- 1Department of Urology, The First Affiliated Hospital of Anhui Medical University, Hefei, Anhui, China
- 2Institute of Urology, Anhui Medical University, Hefei, Anhui, China
- 3Department of Oncology, The First Affiliated Hospital of Anhui Medical University, Hefei, China
- 4Department of Pathology, The First Affiliated Hospital of Anhui Medical University, Hefei, Anhui, China
SMARCA4-deficient tumors represent a highly aggressive subtype of malignancy defined by the loss of SMARCA4 expression, and are associated with rapid progression and poor prognosis. We report the first documented case of SMARCA4-deficient renal cell carcinoma (RCC) in an adult (pT3aN1M1, Stage IV), characterized by sarcomatoid and rhabdomyoblastic differentiation, aggressive clinical behavior, and resistance to standard systemic therapies. This case provides a comprehensive analysis of clinical, pathological, imaging, and genetic findings, offering insights to refine diagnostic, therapeutic, and preventive strategies for this rare malignancy.
Introduction
The SWI/SNF (SWItch/Sucrose non-fermentable) chromatin remodeling complex plays a crucial role in regulating chromatin accessibility and gene expression (1, 2). SMARCA4 encodes BRG1, the core ATPase subunit of this complex that drives chromatin remodeling to regulate gene transcription (3). Recent studies have identified a highly aggressive class of undifferentiated tumors termed SMARCA4-deficient tumors, characterized by SMARCA4 deficiency, and exhibiting rapid proliferation and high metastatic potential, leading to poor clinical outcomes (4). SMARCA4-deficient tumors predominantly arise in the thoracic cavity, sinonasal region, gastrointestinal tract, and female genital tract, but occurrence in the urinary tract, particularly in the kidney, remains exceptionally rare (5–8). Given the significant histological overlap between SMARCA4-deficient tumors and rhabdomyosarcomas, sarcomatoid carcinomas, and other undifferentiated malignancies, accurate diagnosis requires a comprehensive approach incorporating immunohistochemical and genetic evaluation (9–12). Herein, we present the first reported case of SMARCA4-deficient renal cell carcinoma (RCC) in an adult, marked by rapid clinical progression, prominent sarcomatoid and rhabdomyoblastic differentiation, and resistance to standard systemic therapies. Through detailed clinical, pathological, imaging, and genetic analyses, we aim to raise awareness of SMARCA4-deficient RCC, as well as to explore potential diagnostic, therapeutic, and preventive strategies.
Case presentation
A 71-year-old postmenopausal woman was admitted to our urology service reporting progressive left flank pain radiating to the lower abdominal quadrant persisting for 20 days. Digital palpation disclosed a 4cm fixed mass in the left costo-vertebral angle, demonstrating exquisite tenderness upon deep inspiration.
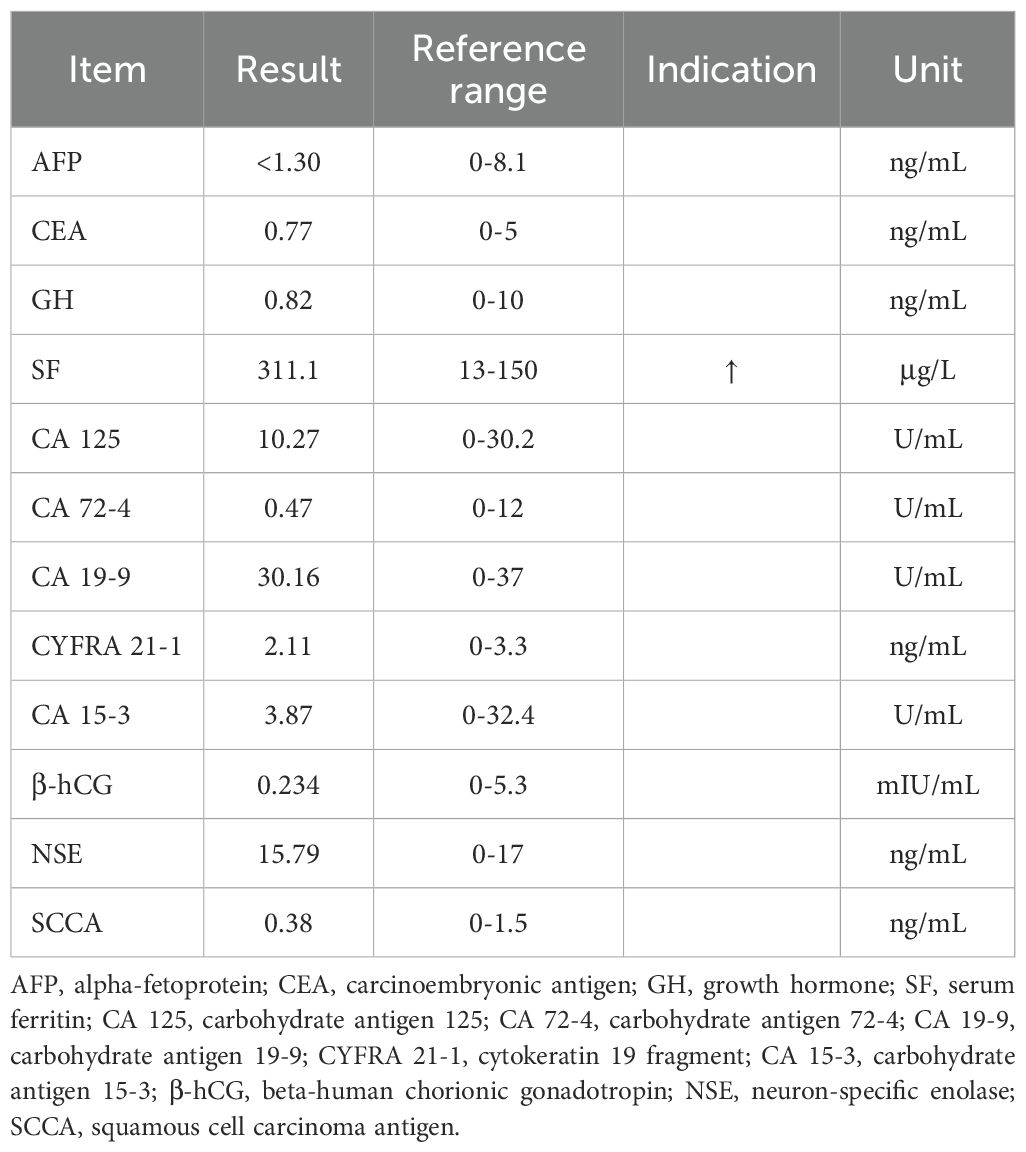
Laboratory tests showed elevated serum ferritin (311.1 µg/L; reference range: 13–150 µg/L), whereas other tumor biomarkers yielded no significant findings (Table 1). Contrast-enhanced abdominal computed tomography (CT) demonstrated a 40 × 36mm heterogeneously enhancing mass at the left renal upper pole, with mild and persistent enhancement accompanied by retroperitoneal lymphadenopathy and subcentimeter hypodense hepatic nodule (Figures 1A–C). Technetium-99m methylene diphosphonate (99mTc-MDP) whole-body scintigraphy revealed focally augmented tracer accumulation at left sixth/seventh anterior ribs, sacral alae, and left sacroiliac joint (Figure 1D). Subsequent standard Fluorine-18 fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT) delineated a hypermetabolic exophytic renal mass (maximum standardized uptake value [SUVmax] of 7.4) in continuity with the left renal upper pole cortex, accompanied by enlarged retroperitoneal lymph nodes and multiple small hypoattenuating hepatic lesions demonstrating FDG avidity (Figure 1F). Osseous metastases were identified in the left sixth and seventh anterior ribs, L3 vertebral body, sacrum, left iliac bone, and left femur, showing metabolically active foci (SUVmax 8.5) with CT evidence of variable bone destruction (Figures 1G, H).
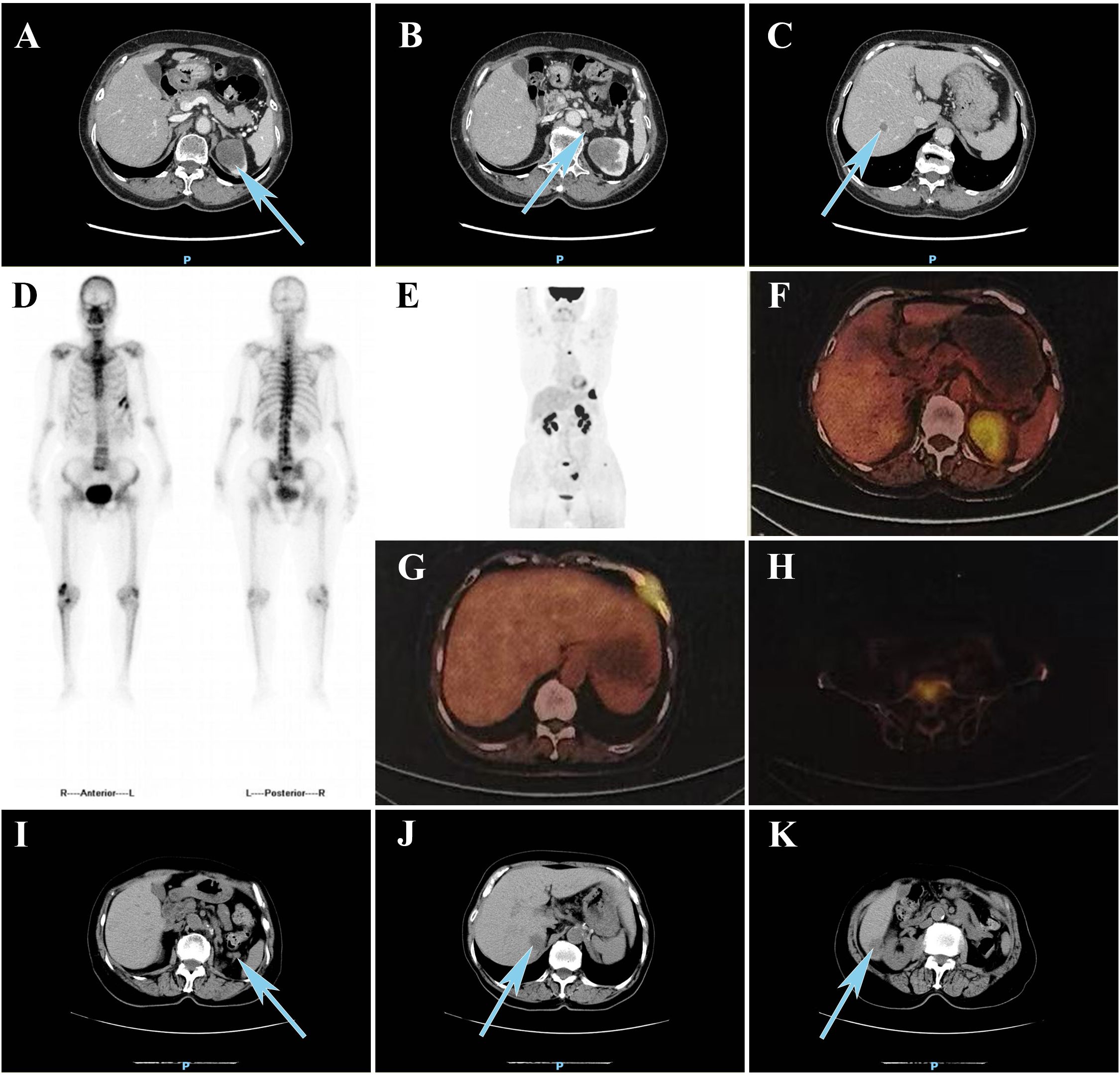
Figure 1. (A) Contrast-enhanced CT reveals (A) a heterogeneously enhancing mass at the left renal upper pole, (B) progressive retroperitoneal lymphadenopathy, and (C) hypodense lesions in the liver. (D) 99mTc-MDP bone scintigraphy demonstrated increased tracer uptake in the left sixth and seventh anterior ribs, sacrum, and left sacroiliac joint. (E) MIP reconstruction of 18F-FDG PET/CT. 18F-FDG PET/CT demonstrated increased fluorodeoxyglucose uptake in (F) the upper pole of the left renal upper pole, (G) left sixth and seventh ribs, and (H) L3 vertebral body. Follow-up CT at 3 months demonstrated newly (I) enlarged retroperitoneal lymph nodes and (J, K) two hypoattenuating hepatic lesions. CT, computed tomography; 99mTc-MDP, technetium-99m methylene diphosphonate; MIP, maximum intensity projection; 18F-FDG, fluorine-18 fluorodeoxyglucose; PET, positron emission tomography.
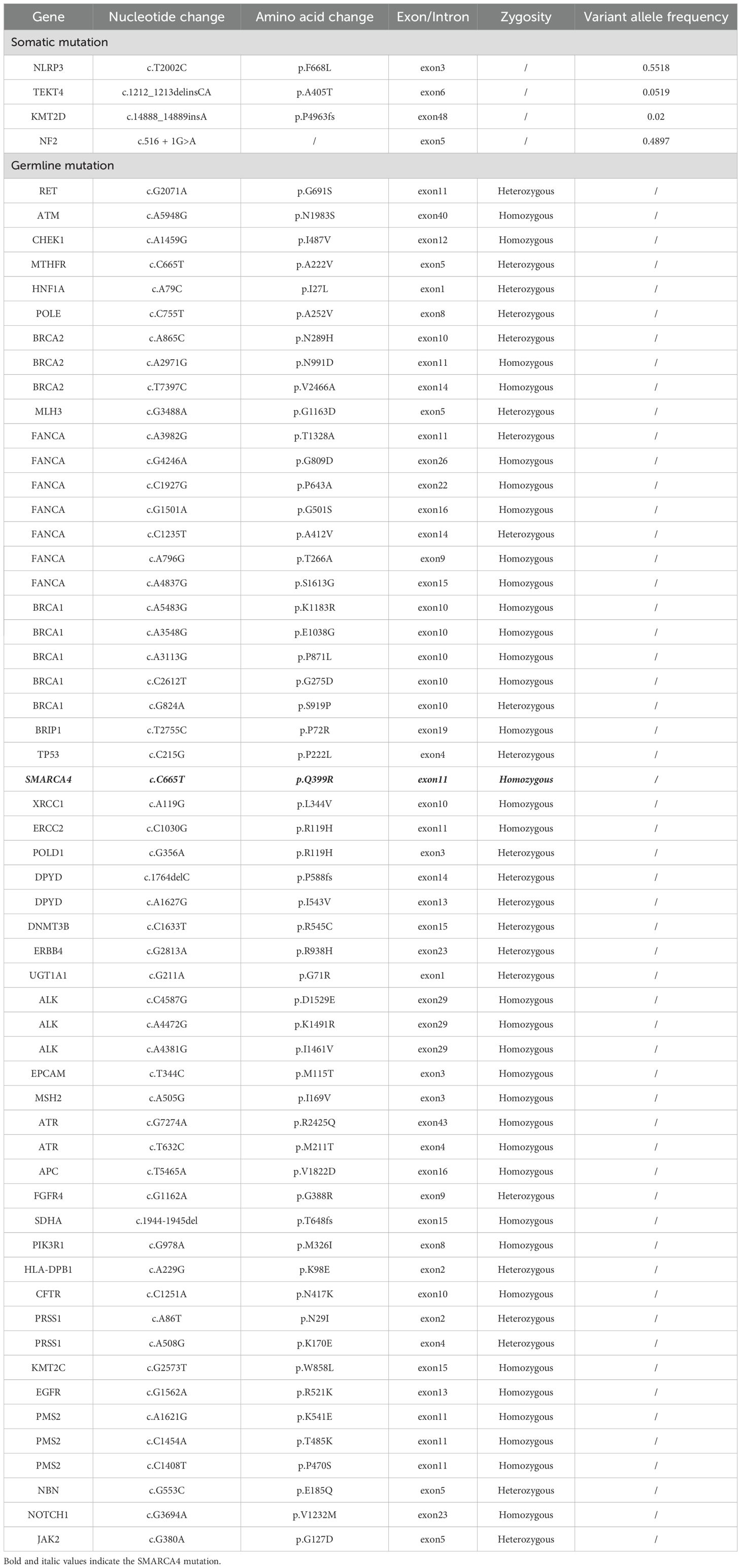
The patient underwent da Vinci Xi robotic-assisted radical left nephrectomy with en bloc retroperitoneal lymph node dissection. Postoperative histology revealed a biphasic neoplasm with eccentrically located nuclei and vacuolated cytoplasm, comprising 20% sarcomatoid differentiation and 10% uniform rhabdomyoblastic morphology (Figure 2A). Immunohistochemistry showed positive staining for PAX-8, with complete loss of SMARCA4/BRG1 staining (Figure 2B). Next-generation sequencing (NGS) identified a heterozygous germline SMARCA4 (NM_005359.5: c.665C>T, p.Pro222Leu) missense mutation. No clinically actionable biomarkers were detected, as PD-L1 was negative, chemotherapy-related single nucleotide polymorphisms (SNPs) showed no predictive value, and no alterations were found in FDA/NCCN-endorsed targets, indicating resistance to current approved therapeutic options (Table 2).
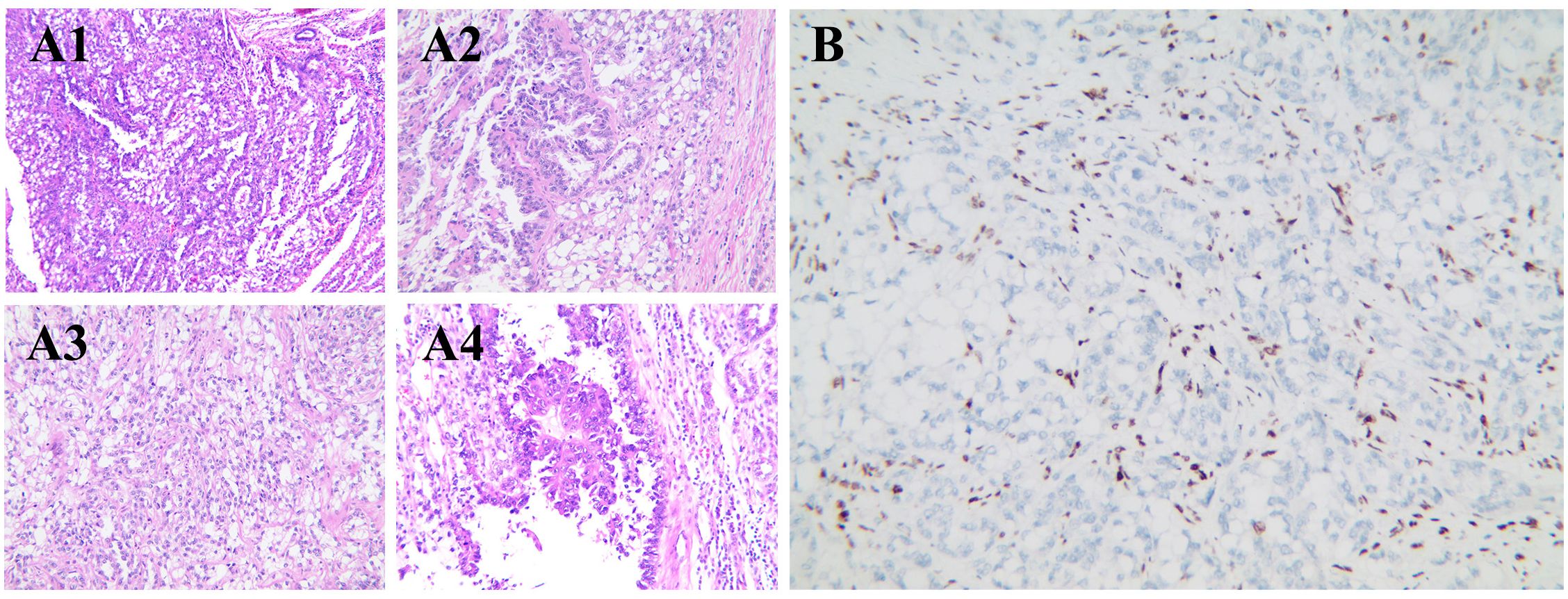
Figure 2. Histologic and immunohistochemical features of SMARCA4-dRCC. (A1) Low-power field with tumor cells showing clear to eosinophilic cytoplasm, arranged in solid and focal tubular patterns, with infiltrative borders. (A2) High-power field with prominent nucleoli, marked nuclear atypia, and architectural disarray. (A3) Sarcomatoid differentiation with spindle-shaped tumor cells in disorganized fascicles. (A4) Rhabdomyoblastic differentiation with eosinophilic cytoplasm and eccentric nuclei. (B) Immunohistochemistry showing complete loss of SMARCA4/BRG1 expression in tumor cells. RCC, renal cell carcinoma.
Postoperatively, the patient was treated with a combination of sunitinib and denosumab. At the 3-month follow-up, the patient was clinically stable with no significant limitations in daily activities. However, CT demonstrated progressive retroperitoneal lymphadenopathy and two new hepatic metastases.
Discussion
The SWI/SNF chromatin remodeling complex governs chromatin structure accessibility and transcriptionally regulates cancer-relevant gene networks by hydrolyzing ATP to mobilize nucleosomes (13). In mammals, the SWI/SNF complex comprises three non-overlapping subtypes, the canonical BAF (cBAF), polybromo-associated BAF (pBAF), and non-canonical BAF (ncBAF) (14). Each subtype contains mutually exclusive ATPase catalytic subunits, either SMARCA4 or SMARCA2, generating mechanical force via ATP hydrolysis for nucleosome mobilization, eviction, or histone exchange (15). This process facilitates the conversion of tightly packed heterochromatin into an accessible, open state, thereby promoting gene transcription. Dysfunctional SMARCA4 impairs SWI/SNF complex assembly, inducing genome-wide enhancer silencing and activating pro-tumorigenic pathways that drive metastasis and therapy resistance (16).
Previous studies have established that SMARCA2 deficiency is a pathognomonic genomic driver in high-grade clear cell renal cell carcinoma (ccRCC), mechanistically linked to epigenetic reprogramming that fuels sarcomatoid differentiation (17). Contrastingly, SMARCA4-deficient tumors predominantly arise in the sinonasal region, thorax, digestive tract, and female genital tract, with occurrences in the urinary tract being exceedingly rare (5–8). Our case represents the first reported instance of SMARCA4-deficient RCC in an adult, pathologically staged as pT3aN1M1 and classified as WHO/ISUP grade 4.
SMARCA4-deficient tumors are typically associated with extremely poor survival outcomes. Carcinoma cells exhibiting hyperactivation of epithelial-mesenchymal transition (EMT), characterized by enhanced migratory and invasive capabilities, which promote the early onset of metastatic spread (18, 19). Clinically, the progression of SMARCA4-deficient tumors occurs at a significantly faster rate compared to other tumor types. The median survival for SMARCA4-deficient gastric carcinoma is less than one year from diagnosis, whereas the median survival for SMARCA4-deficient thoracic sarcomas is only six months from diagnosis (20, 21).
Patients with RCC rarely present with the classic triad of symptoms, including hematuria, flank pain, and abdominal mass in the early stages. More commonly, renal masses are incidentally discovered during abdominal imaging studies conducted for unrelated indications (22). Among imaging modalities, ultrasound is primarily used for tumor screening and adjunctive diagnosis. Contrast-enhanced CT remains the cornerstone for RCC evaluation and multiparametric magnetic resonance imaging (MRI) excels in characterizing cystic/necrotic components and venous invasion, critical for surgical planning (23). Additionally, SMARCA4-deficient tumors and their metastatic lesions typically show a strong affinity for 18F-FDG enabling PET/CT to detect occult metastases (24). Although sufficient case data to characterize the specific imaging features of SMARCA4-deficient undifferentiated RCC are currently lacking, their high metastatic potential and metabolic activity suggest that the integration of CT/MRI with PET/CT should be incorporated into diagnostic workflows and clinical staging.
Histologically, SMARCA4-deficient tumors present as infiltrative, diffuse sheets of undifferentiated cells, frequently accompanied by variable rhabdomyoblastic tumor cell components (9). Due to the high degree of dedifferentiation observed in SMARCA4-deficient undifferentiated RCC, these tumors are morphologically similar to SMARCB1-deficient malignant rhabdoid tumor (MRT), rhabdomyoblastic RCC, and other small round cell undifferentiated tumors, which can lead to misdiagnosis (10–12). Therefore, immunohistochemistry is essential, with complete loss of SMARCA4 expression serving as a reliable marker of SMARCA4-deficient tumors.
It is worth noting that we identified a heterozygous germline mutation in SMARCA4 in this patient. Unlike sporadic tumors driven by somatic mutations, this germline alteration follows an autosomal dominant inheritance pattern with incomplete penetrance, indicating a potential hereditary predisposition to cancer (25, 26). This mechanism closely parallels that observed in small cell carcinoma of the ovary, hypercalcemic type (SCCOHT), wherein more than 40% of cases are driven by germline SMARCA4 mutations (27, 28). Thus, we propose that tumorigenesis in SMARCA4-deficient RCC similarly adheres to Knudson’s classic two-hit model: an initial germline loss-of-function mutation serves as the first hit, followed by somatic inactivation of the remaining wild-type allele, leading to complete loss of BRG1 function (29). Given these implications, germline SMARCA4 testing and cascade screening of at-risk relatives are essential to enable tailored surveillance strategies, including periodic imaging and biochemical assessments for early tumor detection and improved clinical outcomes.
SMARCA4-deficient tumors progress aggressively, with over 60% of patients presenting at stage IV by the time of diagnosis (30). For SMARCA4-deficient RCC amenable to surgery, cytoreductive nephrectomy combined with therapeutic options may improve outcomes. Conventional platinum-based chemotherapy shows limited efficacy in these SWI/SNF-altered malignancies due to intrinsic resistance mechanisms (31). Although our case demonstrated no significant evidence of benefit from immunotherapy, studies have shown that more than 65% of such tumors exhibit high PD-L1 expression in both tumor cells and immune cells within the tumor microenvironment (32, 33). Additionally, targeted therapies involving Enhancer of Zeste Homolog 2 (EZH2) inhibitors and histone deacetylase (HDAC) inhibitors have shown promising therapeutic potential in SMARCA4-deficient undifferentiated thoracic tumors (34). Furthermore, Fang et al. recently reported that SMARCA4-deficient cells exhibit upregulation of the oxidative phosphorylation (OXPHOS) pathway, suggesting that OXPHOS inhibitors may serve as potential therapeutic agents for SMARCA4-deficient tumors (35).
During this study, we collected a comprehensive set of clinical, imaging, pathological and genetic data on SMARCA4-deficient RCC, providing insights into potential diagnostic and therapeutic strategies. This case represents the first reported instance of SMARCA4-deficient undifferentiated RCC in an adult, offering valuable contributions to the refinement of future diagnostic, therapeutic, and preventive protocols.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by the Ethics Committee of the First Affiliated Hospital of Anhui Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
WW: Writing – original draft, Visualization, Writing – review & editing, Validation. SY: Writing – review & editing, Investigation. DX: Supervision, Writing – review & editing, Conceptualization. ST: Data curation, Writing – original draft. XC: Data curation, Writing – review & editing. YC: Writing – review & editing. YF: Writing – review & editing, Investigation. JZ: Writing – review & editing, Supervision, Project administration.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was supported by the Health committee project of Anhui Province in China (No. AHWJ2023BAc10007) and Outstanding Scientific Research and Innovation Team for Male Genitourinary Diseases in Anhui Provincial Universities (2022AH010071).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Glossary
SWI/SNF: SWItch/Sucrose non-fermentable
CT: computed tomography
99mTc-MDP: technetium-99m methylene diphosphonate
18F-FDG PET/CT: fluorine-18 fluorodeoxyglucose positron emission tomography/computed tomography
SUV: standardized uptake value
FH: fumarate hydratase
NGS: next-generation sequencing
SNPs: single nucleotide polymorphisms
BAF: brahma-associated factor
ccRCC: clear cell renal cell carcinoma
WHO: world health organization
EMT: epithelial-mesenchymal transition
MRI: magnetic resonance imaging
MRT: malignant rhabdoid tumor
SCCOHT: small cell carcinoma of the ovary, hypercalcemic type
EZH2: enhancer of zeste homolog 2
HDAC: histone deacetylase
OXPHOS: oxidative phosphorylation
References
1. Wilson BG and Roberts CWM. SWI/SNF nucleosome remodellers and cancer. Nat Rev Cancer. (2011) 11:481–92. doi: 10.1038/nrc3068
2. Kadoch C, Hargreaves DC, Hodges C, Elias L, Ho L, Ranish J, et al. Proteomic and bioinformatic analysis of mammalian SWI/SNF complexes identifies extensive roles in human Malignancy. Nat Genet. (2013) 45:592–601. doi: 10.1038/ng.2628
3. Shen H, Powers N, Saini N, Comstock CE, Sharma A, Weaver K, et al. The SWI/SNF ATPase Brm is a gatekeeper of proliferative control in prostate cancer. Cancer Res. (2008) 68:10154–62. doi: 10.1158/0008-5472.CAN-08-1794
4. Ng CS and Qin J. Switch/sucrose nonfermentable-deficient tumors-morphology, immunophenotype, genetics, epigenetics, nosology, and therapy. Lab Invest. (2025) 105:102185. doi: 10.1016/j.labinv.2024.102185
5. McCluggage WG and Stewart CJR. SWI/SNF-deficient Malignancies of the female genital tract. Semin In Diagn Pathol. (2021) 38:199–211. doi: 10.1053/j.semdp.2020.08.003
6. Agaimy A and Weichert W. SMARCA4-deficient sinonasal carcinoma. Head Neck Pathol. (2017) 11:541–5. doi: 10.1007/s12105-017-0783-4
7. Horton RK, Ahadi M, Gill AJ, Said S, Chen ZE, Bakhshwin A, et al. SMARCA4/SMARCA2-deficient carcinoma of the esophagus and gastroesophageal junction. Am J Surg Pathol. (2021) 45:414–20. doi: 10.1097/PAS.0000000000001599
8. Longo V, Catino A, Montrone M, Montagna ES, Pesola F, Marech I, et al. Treatment of thoracic SMARCA4-deficient undifferentiated tumors: where we are and where we will go. Int J Mol Sci. (2024) 25. doi: 10.3390/ijms25063237
9. Nicholson AG, Tsao MS, Beasley MB, Borczuk AC, Brambilla E, Cooper WA, et al. The 2021 WHO classification of lung tumors: impact of advances since 2015. J Thorac Oncol. (2022) 17:362–87. doi: 10.1016/j.jtho.2021.11.003
10. Ngo C and Postel-Vinay S. Immunotherapy for SMARCB1-deficient sarcomas: current evidence and future developments. Biomedicines. (2022) 10. doi: 10.3390/biomedicines10030650
11. Kinoshita F, Kohashi K, Sugimoto M, Takamatsu D, Kiyozawa D, Eto M, et al. The SWI/SNF chromatin-remodeling complex status in renal cell carcinomas with sarcomatoid or rhabdoid features. Virchows Archiv. (2020) 477:651–60. doi: 10.1007/s00428-020-02839-z
12. Mangray S, Somers GR, He J, Zhong S, Shago M, Treaba DO, et al. Primary undifferentiated sarcoma of the kidney harboring a novel variant of CIC-DUX4 gene fusion. Am J Surg Pathol. (2016) 40:1298–301. doi: 10.1097/PAS.0000000000000688
13. Mittal P and Roberts CWM. The SWI/SNF complex in cancer - biology, biomarkers and therapy. Nat Rev Clin Oncol. (2020) 17:435–48. doi: 10.1038/s41571-020-0357-3
14. Centore RC, Sandoval GJ, Soares LMM, Kadoch C, and Chan HM. Mammalian SWI/SNF chromatin remodeling complexes: emerging mechanisms and therapeutic strategies. Trends In Genetics: TIG. (2020) 36:936–50. doi: 10.1016/j.tig.2020.07.011
15. Field NR, Dickson KA, Nassif NT, and Marsh DJ. SMARCA4 and SMARCA2 co-deficiency: An uncommon molecular signature defining a subset of rare, aggressive and undifferentiated Malignancies associated with defective chromatin remodeling. Cancer Lett. (2024) 605:217282. doi: 10.1016/j.canlet.2024.217282
16. Mardinian K, Adashek JJ, Botta GP, Kato S, and Kurzrock R. SMARCA4: implications of an altered chromatin-remodeling gene for cancer development and therapy. Mol Cancer Ther. (2021) 20:2341–51. doi: 10.1158/1535-7163.MCT-21-0433
17. Xia QY, Rao Q, Cheng L, Shen Q, Shi SS, Li L, et al. Loss of BRM expression is a frequently observed event in poorly differentiated clear cell renal cell carcinoma. Histopathology. (2014) 64:847–62. doi: 10.1111/his.12334
18. Sugimoto M, Kohashi K, Itsumi M, Shiota M, Abe T, Yamada Y, et al. Epithelial to mesenchymal transition in clear cell renal cell carcinoma with rhabdoid features. Pathobiology. (2016) 83:277–86. doi: 10.1159/000445752
19. Matsubara D, Kishaba Y, Ishikawa S, Sakatani T, Oguni S, Tamura T, et al. Lung cancer with loss of BRG1/BRM, shows epithelial mesenchymal transition phenotype and distinct histologic and genetic features. Cancer Sci. (2013) 104:266–73. doi: 10.1111/cas.12065
20. An HR, Kim HD, Ryu MH, and Park YS. SMARCA4-deficient undifferentiated gastric carcinoma: a case series and literature review. Gastric Cancer. (2024) 27:1147–52. doi: 10.1007/s10120-024-01510-9
21. Perret R, Chalabreysse L, Watson S, Serre I, Garcia S, Forest F, et al. SMARCA4-deficient thoracic sarcomas: clinicopathologic study of 30 cases with an emphasis on their nosology and differential diagnoses. Am J Surg Pathol. (2019) 43:455–65. doi: 10.1097/PAS.0000000000001188
22. Capitanio U, Bensalah K, Bex A, Boorjian SA, Bray F, Coleman J, et al. Epidemiology of renal cell carcinoma. Eur Urol. (2019) 75:74–84. doi: 10.1016/j.eururo.2018.08.036
23. Capitanio U and Montorsi F. Renal cancer. Lancet (London England). (2016) 387:894–906. doi: 10.1016/S0140-6736(15)00046-X
24. Crombé A, Alberti N, Villard N, Pilleul F, Buy X, Le Loarer F, et al. Imaging features of SMARCA4-deficient thoracic sarcomas: a multi-centric study of 21 patients. Eur Radiol. (2019) 29:4730–41. doi: 10.1007/s00330-019-06017-x
25. Garber JE and Offit K. Hereditary cancer predisposition syndromes. J Clin Oncol. (2005) 23:276–92. doi: 10.1200/JCO.2005.10.042
26. Rahman N. Realizing the promise of cancer predisposition genes. Nature. (2014) 505:302–8. doi: 10.1038/nature12981
27. Witkowski L, Carrot-Zhang J, Albrecht S, Fahiminiya S, Hamel N, Tomiak E, et al. Germline and somatic SMARCA4 mutations characterize small cell carcinoma of the ovary, hypercalcemic type. Nat Genet. (2014) 46:438–43. doi: 10.1038/ng.2931
28. Tischkowitz M, Huang S, Banerjee S, Hague J, Hendricks WPD, Huntsman DG, et al. Small-cell carcinoma of the ovary, hypercalcemic type-genetics, new treatment targets, and current management guidelines. Clin Cancer Res. (2020) 26:3908–17. doi: 10.1158/1078-0432.CCR-19-3797
29. Knudson AG. Mutation and cancer: statistical study of retinoblastoma. Proc Natl Acad Sci United States America. (1971) 68:820–3. doi: 10.1073/pnas.68.4.820
30. Cooper AJ, Arfe A, Ricciuti B, Gagné A, Sholl LM, Di Federico A, et al. Brief report: clinical characteristics and outcomes of patients with thoracic SMARCA4-deficient undifferentiated tumors. JTO Clin Res Rep. (2025) 6:100759. doi: 10.1016/j.jtocrr.2024.100759
31. Tessier-Cloutier B, Coatham M, Carey M, Nelson GS, Hamilton S, Lum A, et al. SWI/SNF-deficiency defines highly aggressive undifferentiated endometrial carcinoma. J Pathol Clin Res. (2021) 7:144–53. doi: 10.1002/cjp2.188
32. Agaimy A. SWI/SNF-deficient Malignancies: optimal candidates for immune-oncological therapy? Adv In Anatomic Pathol. (2023) 30:211–7. doi: 10.1097/PAP.0000000000000366
33. Agaimy A, Daum O, Michal M, Schmidt MW, Stoehr R, Hartmann A, et al. Undifferentiated large cell/rhabdoid carcinoma presenting in the intestines of patients with concurrent or recent non-small cell lung cancer (NSCLC): clinicopathologic and molecular analysis of 14 cases indicates an unusual pattern of dedifferentiated metastases. Virchows Archiv. (2021) 479:157–67. doi: 10.1007/s00428-021-03032-6
34. Li X, Tian S, Shi H, Ta N, Ni X, Bai C, et al. The golden key to open mystery boxes of SMARCA4-deficient undifferentiated thoracic tumor: focusing immunotherapy, tumor microenvironment and epigenetic regulation. Cancer Gene Ther. (2024) 31:687–97. doi: 10.1038/s41417-024-00732-4
Keywords: SMARCA4-deficient tumor, renal cell carcinoma, sarcomatoid differentiation, rhabdomyoblastic differentiation, germline mutation
Citation: Wang W, Yin S, Xu D, Tai S, Cheng X, Chang Y, Fu Y and Zhou J (2025) Case Report: Recognition and management of SMARCA4-deficient renal cell carcinoma. Front. Oncol. 15:1614796. doi: 10.3389/fonc.2025.1614796
Received: 19 April 2025; Accepted: 30 October 2025;
Published: 12 November 2025.
Edited by:
Hongbing Liu, Tulane University, United StatesReviewed by:
Ziv Radisavljevic, Harvard Medical School, United StatesJad A. Degheili, Ibn Sina Hospital, Kuwait
Copyright © 2025 Wang, Yin, Xu, Tai, Cheng, Chang, Fu and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jun Zhou, dXJvZG9jdG9yemhvdUAxNjMuY29t; Yao Fu, eWFvX2Z1QGFsaXl1bi5jb20=
†These authors have contributed equally to this work
 Weibo Wang1,2†
Weibo Wang1,2† Dandan Xu
Dandan Xu Jun Zhou
Jun Zhou