- 1Clinical Medical College, Southwest Medical University, Luzhou, China
- 2College of First Clinical Medicine, Shandong University of Traditional Chinese Medicine, Jinan, China
- 3Department of Gynecology, Affiliated Hospital of Southwest Medical University, Luzhou, China
Cervical cancer continues to pose a considerable global health challenge, especially in low- and middle-income nations, although progress in screening and vaccine efforts. In recent years, immunotherapy has emerged as a promising treatment option; nevertheless, its efficacy in cervical cancer is constrained by the intricate and heterogeneous tumor immune microenvironment. Reliable biomarkers to predict which patients will benefit from immunotherapy are lacking. The heterogeneity of the immune landscape across patients adds further complexity. This paper offers a thorough examination of the immunological landscape in cervical cancer, highlighting the interactions among tumor cells, immune infiltrates, and stromal elements. Moreover, we investigate how advanced technologies—such as single-cell RNA sequencing, spatial transcriptomics, and multiplex imaging—are transforming our comprehension of immunological heterogeneity and uncovering new therapeutic targets. We seek to delineate present problems and potential pathways in the development of effective, tailored immunotherapies for cervical cancer by integrating genetic analysis with immunological insights.
1 Introduction
Cervical cancer (CC) ranks as the fourth most common malignant neoplasm among women globally, serving as a primary contributor to cancer mortality in females (1, 2). It is a serious threat to women’s health. CC is preventable and treatable, but remains a major global health burden (3). Alarmingly, more than 85% of these cases and deaths occur in low- and middle-income countries, where access to HPV vaccination, routine screening, and timely treatment is often limited. CC can be categorized into squamous cell carcinoma (SCC), adenocarcinoma (AC), and adenosquamous carcinoma (ASC), which represent the three predominant histological variants of CC (4–6). Human papillomavirus (HPV) is a requisite yet insufficient factor in the etiology of CC. HPV is a prevalent sexually transmitted infection; nevertheless, the majority of HPV infections are eradicated by the immune system. Persistent infection of the genital mucous membranes by specific high-risk HPV types (HPV16 and HPV18) induces cellular proliferation and genetic instability, which, if unaddressed, may ultimately progress to malignant tumors (7–10).
In recent years, CC incidence and mortality have declined significantly in some developed countries due to advances in early screening methods and increased HPV vaccine coverage, but CC cannot be completely prevented by this strategy alone (11–16). Presently, commercially accessible preventive HPV vaccinations are prevalent; nevertheless, they do not manage confirmed infections or lesions (17–20). First-line treatment options for CC are still limited to traditional methods such as surgical excision, radiotherapy and chemotherapy (21–25). Treatment alternatives for early and locally invasive CC encompass radical hysterectomy or radical hysterectomy in conjunction with pelvic lymph node dissection, and concomitant chemotherapy and radiotherapy (26–29). Treatment for distant metastatic CC emphasizes systemic therapy. There is no standard treatment for second-line systemic therapy for advanced CC (30, 31). Targeted agents such as tisotumab vedotin and bevacizumab can help some advanced metastatic patients who meet the criteria (32–34), but as the disease worsens or resistance develops, options for further treatment are few and the toxicity and decreased quality of life they cause cannot be ignored. These factors make treating advanced and repeated metastatic CC a very tough task in clinical practice. How to find more accurate biomarkers to help with personalized and accurate treatment has become a major scientific problem that needs to be solved in the current field of CC study.
A key advance in addressing the therapeutic challenges associated with advanced and metastatic CC has been the advent of immunotherapy (35, 36). Immunotherapy is considered a groundbreaking modality in contemporary oncology and offers promising avenues for tumor control (37–42). Immunotherapy, as a novel treatment approach, aims to augment the body’s innate and adaptive immune responses to combat cancer cells. This approach encompasses a range of modalities, including immune checkpoint inhibitors (ICIs), monoclonal antibodies, cancer vaccines, immunomodulatory agents, and adoptive T-cell transfer therapies (43–45). Each of these strategies aims to overcome mechanisms of immune evasion and restore effective antitumor immunity, offering promising avenues for improving outcomes in various malignancies (46, 47). The use of ICIs has brought new hope to patients (48, 49). In clinical practice, inhibitors targeting the immune checkpoints PD-1 and CTLA4 have been shown to improve survival in patients (21, 50–53).
However, the unique histologic features of CC pose a significant challenge to the heterogeneity of immunotherapy (54, 55). Clinical data suggest that only 15-20% of patients benefit from ICIs therapy, and reliable predictors of efficacy are lacking (56). This event really shows how complicated and varied the CC tumor microenvironment (TME) regulatory network is. This means we need to learn more about how it works on the inside and look into how different kinds of immune cells get into the TME in order to make immunotherapies work better and come up with new ways to treat cancer. Emerging tools such as spatial transcriptomics, multiplex imaging, and single-cell RNA sequencing allow high-resolution mapping of the TME (57–62). These approaches are expected to uncover novel immunoregulatory pathways, improve biomarker discovery, and support the development of highly personalized immunotherapy regimens (63–66). Combining multi-omics techniques has become a useful way to study the complexity of TME in recent years (67–70). By correctly looking at the molecular features of each cell group in TME, the dynamic interaction network between tumor cells and immune cells is made clear.
This review aims to systematically examine the immunological landscape of CC, focusing on how tumor–immune interactions contribute to therapeutic resistance and immune evasion. We place particular emphasis on novel immune targets identified through single-cell and spatial transcriptomic technologies, and we explore how these insights may inform next-generation immunotherapeutic strategies (Figure 1).
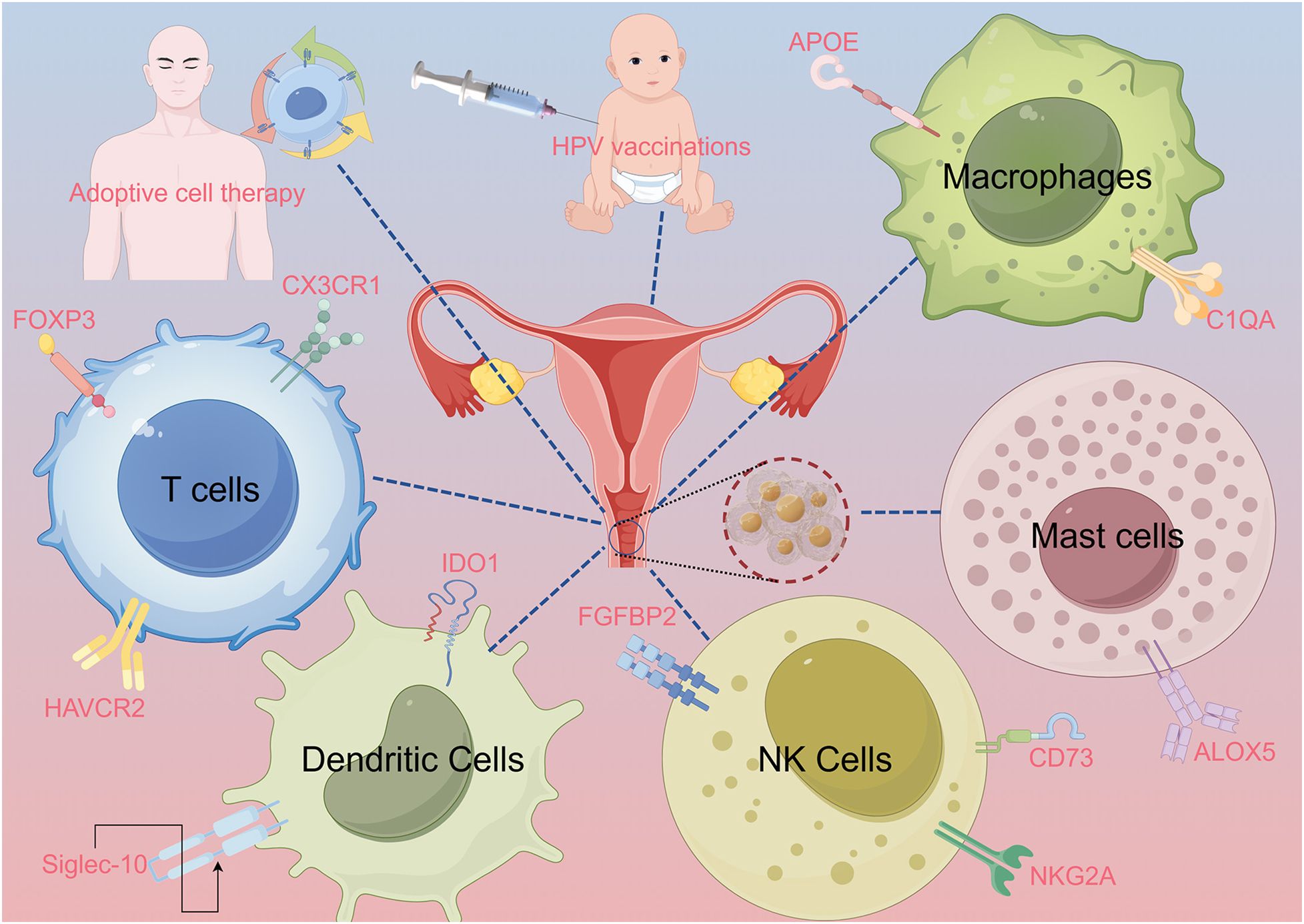
Figure 1. Abstract image. The abstract figure illustrates various scenarios of interactions between immune cells and cervical cancer. These include T cells, dendritic cells (DCs), natural killer (NK) cells, macrophages, and mast cells, with relevant proteins and signaling pathways labeled. The image of a syringe and an infant symbolizes human papillomavirus (HPV) vaccination. Adaptive cell therapy is explained using a human figure. Lines connect the various elements to the central image of the cervix, highlighting their immune interactions.
2 Variability of immune responses in TME of CC
Even though there have been big improvements in CC therapy with ICIs over the last ten years, a lot of patients are still not getting the best immune responses in clinical situations. The heterogeneity of therapeutic response may be influenced by the characteristics of immune cell infiltration in the tumor microenvironment, the integration status of the HPV genome, the expression profiles of immune-related biomarkers, and individual differences in host immune status (21, 71–73). Persistent infection with high-risk HPV, particularly HPV16 and HPV18, plays a central role in cervical carcinogenesis. These viruses have evolved several sophisticated strategies to evade host immune surveillance, which contributes to both viral persistence and progression to malignancy. The HPV oncoproteins E6 and E7 disrupt multiple immune pathways (74). E6 targets interferon regulatory factors (IRFs) and hinders Type I interferon responses, limiting antiviral immunity (75). E7 interferes with antigen processing and presentation, reducing cytotoxic T lymphocyte (CTL) recognition of infected cells (76). Both E6 and E7 reduce the function of dendritic cells (DCs) and Langerhans cells, impairing the priming of HPV-specific T cells (77). The resulting TME becomes immunosuppressive, posing significant barriers to the success of immunotherapies. Cervical tumors, like many solid tumors, create a hostile microenvironment for effective immune activation. PD-L1 is commonly expressed in cervical tumors, especially in HPV-positive cases, leading to T cell exhaustion via the PD-1/PD-L1 axis. Understanding these evasion strategies is critical to improving the response to immune checkpoint inhibitors and other immunotherapies in CC.
The TME displays dynamic and intricate characteristics across different cancer types, where immune cells play a crucial role in the elimination of tumor cells; but, in some cases, they may also promote tumor progression (78–80). In hypermetabolic tumor regions, there were stronger signals from CD56 Natural Killer (NK) cells and immature dendritic cells, while hypometabolic tumor regions exhibited a higher presence of eosinophils, immature B cells, and Treg cells (81). Fan et al. performed a multi-omics investigation demonstrating that bidirectional interactions between malignant epithelial cytokeratin cells and immune-suppressive cancer-associated fibroblasts foster an immune rejection microenvironment in CC through FABP5-mediated transforming growth factor β pathway signalling (82). Additionally, specific molecular processes indicate that NAT10-mediated metabolic reprogramming in cancer cells inhibits the therapeutic efficacy of PD-L1 blockade therapy (83). Insights into the complex immunological landscape of the TME have paved the way for novel treatment strategies (84–88). Finding new immunotherapeutic targets and quickly putting possible biomarkers into clinical trials are big steps forward in improving clinical response rates, making combination therapy strategies work better, and dealing with immunotherapeutic heterogeneity. The following sections will explore current immunotherapeutic approaches and emerging targets that aim to overcome the barriers posed by immune heterogeneity in CC.
3 Summary of contemporary immunotherapy approaches
3.1 Immune checkpoint inhibitors
Immune checkpoints are molecules that inhibit signalling pathways and uphold immune tolerance; however, cancer cells frequently exploit these mechanisms to escape immune surveillance (89, 90). ICIs represent a significant advancement in tumor therapy. Their mode of action primarily involves augmenting the cytotoxic effect of T lymphocytes through the targeted inhibition of inhibitory receptors. ICIs targeting the PD-1/PD-L1 signalling pathway, such as Pembrolizumab, Nivolumab, Cemiplimab, and Balstilimab, have received approval for second-line and subsequent treatment of advanced CC (91–94). While immunotherapy offers a promising avenue for improving outcomes in cervical cancer, it is not without risks. The use of ICIs can lead to a spectrum of immune-related adverse events resulting from excessive immune activation (95). As such, careful patient selection, early detection of toxicity, and proactive management protocols are essential components of clinical immunotherapy use. The therapeutic application of ICIs has progressively transitioned from monotherapy to combination therapy techniques. Research has demonstrated that the dual ICIs regimen exhibits substantial efficacy in patients with metastatic or recurrent CC (96). The combination of ICIs with anti-angiogenic agents, such as Sintilimab and Anlotinib, has demonstrated significant therapeutic efficacy in patients with advanced CC who have not responded to chemotherapy. In patients with advanced CC who have not responded to first-line platinum-based therapy, the overall response rate (ORR) of Sintilimab in conjunction with albumin-paclitaxel is 44.4%, with a median progression-free survival (mPFS) of 5.2 months and a median overall survival (mOS) of 13.1 months (97). The treatment strategy of ICIs combined with concurrent chemoradiotherapy has demonstrated considerable success in patients with high-risk locally advanced CC. Combining radiotherapy and chemotherapy with immunotherapy holds significant promise for improving treatment outcomes in CC. Radiotherapy can enhance tumor immunogenicity by inducing immunogenic cell death, increasing antigen presentation, and modulating the tumor microenvironment to promote immune infiltration. When paired with ICIs, these effects may synergize to overcome immune resistance. Ongoing clinical trials are exploring triplet combinations of radiotherapy, chemotherapy, and PD-1/PD-L1 inhibitors (98). Looking forward, integrating immunotherapy with standard treatments may enable personalized therapeutic strategies.
3.2 Therapeutic vaccinations for HPV
Through the activation of certain T cell immunological responses, the therapeutic vaccine method that is based on HPV oncoprotein E6/E7 attempts to generate anti-tumor effects. A phase II clinical trial (NCT04405349) validated the long-term clinical benefit of the therapeutic DNA vaccine VB10.16 combined with atezolizumab in the population of HPV16-positive patients with recurrent or metastatic CC. The trial demonstrated an overall response rate (ORR) of 19.1%, a median progression-free survival (mPFS) of 4.1 months, and a median overall survival (mOS) that was prolonged to 21.3 months (99). Another phase II clinical trial (NCT04096911) indicated that the combination of Sintilimab and the HPV quadrivalent vaccine could markedly enhance clinical outcomes for individuals with recurrent or metastatic CC who were unresponsive or intolerant to traditional therapies. The treatment regimen had a median progression-free survival (mPFS) of 7.16 months, an overall response rate (ORR) of 53.8%, and a disease control rate of 76.9% on average (100). These research findings emphasize the necessity of spreading HPV preventive vaccination, especially in low-income areas with insufficient medical resources. Although therapeutic vaccines targeting E6/E7 have shown promise in early trials, their integration into standard care is hampered by low immunogenicity and logistical barriers in large-scale application. More rigorous phase III trials are needed to validate their long-term clinical benefit.
3.3 Adoptive cell therapy and application
ACT entails the ex vivo expansion of either autologous or allogeneic tumor-specific T cells, which are then reinfused into the patient to specifically target and eradicate tumor cells (101, 102). ACT is categorized into three primary types: tumor-infiltrating lymphocytes (TIL), TCR-engineered T cells (TCR-T), and chimeric antigen receptor T cells (CAR-T) (103–105). TILs demonstrated superior tumor abrogation compared to lymphocytes produced by vaccine treatment, indicating their efficacy in counteracting immune evasion mechanisms (106). Of nine patients with metastatic CC, two achieved complete remission and one had partial remission according to clinical investigations following TILs (107). Nisha et al. conducted the inaugural human phase 1 clinical study with TCR- T lymphocytes targeting E7 for metastatic human oncovirus-associated epithelial carcinoma, yielding significant tumor reduction and objective clinical responses in 6 out of 12 patients (108). In clinical trials, an NKG2D CAR-T treatment targeted for NKG2DL demonstrated great potential to drastically stop tumor development without appreciable off-target damage (109). These researches show a viable therapeutic method and emphasize the clinical possibilities of using cell therapy for the treatment of CC.
4 Potential novel therapeutic targets for CC in relation to immune infiltration
The extensive application of omics technologies, including scRNA-seq, has enabled researchers to achieve a single-cell comprehension of the TME. Immune infiltration, a significant element of the TME, has been demonstrated to influence tumor progression and the efficacy of immunotherapy. The expansion of options for CC immunotherapies, coupled with the significant functional heterogeneity and plasticity of immune cells, offers novel research avenues for creating personalized diagnostic and therapeutic strategies, as well as enhancing combination therapies.
4.1 T cells
The investigation of T cell-related immune checkpoints, as essential effector cells in tumor immunotherapy, has consistently been a primary focus for biomarker identification. The markers primarily regulate T cell activation, differentiation, and exhaustion.
HAVCR2, a significant member of the TIM family, demonstrates distinct expression characteristics in CC. Research indicates that HAVCR2 expression in exhausted CD8+ T cells within CC tissues is significantly elevated compared to precancerous lesions; however, this expression level declines following chemoradiotherapy (110). The expression level of the ligand-receptor pair LGALS9-HAVCR2 in the TME is significantly elevated in CC tissues compared to high-grade squamous intraepithelial lesions, potentially indicating enhanced immunosuppression (111). Exhausted CD8+ T cells in cervical adenocarcinoma exhibit high expression of HAVCR2 and TIGIT, whereas CD96 is predominantly expressed in exhausted CD8+ T cells in cervical squamous cell carcinoma. These molecules may serve as potential targets for immunotherapy (112, 111).
FOXP3 serves as a critical transcription factor for regulatory T cells (Tregs), with its expression level closely associated with the malignancy of CC. The elevated expression of FOXP3 correlates positively with the advancement of FIGO stage and the differentiation degree of histological subtypes in CC, and is significantly associated with a decreased overall survival rate in patients (113). The elevated expression of FOXP3 in HPV-related CC underscores its significant role in the immune evasion associated with this condition (114).
CX3CR1, a specific receptor for CX3CL1, plays a role in regulating the chemotaxis, adhesion, and cytotoxic functions of immune cells, in conjunction with the perforin-encoding gene PRF1. These two molecules are upregulated in effector memory T cells and cytotoxic T cells and serve a role in early immune activation within CC metastatic lymph nodes (115). The identification of the specific enrichment of CXCL13 in resident memory T cells, along with its immunosuppressive function, offers a novel perspective for a more comprehensive understanding of the complexities within the TME of CC (116).
4.2 Macrophages
The groundbreaking utilization of scRNA-seq technology has fundamentally transformed the conventional binary classification framework of macrophages. Researchers have progressed beyond categorizing macrophages solely as pro-inflammatory (M1 type) or anti-inflammatory (M2 type) (117), now delineating more nuanced functional subtypes based on their multifunctional attributes inside the TME.
APOE, a multifunctional protein released by hepatocytes and macrophages, has been recognized as a signature gene of lipid-associated macrophages due to its crucial role in lipoprotein clearance, lipid transport, and cholesterol metabolism. Macrophage subtypes that overexpress APOE can markedly augment the proliferative activity and migratory capacity of CC cells (118). Mechanistic investigations have demonstrated that this macrophage subtype may secrete immunosuppressive substances via exocytosis (110).
Li et al.’s work demonstrated the probable involvement of C1QA in the spread of CC. In comparison to primary CC tissues, macrophages exhibiting elevated C1QA expression were markedly enriched in metastatic lymph nodes, indicating that C1QA may play a role in the regulation of CC metastasis (119). A separate study arrived at a more intricate conclusion: in patients with advanced CC, macrophages exhibiting reduced SPP1 expression and elevated C1QA expression correlated with improved clinical outcomes (120). CD74 is a crucial regulator of the transport of surface molecules on antigen-presenting cells, and its expression patterns are intimately linked to the functional status of macrophages. Research indicates that CD74-positive macrophages exhibit diminished phagocytic capability and are predisposed to develop into the M2 phenotype (121 ,120). Blocking CD74 can restore the immunosuppressive phenotype and markedly limit the growth of CC cells. The elevated expression levels of IFI30 and TGFBI in macrophages indicate their potential as novel immunological checkpoints (122).
Notably, macrophage subgroups exhibiting elevated HPV16 expression may correlate with favorable prognoses in CC patients. This observation is compounded by the high expression of HPV16 in malignant tumor cells (123, 122). The precise molecular mechanism behind this dual expression pattern and its therapeutic implications require additional investigation.
4.3 Dendritic cells
IDO1 is a pivotal immunoregulatory enzyme mostly expressed in immune cells, astrocytes, and some tumor cells, and it significantly contributes to cancer immunoregulation and cellular metabolism. In comparison to precancerous lesions and normal cervical tissues, dendritic cells in CC tissues exhibited a marked elevation of IDO1 and LAMP3 expression (124). This specific expression pattern may contribute to the formation of the immunosuppressive microenvironment in CC. The combination of IDO1 inhibitors and ICIs significantly suppresses IDO1 overexpression and stimulates the proliferation of effector CD8+ T cells, thereby augmenting the efficacy of anti-tumor immunotherapy.
In the examination of dendritic cell-related immune checkpoints, Siglec-10, as a potential immune checkpoint inhibitor, can suppress the function of adaptive T cells via the Galectin-9-mediated signalling pathway, thereby further augmenting the tumor immunological microenvironment of CC (125).
Moreover, plasmacytoid dendritic cells expressing CLEC4C and LILRA4 are pivotal in modulating the immunological response of CC to HPV infection by secreting IFN-α and suppressing viral genome replication during the initial phase of HPV infection (115). During persistent HPV infection, these dendritic cells may assume a pro-oncogenic function by activating NF-κB and MAPK signalling pathways, underscoring their dual role in CC development.
4.4 NK cells
NK cells are unique innate immune cells that mediate antiviral and antitumor responses. Blocking immune checkpoints not only saves NK cells from depletion, but also enhances their potent anti-tumor activity (126, 127). Monalizumab is a humanized IgG4 antibody that inhibits NKG2A from binding to its HLA-E ligand, which is overexpressed in tumor cells, and also triggers a natural killer cell-mediated immune response against cancer cells (128). Published findings from phase I clinical study NCT02459301 indicate that monalizumab is well tolerated in individuals with advanced gynecological malignancies, exhibiting minimal therapeutic harm (129).
Furthermore, research indicates that the favorable clinical outcome of CC patients is considerably positively linked with the expression levels of FGFBP2. The increase of FGFBP2 expression can markedly augment the cytotoxicity of NK cells, hence improving the body’s anti-tumor immune response (130). This study elucidates the significant function of FGFBP2 in the TME of CC, while also presenting a novel molecular target for the advancement of NK cell-based immunotherapeutic approaches.
NK cells in the TME acquire CD73 molecules and facilitate immunosuppression through the production of adenosine (131). Recent evaluations have assessed the efficacy of anti-CD73 monoclonal antibodies (oleculumab, NZV930), both as monotherapy and in conjunction with other immunosuppressive agents (e.g. anti-PD-1 and A2AR antagonists), for the treatment of various solid tumors in multiple phase I/II studies (NCT03381274, NCT03454451, and NCT03549000) (126). The impact of anti-CD73 treatment on NK cell functionality requires more investigation.
4.5 Mast cells
In the past few years, the regulatory function of MCs in anti-tumor immunity has increasingly garnered interest from the academic community. The study by Zhao et al. demonstrated that elevated ALOX5 expression in MCs is significantly associated with the progression of CC from benign to malignant (132). These MCs may engage in bidirectional contact with CC cells via the TNFRSF12A-mediated signalling pathway. This revelation enhances our comprehension of the mechanism of action of mast cells in the cancer microenvironment and offers a novel research avenue for investigating targeted treatment options centred on mast cell-tumor cell interactions.
5 Discussion
By bridging immunological insights with technological innovation, the future of CC treatment lies in precision immunotherapy tailored to the patient’s unique tumor-immune ecosystem. Immunotherapy is slowly becoming one of the most important ways to treat CC. Immunotherapy has come a long way, but there are still a lot of issues that need to be fixed before it can be used to really target and beat immune resistance. CC generally exhibits a relatively low tumor mutational burden, which limits the generation of neoantigens and reduces immunogenicity (133). Furthermore, upregulation of PD-L1, IDO1 expression, and TGF-β signaling can dampen anti-tumor immune responses and limit the efficacy of ICIs (81). We talked about the new immune markers that have been proven by tests in vitro and in vivo. However, there is still a long way to go before these immune markers can be used in clinical settings.
In summary, the study of immune markers for CC cancer therapy faces many challenges, including little understanding of their interactions in the complex environment of TME and insufficient clinical studies to validate their functionality and potential side effects. We anticipate that with today’s mix of different types of holographic data, such as bulk sequencing, proteomics, spatial transcriptomics, and other technological platforms, to deeply and methodically characterize CC’s immune checkpoints, their functional mechanisms and their biological components will soon become clearer.
Author contributions
XZ: Conceptualization, Writing – review & editing, Validation, Visualization, Data curation, Writing – original draft. WN: Data curation, Conceptualization, Writing – review & editing, Writing – original draft. WS: Visualization, Validation, Writing – review & editing, Writing – original draft. QG: Validation, Data curation, Writing – review & editing, Conceptualization, Writing – original draft, Visualization.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
We appreciate Figdraw for assisting us in creating the summary diagram (ID: YUROYb7a61).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Abu-Rustum NR, Yashar CM, Arend R, Barber E, Bradley K, Brooks R, et al. NCCN guidelines(R) insights: cervical cancer, version 1.2024. J Natl Compr Canc Netw. (2023) 21:1224–33. doi: 10.6004/jnccn.2023.0062
2. Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2024) 74:229–63. doi: 10.3322/caac.21834
3. Mayadev JS, Ke G, Mahantshetty U, Pereira MD, Tarnawski R, and Toita T. Global challenges of radiotherapy for the treatment of locally advanced cervical cancer. Int J Gynecol Cancer. (2022) 32:436–45. doi: 10.1136/ijgc-2021-003001
4. Stolnicu S, Hoang L, and Soslow RA. Recent advances in invasive adenocarcinoma of the cervix. Virchows Arch. (2019) 475:537–49. doi: 10.1007/s00428-019-02601-0
5. Stolnicu S, Barsan I, Hoang L, Patel P, Terinte C, Pesci A, et al. International endocervical adenocarcinoma criteria and classification (IECC): A new pathogenetic classification for invasive adenocarcinomas of the endocervix. Am J Surg Pathol. (2018) 42:214–26. doi: 10.1097/PAS.0000000000000986
6. Stolnicu S, Allison D, Patrichi A, Flynn J, Iasonos A, and Soslow RA. Invasive squamous cell carcinoma of the cervix: A review of morphological appearances encountered in human papillomavirus-associated and papillomavirus-independent tumors and precursor lesions. Adv Anat Pathol. (2024) 31:1–14. doi: 10.1097/PAP.0000000000000411
7. Carse S, Bergant M, and Schafer G. Advances in targeting HPV infection as potential alternative prophylactic means. Int J Mol Sci. (2021) 22(4):2201. doi: 10.3390/ijms22042201
8. McBride AA. Human Malignancies associated with persistent HPV infection. Oncologist. (2024) 29:457–64. doi: 10.1093/oncolo/oyae071
9. Oyouni A. Human papillomavirus in cancer: Infection, disease transmission, and progress in vaccines. J Infect Public Health. (2023) 16:626–31. doi: 10.1016/j.jiph.2023.02.014
10. Hernandez-Silva CD, Ramírez de Arellano A, Pereira-Suárez AL, and Ramírez-López IG. HPV and cervical cancer: molecular and immunological aspects, epidemiology and effect of vaccination in latin american women. Viruses. (2024) 16(3):327. doi: 10.3390/v16030327
11. Perkins RB, Wentzensen N, Guido RS, and Schiffman M. Cervical cancer screening: A review. JAMA. (2023) 330:547–58. doi: 10.1001/jama.2023.13174
12. Wu T, Lucas E, Zhao F, Basu P, and Qiao Y. Artificial intelligence strengthens cervical cancer screening - present and future. Cancer Biol Med. (2024) 21:864–79. doi: 10.20892/j.issn.2095-3941.2024.0198
13. Wang R, Huang H, Yu C, Li X, Wang Y, and Xie L. Current status and future directions for the development of human papillomavirus vaccines. Front Immunol. (2024) 15:1362770. doi: 10.3389/fimmu.2024.1362770
14. Gavinski K and DiNardo D. Cervical cancer screening. Med Clin North Am. (2023) 107:259–69. doi: 10.1016/j.mcna.2022.10.006
15. Zhu X, Yao Q, Dai W, Ji L, Yao Y, Pang B, et al. Cervical cancer screening aided by artificial intelligence, China. Bull World Health Organ. (2023) 101:381–90. doi: 10.2471/BLT.22.289061
16. Canfell K, Smith M, Saville M, and Arbyn M. HPV screening for cervical cancer is reaching maturity. BMJ. (2022) 377:o1303. doi: 10.1136/bmj.o1303
17. Yousefi Z, Aria H, Ghaedrahmati F, Bakhtiari T, Azizi M, Bastan R, et al. An update on human papilloma virus vaccines: history, types, protection, and efficacy. Front Immunol. (2021) 12:805695. doi: 10.3389/fimmu.2021.805695
18. Goldstone SE. Human papillomavirus (HPV) vaccines in adults: Learnings from long-term follow-up of quadrivalent HPV vaccine clinical trials. Hum Vaccin Immunother. (2023) 19:2184760. doi: 10.1080/21645515.2023.2184760
19. Mo Y, Ma J, Zhang H, Shen J, Chen J, Hong J, et al. Prophylactic and therapeutic HPV vaccines: current scenario and perspectives. Front Cell Infect Microbiol. (2022) 12:909223. doi: 10.3389/fcimb.2022.909223
20. Reuschenbach M, Doorbar J, Del Pino M, Joura EA, Walker C, Drury R, et al. Prophylactic HPV vaccines in patients with HPV-associated diseases and cancer. Vaccine. (2023) 41:6194–205. doi: 10.1016/j.vaccine.2023.08.047
21. Francoeur AA, Monk BJ, and Tewari KS. Treatment advances across the cervical cancer spectrum. Nat Rev Clin Oncol. (2025) 22:182–99. doi: 10.1038/s41571-024-00977-w
22. Bizzarri N, Obermair A, Hsu HC, Chacon E, Collins A, Tsibulak I, et al. Consensus on surgical technique for sentinel lymph node dissection in cervical cancer. Int J Gynecol Cancer. (2024) 34:504–9. doi: 10.1136/ijgc-2023-005151
23. Querleu D, Cibula D, Abu-Rustum NR, Fanfani F, Fagotti A, Pedone Anchora L, et al. International expert consensus on the surgical anatomic classification of radical hysterectomies. Am J Obstet Gynecol. (2024) 230:235.e1–8. doi: 10.1016/j.ajog.2023.09.099
24. Mukherjee D, Lahiri D, and Nag M. Therapeutic effects of natural products isolated from different microorganisms in treating cervical cancer: A review. Cancer Insight. (2022) 1:31–46. doi: 10.58567/ci01020003
25. Zhu Y, Liang L, Zhao Y, Li J, Zeng J, Yuan Y, et al. CircNUP50 is a novel therapeutic target that promotes cisplatin resistance in ovarian cancer by modulating p53 ubiquitination. J Nanobiotechnol. (2024) 22:35. doi: 10.1186/s12951-024-02295-w
26. Schubert M, Bauerschlag DO, Muallem MZ, Maass N, and Alkatout I. Challenges in the diagnosis and individualized treatment of cervical cancer. Med (Kaunas). (2023) 59(5):925. doi: 10.3390/medicina59050925
27. Bhatla N, Tomar S, Meena J, Sharma DN, and Kumar L. Adjuvant treatment in cervical, vaginal and vulvar cancer. Best Pract Res Clin Obstet Gynaecol. (2022) 78:36–51. doi: 10.1016/j.bpobgyn.2021.07.005
28. Darin MC, Di Guilmi J, Quiroga Luna J, and Maya AG. Cervical cancer after LACC: a combination of surgical strategies. Int J Gynecol Cancer. (2022) 32:688–9. doi: 10.1136/ijgc-2021-003042
29. Piedimonte S, Helpman L, Pond G, Nelson G, Kwon J, Altman A, et al. Surgical margin status in relation to surgical approach in the management of early-stage cervical Cancer: A Canadian cervical Cancer collaborative (4C) study. Gynecol Oncol. (2023) 174:21–7. doi: 10.1016/j.ygyno.2023.03.005
30. Hiam-Galvez KJ, Allen BM, and Spitzer MH. Systemic immunity in cancer. Nat Rev Cancer. (2021) 21:345–59. doi: 10.1038/s41568-021-00347-z
31. Paulino E, de Melo AC, de Andrade DAP, and de Almeida MS. Systemic therapy for advanced cervical cancer: Leveraging the historical threshold of overall survival. Crit Rev Oncol Hematol. (2023) 183:103925. doi: 10.1016/j.critrevonc.2023.103925
32. Oaknin A, Gladieff L, Martínez-García J, Villacampa G, Takekuma M, De Giorgi U, et al. Atezolizumab plus bevacizumab and chemotherapy for metastatic, persistent, or recurrent cervical cancer (BEATcc): a randomised, open-label, phase 3 trial. Lancet. (2024) 403:31–43. doi: 10.1016/S0140-6736(23)02405-4
33. Tewari KS, Sill MW, Long HJ 3rd, Penson RT, Huang H, Ramondetta LM, et al. Improved survival with bevacizumab in advanced cervical cancer. N Engl J Med. (2014) 370:734–43. doi: 10.1056/NEJMoa1309748
34. Vergote I, González-Martín A, Fujiwara K, Kalbacher E, Bagaméri A, Ghamande S, et al. Tisotumab vedotin as second- or third-line therapy for recurrent cervical cancer. N Engl J Med. (2024) 391:44–55. doi: 10.1056/NEJMoa2313811
35. Ferrall L, Lin KY, Roden RBS, Hung CF, and Wu TC. Cervical cancer immunotherapy: facts and hopes. Clin Cancer Res. (2021) 27:4953–73. doi: 10.1158/1078-0432.CCR-20-2833
36. Cha JH, Chan LC, Song MS, and Hung MC. New approaches on cancer immunotherapy. Cold Spring Harb Perspect Med. (2020) 10(8):a036863. doi: 10.1101/cshperspect.a036863
37. Zhang Y and Zhang Z. The history and advances in cancer immunotherapy: understanding the characteristics of tumor-infiltrating immune cells and their therapeutic implications. Cell Mol Immunol. (2020) 17:807–21. doi: 10.1038/s41423-020-0488-6
38. Ge Q, Zhao Z, Li X, Yang F, Zhang M, Hao Z, et al. Deciphering the suppressive immune microenvironment of prostate cancer based on CD4+ regulatory T cells: Implications for prognosis and therapy prediction. Clin Transl Med. (2024) 14:e1552. doi: 10.1002/ctm2.1552
39. Zhao Z, Zhao Z, Lin Z, Fan L, Xiahou Z, Dong Y, et al. Decoding multiple myeloma: single-cell insights into tumor heterogeneity, immune dynamics, and disease progression. Front Immunol. (2025) 16:1584350. doi: 10.3389/fimmu.2025.1584350
40. Lin L, Zou J, Pei S, Huang W, Zhang Y, Zhao Z, et al. Germinal center B-cell subgroups in the tumor microenvironment cannot be overlooked: Their involvement in prognosis, immunotherapy response, and treatment resistance in head and neck squamous carcinoma. Heliyon. (2024) 10:e37726. doi: 10.1016/j.heliyon.2024.e37726
41. Huang W, Kim BS, Zhang Y, Lin L, Chai G, and Zhao Z. Regulatory T cells subgroups in the tumor microenvironment cannot be overlooked: Their involvement in prognosis and treatment strategy in melanoma. Environ Toxicol. (2024) 39:4512–30. doi: 10.1002/tox.24247
42. Li H, Bian Y, Xiahou Z, Zhao Z, Zhao F, and Zhang Q. The cellular signaling crosstalk between memory B cells and tumor cells in nasopharyngeal carcinoma cannot be overlooked: Their involvement in tumor progression and treatment strategy is significant. J Cancer. (2025) 16:288–314. doi: 10.7150/jca.101420
43. Cappell KM and Kochenderfer JN. Long-term outcomes following CAR T cell therapy: what we know so far. Nat Rev Clin Oncol. (2023) 20:359–71. doi: 10.1038/s41571-023-00754-1
44. Tawbi HA, Schadendorf D, Lipson EJ, Ascierto PA, Matamala L, Castillo Gutiérrez E, et al. Relatlimab and nivolumab versus nivolumab in untreated advanced melanoma. N Engl J Med. (2022) 386:24–34. doi: 10.1056/NEJMoa2109970
45. Jin W, Zhang Y, Zhao Z, and Gao M. Developing targeted therapies for neuroblastoma by dissecting the effects of metabolic reprogramming on tumor microenvironments and progression. Theranostics. (2024) 14:3439–69. doi: 10.7150/thno.93962
46. Yuan Z, Li Y, Zhang S, Wang X, Dou H, Yu X, et al. Extracellular matrix remodeling in tumor progression and immune escape: from mechanisms to treatments. Mol Cancer. (2023) 22:48. doi: 10.1186/s12943-023-01744-8
47. Lin Z, Sui X, Jiao W, Chen C, Zhang X, and Zhao J. Mechanism investigation and experiment validation of capsaicin on uterine corpus endometrial carcinoma. Front Pharmacol. (2022) 13:953874. doi: 10.3389/fphar.2022.953874
48. Nuccio A, Viscardi G, Salomone F, Servetto A, Venanzi FM, Riva ST, et al. Systematic review and meta-analysis of immune checkpoint inhibitors as single agent or in combination with chemotherapy in early-stage non-small cell lung cancer: Impact of clinicopathological factors and indirect comparison between treatment strategies. Eur J Cancer. (2023) 195:113404. doi: 10.1016/j.ejca.2023.113404
49. Liu YT and Sun ZJ. Turning cold tumors into hot tumors by improving T-cell infiltration. Theranostics. (2021) 11:5365–86. doi: 10.7150/thno.58390
50. Lou H, Cai H, Huang X, Li G, Wang L, Liu F, et al. Cadonilimab combined with chemotherapy with or without bevacizumab as first-line treatment in recurrent or metastatic cervical cancer (COMPASSION-13): A phase 2 study. Clin Cancer Res. (2024) 30:1501–8. doi: 10.1158/1078-0432.CCR-23-3162
51. Aggarwal C, Prawira A, Antonia S, Rahma O, Tolcher A, Cohen RB, et al. Dual checkpoint targeting of B7-H3 and PD-1 with enoblituzumab and pembrolizumab in advanced solid tumors: interim results from a multicenter phase I/II trial. J Immunother Cancer. (2022) 10:e004424. doi: 10.1136/jitc-2021-004424
52. Zhao Y, Ma Y, Zang A, Cheng Y, Zhang Y, Wang X, et al. First-in-human phase I/Ib study of QL1706 (PSB205), a bifunctional PD1/CTLA4 dual blocker, in patients with advanced solid tumors. J Hematol Oncol. (2023) 16:50. doi: 10.1186/s13045-023-01445-1
53. Shi J, Liu J, Tu X, Li B, Tong Z, Wang T, et al. Single-cell immune signature for detecting early-stage HCC and early assessing anti-PD-1 immunotherapy efficacy. J Immunother Cancer. (2022) 10:e003133. doi: 10.1136/jitc-2021-003133
54. Sun Q, Wang L, Zhang C, Hong Z, and Han Z. Cervical cancer heterogeneity: a constant battle against viruses and drugs. Biomark Res. (2022) 10:85. doi: 10.1186/s40364-022-00428-7
55. Lin S, Sun Y, Cao C, Zhu Z, Xu Y, Liu B, et al. Single-nucleus RNA sequencing reveals heterogenous microenvironments and specific drug response between cervical squamous cell carcinoma and adenocarcinoma. EBioMedicine. (2023) 97:104846. doi: 10.1016/j.ebiom.2023.104846
56. Xu M, Xu M, Cao C, Wu P, Huang X, and Ma D. Advances in cervical cancer: current insights and future directions. Cancer Commun (Lond). (2025) 45:77–109. doi: 10.1002/cac2.12629
57. Shao Y, Shao Y, Yu X, Shan K, Yan J, and Ye G. Defining the biological functions and clinical significance of AKR1C3 in gastric carcinogenesis through multiomics functional analysis and immune infiltration analysis. J Cancer. (2024) 15:2646–58. doi: 10.7150/jca.94228
58. Xia Y, Wang Y, Shan M, Hao Y, and Liang Z. Decoding the molecular landscape of keloids: new insights from single-cell transcriptomics. Burns Trauma. (2023) 11:tkad017. doi: 10.1093/burnst/tkad017
59. Xu Y, She Y, Li Y, Li H, Jia Z, Jiang G, et al. Multi-omics analysis at epigenomics and transcriptomics levels reveals prognostic subtypes of lung squamous cell carcinoma. BioMed Pharmacother. (2020) 125:109859. doi: 10.1016/j.biopha.2020.109859
60. Lin Z, Fan W, Yu X, Liu J, and Liu P. Research into the mechanism of intervention of SanQi in endometriosis based on network pharmacology and molecular docking technology. Med (Baltimore). (2022) 101:e30021. doi: 10.1097/MD.0000000000030021
61. Lin Z, Sui X, Jiao W, Wang Y, and Zhao J. Exploring the mechanism and experimental verification of puerarin in the treatment of endometrial carcinoma based on network pharmacology and bioinformatics analysis. BMC Complement Med Ther. (2022) 22:150. doi: 10.1186/s12906-022-03623-z
62. Zhang P, Yang Z, Liu Z, Zhang G, Zhang L, Zhang Z, et al. Deciphering lung adenocarcinoma evolution: Integrative single-cell genomics identifies the prognostic lung progression associated signature. J Cell Mol Med. (2024) 28:e18408. doi: 10.1111/jcmm.18408
63. Hu Y, Wang K, Chen Y, Jin Y, Guo Q, and Tang H. Causal relationship between immune cell phenotypes and risk of biliary tract cancer: evidence from Mendelian randomization analysis. Front Immunol. (2024) 15:1430551. doi: 10.3389/fimmu.2024.1430551
64. Cao W, Wang K, Wang J, Chen Y, Gong H, Xiao L, et al. Causal relationship between immune cells and risk of myocardial infarction: evidence from a Mendelian randomization study. Front Cardiovasc Med. (2024) 11:1416112. doi: 10.3389/fcvm.2024.1416112
65. Liu R, Wang K, Guo X, Wang Q, Zhang X, Peng K, et al. A causal relationship between distinct immune features and acute or chronic pancreatitis: results from a mendelian randomization analysis. Pancreatology. (2024) 24:1219–28. doi: 10.1016/j.pan.2024.10.006
66. Nie W, Zhao Z, Liu Y, Wang Y, Zhang J, Hu Y, et al. Integrative single-cell analysis of cardiomyopathy identifies differences in cell stemness and transcriptional regulatory networks among fibroblast subpopulations. Cardiol Res Pract. (2024) 2024:3131633. doi: 10.1155/2024/3131633
67. Nian S, Wang K, Wang J, Wang S, Li C, Li N, et al. Causal associations between immune cell phenotypes and varicose veins: A mendelian randomization analysis. Ann Vasc Surg. (2025) 114:126–32. doi: 10.1016/j.avsg.2025.01.030
68. Cao W, Yang Z, Mo L, Liu Z, Wang J, Zhang Z, et al. Causal relationship between immune cells and risk of heart failure: evidence from a Mendelian randomization study. Front Cardiovasc Med. (2024) 11:1473905. doi: 10.3389/fcvm.2024.1473905
69. Mo L, Pan W, Cao W, Wang K, and Huang L. Immune cells and intracerebral hemorrhage: A causal investigation through mendelian randomization. Brain Behav. (2025) 15:e70263. doi: 10.1002/brb3.70263
70. Chen L, He Y, Duan M, Yang T, Chen Y, Wang B, et al. Exploring NUP62’s role in cancer progression, tumor immunity, and treatment response: insights from multi-omics analysis. Front Immunol. (2025) 16:1559396. doi: 10.3389/fimmu.2025.1559396
71. Ghosh S, O'Hara MP, Sinha P, Mazumdar T, Yapindi L, Sastry JK, et al. Targeted inhibition of Aurora kinase A promotes immune checkpoint inhibition efficacy in human papillomavirus-driven cancers. J Immunother Cancer. (2025) 13:e009316. doi: 10.1136/jitc-2024-009316
72. Lin Z, Wang F, Yin R, Li S, Bai Y, Zhang B, et al. Single-cell RNA sequencing and immune microenvironment analysis reveal PLOD2-driven Malignant transformation in cervical cancer. Front Immunol. (2024) 15:1522655. doi: 10.3389/fimmu.2024.1522655
73. Yu J, Liang LL, Liu J, Liu TT, Li J, Xiu L, et al. Development and validation of a novel gene signature for predicting the prognosis by identifying m5C modification subtypes of cervical cancer. Front Genet. (2021) 12:733715. doi: 10.3389/fgene.2021.733715
74. Trujillo-Cirilo L, Weiss-Steider B, Vargas-Angeles CA, Corona-Ortega MT, and Rangel-Corona R. Immune microenvironment of cervical cancer and the role of IL-2 in tumor promotion. Cytokine. (2023) 170:156334. doi: 10.1016/j.cyto.2023.156334
75. Poirson J, Suarez IP, Straub ML, Cousido-Siah A, Peixoto P, Hervouet E, et al. High-risk mucosal human papillomavirus 16 (HPV16) E6 protein and cutaneous HPV5 and HPV8 E6 proteins employ distinct strategies to interfere with interferon regulatory factor 3-mediated beta interferon expression. J Virol. (2022) 96:e0187521. doi: 10.1128/jvi.01875-21
76. Peng S, Xing D, Ferrall L, Tsai YC, Roden RBS, Hung CF, et al. Development of a spontaneous HPV16 E6/E7-expressing head and neck squamous cell carcinoma in HLA-A2 transgenic mice. mBio. (2022) 13:e0325221. doi: 10.1128/mbio.03252-21
77. Feng J, Liu Y, Zhuang N, Chai Z, Liu L, Qian C, et al. EDA-E7 activated DCs induces cytotoxic T lymphocyte immune responses against HPV expressing cervical cancer in human setting. Vaccines (Basel). (2023) 11(2):320. doi: 10.3390/vaccines11020320
78. Wang L, Zhang L, Zhang Z, Wu P, Zhang Y, and Chen X. Advances in targeting tumor microenvironment for immunotherapy. Front Immunol. (2024) 15:1472772. doi: 10.3389/fimmu.2024.1472772
79. Xiao Y and Yu D. Tumor microenvironment as a therapeutic target in cancer. Pharmacol Ther. (2021) 221:107753. doi: 10.1016/j.pharmthera.2020.107753
80. Shao Y, Chen C, Yu X, Yan J, Guo J, and Ye G. Comprehensive analysis of scRNA-seq and bulk RNA-seq data via machine learning and bioinformatics reveals the role of lysine metabolism-related genes in gastric carcinogenesis. BMC Cancer. (2025) 25:644. doi: 10.1186/s12885-025-14051-w
81. Ou Z, Lin S, Qiu J, Ding W, Ren P, Chen D, et al. Single-nucleus RNA sequencing and spatial transcriptomics reveal the immunological microenvironment of cervical squamous cell carcinoma. Adv Sci (Weinh). (2022) 9:e2203040. doi: 10.1002/advs.202203040
82. Fan J, Lu F, Qin T, Peng W, Zhuang X, Li Y, et al. Multiomic analysis of cervical squamous cell carcinoma identifies cellular ecosystems with biological and clinical relevance. Nat Genet. (2023) 55:2175–88. doi: 10.1038/s41588-023-01570-0
83. Chen X, Hao Y, Liu Y, Zhong S, You Y, Ao K, et al. NAT10/ac4C/FOXP1 promotes Malignant progression and facilitates immunosuppression by reprogramming glycolytic metabolism in cervical cancer. Adv Sci (Weinh). (2023) 10:e2302705. doi: 10.1002/advs.202302705
84. Yu X, Shao Y, Dong H, Yan J, Zhang X, and Ye G. Molecular subtype of gastric cancer based on apoptosis-related genes reveals differential immune microenvironment and intratumoral microorganisms distribution. BMC Cancer. (2025) 25:12. doi: 10.1186/s12885-024-13411-2
85. Zhao Z, Ding Y, Tran LJ, Chai G, and Lin L. Innovative breakthroughs facilitated by single-cell multi-omics: manipulating natural killer cell functionality correlates with a novel subcategory of melanoma cells. Front Immunol. (2023) 14:1196892. doi: 10.3389/fimmu.2023.1196892
86. Zhang Y, Zhao Z, Huang W, Kim BS, Lin L, Li X, et al. Pan-cancer single-cell analysis revealing the heterogeneity of cancer-associated fibroblasts in skin tumors. Curr Gene Ther. (2024) 25:e15665232331353. doi: 10.2174/0115665232331353240911080642
87. Hou M, Zhao Z, Li S, Zhang Z, Li X, Zhang Y, et al. Single-cell analysis unveils cell subtypes of acral melanoma cells at the early and late differentiation stages. J Cancer. (2025) 16:898–916. doi: 10.7150/jca.102045
88. Lin Z, Zou J, Sui X, Yao S, Lin L, Wang J, et al. Necroptosis-related lncRNA signature predicts prognosis and immune response for cervical squamous cell carcinoma and endocervical adenocarcinomas. Sci Rep. (2022) 12:16285. doi: 10.1038/s41598-022-20858-5
89. Archilla-Ortega A, Domuro C, Martin-Liberal J, and Muñoz P. Blockade of novel immune checkpoints and new therapeutic combinations to boost antitumor immunity. J Exp Clin Cancer Res. (2022) 41:62. doi: 10.1186/s13046-022-02264-x
90. Wang SJ, Dougan SK, and Dougan M. Immune mechanisms of toxicity from checkpoint inhibitors. Trends Cancer. (2023) 9:543–53. doi: 10.1016/j.trecan.2023.04.002
91. Marabelle A, Le DT, Ascierto PA, Di Giacomo AM, De Jesus-Acosta A, Delord JP, et al. Efficacy of pembrolizumab in patients with noncolorectal high microsatellite instability/mismatch repair-deficient cancer: results from the phase II KEYNOTE-158 study. J Clin Oncol. (2020) 38:1–10. doi: 10.1200/JCO.19.02105
92. O'Malley DM, Oaknin A, Monk BJ, Selle F, Rojas C, Gladieff L, et al. Phase II study of the safety and efficacy of the anti-PD-1 antibody balstilimab in patients with recurrent and/or metastatic cervical cancer. Gynecol Oncol. (2021) 163:274–80. doi: 10.1016/j.ygyno.2021.08.018
93. Naumann RW, Hollebecque A, Meyer T, Devlin MJ, Oaknin A, Kerger J, et al. Safety and efficacy of nivolumab monotherapy in recurrent or metastatic cervical, vaginal, or vulvar carcinoma: results from the phase I/II checkMate 358 trial. J Clin Oncol. (2019) 37:2825–34. doi: 10.1200/JCO.19.00739
94. Tewari KS, Monk BJ, Vergote I, Miller A, de Melo AC, Kim HS, et al. Survival with cemiplimab in recurrent cervical cancer. N. Engl J Med. (2022) 386:544–55. doi: 10.1056/NEJMoa2112187
95. Ouyang P, Yang W, Sun J, Chen P, He Q, Yan J, et al. Endocrine toxicity of immune checkpoint inhibitors: a network meta-analysis of the current evidence. Acta Mater Med. (2024) 3:1–19. doi: 10.15212/AMM-2023-0037
96. Li H, Xu Y, Jiao X, Xu Q, Peng Z, Tang Y, et al. IBI310 plus sintilimab vs. placebo plus sintilimab in recurrent/metastatic cervical cancer: A double-blind, randomized controlled trial. Med. (2025) 6(5):100573. doi: 10.1016/j.medj.2024.100573
97. Wang Y, Zhao J, Liang H, Liu J, Huang S, Zou G, et al. Efficacy and safety of sintilimab plus albumin-bound-paclitaxel in recurrent or metastatic cervical cancer: a multicenter, open-label, single-arm, phase II trial. EClinicalMedicine. (2023) 65:102274. doi: 10.1016/j.eclinm.2023.102274
98. Huang W, Liu J, Xu K, Chen H, and Bian C. PD-1/PD-L1 inhibitors for advanced or metastatic cervical cancer: From bench to bed. Front Oncol. (2022) 12:849352. doi: 10.3389/fonc.2022.849352
99. Hillemanns P, Zikan M, Forget F, Denys HG, Baurain JF, Rob L, et al. Safety and efficacy of the therapeutic DNA-based vaccine VB10.16 in combination with atezolizumab in persistent, recurrent or metastatic HPV16-positive cervical cancer: a multicenter, single-arm phase 2a study. J Immunother Cancer. (2025) 13(1):e010827. doi: 10.1136/jitc-2024-010827
100. Wang B, Liang Y, Wu Y, Li Q, Zeng Y, Liu L, et al. Sintilimab plus HPV vaccine for recurrent or metastatic cervical cancer. J Immunother Cancer. (2024) 12:e009898. doi: 10.1136/jitc-2024-009898
101. Dabas P and Danda A. Revolutionizing cancer treatment: a comprehensive review of CAR-T cell therapy. Med Oncol. (2023) 40:275. doi: 10.1007/s12032-023-02146-y
102. Granhøj JS, Witness Præst Jensen A, Presti M, Met Ö, Svane IM, and Donia M. Tumor-infiltrating lymphocytes for adoptive cell therapy: recent advances, challenges, and future directions. Expert Opin Biol Ther. (2022) 22:627–41. doi: 10.1080/14712598.2022.2064711
103. Zhu Y, Zhou J, Zhu L, Hu W, Liu B, and Xie L. Adoptive tumor infiltrating lymphocytes cell therapy for cervical cancer. Hum Vaccin Immunother. (2022) 18:2060019. doi: 10.1080/21645515.2022.2060019
104. Zhang X, Zhang H, Lan H, Wu J, and Xiao Y. CAR-T cell therapy in multiple myeloma: Current limitations and potential strategies. Front Immunol. (2023) 14:1101495. doi: 10.3389/fimmu.2023.1101495
105. Baulu E, Gardet C, Chuvin N, and Depil S. TCR-engineered T cell therapy in solid tumors: State of the art and perspectives. Sci Adv. (2023) 9:eadf3700. doi: 10.1126/sciadv.adf3700
106. Wang Y, Wang C, Qiu J, Qu X, Peng J, Lu C, et al. Targeting CD96 overcomes PD-1 blockade resistance by enhancing CD8+ TIL function in cervical cancer. J Immunother Cancer. (2022) 10:e003667. doi: 10.1136/jitc-2021-003667
107. Stevanović S, Draper LM, Langhan MM, Campbell TE, Kwong ML, Wunderlich JR, et al. Complete regression of metastatic cervical cancer after treatment with human papillomavirus-targeted tumor-infiltrating T cells. J Clin Oncol. (2015) 33:1543–50. doi: 10.1200/JCO.2014.58.9093
108. Nagarsheth NB, Norberg SM, Sinkoe AL, Adhikary S, Meyer TJ, Lack JB, et al. TCR-engineered T cells targeting E7 for patients with metastatic HPV-associated epithelial cancers. Nat Med. (2021) 27:419–25. doi: 10.1038/s41591-020-01225-1
109. Zhang Y, Li X, Zhang J, and Mao L. Novel cellular immunotherapy using NKG2D CAR-T for the treatment of cervical cancer. BioMed Pharmacother. (2020) 131:110562. doi: 10.1016/j.biopha.2020.110562
110. Liu C, Li X, Huang Q, Zhang M, Lei T, Wang F, et al. Single-cell RNA-sequencing reveals radiochemotherapy-induced innate immune activation and MHC-II upregulation in cervical cancer. Signal Transduct Target Ther. (2023) 8:44. doi: 10.1038/s41392-022-01264-9
111. Li C, Liu D, Zhao Y, Ding Y, and Hua K. Diverse intratumoral heterogeneity and immune microenvironment of two HPV-related cervical cancer types revealed by single-cell RNA sequencing. J Med Virol. (2023) 95:e28857. doi: 10.1002/jmv.28857
112. Qiu J, Qu X, Wang Y, Guo C, Lv B, Jiang Q, et al. Single-cell landscape highlights heterogenous microenvironment, novel immune reaction patterns, potential biomarkers and unique therapeutic strategies of cervical squamous carcinoma, human papillomavirus-associated (HPVA) and non-HPVA adenocarcinoma. Adv Sci (Weinh). (2023) 10:e2204951. doi: 10.1002/advs.202204951
113. Wang Q, Schmoeckel E, Kost BP, Kuhn C, Vattai A, Vilsmaier T, et al. Higher CCL22+ Cell infiltration is associated with poor prognosis in cervical cancer patients. Cancers (Basel). (2019) 11(12):2004. doi: 10.3390/cancers11122004
114. Wei E, Reisinger A, Li J, French LE, Clanner-Engelshofen B, and Reinholz M. Integration of scRNA-seq and TCGA RNA-seq to analyze the heterogeneity of HPV+ and HPV- cervical cancer immune cells and establish molecular risk models. Front Oncol. (2022) 12:860900. doi: 10.3389/fonc.2022.860900
115. Guo C, Qu X, Tang X, Song Y, Wang J, Hua K, et al. Spatiotemporally deciphering the mysterious mechanism of persistent HPV-induced Malignant transition and immune remodelling from HPV-infected normal cervix, precancer to cervical cancer: Integrating single-cell RNA-sequencing and spatial transcriptome. Clin Transl Med. (2023) 13:e1219. doi: 10.1002/ctm2.1219
116. Wang F, Yue S, Huang Q, Lei T, Li X, Wang C, et al. Cellular heterogeneity and key subsets of tissue-resident memory T cells in cervical cancer. NPJ Precis Oncol. (2024) 8:145. doi: 10.1038/s41698-024-00637-3
117. Yunna C, Mengru H, Lei W, and Weidong C. Macrophage M1/M2 polarization. Eur J Pharmacol. (2020) 877:173090. doi: 10.1016/j.ejphar.2020.173090
118. Sheng B, Pan S, Ye M, Liu H, Zhang J, Zhao B, et al. Single-cell RNA sequencing of cervical exfoliated cells reveals potential biomarkers and cellular pathogenesis in cervical carcinogenesis. Cell Death Dis. (2024) 15:130. doi: 10.1038/s41419-024-06522-y
119. Li C, Liu D, Yang S, and Hua K. Integrated single-cell transcriptome analysis of the tumor ecosystems underlying cervical cancer metastasis. Front Immunol. (2022) 13:966291. doi: 10.3389/fimmu.2022.966291
120. Li X, Zhang Q, Chen G, and Luo D. Multi-omics analysis showed the clinical value of gene signatures of C1QC(+) and SPP1(+) TAMs in cervical cancer. Front Immunol. (2021) 12:694801. doi: 10.3389/fimmu.2021.694801
121. Wang Z, Wang B, Feng Y, Ye J, Mao Z, Zhang T, et al. Targeting tumor-associated macrophage-derived CD74 improves efficacy of neoadjuvant chemotherapy in combination with PD-1 blockade for cervical cancer. J Immunother Cancer. (2024) 12:e009024. doi: 10.1136/jitc-2024-009024
122. Yin R, Zhai X, Han H, Tong X, Li Y, and Deng K. Characterizing the landscape of cervical squamous cell carcinoma immune microenvironment by integrating the single-cell transcriptomics and RNA-Seq. Immun Inflammation Dis. (2022) 10:e608. doi: 10.1002/iid3.608
123. Wang S, Li X, Liu C, Yuan Y, and Ma F. Single-cell transcriptomic analysis of the role of HPV16-positive macrophages in cervical cancer prognosis. J Med Virol. (2023) 95:e28410. doi: 10.1002/jmv.28410
124. Qu X, Wang Y, Jiang Q, Ren T, Guo C, Hua K, et al. Interactions of Indoleamine 2,3-dioxygenase-expressing LAMP3(+) dendritic cells with CD4(+) regulatory T cells and CD8(+) exhausted T cells: synergistically remodeling of the immunosuppressive microenvironment in cervical cancer and therapeutic implications. Cancer Commun (Lond). (2023) 43:1207–28. doi: 10.1002/cac2.12486
125. Wang C, He L, Peng J, Lu C, Zhang M, Qi X, et al. Identification of Siglec-10 as a new dendritic cell checkpoint for cervical cancer immunotherapy. J Immunother Cancer. (2024) 12:e009404. doi: 10.1136/jitc-2024-009404
126. Ghaedrahmati F, Esmaeil N, and Abbaspour M. Targeting immune checkpoints: how to use natural killer cells for fighting against solid tumors. Cancer Commun (Lond). (2023) 43:177–213. doi: 10.1002/cac2.12394
127. Zhang C and Liu Y. Targeting NK cell checkpoint receptors or molecules for cancer immunotherapy. Front Immunol. (2020) 11:1295. doi: 10.3389/fimmu.2020.01295
128. Frohne CC, Llano EM, Perkovic A, Cohen RD, and Luke JJ. Complete response of metastatic melanoma in a patient with Crohn’s disease simultaneously receiving anti-alpha4beta7 and anti-PD1 antibodies. J Immunother Cancer. (2019) 7:1. doi: 10.1186/s40425-018-0484-x
129. Tinker AV, Hirte HW, Provencher D, Butler M, Ritter H, Tu D, et al. Dose-ranging and cohort-expansion study of monalizumab (IPH2201) in patients with advanced gynecologic Malignancies: A trial of the canadian cancer trials group (CCTG): IND221. Clin Cancer Res. (2019) 25:6052–60. doi: 10.1158/1078-0432.CCR-19-0298
130. Li X, Zhang M, Lei T, Zou W, Huang R, Wang F, et al. Single-cell RNA-sequencing dissects cellular heterogeneity and identifies two tumor-suppressing immune cell subclusters in HPV-related cervical adenosquamous carcinoma. J Med Virol. (2022) 94:6047–59. doi: 10.1002/jmv.28084
131. Chambers AM, Wang J, Dao TN, Lupo KB, Veenhuis P, Ayers MG, et al. Functional expression of CD73 on human natural killer cells. Cancer Immunol. Immunother. (2022) 71:3043–56. doi: 10.1007/s00262-022-03219-z
132. Zhao F, Hong J, Zhou G, Huang T, Lin Z, Zhang Y, et al. Elucidating the role of tumor-associated ALOX5+ mast cells with transformative function in cervical cancer progression via single-cell RNA sequencing. Front Immunol. (2024) 15:1434450. doi: 10.3389/fimmu.2024.1434450
Keywords: cervical cancer, tumor immune microenvironment, immunotherapy, PD-1/PD-L1 signaling pathway, immune checkpoint
Citation: Zhang X, Nie W, Shao W and Guo Q (2025) Mapping the immunological landscape and emerging immunotherapeutic strategies in cervical cancer: a comprehensive review. Front. Oncol. 15:1620501. doi: 10.3389/fonc.2025.1620501
Received: 29 April 2025; Accepted: 18 June 2025;
Published: 10 July 2025.
Edited by:
Jing Zhang, University of South Dakota, United StatesReviewed by:
Lei Lei Liang, Chinese Academy of Medical Sciences and Peking Union Medical College, ChinaCopyright © 2025 Zhang, Nie, Shao and Guo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wenwen Shao, c3d3ZW42NjY5OTlAMTYzLmNvbQ==; Qian Guo, Z3VvcWlhbjc4MTEzMG1AMTI2LmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Xinyi Zhang
Xinyi Zhang Wenyang Nie
Wenyang Nie Wenwen Shao
Wenwen Shao Qian Guo3*
Qian Guo3*