- 1Department of Urology, Kunming Municipal Hospital of Traditional Chinese Medicine, Kunming, China
- 2Department of Urology, The Second Affiliated Hospital of Kunming Medical University, Kunming, China
- 3Department of Urology, South Yunnan Central Hospital of Yunnan Province, Yunnan, China
Background: Radical cystectomy (RC) serves as the gold standard treatment for organ-localized bladder cancer; however, postoperative complications diminish the quality of life of patients. Whether male fertility-sparing radical cystectomy(FSRC) with orthotopic neobladder (ONB) surpasses RC and nerve-sparing cystectomy (NSC) remains controversial. The objective of this study is to compare the efficacy and safety of the two surgical approaches.
Methods: In accordance with the PRISMA (Preferred Reporting Items for Systematic Review and Meta-Analysis) statement, PubMed, Web of Science, Embase, CNKI databases, Medline, and Cochrane Library were searched until June 2024. Eligible studies were identified in line with the inclusion and exclusion criteria.
Results: A total of 10 studies encompassing 1104 patients were incorporated in this study. The outcomes demonstrated that fertility-sparing radical cystectomy (FSRC) presented significant superiority in erectile function (EF) (OR: 12.67; 95% CI 3.27-49.03; P<0.001), daytime urinary continence (OR: 5.91; 95% CI, 1.83-19.13; P = 0.003), and nocturnal urinary continence (OR: 5.13; 95% CI, 1.98-13.34; P<0.001) over non-fertility-sparing radical cystectomy (nFSRC). Compared with nFSRC, the incidences of postoperative prostate cancer (RD:−0.10; 95% CI, -0.21-0.10; P = 0.086), tumor local recurrence (OR:0.51; 95% CI, 0.26-1.00; P = 0.052), tumor metastasis (RD:-0.02; 95% CI, -0.09-0.06; P = 0.665) and 2-year survival (OR:1.21; 95% CI, 0.63-2.30; P = 0.567) after surgery were comparable. In the subgroup analysis, some differences in outcome measures were identified based on sample size, study type, control group, and study area.
Conclusion: Under rigorous preoperative screening, male FSRC with ONB demonstrates certain efficacy and safety in the treatment of bladder cancer, particularly among younger patients, warranting broader clinical consideration. More relevant clinical RCTs are required.
Systematic review registration: https://www.crd.york.ac.uk/PROSPERO/view/CRD42024558576
1 Introduction
Bladder cancer (BC) is a prevalent malignant urothelial tumor. It ranks ninth in terms of the incidence of BC worldwide, seventh among men, and thirteenth in terms of mortality (1). BC is predominantly urothelial carcinoma, and the majority of patients are already in muscle-invasive bladder cancer (MIBC) at the time of diagnosis. RC combined with bilateral pelvic lymph node dissection (PLND) constitutes the primary treatment for recurrent high-risk non-muscle-invasive bladder cancer (nMIBC) and MIBC (2), offering a sustained chance of cure, with the 5-year recurrence-free survival rate being 70% (3).
However, in standard RC, the related neurovascular bundles surrounding the prostate will be removed or damaged during the operation. This technique is associated with a considerable incidence and high prevalence of postoperative erectile dysfunction (ED), which may exert a significant influence on the quality of life, especially for younger patients (4, 5). In an attempt to enhance the quality of life of RC patients, Spitz devised and executed fertility-preserving cystectomy in 4 cases in 1989. By attaining a profound comprehension of the anatomical composition of the ejaculatory organ, the traditional surgical approach was modified, and all patients maintained erectile function (EF) after the surgery, among whom 3 cases had anterograde ejaculation and 1 case achieved procreation (6). Subsequently, Prostate capsule-sparing cystectomy (PCSC) was initially depicted by Schilling and Friesen in 1990 (7), subsequently reducing urinary incontinence and preserving EF. Variations exist in the management of male reproductive organs across different surgical approaches, with the primary objective being the preservation of the sexual nerve integrity to enhance postoperative EF. Under physiological conditions, urinary continence is maintained through coordinated action of the internal and external urethral sphincters (8), both of which are innervated by the pelvic autonomic nerves, including the cavernous nerves. Intraoperative damage to these neural structures may result in postoperative urinary incontinence. Nevertheless, oncological safety remains a critical concern. Some clinicians have expressed reservations that preservation of the prostatic capsule might facilitate tumor spillage from the bladder neck or urethral margin, thereby increasing the risk of local recurrence and distant metastasis (9, 10). The risk of prostate adenocarcinoma during prostate tissue-sparing cystectomy has also elicited concerns (11, 12). In this context, FSRC is defined as a surgical procedure involving RC and orthotopic neobladder (ONB), while preserving key male reproductive structures (such as the prostate gland, prostatic capsule, seminal vesicles, and vas deferens). Currently, FSRC can be approximately classified into the following categories (1): cystectomy with the preservation of the prostate capsule (2), cystectomy with the preservation of the prostate (3), cystectomy with the preservation of only the vas deferens and seminal vesicles (2, 9, 12–15).
Currently, some clinical investigations have attested to the favorable outcome of FSRC, and the effect of tumor control is comparable to that of RC (9, 16). Nevertheless, due to the variances in surgical procedures, disputes exist regarding efficacy and safety, and there is a dearth of unified standards that can be universally applied. We have also observed that some researchers have carried out systematic reviews and meta-analyses on the effectiveness of certain surgical methods. However, considering the limited number of controlled studies received and the absence of systematic and comprehensive analysis, it is infeasible to account for the differences in efficacy and safety between FSRC and nFSRC.
Therefore, a systematic review and meta-analysis of the efficacy and safety of FSRC with ONB versus nFSRC for BC was conducted to provide a better reference for clinical practice, to serve clinical treatment more accurately and effectively.
2 Methods
This review was prospectively registered in the PROSPERO database and reported following the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement and AMSTAR (Assessing the methodological quality of systematic reviews) Guidelines (17, 18).
2.1 Search strategy
A literature search was conducted in PubMed, Web of Science, Embase, CNKI databases, Medline and Cochrane Library with the following search terms: ((Urinary Bladder Neoplasms) OR (bladder tumor) OR (bladder cancer) OR (bladder papilloma)) AND ((prostate sparing) OR (capsule sparing) OR (seminal vesicle) OR (erectile) OR (sexual OR ejaculation OR incontinence)) AND ((cystectomy) OR (cystoprostatectomy) OR (neobladder)) AND ((Randomized Controlled Trials) OR (Controlled Clinical Trial) OR (Observational Study)). Appropriate database-specific subject headings were employed where necessary. Results encompass articles published within the period from January 1, 2000, to June 1, 2024. Simultaneously, a manual search was also conducted from the references of relevant studies to broaden the search scope. Each included study was independently evaluated by two reviewers (Y.H. and H.S.), and any dissents were settled through consensus.
2.2 Inclusion/exclusion criteria
Studies meeting the following inclusion criteria were admitted (1): The study conducted a comparison between FSRC and RC or NSC (2). The types of clinical studies encompass randomized controlled studies, prospective controlled studies, and retrospective studies (3). The full text should contain at least one outcome parameter, such as postoperative sexual function, urinary control, tumor control, and survival (4). Language restrictions are limited to English and Chinese. The following studies were excluded: reviews, letters, case reports, low-quality research, and research with no detailed data.
2.3 Types of intervention and comparator
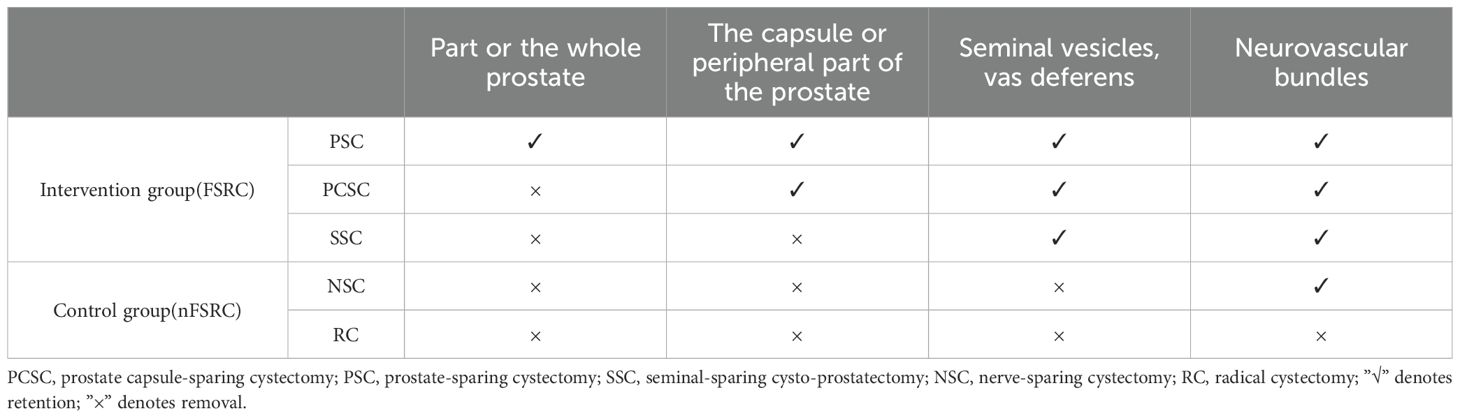
The intervention group comprised the following procedures (1): preservation of the entire or partial prostate tissue (2); preservation of the prostate capsule and adjacent periprostatic tissues (3); preservation of the seminal vesicles and vas deferens. In all three procedures, the relevant neurovascular bundles were preserved and collectively designated as FSRC, in accordance with the prior definition. The control group underwent either conventional RC or NSC, collectively referred to as nFSRC, as detailed in Table 1.
2.4 Data extraction
Our meta-analysis will extract the following data from each study (1): Basic information of the included studies: title, main author, sample size, publication year, and country (2); Research characteristics: research methods, subjects, interventions, and outcome indicators (3); Outcomes: postoperative urinary control, sexual function, oncology outcomes, etc. The data types in this study can be classified into binary variables and continuous variables. For instance, the outcome indicators depicting the urinary control and EF of patients as effective or ineffective, and the tumor recurrence and metastasis, the occurrence of prostate cancer (PCa), and the 2-year survival after surgery, which are counted based on the number of occurrences, are continuous variable data, and we converted them into binary variables. When continuous variables were reported in other forms in the main literature, we calculated the means and standard deviations (19).
2.5 Outcomes
The definitions of urinary incontinence and EF varied across the included studies and existing literature, with each outcome measure defined by the trial investigators to account for inconsistencies in data reporting. Main outcome measures (1): Definition of urinary control: Urinary incontinence was assessed by a valid questionnaire, the quantity of pads or self-impression report at 6 months after surgery, covering both daytime and nighttime (2). Sexual function was defined as sexual activity, EF, and ejaculatory function at 6 to 12 months after surgery, through validated questionnaires or self-impression reports based on preoperative and postoperative evaluations. The secondary outcomes were as follows: the oncologic outcomes were defined as any recurrence of local or metastatic diseases, the occurrence of PCa, and the number of overall survival beyond 2 years during the follow-up period.
2.6 Quality assessment
We assessed the quality of nonrandomized studies by employing the Newcastle-Ottawa Quality Assessment Scale (20). Studies were evaluated in three aspects: selection, comparability, and exposure/outcome. Studies were regarded as of high quality if they obtained at least 7 points. Given the presence of numerous confounding factors in retrospective studies, we excluded studies with a total score exceeding 7 points if the comparability between groups or the outcome assessment score was less than 2 points, to minimize potential bias and enhance the reliability of the study findings. The Cochrane Collaboration’s Risk of Bias Assessment Tool was utilized to evaluate randomized controlled trials (RCTs), and the risk of bias of each trial was classified into high, low, or unclear based on factors such as random sequence generation, allocation concealment, blinding of the study protocol by subjects and researchers, blinding of outcome assessment, incomplete outcome data, and selective reporting (21). All disputes were settled through discussions between the two commentators.
2.7 Statistical analysis
Statistical analysis was conducted using Stata 14.0 software. The weighted mean difference (WMD) and odds ratio (OR), along with 95% confidence interval (CI), were computed for continuous and dichotomous variables, respectively. When the outcome of interest was zero in either the experimental or control groups, the risk difference (RD) was adopted as the pooled statistic. The chi-square test and I2 test were employed to analyze the heterogeneity among studies. A random-effects model was utilized if there was significant heterogeneity (P > 50%); otherwise, a fixed-effects model was applied. Statistical significance was defined as P < 0.05. To further assess the robustness of the final results, sensitivity analyses were carried out.
2.8 Subgroup analysis
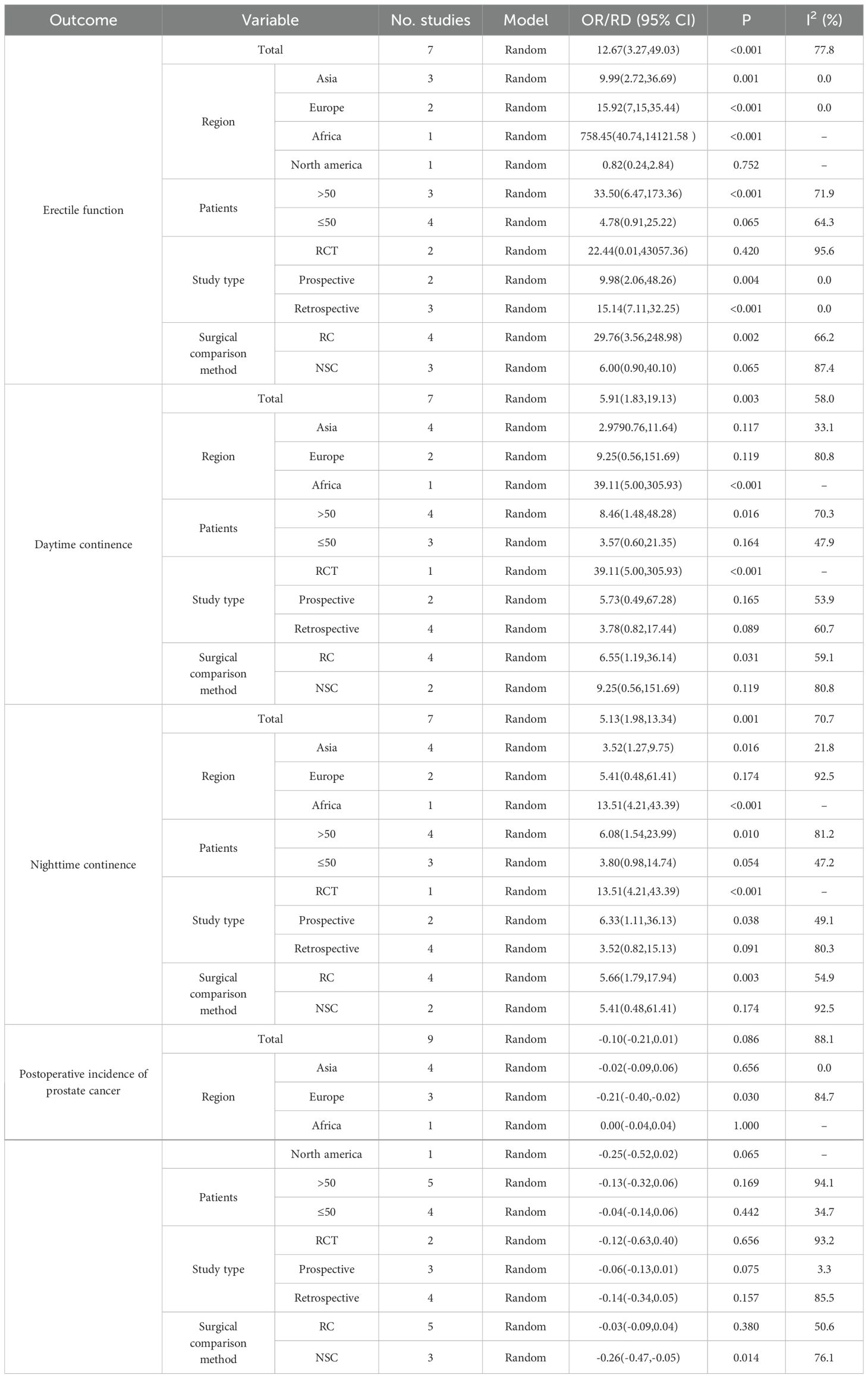
Based on the data extracted from the included literature, subgroups were defined according to region (Europe, Asia, and Africa), sample size (greater than 50 and less than or equal to 50), study type (RCT, retrospective, and prospective), and surgical control group (RC and NSC). Postoperative EF, daytime continence, nighttime continence, and postoperative PCa were analyzed.
2.9 Publication bias
Egger’s test and Begg’s test were employed to evaluate publication bias. In the event of publication bias, a trim and fill approach was utilized to estimate the missing studies and recalculate the results (22).
3 Results
3.1 Search results
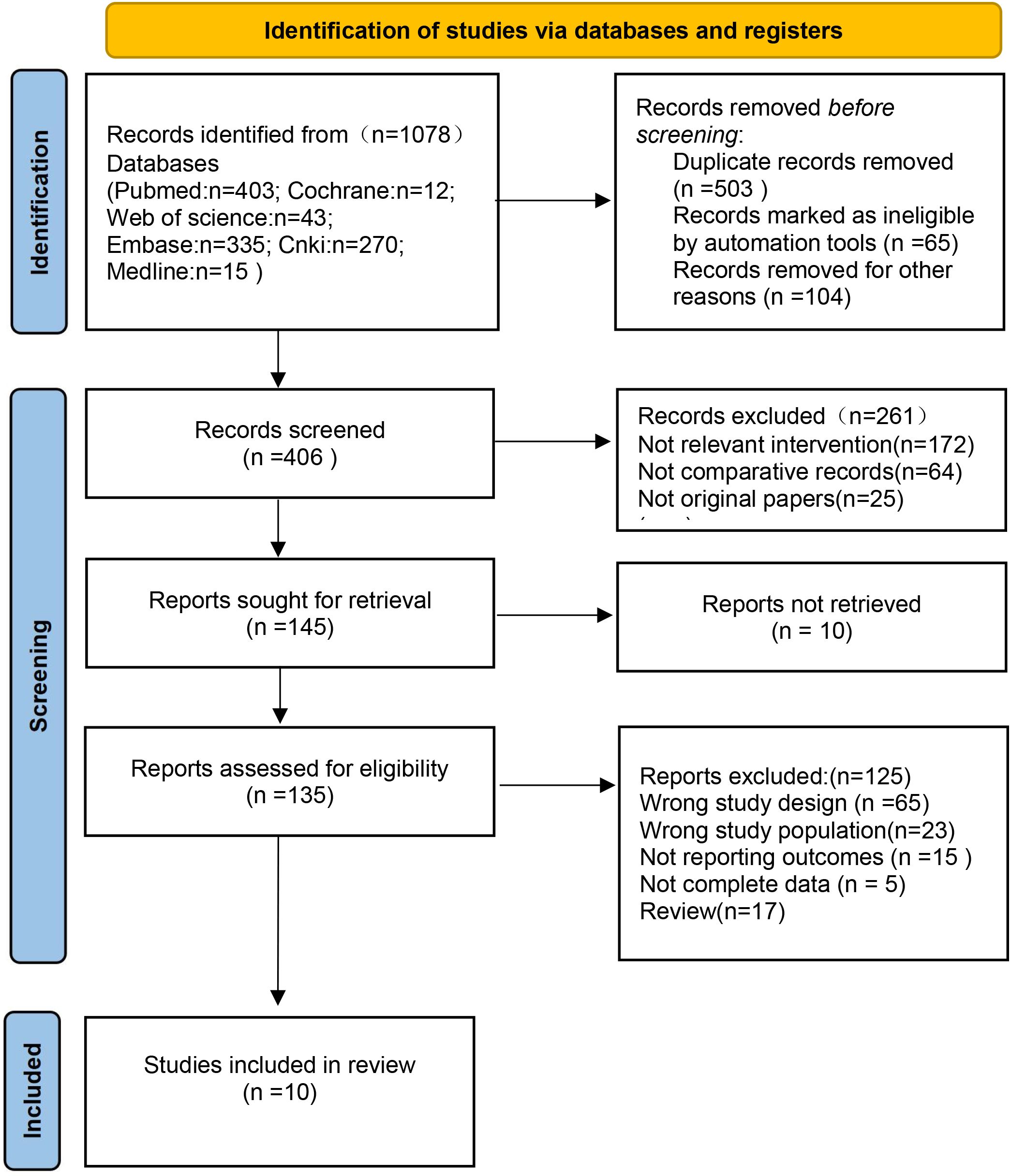
Our search strategy resulted in 1078 articles, among which 503 were duplicates. The rest of the articles were further screened in accordance with strict inclusion and exclusion criteria. Based on the title and abstract, 261 articles were excluded, and a total of 45 full-text articles were retained. Eventually, after eliminating irrelevant articles, a total of 10 articles were selected (Figure 1).
3.2 Characteristics of included studies
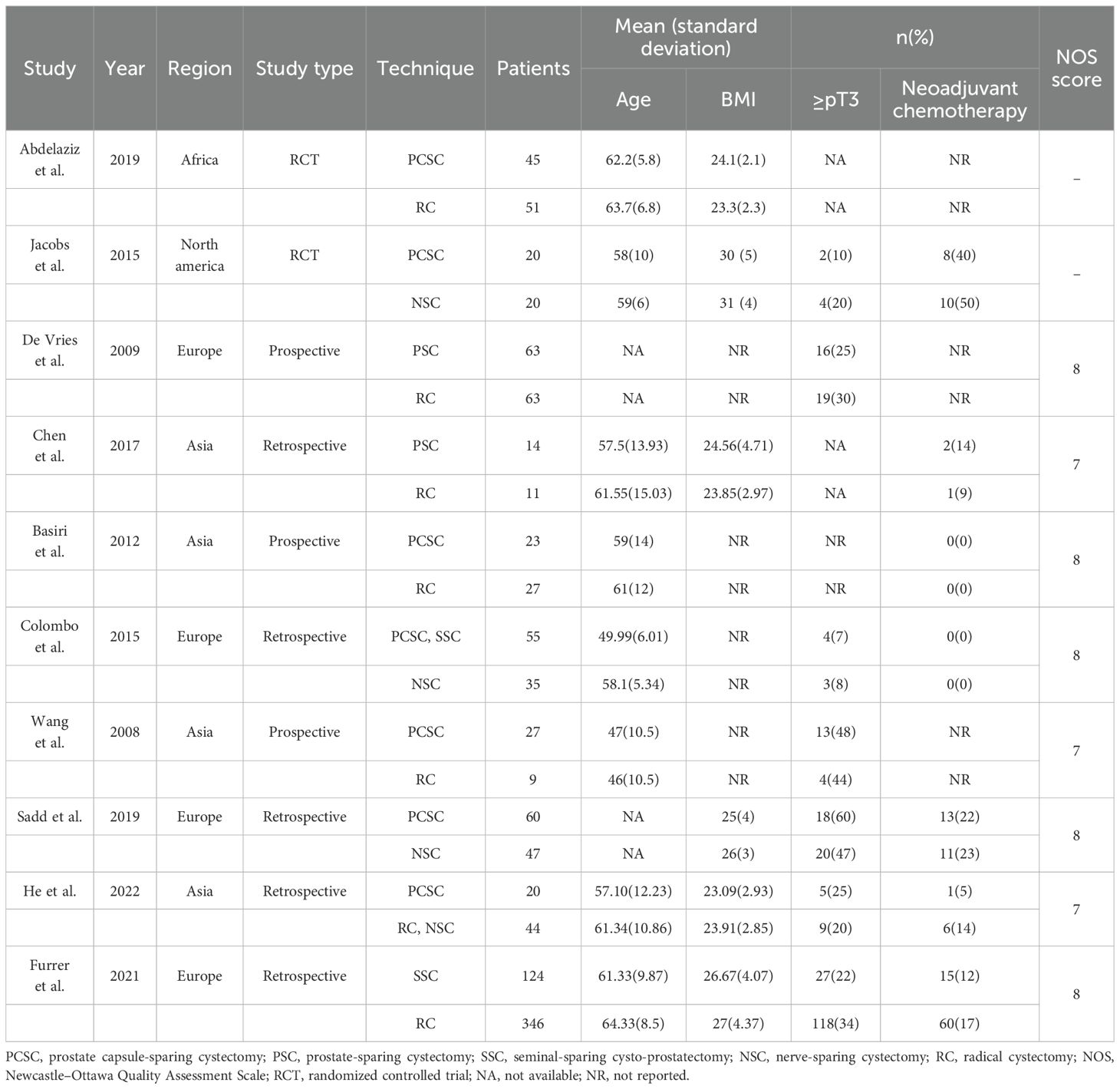
All studies included met the diagnostic criteria for BC and required RC. The reviewed studies comprised two RCTs and eight retrospective or prospective controlled studies. A total of 1, 104 patients were included in these studies, with 451 patients in the intervention group and 653 in the control group. Seven studies compared PCSC with RC or NSC, two studies compared PSC with RC, two studies compared SSC with RC or NSC, and one study compared both PCSC and SSC with NSC. The majority of patients were aged between 50 and 65 years, and the mean follow-up duration exceeded one year. Relevant data have been summarized in Table 2.
3.3 Risk of bias
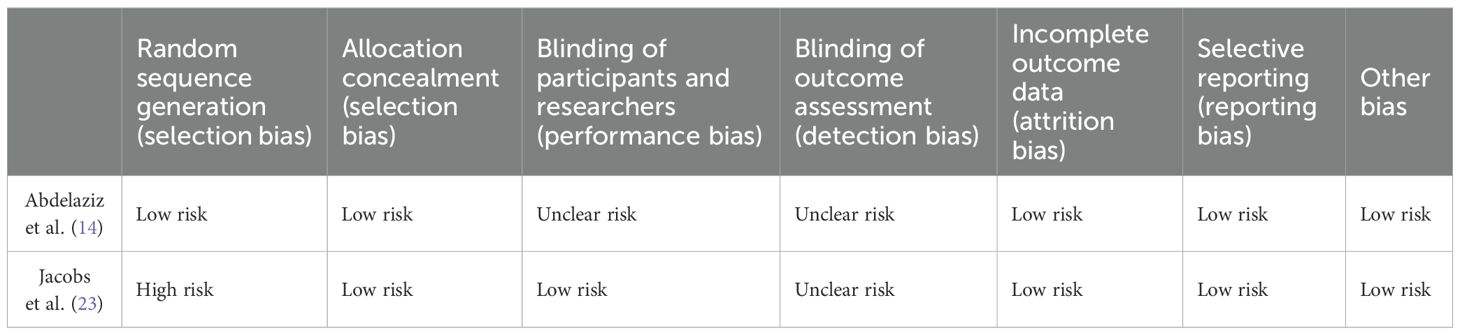
Two RCTs were assessed for risk of bias using the Cochrane Collaboration’s tool, with the results presented in Table 3. Jacobs (23) failed to specify the exact method used for random sequence generation, leading to a high risk of bias in this domain. Both RCTs exhibited unclear risks regarding blinding procedures, while the remaining evaluation criteria indicated low risk. The Newcastle-Ottawa Scale was utilized to assess the quality of nonrandomized studies. Among these, three studies achieved a score of 7, whereas the remaining five scored 8 (Table 2). Notably, some confounding factors were identified in the comparability between groups and outcome measures across the eight controlled studies, with variations observed in follow-up durations. Despite these issues, the overall quality of the eight non-randomized controlled studies was deemed high.
3.4 Demographic variables
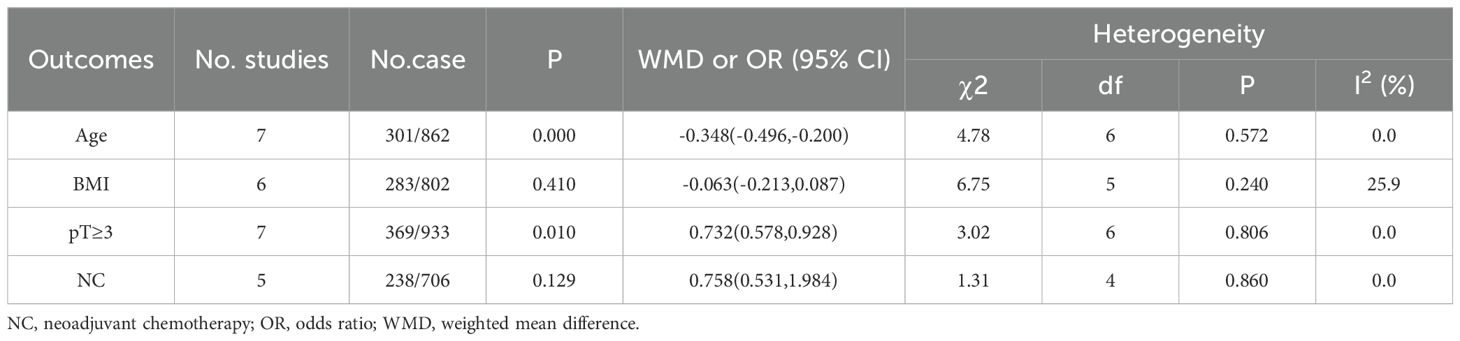
There were no significant differences between the two groups in terms of body mass index (WMD, -0.063;95% CI, -0.213-0.087; P = 0.410) and neoadjuvant chemotherapy (OR, 0.758;95% CI, 0.531-1.984; P = 0.129); There are some differences between age (WMD, -0.348;95% CI, -0.496, -0.200; P<0.001) and pT ≥3 (OR, 0.732;95% CI, 0.578-0.928; P = 0.01; Table 4).
3.5 Functional outcomes
3.5.1 Erectile function
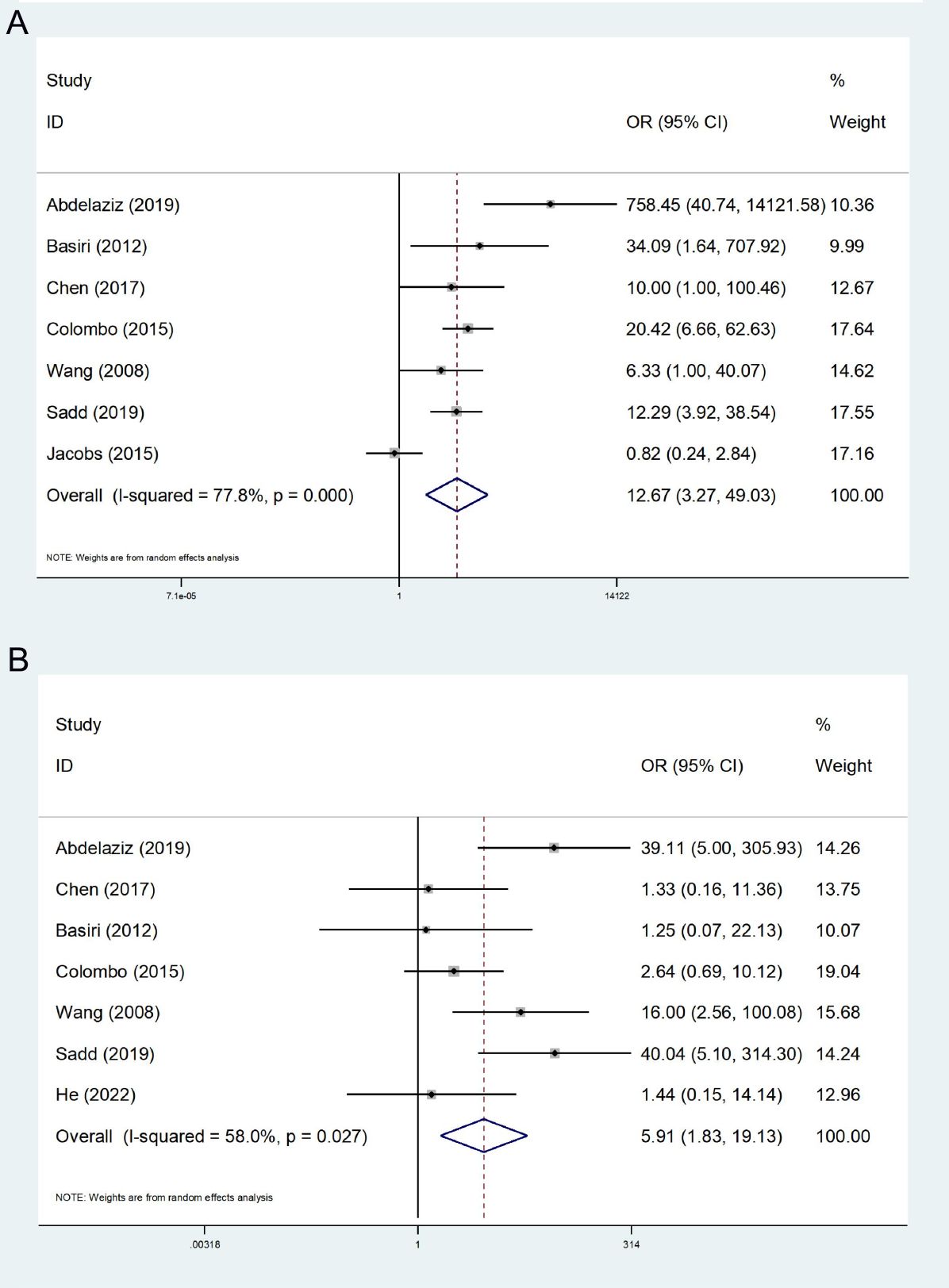
A total of 7 studies concerning EF were incorporated (14, 23–28), presenting high heterogeneity among the studies (I2 = 77.8%). A random effect model was employed, and the ultimate meta-analysis indicated that EF in the FSRC group was higher than that in the nFSRC group (OR: 12.67; 95% CI, 3.27 - 49.03; P < 0.001, Figure 2A).
3.5.2 Daytime continence
In 7 studies regarding daytime urinary continence (14, 24–29), moderate heterogeneity (I2 = 58.0%) was noted. Thus, the random effects model was employed for statistical analysis. Our ultimate results indicated that the FSRC group exhibited superior daytime urinary continence compared to the nFSRC group (OR: 5.91; 95% CI, 1.83 - 19.13; P = 0.003, Figure 2B).
3.5.3 Nighttime continence
7 studies report nighttime continence (14, 24–29). The heterogeneity test revealed a high degree of heterogeneity (I2 = 70.7%), and a random effects model was used. The final results show that the FSRC group had a lower rate of nighttime continence (OR:5.13; 95% CI, 1.98-13.34; P<0.001, Figure 3A).
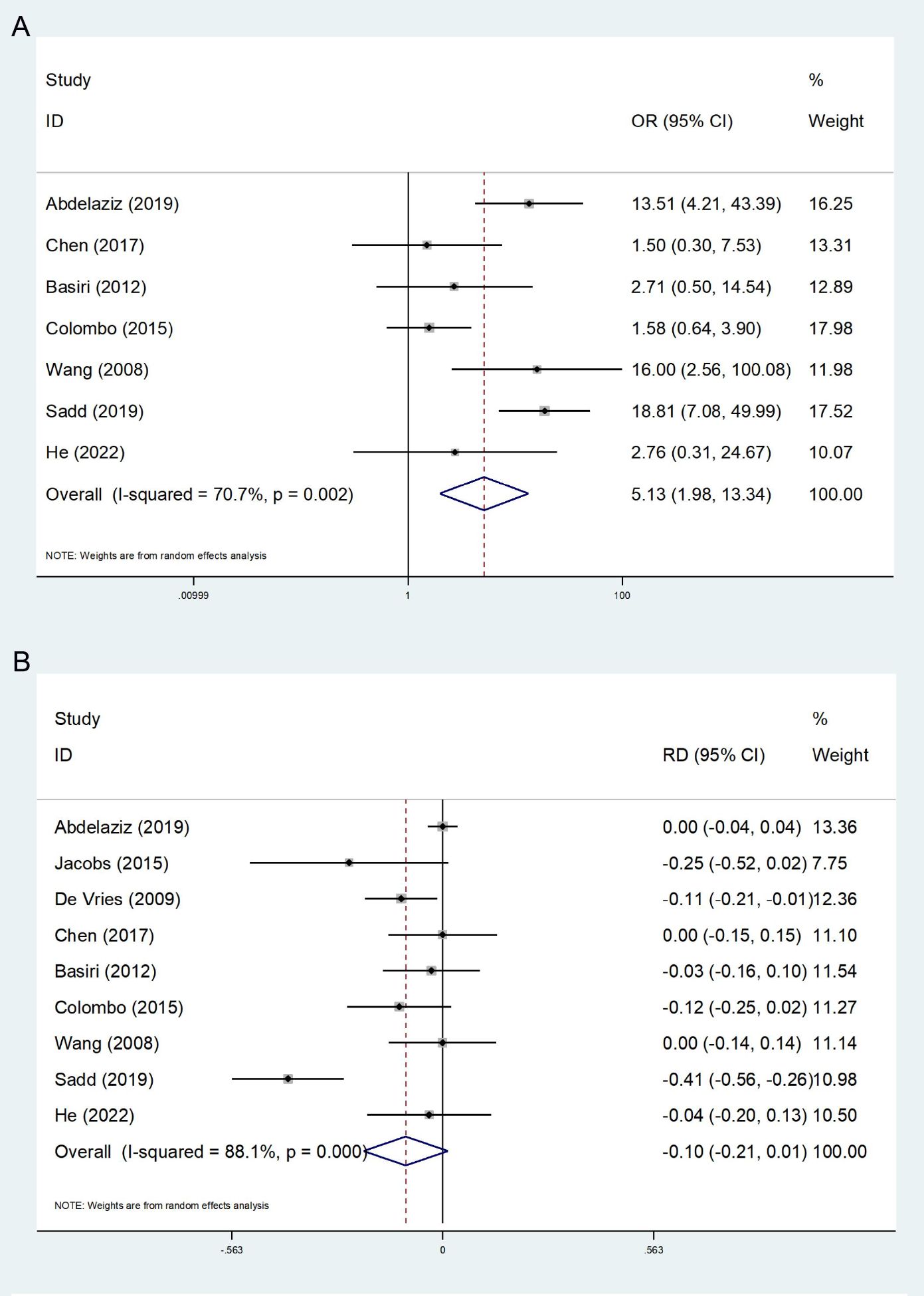
Figure 3. Forest plot and meta-analysis of nighttime continence (A) and postoperative incidence of prostate cancer (B).
3.6 Oncologic outcomes
3.6.1 Postoperative incidence of prostate cancer
A total of 9 studies reported the incidence of postoperative PCa, encompassing the detection of postoperative pathology and the occurrence of follow-up (13, 14, 23–29). The heterogeneity test indicated a high degree of heterogeneity among the studies (I2 = 88.1%); thus, the random effect model was employed in the meta-analysis. The results indicated no statistically significant difference between the FSRC group and the nFSRC group (RD: −0.10; 95% CI, -0.21 - 0.10; P = 0.086, Figure 3B). Despite the observed high heterogeneity, which may be attributed to variations in preoperative screening protocols and surgical techniques, the number of included studies suggests that any true effect difference between the two groups is likely to be modest.
3.6.2 Local recurrence
Five studies on postoperative local recurrence were encompassed (12–14, 25, 26). The heterogeneity test did not indicate significant heterogeneity (I2 = 0.00%). A fixed effect model was employed, and the results revealed that the difference in postoperative local recurrence rates between the two groups was not statistically significant (OR: 0.51; 95% CI, 0.26-1.00; P = 0.052, Figure 4A). Although the p-value approached the conventional threshold for statistical significance, suggesting a potential trend toward reduced local recurrence in the FSRC group compared to the nFERC group, the observed effect should be interpreted with caution. Given the limited follow-up duration and small sample sizes in the included studies, the long-term recurrence risk warrants further investigation in well-designed, large-scale trials.
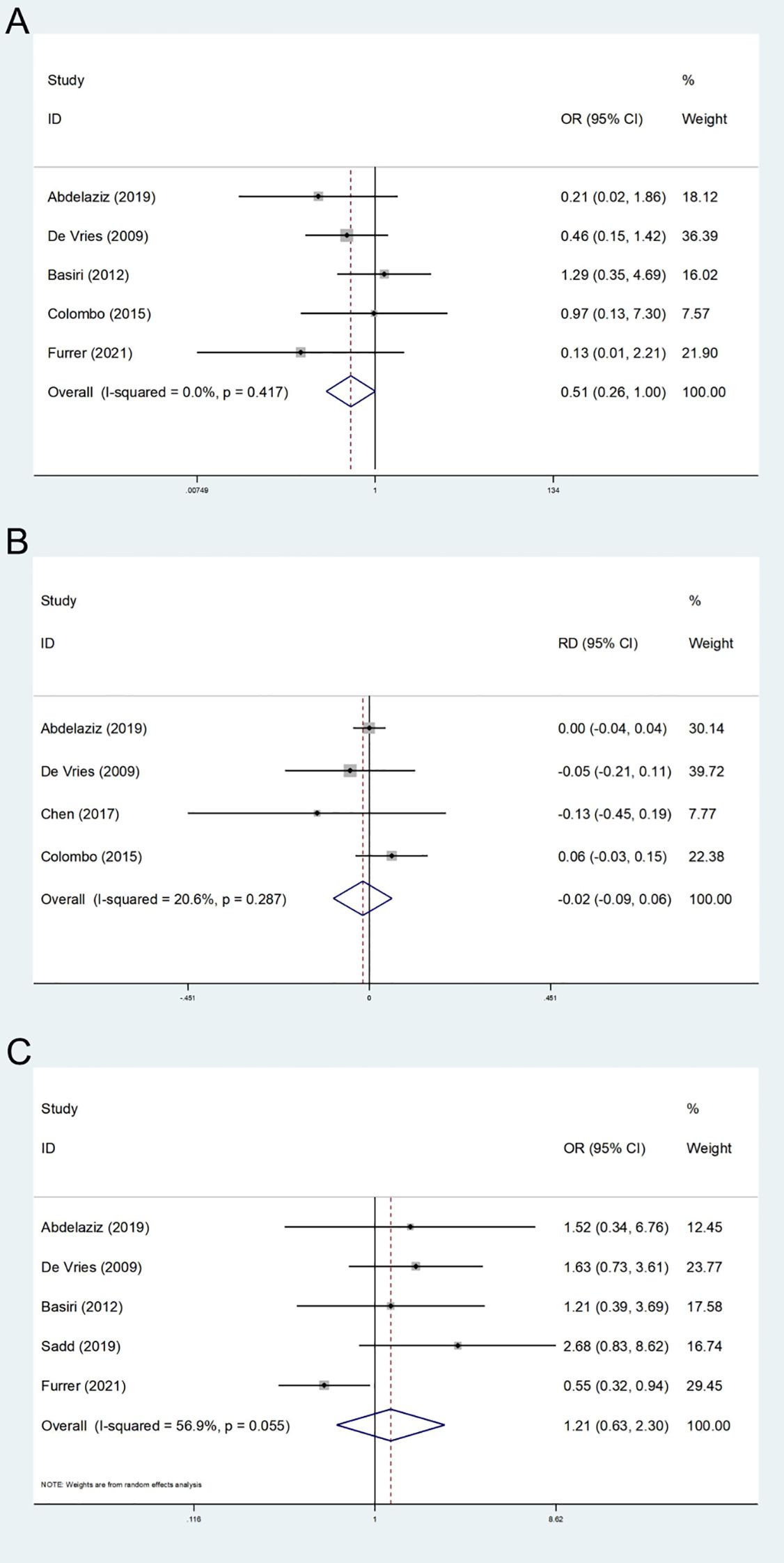
Figure 4. Forest plot and meta-analysis of local recurrence (A), metastatic disease (B), and postoperative survival (C).
3.6.3 Metastatic disease
A total of 4 studies reported distant metastasis during the follow-up period (13, 14, 24, 26). The heterogeneity test indicated low heterogeneity (I2 = 20.6%); therefore, a fixed-effects model was applied. The results showed no statistically significant difference in the risk of postoperative distant metastasis between the two groups (RD: -0.02; 95% CI, -0.09 - 0.06; P = 0.665, Figure 4B); However, given the limited follow-up duration and the small number of included studies, these findings do not rule out the possibility of differences in long-term metastatic risk.
3.6.4 Postoperative survival
We assessed the survival status two years after surgery, and a total of five studies were encompassed (12–14, 25, 27). The results of the heterogeneity test (I2 = 56.9%) indicated that there was moderate heterogeneity. Utilizing the random effects model, the results indicated no statistically significant difference in two-year survival time between the FSRC group and the nFSRC group (OR: 1.21; 95% CI, 0.63 - 2.30; P = 0.567, Figure 4C). Given that the majority of the included studies involved short-term follow-up periods and exhibited heterogeneity, further long-term follow-up studies are warranted to more definitively clarify these findings.
3.7 Publication bias and sensitivity analysis
Sensitivity analysis was performed on EF, daytime continence, nighttime continence, and postoperative incidence of PCa. We failed to observe relative differences after systematically excluding each study, which attests to the stability of our findings (Figure 5). Regarding publication bias, we found no significant publication bias in EF, daytime and nighttime urinary continence, the incidence of postoperative PCa, and postoperative 2-year survival according to Begg’s test (EF: P = 0.764; Daytime urinary continence: P = 1.000; Nighttime urinary continence: P = 1.000; Postoperative PCa: P = 0.707; Local recurrence: P = 0.221; Postoperative 2-year survival: P = 0.462) and Egger’s test (EF: P = 0.99; Daytime urinary continence: P = 0.992; Nighttime urinary continence: P = 0.882; Postoperative PCa: P = 0.425; Local recurrence: P = 0.382; Postoperative 2-year survival: P = 0.113).
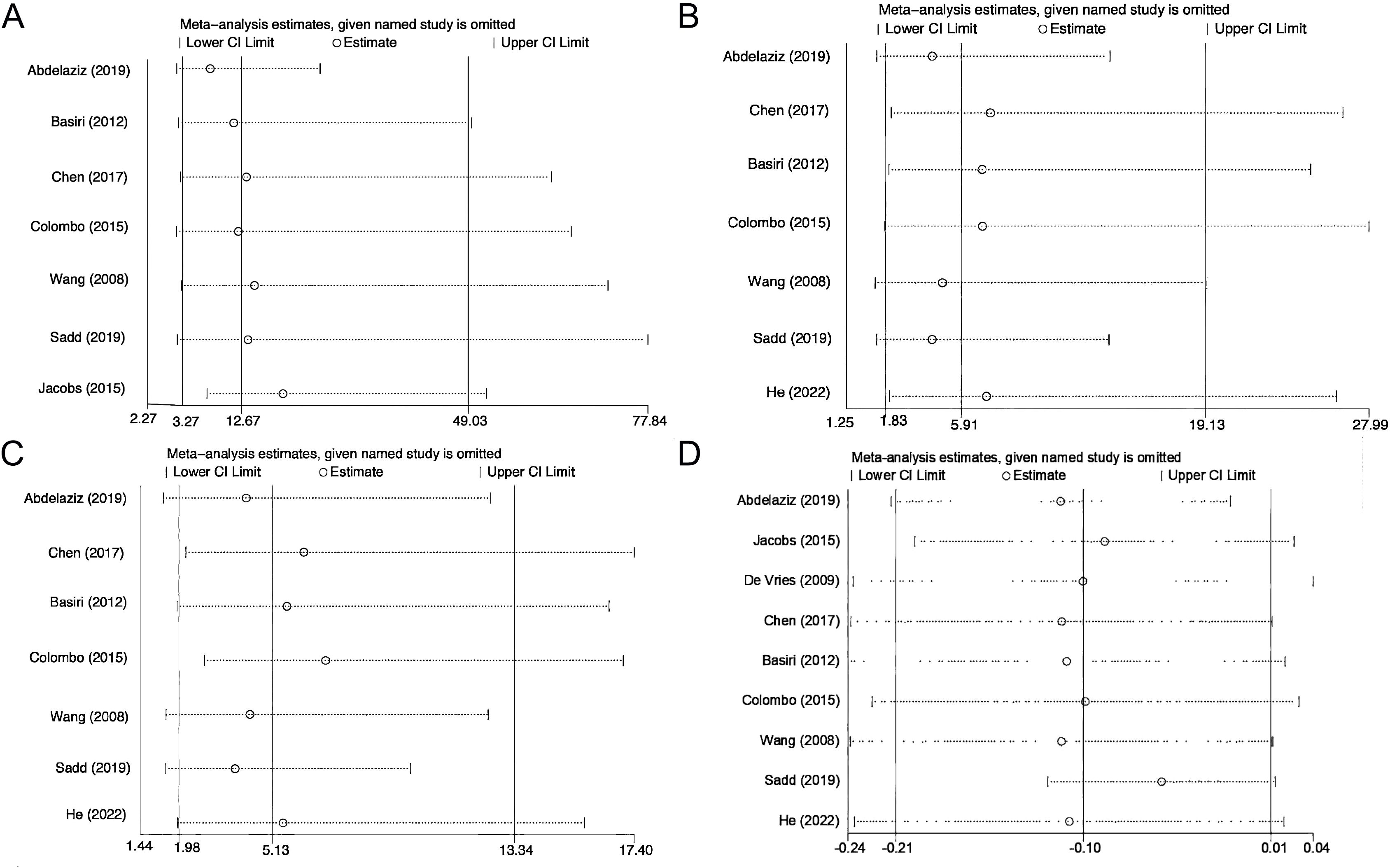
Figure 5. Sensitivity analysis of erectile function (A), daytime continence (B), nighttime continence (C), and postoperative incidence of prostate cancer (D).
3.8 Subgroup analyses
Regarding EF, when the sample size was no more than 50, the study type was RCT, and the surgical control was NSC, no significant difference was observed between the FSRC group and the nFSRC group. Concerning daytime and nighttime urinary continence, when the subgroup was European, the study type was retrospective, the sample size was no more than 50, and the surgical control was NSC, there was no significant difference was found between the FSRC group and the nFSRC group. In terms of the incidence of postoperative PCa, except for the subgroup of the Asian region and the difference between the FSRC group and the nFSRC group when the surgical control was NSC, no significant difference was detected in each group. Table 5 provides detailed statistical data.
4 Discussion
4.1 Characteristics of FSRC
It is imperative to conduct rigorous preoperative screening and carefully evaluate the indications, particularly focusing on patient age, tumor stage, and grade, and the presence of metastasis. Pyrgidis et al. (46) conducted a retrospective study on the long-term health-related quality of life in BC patients following surgery and found that, among patients over 70 years of age, the combination of RC and ONB reconstruction may adversely affect quality of life. Therefore, careful consideration should be given before performing FSRC in this age group.FSRC modifies traditional surgical techniques by preserving the neurovascular bundles surrounding the prostate as well as the seminal vesicle and vas deferens. Compared with RC, FSRC primarily aims to preserve either the prostate or its capsule, or selectively retain the seminal vesicle and vas deferens during surgery. Specific surgical methods differ in terms of how the prostate is removed and how the neobladder is reconstructed. Currently, preserving the prostatic capsule is more commonly adopted in FSRC in clinical practice. Among the included studies, Abdelaziz et al. (14, 23, 25–29) utilized this technique in seven studies. ONB reconstruction was performed using the terminal ileum or sigmoid colon, followed by anastomosis with the residual capsule. Some studies (43) have explored preoperative transurethral resection of the prostate before proceeding with FSRC; however, the risk of implantation and metastasis remains a topic of debate. Only two studies by De Vries et al. (13, 24) employed PSC, PLND, and bladder resection while preserving the prostate tissue, seminal vesicles, vas deferens, and peripheral neurovascular bundles either entirely or partially. Colombo et al. (12, 26) opted for FSRC that preserved only the seminal vesicle and sexual nerves, while completely excising the prostate and bladder, resulting in significant trauma but adhering strictly to tumor control principles. Salem et al. (44) applied this surgical approach to patients with BC and achieved comparable satisfactory outcomes relative to RC. It is noteworthy that ureteroenteric anastomotic stenosis (UAS) may develop following FSRC. Bizzarri et al. (47) reported that patients with preoperative lower albumin serum levels, lower albumin/fibrinogen ratio, and higher fibrinogen levels are at increased risk of developing UAS, thereby providing a potential basis for patient selection before FSRC.
4.2 Functional outcomes
This meta-analysis demonstrates that FSRC may be more efficacious in enhancing postoperative EF and could potentially offer advantages compared to nFSRC. Additionally, we discovered that in contrast to nFSRC, postoperative daytime and nighttime urinary continence was improved. We performed publication bias analysis, sensitivity analysis, and subgroup analysis on the outcomes of the meta-analysis to identify the potential reasons that might influence the results. As of now, no other studies have conducted relevant analyses.
Regarding the preservation of postoperative EF, our study aligns with the outcomes of several previous studies (13, 14, 25–27, 30). The possible mechanism might be associated with the conservation of seminal vesicles and neurovascular bundles during the operation to prevent sexual nerve injury. Nevertheless, the randomized controlled study by Jacobs et al. (23) indicated that there was no significant disparity in the postoperative erectile efficacy between the two groups of PCSC and NSC (50% vs 40%). In the subgroup analysis, we discovered that there was no substantial difference between the FSRC group and the NSC group, which concurred with Jacobs’ results, but the number of studies included in the analysis was limited. Voskuilen et al. (31) demonstrated that 86% of patients in the PSC study maintained EF, which is superior to the results reported after NSC. The preservation rate of EF was 29%-78% after NSC (26, 32), and some patients employed sexual function-improving drugs to enhance EF after the surgery. This also implies that the original nerve was fully preserved; Saad et al. (27) pointed out that without any ED treatment before the surgery, nearly 53% of PCSC patients maintained sexual function without using any drugs, compared with 9% of NSC patients. The latest meta-analysis by Dall et al. (15) also indicated that PCSC is more effective than NSC. Overall, after appropriate selection, FSRC can offer more alternatives for the pursuit of sexual function and a high quality of life after surgery, and holds certain advantages.
Most patients experience urinary incontinence after RC, which might be attributed to the damage to the external urethral sphincter or neurovascular bundle during deep dissection (33). FSRC can better preserve the relevant urinary control structure, and may yield better outcomes (8, 14). Our study suggests that the overall urinary continence of FSRC is superior to nFSRC, and the incidence of daytime urinary incontinence is lower. However, it has been noted that a higher rate of urinary continence is associated with a greater need for catheter insertion secondary to urinary retention (15), which could be caused by strictures at the vesicourethral anastomosis of PCSC and the prostatic urethra. Voskuilen et al. (31) indicated that 95.6% and 70.2% of patients achieved complete recovery of urinary continence during the day and night after PSC, providing better postoperative urinary continence than PCSC and seminal-sparing cysto-prostatectomy (SSC). The randomized controlled trial by Jacobs (23), which aimed at evaluating the control effect, pointed out that there was no difference in urinary continence between PCSC and NSC. Due to the small sample size of the study, the conclusion requires support from more randomized studies with a large sample size. However, Muto et al. (34) indicated that the controllable rate of the PCSC at night was merely 31%, which might be attributed to the disparities in research timing and surgical techniques. Additionally, among RC patients, patients with ONB and nighttime urinary incontinence have lower quality of life and higher depression scores (35). PCSC may enhance the quality of life by improving nighttime sleep and reducing sexual side effects, while Chen et al. (24) indicated that the incidence of nighttime urinary incontinence and the self-catheterization rate in the PCSC group were lower than those in the control group. Furthermore, Volz et al. (48) conducted a propensity score matching analysis over a 4-year follow-up period and found that different types of urinary diversion did not significantly influence disease-specific or general health-related quality of life outcomes, thereby providing indirect support for the functional benefits of FSRC.
4.3 Oncologic outcomes
The deterioration of tumors following surgery is a matter of concern for numerous clinicians and patients. Regarding the treatment of malignant tumors, the ultimate objective of any surgical approach is to achieve complete cure or exert maximum control over tumor recurrence and metastasis. Currently, most FSRC procedures, except for the SSC procedure, carry the risk of PCa. In this meta-analysis, we discovered that there was no significant disparity in the incidence of PCa between FSRC and nFSRC. Dall et al. (15) reported in a recent meta-analysis that only 2% of patients who underwent PCSC were identified to have clinically significant postoperative PCa. Nevertheless, these PCa rates are considerably lower than the rate of incidentally detected PCa during RC, which has been reported to be as high as 40% (36). Studies have indicated that the risk of PCa or urothelial cancer continues to decline in appropriately screened patients (23, 27, 37), and there is no clear evidence that the survival time of patients with occult PCa is affected (34). However, some scholars have pointed out that preoperative risk factors are insufficient to accurately predict clinically significant PCa, and the potential oncological risk of PSC must be taken into account (38). Although there is no significant difference in the incidence of postoperative PCa between the two surgical approaches, it is important to note that the long-term risk of prostate cancer following FSRC remains uncertain, underscoring the necessity of preoperative screening. Incidentally detected PCa does not appear to impact patient survival; the surgical method seems to be safe.
As Smith et al. (39) indicated, the proposition of FSRC represents a novel therapeutic approach for BC, which is highly technical and challenging. Nevertheless, the oncological control outcomes remain controversial and warrant further investigation. The findings of our meta-analysis demonstrated that there was no statistically significant difference in tumor recurrence and metastasis between postoperative FSRC and nFSRC, aligning with the results of certain recent studies (14, 15, 27, 37). Voskuilen et al. (31) noted that the local BC recurrence rate of PSC was 11%. Simone et al. (40) also remarked that patients with T2G3 tumors exhibited extremely poor oncological outcomes, with eight out of ten patients experiencing recurrence; this might be associated with the belated preoperative staging and grading. Botto et al. (41) et al. discovered an augmented risk of distant metastasis (17.6%) in patients who underwent PCSC without local recurrence, suggesting the existence of micrometastatic disease before the intervention. In another study, among 100 patients who underwent PCSC, five had local recurrence and 31 had distant metastasis, but among these patients, 17 had T3 disease and/or nodal disease (42), indicating that the stage rather than the surgery was determining the outcome. Given the limited follow-up duration and the small number of included studies, the current findings do not preclude the potential for differences in long-term recurrence and metastasis; more data are required in the future. Based on the current results, the tumor control of the FSRC is not inferior to that of nFSRC.
The postoperative survival duration of patients is also one of the metrics for evaluating surgical outcomes. We compared the 2-year survival period of FSRC with nFSRC. This is in line with the findings of numerous previous studies (14, 23–28), and the FSRC did not harm the postoperative survival of patients, which is approximately in accordance with nFSRC.
4.4 Subgroup analysis
We observed a significant degree of heterogeneity in the outcomes of EF (I2 = 77.8%). Subgroup analysis suggests that this heterogeneity may be attributed to small sample sizes, varying study designs, and differing surgical controls. Additionally, differences in follow-up periods could contribute to variations in postoperative EF. High heterogeneity was also noted in the analysis of nocturnal urinary continence (I2 = 70.7%). Subgroup analysis revealed that heterogeneity in research findings might be influenced by variations in surgical techniques, geographical regions of the included populations, and the retrospective nature of some studies. Furthermore, differences in postoperative evaluation methods likely contributed to this heterogeneity. For the incidence of postoperative PCa, we identified high heterogeneity as well. Subgroup analysis indicated that disparities in study regions, methodologies, sample sizes, and control groups could account for the heterogeneity in results. In conclusion, these heterogeneous factors reflect variations in medical practices, demographic characteristics, and socioeconomic backgrounds. These include the degree of standardization in surgeons’ experience and techniques, differences in pelvic floor anatomy among the study population, preoperative comorbidities, and postoperative rehabilitation protocols.
Based on our review of the included studies, it is evident that FSRC clinical research, whether RCTs or non-randomized controlled trials (nRCTs), requires adherence to rigorous principles in clinical research design. Careful planning and detailed protocols should be established before study initiation, with active management of confounding factors, rational allocation strategies, and assurance of complete follow-up data. The implementation process must also strictly adhere to the planned protocol to ensure accurate and reliable clinical research outcomes. Despite the heterogeneity observed in some studies, our overall findings provide valuable insights.
4.5 Strengths and limitations
4.5.1 Surgical methods
FSRC is a modified operation of RC. After RC, patients with ED, urinary continence dysfunction, and other complications, especially young patients, will permanently lose their reproductive function and seriously reduce the quality of life of patients with BC. FSRC preserves the relevant male reproductive structure, including the prostate or seminal vesicle or prostate capsule, and improves EF and urinary continence function after surgery, which has been supported by many controlled studies and clinical observations (6, 23–28, 34, 39). However, some researchers have noted that while postoperative complications have decreased, the risk of recurrence of urethral and pelvic tumors, as well as PCa, may have increased (14, 40, 45), and the treatment choice is still controversial. A sufficient volume of a particular surgical procedure is generally associated with improved outcomes in terms of mortality and may also contribute to better functional recovery. Pyrgidis et al. (49) conducted a retrospective analysis of data from all hospitalized patients who underwent BC surgery in Germany, demonstrating that centralization of RC not only reduces hospitalization-related morbidity and mortality but also shortens length of stay and lowers healthcare costs. A threshold of at least 50 RC procedures per year per institution was identified as necessary to achieve optimal outcomes. These findings suggest that variations in treatment outcomes may be influenced by both the treating hospital and its procedural volume. Meanwhile, Milling et al. (50) demonstrated through a cross-sectional study that RC surgery significantly reduces female patients’ satisfaction with sexual function, suggesting that women experience similar postoperative challenges. Our findings may provide a preliminary foundation for future research on female FSRC.
Overall, this study provides evidence in support of the efficacy and safety of FSRC in the treatment of BC.Although FSRC appears promising, there remains insufficient evidence to support its routine clinical implementation, except in carefully selected young patients.
4.5.2 Research result
The main advantage of this meta-analysis lies in the fact that we conducted sensitivity analysis and subgroup analysis for each outcome index. Despite the limited number of included studies and the relatively small sample size of each study, the stability and heterogeneity of the corresponding outcome were explored to a certain extent. In the subgroup analysis, it was discovered that there were some disparities in the outcome indicators when classified based on sample size, study type, control group, and study area, which could also offer some assistance for the design of related research in the future.
We accomplished this meta-analysis in accordance with the strict guidelines of PRISMA; however, certain limitations persist. Firstly, heterogeneity was evident in some outcome measures, such as EF, daytime and nighttime urinary continence, the incidence of postoperative PCa, and 2-year survival after surgery. We identified that the heterogeneity could be attributed to the selection of surgical modalities, inconsistent preoperative baseline characteristics, variations in the definitions of each index, and surgeon proficiency, among other factors. Therefore, our conclusions should be interpreted cautiously. Secondly, some studies were small-scale, retrospective, and non-randomized, entailing a certain risk of bias and confusion, which might influence the overall quality of evidence. Thirdly, several factors could affect the results, including differences in surgical methods, disparities in control groups, and tumor staging.
5 Conclusion
In this meta-analysis, FSRC with ONB is capable of enhancing postoperative EF and urinary continence. There was no statistically significant disparity in the improvement of postoperative survival time between the two groups. It remains unclear whether there are differences in tumor recurrence, metastasis, and PCa occurrence between the two groups. Overall, FSRC may offer a functional benefit in certain patient populations without substantially compromising oncologic safety, thereby supporting its continued evaluation in clinical practice. However, additional high-quality randomized controlled trials are required before broad implementation can be recommended. Although the existing evidence remains limited, current findings provide a compelling rationale for further investigation of this approach in highly selected patient cohorts.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author contributions
YH: Conceptualization, Writing – original draft. HS: Methodology, Writing – original draft. QW: Data curation, Software, Writing – original draft. ZW: Formal Analysis, Validation, Writing – original draft. RW: Resources, Writing – original draft. YZ: Formal Analysis, Validation, Writing – original draft. JW: Investigation, Writing – review & editing. NZ: Visualization, Writing – review & editing. SF: Supervision, Writing – review & editing. HW: Project administration, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. Thanks for the Scientific research project of Yunnan Science and Technology Department (202201AS070085), National Natural Science Foundation of China (grant No. 82260609), Scientific Research Fund Project of Education Department of Yunnan Province (grant No. 2024Y231), and Kunming Medical University graduate Student Innovation Fund (grant No. 2024B025).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
RC, Radical cystectomy; FSRC, fertility-sparing radical cystectomy; NSC, nerve-sparing cystectomy; EF, erectile function; nFSRC, non-fertility-sparing radical cystectomy(including RC and NSC); BC, Bladder cancer; PCSC, Prostate capsule-sparing cystectomy; PCa, prostate cancer; PSC, prostate-sparing cystectomy; SSC, seminal-sparing cysto-prostatectomy; ONB, orthotopic neobladder.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A, et al. Global cancer statistics 2018:GLOBOCAN estinates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2018) 68:394–424. doi: 10.3322/caac.21492
2. Alfred Witjes J, Max Bruins H, Carrión A, Cathomas R, Compérat E, Efstathiou JA, et al. European association of urology guidelines on muscle-invasive and metastatic bladder cancer: summary of the 2023 guidelines. Eur UROL. (2023) 85:17–31. doi: 10.1016/j.eururo.2023.08.016
3. Krishna SR and Konety BR. Current concepts in the management of muscle invasive bladder cancer. Indian J Surg Oncol. (2017) 8:74–81. doi: 10.1007/s13193-016-0586-1
4. Hautmann RE, de Petriconi RC, and Volkmer BG. Lessons learned from 1, 000 neobladders: the 90-day complication rate. J Urol. (2010) 184:990–4. doi: 10.1016/j.juro.2010.05.037
5. Zippe CD, Raina R, Massanyi EZ, Agarwal A, Jones JS, Ulchaker J, et al. Sexual function after male radical cystectomy in a sexually active population. Urology. (2004) 64:682–685-686. doi: 10.1016/j.urology.2004.05.056
6. Spitz A, Stein JP, Lieskovsky G, and Skinner DG. Orthotopic urinary diversion with preservation of erectile and ejaculatory function in men requiring radical cystectomy for nonurothelial Malignancy: a new technique. J Urol. (1999) 161:1761–4. doi: 10.1016/S0022-5347(05)68794-0
7. Schilling A and Friesen A. Transprostatic selective cystectomy with an ileal bladder. Eur Urol. (1990) 18:253–7. doi: 10.1159/000463924
8. Xiong X, Qiu S, Yi X, Jin K, Xu H, Lei H, et al. Effect of neurovascular bundle sparing radical cystectomy on postoperative continence and sexual function: a systematic review and meta-analysis. Andrology. (2020) 2:9–10. doi: 10.1111/andr.12898
9. Muto G, Collura D, Rosso R, Giacobbe A, Muto GL, and Castelli E. Seminal-sparing cystectomy: technical evolution and results over a 20-year period. Urology. (2014) 83:856–61. doi: 10.1016/j.urology.2013.08.093
10. Hautmann RE and Stein JP. Neobladder with prostatic capsule and seminal-sparing cystectomy for bladder cancer: a step in the wrong direction. Urol Clin North Am. (2005) 32:177–85. doi: 10.1016/j.ucl.2005.02.001
11. Kefer JC, Voelzke BB, Flanigan RC, Wojcik EM, Waters WB, and Campbell SC. Risk assessment for occult Malignancy in the prostate before radical cystectomy. UROLOGY. (2005) 66:1251–5. doi: 10.1016/j.urology.2005.07.006
12. Furrer MA, Kiss B, Studer UE, Wuethrich PY, Gahl B, Seiler R, et al. Seminal vesical sparing cystectomy for bladder cancer is feasible with good functional results without impairing oncological outcomes: A longitudinal long-term propensity-matched single center study. J Urol. (2021) 205:1629–40. doi: 10.1097/JU.0000000000001635
13. De Vries RR, Nieuwenhuijzen JA, van Tinteren H, Oddens JR, Visser O, van der Poel HG, et al. Prostate-sparing cystectomy: long-term oncological results. BJU Int. (2009) 104:1239–43. doi: 10.1111/j.1464-410X.2009.08615.x
14. Abdelaziz AY, Shaker H, Seifelnasr M, Elfol H, Nazim M, and Mahmoued M. Early oncological and functional outcomes of prostate capsule sparing cystectomy compared with standard radical cystectomy. Curr Urol. (2019) 13:37–45. doi: 10.1159/000499296
15. Dall CP, Mason JB, Goldman CC, Fabrizio G, Alagha EC, Chou J, et al. Functional and oncologic outcomes of prostate capsule-sparing radical cystectomy: A systematic review and meta-analysis. UROL ONCOL-SEMIN ORI. (2024) 42:121–32. doi: 10.1016/j.urolonc.2024.01.008
16. Mertens LS, Meijer RP, de Vries RR, Nieuwenhuijzen JA, van der Poel HG, Bex A, et al. Prostate sparing cystectomy for bladder cancer: 20-year single center experience. J Urol. (2014) 191:1250–5. doi: 10.1016/j.juro.2013.11.031
17. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Int J Surg (London England). (2021) 88:105906. doi: 10.1016/j.ijsu.2021.105906
18. Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. (2017) 358:j4008. doi: 10.1136/bmj.j4008
19. Wan X, Wang W, Liu J, and Tong T. Estimating the sample mean and standard deviation from the sample size, median, range, and/or interquartile range. BMC Med Res Methodol. (2014) 14:135. doi: 10.1186/1471-2288-14-135
20. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. (2010) 25:603–5. doi: 10.1007/s10654-010-9491-z
21. Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. (2011) 343:d5928. doi: 10.1136/bmj.d5928
22. Duval S and Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. (2000) 56:455–63. doi: 10.1111/j.0006-341X.2000.00455.x
23. Jacobs BL, Daignault S, Lee CT, Hafez KS, Montgomery JS, Montie JE, et al. Prostate capsule sparing versus nerve sparing radical cystectomy for bladder cancer: results of a randomized, controlled trial. J Urol. (2015) 193:64–70. doi: 10.1016/j.juro.2014.07.090
24. Chen PY and Chiang PH. Comparisons of Quality of Life and Functional and Oncological Outcomes after Orthotopic Neobladder Reconstruction: Prostate-Sparing Cystectomy versus Conventional Radical Cystoprostatectomy. BioMed Res Int. (2017) 2017:1983428. doi: 10.1155/2017/1983428
25. Basiri A, Pakmanesh H, Tabibi A, Radfar MH, Tajalli F, Ahadi B, et al. Overall survival and functional results of prostate-sparing cystectomy: a matched case-control study. Urol J. (2012) 9:678–84.
26. Colombo R, Pellucchi F, Moschini M, Gallina A, Bertini R, Salonia A, et al. Fifteen-year single-centre experience with three different surgical procedures of nerve-sparing cystectomy in selected organ-confined bladder cancer patients. World J Urol. (2015) 33:1389–95. doi: 10.1007/s00345-015-1482-y
27. Saad M, Moschini M, Stabile A, Macek P, Lanz C, Prapotnich D, et al. Long-term Functional and Oncologic Outcomes of Nerve Sparing and Prostate Capsule Sparing Cystectomy: a single center experience. BJU Int. (2020) 125:253–9. doi: 10.1111/bju.14850
28. Wang XH, Luo X, and Chen SQ. Impact of preservation of distal prostatic capsule and seminal vesicle on functions of orthotopic ideal neobladder and erectile function of bladder cancer patients. Ai Zheng. (2008) 27:62–5.
29. He W, Yang J, Gao M, Liu H, Li J, Hu J, et al. Pelvic reconstruction and lateral prostate capsule sparing techniques improve early continence of robot-assisted radical cystectomy with orthotopic ileal neobladder. Int UROL Nephrol. (2022) 54:1537–43. doi: 10.1007/s11255-022-03214-4
30. Davila HH, Weber T, Burday D, Herve JM, Yonneau L, and Lebret T. Total or partial prostate sparing cystectomy for invasive bladder cancer: long-term implications on erectile function. BJU Int. (2007) 100:1026–9. doi: 10.1111/j.1464-410X.2007.07169.x
31. Voskuilen CS, Fransen van de Putte EE, Pérez-Reggeti JI, van Werkhoven E, Mertens LS, van Rhijn BWG, et al. Prostate sparing cystectomy for bladder cancer: A two-center study. EJSO-EUR J Surg ONC. (2018) 44:1446–52. doi: 10.1016/j.ejso.2018.05.032
32. Kessler TM, Burkhard FC, Perimenis P, Danuser H, Thalmann GN, Hochreiter WW, et al. Attempted nerve sparing surgery and age have a significant effect on urinary continence and erectile function after radical cystoprostatectomy and ileal orthotopic bladder substitution. J Urol. (2004) 172:1323–7. doi: 10.1097/01.ju.0000138249.31644.ec
33. Wang XJ, Zheng ZF, Yu Q, Li W, Deng Y, Xie ZD, et al. Anatomical and histological investigation of the area anterior to the anorectum passing through the levator hiatus. Zhonghua Wei Chang Wai Ke Za Zhi. (2023) 26:578–87. doi: 10.3760/cma.j.cn441530-20220504-00197
34. Muto G, Bardari F, D'Urso L, and Giona C. Seminal sparing cystectomy and ileocapsuloplasty: long-term follow-up results. J Urol. (2004) 172:76–80. doi: 10.1097/01.ju.0000132130.64727.b6
35. Mohamed NE, Diefenbach MA, Goltz HH, Lee CT, Latini D, Kowalkowski M, et al. Muscle invasive bladder cancer: from diagnosis to survivorship. Adv Urol. (2012) 2012:1–10. doi: 10.1155/2012/142135
36. Bruins HM, Djaladat H, Ahmadi H, Sherrod A, Cai J, Miranda G, et al. Incidental prostate cancer in patients with bladder urothelial carcinoma: comprehensive analysis of 1, 476 radical cystoprostatectomy specimens. J Urol. (2013) 190:1704–9. doi: 10.1016/j.juro.2013.05.034
37. Hernández V, Espinos EL, Dunn J, MacLennan S, Lam T, Yuan Y, et al. Oncological and functional outcomes of sexual function-preserving cystectomy compared with standard radical cystectomy in men: A systematic review. Urol Oncol. (2017) 35:539.e17–539.e29. doi: 10.1016/j.urolonc.2017.04.013
38. Tanaka T, Koie T, Ohyama C, Hashimoto Y, Imai A, Tobisawa Y, et al. Incidental prostate cancer in patients with muscle-invasive bladder cancer who underwent radical cystoprostatectomy. Jpn J Clin Oncol. (2017) 47:1078–82. doi: 10.1093/jjco/hyx119
39. Smith ZL and Soloway MS. Prostate capsule sparing radical cystectomy - a safe procedure for a few. Cent Eur J Urol. (2016) 69:32–3. doi: 10.5173/ceju.2016.e109
40. Simone G, Papalia R, Leonardo C, Sacco R, Damiano R, Guaglianone S, et al. Prostatic capsule and seminal vesicle-sparing cystectomy: improved functional results, inferior oncologic outcome. Urology. (2008) 72:162–6. doi: 10.1016/j.urology.2007.11.077
41. Botto H, Sebe P, Molinie V, Herve JM, Yonneau L, and Lebret T. Prostatic capsule- and seminal-sparing cystectomy for bladder carcinoma: initial results for selected patients. BJU Int. (2004) 94:1021–5. doi: 10.1111/j.1464-410X.2004.05097.x
42. Vallancien G, Abou El Fettouh H, Cathelineau X, Baumert H, Fromont G, and Guillonneau B. Cystectomy with prostate sparing for bladder cancer in 100 patients: 10-year experience. J Urol. (2002) 168:2413–7. doi: 10.1016/S0022-5347(05)64157-2
43. Klotz L. Prostate capsule sparing radical cystectomy: oncologic safety and clinical outcome. Ther Adv Urol. (2009) 1:43–50. doi: 10.1177/1756287209103921
44. Salem HK. Radical cystectomy with preservation of sexual function and fertility in patients with transitional cell carcinoma of the bladder: new technique. Int J Urol. (2007) 14:294–8. doi: 10.1111/j.1442-2042.2007.01607.x
45. Moschini M, Shariat SF, Freschi M, Soria F, Abufaraj M, Gandaglia G, et al. Impact of prostate involvement on outcomes in patients treated with radical cystoprostatectomy for bladder cancer. Urol Int. (2017) 98:290–7. doi: 10.1159/000454736
46. Pyrgidis N, Schulz GB, Ebner B, Jokisch F, Eismann L, Karatas D, et al. Radical cystectomy with ileal orthotopic neobladder after 70 years leads to worse health-related quality of life. J Clin Med. (2024) 13:6102. doi: 10.3390/jcm13206102
47. Bizzarri FP, Campetella M, Russo P, Marino F, Gavi F, Rossi F, et al. Risk factors for benign uretero-enteric anastomotic strictures after open radical cystectomy and ileal conduit. Urologia. (2025) 92:224–30. doi: 10.1177/03915603241303634
48. Volz Y, Eismann L, Pfitzinger P, Westhofen T, Ebner B, Jokisch JF, et al. Long-term health-related quality of life (HRQOL) after radical cystectomy and urinary diversion - A propensity score-matched analysis. Clin GENITOURIN CANC. (2022) 20:e283–90. doi: 10.1016/j.clgc.2022.03.003
49. Pyrgidis N, Volz Y, Ebner B, Kazmierczak PM, Enzinger B, Hermans J, et al. The effect of hospital caseload on perioperative mortality, morbidity, and costs in bladder cancer patients undergoing radical cystectomy: results of the German nationwide inpatient data. World J UROL. (2024) 42:19. doi: 10.1007/s00345-023-04742-z
Keywords: bladder cancer, radical cystectomy, modified, meta-analysis, orthotopic neobladder
Citation: Huang Y, Shi H, Wang Q, Wang Z, Wang R, Zhang Y, Wang J, Zhang N, Fu S and Wang H (2025) Efficacy and safety of male fertility-sparing radical cystectomy with orthotopic neobladder versus radical cystectomy and nerve-sparing cystectomy: a meta-analysis. Front. Oncol. 15:1617812. doi: 10.3389/fonc.2025.1617812
Received: 25 April 2025; Accepted: 17 October 2025;
Published: 31 October 2025.
Edited by:
Sanja Stifter-Vretenar, Skejby Sygehus, DenmarkReviewed by:
Yannic Volz, LMU Munich University Hospital, GermanyFrancesco Pio Bizzarri, Agostino Gemelli University Polyclinic (IRCCS), Italy
Copyright © 2025 Huang, Shi, Wang, Wang, Wang, Zhang, Wang, Zhang, Fu and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Haifeng Wang, d2FuZ2hhaWZlbmdAa21tdS5lZHUuY24=
 Yu Huang
Yu Huang Hongjin Shi2
Hongjin Shi2 Shi Fu
Shi Fu Haifeng Wang
Haifeng Wang