- 1School of Arts and Social Sciences, Moi University, Eldoret, Kenya
- 2Behavioral and Social Science Research Working Group, Academic Model for Providing Access to Healthcare (AMPATH), Moi University, Eldoret, Kenya
- 3Department of Pediatrics, University of Michigan, Ann Arbor, MI, United States
- 4Division of Pediatric Hematology Oncology, University of Michigan, Ann Arbor, MI, United States
- 5Fogarty International Center, National Institute of Health, Bethesda, MD, United States
- 6Department of Global Health, University of Washington, Seattle, WA, United States
- 7Academic Model for Providing Access to Healthcare, Eldoret, Kenya
- 8Emma Children’s Hospital of the Amsterdam UMC, Vrije Universiteit, Amsterdam, Netherlands
- 9Department Pediatrics, Division Hematology-Oncology, University of North Carolina at Chapel Hill, Chapel Hill, NC, United States
- 10Department of Child Health and Pediatrics, Moi Teaching and Referral Hospital, Eldoret, Kenya
- 11Department of Pediatrics, Division of Hematology-Oncology, Riley Hospital for Children, Indiana University School of Medicine, Indianapolis, IN, United States
- 12Department of Learning Health Sciences, University of Michigan Medical School, Ann Arbor, MI, United States
- 13Department of Child Health and Pediatrics, Moi University, Eldoret, Kenya
Children with cancer in lower-middle-income countries (LMICs) are at increased risk of dying from infections. Prompt treatment of fever episodes improves outcomes, yet poorly described challenges impair management. This qualitative study explored healthcare provider perspectives on barriers to and facilitators of inpatient fever management in children with cancer at a public tertiary referral children’s hospital in Kenya. Healthcare providers involved in fever management were recruited. Semi-structured interviews were audio-recorded, transcribed verbatim, and entered into NVivo software. Coding was informed by a theoretical fever framework and the Consolidated Framework for Implementation Research. Thematic analysis and mind mapping identified recurrent themes and subthemes. Strategies were mapped to identified barriers. The sixteen participants included nurses (n = 2), clinicians (n = 6), pharmacists (n = 2), phlebotomists (n = 2), and microbiology laboratory staff (n = 4). We identified three overarching themes: empowerment of healthcare providers and caregivers, the importance of timely management, and teamwork/human resource availability. Healthcare provider attributes served as facilitators: motivation to improve care, eagerness to learn, willingness to change practice, and need for treatment guidance. Factors within the hospital system were barriers, with subthemes including poor communication between cadres, delays in laboratory results, and staffing shortages. Participants suggested knowledge sharing, a treatment guideline, task shifting, and hiring additional healthcare providers as potential interventions. Managing fever episodes in children with cancer is complex, requiring multiple cadres of healthcare providers and caregiver participation. The proposed interventions may overcome barriers, but future studies are needed to assess the effectiveness of these strategies in improving fever management.
Introduction
Children with cancer in lower-middle-income countries (LMICs) are 20–30 times more likely to die from fever episodes and infections (1–3), which is likely multifactorial in etiology (1, 3, 4). A high incidence of bloodstream infections, exceeding 28% (5), with an increased prevalence of multidrug-resistant organisms has been reported (6, 7). Children with cancer in LMICs frequently present with severe illness at the time of infection (1, 3), with up to 55% requiring intensive care (3). Staffing shortages are commonplace and low nurse-to-patient ratios contribute to delays, worse outcomes (8), and higher sepsis-related mortality (9). Many pediatric oncologists in LMICs employ reduced-intensity treatment regimens to mitigate the increased infection and treatment-related mortality. While this approach improves outcomes in LMIC settings, survival remains lower compared with high-income country (HIC) settings (10, 11). Improving infection management—a leading cause of death in children with cancer in LMICs (12),—not only benefits those with infections but also brings oncologists in LMICs closer to safely implementing conventional regimens (11, 12).
Effective fever management in children with cancer—that is, the early detection of severe illness and prompt management with blood cultures and antibiotics—remains challenging at LMIC centers (13, 14). The risk of infection-related mortality increases with moderate (>3 h) and severe (>24 h) treatment delays (1, 15, 16). A multicenter study in Africa revealed that few antibiotics were administered within 3 h of fever detection, almost half were administered after >24 h, and nearly all blood cultures were drawn after antibiotics (1). Early detection and management of fever episodes can reduce cancer mortality risk (17, 18), decrease adverse events (19), lower intensive care unit admissions (20), reduce sepsis-related mortality (15, 18, 20), and improve overall outcomes in pediatric oncology fever episodes in LMICs (4).
Despite the known poor outcomes associated with fever episodes, improving fever management in children with cancer in LMICs is complex. Improved understanding of the barriers and facilitators of a sepsis screening tool in LMICs (21) enabled researchers to identify strategies (22) that enhanced implementation and reduced mortality (23). Conducting a needs assessment that includes the description of barriers and facilitators is a valuable first step before implementing an evidence-based intervention (24–27). However, the barriers and facilitators of timely fever management in children with cancer in LMICs are not well described. Our team applied the Consolidated Framework for Implementation Research (CFIR) (28) and developed the Fever Theoretical Framework (29) (Figure 1) to comprehensively describe fever management, which facilitated the identification of strategies and interventions for future quality improvement initiatives in our large, tertiary public referral hospital in Kenya.

Figure 1. Fever theoretical framework developed during the study planning stage to ensure a comprehensive approach in data collection, describing the main areas of the fever management process (fever detection, blood cultures, antibiotics, and treatment duration). Each area has distinct aspects to consider, denoted by the small light green circles overlapping in the respective areas. HCW, healthcare worker.
Methods
Study design
This phenomenological qualitative study was part of a larger convergent mixed-methods study (29) conducted at Moi Teaching and Referral Hospital (MTRH) in Eldoret, Kenya. The study team included doctoral-trained qualitative researchers (EK, CM); a doctoral-trained implementation scientist (JD); trained qualitative researchers (LN, MN, SK, CNN); and pediatric oncologists and researchers (CNN, KB, TAV, FN, GO), all with experience in the Kenyan healthcare system. The Fever Theoretical Framework (29) was developed by this team to guide the comprehensive evaluation of diagnostic and treatment workflows for fevers in hospitalized children with cancer at MTRH. We also used the Consolidated Framework for Implementation Research (CFIR (24) to guide the evaluation of implementation barriers within the hospital setting. This report followed the Standards for Reporting Qualitative Research (SRQR) where applicable (30) (Appendix 1).
Study setting
Moi Teaching and Referral Hospital (MTRH) is the largest government referral hospital in western Kenya, serving a population of more than 24 million (31). It has strong research capacity through a longstanding partnership with a global consortium of academic universities (32). Children receive care at Shoe4Africa, the largest public children’s hospital in East Africa, which is a 250-bed facility within the MTRH system and includes five beds in the pediatric intensive care unit. The pediatric oncology program manages more than 300 new childhood cancer diagnoses annually (33). The clinical microbiology laboratory has state-of-the-art capabilities to identify bacterial isolates, determine antibiotic susceptibilities, and perform genetic tuberculosis testing. Fever management in children with cancer was adapted from society recommendations, including empiric broad-spectrum antibiotic administration and an infectious disease diagnostic evaluation based on clinical presentation (34). Sepsis screening had not been implemented into routine clinical practice.
Interview participants
Eligible participants were healthcare providers who delivered direct or indirect care for hospitalized pediatric cancer patients with fevers at MTRH. Demographic data were not collected to prevent participant identification. Participants were approached in person by a member of the research team during recruitment activities. Purposive and snowball sampling techniques were used during the 3-month recruitment period (15 March to 30 June 2023) to ensure a wide range of experiences, roles, and job descriptions were represented. Sixteen participants were recruited: nurses (n = 2), pediatric oncology clinicians (n = 6), pharmacists (n = 2), phlebotomists (n = 2), and microbiology laboratory staff (n = 4). Written informed consent was obtained. No financial incentives or reimbursements were provided.
Qualitative data collection: semi-structured interview
The semi-structured interview guide (Appendix 2) was informed by the Fever Theoretical Framework (29) and CFIR 2.0 (28), tailored to the local context, and piloted before data collection. Field notes were documented during the interview process to inform data analysis. A total of 16 interviews were conducted in person in English by CNN and LN and continued until thematic saturation (35) was reached—that is, the point at which additional interviews did not yield new insights, as determined by rapid analysis. Interviews were digitally recorded, de-identified, transcribed verbatim (MN, CNN), entered into NVivo 12.0 for coding, and reviewed (MN, SK, CNN, EK).
Qualitative thematic analysis
Three team members (SK, CNN, EK) first familiarized themselves with the transcript data and the interview guide, then read every sentence and inductively coded relevant segments of data related to fever management in children with cancer to develop the initial codebook. This was done through three transcripts, informed by the study frameworks (28, 29) and the interview guide (Appendix 3). As additional transcripts were analyzed, the codebook was updated to capture new patterns, refine existing themes, and ensure consistency. Changes included adding new codes, adjusting categories, and merging similar themes to improve the analysis. Thematic analysis was conducted (CNN, EK, CM, FN) using mind mapping (36, 37) and the Attride-Sterling method (38) until consensus themes and subthemes were identified through iterative meetings held from 1 July 2023 to June 2024. Mind maps are diagrams that can be an effective method to communicate a comprehensive understanding of key concepts within a subject matter (36, 37). The Attride-Sterling method followed six iterative steps: coding material, identifying themes, constructing thematic networks, exploring thematic networks, summarizing thematic networks, and interpreting patterns. The diverse perspectives of the analysis team, data triangulation, and sharing of insights during meetings supported the rigor of the analysis. Finally, all investigators selected and agreed on vivid de-identified quotes to illustrate the described themes.
Strategy mapping
Informed by the study’s Fever Theoretical Framework (29) and the methodological steps of Fernandez et al. (26), we (CNN, JD, FN) informally mapped (26) expert recommendations for implementing change (ERIC) strategies (39, 40) and behavior change techniques (41) to the recurrent interventions suggested by participants to mitigate identified barriers. Participants were asked to provide solutions to the challenges they described multiple times during the interview process (Appendix 3). During the mapping process, we iteratively revisited the barriers and facilitators based on CFIR domains and the Fever Theoretical Framework to strengthen our understanding of the proposed interventions and strategies. We then selected implementation strategies that best aligned with the proposed solutions, taking into account perceived barriers and facilitators to improve uptake (26, 39, 40). For example, inconsistent antibiotic practice was identified as a barrier, the proposed facilitator was the adoption of a protocol, and the most suitable implementation strategy was a standardized antibiotic treatment protocol communicated through a credible source. Strategies were then arranged by barrier level and briefly described in relation to that barrier. The thematic results and strategy mapping were reflexively reported and shared with the interview participants and other healthcare providers across cadres in a collaborative, multidisciplinary small-group meeting led by FN, CNN, and LN. The goal of this study was to enhance understanding of the barriers and facilitators to fever management, with the aim of informing implementation strategies that address the challenges faced by healthcare providers (29). Therefore, some formal tasks of the implementation mapping process—such as evaluating implementation outcomes, protocol development, and identifying outcomes and objectives (26)—were beyond the scope of this report.
Ethics and dissemination
The study received ethical approval from the institutional review boards of the University of Michigan (HUM0225674), MTRH (0004273), and the MTRH chief executive officer. The study was registered with the National Commission for Science, Technology, and Innovation (P/23/22885).
Results
We recruited a heterogeneous group of 16 healthcare providers: bedside and charge nurses, clinical officers, pediatric oncology medical officers, consultant pediatric oncologists, phlebotomists, pediatric oncology clinical pharmacists, an antimicrobial clinical pharmacist, pharmacy technicians, microbiology laboratory technicians, microbiology laboratory management, and doctoral clinical microbiologists. Participants described multiple barriers, facilitators, and proposed interventions to improve management. The average interview length was 55 min (range: 24–111 min). We identified recurrent themes and organized the barriers, facilitators, and interventions under the most appropriate thematic categories, though the complexities of fever management in children with cancer were evident. Table 1 includes supportive quotations from participants for major themes and subthemes. Participants readily recalled memorable scenarios where multiple challenges in fever management—such as lack of standardization, delayed results reporting, poor communication, and delays in detection and management—resulted in poor outcomes for a child:
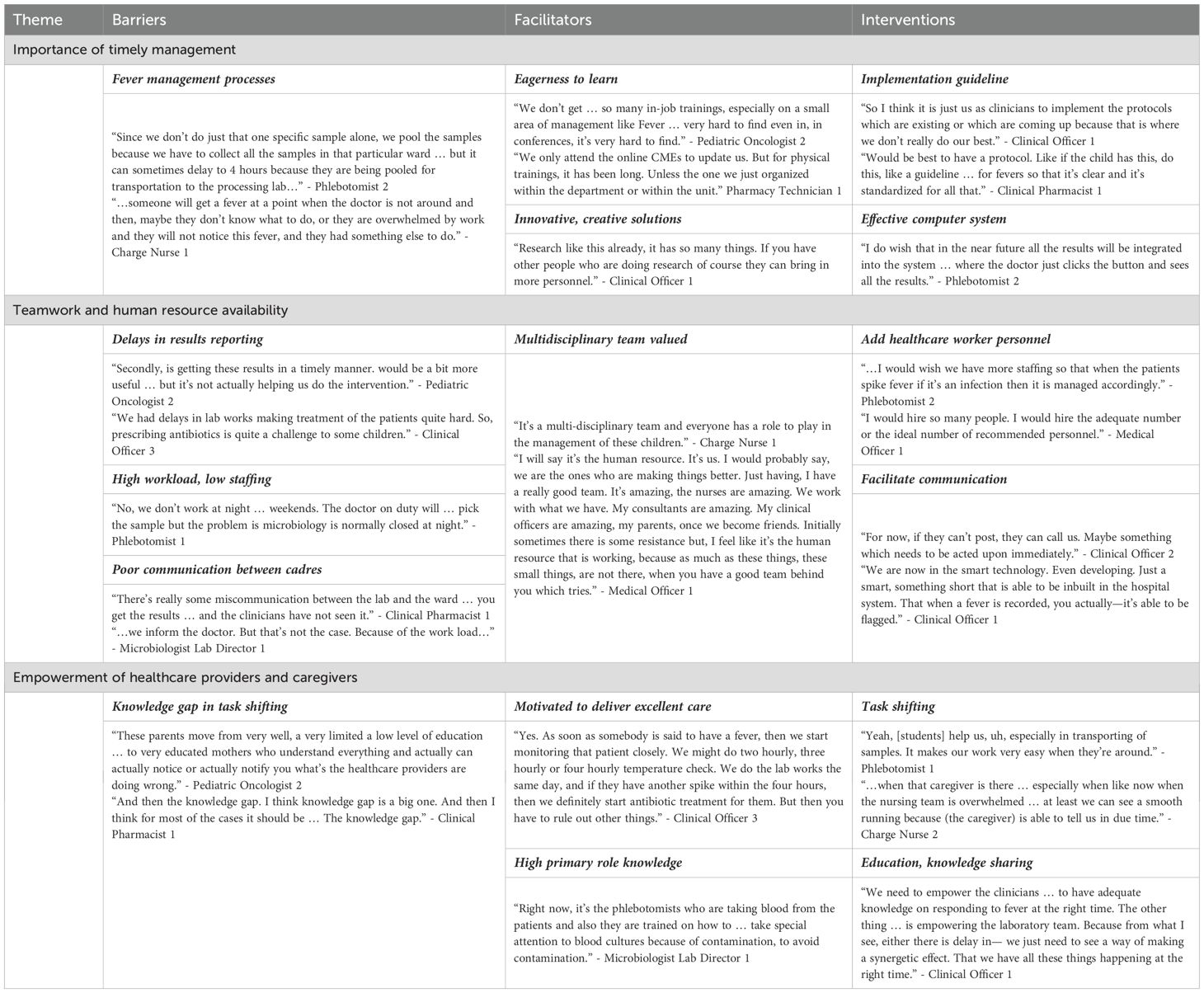
Table 1. Representative quotes from participants regarding fever management in hospitalized Kenyan children with cancer.
“… it’s like we delayed starting antibiotics because we were debating what to give and what to start, because we were waiting for the blood works to come out. Yes, so we were waiting for a hemogram to see what is happening. We took some different samples for different investigations for malaria. Surprisingly it came out to be a neutropenic fever and we didn’t intervene early enough. It was so sad losing that patient.” - Clinical Officer 2
Importance of timely management
Many participants recognized that fever and neutropenia in children with cancer constitute an oncologic emergency associated with poor outcomes, and the importance of timely management was frequently emphasized. Participants described several barriers that prevented prompt management:
“[Fever management is] not done in a [timely] manner. It’s most of the time, I could say up to 70 to 80% is not done in a timely manner. There are some lags that we have a lot because of how the system works, basically.” - Pediatric Oncologist 2
Participants identified challenges across each step of the diagnostic and management continuum—from the accurate detection of a fever episode, to requesting and obtaining a blood culture, ordering and administering an antibiotic, and delays in microbiology results reporting due to the absence of an electronic system:
“The thermal gun [non-contact infrared thermometer] can get this misleading fever … we can take the temperatures but it’s still low and the child is ill, yes.” - Pediatric Oncologist 1
“We were with I.T [information technology] … and they were speaking of one month [to start the electronic system]. But that is the same thing that happened last year.” -Microbiologist Lab Director 1
Several facilitators also fit within this theme. Many participants recommended innovative, creative solutions to the challenges and expressed eagerness to learn how to improve their clinical practice, emphasizing their desire for more training:
“We do have seminars and webinars … exchange ideas with other institutions, other clinicians, other specialties and consultants … We do have trainings very frequently, several times in a year.” -Medical Officer 1
Participants further suggested that successful implementation of a fever treatment guideline and an effective electronic system would substantially improve fever management:
“I think the one single [best] way of doing this … is having protocols, having protocols or job aids for the people. this is how we are going to approach fevers.” - Clinical Pharmacist 1
“…most of those things will be sorted out by the system if it takes effect properly as designed.” -Clinical Officer 1
At least one participant felt that current fever management occurred quickly: “A nurse will come from the ward to pick that drug immediately.” (Pharmacy Technician 1).
Teamwork and human resource availability
Nearly all participants recognized that a multidisciplinary team is valuable in fever management. However, barriers within the hospital system setting limited teamwork effectiveness and led to poor practice habits. When asked to describe a valued aspect of fever management, participants frequently described the team:
“…[the] multidisciplinary team who are involved in fever management. That is the nurses, the clinicians and also the clinical pharmacists … So I think that has really strengthened our intervention in terms of fever management.” - Charge Nurse 2
High workloads faced by healthcare providers due to staffing shortages subsequently caused delays in results reporting and poor communication between cadres, which negatively impacted teamwork:
“The nurses really, I mean like now there is a shortage. We only have three nurses for 64 patients [added emphasis], so that becomes very difficult especially when to do vitals.” - Medical Officer 1
Participants suggested several interventions, such as increasing healthcare personnel and effective facilitation of communication, to enhance teamwork, practice habits, and human resource availability:
“What else can help to also maybe have more clinician at night because it’s usually very few and there’s nothing we can do about the time for rounds [when clinicians must be present due to the workload] because it’s different rounds [at night].” - Clinical Pharmacist 1
“We need to keep talking. Like I said the way the human brains work, once it is used to do things in a particular manner it needs several reminders to change practice.” - Pediatric Oncologist 1
Empowerment of healthcare providers and caregivers
The third recurrent theme was the empowerment of healthcare providers and caregivers. Participants noted that while task shifting from overburdened providers, such as phlebotomists or nurses, to trainees or caregivers may be an ideal intervention, knowledge gaps may exist during task shifting:
“… the nurse is allocating some students to do the vitals … at times cannot give you good information, unless the parent now comes and says ‘my child is feverish, my child is feeling hot’. It’s where you tell the qualified sister nurse, ‘this child is hot, and can we check the temperatures?’ Then you find a very different reading.” - Clinical Officer 2
Despite the challenges faced by healthcare providers involved in the management of fevers in children with cancer, participants consistently demonstrated strong knowledge and eagerness to deliver excellent care:
“[Fever] is constantly given a priority because it’s an oncological emergency … if you don’t respond at the right time, definitely, you will lose that child.” - Clinical Officer 1
“It’s us who make the system work because the system is really confined … So having that team work … working together to try and help you with as little resources that we have, that is the thing that is pushing us forward. Yeah.” - Medical Officer 1
Intricacies of fever management
While we identified three major themes, five barriers, five facilitators, and six interventions, these were interconnected and interacting rather than separate and independent (Figure 2). Each barrier was influenced by the others: low staffing led to high workloads; high workloads contributed to delays in blood culture processing and poor communication; and these, in turn, delayed results reporting. Results retrieval on hard copy, when performed during task shifting by a student or healthcare worker who might not appreciate the urgency, further compounded these delays.
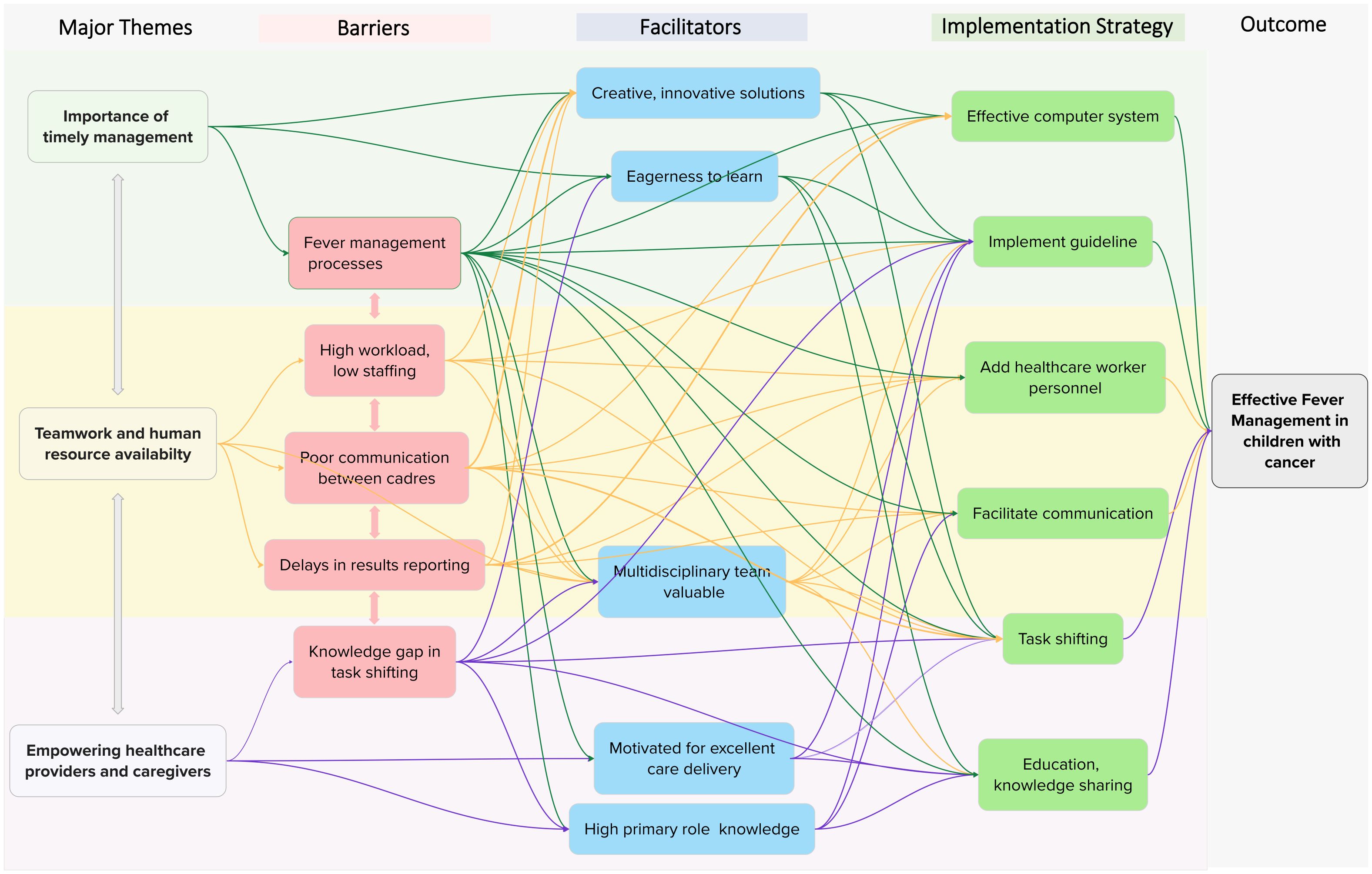
Figure 2. Mind map of the thematic analysis. The mind mapping exercise began following consensus identification of themes and subthemes, at which point we recognized complex relationships between the initial thematic results that were not previously captured. The color scheme is as follows: green = “importance of timely management”; yellow = “teamwork and human resource availability”; purple = “empowering healthcare providers and caregivers.” Subthemes are color-coded by category: red = barrier, blue = facilitator, green = implementation strategy. Branches connect related themes and subthemes to illustrate the complexity of their relationships.
Participants described complex relationships between barriers, facilitators, and interventions, with each factor influencing multiple others within the map. For example, the barriers of high workload and low staffing negatively affected fever management processes and the facilitator of a valued multidisciplinary team. These barriers, however, could be mitigated by several interventions and strategies, such as task shifting, guideline implementation, and adding healthcare personnel. Similarly, each strategy addressed multiple barriers and was shaped by several facilitators. For instance, guideline implementation targeted multiple identified barriers (fever management processes, high workload/low staffing, and knowledge gaps in task shifting) and was facilitated by provider attributes such as eagerness to learn, willingness to adopt innovative solutions, valuing a multidisciplinary approach, motivation to deliver excellent care, and strong role-specific knowledge.
Proposed strategies and interventions
Participants proposed several interventions to comprehensively improve management, summarized in Figure 2. Table 2 describes the ERIC (Expert Recommendations for Implementing Change) implementation strategies (39) and behavior change techniques (41) that were informally mapped to mitigate implementation barriers (26), providing a pragmatic, detailed description of each potential intervention. The most common strategies were use of a credible source, task shifting, use of champions, and facilitating communication. These were organized under the barrier of best fit, although many were applicable across multiple barrier levels.
For example, poor communication between healthcare providers contributed to delays in the reporting of blood culture results. The mapped strategy was “facilitate communication,” and the proposed intervention involved connecting cadres via WhatsApp®, a free and widely used medium. However, facilitating communication—along with changing the record system through an electronic medical record—was also considered an appropriate strategy for hospital system–level barriers.
Many of the proposed strategies in Figure 2 were complex, multilevel interventions likely to require multiple implementation strategies, described in Table 2. Guideline implementation, for instance, was viewed as a multilevel intervention capable of addressing several management barriers. Its effective implementation would likely require multiple strategies, outlined in Table 2, including iterative educational meetings, facilitating communication, hiring additional personnel, issuing managerial directives, engaging champions, and local tailoring.
Discussion
To our knowledge, this is the first qualitative research study to provide novel insights into the complex relationships among barriers and facilitators of fever management in children with cancer in a tertiary referral hospital in Kenya, while also describing interventions and strategies to improve management. Most barriers were located within the hospital system (e.g., high workload/low staffing, delays in results reporting), whereas most facilitators of effective fever management were characteristics of healthcare providers (e.g., eagerness to learn, motivation to deliver excellent care). Participants also suggested interventions that addressed multiple barriers (e.g., guideline implementation, facilitating communication). Task shifting from overburdened healthcare providers to patient caregivers emerged as a potentially cost-effective, impactful, yet underutilized strategy to improve management effectiveness. The complexities of fever management highlighted the importance of an effective healthcare system to support care delivery and the value of a multidisciplinary team approach. Pragmatically, this comprehensive description of fever management practices enabled strategy mapping and identification of interventions directed toward barriers to improve clinical practice. This study established a valuable foundation for our team in selecting implementation strategies to improve aspects of fever management, such as blood culture and antibiotic processes.
This study qualitatively expands upon the intricacies of fever management by describing the interactions of barriers, facilitators, and proposed interventions, which largely align with published quantitative reports. Fever management in children with cancer is challenging, and barriers within healthcare systems (e.g., personnel shortages, budget constraints) have been described in LMICs (3, 13). Specifically, nurse shortages contribute to high workloads and lead to delays in fever detection and management (42). Our thematic findings also support quantitative reports of delays in blood culture and antibiotic processes (1, 14), although antibiotic resistance or shortages were not described as barriers in this study (2, 3, 5, 6). Other LMIC centers have highlighted the need to develop fever treatment guidelines, noted challenges to guideline implementation, and recognized the importance of strategies to support guideline implementation (13, 14). Given the described challenges around guideline implementation (13, 14) and the intricate barriers identified here, the use of multiple implementation strategies (e.g., educational activities, facilitating communication) may improve uptake of evidence-based interventions that enhance fever management. Fever and neutropenia have been described as an oncologic emergency associated with death (1, 3, 4). However, early detection and management of sepsis did not appear as either a barrier or facilitator in our study (21, 23). Poor knowledge during task shifting, especially to trainees, was described (43), whereas a facilitator of effective management was strong role-specific knowledge (e.g., phlebotomists performing phlebotomy tasks (42). Task shifting, educational activities, knowledge sharing, innovative solutions such as mobile technology (44), and guideline implementation have been previously suggested to improve fever management (13, 14). Importantly, our results connect pragmatic implementation strategies to the complex barriers described by healthcare providers.
As our team reflected on this study, we initially underappreciated the scope of challenges faced when managing fever in children with cancer at our center. We found that mapping these strategies was a valuable exercise for planning future efforts to improve our management.
This study has several strengths. Internal validity was supported by reflexive reporting of results to participants, while external validity was reflected in the concordance of our findings with existing literature. The study intentionally recruited a heterogeneous group of participants, including all cadres of healthcare providers with varying levels of experience in fever management. Additionally, because the study was conducted at the largest tertiary public referral hospital in western Kenya, the results may be generalizable to other similarly resourced public hospitals managing fevers in children with cancer in LMICs. The thematic results reported here aligned with the Fever Theoretical Framework and CFIR (28) used in the study planning. However, as our team continued to use these results to inform subsequent interventions, we noted that explicit inclusion of sepsis detection, septic shock management, and intensive care unit access was absent from the Fever Theoretical Framework—important clinical challenges that became evident after this study.
Despite these strengths, we recognize several limitations. Recruitment in certain cadres was limited due to staffing shortages, although thematic saturation was achieved. Semi-structured interviews inherently pose a risk of response bias, which we attempted to mitigate by establishing relationships with participants and ensuring confidentiality of responses. Additionally, aspects of fever management such as sepsis screening and intensive care unit access did not emerge in the analysis, though they may represent important interventions to consider—possibly reflecting response bias. Although members of the study team shared the thematic results and strategy mapping with interview participants and multiple healthcare providers across represented cadres (nursing, phlebotomy, physician, pharmacy, microbiology laboratory), not every participant was available. As a phenomenological qualitative study, the findings highlight perceptions and experiences, but a future study is needed to determine the impact of the proposed strategies on mitigating barriers to effective fever management—for example, improving blood culture and antibiotic processes, reducing time-to-antibiotics, and facilitating prompt detection of severe illness during a fever episode. Other aspects of fever management, including sepsis screening, antibiotic resistance, and caregiver involvement, should also be considered in future studies.
Our study advances knowledge of the intricacies of fever management in children with cancer in a public hospital in an LMIC. More importantly, we mapped implementation strategies and described interventions to address barriers to effective fever management, many of which are not resource intensive (e.g., use of champions, model change, iterative training, development of a guideline, and WhatsApp® to improve communication). Although our public tertiary referral hospital benefits from an advanced clinical research infrastructure (32), MTRH remains a publicly funded hospital with finite resources, similar to other centers (3, 13, 14). While the management barriers may be generalizable to similarly resourced public tertiary referral hospitals in sub-Saharan Africa, the interventions and strategies should be locally tailored.
A multidisciplinary team and comprehensive approach are likely required to improve fever management of children with cancer; a single strategy (e.g., an effective electronic system or hiring more personnel) would be insufficient to advance care delivery without other supportive strategies and interventions (e.g., facilitating communication, task shifting, and increasing healthcare providers). Our team used these qualitative insights to support the implementation of several multilevel interventions, leveraging many of the implementation strategies directed toward the barriers described by participants. We encourage other pediatric cancer centers to evaluate the applicability of these findings—including barriers, facilitators, strategies, and interventions—within their local settings.
Conclusion
A multidisciplinary team must navigate barriers within the healthcare system in the management of fevers in children with cancer in LMICs. Given the complexities in managing fevers in children with cancer in LMICs, a multidisciplinary approach to comprehensively improve clinical practice is likely needed to mitigate the intricate multilevel barriers. Attributes of healthcare providers facilitate effective fever management and should be leveraged in the implementation of strategies and interventions to improve care. Hospital system barriers may be mitigated through knowledge sharing, task shifting, guideline implementation, and effective communication. Future studies should measure the implementation and process outcomes of the proposed strategies mapped to these management barriers.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by University of Michigan Institutional Research Board, the Moi Teaching and Referral Hospital Institutional Research and Ethics Committee, and Moi Teaching and Referral Hospital chief executive officer. This study was registered with National Commission for Science Technology and Innovation. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
EK: Methodology, Formal analysis, Visualization, Writing – original draft, Writing – review & editing. CN: Conceptualization, Data curation, Formal anaylsis, Funding acquisition, Investigation, Methodology, Supervision, Visualization, Writing – original draft, Writing – reviewing & editing. JD: Conceptualization, Methodology, Writing – reviewing & editing. LN: Data curation, Investigation, Writing – reviewing & editing. SK: Data curation, Investigation, Writing – reviewing & editing. MN: Writing – original draft, Writing – review & editing. SL: Project administration, Writing – reviewing & editing. KB: Data curation, Writing – reviewing & editing. GO: Conceptualization, Supervision, Writing – reviewing & editing. TV: Conceptualization, Supervision, Methodology, Writing – reviewing & editing. CM: Conceptualization, Supervision, Methodology, Formal analysis, Visualization, Writing – original draft, Writing – review & editing. FN: Conceptualization, Supervision, Methodology, Formal analysis, Visualization, Resources, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. CNN was supported by the Fogarty International Center and the National Cancer Institute (NCI) of the National Institutes of Health under grant D43TW009345-11S4 awarded to the Northern Pacific Global Health Fellows Program.
Acknowledgments
We thank the children and caregivers on the cancer journey, as well as the healthcare providers who passionately care for them at MTRH. We also appreciate Dr. Amy Kilbourne’s contribution to the description of the implementation strategy mapping methodology.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Author disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
1. Israels T, Afungchwi GM, Klootwijk L, Njuguna F, Hesseling P, Kouya F, et al. Fever and neutropenia outcomes and areas for intervention: A report from SUCCOUR - Supportive Care for Children with Cancer in Africa. Pediatr Blood Cancer. (2021) 68:e29224. doi: 10.1002/pbc.29224
2. Mohammed HB, Yismaw MB, Fentie AM, and Tadesse TA. Febrile neutropenia management in pediatric cancer patients at Ethiopian Tertiary Care Teaching Hospital. BMC Res Notes. (2019) 12:528. doi: 10.1186/s13104-019-4569-5
3. Mukkada S, Melgar M, Bullington C, Chang A, Homsi MR, Gonzalez ML, et al. High morbidity and mortality associated with primary bloodstream infections among pediatric patients with cancer at a Guatemalan tertiary referral hospital. Front Public Health. (2022) 10:1007769. doi: 10.3389/fpubh.2022.1007769
4. Agulnik A, Mora Robles LN, Forbes PW, Soberanis Vasquez DJ, Mack R, Antillon-Klussmann F, et al. Improved outcomes after successful implementation of a pediatric early warning system (PEWS) in a resource-limited pediatric oncology hospital. Cancer. (2017) 123:2965–74. doi: 10.1002/cncr.30664
5. Kipchumba SK, Njuguna FM, and Nyandiko WM. Bacterial isolates and characteristics of children with febrile neutropenia on treatment for cancer at a tertiary hospital in Western Kenya. JCO Glob Oncol. (2024) 10:e2300313. doi: 10.1200/GO.23.00313
6. Agyepong N, Govinden U, Owusu-Ofori A, and Essack SY. Multidrug-resistant gram-negative bacterial infections in a teaching hospital in Ghana. Antimicrob Resist Infect Control. (2018) 7:37. doi: 10.1186/s13756-018-0324-2
7. Lubwama M, Phipps W, Najjuka CF, Kajumbula H, Ddungu H, Kambugu JB, et al. Bacteremia in febrile cancer patients in Uganda. BMC Res Notes. (2019) 12:464. doi: 10.1186/s13104-019-4520-9
8. McHugh MD, Aiken LH, Sloane DM, Windsor C, Douglas C, and Yates P. Effects of nurse-to-patient ratio legislation on nurse staffing and patient mortality, readmissions, and length of stay: a prospective study in a panel of hospitals. Lancet. (2021) 397:1905–13. doi: 10.1016/S0140-6736(21)00768-6
9. Lasater KB, Sloane DM, McHugh MD, Cimiotti JP, Riman KA, Martin B, et al. Evaluation of hospital nurse-to-patient staffing ratios and sepsis bundles on patient outcomes. Am J Infect Control. (2021) 49:868–73. doi: 10.1016/j.ajic.2020.12.002
10. Howard SC, Ortiz R, Baez LF, Cabanas R, Barrantes J, Fu L, et al. Protocol-based treatment for children with cancer in low income countries in Latin America: a report on the recent meetings of the Monza International School of Pediatric Hematology/Oncology (MISPHO)–part II. Pediatr Blood Cancer. (2007) 48:486–90. doi: 10.1002/pbc.20989
11. Lam CG, Howard SC, Bouffet E, and Pritchard-Jones K. Science and health for all children with cancer. Science. (2019) 363:1182–6. doi: 10.1126/science.aaw4892
12. Ehrlich BS, McNeil MJ, Pham LTD, Chen Y, Rivera J, Acuna C, et al. Treatment-related mortality in children with cancer in low-income and middle-income countries: a systematic review and meta-analysis. Lancet Oncol. (2023) 24:967–77. doi: 10.1016/S1470-2045(23)00318-2
13. Mukkada S, Smith CK, Aguilar D, Sykes A, Tang L, Dolendo M, et al. Evaluation of a fever-management algorithm in a pediatric cancer center in a low-resource setting. Pediatr Blood Cancer. (2018) 65. doi: 10.1002/pbc.26790
14. Gulleen EA, Adams SV, Chang BH, Falk L, Hazard R, Kabukye J, et al. Factors and outcomes related to the use of guideline-recommended antibiotics in patients with neutropenic fever at the Uganda cancer institute. Open Forum Infect Dis. (2021) 8:ofab307. doi: 10.1093/ofid/ofab307
15. Daniels LM, Durani U, Barreto JN, O'Horo JC, Siddiqui MA, Park JG, et al. Impact of time to antibiotic on hospital stay, intensive care unit admission, and mortality in febrile neutropenia. Support Care Cancer. (2019) 27:4171–7. doi: 10.1007/s00520-019-04701-8
16. Koenig C, Schneider C, Morgan JE, Ammann RA, Sung L, and Phillips B. Association of time to antibiotics and clinical outcomes in patients with fever and neutropenia during chemotherapy for cancer: a systematic review. Support Care Cancer. (2020) 28:1369–83. doi: 10.1007/s00520-019-04961-4
17. Koenig C, Schneider C, Morgan JE, Ammann RA, Sung L, and Phillips B. Interventions aiming to reduce time to antibiotics (TTA) in patients with fever and neutropenia during chemotherapy for cancer (FN), a systematic review. Support Care Cancer. (2020) 28:2369–80. doi: 10.1007/s00520-019-05056-w
18. Wells T, Thomas C, Watt D, Fountain V, Tomlinson M, and Hilman S. Improvements in the management of neutropenic sepsis: lessons learned from a district general hospital. Clin Med (Lond). (2015) 15:526–30. doi: 10.7861/clinmedicine.15-6-526
19. Pakakasama S, Surayuthpreecha K, Pandee U, Anurathapan U, Maleewan V, Udomsubpayakul U, et al. Clinical practice guidelines for children with cancer presenting with fever to the emergency room. Pediatr Int. (2011) 53:902–5. doi: 10.1111/j.1442-200X.2011.03363.x
20. Salstrom JL, Coughlin RL, Pool K, Bojan M, Mediavilla C, Schwent W, et al. Pediatric patients who receive antibiotics for fever and neutropenia in less than 60 min have decreased intensive care needs. Pediatr Blood Cancer. (2015) 62:807–15. doi: 10.1002/pbc.25435
21. Agulnik A, Gonzalez Ruiz A, Muniz-Talavera H, Carrillo AK, Cardenas A, Puerto-Torres MF, et al. Model for regional collaboration: Successful strategy to implement a pediatric early warning system in 36 pediatric oncology centers in Latin America. Cancer. (2022) 128:4004–16. doi: 10.1002/cncr.34427
22. Agulnik A, Ferrara G, Puerto-Torres M, Gillipelli SR, Elish P, Muniz-Talavera H, et al. Assessment of barriers and enablers to implementation of a pediatric early warning system in resource-limited settings. JAMA Netw Open. (2022) 5:e221547. doi: 10.1001/jamanetworkopen.2022.1547
23. Agulnik A, Muniz-Talavera H, Pham LTD, Chen Y, Carrillo AK, Cardenas-Aguirre A, et al. Effect of paediatric early warning systems (PEWS) implementation on clinical deterioration event mortality among children with cancer in resource-limited hospitals in Latin America: a prospective, multicentre cohort study. Lancet Oncol. (2023) 24:978–88. doi: 10.1016/S1470-2045(23)00285-1
24. 2014 Implementation Reserach Toolkit Workbook TDR WHO. Geneva, Switzerland: World Health Organization (2014).
25. Chambers DA. Implementation Science at a Glance: a Guide for Cancer Control Practitioners. Bethesda, MD: U.S. Department of Health & Human Services, National Institute of Health, National Cancer Institute (2019).
26. Fernandez ME, Ten Hoor GA, van Lieshout S, Rodriguez SA, Beidas RS, Parcel G, et al. Implementation mapping: using intervention mapping to develop implementation strategies. Front Public Health. (2019) 7:158. doi: 10.3389/fpubh.2019.00158
27. Kilbourne AM, Goodrich DE, Miake-Lye I, Braganza MZ, and Bowersox NW. Quality enhancement research initiative implementation roadmap: toward sustainability of evidence-based practices in a learning health system. Med Care. (2019) 57 Suppl 10 Suppl 3:S286–S93. doi: 10.1097/MLR.0000000000001144
28. Damschroder LJ, Reardon CM, Widerquist MAO, and Lowery J. The updated Consolidated Framework for Implementation Research based on user feedback. Implement Sci. (2022) 17:75. doi: 10.1186/s13012-022-01245-0
29. Nessle CN, Njuguna F, Dettinger J, Koima R, Nyamusi L, Kisembe E, et al. Barriers to and facilitators of effective management of fever episodes in hospitalised Kenyan children with cancer: protocol for convergent mixed methods study. BMJ Open. (2023) 13:e078124. doi: 10.1136/bmjopen-2023-078124
30. O'Brien BC, Harris IB, Beckman TJ, Reed DA, and Cook DA. Standards for reporting qualitative research: a synthesis of recommendations. Acad Med. (2014) 89:1245–51. doi: 10.1097/ACM.0000000000000388
32. Mercer T, Gardner A, Andama B, Chesoli C, Christoffersen-Deb A, Dick J, et al. Leveraging the power of partnerships: spreading the vision for a population health care delivery model in western Kenya. Global Health. (2018) 14:44. doi: 10.1186/s12992-018-0366-5
33. Severance TS, Njuguna F, Olbara G, Kugo M, Langat S, Mostert S, et al. An evaluation of the disparities affecting the underdiagnosis of pediatric cancer in Western Kenya. Pediatr Blood Cancer. (2022) 69:e29768. doi: 10.1002/pbc.29768
34. Lehrnbecher T, Robinson PD, Ammann RA, Fisher B, Patel P, Phillips R, et al. Guideline for the management of fever and neutropenia in pediatric patients with cancer and hematopoietic cell transplantation recipients: 2023 update. J Clin Oncol. (2023) 41(9):1774–1785 JCO2202224. doi: 10.1200/JCO.22.02224
35. Hennink MM, Kaiser BN, and Marconi VC. Code saturation versus meaning saturation: how many interviews are enough? Qual Res. (2017) 27:591–608. doi: 10.1177/1049732316665344
36. Meier PS. Mind-mapping: a tool for eliciting and representing knowledge held by diverse informants. Soc Res Update. (2007) 52:1–4.
38. Attride-Stirling J. Thematic networks: an analytic tool for qualitative research. Qual Res. (2001) 1:385–405. doi: 10.1177/146879410100100307
39. Nathan N, Powell BJ, Shelton RC, Laur CV, Wolfenden L, Hailemariam M, et al. Do the Expert Recommendations for Implementing Change (ERIC) strategies adequately address sustainment? Front Health Serv. (2022) 2:905909. doi: 10.3389/frhs.2022.905909
40. Powell BJ, Waltz TJ, Chinman MJ, Damschroder LJ, Smith JL, Matthieu MM, et al. A refined compilation of implementation strategies: results from the Expert Recommendations for Implementing Change (ERIC) project. Implement Sci. (2015) 10:21. doi: 10.1186/s13012-015-0209-1
41. Carey RN, Connell LE, Johnston M, Rothman AJ, de Bruin M, Kelly MP, et al. Behavior change techniques and their mechanisms of action: A synthesis of links described in published intervention literature. Ann Behav Med. (2019) 53:693–707. doi: 10.1093/abm/kay078
42. Gulleen EA, Lubwama M, Komakech A, Krantz EM, Liu C, and Phipps W. Knowledge and perceptions of antimicrobial resistance and antimicrobial stewardship among staff at a national cancer referral center in Uganda. Antimicrob Steward Healthc Epidemiol. (2022) 2:e54. doi: 10.1017/ash.2022.28
43. Al Qadire M, Ballad CAC, Al Omari O, Alkhalaileh M, Sharour LA, Khalaf A, et al. Student nurses' knowledge about the management of chemotherapy-induced neutropenia: Multi-national survey. Nurse Educ Today. (2021) 105:105053. doi: 10.1016/j.nedt.2021.105053
Keywords: febrile neutropenia, pediatric oncology, antibiotic, blood stream infection, guideline, implementation science
Citation: Kisembe E, Nessle CN, Dettinger J, Nyamusi L, Kinja S, Ndung’u M, Langat S, Busby K, Olbara G, Vik TA, Moyer CA and Njuguna F (2025) Improving fever management of hospitalized children with cancer: barriers, facilitators, and proposed interventions from healthcare providers in Kenya. Front. Oncol. 15:1620316. doi: 10.3389/fonc.2025.1620316
Received: 29 April 2025; Accepted: 18 August 2025;
Published: 09 September 2025.
Edited by:
Asya Agulnik, St. Jude Children’s Research Hospital, United StatesReviewed by:
Sheena Mukkada, St. Jude Children’s Research Hospital, United StatesDaniel Ebbs, Yale University, United States
Gita Naidu, Chris Hani Baragwanath Hospital, South Africa
Virginia Mckay, Washington University in St. Louis, United States
Copyright © 2025 Kisembe, Nessle, Dettinger, Nyamusi, Kinja, Ndung’u, Langat, Busby, Olbara, Vik, Moyer and Njuguna. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: C. Nathan Nessle, Y25lc3NsZUBtZWQudW1pY2guZWR1; Festus Njuguna, bXVpZ2FpZmVzMjAwMEBnbWFpbC5jb20=
†These authors have contributed equally to this work and share first authorship
‡These authors have contributed equally to this work and share last authorship
 Everlyn Kisembe1,2†
Everlyn Kisembe1,2† C. Nathan Nessle
C. Nathan Nessle Terry A. Vik
Terry A. Vik Festus Njuguna
Festus Njuguna