- 1Department of Dermatology, University Hospital Tübingen, Tübingen, Germany
- 2Department of Dermatology, Charité - Universitätsmedizin Berlin, Corporate Member of Freie Universität Berlin and Humboldt-Universität zu Berlin, Berlin, Germany
- 3Institute of Medical Genetics and Applied Genomics, University Hospital Tübingen, Tübingen, Germany
- 4Institute of Pathology and Neuropathology, University Hospital Tübingen, Tübingen, Germany
Immune checkpoint inhibitors (ICI) have significantly improved melanoma-specific survival (MSS), particularly in patients with tumors with a high tumor mutational burden (TMB) or BRAF mutation. In the adjuvant setting, ICIs significantly improve relapse-free survival (RFS), but data on MSS are still lacking. Tissue samples from 83 patients with stage IIIC/D/IV melanoma who started adjuvant ICI between March 2018 and September 2019 were examined using a 700 gene panel. TMB and BRAF V600E/K mutation status were analyzed to determine their potential influence on RFS and MSS. TMB levels ≥ 20 Var/Mb were classified as TMB high, corresponding to the top 20% TMB levels in the cohort. RFS and MSS were significantly improved in patients whose tumors had high TMB levels and BRAF V600E/K mutation (p<0.001 and p=0.002, respectively). Patients with BRAF-mutated tumors and high TMB seem to benefit particularly from adjuvant ICI.
Introduction
With the introduction of immune checkpoint inhibitors (ICIs), progression-free survival and melanoma-specific survival (MSS) have improved significantly in advanced melanoma (1, 2). Combined ICIs are recognized as one of the most effective treatments for metastatic or locally advanced melanoma, with a 10-year MSS rate exceeding 50% in patients treated with first-line ipilimumab and nivolumab ICI (1–3).
However, more than half of the patients receiving combined ICI develop severe treatment-related adverse events and only 40-50% of patients respond to ICI (2, 4). Numerous studies have been conducted to determine potential predictive factors of response to ICI. Tumor-specific factors, such as high tumor mutational burden (TMB) and the presence of a BRAF mutation were found to be associated with improved outcome with ICI in the non-adjuvant setting (2, 4–8).
In the adjuvant setting, ICI have also been proven to significantly improve relapse-free survival (RFS) and distant metastasis free survival (DMFS) (9–12). Furthermore, for the CheckMate 238 study, numerically, but not significantly, overall survival was increased with adjuvant anti-PD-1 treatment compared to adjuvant CTLA-4 treatment (10, 11). Recently, the presence of BRAF mutations and high TMB levels were found to be associated with improved RFS in the adjuvant setting with anti-PD-1 treatment (1, 10, 13).
To date, no data demonstrate significant improvements in MSS with adjuvant ICI in advanced melanoma. In a previous study, we reported a significantly improved RFS in a cohort of patients with high-risk melanoma at stage IIIC-IV who received adjuvant ICI. RFS was longer in patients whose tumors had a BRAF mutation and a high TMB (13). With an extended follow-up (FU) of up to five years, we report MSS data from this cohort and examine potential correlations between MSS, BRAF mutation status, and TMB level.
Material and methods
Eligibility criteria and ethical approval
We included all patients with stage IIIC, IIID and IV melanoma who started adjuvant anti-PD-1 therapy at our dermato-oncology department between 01/03/2018 and 30/09/2019, and had tumor tissue available for next-generation sequencing (NGS). As NGS of tumor tissue is more difficult in tumors with low tumor content, such as in stage IIIA and IIIB patients, we only included stage IIIC-IV patients.
The recommendation for adjuvant treatment was based on the German melanoma guidelines and was made by the institutional interdisciplinary tumor board (14).
Demographic and clinical information, including sex, age, melanoma subtype, date of primary diagnosis, stage at the start of adjuvant anti-PD-1 therapy, and details of recurrences (location, type and timing) were extracted from the electronic patient file.
This study complies with the Declaration of Helsinki and was approved by the local Ethics Committee of the University of Tübingen (project number 606/2020BO). All patients included in the study gave written informed consent for the documentation of their clinical data for research purposes and publication.
Next-generation sequencing
NGS was performed on tumor- and normal tissue at the Institute for Medical Genetics and Applied Genomics at the University Hospital Tuebingen. DNA was isolated from tumor FFPE tissue and blood samples using the Maxwell® RSC DNA FFPE kit and the Maxwell® RSC instrument (Promega, Madison, WI, USA) according to the manufacturer’s standard protocols. After isolation, 200 ng of genomic DNA was sequenced using a Covaris ultrasonicator (Covaris, Woburn, MA, USA) and target regions were captured and enriched using the SureSelect XT Low Input Target Enrichment System (Agilent Technologies, Santa Clara, CA, USA). The sequencing panel included 708 cancer-related genes, 7 promoter regions and selected fusion sites.
The sequencing data were analyzed using the in-house bioinformatics pipeline meg-SAP (https://github.com/imgag/megSAP, https://github.com/imgag/ngs-bits). Sequencing reads were aligned to the human reference genome GRCh37 using BWA MEM (15). Small somatic variants (SNVs) and insertion/deletion (indels) were identified using Strelka2 (16) and annotated using variant effect predictor (VEP) (17). ClinCNV was used to detect somatic copy number variants (18). TMB was calculated according to the methodology described previously by Forschner et al. (2020) (19). A ‘high’ TMB was defined as the top 20% of the cohort according to Samstein et al. (2019) (20), which corresponded to TMB levels of ≥ 20 variations per megabase (Var/Mb) in this study.
Statistical analysis
Statistical analyses were performed using IBM® SPSS® Statistics 28 (IBM, Armonk, USA). TMB and BRAF mutation status, sex, stage at the start of adjuvant anti-PD1- treatment and the occurrence of immune-related adverse events were analyzed in view of their potential influence on RFS and MSS using univariate Cox regression analysis. Factors that were significant or near significant in the univariate Cox regression analysis were also tested in a multivariate Cox regression analysis. RFS was defined as the time between administration of the first cycle of anti-PD-1 antibody and recurrence, melanoma-specific death, or censoring on the last date of patient contact. MSS was defined as the time between the first application of anti-PD-1 antibody and melanoma-specific death or censoring on the last date of patient contact.
In addition, combined variables were created to analyze the potential influence of TMB and BRAF mutation status (TMB-high + BRAF mutation, TMB-high + BRAF wildtype, TMB-low + BRAF mutation, and TMB-low + BRAF wildtype). According to Samstein et al. (20) we classified the top 20% of the cohort as ‘high’ TMB, which in this study corresponded to TMB levels of at least 20 variants per megabase (Var/Mb). RFS and MSS were analyzed according to these combined variables using Kaplan-Meier estimator. Differences between groups were tested for significance using the log-rank-test, with a significance threshold of 0.05 (two-sided). Thus, p-values < 0.05 were considered as significant.
Results
Patient characteristics
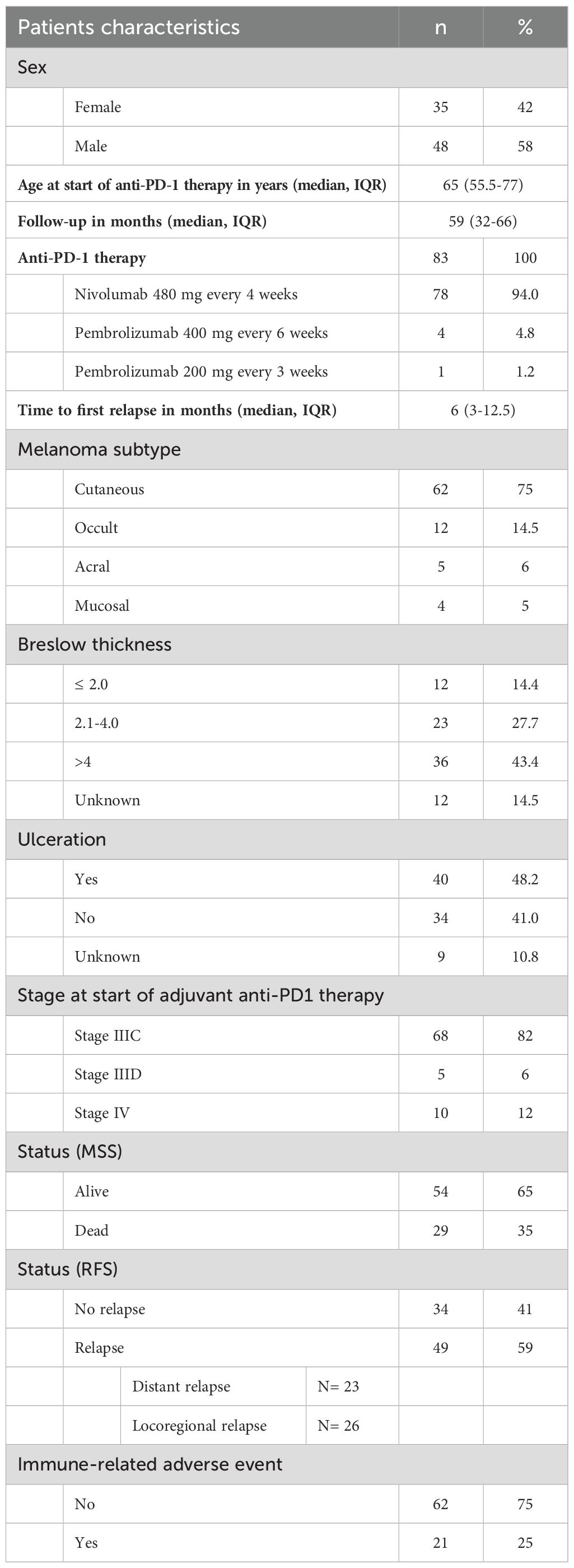
A total of 165 patients started adjuvant anti-PD-1 therapy between March 1, 2018, and September 30, 2019, at the dermato-oncology outpatient department of the University Hospital Tübingen. Of these, 85 patients had melanoma at stage IIIC-IV. Two patients were excluded because NGS of the tumor tissue could not be successfully performed. Consequently, 83 patients were included in this study: 35 female patients (42%) and 42 male patients (58%). The median age at the start of anti-PD-1 therapy was 65 years, interquartile range (IQR) was 55.5–77 years (Table 1). Further information can be found in Table 1 and Supplementary Table 1.
The median FU time was 59 months (IQR 32–66 months). Cutaneous melanoma was the most common subtype (n=62, 75%), followed by occult melanoma (n=12, 15%), acral melanoma (n=5, 6%), and mucosal melanoma (n=4, 5%). At the beginning of adjuvant anti-PD-1 therapy, most patients (n=68; 81,9%) were stage IIIC. Five patients (6%) had stage IIID melanoma and 10 patients (12%) had stage IV melanoma with no evidence of disease (Table 1).
After five years of FU, most patients were still alive (n=54, 65%), and 29 patients died of melanoma (35%). The median time to first relapse was 6 months (IQR 3-12.5 months). A total of 34 patients (41%) remained relapse-free, and 49 patients (59%) relapsed, of which 23 patients had a distant relapse and 26 patients had a locoregional relapse. Immune-related adverse events occurred in 21 patients (25%) (Table 1).
TMB and BRAF mutation status had a significant effect on RFS and MSS
RFS was significantly improved in tumors with high TMB and presence of BRAF mutation (p<0.001) (Figure 1). Multivariate Cox regression analysis revealed a significantly higher risk for relapse in BRAF wild-type tumors than in BRAF-mutated tumors (HR 2.279; CI 1.218-4.427; p= 0.010). Tumors with low TMB were at a significantly higher risk for relapse compared to high TMB tumors (HR 6.240; CI 2.222-17.519; p<0.001). Both factors, TMB and BRAF mutation status, were independent significant factors influencing RFS in the multivariate Cox regression analysis.
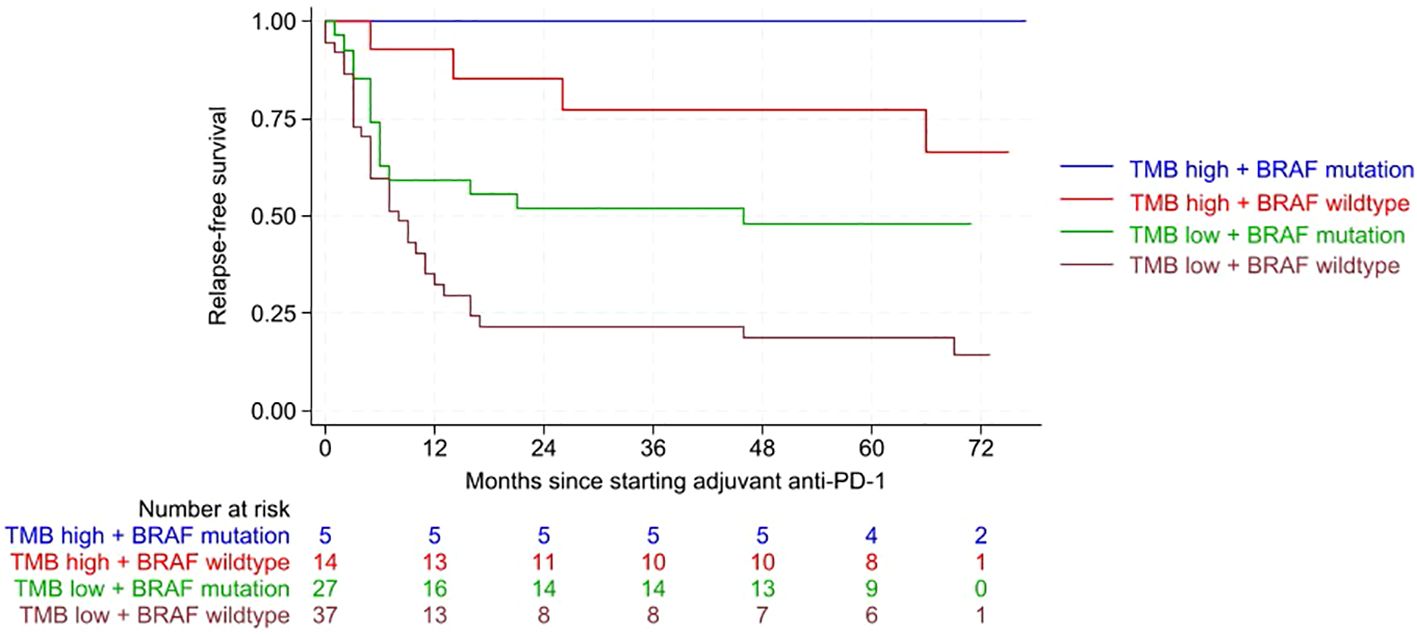
Figure 1. Kaplan-Meier curves showing relapse-free survival (RFS) according to the combined variables of tumor mutational burden (TMB) and BRAF mutation. RFS was significantly improved for tumors with high TMB and presence of a BRAF mutation (p<0.001). Following abbreviations are used in the graph: Tumor mutational burden (TMB), anti-Programmed Death-1 (anti-PD-1).
In terms of MSS, patients whose tumors had a BRAF mutation and a high TMB had significantly better outcomes (p=0.002) (Figure 2). In multivariate Cox regression analysis absence of BRAF mutation doubled the risk of death from melanoma (HR 2.341; CI 0.997-5.498; p=0.051). Even more important, however, is that multivariate Cox regression analysis revealed a significantly increased risk of death from melanoma in tumors with low TMB levels compared to those with high TMB (HR 5.696; CI 1.349-24.055; p=0.018). This means that TMB is a strong and independent influencing factor on MSS. The risk to die from melanoma is about 6-fold increased for patients with TMB-low (HR 5.7). In case of BRAF wildtype, the risk to die from melanoma was doubled (HR 2.3). Other patient characteristics, such as sex, tumor stage at the start of adjuvant PD-1 treatment, and the occurrence of immune-related adverse events, did not have a significant impact on RFS or MSS in the univariate Cox regression analysis (Supplementary Tables 2, 3).
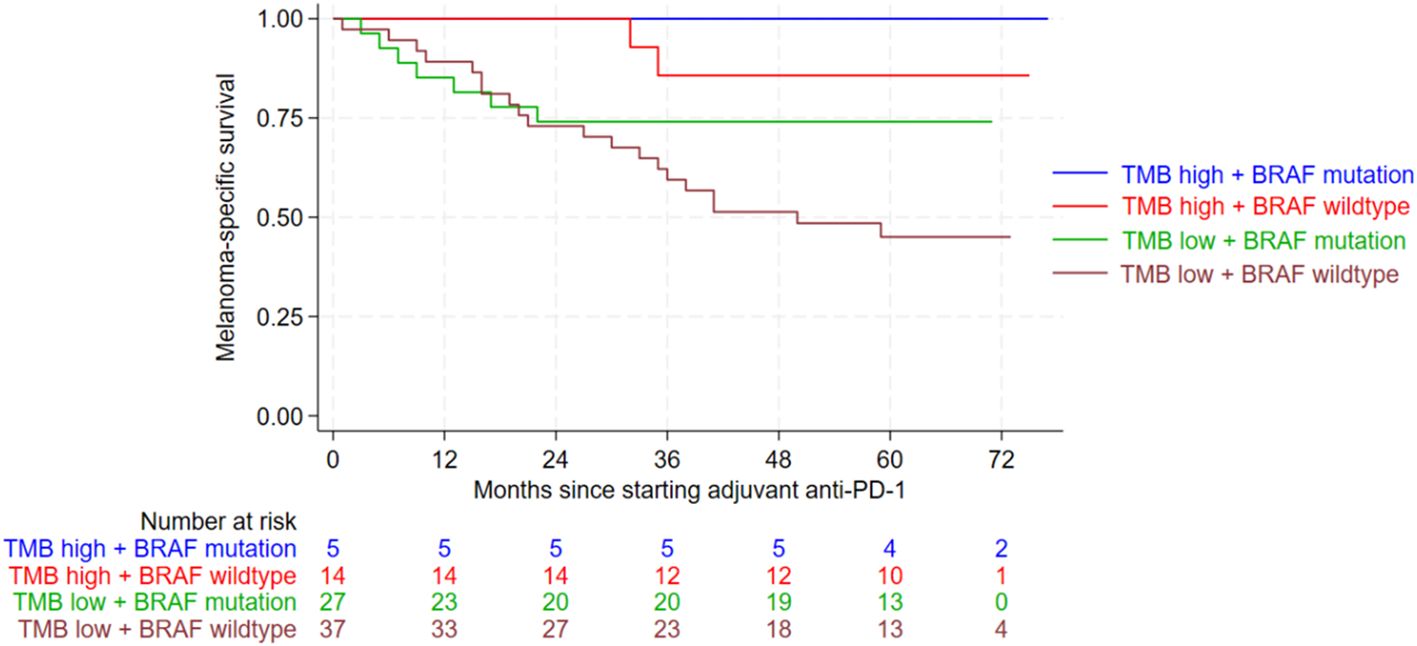
Figure 2. Kaplan-Meier curves showing melanoma-specific survival (MSS) according to the combined variables of tumor mutational burden (TMB) and BRAF mutation. MSS was significantly improved for patients with BRAF mutation and a high TMB level (p=0.002). Following abbreviations are used in the graph: Tumor mutational burden (TMB), anti-Programmed Death-1 (anti-PD-1).
Discussion
Adjuvant ICI provides significant improvements in RFS and DMFS in patients with stage III melanoma (9, 11). However, despite a FU times of seven years, no significant improvement in MSS has been achieved with adjuvant ICI so far (9).
In this study, we identified significant tumor-specific influence factors associated with improved RFS and MSS in patients with stage IIIC-IV melanoma. The strongest influence factor was a high TMB level, that was independent in multivariate testing for MSS and RFS. The combination of TMB high and presence of BRAF mutation was associated with the best MSS and RFS.
In the non-adjuvant setting, a positive correlation between BRAF mutation status and survival has already been observed with combined ipilimumab and nivolumab treatment (2). Other studies have revealed a positive association between high TMB values and improved outcomes in advanced melanoma treated with ICI (8, 21). In the adjuvant setting, of the CheckMate 238 study, high TMB and BRAF-mutated tumors were associated with improved RFS (10). In a previous publication of this cohort, we reported a significantly improved RFS for patients with high TMB and presence of BRAF mutation (13). However, data on improved MSS for BRAF-mutated tumors and high TMB were lacking. To our knowledge, this is the first study to report significantly improved MSS after adjuvant ICI in patients with BRAF-mutated tumors and high TMB.
The risk of melanoma relapse or death was approximately twice as high for BRAF wildtype tumors and an even stronger influence was found for TMB. The risk of melanoma recurrence or death was almost six times higher for tumors with low TMB
In our study, 59% of the patients relapsed, 31% locoregional and 28% distant. Comparing these results with those of the KEYNOTE-054 study, we had a higher overall relapse rate. In the KEYNOTE-054 study, there were 244 relapses, corresponding to 47% of the cohort receiving adjuvant pembrolizumab. The percentage of patients with distant metastasis was approximately one third (28%) in our cohort, which is similar to the pembrolizumab arm of the KEYNOTE-054 study (28%) and the nivolumab arm of the CheckMate 238 study (27%). The rate of locoregional relapses in our study was twice (31%) as high than in the KEYNOTE-054 study (15%) and the nivolumab arm of the CheckMate 238 study (17%) (9, 10).
The higher recurrence rate in our cohort can be explained with the exclusion of stage IIIA and IIIB melanoma patients and the consequently higher percentage of stage IIIC-IV melanoma patients included in our study. The KEYNOTE-054 study enrolled patients with stage IIIA or IIIB melanoma, while the CheckMate 238 study included patients with stage IIIB melanoma. As a result, both studies focused also on patients with lower risk of relapse. The percentage of distant metastases was almost identical to that observed in these two studies. However, the percentage of locoregional relapses was twice as high compared to the KEYNOTE-054 and CheckMate 238 study. It has to be considered, that the FU time in the KEYNOTE-054 study was longer with seven years, compared to our study, which had a FU of five years. Therefore, another FU at a later time point may have further increased the difference.
The positive correlation between high TMB and BRAF mutation on MSS in patients treated with ICI has already been described in the non-adjuvant setting (4, 8). Therefore, our results confirm the data of the non-adjuvant setting in the adjuvant setting treated with ICI. Additional factors have been identified as predictive markers for patients undergoing adjuvant treatment, such as T and NK cell subsets as negative predictive indicators (22) or the presence of circulating tumor DNA (ctDNA) post-surgery (23). In this cohort of advanced melanoma patients, we considered two factors, that were easy to obtain: TMB and BRAF mutation status based on the NGS results. However, a multifactorial approach, incorporating more predictive factors could further enhance the significance of the findings. Furthermore, it cannot be ruled out whether tumors with a high TMB value have a better prognosis independent of ICI, i.e. TMB may not only be a predictive marker for response to ICI (20), but also be generally regarded as a favorable prognostic factor (24). Although the BRAF mutation status is a well-known marker that predicts the response to BRAF- and MEK-targeted therapies, its potential as an additional prognostic marker should be considered, particularly in the adjuvant setting. Indeed, the presence of a BRAF mutation has been discussed in several publications as a potential negative prognostic factor in melanoma (25, 26).
It should also be considered, that we only report on adjuvant ICI and not on adjuvant targeted therapy. Most recently, two retrospective, real-world studies have been published, indicating that in the case of a BRAF mutation, adjuvant BRAF MEK inhibitor therapy with dabrafenib and trametinib may be superior to adjuvant ICI (27, 28). Our study does not answer the question of whether one adjuvant treatment regime is superior to the other. However, our findings suggest that TMB high tumors may derive particularly benefit from adjuvant ICI. Prospective trials are needed to evaluate whether TMB-high, BRAF-mutant patients benefit more from adjuvant BRAF/MEK inhibition or from adjuvant ICI.
Another aspect as to be considered due to most recently published studies:
in the case of macrometastasis, a neoadjuvant-adjuvant approach with pembrolizumab according to the SWOG regime (29) or a neoadjuvant treatment with 2 cycles of combined ipilimumab and nivolumab according to the NADINA regime has been proven to be superior to adjuvant-only procedures (30). However, neoadjuvant ICI are not approved in this indication and can usually not be carried out without prior clarification of costs with the health insurance companies.
It is the strength of our study that all patients included were treated within one single center, thereby minimizing the potential bias that could arise from variations in follow-up procedures or medical documentation. Additionally, the use of a 700-gene sequencing panel enabled a robust and accurate calculation of TMB values. However, it is important to consider the limited sample size of or cohort with even smaller numbers in each subgroup. Nevertheless we found out, that patients with stage IIIC-IV melanoma and low TMB values were six times more likely to relapse or die from melanoma than those with high TMB values. Similarly, patients with BRAF wildtype tumors had twice the risk of relapse or death from melanoma compared to patients with BRAF-mutated tumors. In patients with TMB-low and BRAF wild-type tumors, adjuvant radiotherapy could be recommended with a higher priority and close follow-up including ultrasound and more frequent radiologic staging intervals may be reasonable to be able to detect relapse as early as possible. For the future, larger and prospective studies are needed to verify our results and also to find out whether TMB-high, BRAF-mutant patients benefit more from adjuvant BRAF/MEK inhibition or from adjuvant ICI.
Author’s Note
This study had been selected for poster presentation at the 11th World Congress of Melanoma in Athens in April 2025. Therefore parts of this work had been presented there.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the corresponding author, without undue reservation.
Ethics statement
The studies involving humans were approved by Ethics Committee of the University of Tübingen (project number 606/2020BO). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
JF: Writing – original draft, Investigation, Software, Data curation, Conceptualization, Visualization, Methodology. JH: Writing – review & editing, Data curation. CS: Formal analysis, Writing – review & editing, Software. SA-E: Formal analysis, Software, Writing – review & editing, Methodology. OS-K: Software, Methodology, Writing – review & editing, Formal analysis. AG: Formal analysis, Writing – review & editing, Software, Methodology. IB: Methodology, Software, Writing – review & editing, Formal analysis. TE: Writing – review & editing. TA: Writing – review & editing. SO: Writing – review & editing. LF: Writing – review & editing. CG: Writing – review & editing. AF: Data curation, Writing – review & editing, Resources, Project administration, Supervision. MR: Formal analysis, Software, Validation, Data curation, Supervision, Writing – review & editing, Writing – original draft.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. We would like to thank the University of Tübingen for funding through the Junior Clinician Scientists Program (application no. 523-0-0). Otherwise, this research did not receive any specific grants from public, commercial, or non-profit organizations.
Conflict of interest
JH reports travel grants/speaker fees from MSD and Kyowa Kirin, outside the submitted work. CS reports institutional grants from Illumina and research grants from BMS Stiftung Immunonkologie and Westdeutsche Studiengruppe GmbH outside the submitted work.
IB received speaker fees from Novartis, Bayer, Pfizer, Takeda, Stemline and AstraZeneca and honoraria for advisory board participation from BMS and Novartis, outside the submitted work. TE served as a consultant to Novartis, Merck Sharp & Dohme, Bristol-Myers Squibb, Pierre-Fabre, Sun Pharma, Anaveon, CureVac, Sanofi, Regeneron, outside the submitted work. TA reports personal fees for advisory board membership from Delcath and Philogen; personal fees as an invited speaker from BMS, Neracare, Novartis and Pierre Fabre; personal fees for a writing engagement from CeCaVa and Medtrix; institutional fees as local principal investigator PI from Agenus Inc., AstraZeneca, BioNTech, BMS, HUYA Bioscience, Immunocore, IO Biotech, MSD, Pfizer, Philogen, Regeneron, Roche and University Hospital Essen; institutional fees as coordinating PI from Unicancer; institutional research grants from iFIT and Novartis; institutional funding from MNI - Naturwissenschaftliches und Medizinisches Institut, Neracare, Novartis, Pascoe, Sanofi and Skyline-Dx; non-remunerated membership of the American Society of Clinical Oncology ASCO and the Portuguese Society for Medical Oncology; and a role as clinical expert in the area of medical oncology for Infarmed, all outside the submitted work. SO received speaker fees from Illumina and ONT, and is founder of the company dxOmics for NGS-based diagnostics, outside the submitted work. LF received grants from Hookipa Pharma, Swiss Cancer League, German Research Foundation, Immunophotonics, Mundipharma. LF received consulting fees from Philogen and support for attending meetings or travel from Philogen, Hookipa Pharma. LF participates on board for the University of Basel TIL trial, unpaid and is founder of Hookipa Pharma, Schmelzberg, Humion and Abtherix – all outside the submitted work. CG reports personal fees from CeCaVa, personal fees from MSD, personal fees from NeraCare, personal fees from Philogen, outside the submitted work. AF served as a consultant to Novartis, Merck Sharp & Dohme, Bristol-Myers Squibb, Pierre-Fabre, Delcath, Immunocore; received travel support from Novartis, Merck Sharp & Dohme, BMS, Pierre-Fabre, and received speaker fees from Novartis, Bristol-Myers Squibb, Delcath and Merck Sharp & Dohme and reports institutional research grants from Bristol-Myers Squibb Stiftung Immunonkologie, outside the submitted work. MR received funding as part of the Clinician Scientist Program of the University of Tuebingen application no. 523-0-0 and travel support from Almirall Hermal, Galderma and Pierre-Fabre, outside the submitted work.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer MH declared a past co-authorship with the author AF to the handling editor.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2025.1618596/full#supplementary-material
References
1. Long GV, Carlino MS, McNeil C, Ribas A, Gaudy-Marqueste C, Schachter J, et al. Pembrolizumab versus ipilimumab for advanced melanoma: 10-year follow-up of the phase III KEYNOTE-006 study. Ann Oncol. (2024) 35:1191–9. doi: 10.1016/j.annonc.2024.08.2330
2. Wolchok JD, Chiarion-Sileni V, Rutkowski P, Cowey CL, Schadendorf D, Wagstaff J, et al. Final, 10-year outcomes with nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. (2024) 392:11–22. doi: 10.1056/NEJMoa2407417
3. Ziogas DC, Theocharopoulos C, Lialios PP, Foteinou D, Koumprentziotis IA, Xynos G, et al. Beyond CTLA-4 and PD-1 inhibition: novel immune checkpoint molecules for melanoma treatment. Cancers (Basel). (2023) 15. doi: 10.3390/cancers15102718
4. Wolchok JD, Chiarion-Sileni V, Gonzalez R, Grob JJ, Rutkowski P, Lao CD, et al. Long-term outcomes with nivolumab plus ipilimumab or nivolumab alone versus ipilimumab in patients with advanced melanoma. J Clin Oncol. (2022) 40:127–37. doi: 10.1200/JCO.21.02229
5. Hodi FS, Wolchok JD, SChadendorf D, Larkin J, Long GV, Qian X, et al. TMB and inflammatory gene expression associated with clinical outcomes following immunotherapy in advanced melanoma. Cancer Immunol Res. (2021) 9:1202–13. doi: 10.1158/2326-6066.CIR-20-0983
6. Wu Y, Xu J, Du C, Wu Y, Xia D, Lv W, et al. The predictive value of tumor mutation burden on efficacy of immune checkpoint inhibitors in cancers: A systematic review and meta-analysis. Front Oncol. (2019) 9:1161. doi: 10.3389/fonc.2019.01161
7. Goodman AM, Kato S, Bazhenova L, Patel SP, Frampton GM, Miller V, et al. Tumor mutational burden as an independent predictor of response to immunotherapy in diverse cancers. Mol Cancer Ther. (2017) 16:2598–608. doi: 10.1158/1535-7163.MCT-17-0386
8. Forschner A, Battke F, Hadaschik D, Schulze M, Weissgraeber S, Han CT, et al. Tumor mutation burden and circulating tumor DNA in combined CTLA-4 and PD-1 antibody therapy in metastatic melanoma - results of a prospective biomarker study. J Immunother Cancer. (2019) 7:180. doi: 10.1186/s40425-019-0659-0
9. Eggermont AM, Kicinski M, Blank CU, Mandala M, Long GV, Atkinson V, et al. Seven-year analysis of adjuvant pembrolizumab versus placebo in stage III melanoma in the EORTC1325/KEYNOTE-054 trial. Eur J Cancer. (2024) 211:114327. doi: 10.1016/j.ejca.2024.114327
10. Larkin J, Del Vecchio M, Mandalá M, Gogas H, Arance Fernandez AM, Dalle S, et al. Adjuvant nivolumab versus ipilimumab in resected stage III/IV melanoma: 5-year efficacy and biomarker results from checkMate 238. Clin Cancer Res. (2023) 29:3352–61. doi: 10.1158/1078-0432.CCR-22-3145
11. Ascierto PA, Del Vecchio M, Mandalá M, Gogas H, Arance AM, Dalle S, et al. Adjuvant nivolumab versus ipilimumab in resected stage IIIB-C and stage IV melanoma (CheckMate 238): 4-year results from a multicentre, double-blind, randomised, controlled, phase 3 trial. Lancet Oncol. (2020) 21:1465–77. doi: 10.1016/S1470-2045(20)30494-0
12. Eggermont AMM, Blank CU, Mandala M, Long GV, Atkinson V, Dalle S, et al. Adjuvant pembrolizumab versus placebo in resected stage III melanoma. N Engl J Med. (2018) 378:1789–801. doi: 10.1056/NEJMoa1802357
13. Eckardt J, Schroeder C, Martus P, Armeanu-Ebinger S, Kelemen O, Gschwind A, et al. TMB and BRAF mutation status are independent predictive factors in high-risk melanoma patients with adjuvant anti-PD-1 therapy. J Cancer Res Clin Oncol. (2023) 149:833–40. doi: 10.1007/s00432-022-03939-w
14. Eigentler T, Hoge J, Garbe C, and Schadendorf D. Deutsche dermatologische gesellschaft DK. In: S3-Leitlinie Diagnostik, Therapie und Nachsorge des Melanoms. AWMF. (2020). Available online at: https://register.awmf.org/de/leitlinien/detail/032-024OL. 07.202005.05.2024 (Accessed May 5, 2024).
15. Li H and Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. (2009) 25:1754–60. doi: 10.1093/bioinformatics/btp324
16. Kim S, Scheffler K, Halpern AL, Bekritsky MA, Noh E, Källberg M, et al. Strelka2: fast and accurate calling of germline and somatic variants. Nat Methods. (2018) 15:591–4. doi: 10.1038/s41592-018-0051-x
17. McLaren W, Gil L, Hunt SE, Riat HS, Ritchie GR, Thormann A, et al. The ensembl variant effect predictor. Genome Biol. (2016) 17:122. doi: 10.1186/s13059-016-0974-4
18. Demidov G and Ossowski S. ClinCNV: novel method for allele-specific somatic copy-number alterations detection. bioRxiv. (2019). doi: 10.1101/837971
19. Forschner A, Hilke FJ, Bonzheim I, Gschwind A, Demidov G, Amaral T, et al. MDM2, MDM4 and EGFR amplifications and hyperprogression in metastatic acral and mucosal melanoma. Cancers (Basel). (2020) 12. doi: 10.3390/cancers12030540
20. Samstein RM, Lee CH, Shoushtari AN, Hellmann MD, Shen R, Janjigian YY, et al. Tumor mutational load predicts survival after immunotherapy across multiple cancer types. Nat Genet. (2019) 51:202–6. doi: 10.1038/s41588-018-0312-8
21. Snyder A, Makarov V, Merghoub T, Yuan J, Zaretsky JM, Desrichard A, et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med. (2014) 371:2189–99. doi: 10.1056/NEJMoa1406498
22. Moschella F, Buccione C, Ruspantini I, Castiello L, Rozo Gonzalez A, Iacobone F, et al. Blood immune cells as potential biomarkers predicting relapse-free survival of stage III/IV resected melanoma patients treated with peptide-based vaccination and interferon-alpha. Front Oncol. (2023) 13:1145667. doi: 10.3389/fonc.2023.1145667
23. Genta S, Araujo DV, Hueniken K, Pipinikas C, Ventura R, Rojas P, et al. Bespoke ctDNA for longitudinal detection of molecular residual disease in high-risk melanoma patients. ESMO Open. (2024) 9:103978. doi: 10.1016/j.esmoop.2024.103978
24. Kött J and Gebhardt C. Biomarkers in adjuvant and neoadjuvant treatment of melanoma. Dermatologie (Heidelb). (2025) 76:361–4. doi: 10.1007/s00105-025-05506-z
25. Ellerhorst JA, Greene VR, Ekmekcioglu S, Warneke CL, Johnson MM, Cooke CP, et al. Clinical correlates of NRAS and BRAF mutations in primary human melanoma. Clin Cancer Res. (2011) 17:229–35. doi: 10.1158/1078-0432.CCR-10-2276
26. Moreau S, Saiag P, Aegerter P, Bosset D, Longvert C, Hélias-Rodzewicz Z, et al. Prognostic value of BRAF(V600) mutations in melanoma patients after resection of metastatic lymph nodes. Ann Surg Oncol. (2012) 19:4314–21. doi: 10.1245/s10434-012-2457-5
27. Haist M, Stege H, Rogall F, Tan Y, von Wasielewski I, Klespe KC, et al. Treatment management for BRAF-mutant melanoma patients with tumor recurrence on adjuvant therapy: a multicenter study from the prospective skin cancer registry ADOREG. J Immunother Cancer. (2023) 11. doi: 10.1136/jitc-2023-007630
28. Lodde GC, Hassel JC, von Wasielewski I, Meier F, Mohr P, Kähler K, et al. Long-term follow-up of real-world adjuvant anti-PD1 checkpoint inhibition and targeted therapy in stage III melanoma patients. J Clin Oncol. (2025), jco2402776. doi: 10.1200/JCO-24-02776
29. Patel SP, Othus M, Chen Y, Wright GP, Yost KJ, Hyngstrom JR, et al. Neoadjuvant–adjuvant or adjuvant-only pembrolizumab in advanced melanoma. New Engl J Med. (2023) 388:813–23. doi: 10.1056/NEJMoa2211437
Keywords: adjuvant immune checkpoint inhibitors, relapse-free survival, melanoma-specific survival, tumor mutational burden, BRAF V600E/K mutation
Citation: Forschner J, Huynh J, Schroeder C, Armeanu-Ebinger S, Seibel-Kelemen O, Gschwind A, Bonzheim I, Eigentler TK, Amaral T, Ossowski S, Flatz L, Garbe C, Forschner A and Reitmajer M (2025) Adjuvant anti-PD-1 therapy improves melanoma-specific survival in stage IIIC-IV melanoma patients with high tumor mutation burden and BRAF V600 mutation. Front. Oncol. 15:1618596. doi: 10.3389/fonc.2025.1618596
Received: 26 April 2025; Accepted: 28 July 2025;
Published: 12 August 2025.
Edited by:
Henner Stege, Johannes Gutenberg University Mainz, GermanyReviewed by:
Maximilian Haist, Johannes Gutenberg University Mainz, GermanyBerenice Lang, University Hospital Frankfurt, Germany
Copyright © 2025 Forschner, Huynh, Schroeder, Armeanu-Ebinger, Seibel-Kelemen, Gschwind, Bonzheim, Eigentler, Amaral, Ossowski, Flatz, Garbe, Forschner and Reitmajer. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Markus Reitmajer, bWFya3VzLnJlaXRtYWplckBtZWQudW5pLXR1ZWJpbmdlbi5kZQ==
 Julia Forschner1
Julia Forschner1 Christopher Schroeder
Christopher Schroeder Irina Bonzheim
Irina Bonzheim Teresa Amaral
Teresa Amaral Stephan Ossowski
Stephan Ossowski Andrea Forschner
Andrea Forschner Markus Reitmajer
Markus Reitmajer