- 1Department of Pharmacy, The First People’s Hospital of Nanning, Nanning, China
- 2Department of Pharmacy, Shengli Clinical Medical College of Fujian Medical University, Fujian Provincial Hospital, Fuzhou University Affiliated Provincial Hospital, Fuzhou, Fujian, China
- 3Department of Pharmacy, The First Affiliated Hospital, Fujian Medical University, Fuzhou, China
- 4Department of Pharmacy, National Regional Medical Center, Binhai Campus of the First Affiliated Hospital, Fujian Medical University, Fuzhou, China
- 5Department of Pharmacy, Pingtan Comprehensive Experimental Area Hospital, Fuzhou, China
Introduction: Pacritinib, a selective Janus kinase (JAK) inhibitor, is approved for the treatment of myelofibrosis in adults with severe thrombocytopenia. However, its safety profile in real-world populations remains unclear. The aim of study is provided a comprehensive profile of pacritinib's safety by evaluating the adverse events (AEs) using a real-world pharmacovigilance database.
Methods: Data from the FDA Adverse Event Reporting System (FAERS) database, spanning from the first quarter of 2022 to the second quarter of 2024, served as the basis for this analysis. To identify potential AE risk signals, several disproportionality analysis methods were applied, including the reporting odds ratio, the proportional reporting ratio, the multi-item gamma Poisson shrinker, and the Bayesian confidence propagation neural network.
Results: A total of 4,304,335 AE reports were collected from the FAERS, with 1,940 reports identifying pacritinib as the primary suspect drug. Significant disproportionality was observed in the following system organ classes: gastrointestinal disorders, investigations, and surgical and medical procedures. Common preferred terms were identified, including diarrhea, fatigue, death, nausea, platelet count decreased, and hemoglobin decreased. Notably, 26 off-label AEs were also identified.
Discussion: Our study would provide valuable insights for the post-marketing safety surveillance and assessment of pacritinib, and guide its clinical practice.
1 Introduction
Myelofibrosis (MF), a type of myeloproliferative neoplasm (MPN), encompasses primary myelofibrosis and secondary myelofibrosis (1). Clinically, MF is characterized by splenomegaly, constitutional symptoms, anemia, thrombocytosis, or thrombocytopenia (2). It is a rare disease, with an incidence of 4 to 6 cases per 100,000 individuals in the United States (3). While conventional treatments can improve or alleviate the clinical symptoms of MF, they fall short of delivering a definitive cure. Allogeneic stem cell transplantation is a potentially curative treatment for MF, but it is hindered by limitations and a low success rate. In recent years, the discovery of pathogenic genes in MPNs has spurred the development of Janus kinase (JAK) inhibitors, offering a novel therapeutic avenue for MF. JAK is a family of non-receptor protein-tyrosine kinases, comprising JAK1, JAK2, JAK3, and tyrosine kinases-2, among which JAK2 mutation (JAK2V617F) plays a pivotal role in the pathogenesis of MPNs (4, 5). JAK inhibitors, specifically targeting JAK2, have demonstrated efficacy in reducing spleen size and alleviating disease-related symptoms (6), thus providing a promising treatment strategy for patients with MF.
Pacritinib is an oral JAK2/FLT3 inhibitor that was approved by the U.S. Food and Drug Administration (FDA) on February 28, 2022, for the treatment of intermediate or high-risk primary or secondary (post-polycythemia vera or post-essential thrombocythemia) myelofibrosis with platelet counts below 50×10^9/L (7). Studies have indicated that pacritinib significantly alleviates clinical symptoms in MF patients with cytopenias, particularly by reducing spleen size, thereby improving patients’ quality of life and extending survival (8). However, several adverse events (AEs) have been reported during pacritinib administration, including diarrhea, nausea, vomiting, thrombocytopenia, anemia, peripheral edema, dizziness, and fever, with the potential for severe AEs to occur (9, 10). With the widespread use of pacritinib, it is necessary to conduct post-marketing surveillance and evaluation.
The FDA Adverse Event Reporting System (FAERS) database serves as a spontaneous reporting system for the collection of AE information and medication error information worldwide, covering millions of drug AE reports. It provides support for the FDA’s post-marketing drugs monitor and therapeutic products. This system plays an essential role in identifying and evaluating potential signals while quantifying the associations between specific drugs and AEs reported by individuals. The database is regularly updated every quarter, encompassing a wide range of AE records, documentation of medication errors, and instances of product quality complaints (11, 12). The aim of study is perform signal detection of pacritinib-related AEs in the FAERS database, evaluating the real-world incidence of AEs, identifying new and serious AE signals, and providing reference for the long-term application management of pacritinib.
2 Methods
The methods section was based on our previous study (13).
2.1 Data source
This pharmacovigilance investigation evaluated the AEs associated with pacritinib, as documented in the FAERS database. Data entries made between the first quarter of 2022 and the second quarter of 2024 were included in the analysis to coincide with the timing of FDA’s approval of pacritinib. The FAERS data were obtained from the official FDA website, accessed at https://fis.fda.gov/extensions/FPD-QDE-FAERS/FPD-QDE-FAERS.html.
The FAERS data package can be downloaded from the website as a compressed file, comprising seven distinct sections that encompass demographic and management details, drug-related information, AEs, patient outcomes, sources of reporting, treatment duration with reported drugs, indications, and removed cases (14). The intricate process of data manipulation was proficiently executed through the utilization of Python 3.10 (Python Software Foundation, Holland) and Microsoft Excel 2019, collectively contributing to the meticulous data processing endeavors. Fuzzy matching techniques were utilized to filter reports referencing the drug’s generic name, pacritinib, and its product name, Vonjo®, specifically tagged as “primary suspect” drug names in the database. Given that this study hinged on a globally accessible database containing de-identified information without direct treatment intervention or the collection of human samples, the requirement for informed consent was deemed unnecessary.
2.2 Data extraction
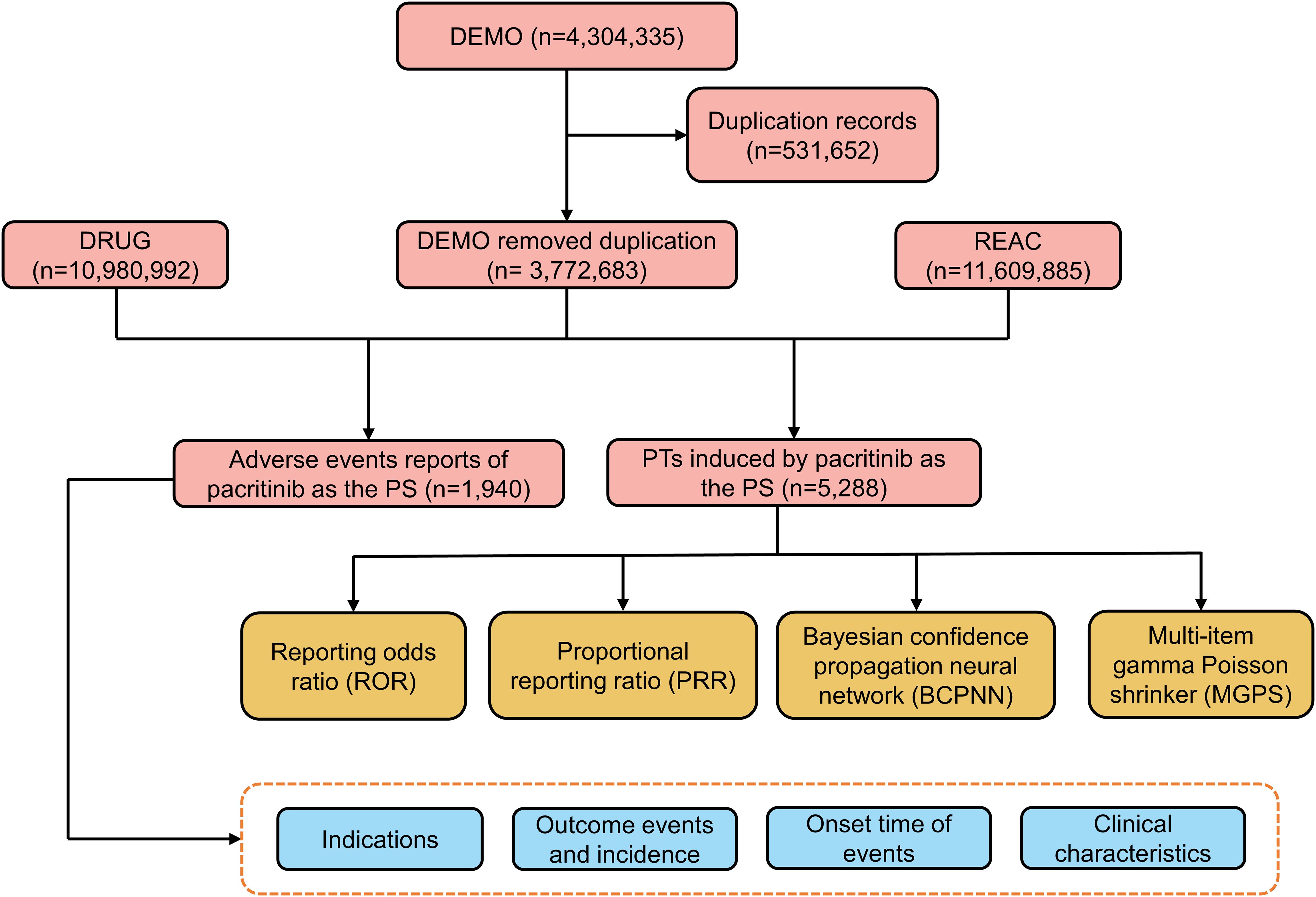
Due to the inherent spontaneity of the reports, duplication was likely to occur. Therefore, a deduplication process was performed before the analysis, per the FDA guidelines (15). We manually reviewed the reports to exclude instances where the PRIMARYID was lower when the CASEID matched. Additionally, we excluded any CASEID listed in the deleted cases file. AEs in the FAERS are classified based on the preferred terms (PTs) from the standardized Medical Dictionary for Regulatory Activities 26.0 (MedDRA 26.0). The classification is made into five levels: system organ class (SOC), high-level group term (HLGT), high-level term (HLT), PT, and lowest-level term (LLT) (15, 16). Figure 1 depicts a flowchart to illustrate the sequential procedures involved in extracting, processing, and analyzing data.
In the context of AEs in drug-related reports, it is the responsibility of the individuals reporting these events to assign appropriate codes. The available codes for assignment were: 1 = suspect, 2 = concomitant, and 3 = interacting. To ensure utmost precision and accuracy in our analysis, we made a strategic decision to designate code “1” as “PS” (primary suspected) within the DRUG files with the aim of enhancing analytical accuracy and overall conclusions (11).
2.3 Data mining
Disproportionality analysis is a vital technique employed in pharmacovigilance studies, playing a pivotal role in the identification of potential signals indicating AEs associated with a drug. This approach entails comparing the frequency of AEs linked to a specific medication against the occurrence of AEs related to all other drugs. Essentially, it relies on the concept that a signal emerges during data extraction when the incidence rate of a particular AE for a given drug significantly surpasses the background occurrence rate observed across the entire database. This deviation from normality must exceed a predetermined threshold or set of criteria to be considered statistically significant (11). To explore the association between pacritinib and AEs, we applied both Bayesian and frequentist approaches, employing various statistical measures such as the reporting odds ratio (ROR), proportional reporting ratio (PRR), information component (IC), and empirical Bayes geometric mean (EBGM) (17–19). The equations and criteria for these four algorithms are detailed in Supplementary Tables S1 and S2.
3 Results
3.1 Descriptive analysis
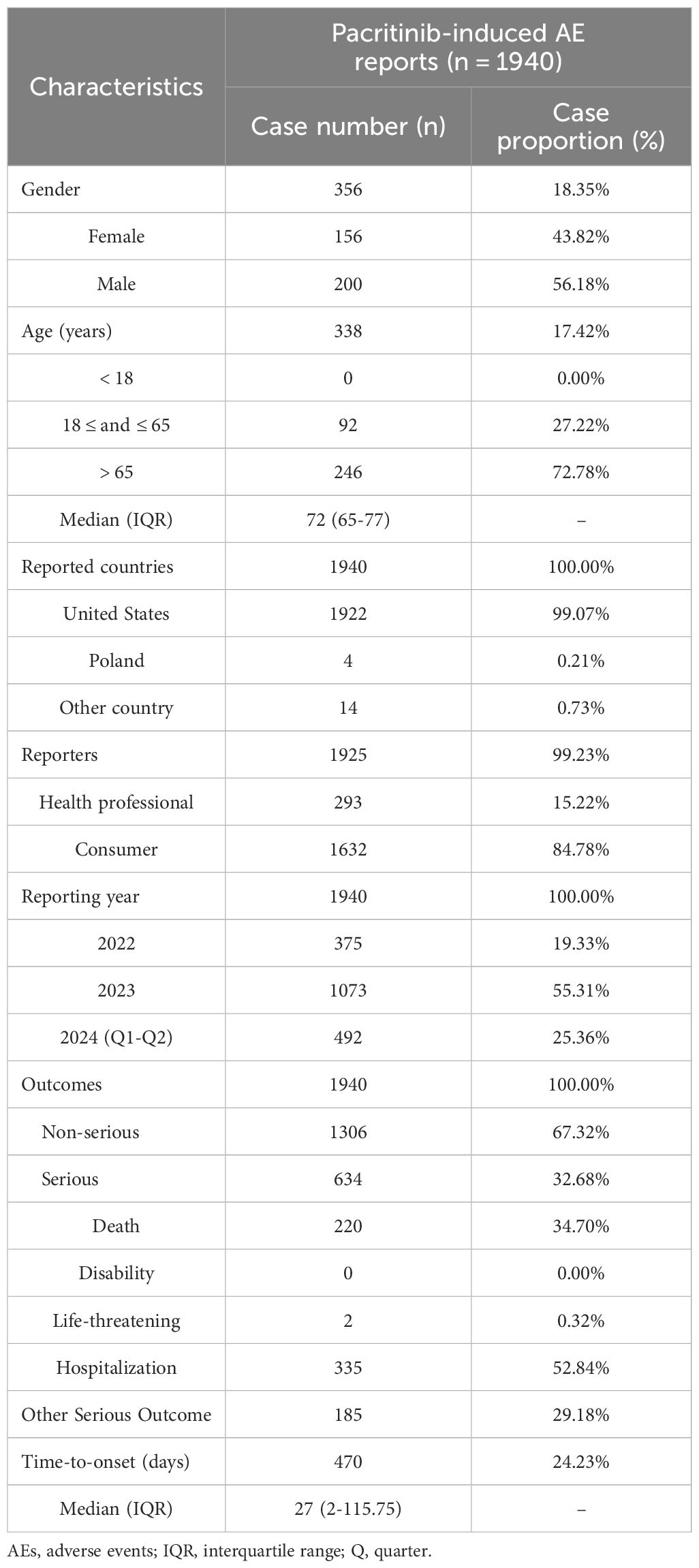
A total of 4,304,335 AE reports were collected from the FAERS database from Q1 2022 to Q2 2024. After removing duplicate reports, 1,940 reports of pacritinib as the PS drug were obtained (Figure 1). The clinical characteristics of AEs associated with pacritinib were summarized in Table 1. Among the recorded AEs, the proportion of males (56.18%) was slightly higher than that of females (43.82%); most of the patients who experienced AEs were aged 65 years or older (72.78%), with a median age of approximately 72 years (range: 65-77 years). A total of 634 (32.68%) serious AEs were reported, including 335 (52.84%) cases of hospitalization and 220 (34.70%) cases of death. The majority of these AE reports originated from the United States (99.07%), and most were reported by consumers (84.78%). Since the drug’s market introduction, the highest number of AEs was reported in 2023 (55.31%).
3.2 Disproportionality analysis
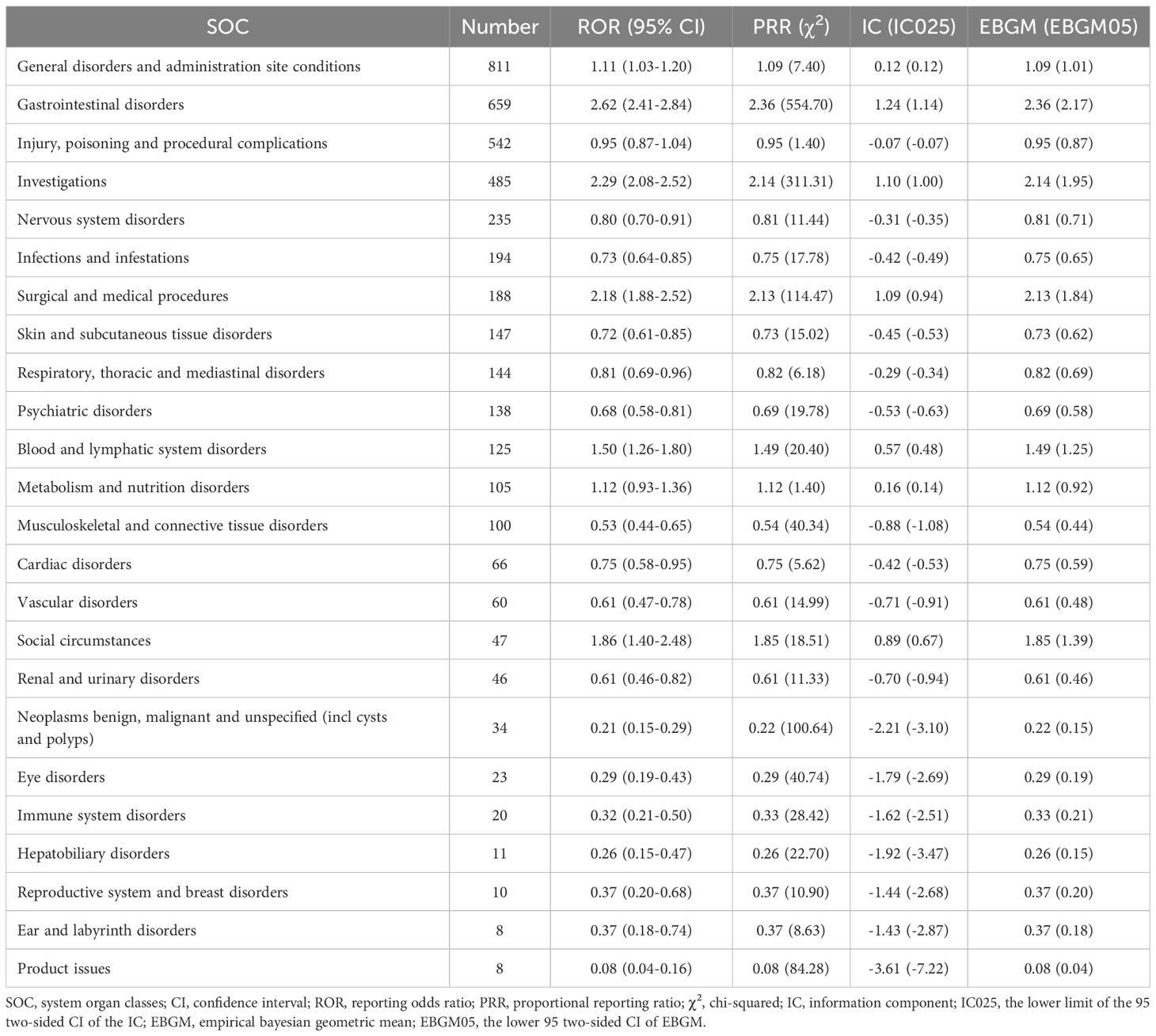
The AEs associated with pacritinib detected in this study mainly involved 24 SOCs. The disproportionality analysis signals at the SOC level were described in Table 2. The SOCs meeting all four algorithm criteria were gastrointestinal disorders (n=659, ROR 2.62, PRR 2.36, IC 1.24, EBGM 2.36), investigations (n=485, ROR 2.29, PRR 2.14, IC 1.10, EBGM 2.14), and surgical and medical procedures (n=188, ROR 2.18, PRR 2.13, IC 1.09, EBGM 2.13). Additionally, SOCs meeting one of the four algorithm criteria included general disorders and administration site conditions (n=811, ROR 1.11, IC 0.12), blood and lymphatic system disorders (n=125, ROR 1.50, IC 0.57), metabolism and nutrition disorders (n=105, ROR 1.12, IC 0.16), and social circumstances (n=47, ROR 1.86, IC 0.89). Of note, some signals unrelated to drug AEs were also collected in this study, including injury, poisoning and procedural complications, surgical and medical procedures, and social circumstances.
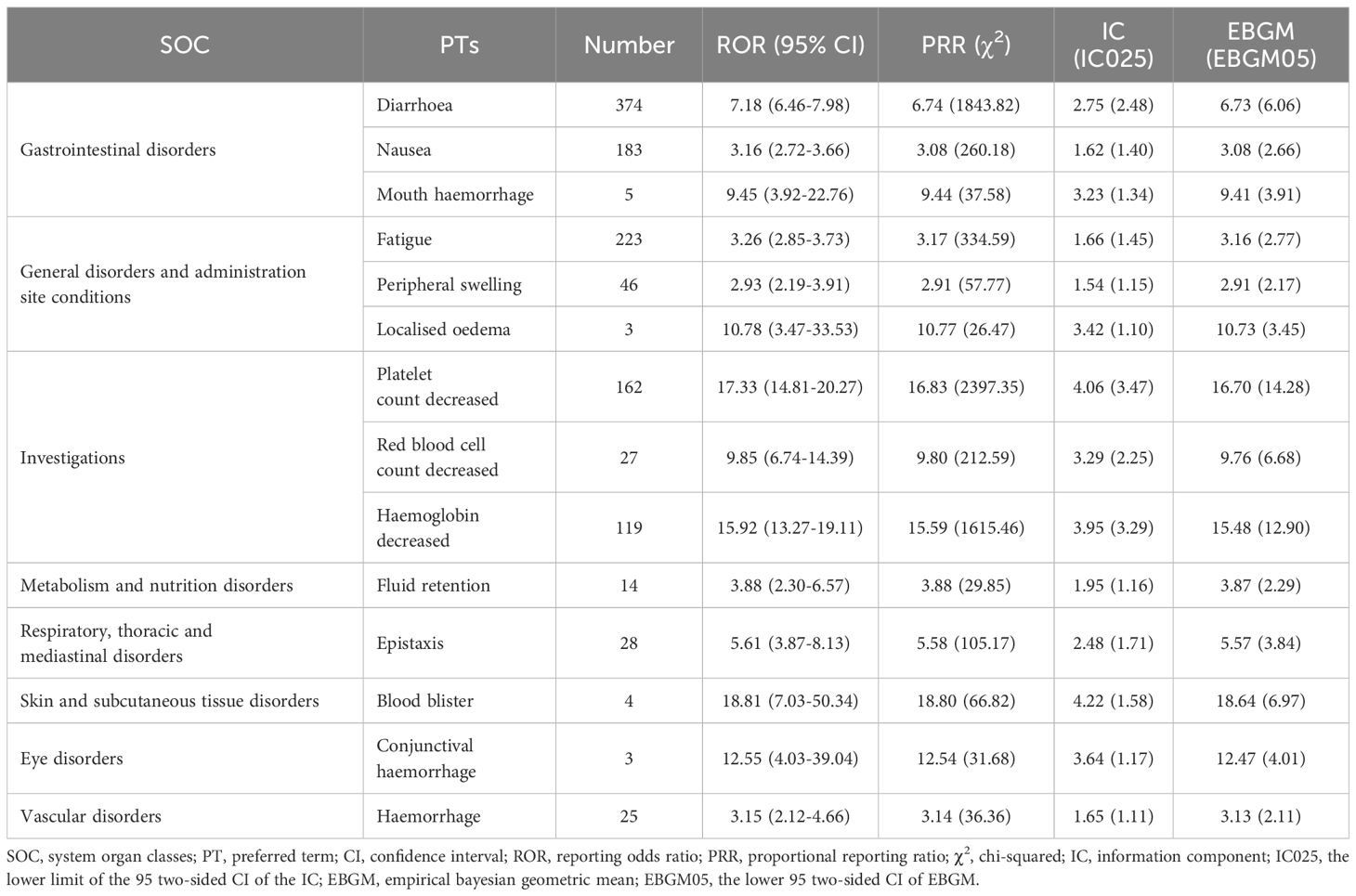
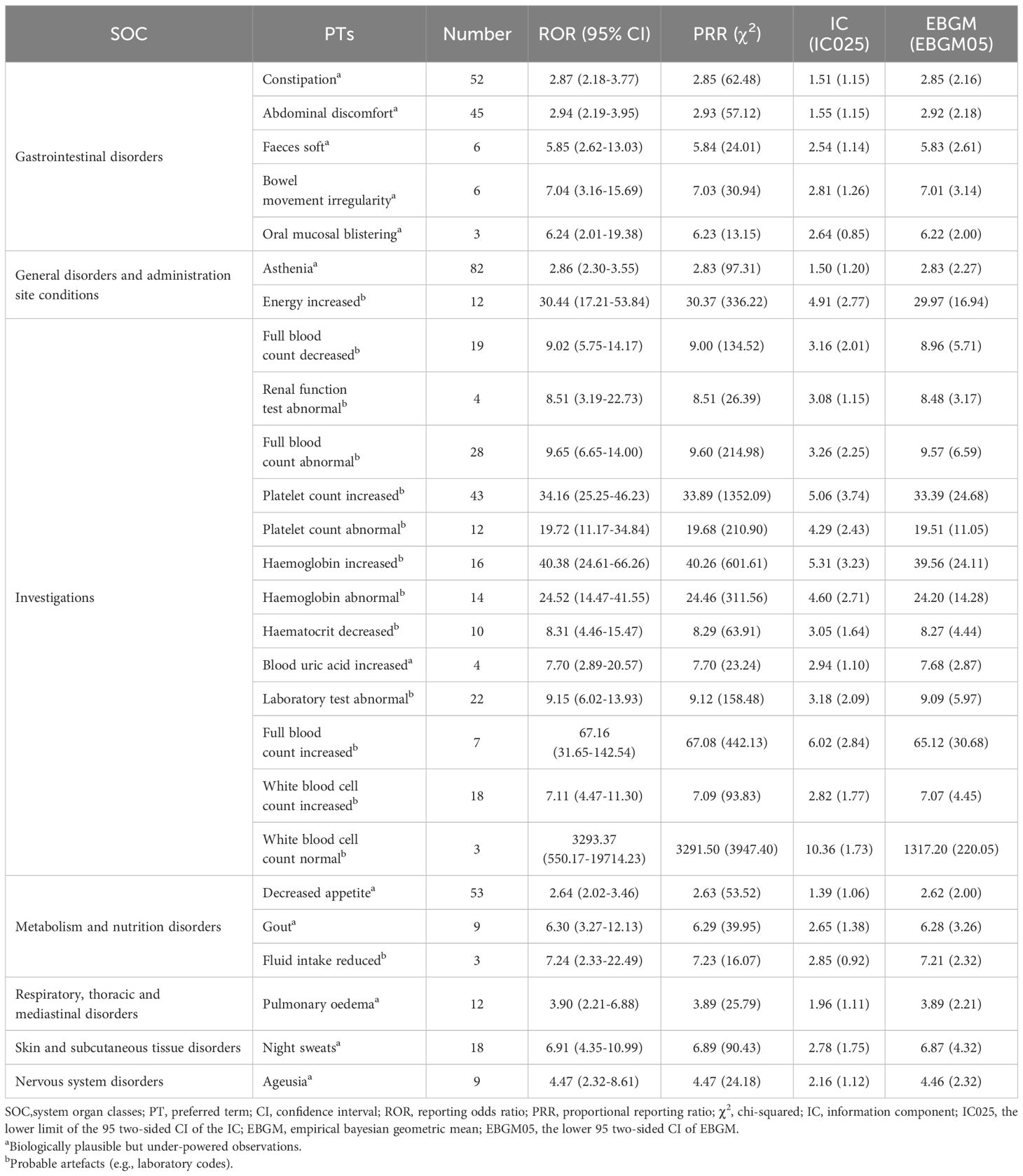
A total of 60 significant disproportionality PTs related to pacritinib were detected across 14 SOCs by applying all four algorithms in combination. The top 10 most common reports were diarrhea (n=374), fatigue (n=223), death (n=194), nausea (n=183), platelet count decreased (n=162), hemoglobin decreased (n=119), asthenia (n=82), splenomegaly (n=61), decreased appetite (n=53), and constipation (n=52). Table 3 presents the signal strength of AEs listed in the pacritinib label. Among these, diarrhea, nausea, platelet count decreased, hemoglobin decreased, and peripheral swelling were consistent with the common AEs enumerated in the drug label. Notably, after comparing with the drug label, our study identified 26 PTs not listed in the label (Table 4), such as asthenia (n=82), decreased appetite (n=53), constipation (n=52), abdominal discomfort (n=45), night sweats (n=18), pulmonary oedema (n=12), which may represent new potential AEs of pacritinib. Furthermore, Supplementary Table S4 presents PTs that are unrelated to AEs of pacritinib.
3.3 Onset time of events
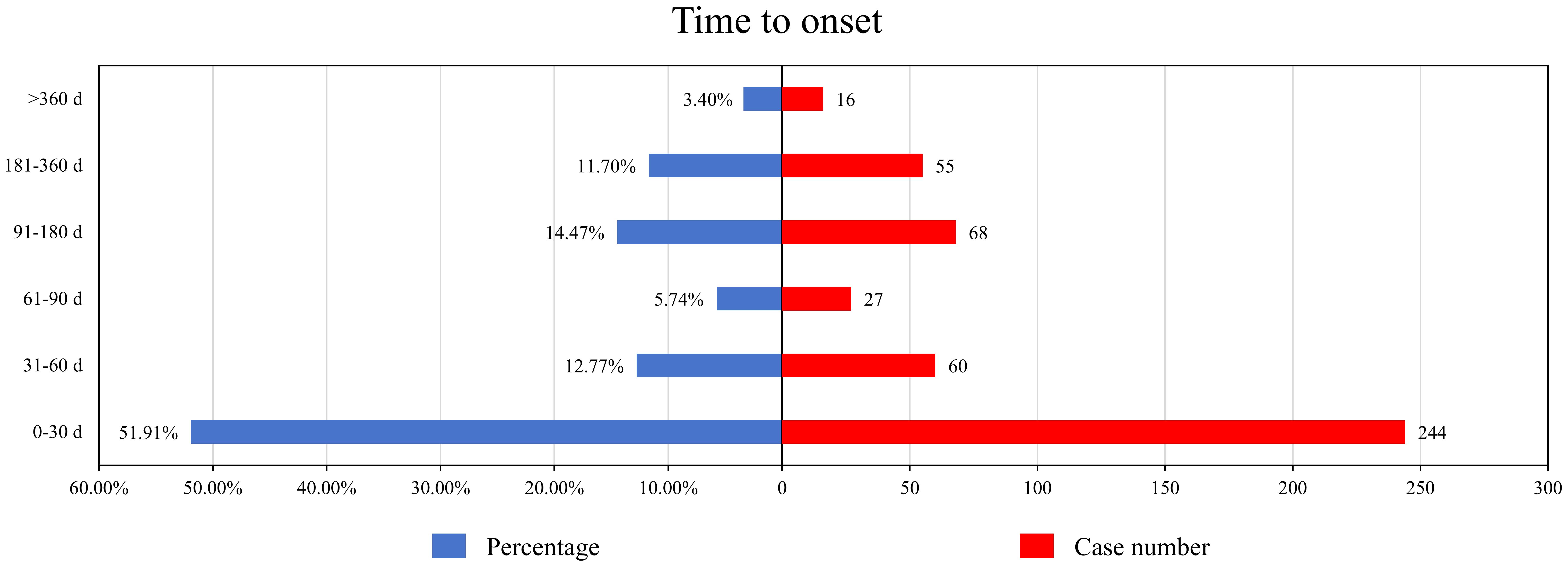
After excluding reports with missing data and duplicates, a total of 470 AEs included the time to onset. The median onset time for pacritinib-related AEs was 27 days (interquartile range 2-115.75 days). As shown in Figure 2, more than half of the AEs occurred within the first month (n=244, 51.91%) after starting pacritinib treatment. The frequency of AEs decreased over time, but there was a resurgence in AEs during the 3 to 12-month period (n=123, 26.17%) after initiation. Remarkably, even after one year of pacritinib treatment, 3.40% (n=16) of the AEs were still reported.
4 Discussion
Current studies have indicated that the pathogenesis of MF is frequently linked to dysregulation of the JAK2 signaling pathway. Specifically, the V617F mutation in the JH2 domain of JAK2 causes a loss of the negative regulatory function of JH1, resulting in excessive collagen proliferation in the bone marrow hematopoietic tissue, thereby triggering MF (20). The safety of pacritinib, a novel JAK inhibitor used for the treatment of MF with severe thrombocytopenia, raises critical concern for healthcare providers and patients. Our study provides the AEs profile of pacritinib in real-world settings, which would help clinicians in making more comprehensive and accurate treatment decisions during the treatment of pacritinib in MF.
Our study revealed a male-to-female ratio of 1.28:1 in pacritinib-related AE reports, with the majority of events involving individuals aged 65 or older. This finding is consistent with the epidemiological characteristics of MF, which exhibits a slightly higher incidence in males, and a median age of initial diagnosis at 65 years (21, 22). However, the substantial proportion of missing data on gender (81.65%) and age (82.58%) may affect these results. Notably, 99.07% of AE reporting country is the United States, a consequence of pacritinib’s lack of approval outside North America. Therefore, the potential differences in disease incidence and AE rates among different ethnic groups cannot be excluded.
The most frequently reported AEs associated with pacritinib in our findings were gastrointestinal disorders, notably diarrhea (n=374), nausea (n=183), and constipation (n=52), which was consistent with earlier studies. Previous phase II clinical trials indicated that the most common AEs in patients receiving pacritinib were grade 1 and 2 diarrhea, nausea, and vomiting (23, 24). Similarly, the most common AEs in the pacritinib group were observed in a larger randomized, controlled, phase III trials (PERSIST-1), including diarrhea (n=120, 55%), nausea (n=60, 27%), and vomiting (n=36, 16%), and 9 (4%) patients discontinued treatment due to gastrointestinal AEs (25). Diarrhea emerged as the most frequent gastrointestinal AE associated with pacritinib and was generally manageable with standard antidiarrheal medications, typically resolving within 1 to 2 weeks (9). Current evidence suggests that pacritinib-related gastrointestinal AEs may be related to FLT3 inhibition (6), with most events being grade 1 or 2 and within manageable limits. However, severe gastrointestinal events, such as diarrhea, warrant still be monitored closely.
Hematologic toxicity is a common AE of JAK inhibitors, often manifesting as febrile neutropenia, anemia, and thrombocytopenia (26). Pacritinib, which selectively inhibits JAK2, FLT3, c-fms, and IRAK1, but not inhibit JAK1 (27), is associated with less bone marrow suppression compared to other JAK inhibitors such as ruxolitinib and fedratinib. This reduced bone marrow suppression is likely due to the selective inhibition of JAK2 while sparing JAK1, and the reduction of hematopoietic suppressive cytokines through inhibition of interleukin-1 receptor-associated kinase 1 and colony-stimulating factor 1 receptor (28). Thus, pacritinib is a safer option for MF patients with severe thrombocytopenia. A phase III trials observed hematologic AEs including anemia and thrombocytopenia (9, 25), and a retrospective analysis of safety data from 189 patients in a phase III trial showed that the most common grade 3 or 4 AEs were thrombocytopenia (34.8%) and anemia (31.8%), though these rarely necessitated dose reductions or discontinuation (29). Our study also identified a substantial number of reports related to thrombocytopenia and hemoglobin decreased, but the detailed information on the severity and correlation of these AEs were lacked. Additionally, our results indicated strong signal strength for hematologic-related investigations, such as hemoglobin increased (ROR 40.38, PRR 40.26, IC 5.31, EBGM 39.56) and platelet count increased (ROR 34.16, PRR 33.89, IC 5.06, EBGM 33.39), suggesting that necessary for intensive monitoring of hematologic parameters during pacritinib treatment.
The PERSIST-1 trial identified the most common severe AEs associated with pacritinib as grade 3 or 4 bleeding (3%) and cardiac events (8%) (25). Similarly, the multicenter randomized phase III study (PERSIST-2) reported grade 3 or 4 bleeding (10.5%) and cardiac events (9.5%), rates comparable to those observed in the Best Available Therapy (BAT) group (9). Risk-adjusted analyses of early-phase clinical trials have demonstrated that the incidence of fatal AEs with pacritinib is lower than that associated with BAT, with no increased occurrences of bleeding, cardiac events, secondary malignancies, or thrombosis (30). Concerns regarding these severe AEs have prompted adjustments in patient exclusion criteria in subsequent dose-exploration PAC203 clinical trials. Patients receiving pacritinib did not experience grade 3 or higher bleeding or cardiac events (31). In our study, while we did not observe AEs such as cardiac failure or prolonged QT interval, we did identify bleeding-related AEs, including hemorrhage, conjunctival hemorrhage, epistaxis, mouth hemorrhage, and blood blister. Although severe AEs like intracerebral hemorrhage or meningeal bleeding were not reported, it is important to monitor and assess these potential AEs during pacritinib treatment in MF patients.
In this study, we not only identified common AEs labeled in pacritinib’s package insert, including diarrhea, thrombocytopenia, nausea, peripheral edema, and hemorrhage, but also discovered 26 potential AEs not listed in the label. Among them, constipation (n=52), abdominal discomfort (n=45), decreased appetite (n=53), and asthenia (n=82) are of significant clinical concern. Literature comparison analysis shows that these newly identified AEs have been clearly reported in similar JAK2/FLT3 inhibitors: phase III trial data of fedratinib indicates that 27% of patients experienced constipation and 24% asthenia (32); in ruxolitinib treatment, 5.5% of patients had constipation, 3.2% had decreased appetite, and 15.4% presented with asthenia (33). Such consistency suggests that these AEs may be associated with the pharmacological mechanisms of JAK inhibitors. It is noteworthy that constipation, decreased appetite, and abdominal discomfort have been documented in the phase I/II studies of pacritinib, and this study further confirms their clinical relevance by expanding the sample size. Future research should combine pharmacogenomic analysis to explore their molecular mechanisms.
Our study also detected some AE signals associated with primary diseases, such as splenomegaly and splenic disorders. Additionally, a total of 194 death reports were collected in this study (ROR 2.75, PRR 2.69, IC 1.43, EBGM 2.69). Given that this study cannot exclude the influence of primary disease, concomitant medications, dosage, and treatment duration, the findings only suggest a statistical association between the drug and AEs. The determination of causality between pacritinib and these events must be assessed in the context of evidence-based medicine. It is emphasized that studies have shown pacritinib may have a lower risk of death compared to the best available therapy (BAT) (hazard ratio [HR] = 0.68, 95% CI: 0.30–1.53) (25). Based on this evidence, the “death” signals identified in FAERS may partially reflect progression of patients’ underlying diseases (e.g., myelofibrosis) rather than direct drug toxicity.
In addition to potential disease progression or comorbidities, concomitant medications are a significant confounding factor in attributing AEs. Patients in the study commonly used multiple concomitant medications, whose potential drug interactions may significantly impact the safety profile of pacritinib. Pacritinib is primarily metabolized by the cytochrome P450 3A (CYP3A) enzyme system, making it susceptible to pharmacokinetic influences from CYP3A inducers or inhibitors (4). For example, co-administration with potent CYP3A inhibitors (such as itraconazole, ketoconazole, voriconazole, clarithromycin) can significantly increase the concentration curve of pacritinib, resulting in elevated plasma drug concentrations (34). This may consequently increase the risk of serious AEs such as myelosuppression and hemorrhage. Pacritinib has been shown in vitro to be an inhibitor of P-glycoprotein (P-gp), breast cancer resistance protein (BCRP), and organic cation transporter 1 (OCT1). When co-administered with substrates of P-gp, BCRP, or OCT1, pacritinib may increase the plasma concentrations of these substrates by inhibiting transporter activity, potentially leading to serious adverse events (35).
However, this study acknowledges several limitations inherent in using data from the FAERS spontaneous reporting system. First, as a spontaneous reporting system, FAERS may have cases of underreporting or duplicate reporting of adverse events. Second, as a newly approved drug, pacritinib tends to receive heightened attention from the media and clinicians, which may lead to an increase in the number of AE reports associated with it. Third, the data analyzed in this study show that a substantial number of AE reports originate from consumers, and the information provided in such reports may be inaccurate or incomplete, potentially introducing biases into the data. Fourth, the occurrence of AEs described in the reports may be influenced by multiple factors such as progression of the underlying disease, concomitant diseases and medications, off-label use, and drug interactions, rather than being directly caused by pacritinib. Meanwhile, insufficient reporting information limits comprehensive causal assessment, making it impossible to clearly attribute the AEs in the FAERS database to pacritinib. Finally, the FAERS database does not include all AE reports related to pacritinib, so the true incidence of pacritinib-related AEs in the medication population cannot be calculated. Despite the above-mentioned multiple limitations, this study enhances the reliability of the identified potential AEs through cross-validation of four data mining algorithms, providing valuable supplementary evidence for the safety monitoring of pacritinib.
5 Conclusion
Using the FAERS database, a comprehensive analysis concerning pacritinib and AEs among MF patients was conducted. Several common AEs that align with those listed in the prescribing information were identified, such as diarrhea, vomiting, nausea, anemia, and thrombocytopenia. Moreover, 26 new AE signals not included in the prescribing information were detected. These findings highlight the necessity of continuing monitoring and further investigation into these emerging risk signals. Overall, our research provides valuable insights for the post-marketing safety surveillance and assessment of pacritinib.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Author contributions
HZ: Conceptualization, Data curation, Formal Analysis, Methodology, Visualization, Writing – original draft. YH: Conceptualization, Data curation, Formal Analysis, Methodology, Visualization, Writing – original draft. CK: Conceptualization, Formal Analysis, Methodology, Supervision, Writing – original draft, Writing – review & editing. MC: Conceptualization, Formal Analysis, Methodology, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2025.1618267/full#supplementary-material
Supplementary Table 1 | Two-by-two contingency table for disproportionality analyses. Equation: a, number of reports containing both the target drug and target adverse drug reaction; b, number of reports containing other adverse drug reaction of the target drug; c, number of reports containing the target adverse drug reaction of other drugs; d, number of reports containing other drugs and other adverse drug reactions.
Supplementary Table 2 | Summary of major algorithms used for signal detection. N, number of adverse event reports; CI, confidence interval; ROR, reporting odds ratio; ROR05, the lower limit of the 95 two-sided CI of the ROR; N, the number of co-occurrences; PRR, proportional reporting ratio; χ2, chi-squared; BCPNN, bayesian confidence propagation neural network; IC, information component; IC025, the lower limit of the 95 two-sided CI of the IC; MGPS, multi-item gamma Poisson shrinker; EBGM, empirical bayesian geometric mean; EBGM05, the lower 95 two-sided CI of EBGM.
Supplementary Table 3 | The signal strength of PTs associated with diseases *. SOC, system organ classes; PT, preferred terms;ROR, CI, confidence interval; ROR, reporting odds ratio; ROR05, the lower limit of the 95 two-sided CI of the ROR; N, the number of co-occurrences; PRR, proportional reporting ratio; χ2, chi-squared; BCPNN, bayesian confidence propagation neural network; IC, information component; IC025, the lower limit of the 95 two-sided CI of the IC; MGPS, multi-item gamma Poisson shrinker; EBGM, empirical bayesian geometric mean; EBGM05, the lower 95 two-sided CI of EBGM. * PTs associated with the natural disease course, pathophysiological mechanisms, or manifestations of complications.
Supplementary Table 4 | The signal strength of AEs of pacritinib-unrelated at the PT level. SOC, system organ classes; PT, preferred terms; ROR, CI, confidence interval; ROR, Abbreviations: reporting odds ratio; ROR05, the lower limit of the 95 two-sided CI of the ROR; N, the number of co-occurrences; PRR, proportional reporting ratio; χ2, chi-squared; BCPNN, bayesian confidence propagation neural network; IC, information component; IC025, the lower limit of the 95 two-sided CI of the IC; MGPS, multi-item gamma Poisson shrinker; EBGM, empirical bayesian geometric mean; EBGM05, the lower 95 two-sided CI of EBGM.
References
1. Wang F, Qiu T, Wang H, and Yang Q. State-of-the-art review on myelofibrosis therapies. Clin Lymphoma Myeloma Leuk. (2022) 22:e350–62. doi: 10.1016/j.clml.2021.11.007, PMID: 34903489
2. Genthon A, Killian M, Mertz P, Cathebras P, Gimenez De Mestral S, Guyotat D, et al. Myelofibrosis: A review. Rev Med Interne. (2021) 42:101–9. doi: 10.1016/j.revmed.2020.08.018, PMID: 33243417
3. Mehta J, Wang H, Iqbal SU, and Mesa R. Epidemiology of myeloproliferative neoplasms in the United States. Leuk Lymphoma. (2014) 55:595–600. doi: 10.3109/10428194.2013.813500, PMID: 23768070
4. Shawky AM, Almalki FA, Abdalla AN, Abdelazeem AH, and Gouda AM. A comprehensive overview of globally approved JAK inhibitors. Pharmaceutics. (2022) 14:1001. doi: 10.3390/pharmaceutics14051001, PMID: 35631587
5. Levine RL. Mechanisms of mutations in myeloproliferative neoplasms. Best Pract Res Clin Haematol. (2009) 22:489–94. doi: 10.1016/j.beha.2009.08.006, PMID: 19959098
6. Gerds AT, Gotlib J, Ali H, Bose P, Dunbar A, Elshoury A, et al. Myeloproliferative neoplasms, version 3.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. (2022) 20:1033–62. doi: 10.6004/jnccn.2022.0046, PMID: 36075392
7. Lamb YN. Pacritinib: first approval. Drugs. (2022) 82:831–8. doi: 10.1007/s40265-022-01718-y, PMID: 35567653
8. Gagelmann N, Bose P, Gupta V, McLornan DP, Vachhani P, Al-Ali HK, et al. Consistency of spleen and symptom reduction regardless of cytopenia in patients with myelofibrosis treated with pacritinib. Clin Lymphoma Myeloma Leuk. (2024) 24:796–803. doi: 10.1016/j.clml.2024.06.012, PMID: 39034203
9. Mascarenhas J, Hoffman R, Talpaz M, Gerds AT, Stein B, Gupta V, et al. Pacritinib vs best available therapy, including ruxolitinib, in patients with myelofibrosis: A randomized clinical trial. JAMA Oncol. (2018) 4:652–9. doi: 10.1001/jamaoncol.2017.5818, PMID: 29522138
10. Mascarenhas J. Pacritinib for the treatment of patients with myelofibrosis and thrombocytopenia. Expert Rev Hematol. (2022) 15:671–84. doi: 10.1080/17474086.2022.2112565, PMID: 35983661
11. Lin Y, Zheng X, Chen Y, Nian Q, Lin L, and Chen M. A real-world disproportionality analysis of FDA adverse event reporting system (FAERS) events for alpelisib. Heliyon. (2024) 10:e27529. doi: 10.1016/j.heliyon.2024.e27529, PMID: 38496864
12. Khaleel MA, Khan AH, Ghadzi SMS, Adnan AS, and Abdallah QM. A standardized dataset of a spontaneous adverse event reporting system. Healthcare (Basel). (2022) 10:420. doi: 10.3390/healthcare10030420, PMID: 35326898
13. Liu Z, Wu D, Ke C, Nian Q, Chen Y, Huang Y, et al. Real-world disproportionality analysis of the food and drug administration adverse event reporting system database for asciminib. Oncology. (2024) 102:1–13. doi: 10.1159/000540542, PMID: 39102794
14. Huang L, Guo T, Zalkikar JN, and Tiwari RC. A review of statistical methods for safety surveillance. Ther Innov Regul Sci. (2014) 48:98–108. doi: 10.1177/2168479013514236, PMID: 30231423
15. Shu Y, He X, Liu Y, Wu P, and Zhang Q. A real-world disproportionality analysis of olaparib: data mining of the public version of FDA adverse event reporting system. Clin Epidemiol. (2022) 14:789–802. doi: 10.2147/clep.S365513, PMID: 35789689
16. Zhang Q, Ding Y, Shu Y, and Chen J. A real-world disproportionality analysis of Rucaparib: Post-marketing Pharmacovigilance Data. BMC Cancer. (2023) 23:745. doi: 10.1186/s12885-023-11201-w, PMID: 37568126
17. Subeesh V, Maheswari E, Singh H, Beulah TE, and Swaroop AM. Novel adverse events of iloperidone: A disproportionality analysis in US food and drug administration adverse event reporting system (FAERS) database. Curr Drug Saf. (2019) 14:21–6. doi: 10.2174/1574886313666181026100000, PMID: 30362421
18. Hu Y, Gong J, Zhang L, Li X, Li X, Zhao B, et al. Colitis following the use of immune checkpoint inhibitors: A real-world analysis of spontaneous reports submitted to the FDA adverse event reporting system. Int Immunopharmacol. (2020) 84:106601. doi: 10.1016/j.intimp.2020.106601, PMID: 32422528
19. Kinoshita S, Hosomi K, Yokoyama S, and Takada M. Time-to-onset analysis of amiodarone-associated thyroid dysfunction. J Clin Pharm Ther. (2020) 45:65–71. doi: 10.1111/jcpt.13024, PMID: 31400296
20. Vainchenker W, Dusa A, and Constantinescu SN. JAKs in pathology: role of Janus kinases in hematopoietic Malignancies and immunodeficiencies. Semin Cell Dev Biol. (2008) 19:385–93. doi: 10.1016/j.semcdb.2008.07.002, PMID: 18682296
21. Breccia M, Palandri F, Polverelli N, Caira M, Berluti M, Palumbo GA, et al. Epidemiology and disease characteristics of myelofibrosis: a comparative analysis between Italy and global perspectives. Front Oncol. (2024) 14:1382872. doi: 10.3389/fonc.2024.1382872, PMID: 39114304
22. Verstovsek S, Odenike O, Singer JW, Granston T, Al-Fayoumi S, and Deeg HJ. Phase 1/2 study of pacritinib, a next generation JAK2/FLT3 inhibitor, in myelofibrosis or other myeloid Malignancies. J Hematol Oncol. (2016) 9:137. doi: 10.1186/s13045-016-0367-x, PMID: 27931243
23. Diaz AE and Mesa RA. Pacritinib and its use in the treatment of patients with myelofibrosis who have thrombocytopenia. Future Oncol. (2018) 14:797–807. doi: 10.2217/fon-2017-0494, PMID: 29235894
24. Komrokji RS, Seymour JF, Roberts AW, Wadleigh M, To LB, Scherber R, et al. Results of a phase 2 study of pacritinib (SB1518), a JAK2/JAK2(V617F) inhibitor, in patients with myelofibrosis. Blood. (2015) 125:2649–55. doi: 10.1182/blood-2013-02-484832, PMID: 25762180
25. Mesa RA, Vannucchi AM, Mead A, Egyed M, Szoke A, Suvorov A, et al. Pacritinib versus best available therapy for the treatment of myelofibrosis irrespective of baseline cytopenias (PERSIST-1): an international, randomised, phase 3 trial. Lancet Haematol. (2017) 4:e225–36. doi: 10.1016/s2352-3026(17)30027-3, PMID: 28336242
26. Zhao Y, Zhang X, Ding X, Wang Y, Li Z, Zhao R, et al. Efficacy and safety of FLT3 inhibitors in monotherapy of hematological and solid Malignancies: a systemic analysis of clinical trials. Front Pharmacol. (2024) 15:1294668. doi: 10.3389/fphar.2024.1294668, PMID: 38828446
27. Tremblay D and Mascarenhas J. Pacritinib to treat myelofibrosis patients with thrombocytopenia. Expert Rev Hematol. (2018) 11:707–14. doi: 10.1080/17474086.2018.1500456, PMID: 30001163
28. Singer JW, Al-Fayoumi S, Ma H, Komrokji RS, Mesa R, and Verstovsek S. Comprehensive kinase profile of pacritinib, a nonmyelosuppressive Janus kinase 2 inhibitor. J Exp Pharmacol. (2016) 8:11–9. doi: 10.2147/jep.S110702, PMID: 27574472
29. Verstovsek S, Mesa R, Talpaz M, Kiladjian JJ, Harrison CN, Oh ST, et al. Retrospective analysis of pacritinib in patients with myelofibrosis and severe thrombocytopenia. Haematologica. (2022) 107:1599–607. doi: 10.3324/haematol.2021.279415, PMID: 34551507
30. Gerds AT, Savona MR, Scott BL, Talpaz M, Egyed M, Harrison CN, et al. Determining the recommended dose of pacritinib: results from the PAC203 dose-finding trial in advanced myelofibrosis. Blood Adv. (2020) 4:5825–35. doi: 10.1182/bloodadvances.2020003314, PMID: 33232476
31. Pemmaraju N, Harrison C, Gupta V, Verstovsek S, Scott B, Oh ST, et al. Risk-adjusted safety analysis of the oral JAK2/IRAK1 inhibitor pacritinib in patients with myelofibrosis. EJHaem. (2022) 3:1346–51. doi: 10.1002/jha2.591, PMID: 36467816
32. Pardanani A, Harrison C, Cortes JE, Cervantes F, Mesa RA, Milligan D, et al. Safety and efficacy of fedratinib in patients with primary or secondary myelofibrosis: A randomized clinical trial. JAMA Oncol. (2015) 1:643–51. doi: 10.1001/jamaoncol.2015.1590, PMID: 26181658
33. Al-Ali HK, Griesshammer M, Foltz L, Palumbo GA, Martino B, Palandri F, et al. Primary analysis of JUMP, a phase 3b, expanded-access study evaluating the safety and efficacy of ruxolitinib in patients with myelofibrosis, including those with low platelet counts. Br J Haematol. (2020) 189:888–903. doi: 10.1111/bjh.16462, PMID: 32017044
34. De SK. First approval of pacritinib as a selective janus associated kinase-2 inhibitor for the treatment of patients with myelofibrosis. Anticancer Agents Med Chem. (2023) 23:1355–60. doi: 10.2174/1871520623666230320120915, PMID: 36959157
Keywords: pacritinib, adverse event, disproportionality analysis, FAERS, real-world study
Citation: Zhang H, Huang Y, Ke C and Chen M (2025) Disproportionality analysis of adverse events associated with pacritinib: a real-world study based on FDA Adverse Event Reporting System (FAERS) database. Front. Oncol. 15:1618267. doi: 10.3389/fonc.2025.1618267
Received: 25 April 2025; Accepted: 04 July 2025;
Published: 28 July 2025.
Edited by:
Massimiliano Bonifacio, University of Verona, ItalyReviewed by:
Gianni Binotto, University of Padua, ItalyAndrea Duminuco, Gaspare Rodolico Ospedale, Italy
Copyright © 2025 Zhang, Huang, Ke and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chengjie Ke, a2NqMTk5NEBmam11LmVkdS5jbg==; Maohua Chen, bWhjcHR5eUAxMjYuY29t
†These authors share first authorship
‡ORCID: Huiling Zhang, orcid.org/0009-0006-4749-9322
Yaping Huang, orcid.org/0009-0008-8548-0555
Chengjie Ke, orcid.org/0009-0006-4160-1875
Maohua Chen, orcid.org/0000-0002-0812-2729
 Huiling Zhang1†‡
Huiling Zhang1†‡ Maohua Chen
Maohua Chen