- Department of Urology, The First Affiliated Hospital of Hunan Normal University/Hunan Provincial People’s Hospital, Changsha, Hunan, China
Early and accurate diagnosis of prostate cancer is critical for optimizing patient prognosis. However, traditional transrectal ultrasound-guided systematic biopsy (TRUS-Bx) has a relatively high false-negative rate. This is attributed to limitations such as insufficient anatomical coverage and inadequate assessment of tumor heterogeneity. Multiparametric magnetic resonance imaging (mpMRI), when combined with the Prostate Imaging Reporting and Data System (PI-RADS), has substantially improved the diagnostic specificity of clinically significant prostate cancer (csPCa; Gleason grade ≥ 3 + 4). Nevertheless, its discriminatory ability for PI-RADS 3 lesions remains restricted. In recent years, multimodal image fusion technology has boosted the detection rate of csPCa by 10%-15% via precise lesion localization. Molecular imaging exhibits a sensitivity of up to 95% (range: 90-98%) in the whole-body staging of high-risk patients, particularly for nodal metastases. Artificial intelligence (AI), through deep-learning algorithms, optimizes lesion segmentation and image texture analysis, thereby significantly enhancing the detection rate of csPCa in targeted biopsies. Looking ahead, it is essential to integrate multimodal imaging and genomic data, construct individualized risk-stratification models, and facilitate the clinical translation of low-cost and standardized technologies. This article comprehensively examines the synergistic mechanisms of imaging and AI technologies in the diagnosis and biopsy guidance of prostate cancer, offering a theoretical foundation for precision medicine practice.
1 Introduction
PCa is the second most prevalent malignant tumor among men globally, accounting for approximately 14.2% (1). Early and accurate diagnosis of prostate cancer is a pivotal aspect in enhancing prognosis (2). At present, prostate biopsy remains the “gold standard” for diagnosing prostate cancer. However, traditional systematic biopsy techniques are characterized by both a high false-negative rate and overdiagnosis, defined as the detection of indolent cancers that may not progress, potentially leading to unnecessary treatment. This is due to insufficient anatomical coverage and limitations in evaluating tumor heterogeneity. Additionally, these techniques are associated with risks such as infection and bleeding (2). Notably, for patients under active surveillance, false-negative results may delay the treatment opportunity and increase the reclassification risk (10%-25%) (3).
The advent of multiparametric magnetic resonance imaging (mpMRI) represents a significant breakthrough in image-guided techniques. When integrated with the PI-RADS, it can substantially improve the diagnostic specificity of clinically significant prostate cancer (csPCa, Gleason grade ≥ 3 + 4) to approximately 0.83 (range: 0.76-0.89) in high-risk cohorts, compared to historical values around 0.248 (4). However, mpMRI has restricted discriminatory capacity for PI-RADS 3 lesions, with a false-negative rate of 20% (2). Consequently, multimodal image fusion technology has substantially optimized the detection efficiency through precise lesion localization. Molecular imaging modalities, such as prostate- specific membrane antigen positron emission tomography/computed tomography (PSMA PET/CT), through the quantification of tumor metabolic heterogeneity, provide high-sensitivity support for the staging of high-risk patients (5). Moreover, artificial intelligence-driven image analysis techniques, by integrating multimodal data (radiomics, genomics, clinical parameters), construct individualized prediction models, gradually attaining optimization of the entire process from diagnosis to prognosis (6).
This article comprehensively reviews the progress in the application of imaging techniques and artificial intelligence in prostate biopsy, analyzes their synergistic mechanisms, and explores the future directions of development.
Contribution of this review: This narrative review synthesizes the latest evidence on imaging and AI technologies in prostate biopsy, highlights their synergistic mechanisms, and proposes future directions for integrating multimodal data into clinical practice. It serves as a comprehensive reference for urologists and radiologists seeking to implement precision biopsy strategies.
2 Limitations of traditional prostate biopsy techniques
2.1 Traditional technique: transrectal ultrasound-guided systematic biopsy
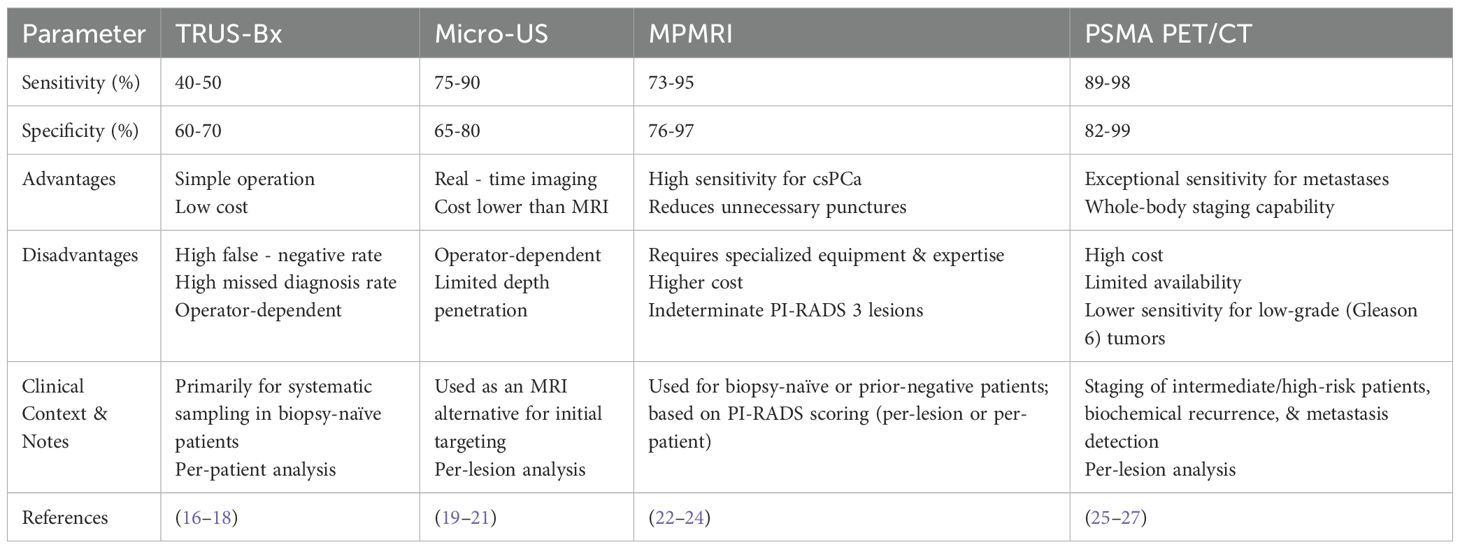
The cornerstone of traditional prostate biopsy is transrectal ultrasound-guided systematic biopsy (TRUS-Bx). This approach utilizes transrectal ultrasound to localize the prostate and, following a standardized protocol, systematically samples from regions such as the peripheral zone and the middle lobe of the prostate (typically with 10–12 needles). The objective is to cover the potentially cancer-prone areas of the prostate through randomly distributed tissue cores (7). For a long time, its ease of operation and relatively low cost have led to its being regarded as the “gold standard” for prostate cancer diagnosis (7). Nevertheless, as clinical evidence has accumulated, the limitations of TRUS-Bx have gradually become more pronounced. Traditional systematic biopsy is associated with a high false-negative rate (10–30% for csPCa) and overdiagnosis, defined as the detection of indolent cancers (e.g., Gleason score 6) that may not progress, potentially leading to unnecessary treatment. In contrast, oversampling refers to excessive biopsy cores that do not improve detection. These rates vary by clinical context (e.g., biopsy-naïve vs. repeat biopsy, transrectal vs. transperineal approach) (8–10). The limitations are primarily manifested in three aspects: inadequate anatomical coverage, limited detection efficiency, and suboptimal assessment of tumor heterogeneity.
2.2 Limitation analysis
2.2.1 Insufficient anatomical coverage and lesion missed diagnosis
Prostate-specific studies have quantified the risk of missing anterior tumors. In patients with low-risk prostate cancer, up to 16% of csPCa lesions are located in the anterior part and are frequently missed by standard TRUS-Bx templates (11). Importantly, this risk is context-dependent. While expanding the biopsy scope to include anterior sampling can increase the detection rate of csPCa, the net benefit varies. For instance, in the biopsy-naïve population, anterior sampling may increase the detection rate of csPCa by approximately 5.7% (p=0.09, not statistically significant in that cohort), whereas in men on active surveillance (AS) with prior negative biopsies, the incremental yield can be higher, underscoring the need for risk-adapted sampling strategies (11).
2.2.2 Bottleneck in the detection rate of random sampling
Traditional techniques rely on random sampling, which makes it arduous to effectively detect tumors that are small in volume (<0.5 cm³) or have an atypical distribution. The random sampling nature of TRUS-Bx creates a significant detection bottleneck, particularly for small or atypically located tumors. The false-negative rate is not uniform across all patient groups. For example, in patients under AS, a 12-core systematic biopsy may miss approximately 10% of csPCa (12). Notably, the reclassification risk following a negative biopsy is a key metric of this limitation. Cohort studies focused on AS populations report that patients with an initial negative biopsy harbor a 10%-25% risk of being reclassified to higher-risk disease upon subsequent surveillance biopsies, a figure directly attributable to sampling error and tumor multifocality (13). This phenomenon is closely associated with the multifocality and spatial heterogeneity of tumors (13).
2.2.3 Inadequate assessment of tumor biological heterogeneity
The limited number of biopsy samples (usually 12 cores) may not comprehensively represent the genomic diversity of tumors. For instance, the correlation between the genomic risk score of low-risk patients and postoperative pathological upgrading and biochemical recurrence suggests that traditional biopsies may underestimate tumor aggressiveness (14). Furthermore, the Gleason scoring system’s disregard for minor Gleason 5 components can impact the accuracy of prognostic assessment (15).
2.2.4 Operator dependence and standardization variations
The operator-dependent nature of TRUS-Bx is a well-documented limitation. Discrepancies in the definition of the ‘standard 12-core’ distribution among different institutions (e.g., inclusion of anterior or apical sampling) contribute significantly to inter-institutional variability in detection rates and limit result consistency (7). Furthermore, the reliance on ultrasound alone (resolution ~1–2 mm) for targeting is a fundamental constraint. Studies quantifying operator performance suggest that insufficient experience can reduce the detection rate of csPCa by a relative margin of up to 15-20% compared to expert operators, highlighting the critical impact of expertise on procedural efficacy (12).
2.3 Clinical impact and improvement directions
The aforementioned limitations directly influence the accuracy of clinical decision-making. Missed diagnosis of anterior cancer may misclassify low-risk patients as “benign” or “very low-risk,” thereby delaying the opportunity for radical treatment (11). False-negative results may extend the monitoring period, increasing patients’ psychological burden and the risk of complications associated with repeated biopsies (13). Misjudgment of tumor heterogeneity can interfere with the formulation of genomic risk stratification and personalized treatment decisions (such as the choice between AS and aggressive treatment) (14, 15).
To overcome the bottleneck of traditional techniques, targeted biopsy guided by new imaging techniques has significantly enhanced the detection rate of anterior and high-risk lesions through image-pathology fusion techniques (the detection rate of csPCa has increased by 10%-15%) (7, 12). Additionally, expanding the number of cores (such as 24-core extensive biopsy) combined with anterior sampling has also been shown to optimize the detection rate, but considerations such as an increased risk of infection need to be taken into account (11). In the future, it will be essential to further integrate imaging and genomic data to develop a precise sampling strategy for more individualized risk stratification and management. A comparative analysis of prostate biopsies that are guided by various imaging techniques is presented in Table 1.
3 Innovation and clinical application of imaging technologies
3.1 Ultrasound technologies: from conventional ultrasound to micro-ultrasound
TRUS-Bx is plagued by low sensitivity to early cancerous lesions. The advent of micro-ultrasound technology has substantially enhanced the detection capacity of blood flow signals.
3.1.1 Super-microvascular imaging
Super-microvascular imaging (SMI) is capable of real-time visualization of abnormal microvessels within the prostate (such as tortuous and increased branching patterns), facilitating the identification of suspicious areas and guiding targeted biopsy. Studies have indicated that, in comparison to traditional color Doppler flow imaging (CDFI) and power Doppler ultrasound (PDUS), SMI exhibits a greater aptitude for detecting low-velocity blood flow and can more sensitively discern the neovascularization signals of tumors <mark>in the prostate (28). Prostate biopsy guided by SMI can significantly elevate the positive rate of tissue sampling (29). When integrated with other techniques like ultrasound elastography, SMI can dynamically monitor changes in blood flow signals, optimize the puncture trajectory, and mitigate accidental damage to normal blood vessels (30). SMI’s ability to display microcirculation without the need for contrast agent injection circumvents contrast-related risks, rendering it particularly suitable for patients with renal insufficiency.
3.1.2 Contrast-enhanced ultrasound
Through the utilization of specific contrast agents, contrast-enhanced ultrasound (CEUS) can generate high-resolution images of tissue microvessels, enabling the observation of lesion characteristics, such as those of tumors and inflammations. Prostate cancer demonstrates rapid and high-intensity contrast enhancement attributed to neovascularization. CEUS can precisely delineate the tumor boundary and direct targeted biopsy (31, 32). Research reveals that targeted biopsy guided by CEUS has a 15%-20% higher cancer detection rate compared to systematic biopsy (31), while simultaneously reducing the number of unnecessary biopsies.
3.2 Magnetic resonance technologies: from biparametric to multiparametric
Biparametric magnetic resonance imaging (bpMRI) and mpMRI each possess distinct advantages in clinical diagnosis.
3.2.1 Simplified efficacy of bpMRI
BpMRI encompasses only T2-weighted imaging (T2WI) and diffusion-weighted imaging (DWI)/apparent diffusion coefficient (ADC) sequences, excluding the dynamic contrast-enhanced (DCE) sequence. This not only substantially shortens the examination duration and reduces costs by approximately 50% but also eliminates risks associated with contrast agents (such as allergies, renal impairment, etc.) (33). Multiple meta-analyses indicate that the sensitivity (0.74-0.79 vs. 0.76-0.84) and specificity (0.88-0.90 vs. 0.89-0.89) of bpMRI and mpMRI are comparable, with no statistically significant difference (22, 23). Moreover, outside the prostate-specific antigen (PSA) range of 10–20 ng/ml, there is no marked difference in the cancer detection rates between bpMRI and mpMRI (24). Nevertheless, its sensitivity to certain lesions (such as tumors in the transition zone) may be lower than that of mpMRI, particularly when differentiating PI-RADS 3–4 lesions (33).
3.2.2 Comprehensiveness of MpMRI
By integrating T2WI, DWI, DCE, and magnetic resonance spectroscopy (MRS), MpMRI can more comprehensively assess prostate lesions, including complex manifestations such as seminal vesicle invasion and pelvic lymph node metastasis. Studies demonstrate that the predictive accuracy of mpMRI for prostate extracapsular extension reaches 89%, significantly superior to traditional imaging (34). Its targeted biopsy (TB) in combination with ultrasound fusion exhibits higher sensitivity (81%-86%) and specificity (69%-84%) in the detection of csPCa, especially in lesions with PI-RADS ≥ 4, where its diagnostic efficacy is markedly better than that of traditional 12-core systematic biopsy (SB) (35–37). Combining TB and SB can further optimize the detection rate, particularly for multifocal lesions or in the anterior prostate region, with an incremental csPCa detection rate of 10%-15% (37–39). It is noteworthy that approximately 19%-31% of csPCa are detected solely in the second or third targeted biopsy, suggesting the necessity of a multi-core sampling strategy (36). Although several studies have shown that the sensitivity and specificity of bpMRI and mpMRI in detecting prostate cancer are marginally different, mpMRI demonstrates higher diagnostic accuracy in certain cases, especially in the detection of csPCa, where its sensitivity is significantly greater than that of bpMRI (22). Additionally, the enhanced imaging capabilities of mpMRI facilitate more precise prostate cancer localization, thereby reducing the false-positive rate (33). When the mpMRI result is negative, the negative predictive value for significant prostate cancer is as high as 95% (40). In AS, mpMRI can detect tumor progression (such as a volume change of ≥ 50%) at an earlier stage and guide the appropriate timing of treatment (41).
PI-RADS, through the interpretation of standard mpMRI images, has significantly enhanced the detection efficiency of csPCa. Research has validated that PI-RADS 4–5 lesions are strongly correlated with csPCa, with positive predictive values (PPV) of up to 48.1% and 68.3% respectively (42, 43). However, the clinical management of PI-RADS 3 lesions remains a subject of controversy, with a csPCa detection rate of only 12.5%-20.8%. Individualized decisions should be made by incorporating high-risk factors such as prostate-specific antigen density (PSAD > 0.15 ng/ml/cm³) or abnormal digital rectal examination (44, 45). It is important to note that even though PI-RADS 5 lesions have a high predictive value, 18% of cases yield benign pathological results following targeted biopsy, suggesting that integration of other imaging or molecular markers is necessary to optimize diagnostic efficacy (46, 47). In response to the clinical challenges posed by PI-RADS 3 lesions, the latest guidelines advocate a dynamic risk-stratification strategy: for single-focus lesions with a low PSAD (PSAD < 0.12 ng/ml/cm³), short-term imaging follow-up can be implemented, while for multiple-focus lesions or those with high-risk factors, MRI-ultrasound fusion-guided targeted biopsy is recommended (44, 45, 48). Moreover, elastography technology, by quantifying tissue hardness disparities, can assist in locating sclerotic areas not visualized by MRI. When combined with mpMRI, it can increase the csPCa detection rate by 8%-12%, particularly providing supplementary value in transition-zone lesions (45, 46).
3.3 Molecular imaging breakthrough of PSMA PET/CT
3.3.1 Precise targeting and tumor heterogeneity assessment of PSMA PET/CT
PSMA PET/CT enables highly sensitive detection (sensitivity 90-98%, specificity 82%-99%) of prostate cancer lesions by targeting the expression of the PSMA protein (49, 50). It can also furnish information regarding the body-wide distribution of tumors, assisting doctors in comprehensively evaluating disease spread and thereby formulating more precise treatment plans (51, 52). Studies have indicated that PSMA PET/CT demonstrates high sensitivity and specificity in identifying local and distant metastases (49, 53), particularly in high-risk prostate cancer patients, where its diagnostic accuracy surpasses that of traditional imaging methods (CT and MRI) (54). The maximum standardized uptake value (SUVmax) of PSMA PET/CT is significantly positively correlated with tumor aggressiveness (such as Gleason score, pathological stage) (p=0.007) (55, 56). In instances where mpMRI results are negative or equivocal, PSMA PET/CT can function as an effective supplementary tool.
3.3.2 Challenges of PSMA-negative tumors and exploration of new targets
Approximately 5%-10% of prostate cancers (notably those with neuroendocrine differentiation) display low PSMA expression (SUVmax < 10), and a missed diagnosis could potentially delay treatment (57, 58). For such cases, alternative biomarkers like KLF8, CHST11, or functional imaging (such as FDG-PET) must be incorporated (56, 57). Research reveals that the combined use of mpMRI and PSMA PET can elevate the negative predictive value (NPV) of biopsy to 96%, diminishing the necessity for systematic biopsy (58). The detection rate of PSMA-PET for tumors with a high Gleason score (≥8) is markedly higher than that for tumors with a low score (90% vs 60%), yet its sensitivity to Gleason 6 tumors is inadequate (<50%) (57, 58). Additionally, antibody probes targeting KLF8 have manifested potential for specific binding to poorly differentiated tumors in preclinical studies, and future endeavors are required to facilitate their clinical translation (57).
4 AI-driven multimodal image fusion and target region identification
4.1 Artificial intelligence and image analysis technologies
4.1.1 Lesion segmentation based on deep learning
AI, leveraging deep-learning algorithms, has empowered automated analysis of prostate imaging data and extraction of quantitative features, thereby significantly enhancing the accuracy and efficiency of lesion localization. AI models, such as U-Net and nnUNet, are capable of automatically segmenting prostate anatomical structures and suspicious lesions by analyzing mpMRI and PET images (59). For instance, FocalNet, through the integration of convolutional neural network and Gleason score data, has accomplished joint detection of prostate cancer and prediction of its aggressiveness. Its sensitivity and specificity in the detection of clinically significant prostate cancer attain 89.7% and 87.9% respectively (60). In contrast, traditional image segmentation hinges on manual delineation, which is not only time-consuming but also prone to subjective bias. Deep-learning models can mitigate the subjective discrepancies among radiologists and enhance the consistency of segmentation results. For example, a study encompassing 976 cases demonstrated that the consistency between the AI segmentation model based on ADC maps and the manual annotations of multiple radiologists reached 0.96 (95% CI 0.95-0.97) (61).
4.1.2 Quantification of image texture features
Subsequent to lesion segmentation, the technique of image texture feature quantification can be further employed to conduct a more in-depth analysis of the segmented lesion area. Texture feature quantification has the capacity to capture subtle alterations in the lesion area, such as cell arrangement and blood vessel distribution. This information aids in the evaluation of the malignancy and aggressiveness of the lesion. For example, regarding PI-RADS 3 lesions, the AI classification model founded on the texture features of T2-weighted images exhibits a sensitivity and specificity of 83% and 96% respectively for csPCa, markedly outperforming traditional visual assessment (Area Under Curve [AUC] 0.89 vs 0.72, p < 0.001) (62). Three-dimensional morphological analysis techniques, like light-sheet microscopy, further augment the model’s ability to capture heterogeneous structures and enhance diagnostic robustness (63).
4.2 Multimodal image fusion
Multimodal image fusion technology significantly enhances the precision and efficiency of prostate biopsy by integrating the complementary advantages of MRI, US, and CT.
4.2.1 MRI-TRUS fusion
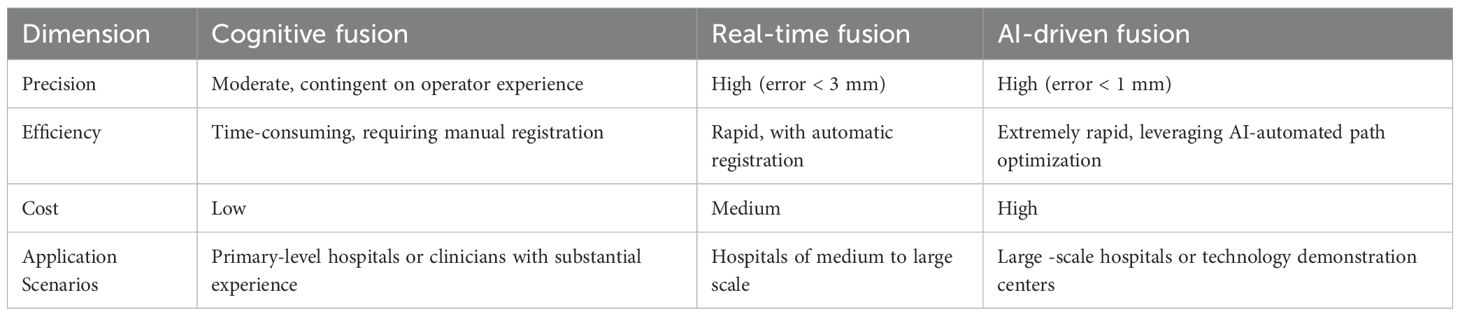
By integrating the high-resolution anatomical information of MRI with the real-time navigation functionality of ultrasound, a three-dimensional model is constructed to facilitate puncture positioning. Targeted biopsy using MRI-TRUS fusion in conjunction with systematic biopsy (TB + SB) can significantly boost the detection rate of csPCa. Compared to traditional 12-core TRUS-Bx, fusion biopsy has demonstrated superiority in large-scale clinical trials such as PRECISION and PRECISE, particularly in the detection of high-risk tumors with an International Society of Urological Pathology (ISUP) grade of ≥ 2 (16–18). Prospective multicenter trials have revealed that mpMRI combined with TRUS fusion biopsy yields a significantly higher csPCa detection rate than simple systematic biopsy. The biopsy positive rate is increased by 20%-30%, while the overdiagnosis of low-risk tumors is reduced (64–66). In comparison to the transrectal approach, transperineal MRI-TRUS fusion biopsy exhibits a lower infection complication rate (0.8% vs. 3.5%) (2, 67), and antibiotic use is more standardized. Although pain and anxiety persist in fusion biopsy, advancements in electromagnetic tracking and local anesthesia techniques (such as the Vector electromagnetic needle tracking system) have improved patient tolerance (68, 69). A comparative analysis of different fusion methods of MRI - TRUS is presented in Table 2.
4.2.2 Exploration of fusion of other imaging technologies
PSMA-PET/CT and Ultrasound Fusion: PSMA-PET/CT can specifically identify the molecularly metabolically active regions of prostate cancer lesions, while ultrasound offers real-time anatomical guidance. The integration of these two modalities combines highly sensitive molecular imaging with real-time puncture navigation, significantly increasing the detection rate of csPCa (70–72). PSMA-PET/CT can also pinpoint multifocal or occult lesions, thereby avoiding blind punctures in non-metabolically active areas. For instance, in PSMA-PET-negative areas, the csPCa missed diagnosis rate is merely 5%-8% (25, 73). whereas the missed diagnosis rate of TRUS-Bx is as high as 20%-30% (71).
PET and MRI Fusion: The PET-MRI fusion technology combines the functional metabolic information of PET with the anatomical structure information of MRI, surmounting the limitations of single-technology approaches. By simultaneously acquiring metabolic and anatomical data, doctors can more comprehensively assess the location, size, and activity of lesions. For example, PSMA-PET/MRI can identify metabolically active lesions that MRI might overlook, and MRI can supply anatomical details to aid in targeted biopsy (74, 75).
4.3 Target region identification
In cognitive fusion, doctors visually incorporate the information of suspicious lesions in mpMRI images (such as those with a PI-RADS score ≥ 3), subjectively localize them under the guidance of TRUS, and subsequently direct the biopsy. Cognitive fusion does not necessitate special software or equipment; rather, it only requires a conventional TRUS biopsy system (17, 76). This approach circumvents the costs associated with developing or procuring complex image fusion algorithms (77, 78). Nevertheless, there are substantial disparities among different physicians in the identification and spatial registration of MRI lesions, leading to low detection consistency (77, 79). If the surgeon has not undergone professional MRI image-reading training or solely relies on the imaging report without independently assessing the images, inexperienced doctors may overlook small lesions or misinterpret the lesion boundaries, significantly augmenting the risk of biopsy errors (76, 78). Studies have indicated that approximately 5%-13% of csPCa might be undetected in cognitive targeted biopsies, and systematic biopsy must be combined to enhance sensitivity (69, 80).
AI software fusion: Deep-learning algorithms, like convolutional neural networks, are utilized to automatically delineate the prostate region and lesions within mpMRI or bpMRI. Coupled with elastic registration technology, the MRI and TRUS images are precisely merged to accomplish automatic target-region marking and navigation. AI algorithms mitigate the variability of human interpretation, particularly demonstrating stable performance in multi-center and multi-scanner scenarios (79, 81). Automated segmentation and registration decrease the reliance on physician experience and shorten the learning curve (82, 83). AI models can optimize the interpretation of bpMRI (T2WI + DWI) to reduce costs while sustaining accuracy. For instance, the bpMRI AI model maintained a PPV comparable to that of mpMRI in external validation (79, 84). Compared to cognitive fusion technology, targeted biopsy guided by AI software (such as Biopsee, UroNav) elevates the csPCa detection rate by approximately 10%-15% and exhibits a greater capacity to distinguish PI-RADS 3-score lesions (77, 85, 86). AI software fusion is characterized by high precision and a low learning threshold, yet the equipment cost is relatively high, rendering it suitable for centers that prioritize standardized diagnosis (82, 87). In the foreseeable future, AI-assisted prostate biopsy will realize a fully automated closed-loop process encompassing pre-operative, intra-operative, and post-operative phases. Specifically, during the pre-operative phase, state-of-the-art AI algorithms will automatically analyze DICOM imaging data to generate a personalized biopsy plan. This plan will specify the optimal number of needles, precise puncture paths, and potential risk warnings based on anatomical landmarks and lesion localization. In the intra-operative stage, a robotic system will execute the biopsy procedure, whereas AI-driven real - time tracking will compensate for spatial deviations caused by respiratory motion or patient body movements. This will ensure millimeter-level accuracy through dynamic path correction algorithms. Post-procedure, the system will autonomously generate a comprehensive structured clinical report that integrates histopathological findings with quantitative imaging biomarkers. Supported by AI-powered analysis, the report will recommend patient-specific follow-up intervals aligned with clinical guidelines. This end-to-end automation framework aims to standardize biopsy workflows, minimize operator dependency, enhance diagnostic consistency in prostate cancer management, thereby improving the overall quality of prostate cancer diagnosis and treatment. As depicted in Figure 1, a schematic diagram illustrates the specific biopsy procedure in detail.
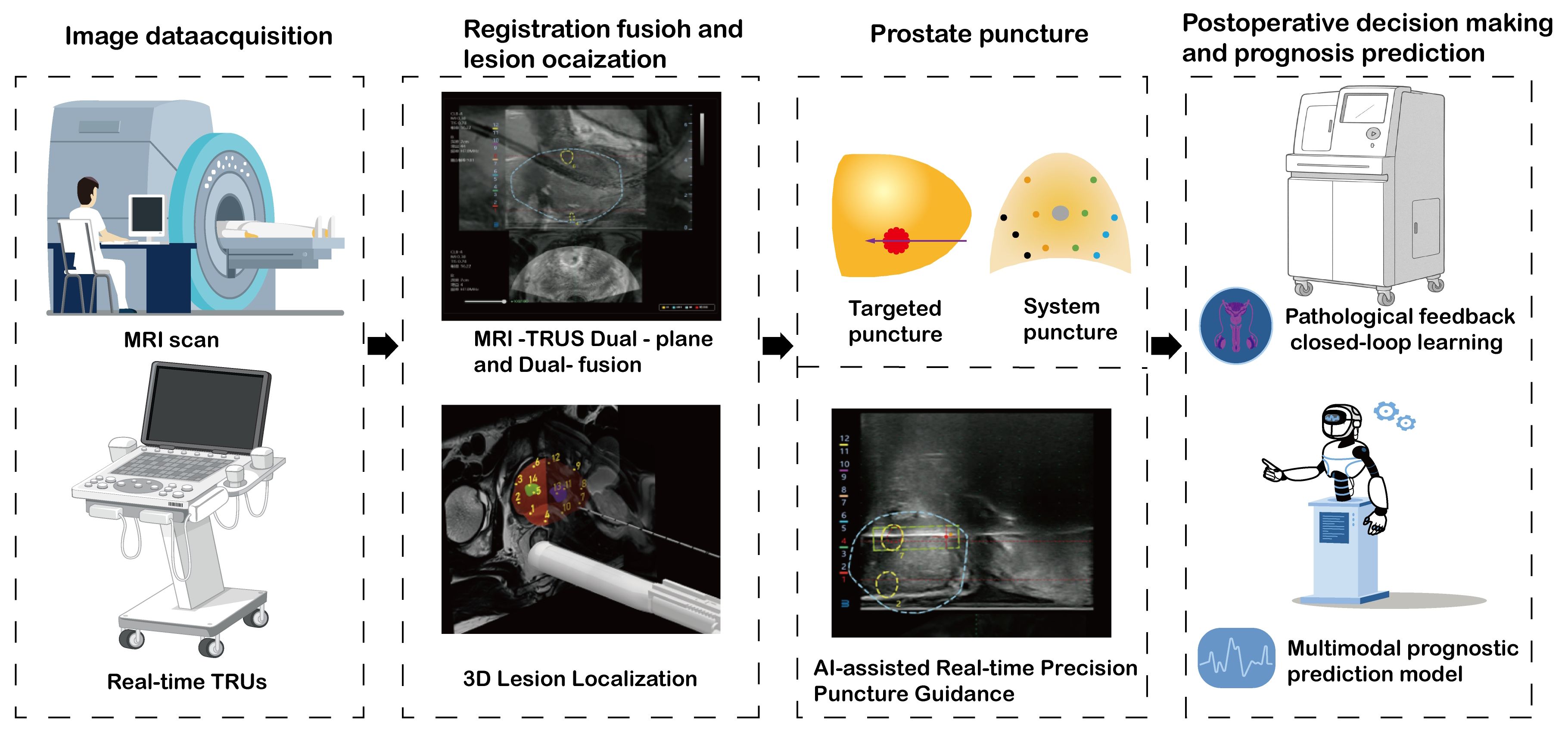
Figure 1. A comprehensive workflow of artificial intelligence-assisted prostate biopsy: from image acquisition to prognosis prediction.
4.4 Limitations and challenges of AI in prostate biopsy
Despite promising results, the widespread adoption of AI-driven biopsy systems faces several challenges:
Generalizability: AI models trained on single-center data may underperform in external validation due to variations in MRI scanners, protocols, and patient populations (88). Data Dependency: High-quality annotated datasets are scarce, limiting the development of robust models (89, 90). Cost and Accessibility: AI software and robotic biopsy systems require significant investment, hindering deployment in resource-limited settings. Future efforts should focus on multi-center collaborations, federated learning, and cost-effective AI solutions to enhance accessibility (89, 91).
5 Conclusion
The collaborative utilization of imaging technologies and artificial intelligence has substantially enhanced the precision of prostate cancer biopsy and diagnosis. However, these precision diagnostic tools directly influence treatment decisions, such as qualifying patients for active surveillance or guiding focal therapy. The combination of MRI-TURS targeted biopsy and systematic biopsy can effectively lower the missed-diagnosis rate. Meanwhile, PSMA PET/CT compensates for the limitations of traditional imaging by providing molecular metabolic information. AI technology, via automated segmentation, texture-feature quantification, and multimodal data fusion, has decreased operator dependence. Nevertheless, its clinical dissemination is constrained by equipment costs and the generalization ability of algorithms. Future research directions should center on:
1. Technology Integration and Standardization: Promote the in-depth integration of multimodal imaging (MRI, PET, ultrasound) with genomic data to construct individualized risk-prediction models. Develop cross-platform AI algorithms and enhance the robustness and clinical applicability of these models through multi-center validation.
2. Optimization of Precise Diagnosis: For PI-RADS 3 lesions, create dynamic risk-stratification AI tools and achieve precise differentiation by integrating molecular markers and radiomic features. Explore novel molecular probes for PSMA-negative tumors and multimodal imaging complementary strategies.
3. Clinical Translation and Accessibility: Simplify the AI-assisted diagnosis procedure and reduce hardware reliance. Promote the low-cost screening approach of bpMRI combined with AI to facilitate the dissemination of this technology in resource-constrained regions.
Through interdisciplinary collaboration and technological evolution, the collaborative application of imaging and AI will propel the diagnosis and treatment of prostate cancer toward intelligent whole-process management, ultimately leading to a comprehensive improvement in patient prognosis and efficient utilization of medical resources.
Author contributions
YW: Investigation, Writing – review & editing, Formal Analysis, Writing – original draft, Conceptualization. QL: Writing – review & editing, Formal Analysis, Investigation, Writing – original draft, Conceptualization. ZL: Writing – review & editing, Writing – original draft. JW: Writing – original draft, Writing – review & editing. XH: Writing – original draft, Writing – review & editing. YY: Writing – review & editing, Writing – original draft. YL: Funding acquisition, Writing – review & editing, Writing – original draft.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This article was supported by National Key Clinical Specialty Scientific Research Project (grant number: Z2023088).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
PI-RADS, Prostate Imaging Reporting and Data System; AI, Artificial intelligence; csPCa, Clinically significant prostate cancer; MpMRI, Multiparametric magnetic resonance imaging; PSMA, Prostate-Specific Membrane Antigen; PET, Positron Emission Tomography; CT, Computed Tomography; TRUS – Bx, Transrectal Ultrasound-Guided Systematic Biopsy; AS, Active surveillance; SMI, Super-Microvascular Imaging; CDFI, Color Doppler flow imaging; PDUS, Power Doppler ultrasound; CEUS, Contrast-Enhanced Ultrasound; BpMRI, Biparametric magnetic resonance imaging; T2WI, T2-weighted imaging; DWI, Diffusion-weighted imaging; ADC, Apparent diffusion coefficient; DCE, Dynamic contrast – enhanced; PSA, Prostate-specific antigen; MRS, Magnetic resonance spectroscopy; TB, Targeted biopsy; SB, Systematic biopsy; PPV, Positive predictive values; PSAD, Prostate-specific antigen density; SUVmax, Maximum standardized uptake value; NPV, Negative predictive value; AUC, Area Under Curve; ISUP, International Society of Urological Pathology, DICOM, Digital Imaging and Communications in Medicine.
References
1. Jokhadze N, Das A, and Dizon DS. Global cancer statistics: A healthy population relies on population health. CA Cancer J Clin. (2024) 74:224–6. doi: 10.3322/caac.21838
2. Romero-Selas E, Cuadros V, Montans J, Sanchez E, Lopez-Alcorocho JM, and Gomez-Sancha F. Transperineal prostate biopsy with echo-mri fusion. Biopsee system. Initial experience. Actas Urol Esp. (2016) 40:295–302. doi: 10.1016/j.acuro.2015.12.002
3. Peters M, Eldred-Evans D, Kurver P, Falagario UG, Connor MJ, Shah TT, et al. Predicting the need for biopsy to detect clinically significant prostate cancer in patients with a magnetic resonance imaging-detected prostate imaging reporting and data system/likert >/=3 lesion: development and multinational external validation of the imperial rapid access to prostate imaging and diagnosis risk score. Eur Urol. (2022) 82:559–68. doi: 10.1016/j.eururo.2022.07.022
4. Choi MH, Ha US, Park YH, Hong SH, Lee JY, Lee YJ, et al. Combined mri and psa strategy improves biopsy decisions compared with psa only: longitudinal observations of a cohort of patients with a psa level less than 20 ng/ml. Acad Radiol. (2023) 30:509–15. doi: 10.1016/j.acra.2022.07.020
5. Wong LM, Koschel S, Whish-Wilson T, Farag M, Bolton D, Zargar H, et al. Investigating psma-pet/ct to resolve prostate mri pirads4–5 and negative biopsy discordance. World J Urol. (2023) 41:463–9. doi: 10.1007/s00345-022-04243-5
6. Li J, Wang K, Li S, Wu P, Wang X, He Y, et al. Clinical study of multifactorial diagnosis in prostate biopsy. Prostate. (2023) 83:1494–503. doi: 10.1002/pros.24608
7. Alberts AR, Roobol MJ, Verbeek JFM, Schoots IG, Chiu PK, Osses DF, et al. Prediction of high-grade prostate cancer following multiparametric magnetic resonance imaging: improving the rotterdam european randomized study of screening for prostate cancer risk calculators. Eur Urol. (2019) 75:310–8. doi: 10.1016/j.eururo.2018.07.031
8. Desmond C, Kaul S, Fleishman A, Korets R, Chang P, Wagner A, et al. The association of patient and disease characteristics with the overtreatment of low-risk prostate cancer from 2010 to 2016. Prostate Cancer Prostatic Dis. (2025) 28:385–93. doi: 10.1038/s41391-024-00822-2
9. Baboudjian M, Diamand R, Uleri A, Beauval JB, Touzani A, Roche JB, et al. Does overgrading on targeted biopsy of magnetic resonance imaging-visible lesions in prostate cancer lead to overtreatment? Eur Urol. (2024) 86:232–7. doi: 10.1016/j.eururo.2024.02.003
10. Chandra Engel J, Eklund M, Jäderling F, Palsdottir T, Falagario U, Discacciati A, et al. Diagnostic effects of omitting systematic biopsies in prostate cancer screening. Eur Urol Oncol. (2025) 8:435–43. doi: 10.1016/j.euo.2024.10.002
11. Bratt O, Holmberg E, Andrén O, Carlsson S, Drevin L, Johansson E, et al. The value of an extensive transrectal repeat biopsy with anterior sampling in men on active surveillance for low-risk prostate cancer: A comparison from the randomised study of active monitoring in Sweden (Sams). Eur Urol. (2019) 76:461–6. doi: 10.1016/j.eururo.2019.02.035
12. Klotz L, Loblaw A, Sugar L, Moussa M, Berman DM, van der Kwast T, et al. Active surveillance magnetic resonance imaging study (Asist): results of a randomized multicenter prospective trial. Eur Urol. (2019) 75:300–9. doi: 10.1016/j.eururo.2018.06.025
13. Kearns JT, Faino AV, Newcomb LF, Brooks JD, Carroll PR, Dash A, et al. Role of surveillance biopsy with no cancer as a prognostic marker for reclassification: results from the canary prostate active surveillance study. Eur Urol. (2018) 73:706–12. doi: 10.1016/j.eururo.2018.01.016
14. Cooperberg MR, Erho N, Chan JM, Feng FY, Fishbane N, Zhao SG, et al. The diverse genomic landscape of clinically low-risk prostate cancer. Eur Urol. (2018) 74:444–52. doi: 10.1016/j.eururo.2018.05.014
15. Sauter G, Clauditz T, Steurer S, Wittmer C, Büscheck F, Krech T, et al. Integrating tertiary gleason 5 patterns into quantitative gleason grading in prostate biopsies and prostatectomy specimens. Eur Urol. (2018) 73:674–83. doi: 10.1016/j.eururo.2017.01.015
16. Schlemmer HP, Krause BJ, Schütz V, Bonekamp D, Schwarzenböck SM, and Hohenfellner M. Imaging of prostate cancer. Dtsch Arztebl Int. (2021) 118:713–9. doi: 10.3238/arztebl.m2021.0309
17. Klotz L, Chin J, Black PC, Finelli A, Anidjar M, Bladou F, et al. Comparison of multiparametric magnetic resonance imaging-targeted biopsy with systematic transrectal ultrasonography biopsy for biopsy-naive men at risk for prostate cancer: A phase 3 randomized clinical trial. JAMA Oncol. (2021) 7:534–42. doi: 10.1001/jamaoncol.2020.7589
18. Kasivisvanathan V, Wai-Shun Chan V, Clement KD, Levis B, Ng A, Asif A, et al. Vision: an individual patient data meta-analysis of randomised trials comparing magnetic resonance imaging targeted biopsy with standard transrectal ultrasound guided biopsy in the detection of prostate cancer. Eur Urol. (2024). doi: 10.1016/j.eururo.2024.08.022
19. Rodríguez Socarrás ME, Gomez Rivas J, Cuadros Rivera V, Reinoso Elbers J, Llanes González L, Michel Mercado I, et al. Prostate mapping for cancer diagnosis: the madrid protocol. Transperineal prostate biopsies using multiparametric magnetic resonance imaging fusion and micro-ultrasound guided biopsies. J Urol. (2020) 204:726–33. doi: 10.1097/ju.0000000000001083
20. Kinnaird A, Luger F, Cash H, Ghai S, Urdaneta-Salegui LF, Pavlovich CP, et al. Microultrasonography-guided vs mri-guided biopsy for prostate cancer diagnosis: the optimum randomized clinical trial. Jama. (2025). doi: 10.1001/jama.2025.3579
21. Sountoulides P, Pyrgidis N, Polyzos SA, Mykoniatis I, Asouhidou E, Papatsoris A, et al. Micro-ultrasound-guided vs multiparametric magnetic resonance imaging-targeted biopsy in the detection of prostate cancer: A systematic review and meta-analysis. J Urol. (2021) 205:1254–62. doi: 10.1097/ju.0000000000001639
22. Liang Z, Hu R, Yang Y, An N, Duo X, Liu Z, et al. Is dynamic contrast enhancement still necessary in multiparametric magnetic resonance for diagnosis of prostate cancer: A systematic review and meta-analysis. Transl Androl Urol. (2020) 9:553–73. doi: 10.21037/tau.2020.02.03
23. Pesapane F, Acquasanta M, Meo RD, Agazzi GM, Tantrige P, Codari M, et al. Comparison of sensitivity and specificity of biparametric versus multiparametric prostate mri in the detection of prostate cancer in 431 men with elevated prostate-specific antigen levels. Diagnostics (Basel). (2021) 11. doi: 10.3390/diagnostics11071223
24. Pan Y, Shen C, Chen X, Cao D, Jiang J, Xu W, et al. Bpmri and mpmri for detecting prostate cancer: A retrospective cohort study. Front Surg. (2022) 9:1096387. doi: 10.3389/fsurg.2022.1096387
25. Kawada T, Yanagisawa T, Rajwa P, Sari Motlagh R, Mostafaei H, Quhal F, et al. Diagnostic performance of prostate-specific membrane antigen positron emission tomography-targeted biopsy for detection of clinically significant prostate cancer: A systematic review and meta-analysis. Eur Urol Oncol. (2022) 5:390–400. doi: 10.1016/j.euo.2022.04.006
26. Margel D, Bernstine H, Groshar D, Ber Y, Nezrit O, Segal N, et al. Diagnostic performance of (68)Ga prostate-specific membrane antigen pet/mri compared with multiparametric mri for detecting clinically significant prostate cancer. Radiology. (2021) 301:379–86. doi: 10.1148/radiol.2021204093
27. True LD and Chen DL. How accurately does psma inhibitor 18f-dcfpyl-pet-ct image prostate cancer? Clin Cancer Res. (2021) 27:3512–4. doi: 10.1158/1078-0432.Ccr-21-0749
28. McCall JR, Santibanez F, Belgharbi H, Pinton GF, and Dayton PA. Non-invasive transcranial volumetric ultrasound localization microscopy of the rat brain with continuous, high volume-rate acquisition. Theranostics. (2023) 13:1235–46. doi: 10.7150/thno.79189
29. Wang C and Tao Y. Superb microvascular imaging in guiding targeted biopsy of prostate cancer: A protocol for systematic review and meta analysis. Med (Baltimore). (2020) 99:e23604. doi: 10.1097/md.0000000000023604
30. Huang X, Ye H, Hu Y, Lei Y, Tian Y, Huang X, et al. Ultrasound super-resolution imaging for non-invasive assessment of microvessel in prostate lesion. Cancer Imaging. (2025) 25:1. doi: 10.1186/s40644-024-00819-z
31. Kaneko M, Lenon MSL, Storino Ramacciotti L, Medina LG, Sayegh AS, La Riva A, et al. Multiparametric ultrasound of prostate: role in prostate cancer diagnosis. Ther Adv Urol. (2022) 14:17562872221145625. doi: 10.1177/17562872221145625
32. Sparchez Z. Contrast enhanced ultrasound of the prostate. New role in the evaluation of loco-regional therapy of prostate tumors. Med Ultrason. (2018) 20:125–6. doi: 10.11152/mu-1536
33. Iacob R, Manolescu D, Stoicescu ER, Cerbu S, Bardan R, Ghenciu LA, et al. The diagnostic value of bpmri in prostate cancer: benefits and limitations compared to mpmri. Bioengineering (Basel). (2024) 11. doi: 10.3390/bioengineering11101006
34. Chen X, Li W, Yang J, Huang C, Zhou C, Chen Y, et al. Extracapsular extension of transitional zone prostate cancer miss-detected by multiparametric magnetic resonance imaging. J Cancer Res Clin Oncol. (2023) 149:6943–52. doi: 10.1007/s00432-023-04573-w
35. Febres-Aldana CA, Alghamdi S, Weppelmann TA, Lastarria E, Bhandari A, Omarzai Y, et al. Magnetic resonance imaging-ultrasound fusion-targeted biopsy combined with systematic 12-core ultrasound-guided biopsy improves the detection of clinically significant prostate cancer: are we ready to abandon the systematic approach? Urol Ann. (2020) 12:366–72. doi: 10.4103/ua.Ua_123_19
36. Tewes S, Peters I, Tiemeyer A, Peperhove M, Hartung D, Pertschy S, et al. Evaluation of mri/ultrasound fusion-guided prostate biopsy using transrectal and transperineal approaches. BioMed Res Int. (2017) 2017:2176471. doi: 10.1155/2017/2176471
37. Preisser F, Theissen L, Wenzel M, Humke C, Bodelle B, Köllermann J, et al. Performance of combined magnetic resonance imaging/ultrasound fusion-guided and systematic biopsy of the prostate in biopsy-naïve patients and patients with prior biopsies. Eur Urol Focus. (2021) 7:39–46. doi: 10.1016/j.euf.2019.06.015
38. In de Braekt T, van Rooij SBT, Daniels-Gooszen AW, Scheepens WA, de Jongh R, Bosch SL, et al. Accuracy of mri-ultrasound fusion-guided and systematic biopsy of the prostate. Br J Radiol. (2024) 97:1132–8. doi: 10.1093/bjr/tqae080
39. Sterling J, Smith K, Farber N, Nagaya N, Jang TL, Singer EA, et al. Fourteen-core systematic biopsy that includes two anterior cores in men with pi-rads lesion ≥ 3 is comparable with magnetic resonance imaging-ultrasound fusion biopsy in detecting clinically significant prostate cancer: A single-institution experience. Clin Genitourin Cancer. (2021) 19:275–9. doi: 10.1016/j.clgc.2020.09.006
40. Nazim SM, Ather MH, and Salam B. Role of multi-parametric (Mp) mri in prostate cancer. J Pak Med Assoc. (2018) 68:98–104.
41. Stabile A, Giganti F, Rosenkrantz AB, Taneja SS, Villeirs G, Gill IS, et al. Multiparametric mri for prostate cancer diagnosis: current status and future directions. Nat Rev Urol. (2020) 17:41–61. doi: 10.1038/s41585-019-0212-4
42. Yamaya N, Kimura K, Ichikawa R, Kawanishi M, Kawasaki Y, Higuchi S, et al. Prospective evaluation of pi-radsv2.1 using multiparametric and biparametric mri for detecting clinically significant prostate cancer based on mri/us fusion-guided biopsy. Jpn J Radiol. (2025) 43:472–82. doi: 10.1007/s11604-024-01675-4
43. Syed JS, Nguyen KA, Nawaf CB, Bhagat AM, Huber S, Levi A, et al. Prostate zonal anatomy correlates with the detection of prostate cancer on multiparametric magnetic resonance imaging/ultrasound fusion-targeted biopsy in patients with a solitary pi-rads V2-scored lesion. Urol Oncol. (2017) 35:542.e19–.e24. doi: 10.1016/j.urolonc.2017.04.011
44. Kwe J, Baunacke M, Boehm K, Platzek I, Thomas C, and Borkowetz A. Pi-rads upgrading as the strongest predictor for the presence of clinically significant prostate cancer in patients with initial pi-rads-3 lesions. World J Urol. (2024) 42:84. doi: 10.1007/s00345-024-04776-x
45. Apfelbeck M, Pfitzinger P, Bischoff R, Rath L, Buchner A, Mumm JN, et al. Predictive clinical features for negative histopathology of mri/ultrasound-fusion-guided prostate biopsy in patients with high likelihood of cancer at prostate mri: analysis from a urologic outpatient clinic1. Clin Hemorheol Microcirc. (2020) 76:503–11. doi: 10.3233/ch-209225
46. Sheridan AD, Nath SK, Aneja S, Syed JS, Pahade J, Mathur M, et al. Mri-ultrasound fusion targeted biopsy of prostate imaging reporting and data system version 2 category 5 lesions found false-positive at multiparametric prostate mri. AJR Am J Roentgenol. (2018) 210:W218–w25. doi: 10.2214/ajr.17.18680
47. Apfelbeck M, Schlenker B, Chaloupka M, Stief CG, and Clevert DA. Multiparametric mri lesion classified as prostate imaging-reporting and data system 5 but histopathologically described as benign: A case report and review of literature. Urol Int. (2021) 105:520–4. doi: 10.1159/000512378
48. Ziayee F, Schimmöller L, Boschheidgen M, Kasprowski L, Al-Monajjed R, Quentin M, et al. Benefit of dynamic contrast-enhanced (Dce) imaging for prostate cancer detection depending on readers experience in prostate mri. Clin Radiol. (2024) 79:e468–e74. doi: 10.1016/j.crad.2023.11.026
49. Chow KM, So WZ, Lee HJ, Lee A, Yap DWT, Takwoingi Y, et al. Head-to-head comparison of the diagnostic accuracy of prostate-specific membrane antigen positron emission tomography and conventional imaging modalities for initial staging of intermediate- to high-risk prostate cancer: A systematic review and meta-analysis. Eur Urol. (2023) 84:36–48. doi: 10.1016/j.eururo.2023.03.001
50. Hope TA, Eiber M, Armstrong WR, Juarez R, Murthy V, Lawhn-Heath C, et al. Diagnostic accuracy of 68ga-psma-11 pet for pelvic nodal metastasis detection prior to radical prostatectomy and pelvic lymph node dissection: A multicenter prospective phase 3 imaging trial. JAMA Oncol. (2021) 7:1635–42. doi: 10.1001/jamaoncol.2021.3771
51. Eiber M, Herrmann K, Calais J, Hadaschik B, Giesel FL, Hartenbach M, et al. Prostate cancer molecular imaging standardized evaluation (Promise): proposed mitnm classification for the interpretation of psma-ligand pet/ct. J Nucl Med. (2018) 59:469–78. doi: 10.2967/jnumed.117.198119
52. Lenzo NP, Meyrick D, and Turner JH. Review of gallium-68 psma pet/ct imaging in the management of prostate cancer. Diagnostics (Basel). (2018) 8. doi: 10.3390/diagnostics8010016
53. de Feria Cardet RE, Hofman MS, Segard T, Yim J, Williams S, Francis RJ, et al. Is prostate-specific membrane antigen positron emission tomography/computed tomography imaging cost-effective in prostate cancer: an analysis informed by the propsma trial. Eur Urol. (2021) 79:413–8. doi: 10.1016/j.eururo.2020.11.043
54. Li Y, Chen J, Wang X, Yang P, Yang J, Zhao Q, et al. Predictive value of volumetric parameters based on (18)F-psma-1007 pet/ct for prostate cancer metastasis. Front Oncol. (2024) 14:1335205. doi: 10.3389/fonc.2024.1335205
55. Surasi DS, Eiber M, Maurer T, Preston MA, Helfand BT, Josephson D, et al. Diagnostic performance and safety of positron emission tomography with (18)F-rhpsma-7.3 in patients with newly diagnosed unfavourable intermediate- to very-high-risk prostate cancer: results from a phase 3, prospective, multicentre study (Lighthouse). Eur Urol. (2023) 84:361–70. doi: 10.1016/j.eururo.2023.06.018
56. Devos G, Tosco L, Baldewijns M, Gevaert T, Goffin K, Petit V, et al. Arneo: A randomized phase ii trial of neoadjuvant degarelix with or without apalutamide prior to radical prostatectomy for high-risk prostate cancer. Eur Urol. (2023) 83:508–18. doi: 10.1016/j.eururo.2022.09.009
57. Bukavina L, Luckenbaugh AN, Hofman MS, Hope T, Kamran SC, Murphy DG, et al. Incorporating prostate-specific membrane antigen positron emission tomography in management decisions for men with newly diagnosed or biochemically recurrent prostate cancer. Eur Urol. (2023) 83:521–33. doi: 10.1016/j.eururo.2022.10.024
58. Emmett L, Buteau J, Papa N, Moon D, Thompson J, Roberts MJ, et al. The additive diagnostic value of prostate-specific membrane antigen positron emission tomography computed tomography to multiparametric magnetic resonance imaging triage in the diagnosis of prostate cancer (Primary): A prospective multicentre study. Eur Urol. (2021) 80:682–9. doi: 10.1016/j.eururo.2021.08.002
59. Schrader A, Netzer N, Hielscher T, Görtz M, Zhang KS, Schütz V, et al. Prostate cancer risk assessment and avoidance of prostate biopsies using fully automatic deep learning in prostate mri: comparison to pi-rads and integration with clinical data in nomograms. Eur Radiol. (2024) 34:7909–20. doi: 10.1007/s00330-024-10818-0
60. Cao R, Mohammadian Bajgiran A, Afshari Mirak S, Shakeri S, Zhong X, Enzmann D, et al. Joint prostate cancer detection and gleason score prediction in mp-mri via focalnet. IEEE Trans Med Imaging. (2019) 38:2496–506. doi: 10.1109/tmi.2019.2901928
61. Ström P, Kartasalo K, Olsson H, Solorzano L, Delahunt B, Berney DM, et al. Artificial intelligence for diagnosis and grading of prostate cancer in biopsies: A population-based, diagnostic study. Lancet Oncol. (2020) 21:222–32. doi: 10.1016/s1470-2045(19)30738-7
62. Saha A, Bosma JS, Twilt JJ, van Ginneken B, Bjartell A, Padhani AR, et al. Artificial intelligence and radiologists in prostate cancer detection on mri (Pi-cai): an international, paired, non-inferiority, confirmatory study. Lancet Oncol. (2024) 25:879–87. doi: 10.1016/s1470-2045(24)00220-1
63. Song AH, Williams M, Williamson DFK, Chow SSL, Jaume G, Gao G, et al. Analysis of 3d pathology samples using weakly supervised ai. Cell. (2024) 187:2502–20.e17. doi: 10.1016/j.cell.2024.03.035
64. Hamid S, Donaldson IA, Hu Y, Rodell R, Villarini B, Bonmati E, et al. The smarttarget biopsy trial: A prospective, within-person randomised, blinded trial comparing the accuracy of visual-registration and magnetic resonance imaging/ultrasound image-fusion targeted biopsies for prostate cancer risk stratification. Eur Urol. (2019) 75:733–40. doi: 10.1016/j.eururo.2018.08.007
65. Kaneko M, Sugano D, Lebastchi AH, Duddalwar V, Nabhani J, Haiman C, et al. Techniques and outcomes of mri-trus fusion prostate biopsy. Curr Urol Rep. (2021) 22:27. doi: 10.1007/s11934-021-01037-x
66. Kesch C, Schütz V, Dieffenbacher S, Bonekamp D, Hadaschik BA, Hohenfellner M, et al. Multiparametric mri fusion-guided biopsy for the diagnosis of prostate cancer. Curr Opin Urol. (2018) 28:172–7. doi: 10.1097/mou.0000000000000461
67. Briggs LG, Kim M, Gusev A, Rumpf F, Feldman A, McGovern F, et al. Evaluation of in-Office Mri/Us Fusion Transperineal Prostate Biopsy Via Free-Hand Device during Routine Clinical Practice. Urology. (2021) 155:26–32. doi: 10.1016/j.urology.2021.04.040
68. Deivasigamani S, Adams ES, Kotamarti S, Mottaghi M, Taha T, Aminsharifi A, et al. Comparison of procedural anxiety and pain associated with conventional transrectal ultrasound prostate biopsy to magnetic resonance imaging-ultrasound fusion-guided biopsy: A prospective cohort trial. Prostate Cancer Prostatic Dis. (2024) 27:294–9. doi: 10.1038/s41391-023-00760-5
69. Bryant RJ, Marian IR, Williams R, Lopez JF, Mercader C, Raslan M, et al. Local anaesthetic transperineal biopsy versus transrectal prostate biopsy in prostate cancer detection (Translate): A multicentre, randomised, controlled trial. Lancet Oncol. (2025). doi: 10.1016/s1470-2045(25)00100-7
70. Kumar R, Singh SK, Mittal BR, Vadi SK, Kakkar N, Singh H, et al. Safety and diagnostic yield of (68)Ga prostate-specific membrane antigen pet/ct-guided robotic-assisted transgluteal prostatic biopsy. Radiology. (2022) 303:392–8. doi: 10.1148/radiol.204066
71. Zhang LL, Li WC, Xu Z, Jiang N, Zang SM, Xu LW, et al. (68)Ga-psma pet/ct targeted biopsy for the diagnosis of clinically significant prostate cancer compared with transrectal ultrasound guided biopsy: A prospective randomized single-centre study. Eur J Nucl Med Mol Imaging. (2021) 48:483–92. doi: 10.1007/s00259-020-04863-2
72. Liu Y, Yu H, Liu J, Zhang X, Lin M, Schmidt H, et al. A pilot study of (18)F-dcfpyl pet/ct or pet/mri and ultrasound fusion targeted prostate biopsy for intra-prostatic pet-positive lesions. Front Oncol. (2021) 11:612157. doi: 10.3389/fonc.2021.612157
73. Bodar YJL, Boevé LMS, van Leeuwen PJ, Baars PC, Nieuwenhuijzen JA, van Haarst EP, et al. Using prostate-specific membrane antigen positron-emission tomography to guide prostate biopsies and stage men at high-risk of prostate cancer. BJU Int. (2023) 132:705–12. doi: 10.1111/bju.16167
74. Niu S, Liu Y, Ao L, Ding X, Chang X, Li J, et al. Comparison between (18)F-dcfpyl pet/mri-guided ultrasound fusion targeted biopsy and systematic biopsy for tumor detection and grading in selected patients: A prospective randomized controlled trial. Asian J Urol. (2025) 12:43–50. doi: 10.1016/j.ajur.2024.07.006
75. Wong LM, Sutherland T, Perry E, Tran V, Spelman T, Corcoran N, et al. Fluorine-18-labelled prostate-specific membrane antigen positron emission tomography/computed tomography or magnetic resonance imaging to diagnose and localise prostate cancer. A Prospective Single-Arm Paired Comparison (Pedal) Eur Urol Oncol. (2024) 7:1015–23. doi: 10.1016/j.euo.2024.01.002
76. Izadpanahi MH, Elahian A, Gholipour F, Khorrami MH, Zargham M, Mohammadi Sichani M, et al. Diagnostic yield of fusion magnetic resonance-guided prostate biopsy versus cognitive-guided biopsy in biopsy-naive patients: A head-to-head randomized controlled trial. Prostate Cancer Prostatic Dis. (2021) 24:1103–9. doi: 10.1038/s41391-021-00366-9
77. Deng R, Liu Y, Wang K, Ruan M, Li D, Wu J, et al. Comparison of mri artificial intelligence-guided cognitive fusion-targeted biopsy versus routine cognitive fusion-targeted prostate biopsy in prostate cancer diagnosis: A randomized controlled trial. BMC Med. (2024) 22:530. doi: 10.1186/s12916-024-03742-z
78. Falagario UG, Pellegrino F, Fanelli A, Guzzi F, Bartoletti R, Cash H, et al. Prostate cancer detection and complications of mri-targeted prostate biopsy using cognitive registration, software-assisted image fusion or in-bore guidance: A systematic review and meta-analysis of comparative studies. Prostate Cancer Prostatic Dis. (2024). doi: 10.1038/s41391-024-00827-x
79. Lin Y, Yilmaz EC, Belue MJ, Harmon SA, Tetreault J, Phelps TE, et al. Evaluation of a cascaded deep learning-based algorithm for prostate lesion detection at biparametric mri. Radiology. (2024) 311:e230750. doi: 10.1148/radiol.230750
80. Novara G, Zattoni F, Zecchini G, Aceti A, Pellizzari A, Ferraioli G, et al. Role of targeted biopsy, perilesional biopsy, random biopsy, and their combination in the detection of clinically significant prostate cancer by mpmri/transrectal ultrasonography fusion biopsy in confirmatory biopsy during active surveillance program. Prostate Cancer Prostatic Dis. (2024) 27:129–35. doi: 10.1038/s41391-023-00733-8
81. Couchoux T, Jaouen T, Melodelima-Gonindard C, Baseilhac P, Branchu A, Arfi N, et al. Performance of a region of interest-based algorithm in diagnosing international society of urological pathology grade group ≥r prostate cancer on the mri-first database-cad-first study. Eur Urol Oncol. (2024) 7:1113–22. doi: 10.1016/j.euo.2024.03.003
82. Harder C, Pryalukhin A, Quaas A, Eich ML, Tretiakova M, Klein S, et al. Enhancing prostate cancer diagnosis: artificial intelligence-driven virtual biopsy for optimal magnetic resonance imaging-targeted biopsy approach and gleason grading strategy. Mod Pathol. (2024) 37:100564. doi: 10.1016/j.modpat.2024.100564
83. Pantanowitz L, Quiroga-Garza GM, Bien L, Heled R, Laifenfeld D, Linhart C, et al. An artificial intelligence algorithm for prostate cancer diagnosis in whole slide images of core needle biopsies: A blinded clinical validation and deployment study. Lancet Digit Health. (2020) 2:e407–e16. doi: 10.1016/s2589-7500(20)30159-x
84. Schieda N, Nisha Y, Hadziomerovic AR, Prabhakar S, Flood TA, Breau RH, et al. Comparison of positive predictive values of biparametric mri and multiparametric mri-directed transrectal us-guided targeted prostate biopsy. Radiology. (2024) 311:e231383. doi: 10.1148/radiol.231383
85. Lenfant L, Seisen T, Rouprêt M, Pinar U, and Mozer PC. Unleashing the power of artificial intelligence and fusion magnetic resonance imaging-targeted biopsy: transforming prostate cancer diagnosis. Eur Urol Oncol. (2023) 6:541–2. doi: 10.1016/j.euo.2023.06.013
86. Wu J, Ji A, Xie B, Wang X, Zhu Y, Wang J, et al. Is magnetic resonance/ultrasound fusion prostate biopsy better than systematic prostate biopsy? An updated meta- and trial sequential analysis. Oncotarget. (2015) 6:43571–80. doi: 10.18632/oncotarget.6201
87. Chatterjee A, Yousuf AN, Engelmann R, Harmath C, Lee G, Medved M, et al. Prospective validation of an automated hybrid multidimensional mri tool for prostate cancer detection using targeted biopsy: comparison with pi-rads-based assessment. Radiol Imaging Cancer. (2025) 7:e240156. doi: 10.1148/rycan.240156
88. Bulten W, Kartasalo K, Chen PC, Ström P, Pinckaers H, Nagpal K, et al. Artificial intelligence for diagnosis and gleason grading of prostate cancer: the panda challenge. Nat Med. (2022) 28:154–63. doi: 10.1038/s41591-021-01620-2
89. Satturwar S and Parwani AV. Artificial intelligence-enabled prostate cancer diagnosis and prognosis: current state and future implications. Adv Anat Pathol. (2024) 31:136–44. doi: 10.1097/pap.0000000000000425
90. Martinez-Marroquin E, Chau M, Turner M, Haxhimolla H, and Paterson C. Use of artificial intelligence in discerning the need for prostate biopsy and readiness for clinical practice: A systematic review protocol. Syst Rev. (2023) 12:126. doi: 10.1186/s13643-023-02282-6
Keywords: artificial intelligence, image technology, multimodal image fusion, prostate biopsy, research progress
Citation: Wu Y, Lu Q, Liu Z, Wu J, He X, Yang Y and Li Y (2025) Advances in imaging and artificial intelligence for precision diagnosis and biopsy guidance in prostate cancer. Front. Oncol. 15:1614891. doi: 10.3389/fonc.2025.1614891
Received: 20 April 2025; Accepted: 22 September 2025;
Published: 13 October 2025.
Edited by:
Angelo Naselli, MultiMedica Holding SpA (IRCCS), ItalyReviewed by:
Federico Mastroleo, European Institute of Oncology (IEO), ItalyAdnan Mohsin Abdulzeez, University of Duhok, Iraq
Copyright © 2025 Wu, Lu, Liu, Wu, He, Yang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuanwei Li, bGl5dWFud2VpQGh1bm51LmVkdS5jbg==; Yongjun Yang, eXlqdXJvbG9neUAxNjMuY29t
†These authors have contributed equally to this work and share first authorship
 Ye Wu
Ye Wu Qiang Lu
Qiang Lu Zhifu Liu
Zhifu Liu Jianhe Wu
Jianhe Wu