- 1Division of Biliary Tract Surgery, Department of General Surgery, West China Hospital, Sichuan University, Chengdu, Sichuan, China
- 2Department of Medical Ultrasound, West China Hospital, Sichuan University, Chengdu, China
Background: Biliary drainage for advanced malignant hilar biliary obstruction (MHBO) remains a significant challenge in current clinical practice.
Case description: A 58-year-old male diagnosed with unresectable advanced intrahepatic cholangiocarcinoma with hilar obstruction and required palliative biliary drainage. Imaging revealed obstruction of the common bile duct, left hepatic duct, right anterior hepatic duct, and right posterior hepatic duct (Bismuth–Corlette type IV). Due to the failure of ERCP, we decided to bridge biliary drainage with percutaneous transhepatic cholangioscopy (PTCS) after multidisciplinary discussion. First, one-step PTCS was used to establish a channel between the skin and the right anterior hepatic duct. Then a puncture needle was used to puncture the right anterior hepatic duct to the distal common bile duct, and the first stent was inserted for bridging. Next, a puncture needle was used from the right anterior hepatic duct to the left hepatic duct and a second stent was inserted for bridging. Finally, a puncture needle was used to puncture the right anterior hepatic duct to the right posterior hepatic duct, and a third stent was inserted for bridging. Intraoperative X-ray examination with contrast agent injected through the sinus confirmed successful bridging. The jaundice disappeared a few days after surgery, and no post-procedure-related adverse events occurred.
Conclusion: This case demonstrates that ultrasound-guided PTCS triple-bridge biliary drainage connecting multiple bile ducts is a feasible palliative option for MHBO and warrants further clinical investigation.
1 Introduction
Malignant hilar biliary obstruction (MHBO) can arise from intrahepatic and extrahepatic bile duct cancer, ampulla cancer, hepatocellular carcinoma, pancreatic cancer, or metastatic tumors from other primary sites (1, 2). MHBO often leads to obstructive jaundice and impaired liver function, and the prognosis of patients with unresectable MHBO is poor. In clinical practice, biliary drainage is widely used to relieve jaundice and improve quality of life (3, 4). Adequate biliary drainage is associated with prolonged survival (3, 5). However, effective biliary drainage in advanced MHBO (particularly Bismuth-Corlette type III–IV) remains a significant technical challenge (6, 7).
Percutaneous transhepatobiliary drainage (PTBD) and/or endoscopic retrograde cholangiopancreatography (ERCP) are well-known palliative biliary drainage strategies recommended by the guidelines of the European Society for Gastrointestinal Endoscopy (8). Unfortunately, the strategies currently available are difficult to achieve the desired drainage effect. Complicated MHBO often requires multiple PTBD. However, multiple PTBDs may increase the risk of complications and discomfort compared to unilateral PTBD. Biliary bridge drainage offers a potential alternative for complex MHBO. However, ultrasound guided hepaticogastrostomy bridge drainage can only establish a single bridge between two bile ducts (the left and right hepatic duct), and there is still a lack of technology to achieve multiple bridge drainage between multiple intrahepatic ducts for the treatment of multiple intrahepatic bile duct occlusion (6).
Herein, we report a novel technique using PTCS to achieve triple-bridge drainage among multiple hepatic ducts, enabling bilateral drainage in patients with Bismuth Corlette III and IV MHBO.
2 Case presentation
2.1 Patient presentation
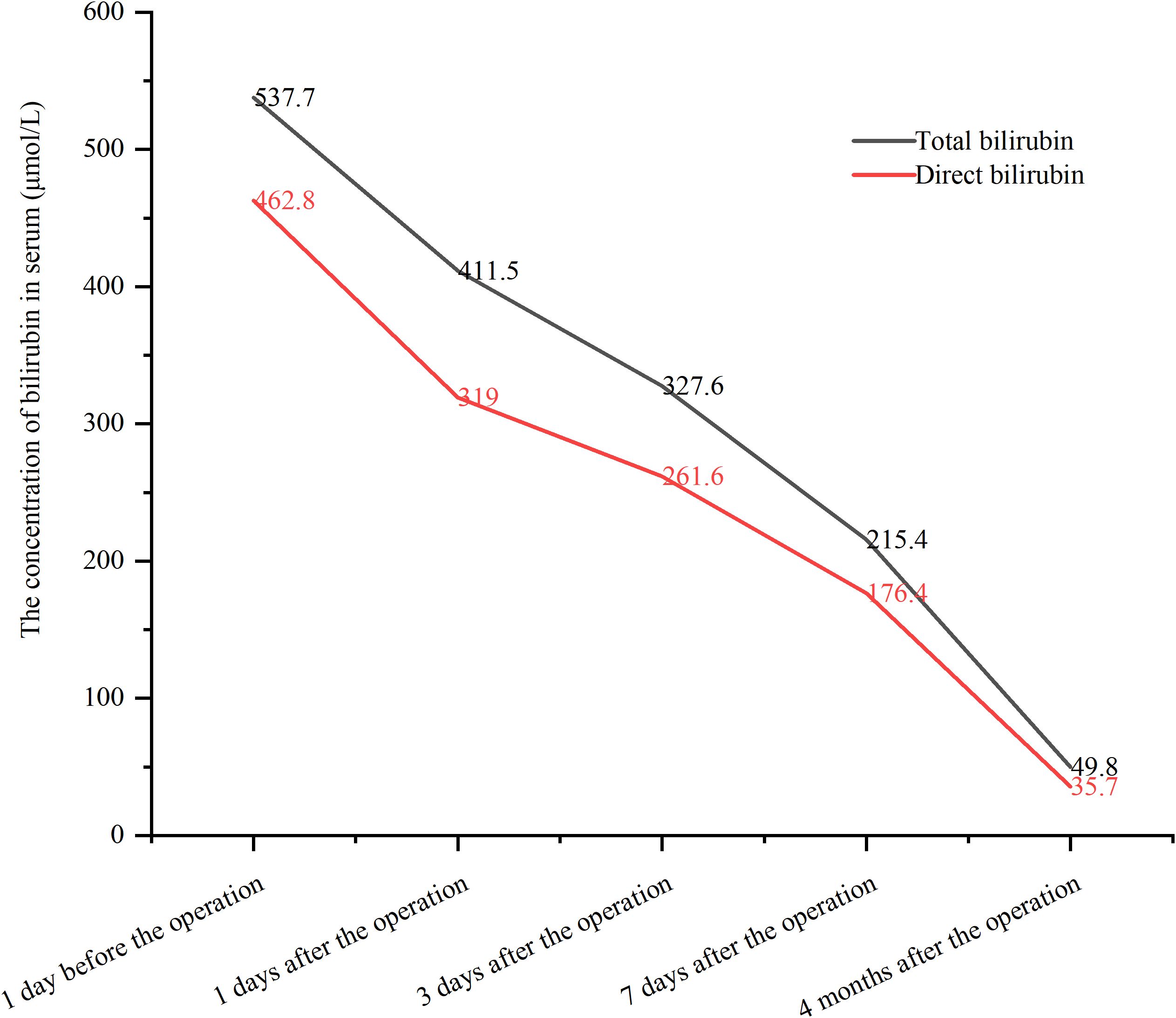
A 58-year-old male presented with jaundice, no systemic disease, comorbidities, or prior abdominal surgery. There were no clinical manifestations related to cholangitis such as fever and abdominal pain. Laboratory tests revealed markedly elevated serum bilirubin levels: total bilirubin 537.7μmol/L and direct bilirubin 462.8μmol/L. CT showed advanced intrahepatic cholangiocarcinoma with hilar bile duct invasion, rendering the tumor unresectable. Obstruction was noted in the common bile duct, left hepatic duct, right anterior hepatic duct, and right posterior hepatic duct, corresponding to Bismuth-Corlette type IV (Supplementary Figure S1). The pathological diagnosis was intrahepatic cholangiocarcinoma.
2.2 Diagnostic findings and surgical treatment
The patient was diagnosed with advanced intrahepatic cholangiocarcinoma with hilar obstruction and required palliative biliary drainage. We decided to perform PTCS guided bridge drainage between multiple biliary ducts after multidisciplinary discussion.
One-step PTCS was adopted (9, 10) (Supplementary Video 1). Step 1: After successful general anesthesia through tracheal intubation, the patients were tilted to the right by 15 degrees in supine position. Intraoperative ultrasonography guided puncture of the right anterior hepatic duct, through which a zebra guidewire was advanced into the intrahepatic biliary system. With the guidance of the zebra guidewire, the tract was immediately expanded by the biliary expanders step-by-step until it could hold an 18-Fr protective sheath. In this way, a working channel for the rigid choledochoscopy was established.
Step 2: Choledochoscopy revealed distal obstruction of the right anterior hepatic duct, preventing guidewire passage. Then, a puncture needle was used to puncture the right anterior hepatic duct to the distal common bile duct, and a balloon was used to dilate the channel. A self-expanding metal stent was deployed, creating the first bridge. Intraoperative cholangiography confirmed successful bridging between the right anterior hepatic duct and the distal common bile duct (the first bridge).
Step 3: Under combined PTCS and ultrasound guidance, the right anterior hepatic duct was punctured into the left hepatic duct. The tract was re-dilated, and an 8 x 60 mm metal biliary stent was deployed, forming the second bridge. X-ray examination by injecting contrast agent showed successful bridging of the right anterior and left hepatic ducts (the second bridge), but poor imaging of the right posterior hepatic duct suggested that the right posterior hepatic duct may still be narrow.
Step 4: Finally, ultrasound-guided puncture from right anterior hepatic duct into right posterior hepatic duct. The passage between the right anterior and right posterior hepatic ducts was re-dilated using the guidewire. The right anterior hepatic duct was bridged with an 8 x 60 mm metal biliary stent to the right posterior hepatic duct. Cholangiography confirmed successful communication between the right anterior and right posterior hepatic ducts (the third bridge) (Figure 1).
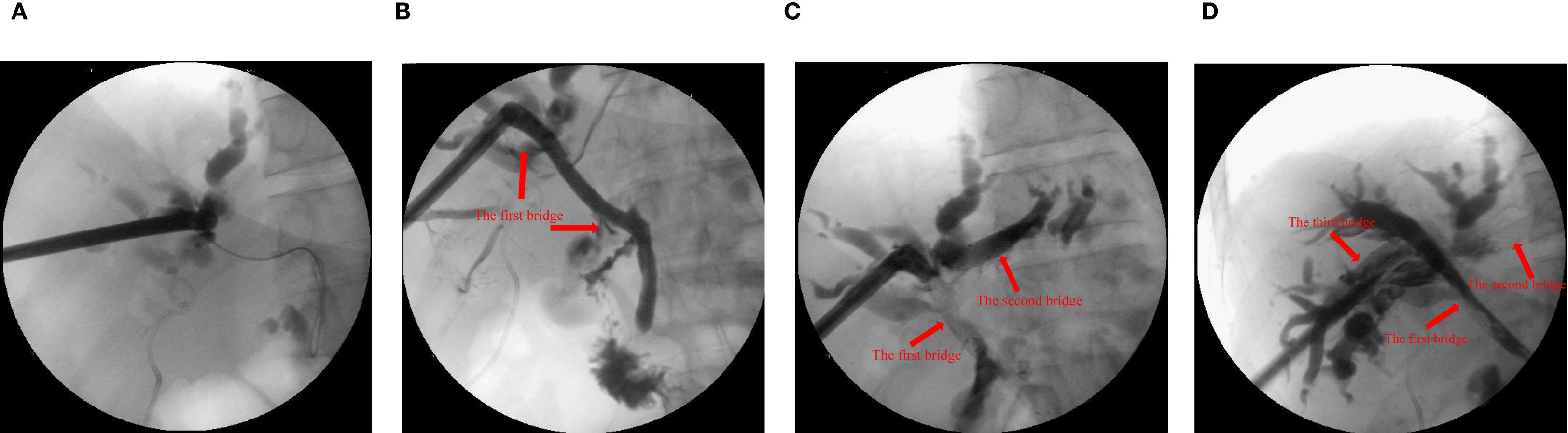
Figure 1. X-ray examination with contrast agent injected through the sinus. (A) the common bile duct before guide wire puncture and balloon dilation. (B) the right anterior intrahepatic bile duct and the common bile duct were bridged with metal stent (10x60 mm, the first bridge). (C) the left and right anterior intrahepatic bile ducts were bridged with metal stent (8x60 mm, the second bridge). (D) the right anterior and right posterior intrahepatic bile ducts were bridged with metal stent (8x60 mm, the third bridge).
2.3 Outcome
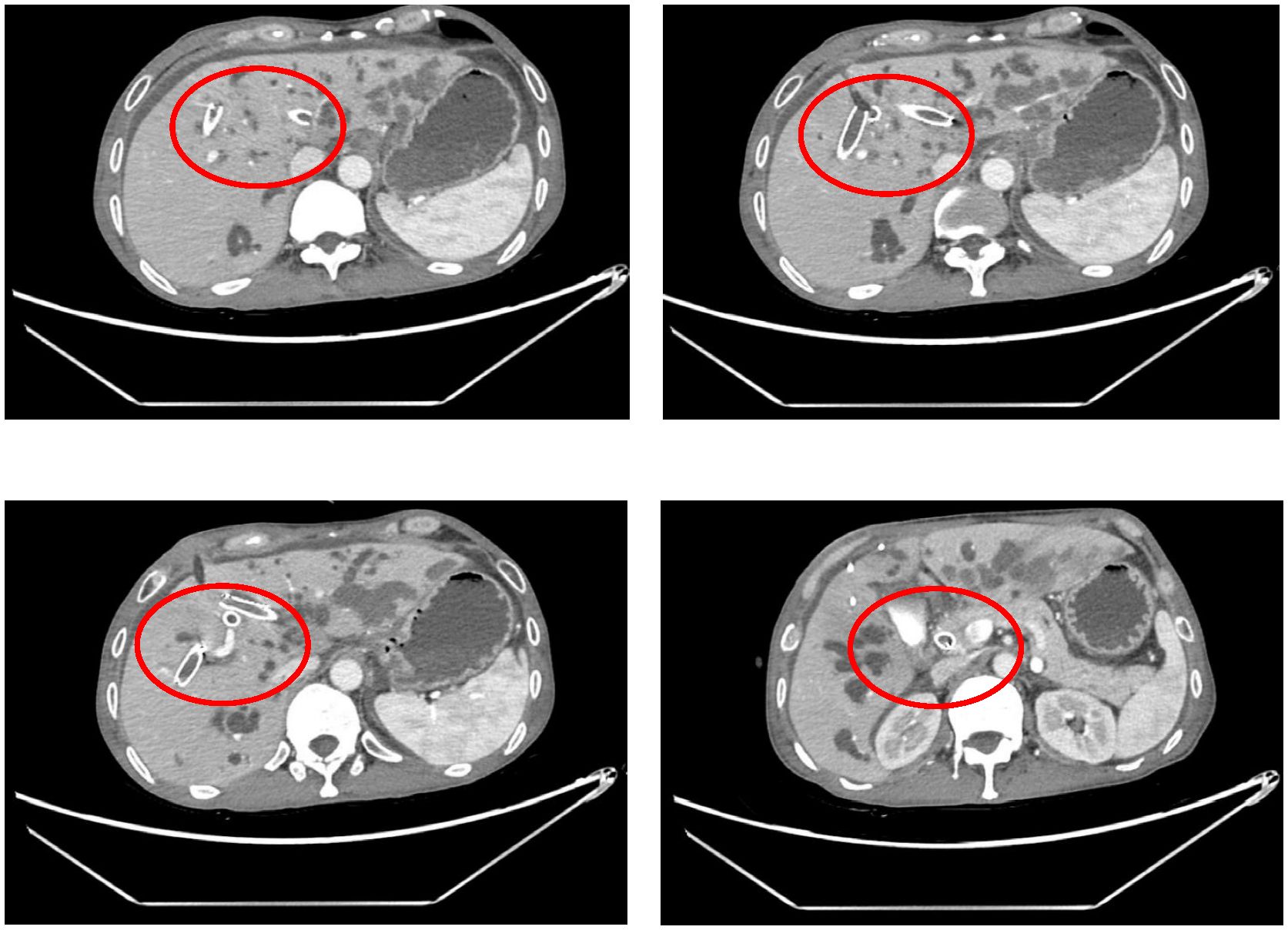
The procedure time was 239 minutes. Jaundice resolved within several days, with total and direct bilirubin levels decreasing to 215.4 μmol/L and 176.4 μmol/L, respectively, within one week (Figure 2). No post-procedure-related adverse events occurred. The patient’s bilirubin decreased by 60% within one week after the procedure compared with the preoperative value, indicating clinical success. Post-procedure CT (Figure 3) at one week showed that bilateral hepatic duct drainage was smooth. The hospital stay was 5 days (ICU stay: 0 days). Palliative adjuvant therapy commenced three weeks post-procedure. During the four-month follow-up period, no reintervention (like another PTBD or surgery) was required.
3 Discussion
PTBD remains the primary palliative drainage option for MHBO when ERCP fails (11, 12). However, PTBD is limited by the small caliber of drainage catheters, which restricts drainage efficiency, and is associated with high re-intervention rates and complication risks (13–15). Páez-Carpio et al. reported a major complication rate of up to 21% (16). PTBD cannot achieve bilateral bile duct bridging, and complex MHBO often necessitates multiple drainage catheters. Additional catheters not only increase postoperative complication risk but also worsen patient discomfort. Moreover, due to biliary atresia, internal biliary drainage is often unachievable with PTBD. Compared with internal biliary drainage, external biliary drainage not only affects the nutritional status and immune function of patients, but also may damage the long-term prognosis of patients (17, 18). Kumar et al. demonstrated that internal drainage significantly improves overall survival compared with external drainage (18). Endoscopic ultrasound-guided biliary drainage (EUS-BD) has received extensive attention in recent years as a novel minimally invasive treatment method (19–22). Initially developed for distal bile duct strictures (23), technological advances have extended its application to MHBO (20, 24, 25). Compared with PTBD, EUS-BD can achieve intraluminal drainage and keep the stent away from the tumor, providing a longer stent patency time and a lower re-intervention rate (26). EUS-BD includes EUS-guided hepaticogastrostomy (EUS-HGS), EUS-guided choledochoduodenostomy (EUS-CDS) and EUS-guided hepaticoduodenostomy (EUS-HDS). EUS-guided rendezvous can be considered after ERCP failure (25). Kongkam et al. (7) reported a new strategy combining ERCP and EUS-BD for the treatment of MHBO (CERES). If the self-expandable metal stent is placed in the right biliary system, EUS-HGS can be performed subsequently. However, if the stent is placed in the left biliary system, EUS-HDS is performed. For MHBO with no function in the right liver, EUS-HGS can be performed after ERCP failure, or primary HGS can be performed in the left liver. A multicenter retrospective study (7) involving 36 patients with native high grade-MHBO demonstrated that compared with bilateral PTBD, combined ERCP and EUS-BD significantly reduced the recurrent biliary obstruction rate at 3 months and 6 months. And there was no significant difference between the two groups in terms of the incidence of complications and mortality. Its ability to achieve internal drainage offers clear advantages over PTBD, and its higher technical success rate makes it favorable compared with bilateral self-expandable metal stent via ERCP (7, 25).
Interbiliary bridging drainage has recently gained attention as a potential internal drainage strategy for complex MHBO. However, bilateral intrahepatic bridging remains rarely reported, with only four studies published to date (27–30) (Table 1). In 2014, Ogura et al. (27) reported the first case of EUS-guided hepaticogastrostomy (EUS-HGS) combined biliary bridge procedure. They placed two metal stents in sequence (one connecting the left and right ducts, and another for hepaticogastrostomy) to treat a patient with hilar obstruction caused by colorectal cancer metastasis. In the same year, Reimao et al. (28) reported nine patients undergoing EUS-HGS biliary bridging for MHBO. All patients were treated with three-step drainage. Step 1: EUS-guided left duct puncture with a 19-gauge needle. Step 2: Insert 0.0035 inch guide wire located on the right biliary tree where it crosses the bile duct bifurcation. After expansion, a self-expanding metal stent without cover was placed to connect the left and right bile ducts. Step 3: A second stent is inserted into the left bile duct, with the distal part in the previous stent and the proximal edge in the stomach. Drainage failed in 2/9 patients, and complications occurred in 33% (4 cases). Postoperative mortality was 8%, and 70% of patients proceeded to chemotherapy (7). In 2020, Atalla et al. (29) described a patient who had previously undergone distal gastrectomy and Roux-en-Y surgery for gastric cancer and developed postoperative liver metastases with hilar obstruction. They performed EUS-HGS bile duct bridging and common bile duct stent implantation for the patient. In addition, in 2023, Niiya et al. (30) reported a patient with gallbladder cancer who received multiple ERCP treatment due to MHBO, and was admitted to hospital again due to cholangitis after five stents were placed successively. CT showed dilated intrahepatic and right posterior bile duct (RPD) in this patient. ERCP failed because tumor obstruction prevented the punctured RPD from guiding RPD drainage from the duodenum. Therefore, they used EUS-HGS combined bile duct bridging method. Hence, bile duct bridging procedure is an important strategy for the treatment of complex MHBO. However, due to the difficulty of EUS-HGS bile duct bridging and the need to be performed by an experienced physician in a high-volume center, further promotion of this method is limited (6).
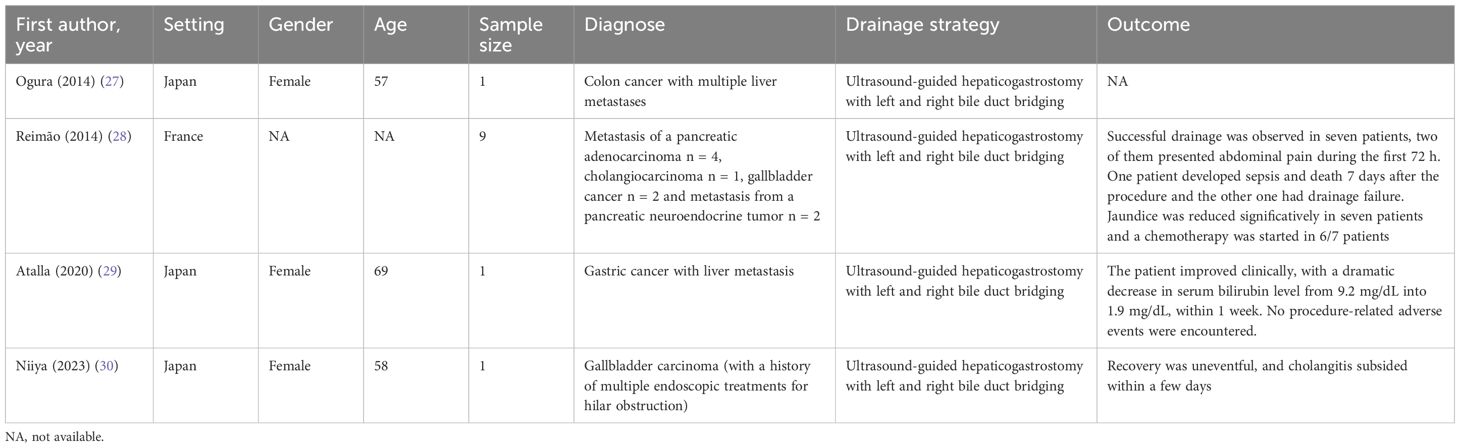
Table 1. Study characteristics of the studies using ultrasound-guided hepaticogastrostomy with bridging.
PTCS, with its ability to provide direct intraductal visualization, may overcome some limitations of EUS-HGS in interbiliary bridging. PTCS is well established in the management of intrahepatic bile duct stones and strictures (31–33) and in diagnosing malignant biliary obstruction (34), and has shown therapeutic potential in unresectable MHBO (35). PTCS-guided bridging offers several advantages. First, PTCS can be used to observe the lesions directly, and the direction could be adjusted by cholangioscope (36). After ultrasound-guided puncture, the contralateral intrahepatic bile duct can be observed by PTCS, which ensured safety of the puncture. In addition, when complications such as bleeding occur, PTCS can quickly find the bleeding point and carry out effective hemostasis. We previously reported two cases of successful bilateral drainage using PTCS combined with ultrasound-guided interbiliary bridging (37, 38). In the present case, however, multiple intrahepatic duct occlusions required more than a single bridge to achieve adequate drainage. Therefore, we carried out the first case of three-bridge connection between bile ducts and successfully achieved adequate biliary drainage. In addition, our novel technique achieves internal drainage by bridging the right anterior bile duct and the common bile duct to imitate the endoscopic method. In this study, the patient’s bilirubin decreased rapidly in the days after procedure. In addition, the CT examination one week post-procedure indicated that the three stents were in place, and the bilirubin level decreased significantly. All these suggest that our drainage strategy is effective. Previous studies have reported postoperative complication rates of PTCS ranging from 22% to 65%, with the most common adverse events being cholangitis, bleeding, pleural effusion, and bile leakage (33, 39, 40). In addition, long-term stent-related complications include re-occlusion and migration. Caillol et al. (41) reported a 33% (4/12) complication rate for EUS-bridging, with ≥ CD grade III events in 8.3% (1/12). Although our patient experienced no adverse events during 4-month follow-up, larger studies are required to determine the safety of PTCS-guided bridge biliary drainage.
In conclusion, ultrasound-guided PTCS triple-bridge biliary drainage between multiple bile ducts represents a feasible and effective strategy for complex MHBO, offering a novel internal drainage option. It is necessary to accumulate more cases to further explore the potential benefits of this approach.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by Ethics Committee of West China Hospital, Sichuan University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
GT: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Software, Supervision, Visualization, Writing – original draft, Writing – review & editing. JieZ: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Software, Supervision, Visualization, Writing – original draft, Writing – review & editing. RC: Conceptualization, Data curation, Investigation, Visualization, Writing – original draft, Writing – review & editing, Project administration. JinZ: Conceptualization, Data curation, Investigation, Visualization, Writing – original draft, Writing – review & editing, Formal Analysis, Software. RZ: Conceptualization, Data curation, Formal Analysis, Investigation, Software, Visualization, Writing – original draft, Writing – review & editing, Funding acquisition, Methodology, Project administration, Resources, Supervision, Validation.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was financially supported by National Natural Science Foundation of China (22004088) and Science & Technology Support Project of Sichuan Province (2023YFS0183).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2025.1620937/full#supplementary-material
Supplementary Figure 1 | Computed tomography showed advanced intrahepatic cholangiocarcinoma, hilar bile duct invasion, and radical surgery is not possible. The patient's common bile duct, left, right anterior, and right posterior hepatic ducts were obstructed.
Supplementary Video 1 | We successfully performed biliary drainage of the bilateral hepatic duct system using a triple bridge drainage method from the ultrasound-guided percutaneous transhepatic cholangioscopy in malignant hilar biliary obstruction.
References
1. Bezabih YS and Gebremariam SN. Perioperative outcomes after open biliary bypass for Malignant biliary obstruction (MBO) in resource-limited setups; a multicenter prospective cohort study, 2023. Eur J Surg Oncol. (2024) 50:108254. doi: 10.1016/j.ejso.2024.108254
2. Pietrzak J and Przybyłkowski A. Endoscopic treatment of Malignant hilar biliary obstruction. Cancers (Basel). (2023) 15:5819. doi: 10.3390/cancers15245819
3. Imagawa N, Fukasawa M, Takano S, Kawakami S, Fukasawa Y, Hasegawa H, et al. A novel method of calculating the drained liver volume using a 3D volume analyzer for biliary drainage of unresectable Malignant hilar biliary obstruction. Dig Dis Sci. (2024) 69:969–77. doi: 10.1007/s10620-024-08294-z
4. Yue Q, Han W, and Liu ZL. Endoscopic reintervention after unilateral metal stent deployment for MHBO using SIS method. Med (Baltimore). (2023) 102:e34467. doi: 10.1097/md.0000000000034467
5. Vienne A, Hobeika E, Gouya H, Lapidus N, Fritsch J, Choury AD, et al. Prediction of drainage effectiveness during endoscopic stenting of Malignant hilar strictures: the role of liver volume assessment. Gastrointest Endosc. (2010) 72:728–35. doi: 10.1016/j.gie.2010.06.040
6. Pal P and Lakhtakia S. Endoscopic ultrasound-guided intervention for inaccessible papilla in advanced Malignant hilar biliary obstruction. Clin Endosc. (2023) 56:143–54. doi: 10.5946/ce.2022.198
7. Kongkam P, Orprayoon T, Boonmee C, Sodarat P, Seabmuangsai O, Wachiramatharuch C, et al. ERCP plus endoscopic ultrasound-guided biliary drainage versus percutaneous transhepatic biliary drainage for Malignant hilar biliary obstruction: a multicenter observational open-label study. Endoscopy. (2021) 53:55–62. doi: 10.1055/a-1195-8197
8. van der Merwe SW, van Wanrooij RLJ, Bronswijk M, Everett S, Lakhtakia S, Rimbas M, et al. Therapeutic endoscopic ultrasound: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. (2022) 54:185–205. doi: 10.1055/a-1717-1391
9. Wang P, Tao H, Liu C, Zhou X, Sun B, Zhu C, et al. One-step percutaneous transhepatic cholangioscopic lithotripsy in patients with choledocholithiasis. Clin Res Hepatol Gastroenterol. (2021) 45:101477. doi: 10.1016/j.clinre.2020.06.003
10. Yan Q, Zhang J, Chen R, Zhang J, and Zhou R. Percutaneous transhepatic cholangioscopy in hepatolithiasis associated with decompensated cirrhosis: A retrospective cohort study. J Evid Based Med. (2024) 17:843–50. doi: 10.1111/jebm.12673
11. Nikolić I, Radić J, Petreš A, Djurić A, Protić M, Litavski J, et al. The clinical benefit of percutaneous transhepatic biliary drainage for Malignant biliary tract obstruction. Cancers (Basel). (2022) 14:4673. doi: 10.3390/cancers14194673
12. Paik WH, Lee NK, Nakai Y, Isayama H, Oh D, Song TJ, et al. Conversion of external percutaneous transhepatic biliary drainage to endoscopic ultrasound-guided hepaticogastrostomy after failed standard internal stenting for Malignant biliary obstruction. Endoscopy. (2017) 49:544–8. doi: 10.1055/s-0043-102388
13. Wu SZ. Endoscopic ultrasound-guided biliary drainage using electrocautery-enhanced lumen-apposing metal stent for Malignant biliary obstruction: A promising procedure. World J Clin cases. (2024) 12:5859–62. doi: 10.12998/wjcc.v12.i26.5859
14. Troncone E, Amendola R, Moscardelli A, De Cristofaro E, De Vico P, Paoluzi OA, et al. Endoscopic gallbladder drainage: A comprehensive review on indications, techniques, and future perspectives. Med (Kaunas). (2024) 60:633. doi: 10.3390/medicina60040633
15. Westwood DA, Fernando C, and Connor SJ. Internal-external percutaneous transhepatic biliary drainage for Malignant biliary obstruction: a retrospective analysis. J Med Imaging Radiat Oncol. (2010) 54:108–10. doi: 10.1111/j.1754-9485.2010.02147.x
16. Páez-Carpio A, Hessheimer A, Bermúdez P, Zarco FX, Serrano E, Moreno J, et al. Percutaneous transhepatic biliary drainage for biliary obstruction in perihilar cholangiocarcinoma: a 10-year analysis of safety and outcomes using the CCI index. Langenbecks Arch Surg. (2023) 408:109. doi: 10.1007/s00423-023-02852-1
17. An J, Dong Y, Li Y, Han X, Sha J, Zou Z, et al. Retrospective analysis of T-lymphocyte subsets and cytokines in Malignant obstructive jaundice before and after external and internal biliary drainage. J Int Med Res. (2021) 49:300060520970741. doi: 10.1177/0300060520970741
18. Kumar S, Singh P, Kumar V, Kumar M, and Mahto M. Survival benefit of percutaneous transhepatic biliary drainage for Malignant biliary tract obstruction-a prospective study comparing external and internal drainage techniques. Abdom Radiol (NY). (2021) 46:5408–16. doi: 10.1007/s00261-021-03215-4
19. Choi JH, Kim HW, Lee JC, Paik KH, Seong NJ, Yoon CJ, et al. Percutaneous transhepatic versus EUS-guided gallbladder drainage for Malignant cystic duct obstruction. Gastrointest Endosc. (2017) 85:357–64. doi: 10.1016/j.gie.2016.07.067
20. Dhar J, Gupta P, and Samanta J. The role of endoscopy in Malignant hilar obstruction. Ann Gastroenterol. (2023) 36:347–59. doi: 10.20524/aog.2023.0810
21. Choudhury A, Samanta J, Muktesh G, Dhar J, Kumar A, Shah J, et al. Endoscopic ultrasound-guided rendezvous technique versus precut sphincterotomy as salvage technique in patients with benign biliary disease and difficult biliary cannulation: A randomized controlled trial. Ann Intern Med. (2024) 177:1361–9. doi: 10.7326/m24-0092
22. Samanta J, Sundaram S, Dhar J, Mane K, Gupta P, Gupta V, et al. EUS-guided biliary drainage in patients with moderate-severe cholangitis is safe and effective: a multi-center experience. Surg Endosc. (2023) 37:298–308. doi: 10.1007/s00464-022-09495-1
23. Khashab MA, Valeshabad AK, Afghani E, Singh VK, Kumbhari V, Messallam A, et al. A comparative evaluation of EUS-guided biliary drainage and percutaneous drainage in patients with distal Malignant biliary obstruction and failed ERCP. Dig Dis Sci. (2015) 60:557–65. doi: 10.1007/s10620-014-3300-6
24. Hijioka S, Nagashio Y, Maruki Y, Harai S, Agarie D, Yamashige D, et al. Novel approach to bilateral biliary drainage: EUS-guided hepaticoduodenodenostomy plus hepaticogastrostomy in Malignant hilar biliary obstruction. Endosc Int Open. (2025) 13:a25658206. doi: 10.1055/a-2565-8206
25. Kongkam P, Tasneem AA, and Rerknimitr R. Combination of endoscopic retrograde cholangiopancreatography and endoscopic ultrasonography-guided biliary drainage in Malignant hilar biliary obstruction. Dig Endosc. (2019) 31 Suppl 1:50–4. doi: 10.1111/den.13371
26. Cho SH, Song TJ, Oh D, Park DH, Seo DW, and Lee SK. Endoscopic ultrasound-guided hepaticoduodenostomy versus percutaneous drainage for right intrahepatic duct dilatation in Malignant hilar obstruction. J Gastroenterol Hepatol. (2024) 39:552–9. doi: 10.1111/jgh.16442
27. Ogura T, Masuda D, Imoto A, Umegaki E, and Higuchi K. EUS-guided hepaticogastrostomy for hepatic hilar obstruction. Endoscopy. (2014) 46 Suppl 1 UCTN:E32–3. doi: 10.1055/s-0033-1359133
28. Reimão S, Francioni E, Bories E, Caillol F, Pesenti C, and Giovannini M. Endoscopic ultrasonography-guided bi-lateral biliary drainage: A case series study. Endosc Ultrasound. (2014) 3:S18. doi: 10.4103/2303-9027.129533
29. Atalla H, Shiomi H, Sakai A, Masuda A, and Kodama Y. Combined bridging and antegrade stent placement during transmural treatment for Malignant hilar biliary obstruction in a patient with surgically altered anatomy. VideoGIE. (2021) 6:87–9. doi: 10.1016/j.vgie.2020.09.011
30. Niiya F, Ishiwatari H, Sato J, Matsubayashi H, and Ono H. Endoscopic ultrasound-guided hepaticogastrostomy with bridging as reintervention for stent occlusion in Malignant hilar biliary obstruction. Endoscopy. (2023) 55:E1213–e4. doi: 10.1055/a-2186-4941
31. Wang P, Sun B, Huang B, Xie J, Liu Y, Zhu C, et al. Comparison between percutaneous transhepatic rigid cholangioscopic lithotripsy and conventional percutaneous transhepatic cholangioscopic surgery for hepatolithiasis treatment. Surg Laparosc Endosc Percutan Tech. (2016) 26:54–9. doi: 10.1097/sle.0000000000000222
32. Jeng KS, Sheen IS, and Yang FS. Percutaneous transhepatic cholangioscopy in the treatment of complicated intrahepatic biliary strictures and hepatolithiasis with internal metallic stent. Surg Laparosc Endosc Percutan Tech. (2000) 10:278–83. doi: 10.1097/00129689-200010000-00004
33. Yasen A, Feng J, Liang RB, Zhu CH, Li J, Liu AZ, et al. Efficiency of percutaneous transhepatic cholangioscopy in the treatment of biliary complications after liver transplantation. HPB (Oxford). (2023) 25:463–71. doi: 10.1016/j.hpb.2023.01.010
34. Yang HJ, Kim JH, Chun JY, Kim SJ, Lee SH, Kim H, et al. A case of adenocarcinoma in situ of the distal common bile duct diagnosed by percutaneous transhepatic cholangioscopy. Korean J Intern Med. (2012) 27:211–5. doi: 10.3904/kjim.2012.27.2.211
35. Jung JY, Lee SK, Oh HC, Lee TY, Kwon SH, Lee SS, et al. The role of percutaneous transhepatic cholangioscopy in patients with hilar strictures. Gut Liver. (2007) 1:56–62. doi: 10.5009/gnl.2007.1.1.56
36. Zhang W, Sun H, Dong D, and Li Y. Safety and feasibility of a novel recanalization technique using guidewire puncture under cholangioscopy for complete biliary stricture after liver transplantation. Sci Rep. (2023) 13:4874. doi: 10.1038/s41598-023-31475-1
37. Tang G, Zhang J, Chen R, Zhang J, and Zhou R. Percutaneous transhepatic cholangioscopy combined with endoscopic retrograde cholangiopancreatography for bilateral biliary bridge drainage for Malignant biliary obstruction. Endoscopy. (2024) 56:E724–e5. doi: 10.1055/a-2375-0187
38. Tang G, Zhang J, Zhang J, and Zhou R. First case of ultrasound-guided percutaneous transhepatic cholangioscopy with bridging for bilateral biliary drainage of Malignant hilar biliary obstruction (with video). Asian J Surg. (2025). doi: 10.1016/j.asjsur.2025.05.140
39. Qin J, He Y, Ma L, Duan J, Duan R, Liu R, et al. Efficacy of 3D-printed assisted percutaneous transhepatic one-step biliary fistulation combined with rigid choledochoscopy for intrahepatic bile duct stones. Dig Liver Dis. (2023) 55:1699–704. doi: 10.1016/j.dld.2023.05.030
40. Yasen A, Feng J, Dai TX, Zhu CH, Liang RB, Liao ZH, et al. Management of anastomotic biliary stricture through utilizing percutaneous transhepatic cholangioscopy. Clin Radiol. (2024) 79:e868–e77. doi: 10.1016/j.crad.2024.02.004
Keywords: malignant hilar biliary obstruction, percutaneous transhepaticcholangioscopy, bridging technique, biliary drainage, case report
Citation: Tang G, Zhang J, Chen R, Zhang J and Zhou R (2025) Case Report: First case of percutaneous transhepatic cholangioscopy guided triple bridge drainage between multiple bile ducts for malignant hilar biliary obstruction. Front. Oncol. 15:1620937. doi: 10.3389/fonc.2025.1620937
Received: 30 April 2025; Accepted: 22 September 2025;
Published: 02 October 2025.
Edited by:
Zhaohui Tang, Shanghai Jiao Tong University, ChinaReviewed by:
Jayanta Samanta, Post Graduate Institute of Medical Education and Research (PGIMER), IndiaJahnvi Dhar, Post Graduate Institute of Medical Education and Research (PGIMER), India
Delong Qin, Shanghai Jiao Tong University, China
Copyright © 2025 Tang, Zhang, Chen, Zhang and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rongxing Zhou, cm9uZ3hpbmd6aG91QDEyNi5jb20=; Jingyi Zhang, amluZ3lpMjIzQDE2My5jb20=
†These authors have contributed equally to this work and share first authorship
 Gang Tang
Gang Tang Jie Zhang1†
Jie Zhang1† Rongxing Zhou
Rongxing Zhou