suggesting compromised treatment tolerance. In contrast, the BRCA1/BRCA2 GDH patient exhibited better chemotherapy tolerance. Both patients experienced clinical benefit from Olaparib maintenance therapy, achieving disease stabilization for 17 months in the BRCA1/BRIP1 GDH patient and 53 months in the BRCA1/BRCA2 GDH patient, respectively. Continued follow-up may reveal differences in long-term therapeutic outcomes. These findings highlight the influence of different GDH combinations on chemotherapy tolerance and efficacy. However, due to the extremely small sample size, these observations should be interpreted as hypothesis-generating and require validation in larger patient cohorts to elucidate their underlying biological and clinical implications. In addition to genetic factors, differences in clinical presentation and prior treatments may have contributed to the distinct outcomes observed in our two patients. Patient 1 was diagnosed at a more advanced FIGO stage with evidence of bone metastases and had received prior bevacizumab therapy, which may have negatively impacted treatment tolerance and increased the risk of severe toxicity. Patient 2 was diagnosed at an earlier stage and did not have bone metastases or prior exposure to bevacizumab, potentially contributing to better treatment tolerance and efficacy.
Among OC patients receiving first-line platinum-based chemotherapy, germline BRCA gene variants have been associated with an increased incidence of grade 2/3 anemia (18). The present case, which presented with rare postoperative chylous ascites and progressed to grade 4 anemia. These observations raise the possibility that combined BRCA1/BRIP1 gene deficiency could be associated with increased platinum-induced toxicity. The RING domain (amino acids 8–96) and BRCT domain (1646–1859) of BRCA1 are essential for homologous recombination (HR) (19). BRIP1, through its BRCT domain (888–1063), interacts with BRCA1 and plays a pivotal role in the Fanconi anemia (FA) and HR repair pathways (5). FA pathway is a crucial DNA repair pathway primarily responsible for recognizing and repairing DNA interstrand cross-link (ICL) damage, thereby maintaining genomic stability (20). The BRCA1 (NM_007294.4) c.3288_3289del (p.Leu1098SerfsTer4) variant is predicted to disrupt the functionality of both its RING and BRCT domains, while the BRIP1 (NM_032043.3) c.3072del (p.Ser1025HisfsTer34) variant may impair its interaction with BRCA1. This dual defect could significantly impair the ability to repair DNA damage induced by chemotherapeutic agents, such as platinum compounds. Furthermore, variation in BRIP1 may affect hematopoietic function, thereby increasing the risk of hematological toxicity following treatment. Our findings provide new insight into the severe gastrointestinal and hematological toxicities observed in this patient. However, as this observation is derived from a single case and this study did not experimentally investigate the functional impact of these genetic alterations, it should be considered hypothesis-generating. Future research confirmation in larger, well-characterized patient cohorts is warranted
A narrative review of reported cases suggests that the average age of diagnosis for OC patients with GDH is 46.7 years (Table 1). Compared to patients with a single BRCA1/2 germline variant, GDH carriers tend to develop OC at a younger age (35). This observation aligns with our finding of early onset in GDH patients. While GDH involving BRCA1/2 has been associated with younger age at diagnosis and poorer prognosis in breast cancer patients (36), other studies have found no statistically significant differences in prognosis (37, 38). In our study, Patient 2 exhibited a stable disease course, supporting the latter perspective, whereas Patient 1 had a more aggressive disease trajectory with heightened chemotherapy-related toxicity. This suggests that different GDH combinations may differentially impact clinical outcomes. Notably, analysis of the cases summarized in Table 1 reveals that most GDH-associated OC patients present with advanced-stage disease, predominantly FIGO stage III, and the majority of these cases are reported from European populations. Data from Asian cohorts remain limited. Furthermore, while prior literature has primarily focused on the genetic characterization of GDH, detailed descriptions of treatment strategies are relatively scarce. Nevertheless, available follow-up data suggest that the overall survival in these patients may be generally favorable. Given the pronounced chemotherapy-related toxicity observed in patients harboring BRCA1/BRIP1 GDH, it is clinically prudent to consider early implementation of individualized supportive care strategies. These may include the prophylactic administration of hematopoietic growth factors and tailored nutritional support to mitigate hematologic and gastrointestinal adverse events. Furthermore, in cases of poor chemotherapy tolerance, reduced-intensity regimens or earlier initiation of targeted therapies, such as PARP inhibitors, may serve as feasible alternatives to enhance tolerability without compromising therapeutic efficacy. Although these considerations remain preliminary, they underscore the necessity of developing personalized treatment approaches informed by specific GDH genotypes. However, the rarity of GDH cases, particularly within Asian populations, poses a challenge in drawing generalized conclusions. Future studies with larger cohorts are necessary to systematically compare the effects of different GDH combinations and their implications for patient management.
Notably, individuals from two families carrying germline variants in BRCA and BRIP1 exhibited a spectrum of malignancies, including esophageal cancer, renal cancer, ovarian cancer, laryngeal cancer, and lung cancer. While the association between OC and BRCA/BRIP1 germline variants is well established, the relationship between these variants and other malignancies requires further investigation. Our findings provide additional insights that may inform future research in this area.
2.4 Conclusion
This study is the first to characterize BRCA1/BRIP1 GDH variants in OC and compare their clinical impact with BRCA1/BRCA2 GDH variants. Our findings highlight the differential impact of GDH combinations on chemotherapy tolerance and therapeutic outcomes. The results suggest that patients with BRCA1/BRIP1 GDH variants may require personalized dose modifications for platinum-based chemotherapy to minimize toxicity while maximizing efficacy. Simultaneously, more frequent complete blood count monitoring and early or prophylactic administration of hematopoietic growth factors, along with nutritional support, may be necessary to mitigate hematologic toxicity. However, given the limited sample size (n=2) and the absence of functional validation, these results should be interpreted with caution and considered as hypothesis-generating. Further functional and clinical studies are necessary to further elucidate the underlying molecular mechanisms and refine treatment strategies for GDH-associated OC. Expanding the sample size and integrating functional analyses will be essential in optimizing individualized therapeutic approaches for patients with GDH variant.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
LZ: Writing – review & editing, Methodology, Investigation, Data curation, Validation. QZ: Visualization, Formal analysis, Writing – original draft. FZ: Writing – review & editing. HQ: Writing – review & editing. LQ: Writing – review & editing. JY: Writing – review & editing, Conceptualization, Project administration, Data curation. MQ: Supervision, Conceptualization, Writing – review & editing, Project administration.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Scientific Research Project of the Zhejiang Provincial Department of Education (Grant No. Y202454312).
Acknowledgments
We are thankful to the families and clinical staff for participation in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2025.1614373/full#supplementary-material
References
1. Huang J, Chan WC, Ngai CH, Lok V, Zhang L, Lucero-Prisno DE, et al. Worldwide burden, risk factors, and temporal trends of ovarian cancer: A global study. Cancers (Basel). (2022) 14:2230. doi: 10.3390/cancers14092230
2. Wu X, Chen Z, Ren P, Zhao X, Tang D, Geng H, et al. Identifying sequence variants of 18 hereditary ovarian cancer-associated genes in chinese epithelial ovarian cancer patients. BioMed Res Int. (2021) 2021:5579543. doi: 10.1155/2021/5579543
3. Roy R, Chun J, and Powell SN. BRCA1 and BRCA2: different roles in a common pathway of genome protection. Nat Rev Cancer. (2011) 12:68–78. doi: 10.1038/nrc3181
4. Tan DS, Rothermundt C, Thomas K, Bancroft E, Eeles R, Shanley S, et al. “BRCAness” syndrome in ovarian cancer: a case-control study describing the clinical features and outcome of patients with epithelial ovarian cancer associated with BRCA1 and BRCA2 mutations. J Clin Oncol. (2008) 26:5530–6. doi: 10.1200/JCO.2008.16.1703
5. Milano L, Alenezi WM, Fierheller CT, Serruya C, Revil T, Oros KK, et al. Genetic and molecular analyses of candidate germline BRIP1/FANCJ variants implicated in breast and ovarian cancer. medRxiv. (2023) 07:03.23290133. doi: 10.1101/2023.07.03.23290133
6. Palmirotta R, Lovero D, Stucci LS, Silvestris E, Quaresmini D, Cardascia A, et al. Double heterozygosity for BRCA1 pathogenic variant and BRCA2 polymorphic stop codon K3326X: A case report in a southern italian family. Int J Mol Sci. (2018) 19:285. doi: 10.3390/ijms19010285
7. Concolino P, Gelli G, Rizza R, Costella A, Scambia G, and Capoluongo E. BRCA1 and BRCA2 testing through next generation sequencing in a small cohort of italian breast/ovarian cancer patients: novel pathogenic and unknown clinical significance variants. Int J Mol Sci. (2019) 20:3442. doi: 10.3390/ijms20143442
8. Noh JM, Choi DH, Nam SJ, Lee JE, Kim JW, Kim SW, et al. Characteristics of double heterozygosity for BRCA1 and BRCA2 germline mutations in Korean breast cancer patients. Breast Cancer Res Treat. (2012) 131:217–22. doi: 10.1007/s10549-011-1718-5
9. Vietri MT, Caliendo G, D’Elia G, Resse M, Casamassimi A, Minucci PB, et al. Five italian families with two mutations in BRCA genes. Genes (Basel). (2020) 11:1451. doi: 10.3390/genes11121451
10. Madar L, Majoros V, Szűcs Z, Nagy O, Babicz T, Butz H, et al. Double heterozygosity for rare deleterious variants in the BRCA1 and BRCA2 genes in a hungarian patient with breast cancer. IJMS. (2023) 24:15334. doi: 10.3390/ijms242015334
11. Colombo M, Mondini P, Minenza E, Foglia C, Mosconi A, Molica C, et al. A novel BRCA1 splicing variant detected in an early onset triple-negative breast cancer patient additionally carrying a pathogenic variant in ATM: A case report. Front Oncol. (2023) 13:1102184. doi: 10.3389/fonc.2023.1102184
12. Hur JY, Kim JY, Ahn JS, Im YH, Lee J, Kwon M, et al. Clinical characteristics of korean breast cancer patients who carry pathogenic germline mutations in both BRCA1 and BRCA2: A single-center experience. Cancers. (2020) 12:1306. doi: 10.3390/cancers12051306
13. Bang YJ, Kwon WK, Nam SJ, Kim SW, Chae BJ, Lee SK, et al. Clinicopathological characterization of double heterozygosity for BRCA1 and BRCA2 variants in korean breast cancer patients. Cancer Res Treat. (2022) 54:827–33. doi: 10.4143/crt.2021.791
14. Zhang C, Zhu D, Qu Y, Shi M, Ma J, Peng Y, et al. Profiling of the genetic features of Chinese patients with gastric cancer with HRD germline mutations in a large-scale retrospective study. J Med Genet. (2023) 60:760–8. doi: 10.1136/jmg-2022-108816
15. Wen L, Li X, Shi J, Zhang S, Wang R, Yao M, et al. Allele-specific expression mediates primary resistance to poly (ADP-ribose) polymerase inhibitor therapy in a case of BRCA1/2 double-germline mutant gastric cancer. J Int Med Res. (2019) 48:0300060519886226. doi: 10.1177/0300060519886226
16. Kwong A, Ho CYS, Au CH, and Ma ESK. Double heterozygosity for germline mutations in chinese breast cancer patients. Cancers. (2024) 16:2547. doi: 10.3390/cancers16142547
17. Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. (2015) 17:405–23. doi: 10.1038/gim.2015.30
18. Lee IH, Lee SJ, Kim J, Lee YH, Chong GO, Kim JM, et al. Exploring the effect of BRCA1/2 status on chemotherapy-induced hematologic toxicity in patients with ovarian cancer[J. Cancer Chemotherapy Pharmacol. (2024) 94:103–8. doi: 10.1007/s00280-024-04670-8
19. Fu X, Tan W, Song Q, Pei H, and Li J. BRCA1 and breast cancer: molecular mechanisms and therapeutic strategies. Front Cell Dev Biol. (2022) 10:813457. doi: 10.3389/fcell.2022.813457
20. Rodríguez A and D’Andrea A. Fanconi anemia pathway. Curr Biol. (2017) 27:R986–8. doi: 10.1016/j.cub.2017.07.043
21. Ramus SJ, Friedman LS, Gayther SA, Ponder BA, Bobrow LG, van der Looji M, et al. A breast/ovarian cancer patient with germline mutations in both BRCA1 and BRCA2. Nat Genet. (1997) 15:14–5. doi: 10.1038/ng0197-14
22. Friedman E, Bar-sade Bruchim R, Kruglikova A, Risel S, Levy-Lahad E, Halle D, et al. Double heterozygotes for the ashkenazi founder mutations in BRCA1 and BRCA2 genes. Am J Hum Genet. (1998) 63:1224–7. doi: 10.1086/302040
23. Randall TC, Bell KA, Rebane BA, Rubin SC, and Boyd J. Germline mutations of theBRCA1andBRCA2Genes in a breast and ovarian cancer patient. Gynecologic Oncol. (1998) 70:432–4. doi: 10.1006/gyno.1998.5081
24. Leegte B, van der Hout AH, Deffenbaugh AM, Bakker MK, Mulder IM, Ten Berge A, et al. Phenotypic expression of double heterozygosity for BRCA1 and BRCA2 germline mutations. J Med Genet. (2005) 42:e20–0. doi: 10.1136/jmg.2004.027243
25. Spannuth WA, Thaker PH, and Sood AK. Concomitant BRCA1 and BRCA2 gene mutations in an Ashkenazi Jewish Woman with primary breast and ovarian cancer. Am J Obstetrics Gynecology. (2007) 196:e6–9. doi: 10.1016/j.ajog.2007.01.026
26. Pilato B, De Summa S, Danza K, Lambo R, Paradiso A, and Tommasi S. Maternal and paternal lineage double heterozygosity alteration in familial breast cancer: a first case report. Breast Cancer Res Treat. (2010) 124:875–8. doi: 10.1007/s10549-010-1125-3
27. Steffensen AY, Jønson L, Ejlertsen B, Gerdes AM, Nielsen FC, and Hansen TV. Identification of a Danish breast/ovarian cancer family double heterozygote for BRCA1 and BRCA2 mutations. Familial cancer. (2010) 9:283–7. doi: 10.1007/s10689-010-9345-6
28. Zuradelli M, Peissel B, Manoukian S, Zaffaroni D, Barile M, Pensotti V, et al. Four new cases of double heterozygosity for BRCA1 and BRCA2 gene mutations: clinical, pathological, and family characteristics. Breast Cancer Res Treat. (2010) 124:251–8. doi: 10.1007/s10549-010-0853-8
29. Augustyn AM, Agostino NM, Namey TL, Nair S, and Martino MA. Two patients with germline mutations in both BRCA1 and BRCA2 discovered unintentionally: a case series and discussion of BRCA testing modalities. Breast Cancer Res Treat. (2011) 129:629–34. doi: 10.1007/s10549-011-1597-9
30. Pedroni M, Di Gregorio C, Cortesi L, Reggiani Bonetti L, Magnani G, Simone ML, et al. Double heterozygosity for BRCA1 and hMLH1 gene mutations in a 46-year-old woman with five primary tumors. Tech Coloproctol. (2014) 18:285–9. doi: 10.1007/s10151-013-1030-y
31. Li W, Shao D, Li L, Wu M, Ma S, Tan X, et al. Germline and somatic mutations of multi-gene panel in Chinese patients with epithelial ovarian cancer: a prospective cohort study. J Ovarian Res. (2019) 12:80. doi: 10.1186/s13048-019-0560-y
32. Vietri MT, D’Elia G, Caliendo G, Casamassimi A, Resse M, Passariello L, et al. Double mutation of APC and BRCA1 in an Italian family. Cancer Genet. (2020) 244:32–5. doi: 10.1016/j.cancergen.2020.04.074
33. Ferrer-Avargues R, Castillejo MI, Dámaso E, Díez-Obrero V, Garrigos N, Molina T, et al. Co-occurrence of germline pathogenic variants for different hereditary cancer syndromes in patients with Lynch syndrome. Cancer Commun. (2021) 41:218–28. doi: 10.1002/cac2.12134
34. Harada R, Matsubayashi H, Kiyozumi Y, Kobayashi H, Mitsuya K, Imai K, et al. Japanese case of ovarian mucinous adenocarcinoma with germline double variants of MSH2 and BRCA2. J Hum Genet. (2023) 68:783–7. doi: 10.1038/s10038-023-01178-6
35. Wu X, Wu L, Kong B, Liu J, Yin R, Wen H, et al. The first nationwide multicenter prevalence study of germline BRCA1 and BRCA2 mutations in chinese ovarian cancer patients. Int J Gynecologic Cancer. (. 2017) 27:1650–57. doi: 10.1097/igc.0000000000001065
36. Heidemann S, Fischer C, Engel C, Fischer B, Harder L, Schlegelberger B, et al. Double heterozygosity for mutations in BRCA1 and BRCA2 in German breast cancer patients: implications on test strategies and clinical management. Breast Cancer Res Treat. (2012) 134:1229–39. doi: 10.1007/s10549-012-2050-4
37. Lavie O, Narod S, Lejbkowicz F, Dishon S, Goldberg Y, Gemer O, et al. Double heterozygosity in the BRCA1 and BRCA2 genes in the Jewish population. Ann Oncol. (2011) 22:964–6. doi: 10.1093/annonc/mdq460
Keywords: BRCA1, BRCA2, BRIP1, germline double heterozygosity, ovarian cancer, case report
Citation: Zheng L, Zhu Q, Zhang F, Qiu H, Qin L, Yang J and Qi M (2025) Case Report: Clinical impact of BRCA1 and BRIP1 vs. BRCA1 and BRCA2 germline double heterozygosity in ovarian cancer: a comparative case study. Front. Oncol. 15:1614373. doi: 10.3389/fonc.2025.1614373
Received: 18 April 2025; Accepted: 04 July 2025;
Published: 24 July 2025.
Edited by:
Robert Fruscio, University of Milano Bicocca, ItalyCopyright © 2025 Zheng, Zhu, Zhang, Qiu, Qin, Yang and Qi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jianhua Yang, eWpoMjAwNkB6anUuZWR1LmNu; Ming Qi, bWluZ3FpQHpqdS5lZHUuY24=
†These authors have contributed equally to this work and share first authorship
 Limei Zheng1†
Limei Zheng1† Qianyuan Zhu
Qianyuan Zhu Fenglan Zhang
Fenglan Zhang Jianhua Yang
Jianhua Yang Ming Qi
Ming Qi