- 1Department of Advanced Diagnostics, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milano, Italy
- 2Medical Oncology and Hematology Unit, IRCCS Humanitas Research, Milan, Italy
- 3Department of Experimental Oncology, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milano, Italy
- 4Isinnova s.r.l, Brescia, Italy
- 5Breast Unit, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milano, Italy
- 6Radiation Oncology 1, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milano, Italy
- 7Breast Imaging Unit, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milano, Italy
- 8Bioinformatics and Biostatistics Unit, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milano, Italy
Background: Loco-regional recurrence (LRR) poses a clinical challenge for the follow-up of patients treated with curative intent for early-stage breast cancer (EBC). While circulating tumor DNA (ctDNA) has been shown to predict distant metastases, its value for LRR is less characterized.
Methods: Starting from an index case with documented LRR and available tumor and plasma samples, we report the analysis of the prospective phase III fenretinide prevention trial, which primarily aimed to assess the incidence of second malignancy in women with T1-T2 N0 EBC. Patients were eligible if they had FFPE and/or frozen tissue from primary or recurrent invasive tumor for next generation sequencing, and at least three serial plasma samples for ctDNA analysis by digital PCR.
Results: The TP53 R196* mutation was identified in the primary tumor of the index case with a variant allele frequency (VAF) of 29%, and in the LRR with a VAF of 58%. The same mutation was also detected in plasma prior to both the primary and LRR surgeries with VAFs of 0.19% and 0.12%, respectively. Following treatment, the mutation became undetectable in plasma samples during follow-up, consistent with the absence of recurrence. Among 40 eligible patients from the fenretinide prevention trial, 27 (67.5%) had primary tumor somatic variants trackable in plasma. Median age was 55 years (range, 35-78); stage I (16, 59%) and stage II (11, 41%); mostly luminal-like (19, 70%); median follow-up 173 months (range, 98-193); common mutations included PIK3CA (50%), TP53 (30.7%), and PTEN (5.9%). Six patients developed LRR as first event; 4 distant metastases. In all LRR cases, except one, ctDNA was detected prior to surgery and anticipated the clinical diagnosis up to 28 months. Three patients with LRR developed distant metastases 1 to 2 years later.
Conclusion: These findings show the potential of ctDNA for the early detection of LRR in EBC, and its promise as a tool for timely interventions and personalized surveillance strategies.
Introduction
Breast cancer accounts for nearly a quarter of all female malignancy and represents the leading cause of cancer-related mortality among women worldwide (1). Although substantial advances in early diagnosis and treatment have contributed to ameliorate survival, disease recurrence remains a major clinical challenge.
Loco-regional recurrence (LRR) occurs in approximately 5-15% of patients with early-stage disease and is associated with an increased risk of subsequent systemic spread (2–4). Since LRR is strongly associated with disease-specific mortality (5, 6), its early identification is crucial to improving patient outcomes. However, current surveillance protocols relying on physical examinations and imaging have limited sensitivity in detecting subclinical disease, highlighting the need for more dynamic and specific biomarkers.
Circulating tumor DNA (ctDNA), a component of cell-free DNA shed by tumor cells, has emerged as a promising and minimally invasive biomarker, providing a unique source to monitor disease in real time (7).
In advanced breast cancer, ctDNA is increasingly integrated into clinical decision-making to identify actionable mutations for targeted therapy (8). As an example, plasma PIK3CA mutations can guide the use of PI3K inhibitors in patients with hormone receptor-positive/HER2-negative disease; while ESR1 mutations inform resistance to aromatase inhibitors and the use of novel oral SERDs (9).
In the early-stage breast cancer (EBC), ctDNA is technically more challenging due to its lower levels. Nevertheless, ctDNA has shown clinical potential for prognosis, prediction of pathological complete response after neoadjuvant therapy, detection of residual disease after surgery, and early identification of relapse during follow-up (10).
Plasma serial monitoring for individual tumor mutations in TP53, PIK3CA, GATA3, ARID1A, AKT which are the most commonly found altered genes in breast cancer (11–13), has shown to detect minimal residual disease even months before clinical or radiologic evidence of overt metastases (14–16). These studies have reported the potential of ctDNA in predicting distant recurrence in operable breast cancer, with encouraging results in terms of sensitivity and specificity and supported prospective trials that aim to assess the utility of ctDNA for EBC (17–19). Nonetheless, most ctDNA research has focused on distant recurrence or treatment monitoring, whereas data on the use of ctDNA for detecting LRR, particularly in early-stage disease, remain limited.
In this study, we explored the role of ctDNA for the early identification of LRR in EBC patients. As a first step, we retrospectively analyzed ctDNA dynamics in an index case with a documented LRR and available tumor and plasma samples. Building on this observation, we report a post hoc analysis of a prospective phase III prevention trial enrolling patients with surgically treated for stage I-II breast cancer (20), for whom serial plasma samples were prospectively collected during follow-up. Our goal was to evaluate whether ctDNA monitoring could anticipate LRR, thus envisaging its integration into tailored post-treatment surveillance protocols.
Materials and methods
Study design and patient population
This study was based on a single breast cancer patient, referred to as the index case, and evaluable EBC patients from the prospective phase III fenretinide prevention trial (20).
For the index case, clinico-pathological data, sequencing of tumor tissue and profiling of plasma samples were evaluated at diagnosis, at the time of LRR, and during follow-up.
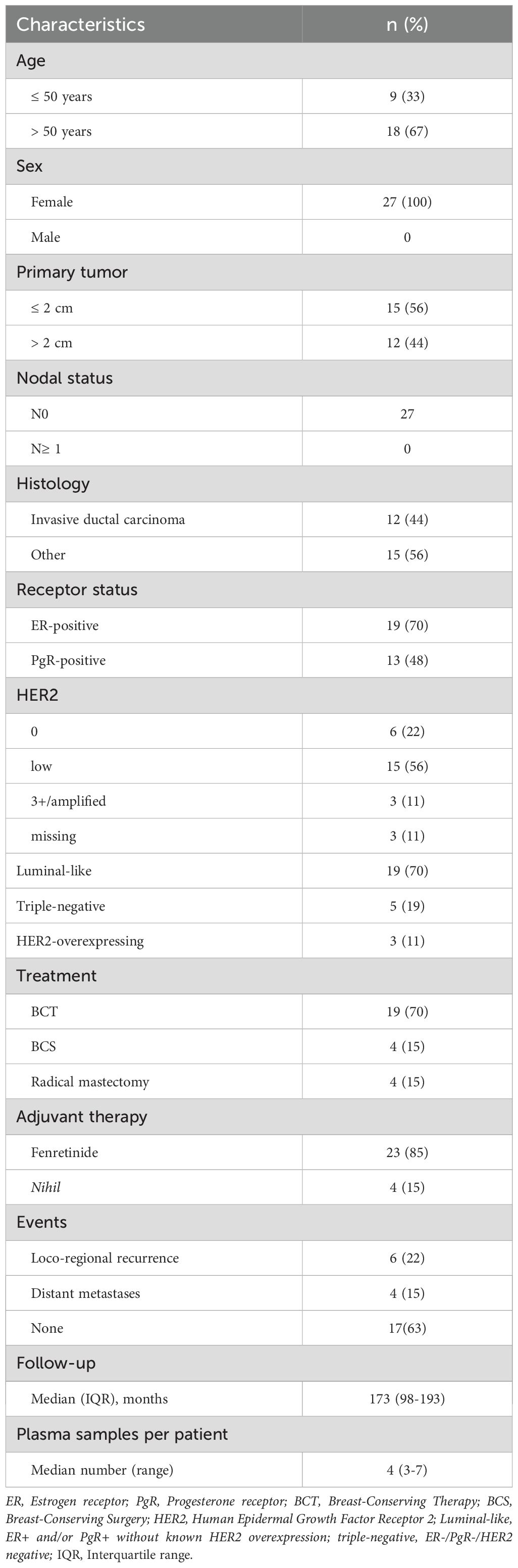
Patients from the prospective phase III fenretinide prevention trial were eligible if they fulfilled the following criteria: i) FFPE or frozen tumor tissue suitable for somatic single-nucleotide variants (SNV) identification by next generation sequencing (NGS), and ii) at least three plasma samples prospectively collected during follow-up. It is worth noting that the trial included women aged 30–70 years with T1-T2 N0 breast cancer who were treated with surgery ± radiotherapy, without adjuvant systemic chemo- or endocrine-therapy. Participants underwent semi-annual clinical evaluations, annual mammography and chest X-rays, and biennial bone scans. During trial follow-up serial plasma samples were collected every 6 months until relapse. These samples had been previously used for ancillary studies (reviewed in 21). Written informed consent and Institutional Review Board approval were obtained for the original and current analyses. As original assessment did not include HER2 status, HER2 was evaluated for the purpose of the current analysis by immunohistochemistry (IHC) and in situ hybridization as per standard practice (22).
Tumor tissue DNA extraction and sequencing
DNA was isolated from four sections of primary tumor FFPE tissues (10 μm thick slides with tumor cellularity ≥50%) using the GeneRead DNA FFPE Kit (Qiagen, Valencia, CA, USA) according to the manufacturer instructions. For DNA extraction from frozen samples the QIAamp DNA Mini Kit (Qiagen) was used following the manufacturer protocol. DNA quantity was assessed using Qubit dsDNA HS Assay Kit (Thermo Fisher Scientific, Waltham, MA, USA). Targeted NGS was performed using the Ion AmpliSeq™ Cancer Hotspot Panel v2 (Thermo Fisher Scientific), which includes 207 amplicons covering of 50 oncogenes and tumor suppressor genes. Cases with no detectable somatic variants using this panel and with available matched germline DNA from normal (non-tumoral) lymph nodes or breast tissue were subsequently analyzed with the Ion AmpliSeq™ Comprehensive Cancer Panel (Thermo Fisher Scientific), which targets all exons of 409 cancer-related genes. Detailed procedures are described in the Supplementary Materials.
Plasma collection and cell-free DNA extraction
For the index case, blood was collected in K2EDTA tubes preoperatively at both surgical time points and during follow-up. For the fenretinide cohort, blood was collected in heparin tubes at baseline, follow-up visits, and until relapse. Plasma was separated by centrifugation and stored at −80°C. cell-free DNA (cfDNA) was extracted using the QIAamp Circulating Nucleic Acid Kit (Qiagen), eluted in 35 µL of AVE buffer, and quantified using Qubit dsDNA HS Assay Kit (Thermo Fisher Scientific). For heparinized samples, eluates were treated with heparinase I (1u/µl) for 1 hour at room temperature (23). Spike-in experiments using a 125 bp Lambda DNA fragment confirmed successful dPCR performance after heparinase treatment (Supplementary Material, Supplementary Figure S1A).
digital polymerase chain reaction
dPCR assays (TaqMan SNP Genotyping, Thermo Fisher) were developed to validate somatic variants in tumor tissue and to track them in plasma. When dPCR assays wet-lab validated by the manufacturer were not available custom mutation-specific dPCR assays were designed using the Thermo Fisher Scientific custom SNP genotyping assay tool.
PCR reactions were run on the ProFlex™ 2x Flat PCR System thermal cycler (Thermo Fisher), incubating the chips at 96°C for 10 minutes, followed by 45 cycles of 56°C for 2 minutes, 98°C for 30 seconds and 60°C for 2 minutes. Chips were read on the QuantStudio® 3D Digital PCR Instrument (Thermo Fisher) and analyzed using QuantStudio® 3D AnalysisSuite™ Server (Thermo Fisher). Negative controls were performed with wild type (wt) genome (Promega Corporation, Madison, WI, USA) and no DNA template (NTC) were included in every run.
Plasma DNA pre-amplification
Plasma DNA was pre-enriched by amplification using TaqMan® PreAmp Master Mix Kit (Thermo Fisher Scientific), as previously described (24). Briefly, sample volume was reduced by Eppendorf Concentrator 5301 (Epperdorf Srl, Milano, Italy) to 14 µl. Pre-amplification reaction was performed in a volume of 10 µl containing 4 µl of DNA template, 5 µl of pre-amplification mastermix, and 1 µl of the same specific primers and probes designed for dPCR (at a final dilution of 0.05x). The amplification reaction was initiated by incubation of samples at 95°C for 10 minutes followed by 12 cycles of 95°C for 15 seconds, 60°C for 4 minutes. The pre-amplified PCR products were then diluted 1:100-1:500 and 7 µl of dilutions were used to perform dPCR.
As negative controls, wild-type genome and NTC, in place of DNA template, were included in each pre-amplification reaction, and assayed by dPCR. VAFs estimated by dPCR with or without pre-amplification showed a strong linear correlation, with an r² = 0.96 (Supplementary Figure S1B), indicating that pre-amplification does not impair the evaluation of VAFs.
Results
Presentation of the case
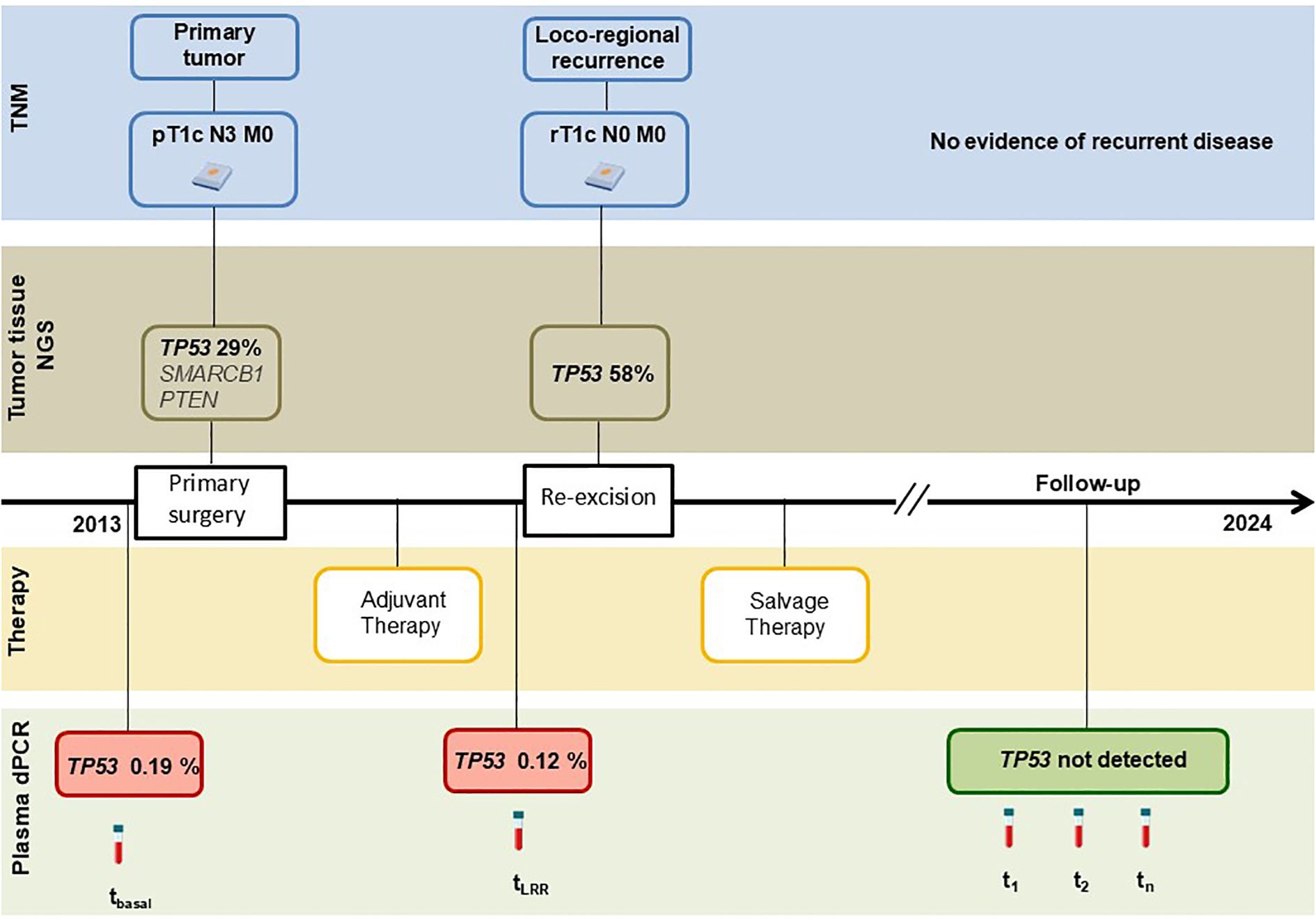
A 37-year-old premenopausal woman, with no family history of breast or ovarian cancer, and confirmed germline BRCA1 and BRCA2 wild-type, was referred to our institution following a left breast tumorectomy performed at another hospital.
Physical examination revealed a well-healed surgical scar on the left breast and a palpable lymph node approximately 1 cm in diameter in the left axilla. Mammography and ultrasound showed both hyperplastic and suspicious nodes in the left axilla. The patient underwent left upper-outer quadrantectomy and axillary lymph node dissection 5 weeks after the initial surgery.
Histological examination of the definitive surgical specimen showed an invasive breast carcinoma, grade III, measuring 18 mm in greatest dimension (pT1c), with metastatic involvement of 15 out of 30 axillary lymph nodes (pN3). Immunohistochemistry was negative for estrogen receptor (ER), progesterone receptor (PgR), and HER2, consistent with a triple-negative phenotype; Ki-67 proliferation index was 90%.
Adjuvant chemotherapy was initiated with four cycles of doxorubicin and paclitaxel (AT regimen) every 21 days, followed by four cycles of cyclophosphamide, methotrexate, and fluorouracil (CMF) administered on days 1 and 8 every 28 days. The patient completed the treatment without major complications.
Approximately one year after the initial surgery, the patient developed local recurrence at the site of the primary tumor. Imaging studies confirmed the absence of distant metastases. She underwent radical mastectomy. Histological evaluation confirmed a triple-negative LRR, spanning at least 1.7 cm in greatest dimension (rpT1c). Following surgery, the patient received six cycles of carboplatin and gemcitabine administered on days 1 and 8, every 21 days, followed by adjuvant radiotherapy to the chest wall and supraclavicular lymph nodes, for a total dose of 50.4 Gy delivered in 28 fractions of 1.8 Gy per day.
The patient has remained under routine surveillance. At her most recent follow-up in June 2024, clinical examination and imaging studies showed no evidence of local, regional, or distant disease recurrence (Figure 1).
Tumor targeted NGS identified multiple somatic variants, specifically TP53 (c.586C>T, p.R196*, VAF 29%), SMARCB1 (c.215C>A, p.T72K, VAF 38%), and PTEN (c.203A>G, p.Y68C, VAF 8%). Among these, only the TP53 p.R196* mutation was detected in both the primary tumor and the LRR, and was therefore selected for subsequent plasma analysis. SMARCB1 and PTEN mutations were not detected in the recurrent lesion and were not assessed in plasma samples. TP53 p.R196* mutation was confirmed by dPCR in both lesions, including the recurrent tumor, where it was present at a VAF of 58%. In addition, the same mutation was found in preoperative plasma samples of both primary and LRR surgeries with values of VAF of 0.19% and 0.12%, respectively. Notably, it was undetectable in all five samples prospectively collected during follow-up, when the patient remained disease-free.
ctDNA monitoring in the trial cohort
Based on these results, we report a post hoc analysis of patients enrolled in the prospective phase III fenretinide prevention trial. A total of 40 patients were considered eligible based on the prefixed criteria reported above (Figure 2).
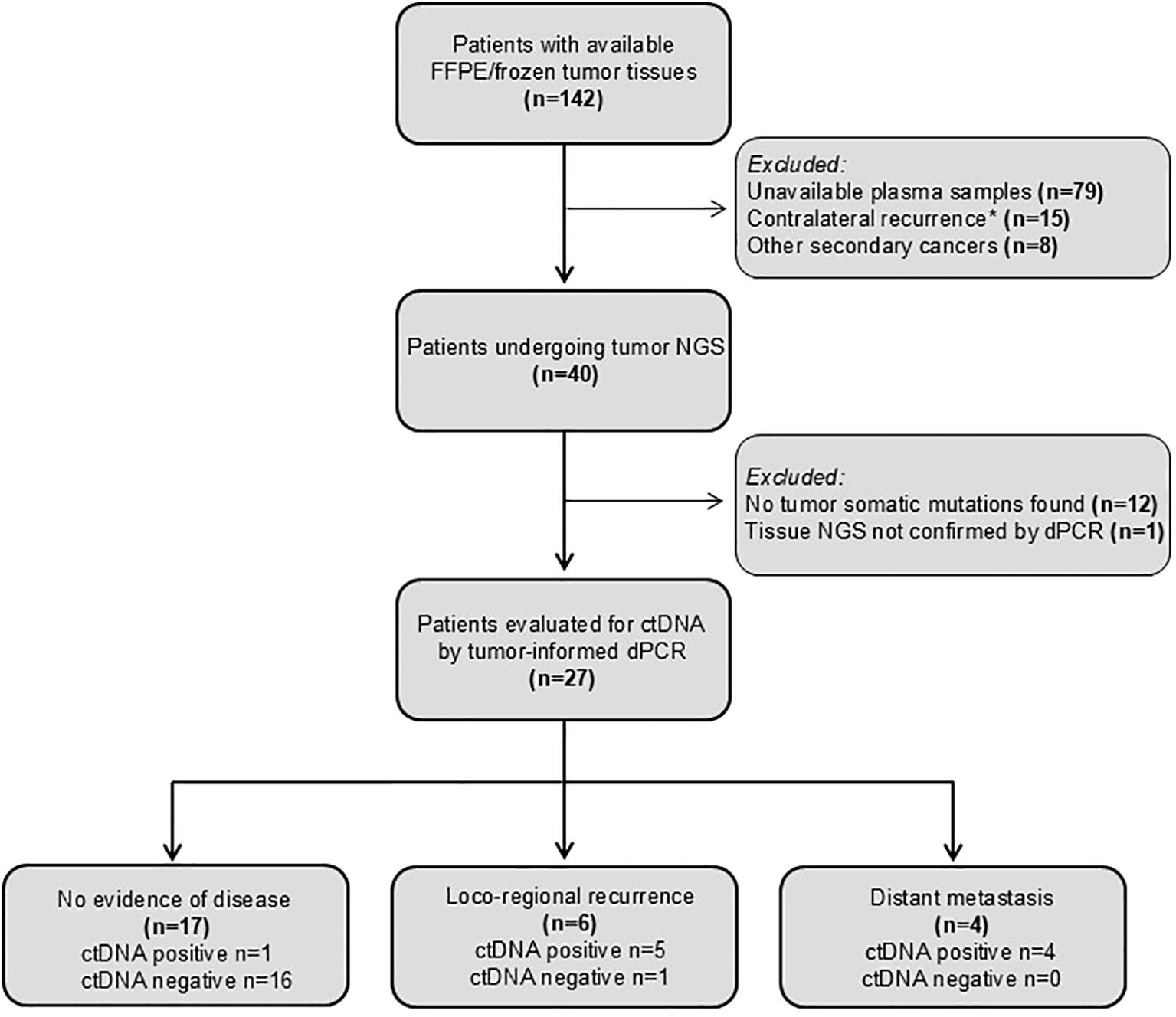
Figure 2. CONSORT diagram showing patients analyzed and reasons for their exclusion. Contralateral recurrences was excluded as it may represent a distinct primary tumor.
Even though DNA was extracted from specimens more than 20 years old, one or more somatic mutations suitable for ctDNA tracking were identified in tumor samples from 27 patients of the 40 analyzed (67.5%), enabling the development of tumor-informed dPCR assays for plasma analysis. Study patients, tumor and treatment characteristics are summarized in Table 1.
A total of 34 tumor mutations were found by NGS and validated by dPCR: 23 patients had 1 mutation (85%), 3 had 2 (11%), and 1 patient had 3 (4%). The most frequently mutated genes were PIK3CA (17/34, 50%), TP53 (8/34, 23.5%), and PTEN (2/34, 5.9%). VAFs estimated by NGS and dPCR showed a strong linear correlation, with an r² = 0.90 (Supplementary Figure S1C). Details on validated mutations are provided in Supplementary Table S1.
A total of 112 plasma samples were analyzed for the identified tumor mutations by dPCR. Among the recurrent patients, 6 with LRR and 4 with distant metastases, ctDNA was detectable at the time of clinical diagnosis in all the cases except one with VAF values ranging from 0.113 to 4.69 (Figures 3, 4, Supplementary Figure S2). Detection of ctDNA prior to clinical diagnosis was observed in both LRR and distant relapses with a median lead time of 24.5 months (IQR: 17.9 - 27.9) for LRR and 12.25 months (IQR: 7.4 - 22.8) for metastatic disease compared with clinical relapse. In patients #2, #7, and #8, LRR was eventually followed by metastasis (Supplementary Table S2). As the protocol stopped plasma sampling after the initial event, ctDNA monitoring until progression was precluded.

Figure 3. Swimmer plot representing longitudinal ctDNA tracking for study patients. For each patient, times of surgical resection and relapse are indicated by a green line and a blue asterisk, respectively.
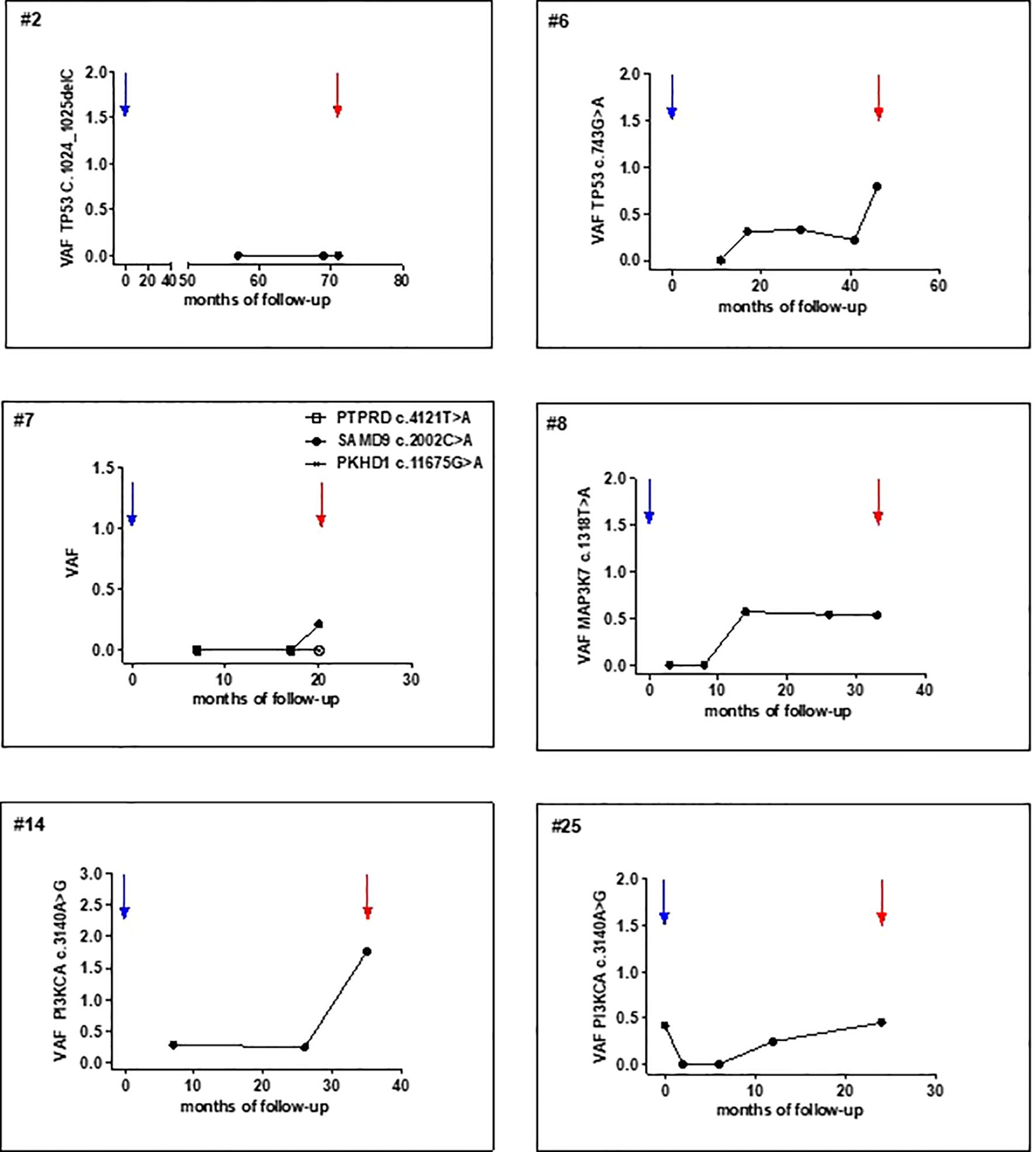
Figure 4. Post-operative tumor mutation tracking in plasma samples of patients surgically treated for primary T1-T2 N0. x-axis, time of follow-up (months from primary surgery); y-axis, mutation VAF (%). Blue and red arrows indicate primary tumor resection and clinical detection of LRR, respectively.
In 17 patients without evidence of clinical recurrence, ctDNA was undetectable in all 67 samples except in patient #19 that showed ctDNA in three consecutive samples which turned negative in the last sampling (Supplementary Figure S3). No documented clinical breast relapse was available. The patient died 52 days after last blood draw, at the age of 79 years.
Even considering the limited number of cases, ctDNA showed an overall accuracy of 92% (95% CI: 75-99), with a sensitivity of 90% (95% CI: 55-99), and specificity of 94% (95% CI: 71-99) (Supplementary Table S3).
Discussion
Tumor-specific mutations in post-operative plasma samples of patients with EBC may serve as a monitoring tool for detecting LRR. In the index case, a TP53 mutation shared by primary and LRR was detected in plasma at diagnosis and at the time of relapse but remained undetectable during follow-up consistent with the patient remaining disease-free. In a prevention trial cohort, ctDNA not only identified LRR, but also anticipated its clinical diagnosis.
These findings expand the evidence supporting the potential applications of ctDNA for breast cancer management, complementing its established role in predicting distant recurrence (14). While prior research focused on ctDNA for monitoring advanced disease or therapy response, recent advances suggest we should reconsider the full potential of ctDNA. Beyond simply detecting recurrence, ctDNA can assess surgical efficacy, identify residual disease, and monitor evolution during remission (25, 26), as a dynamic biomarker guiding personalized care across the treatment continuum. Our earlier study detecting primary tumor mutations in blood from patients with ductal carcinoma in situ (27) further reinforced the potential of ctDNA analysis across all breast cancer stages, from pre-invasive to recurrent disease.
Early diagnosis of LRR in breast cancer remains a cornerstone of post-treatment management, given its critical implications for prognosis and therapeutic strategy. LRR not only signals potential treatment failure but may also precede distant metastases, thereby influencing both disease-free and overall survival (4, 28). Traditional mammographic surveillance after breast-conserving therapy aims to detect ipsilateral recurrences and contralateral breast cancer, which occur with an annual risk of 0.2-2% and 0.4%, respectively (1).
While annual diagnostic mammography is commonly performed during the first three to five years to identify residual or recurrent disease and to establish a reliable post-treatment baseline (29), high-quality evidence regarding its optimal frequency, methodology, and survival benefit remains limited (30, 31). Nonetheless, retrospective studies suggest a survival advantage for mammographically detected recurrences (32), despite the lower sensitivity and specificity observed in women with a personal history of breast cancer (33).
Hence, ctDNA holds the potential to assist the diagnosis of LRR still at a potentially curable status by integrating both imaging and molecular tools to optimize early detection and management of LRR in breast cancer survivors.
In addition, our observation that ctDNA-negative patients remained recurrence-free supports the personalization of imaging assessment based on individual risk. We have to recognize that one patient (out of 17) had detectable ctDNA without developing overt recurrence during the follow-up period. Although patient #19 had a false positive result, given the absence of recurrence and subsequent ctDNA clearance, this was the only such case in the study population, with ctDNA demonstrating a high positive predictive value of 90% (95% CI: 57-98%) and a similarly high negative predictive value of 94% (95% CI: 73-99%). The patient died 52 days after the last blood draw. Unfortunately, attempts to gather further clinical information on the cause of death through possible means were unsuccessful. Therefore, we cannot exclude the possibilities of an undiagnosed malignancy, a subclinical disease, or other unrelated causes. These findings underscore the importance of maintaining high specificity in the development of new ctDNA assays, both to prevent unnecessary psychological distress due to false positive results and to address technical challenges such as background signals potentially related to ineffective erythropoiesis (34).
It is worth noting that, in current clinical practice, international guidelines recommend a rational use of NGS in advanced breast cancer, prioritizing targeted testing for known actionable mutations (e.g., PIK3CA, BRCA1/2, ESR1, etc.). Conversely, the assessment of minimal residual disease in the post-operative setting for localized disease is not yet standard practice and should be limited to patients enrolled in clinical research protocols.
Studies have shown that ctDNA detection after surgery can predict early relapse and a worse prognosis in breast cancer, with a median lead time of 7.9 to 18.9 months before clinical recurrence (reviewed in 35). Most of these studies assess ctDNA using a tumor-informed approach, often through digital PCR, as in our case, or with assays such as Signatera, which offer high sensitivity with a limit of detection 0.01%. More recently, novel methods for ctDNA analysis have emerged. Invitae PCM tracks 18–50 tumor-specific variants and detected ctDNA in 10 of 13 patients who experienced relapse, with a median lead time of 13.7 months and no false positives among patients who did not relapse. NeXT Personal combines whole-genome sequencing-based tumor-informed panels with a fixed panel of clinically relevant variants, allowing the tracking of up to 1,800 tumor-specific mutations with a level of detection as low as 1 part per million, and a reported lead time for relapse of 11.7 months (36).
Our study contributes to this expanding field by specifically focusing the potential of ctDNA to predict loco-regional recurrence, an aspect that has received limited attention to date. The strength of our work lies in the use of a well-defined patient population enrolled in a prospective clinical trial designed to evaluate loco-regional relapse, offering a robust framework for analyzing ctDNA dynamics in this setting. Moreover, our findings strengthen the case for using ctDNA to detect disease early and to initiate treatment sooner, particularly when the recurrence is still localized and therefore amenable to treatment with curative intent. While the findings of this study advance the understanding of the potential of ctDNA in LRR detection, several limitations should be acknowledged. The study sample size was relatively small, the use of historical tissue and plasma samples may affect the applicability of our results to a contemporary clinical setting, and the variable timing for post-surgical blood drawings could affect the evaluation of relapse/progression anticipation. In addition, the extraction of DNA from frozen recurrent tissue specimens in five cases represents a technical limitation. However, as direct plasma sequencing technologies continue to evolve and increase in level of detection, tumor-informed approaches may eventually be complemented, or even replaced, by direct, tumor agnostic ctDNA profiling, particularly in settings where tissue is unavailable or archival material is suboptimal. Future investigations should include prospective clinical trials to determine whether ctDNA-guided interventions improve patient management. Key questions remain about optimal sampling frequency and the clinical utility of quantitative ctDNA monitoring over time.
Conclusions
Our findings suggest that ctDNA represents a promising tool for the detection of LRR following curative treatment of EBC. The strengths of this study include the use of highly sensitive, tumor-informed digital PCR technology; the availability of samples from a cohort enrolled in a study specifically designed to monitor second primary breast cancers; and the presence of multiple prospectively collected longitudinal plasma samples. Furthermore, ctDNA analysis was conducted retrospectively in patients who were regularly monitored with breast imaging as part of the study protocol, thereby minimizing the confounding bias that has historically affected ctDNA studies in metastatic settings, where imaging was often irregular. Nonetheless, several limitations must be acknowledged, particularly the detection of VAFs frequently below 0.2%, highlighting the need to enhance the sensitivity and specificity of direct profiling assays to render ctDNA assessment sufficiently practical for wide routine clinical application. Further confirmation by independent studies will be essential to corroborate these findings and support the integration of ctDNA analysis into the multidisciplinary management of patients treated for early-stage breast cancer.
Data availability statement
All data supporting the findings of this study, including somatic variants, are reported in the manuscript and its Supplementary Material. Further inquiries and additional details may be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Comitato Etico dell’Istituto Nazionale dei Tumori di Milano, Fondazione IRCCS Istituto Nazionale dei Tumori, Milano, Italy. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
VA: Conceptualization, Data curation, Funding acquisition, Investigation, Writing – original draft. ET: Investigation, Writing – review & editing. PT: Investigation, Writing – review & editing. AB: Investigation, Writing – review & editing. LD: Investigation, Writing – review & editing. MS: Data curation, Writing – review & editing. CD: Investigation, Writing – review & editing. EC: Investigation, Writing – review & editing. MD: Investigation, Writing – review & editing. SF: Investigation, Writing – review & editing. GS: Investigation, Writing – review & editing. RM: Formal analysis, Writing – review & editing. AV: Investigation, Writing – review & editing. GP: Investigation, Writing – review & editing. SD: Conceptualization, Data curation, Investigation, Supervision, Writing – original draft. RM:.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was supported by the Scientific Directorate of the IRCCS Istituto Nazionale dei Tumori di Milano, through funds from the Italian Ministry of Education, University and Research (MIUR, 5×1000), allocated to the Project INT D302456 “Identificazione e monitoraggio delle recidive da carcinoma mammario, mediante sequenziamento del DNA plasmatico”), granted to Valentina Appierto, as Principal Investigator. This work was also supported by Ministry of Health, Ricerca Corrente funds.
Acknowledgments
We acknowledge Dr. Maria Grazia Daidone for initiating liquid biopsy research at our Institute and for the impetus and vision she provided, inspiring this study and the development of the field until her retirement as Head of the Department of Applied Research and Technological Development in 2022. We thank Dr. Matteo Dugo for his expertise in bioinformatics for NGS data, Filippo Cascone for his dedicated work as clinical research nurse, and Dr. Silvia Veneroni for her commitment to plasma repository linked to breast cancer registry until her recent retirement.
Conflict of interest
Author MS was employed by the company Isinnova s.r.l.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2025.1621322/full#supplementary-material
References
1. Arnold M, Morgan E, Rumgay H, Jemal A, Bray F, Ferlay J, et al. Current and future burden of breast cancer: global statistics for 2020 and 2040. Breast. (2022) 66:15–23. doi: 10.1016/j.breast.2022.08.010
2. Habbous S, Barisic A, Homenauth E, Hall S, Hofer S, Ireland C, et al. Estimating the incidence of breast cancer recurrence using administrative data. Breast Cancer Res Treat. (2023) 198:509–22. doi: 10.1007/s10549-023-06984-9
3. Kaufmann M, Morrow M, von Minckwitz G, Harris JR, Hellman S, Mamounas EP, et al. Locoregional treatment of primary breast cancer: consensus recommendations from an international expert panel. Cancer. (2010) 116:1184–91. doi: 10.1002/cncr.24874
4. Wapnir IL, Anderson SJ, Mamounas EP, Geyer CE Jr, Jeong JH, Tan-Chiu E, et al. Prognosis after ipsilateral breast tumor recurrence and locoregional recurrences in five National Surgical Adjuvant Breast and Bowel Project node-positive adjuvant breast cancer trials. J Clin Oncol. (2006) 24:2028–37. doi: 10.1200/JCO.2005.04.3273
5. Reddy JP, Hwang ES, Euhus DM, and Mittendorf EA. The role of salvage surgery in local-regional recurrent breast cancer: a review. Int J Surg. (2015) 21:1–5. doi: 10.1016/j.ijsu.2015.06.063
6. Punglia RS, Morrow M, Winer EP, and Harris JR. Local therapy and survival in breast cancer. N Engl J Med. (2007) 356:2399–405. doi: 10.1056/NEJMoa066541
7. Wan JCM, Massie C, Garcia-Corbacho J, Mouliere F, Brenton JD, Caldas C, et al. Liquid biopsies come of age: towards implementation of circulating tumour DNA. Nat Rev Cancer. (2017) 17:223–38. doi: 10.1038/nrc.2017
8. Mosele MF, Westphalen CB, Stenzinger A, Barlesi F, Bayle A, Bièche I, et al. Recommendations for the use of next-generation sequencing (NGS) for patients with advanced cancer in 2024: a report from the ESMO Precision Medicine Working Group. Ann Oncol. (2024) 35:588–606. doi: 10.1016/j.annonc.2024.04.005
9. Carnevale MG, Colciago RR, De Santis MC, Cortesi L, De Marco C, Marra A, et al. Advancing breast cancer therapy in the era of molecular diagnostics. Breast. (2025) 82:104488. doi: 10.1016/j.breast.2025.104488
10. Panet F, Papakonstantinou A, Borrell M, Vivancos J, Vivancos A, Oliveira M, et al. Use of ctDNA in early breast cancer: analytical validity and clinical potential. NPJ Breast Cancer. (2024) 10:50. doi: 10.1038/s41523-024-00653-3
11. Bharde A, Nadagouda S, Dongare M, Hariramani K, Basavalingegowda M, Haldar S, et al. ctDNA-based liquid biopsy reveals wider mutational profile with therapy resistance and metastasis susceptibility signatures in early-stage breast cancer patients. J Liq Biopsy. (2024) 7:100284. doi: 10.1016/j.jlb.2024.100284
12. Curtis C, Shah SP, Chin SF, Turashvili G, Rueda OM, Dunning MJ, et al. The genomic and transcriptomic architecture of 2,000 breast tumours reveals novel subgroups. Nature. (2012) 486:346–52. doi: 10.1038/nature10983
13. Kingston B, Cutts RJ, Bye H, Beaney M, Walsh-Crestani G, Hrebien S, et al. Genomic profile of advanced breast cancer in circulating tumour DNA. Nat Commun. (2021) 12:2423. doi: 10.1038/s41467-021-22605-2
14. Nader-Marta G, Monteforte M, Agostinetto E, Piccart-Gebhart M, Sotiriou C, Ignatiadis M, et al. Circulating tumor DNA for predicting recurrence in patients with operable breast cancer: a systematic review and meta-analysis. ESMO Open. (2024) 9:102390. doi: 10.1016/j.esmoop.2024.102390
15. Coombes RC, Page K, Salari R, Hastings RK, Armstrong A, Ahmed S, et al. Personalized detection of circulating tumor DNA antedates breast cancer metastatic recurrence. Clin Cancer Res. (2019) 25:4255–63. doi: 10.1158/1078-0432.CCR-18-3663
16. Garcia-Murillas I, Chopra N, Comino-Mendez I, Beaney M, Tovey H, Cutts RJ, et al. ctDNA analysis for detection of minimal residual disease in breast cancer patients. Ann Oncol. (2019) 30:1804–10. doi: 10.1093/annonc/mdz410
17. Turner NC, Swift C, Jenkins B, Pearce S, Alfaro J, Symeonides S, et al. Results of the c-TRAK TN trial: a clinical trial utilising ctDNA mutation tracking to detect molecular residual disease and trigger intervention in patients with moderate- and high-risk early-stage triple-negative breast cancer. Ann Oncol. (2023) 34:200–11. doi: 10.1016/j.annonc.2022.11.005
18. RADAR Trial Investigators. Circulating tumour DNA analysis in early breast cancer: results from the RADAR trial. Lancet Oncol. (2023) 24:293–304. doi: 10.1016/S1470-2045(22)00772-7
19. Duffy MJ, Elez E, Azam F, Bergh J, Clarke RB, Cristofanilli M, et al. Clinical utility of circulating tumor DNA in breast cancer: update of recommendations from an ESMO expert panel. Ann Oncol. (2022) 33:999–1013. doi: 10.1016/j.annonc.2022.07.003
20. Veronesi U, De Palo G, Marubini E, Formelli F, Camerini T, Del Vecchio M, et al. Randomized trial of fenretinide to prevent second breast Malignancy in women with early breast cancer. J Natl Cancer Inst. (1999) 91:1847–56. doi: 10.1093/jnci/91.21.1847
21. Albini A, Noonan DM, Corradino P, Cappelletti M, Giovannoni R, Anselmi L, et al. The past and future of angiogenesis as a target for cancer therapy and prevention. Cancer Prev Res (Phila). (2024) 17:289–303. doi: 10.1158/1940-6207.CAPR-24-0085
22. Wolff AC, Hammond MEH, Hicks DG, Dowsett M, McShane LM, Allison KH, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol. (2013) 31:3997–4013. doi: 10.1200/JCO.2013.50.9984
23. Tiberio P, De Cecco L, Callari M, Cavadini E, and Daidone MG. MicroRNA detection in plasma samples: how to treat heparinized plasma. J Mol Diagn. (2013) 15:138–9. doi: 10.1016/j.jmoldx.2012.09.003
24. Ortolan E, Appierto V, Silvestri M, Daidone MG, and Cappelletti V. Blood-based genomics of triple-negative breast cancer progression in patients treated with neoadjuvant chemotherapy. ESMO Open. (2021) 6:100086. doi: 10.1016/j.esmoop.2021.100086
25. Pascual J, Attard G, Bidard FC, Curigliano G, De Mattos-Arruda L, Diehn M, et al. ESMO recommendations on the use of circulating tumour DNA assays for patients with cancer: a report from the ESMO Precision Medicine Working Group. Ann Oncol. (2022) 33:750–68. doi: 10.1016/j.annonc.2022.05.520
26. Febbo PG, Allo M, Alme EB, Cuyun Carter G, Dumanois R, Essig A, et al. Recommendations for the equitable and widespread implementation of liquid biopsy for cancer care. JCO Precis Oncol. (2024) 8:e2300382. doi: 10.1200/PO.23.00382
27. Di Cosimo S, Appierto V, Silvestri M, Ortolan E, De Cecco L, Veneroni S, et al. Primary tumor somatic mutations in the blood of women with ductal carcinoma in situ of the breast. Ann Oncol. (2020) 31:435–7. doi: 10.1016/j.annonc.2019.11.022
28. Van Laar C, van der Sangen MJ, Poortmans PM, Nieuwenhuijzen GA, Roukema JA, Roumen RM, et al. Local recurrence following breast-conserving treatment in women aged 40 years or younger: trends in risk and the impact on prognosis in a population-based cohort of 1143 patients. Eur J Cancer. (2013) 49:3093–101. doi: 10.1016/j.ejca.2013.05.030
29. Expert Panel on Breast Imaging, Lewin AA, Moy L, Baron P, Didwania AD, diFlorio-Alexander RM, et al. ACR Appropriateness Criteria® Stage I breast cancer: initial workup and surveillance for local recurrence and distant metastases in asymptomatic women. J Am Coll Radiol. (2019) 16(11S):S428–35. doi: 10.1016/j.jacr.2019.05.024
30. Lash TL, Fox MP, and Silliman RA. Reduced mortality rate associated with annual mammograms after breast cancer therapy. Breast J. (2006) 12:2–10. doi: 10.1111/j.1075-122X.2006.00177.x
31. Dunn JA, Donnelly P, Elbeltagi N, Marshall A, Hopkins A, Thompson AM, et al. Annual versus less frequent mammographic surveillance in people with breast cancer aged 50 years and older in the UK (Mammo-50): a multicentre, randomised, phase 3, non-inferiority trial. Lancet. (2025) 405:396–407. doi: 10.1016/S0140-6736(24)02715-6
32. Schootman M, Jeffe DB, Lian M, Aft R, and Gillanders WE. Surveillance mammography and the risk of death among elderly breast cancer patients. Breast Cancer Res Treat. (2008) 111:489–96. doi: 10.1007/s10549-007-9811-5
33. Houssami N, Abraham LA, Miglioretti DL, Sickles EA, Kerlikowske K, Buist DS, et al. Accuracy and outcomes of screening mammography in women with a personal history of early-stage breast cancer. JAMA. (2011) 305:790–9. doi: 10.1001/jama.2011.164
34. Razavi P, Li BT, Brown DN, Jung B, Hubbell E, Shen R, et al. High-intensity sequencing reveals the sources of plasma circulating cell-free DNA variants. Nat Med. (2019) 25:1928–37. doi: 10.1038/s41591-019-0652-7
35. Medford AJ, Carmeli AB, Ritchie A, Wagle N, Garraway L, Lander ES, et al. A standing platform for cancer drug development using ctDNA-based evidence of recurrence. Nat Rev Cancer. (2024) 24:810–21. doi: 10.1038/s41568-024-00742-2
Keywords: circulating tumor DNA, loco-regional recurrence (LRR), early breast cancer (EBC), follow-up, next generation sequencing (NGS), digital PCR (dPCR), somatic mutation
Citation: Appierto V, Tamborini E, Tiberio P, Busico A, De Cecco L, Silvestri M, De Marco C, Cavadini E, De Santis MC, Folli S, Scaperrotta G, Manitto R, Vingiani A, Pruneri G and Di Cosimo S (2025) Circulating tumor DNA to anticipate loco-regional recurrence in early-stage breast cancer: a proof-of-concept study. Front. Oncol. 15:1621322. doi: 10.3389/fonc.2025.1621322
Received: 30 April 2025; Accepted: 28 August 2025;
Published: 11 September 2025.
Edited by:
Lorenzo Gerratana, University of Udine, ItalyReviewed by:
Emer Bourke, University of Galway, IrelandCarlo Bosi, Cornell University, United States
Copyright © 2025 Appierto, Tamborini, Tiberio, Busico, De Cecco, Silvestri, De Marco, Cavadini, De Santis, Folli, Scaperrotta, Manitto, Vingiani, Pruneri and Di Cosimo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Serena Di Cosimo, c2VyZW5hLmRpY29zaW1vQGlzdGl0dXRvdHVtb3JpLm1pLml0
 Valentina Appierto
Valentina Appierto Elena Tamborini
Elena Tamborini Paola Tiberio2
Paola Tiberio2 Adele Busico
Adele Busico Loris De Cecco
Loris De Cecco Marco Silvestri
Marco Silvestri Elena Cavadini
Elena Cavadini Maria Carmen De Santis
Maria Carmen De Santis Gianfranco Scaperrotta
Gianfranco Scaperrotta Giancarlo Pruneri
Giancarlo Pruneri Serena Di Cosimo
Serena Di Cosimo