- 1West China School of Medicine, West China Hospital, Sichuan University, Chengdu, China
- 2Department of Neurosurgery and Neurosurgery Research Laboratory, West China Hospital, Sichuan University, Chengdu, China
- 3Department of Radiation Oncology, Shandong Cancer Hospital and Institute, Shandong First Medical University and Shandong Academy of Medical Sciences, Jinan, Shandong, China
- 4Department of Radiation Oncology, The Affiliated Hospital of Qingdao University, Qingdao, Shandong, China
- 5Key Laboratory of Organ Regeneration and Transplantation of Ministry of Education, Institute of Immunology, The First Hospital, Jilin University, Changchun, China
- 6State Key Laboratory of Oncology in South People's Republic of China, Collaborative Innovation Center for Cancer Medicine, Sun Yat-sen University Cancer Center, Guangzhou, China
- 7Department of Respiratory and Critical Care Medicine, West China Hospital, Sichuan University, Chengdu, China
- 8Precision Medicine Research Center, Precision Medicine Key Laboratory of Sichuan Province, State Key Laboratory of Respiratory Health and Multimorbidity, West China Hospital, Sichuan University, Chengdu, Sichuan, China
Importance: Immune checkpoint inhibitors (ICIs) have become the standard treatment for advanced non-small cell lung cancer (NSCLC). However, their prognostic role in NSCLC patients remains controversial. Hypertension (HTN) is an important risk factor for many cancers, but the pathogenesis underlying HTN in relation to cancer prognosis remains unclear.
Objective: We aimed to investigate the possible association between HTN and prognosis in advanced NSCLC patients.
Data sources: Data on advanced NSCLC patients receiving immunotherapy at stages IIIb, IIIc or IV were included.
Study selection: Multicenter retrospective studies and trials reporting the use of immunotherapy were included. Main outcomes and measures: Progression-free survival (PFS) and overall survival (OS) were analyzed using Cox proportional hazards models and were estimated by the Kaplan-Meier method. Subgroup analysis on NSCLC hypertensive patients was pre-planned and was presented in the form of Forest Plot. Statistical software utilized for all analyses included statistical analysis system (SAS) V.9.4 and R version 4.2.2 (R Foundation for Statistical Computing).
Results: Between January 2016 and June 2024, 1175 NSCLC patients receiving immunotherapy were enrolled, with 219 (18.6%) classified as hypertensive group and 956 (81.4%) classified as non-hypertensive group. Neutrophil count and ECOG = 2 showed a significant association with OS in univariate analysis (HR = 0.69, 95%Cl: 0.51 - 0.92, P = 0.012, and HR = 1.02, 95%Cl: 1.00 to 1.03, P = 0.008 respectively). In multivariate analysis, ECOG = 2 was significantly correlated with OS (HR = 0.73, 95%Cl: 0.54 to 0.98, P = 0.037) and PD - 1/PD-L1 had significant association with PFS (HR = 1.27, 95%Cl: 1.00 to 1.61, P = 0.050). OS was found significantly longer in non-hypertensive group than in hypertensive group (P = 0.049). No baseline indicator was found significant correlated with the survival prognosis of patients receiving immunotherapy in subgroup analysis.
Conclusion and relevance: The non-hypertensive group was associated with a lower risk of mortality than hypertensive group. In subgroup analysis, no baseline indicator was observed a significant correlation with survival prognosis on OS and PFS in hypertensive patients. Our findings provided an important prognostic factor to improve the prognosis of advanced NSCLC patients receiving immunotherapy. Prospective randomized trials are needed to further validate these findings.
1 Background
Lung cancer is the malignant tumor with the highest morbidity and mortality in China, and indeed worldwide (1). Non-Small Cell Lung Cancer (NSCLC) is the most common pathological subtype, accounting for 85% of all lung cancers (2). However, 30%-40% of NSCLC patients are diagnosed at an advanced stage, having a poor prognosis (3). Immunotherapy plays an important role in the treatment of NSCLC patients. Recently, immune checkpoint inhibitors (ICIs) have brought a major breakthrough, especially those targeting the programmed death receptor (PD - 1) and programmed death ligand 1 (PD-L1), which have become the standard treatment for driver-negative advanced NSCLC. According to the American Society of Clinical Oncology (ASCO), continuing the exploration of immune strategies, optimization of existing immunotherapy protocols, and reduction of adverse outcomes of cancer treatment would be the focus of future research (4, 5).
Although immunotherapy has made a tremendous impact on patient survival in many high-incidence cancer indications (6), the response of patients to ICIs varies considerably. Patient-intrinsic factors (such as age, sex, body mass index (BMI) and human leukocyte antigen (HLA) genotype), tumor-intrinsic factors (such as the tumor-associated stroma) and environmental factors (such as the gut microbiota) may lead to the success or failure of the blockade of ICIs (7–10). In addition, controlling the gut microbiome may modulate the effectiveness of tumor immunotherapy (11). The key drivers of ICIs efficacy and patient survival have been summarized into five separate underlying factors: tumor mutation burden (TMB), T cell effective infiltration, transforming growth factor-beta (TGF-β) activity, prior treatment and proliferative potential (12). Therefore, different types of tumors can be systematically classified to better predict how patients would respond to ICIs.
The development of PD - 1 and PD-L1 has significantly extended the survival time of NSCLC patients, with higher response rates and lower incidence of side effects than anti-cytotoxic T lymphocyte-associated antigen-4 (CTLA - 4) (13, 14). Recent studies have shown that a variety of factors could affect the immunotherapy prognosis of advanced NSCLC patients. High BMI appeared to be associated with improved survival (15). CD161+CD127+CD8+T cells may be a key indicator of the poor prognosis in NSCLC patients with diabetes (16). ICIs, especially nivolumab combined with ipilimumab, might become one of the options for NSCLC patients with chronic kidney disease (CKD) (17). Moreover, the two most common adverse events of immunotherapy-related cardiotoxicity were myocarditis and pericarditis, which can lead to a high mortality rate (18). Beyond comorbidities, baseline inflammatory markers may critically guide ICI decisions. CRP level and neutrophil to lymphocyte ratio (NLR) may serve as a marker for the prognosis of advanced NSCLC patients receiving immunotherapy (19–21). Paradoxically, leukopenia (WBC<4×109/L), which may have an association with malignancies, usually disqualified patients from clinical trials (22), though real-world data suggest comparable benefit (23–25).
Hypertension (HTN) remains a major chronic disease morbidity across the world. The prevalence of HTN continues to increase worldwide. The incidence of HTN in people aged 30–79 years doubled from 1990 to 2019, with 59% of women and 49% of men globally having previously diagnosed HTN in 2019 (26). Due to the common risk factors HTN and cancer shared, it might mask a causal relationship between high blood pressure (BP) and cancer (27). Some of the risk factors associated with HTN, such as obesity, type II diabetes mellitus, smoking, and a sedentary life-style, also increased the risk of cancer (28). Many studies have indicated that HTN was an important risk factor for certain cancers (29), such as breast cancer (30), lung cancer (31) and kidney cancer (32), which contributed to a larger mortality burden (33). The pathogenesis underlying HTN in relation to cancer prognosis remains unclear. Preclinical evidence suggested HTN may impair anti-PD-1 response through angiotensin II-mediated CD8+T cell suppression and vascular endothelial growth factor-driven myeloid-derived suppressor cell accumulation (34, 35). Moreover, previous murine models demonstrated calcium channel blockers (e.g., amlodipine) reverse tumor vasculature abnormalities and improve PD - 1 inhibitor efficacy (36). Yet human translational data remain conflicting: while renin-angiotensin-aldosterone system inhibitors correlate with prolonged OS in retrospective NSCLC cohorts (37), randomized trials show no benefit (38). This mechanistic uncertainty underscored the clinical importance to clarify HTN’s real-world impact in ICI-treated patients.
Nowadays, some studies have confirmed that HTN can affect the prognosis of patients receiving targeted therapy while few studies have focused on the correlation between HTN and immunotherapy of lung cancer. Therefore, in this study, we conducted a comparative analysis of 1175 patients diagnosed with advanced NSCLC and received immunotherapy at West China Hospital, West China Lung Cancer Center, Sichuan Cancer Hospital, Sichuan People’s Hospital, and Shandong Cancer Hospital from January 2016 to June 2024 to investigate the possible association between HTN and prognosis in advanced NSCLC patients.
2 Materials and methods
2.1 Patient materials
This multicenter prospective, observational study included all patients with pathologically confirmed advanced-stage NSCLC who were treated immunotherapy at West China Hospital of Sichuan University, West China Lung Cancer Center, Sichuan Cancer Hospital, Sichuan People’s Hospital, and Shandong Cancer Hospital from January 2016 to June 2024. Advanced-stage disease was defined as stage IIIb (N3M0), IIIc (N3M0) or IV (NanyM1) according to the AJCC Version 8 staging system. Pathologic confirmation was required, and the eligible histologies were as follows: squamous cell carcinoma, adenocarcinoma, large cell carcinoma, neuroendocrine carcinoma (NSCLC with neuroendocrine features or atypical carcinoids, but not small cell carcinoma) or NSCLC not otherwise specified. Data were collected into specific databases, with information on pathological type and features, previous medical and surgical history, diagnosis, metastasis and follow-up. The exclusion criteria included (1): incomplete treatment cycle (2); lack of available data on HTN (3); immunotherapy received after third-line treatment (4); no follow-up data (5); receipt of ICIs other than PD - 1/PD-L1 and (6) receipt of only neoadjuvant immunotherapy.
This study was approved by the Institutional Review Board of West China Hospital of Sichuan University (approval number 20241410), and all patients provided written informed consent.
2.2 Statistical methods
The main objectives were to assess the association between HTN and the prognosis of immunotherapy in advanced NSCLC patients with progression-free survival (PFS) and overall survival (OS) as the primary endpoints. Additionally, the direct efficacy of immunotherapy was evaluated, using complete response (CR), partial response (PR), stable disease (SD), progressive disease (PD), objective response rate (ORR) and disease control rate (DCR). PFS was defined as the time from the date of randomized treatment to the date of progression or death, whichever came first, while OS was defined as the time from the date of randomization to the date of death from any cause.
Baseline variables such as age, BMI, sex, underlying conditions, histology, smoking history, tumor stages, metastasis, medication, blood routine and PD-L1 TPS were included in patient characteristics. The chi-squared test (for categorical variables) or Wilcoxon rank sum test (for continuous variables) was used to observe the differences in baseline indicators between groups.
Patients with at least one evaluable endpoint (i.e. PFS or OS) were included in the present analysis. A possible different impact of HTN on prognosis was evaluated by means of survival curves. The Kaplan-Meier method was used to estimate OS and PFS, which was compared with log-rank tests.
Univariate and multivariate analyses cox proportional hazards models were used to determine the baseline indicators on OS and PFS without or with adjustment for other prognostic factors. All multivariate models included as covariates age, BMI, diabetes, coronary heart disease (CHD), lung metastasis, neutrophil count and medication. All the fitted models were stratified according to the enrolling center and results were presented as HR with their 95% Cl.
A further subgroup analysis was performed on NSCLC patients with HTN, including indicators such as age, BMI, diabetes, CHD, metastasis and type of immunotherapy drugs. The results of subgroup analysis were presented in the form of Forest Plot. Statistical software utilized for all analyses were statistical analysis system (SAS) V.9.4 and R version 4.2.2 (R Foundation for Statistical Computing). Statistical significance was defined as two-sided P <0.05. Our study fully aligned with methodological consensus (NCCN Biostatistics Guidelines 2023, https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1450).
3 Result
3.1 Patients characteristics
A total of 4896 patients were enrolled in the present study. 3811 patients (77.8%) were excluded based on the exclusion criteria (Figure 1). The remaining 1175 patients were included in the final analyses, classified into two groups: hypertensive group (n=219) and non-hypertensive group (n=956).
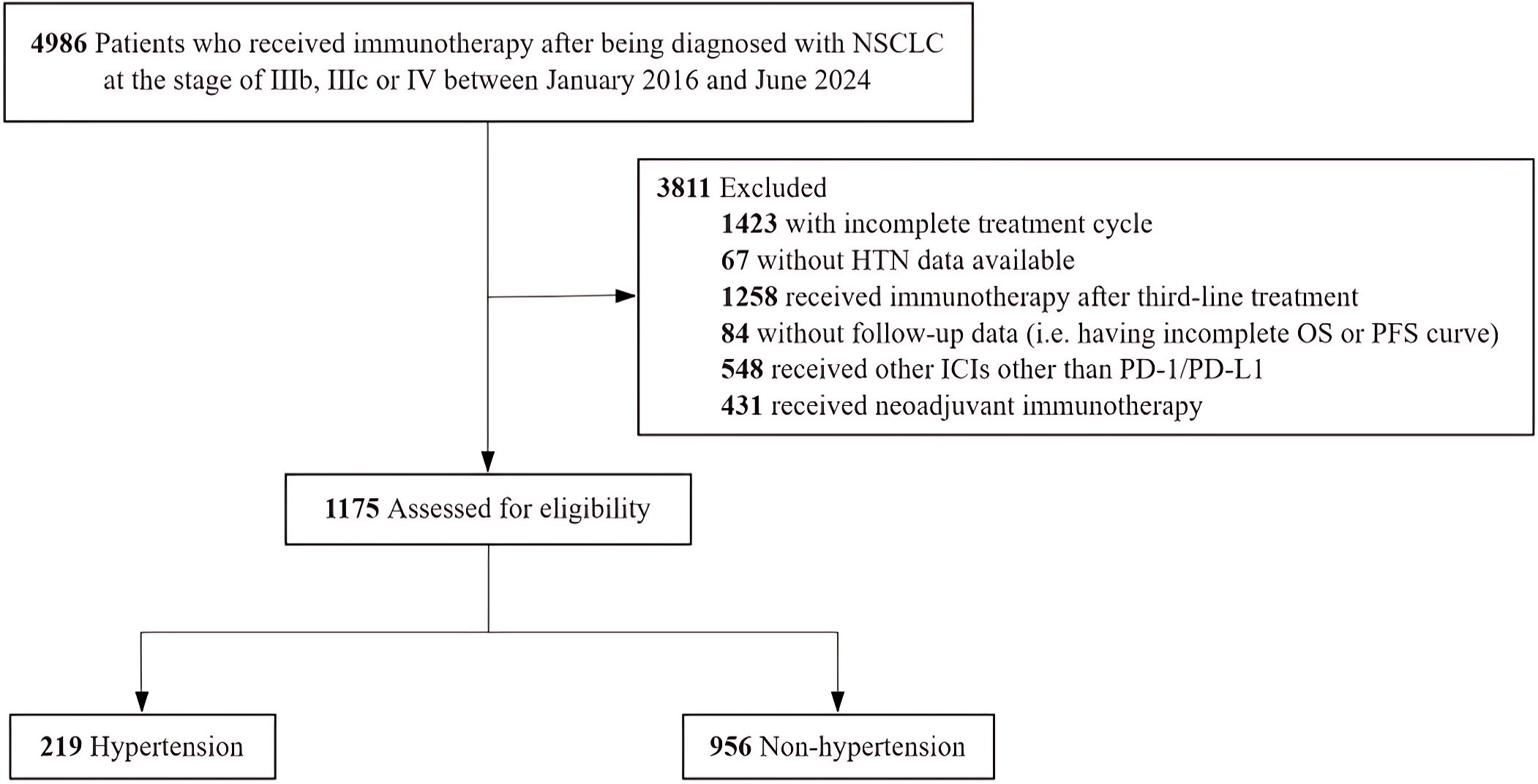
Figure 1. Flowchart of the Study Population. NSCLC indicates non-small cell lung cancer; HTN, hypertension; OS, overall survival; PFS, progression-free survival; ICIs, immune checkpoint inhibitors; PD-1, programmed death receptor and PD-L1, programmed death ligand1.
The demographic characteristics of the two groups were presented in Table 1. The hypertensive group had higher age and BMI than the non-hypertensive group (P<0.001). Among underlying conditions, the rates of diabetes and CHD in hypertensive group were higher than non-hypertensive groups (21.5% vs. 8.1%, P<0.001; 13.7% vs. 5.3%, P<0.001, respectively). Non-hypertensive group had a lower rate of metastasis than hypertensive group (14.4% vs. 6.8%, P = 0.011). Furthermore, a large proportion of the patients were male (78.7%), with the majority exhibiting ECOG scores of 1 - 2, and 645 patients (54.9%) were current or former smokers.
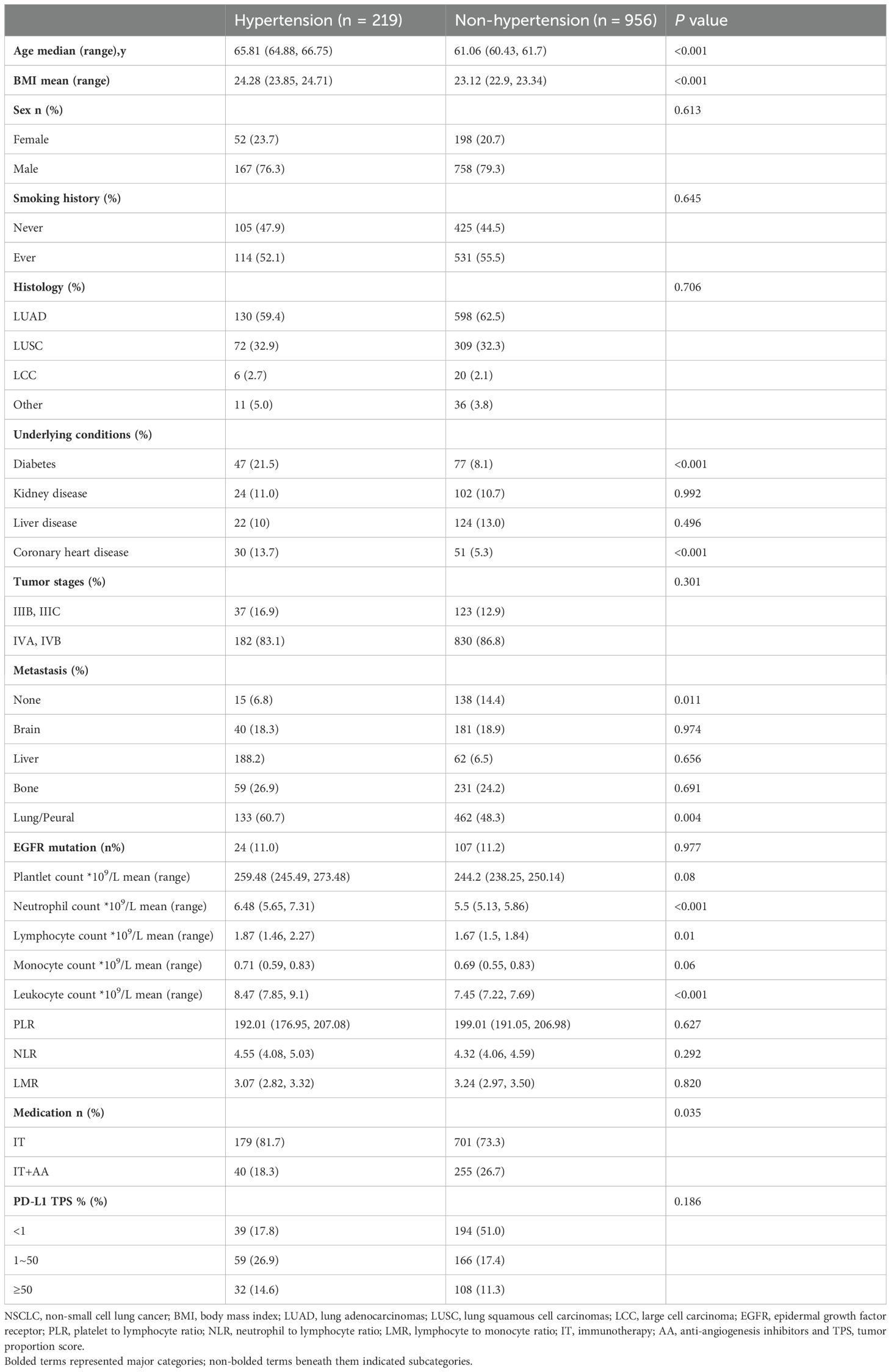
Table 1. Baseline characteristics of NSCLC patients categorized by hypertension and non-hypertension.
3.2 Impact of univariate on overall survival and progression-free survival
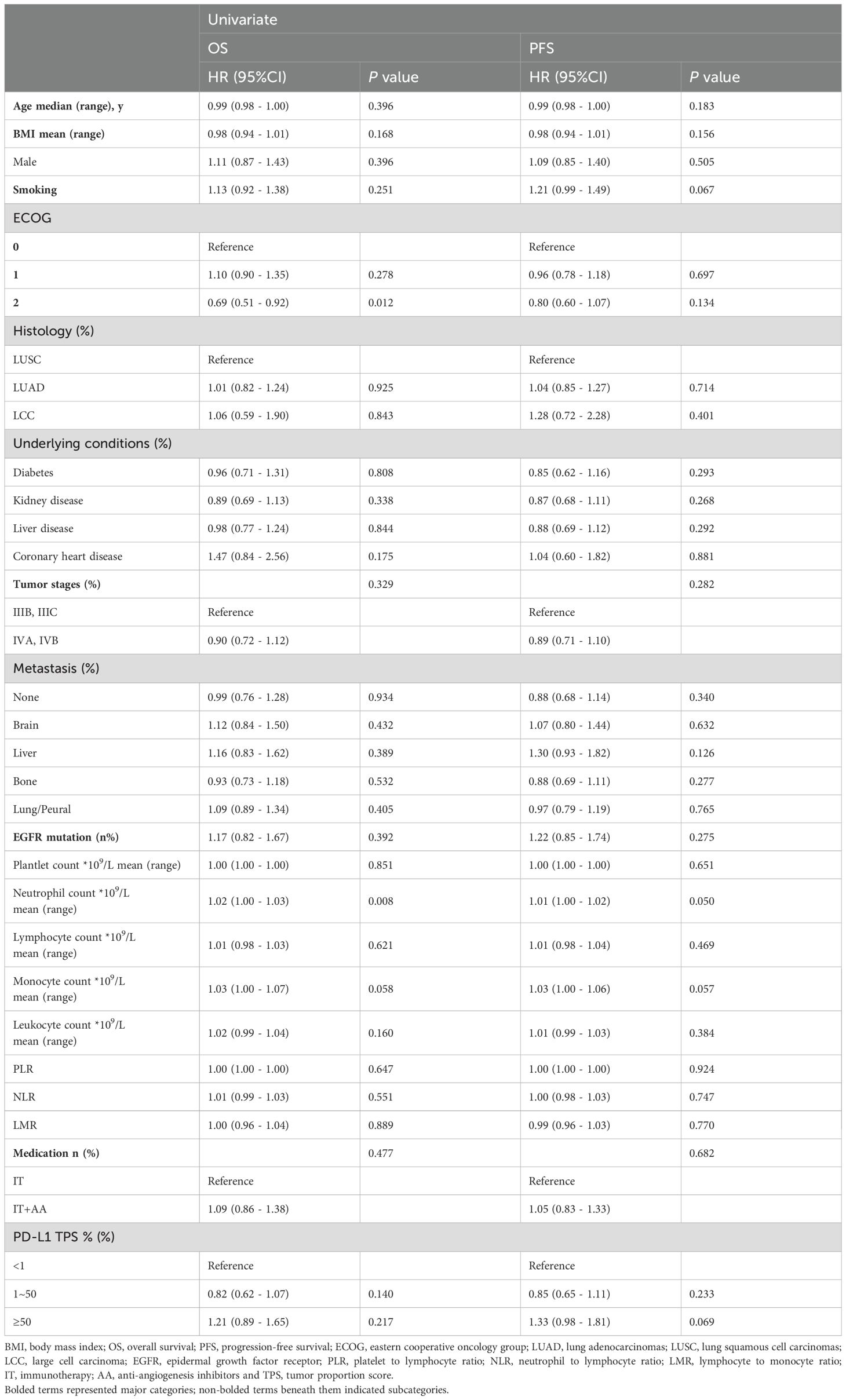
The results of the univariate analysis in OS and PFS were summarized in Table 2. Neutrophil count and ECOG = 2 showed a significant association with OS (HR = 0.69, 95%Cl: 0.51 - 0.92, P = 0.012, and HR = 1.02, 95%Cl: 1.00 - 1.03, P = 0.008 respectively). Neutrophil count was also the only one indicator that was associated with PFS (HR = 1.01, 95%Cl: 1.00 - 1.02, P = 0.050).
However, the PD-L1 TPS between 1% and 50% and more than 50% were associated with increased HR of 0.82 (95%Cl: 0.62 - 1.07, P = 0.140) and 1.21 (95%Cl: 0.89 - 1.65, P = 0.217), respectively, suggesting decreased OS compared to the PD-L1 TPS less than 1%.
3.3 Impact of multivariate on overall survival and progression-free survival
The results of the multivariable Cox model on OS and PFS were illustrated in Table 3, including indicators such as age, BMI, ECOG = 2, underlying condition (diabetes and CHD), lung/pleural metastasis, neutrophil count, medication and PD - 1/PD-L1.
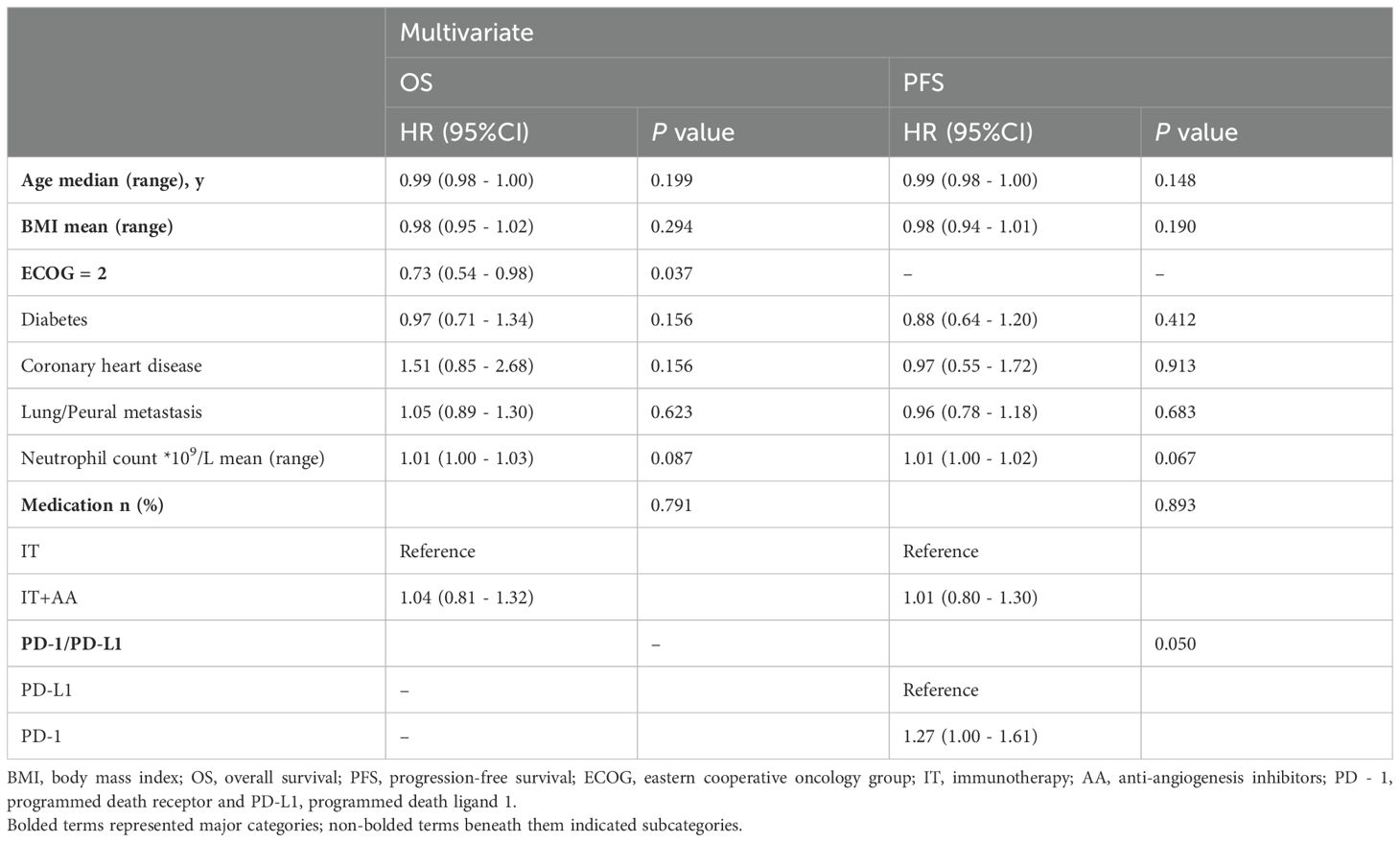
Table 3. Multivariable Cox model for overall survival and progression-free survival in immunotherapy.
A multivariate logistic regression analysis demonstrated that ECOG = 2 was significantly correlated with OS (HR = 0.73, 95%Cl: 0.54 to 0.98, P = 0.037) while PD - 1/PD-L1 had significant association with PFS (HR = 1.27, 95%Cl: 1.00 to 1.61, P = 0.050). No impact of other variables was detected on OS and PFS.
3.4 Immunotherapy survival analysis on overall survival and progression-free survival between hypertensive group and non-hypertensive group
We employed the Kaplan-Meier method to examine survival differences between hypertensive and non-hypertensive groups. OS was significantly longer in non-hypertensive group than in hypertensive group (P = 0.049, Figure 2A). Conversely, survival prognosis of immunotherapy of hypertensive group was not better than non-hypertensive group (P = 0.084, Figure 2B).

Figure 2. Kaplan_meier curves for survival prognosis between hypertensive group and non-hypertensive group were shown. (A) OS was significantly longer in non-hypertensive group than the hypertensive group. (B) PFS in hypertensive group was not better group. OS, overall survival.
The direct effects of immunotherapy in hypertensive and non-hypertensive groups were illustrated respectively in the pie charts (Figure 3A). ORR in hypertensive group (25%) was far lower than that in non-hypertensive group (39%), which indicated a potential of better treatment in non-hypertensive group. Furthermore, stacked bar plot presented the PD - 1/PD-L1 proportion in hypertensive and non-hypertensive groups (Figure 3B). PD - 1 dominated both in hypertensive (69.3%) and non-hypertensive group (77.7%), which may suggest that PD - 1 had a stronger effect on immunotherapy prognosis than PD-L1.
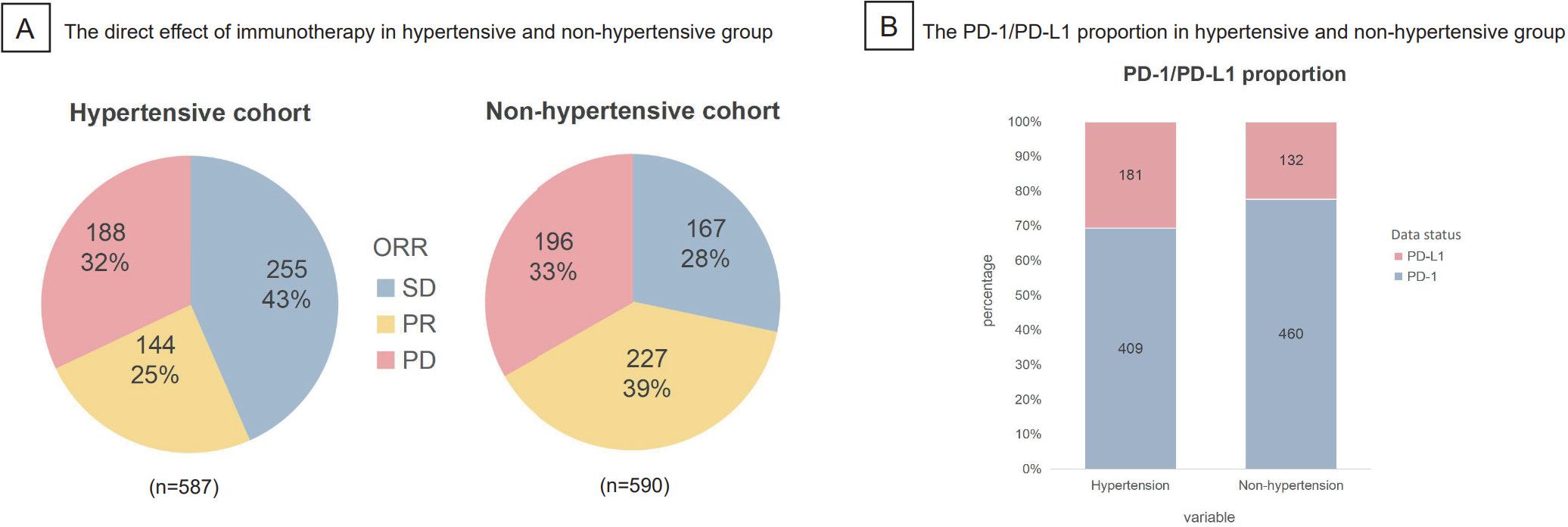
Figure 3. The direct effect and PD-1/PD-L1 proportion in hypertensive and non-hypertensive group were shown. (A) ORR in hypertensive group was 25%, much lower than the 39% in non-hypertensive group. (B) Staked bar plot presented the distribution of PD-1/PD-L1 in hypertensive and non-hypertensive group, indication that PD-1 made up the majority both in hypertensive (69.3%) and non hypertensive group (77.7%). ORR, objective response rate (SD, stable disease; PR, partial response; PD, progressive disease.
3.5 Subgroup analysis on overall survival and progression-free survival
A cohort of 564 NSCLC hypertensive patients were included in the subgroup analysis on OS and PFS. However, there was no significance in OS (Figure 4A) and PFS (Figure 4B) analysis between the baseline indicators and the survival prognosis of patients receiving immunotherapy.
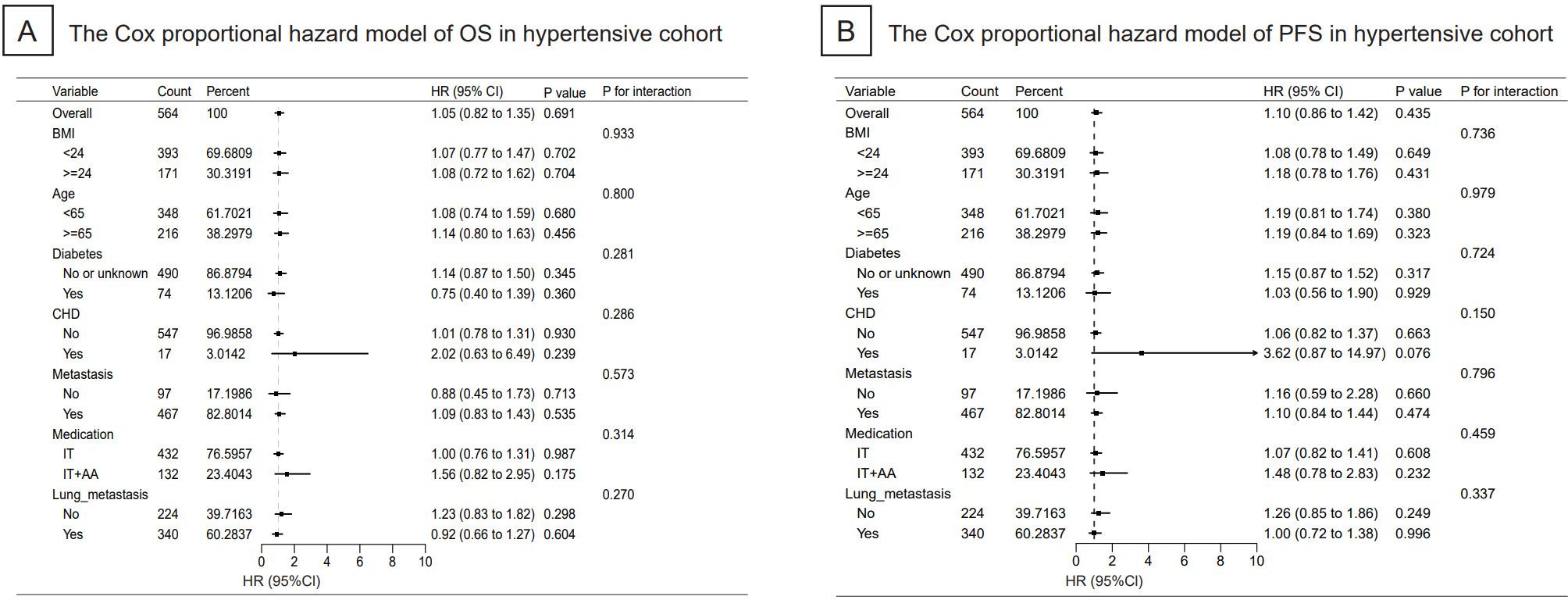
Figure 4. Cox proportional hazard models of significant independent predictive factors associated with progression-free survival and overall survival were shown. As illustrated in the form of Forest Plot, there was no significance in OS and PFS analysis between the indicators and the survival prognosis of immunotherapy. OS, overall survival; PFS, progression-free-survival; BMI, body mass index; CHD, coronary heart disease; IT, immunotherapy and AA, anti-angiogenesis inhibitors.
4 Discussion
With the emergence of ICIs, these drugs have made significant breakthroughs in the treatment of NSCLC and have rapidly become a standard treatment for driver-negative patients. Currently, PD - 1/PD-L1 is recognized as an important biomarker used in clinical practice (39, 40). However, complex clinical conditions, such as baseline characteristics and underlying diseases, may influence the effectiveness of immunotherapy and affect the identification of NSCLC patients who benefit from it. Indeed, identifying indicators that influence the prognosis of immunotherapy in NSCLC patients may be a promising strategy to improve survival and reduce mortality.
In our univariate and multivariate analyses, PD - 1/PD-L1 was significantly associated with PFS, while ECOG = 2 was associated with OS, consistent with previous studies that ECOG PS score≥2 has important prognostic value in NSCLC patients receiving ICIs treatment (41). In a previous single-arm trial assessing the safety and efficacy of PD - 1 inhibitors in advanced NSCLC, PD - 1 blockade (Camrelizumab)-activated neoantigen-specific cellular therapy (aNASCT) demonstrated safety and immunogenicity in NSCLC patients, suggesting its promising potential in cancer immunotherapy (42). Another observational study indicated that PD - 1 inhibitors were widely administered to NSCLC patients with stage III and IV extensively metastatic lung cancer, showing significantly longer PFS and OS (43–46). Anti-PD-1 antibody alone or combined with chemotherapy has become the standard of care for advanced NSCLC patients (47). Consistent with previous studies, our study showed a significant correlation between PD - 1/PD-L1 and PFS. However, we did not observe a significant association between PD - 1/PD-L1 and OS. This discrepancy may be due to variations in the study population, the type of PD - 1/PD-L1 utilized, the sample size, and the method of obtaining the results.
Our study found that, among advanced NSCLC patients who received immunotherapy, the non-hypertensive group was associated with a lower risk of mortality than hypertensive group. Additionally, no baseline indicator was observed a significant correlation with survival prognosis in hypertensive patients. HTN was reported that it may lead to a poor survival for advanced NSCLC patients with type 2 diabetes mellitus (48). Nevertheless, in our study, we didn’t see this correlation, which was not consistent with previous research. The results of studies on the correlation between HTN and the prognosis of targeted therapy in NSCLC patients varies. Some indicated some targeted drug (such as bevacizumab) was associated with an increased risk of HTN development in NSCLC patients (49), which led to the poor prognosis of treatment. Others showed that HTN in bevacizumab-treated patients with metastatic NSCLC led to significantly improved responses (50). The occurrence of HTN may be an important clinical indicator predicting the efficacy of third-line targeted drug treatment in NSCLC patients (51). However, no specific large-scale studies of the correlation between HTN and the prognosis of immunotherapy in NSCLC patients was observed.
Due to the fact that there was no previous studies on the relevant pathogenesis, we hypothesized the reasons why there was a significant correlation between HTN and the prognosis of immunotherapy in NSCLC patients were as follows. HTN was reported to be independently associated with increased risk of both VEGF-related adverse vascular events (AVEs) and discontinuation due to VEGF-related AVEs, which may be an important factor to affect the prognosis of immunotherapy (52).
Voltage-gated calcium channels (VGCCs) increased BP in HTN by promotin vasoconstriction, while abnormally regulated calcium channels in cancer may drive tumor cell’s proliferation, migration, and immune escape (36). In other words, some anti-hypertensive drugs may affect the prognosis of NSCLC immunotherapy, which needed to be confirmed by further studies.
Overall, our data suggested that further research would be needed to elucidate the relationship between HTN and the prognosis of immunotherapy in NSCLC patients.
5 Limitations
The findings of this study had to be seen in light of the following limitations. Firstly, this was a retrospective study, and the inherent drawbacks of retrospective studies made some selection bias inevitable, while the lack of original imaging data in a proportion of patients made it difficult to further explore the prognostic impact of NSCLC patients receiving immunotherapy. Secondly, the data used were not specifically collected for the purposes of this study, but were derived from existing clinical practice information, not allowing for a randomized clinical trial (RCT). Thirdly, the results of this study were based on data from the population in five hospitals. The moderate sample size of NSCLC patients receiving immunotherapy limited the ability to draw a definitive conclusion and the generalizability to other populations. However, our study provided the preliminary evidence for future large-scale and prospective trials. Fourthly, data of PD-L1 expression were sparse and some of the PD-L1 status could not be determined from this database. There was a possibility that patients with low or negative PD-L1 expression were more likely to receive conventional chemotherapy, which were excluded from this study. Finally, while CRP, ESR and cytokines were not measured due to retrospective constraints, we analyzed guideline-recommended hematologic indices (e.g., NLR, PLR and LMR) and adjusting for direct inflammation proxies (e.g., neutrophil count, ECOG). Future studies should integrate multiplex cytokine profiling. At the same time, this study did not further investigate the effects of different types of anti-hypertensive drugs on the prognosis of NSCLC patients receiving immunotherapy. Further studies should be based on samples using the same ICI drugs (PD - 1/PD-L1 inhibitors) and classified according to the types of anti-hypertensive drugs to investigate its impact on immunotherapy prognosis.
6 Conclusion
Our study is the first large-scale retrospective multicenter study to investigate the association between HTN and the prognosis of immunotherapy in advanced NSCLC patients. In our study, the non-hypertensive group was associated with a lower risk of mortality compared to the hypertensive group. In subgroup analysis, no baseline indicator was observed a significant correlation with survival prognosis on OS and PFS in hypertensive patients. Our findings provide an important prognostic factor to improve the prognosis of advanced NSCLC patients receiving immunotherapy. Prospective randomized trials are warranted to further validate these findings.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author Jiadi Gan (TWVkX2dhbmpkQDE2My5jb20=).
Ethics statement
The studies involving humans were approved by West China Hospital of Sichuan University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
JY: Formal Analysis, Validation, Writing – original draft. KL: Data curation, Writing – original draft, Formal Analysis. QK: Formal Analysis, Investigation, Methodology, Writing – original draft. TC: Methodology, Project administration, Writing – original draft. XG: Methodology, Project administration, Resources, Writing – original draft. JW: Project administration, Resources, Validation, Writing – original draft. L-NH: Methodology, Supervision, Writing – original draft. JG: Conceptualization, Data curation, Writing – original draft, Writing – review & editing. BC: Conceptualization, Methodology, Project administration, Validation, Writing – original draft, Writing – review & editing. WM: Methodology, Supervision, Writing – original draft.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was supported with a Science Fund for Distinguished Young Scholars grant to Bojiang Chen from the Sichuan Natural Science Foundation of Science Fund (2024NSFJQ0051); a Noncommunicable Chronic Diseases-National Science and Technology Major Project grant to Bojiang Chen from the National Natural Science Foundation of China (2023ZD0506101/2023ZD0506100).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Han B, Zheng R, Zeng H, Wang S, Sun K, Chen R, et al. Cancer incidence and mortality in China, 2022. Zhonghua Zhong Liu Za Zhi. (2024) 46:221–31. doi: 10.3760/cma.j.cn112152-20240119-00035
2. Herbst R, Morgensztern D, and Boshoff C. The biology and management of non-small cell lung cancer. Nature. (2018) 553:446–54. doi: 10.1038/nature25183
3. Travis WD, Brambilla E, Nicholson AG, Yatabe Y, Austin JHM, and Beasley MB. The 2015 world health organization classification of lung tumors: impact of genetic, clinical and radiologic advances since the 2004 classification. J Thorac Oncol. (2015) 10:1243–60. doi: 10.1097/JTO.0000000000000630
4. Remon J, Passiglia F, Ahn MJ, Barlesi F, Forde PM, Garon EB, et al. Immune checkpoint inhibitors in thoracic Malignancies: review of the existing evidence by an IASLC expert panel and recommendations. J Thorac Oncol. (2020) 15:914–47. doi: 10.1016/j.jtho.2020.03.006
5. Hendriks LE, Kerr KM, Menis J, Mok TS, Nestle U, Passaro A, et al. Electronic address:Y2xpbmljYWxndWlkZWxpbmVzQGVzbW8ub3Jn. Oncogene-addicted metastatic non-small-cell lung cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. (2023) 34:339–57. doi: 10.1016/j.annonc.2022.12.009
6. Korman AJ, Garrett-Thomson SC, and Lonberg N. The foundations of immune checkpoint blockade and the ipilimumab approval decennial. Nat Rev Drug Discov. (2022) 21:509–28. doi: 10.1038/s41573-021-00345-8
7. Kalbasi A and Ribas A. Tumour-intrinsic resistance to immune checkpoint blockade. Nat Rev Immunol. (2020) 20:25–39. doi: 10.1038/s41577-019-0218-4
8. Chowell D, Morris LGT, Grigg CM, Weber JK, Samstein RM, Makarov V, et al. Patient HLA class I genotype influences cancer response to checkpoint blockade immunotherapy. Science. (2018) 359:582–7. doi: 10.1126/science.aao4572
9. Pitt JM, Vétizou M, Daillère R, Roberti MP, Yamazaki T, Routy B, et al. Resistance mechanisms to immune-checkpoint blockade in cancer: tumor-intrinsic and -extrinsic factors. Immunity. (2016) 44:1255–69. doi: 10.1016/j.immuni.2016.06.001
10. Fessler J, Matson V, and Gajewski TF. Exploring the emerging role of the microbiome in cancer immunotherapy. J Immunother Cancer. (2019) 7:108. doi: 10.1186/s40425-019-0574-4
11. Sivan A, Corrales L, Hubert N, Williams JB, Aquino-Michaels K, Earley ZM, et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti–PD-L1 efficacy. Science. (2015) 350:1084–9. doi: 10.1126/science.aac4255
12. Usset J, Rosendahl Huber A, Andrianova MA, Batlle E, Carles J, Cuppen E, et al. Five latent factors underlie response to immunotherapy. Nat Genet. (2024) 56:2112–20. doi: 10.1038/s41588-024-01899-0
13. Zhang D, Huang J, Zhang C, Guan Y, and Guo Q. Progress on PD - 1/PD-L1 checkpoint inhibitors in lung cancer. Zhongguo Fei Ai Za Zhi. (2019) 22:369–79. doi: 10.3779/j.issn.1009-3419.2019.06.07
14. Havel JJ, Chowell D, and Chan TA. The evolving landscape of biomarkers for checkpoint inhibitor immunotherapy. Nat Rev Cancer. (2019) 19:133–50. doi: 10.1038/s41568-019-0116-x
15. Kichenadasse G, Miners JO, Mangoni AA, Rowland A, Hopkins AM, and Sorich MJ. Association between body mass index and overall survival with immune checkpoint inhibitor therapy for advanced non-small cell lung cancer. JAMA Oncol. (2020) 6:512–8. doi: 10.1001/jamaoncol.2019.5241
16. Qu J, Li Y, Wu B, Shen Q, Chen L, Sun W, et al. CD161+CD127+CD8+ T cell subsets can predict the efficacy of anti-PD-1 immunotherapy in non-small cell lung cancer with diabetes mellitus. Oncoimmunology. (2024) 13:2371575. doi: 10.1080/2162402X.2024.2371575
17. Matsumoto H, Maezawa Y, Ohara G, Shiozawa T, Masuko H, and Satoh H. Long-term control by immune checkpoint inhibitors in a lung cancer patient with chronic kidney disease. Klin Onkol. (2024) 38:375–9. doi: 10.48095/ccko2024375
18. Sławiński G, Wrona A, Dąbrowska-Kugacka A, Raczak G, and Lewicka E. Immune checkpoint inhibitors and cardiac toxicity in patients treated for non-small lung cancer: A review. Int J Mol Sci. (2020) 21:7195. doi: 10.3390/ijms21197195
19. Li Y, Zhang Z, Hu Y, Yan X, Song Q, Wang G, et al. Pretreatment neutrophil-to-lymphocyte ratio (NLR) may predict the outcomes of advanced non-small-cell lung cancer (NSCLC) patients treated with immune checkpoint inhibitors (ICIs). Front Oncol. (2020) 10:654. doi: 10.3389/fonc.2020.00654
20. Tong W, Xu H, Tang J, Zhao N, Zhou D, Chen C, et al. Inflammatory factors are associated with prognosis of non-small cell lung cancer patients receiving immunotherapy: a meta-analysis. Sci Rep. (2024) 14:26102. doi: 10.1038/s41598-024-76052-2
21. Fujimoto A, Toyokawa G, Koutake Y, Kimura S, Kawamata Y, Fukuishi K, et al. Association between pretreatment neutrophil-to-lymphocyte ratio and immune-related adverse events due to immune checkpoint inhibitors in patients with non-small cell lung cancer. Thorac Cancer. (2021) 12:2198–204. doi: 10.1111/1759-7714.14063IF:2.3Q2
22. Weber JS, Hodi FS, Wolchok JD, Topalian SL, SChadendorf D, Larkin J, et al. Safety profile of nivolumab monotherapy: A pooled analysis of patients with advanced melanoma. J Clin Oncol. (2017) 35:785–92. doi: 10.1200/JCO.2015.66.1389
23. Hara H, Mizusawa J, Hironaka S, Kato K, Daiko H, Abe T, et al. Influence of preoperative chemotherapy-induced leukopenia on survival in patients with esophageal squamous cell carcinoma: exploratory analysis of JCOG9907. Esophagus. (2021) 18:41–8. doi: 10.1007/s10388-020-00752-7
24. Dechow T, Riera-Knorrenschild J, Hackanson B, Janssen J, Schulz H, Oppermann U, et al. First-line nab-paclitaxel plus carboplatin for patients with advanced non-small cell lung cancer: Final results of the NEPTUN study. Int J Cancer. (2023) 153:141–52. doi: 10.1002/ijc.34467
25. Kha VV, Ly TH, Duy PDT, Hoa PT, and Cong BT. The prognostic significance of pretreatment white blood cell and platelet counts for survival outcome in primary lung cancer. Mater Sociomed. (2024) 36:97–102. doi: 10.5455/msm.2024.36.97-102
26. NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: a pooled analysis of 1201 population-representative studies with 104 million participants. Lancet. (2021) 398:957–80. doi: 10.1016/S0140-6736(21)01330-1
27. Kidoguchi S, Sugano N, Tokudome G, Yokoo T, Yano Y, Hatake K, et al. New concept of onco-hypertension and future perspectives. Hypertension. (2021) 77:16–27. doi: 10.1161/HYPERTENSIONAHA.120.16044
28. Cohen JB, Geara AS, Hogan JJ, and Townsend RR. Hypertension in cancer patients and survivors: epidemiology, diagnosis, and management. JACC CardioOncol. (2019) 1:238–51. doi: 10.1016/j.jaccao.2019.11.009
29. Sahni G. Onco-hypertension: changing paradigm of treating hypertension in patients with cancer. J Clin Oncol. (2023) 41:958–63. doi: 10.1200/JCO.22.01875
30. Han H, Guo W, Shi W, Yu Y, Zhang Y, Ye X, et al. Hypertension and breast cancer risk: a systematic review and meta-analysis. Sci Rep. (2017) 7:44877. doi: 10.1038/srep44877
31. Lindgren A, Pukkala E, Nissinen A, and Tuomilehto J. Blood pressure, smoking, and the incidence of lung cancer in hypertensive men in North Karelia, Finland. Am J Epidemiol. (2003) 158:442–7. doi: 10.1093/aje/kwg179
32. Everatt R, Kuzmickienė I, Brasiūnienė B, Vincerževskienė I, Intaitė B, Cicėnas S, et al. Postdiagnostic use of antihypertensive medications and survival in colorectal, lung, corpus uteri, melanoma and kidney cancer patients with hypertension. BMC Cancer. (2025) 25:38. doi: 10.1186/s12885-024-13273-8
33. Arima H, Barzi F, and Chalmers J. Mortality patterns in hypertension. J Hypertens. (2011) 29 Suppl 1:S3–7. doi: 10.1097/01.hjh.0000410246.59221.b1
34. Zhang R, Yin H, Yang M, Liu J, Zhen D, and Zhang Z. Advanced progress of the relationship between renin-angiotensin-aldosterone system inhibitors and cancers. J Hypertens. (2024) 42:1862–73. doi: 10.1097/HJH.0000000000003836
35. Deluce J, Maj D, Verma S, Breadner D, Boldt G, and Raphael J. Efficacy and toxicity of combined inhibition of EGFR and VEGF in patients with advanced non-small cell lung cancer harboring activating EGFR mutations: A systematic review and meta-analysis. Am J Clin Oncol. (2023) 46:87–93. doi: 10.1097/COC.0000000000000976
36. Sheng Y, Qiao C, Zhang Z, Shi X, Yang L, Xi R, et al. Calcium channel blocker lacidipine promotes antitumor immunity by reprogramming tryptophan metabolism. Adv Sci (Weinh). (2025) 12:e2409310. doi: 10.1002/advs.202409310
37. Wei J, Zhou Z, Xu Z, Zeng S, Chen X, Wang X, et al. Retrospective clinical study of renin-angiotensin system blockers in lung cancer patients with hypertension. PeerJ. (2019) 7:e8188. doi: 10.7717/peerj.8188
38. Rachow T, Schiffl H, and Lang SM. Risk of lung cancer and renin-angiotensin blockade: a concise review. J Cancer Res Clin Oncol. (2021) 147:195–204. doi: 10.1007/s00432-020-03445-xIF:2.8Q2
39. Carbone DP, Reck M, and Paz-Ares L. CheckMate 026 investigators. First-Line Nivolumab Stage IV Recurrent Non-Small-Cell Lung Cancer. N Engl J Med. (2017) 376:2415–26. doi: 10.1056/NEJMoa1613493
40. Reck M. Pembrolizumab as first-line therapy for metastatic non-small-cell lung cancer. Immunotherapy. (2018) 10:93–105. doi: 10.2217/imt-2017-0121
41. Dall’Olio FG, Maggio I, Massucci M, et al. ECOG performance status ≥2 as a prognostic factor in patients with advanced non small cell lung cancer treated with immune checkpoint inhibitors-A systematic review and meta-analysis of real world data. Lung Cancer. (2020) 145:95–104. doi: 10.1016/j.lungcan.2020.04.027
42. Qiao Y, Hui K, Hu C, Wang M, Sun W, Liu L, et al. Efficacy and safety of PD - 1 blockade-activated neoantigen specific cellular therapy for advanced relapsed non-small cell lung cancer. Cancer Immunol Immunother. (2025) 74:60. doi: 10.1007/s00262-024-03906-z
43. Wang C, Liang N, Qiao L, Wu Y, Zhang J, and Zhang Y. Clinical features and prognosis analysis of stage III/IV patients with lung cancer after treatment with toripalimab: A real-world retrospective. J Cancer Res Ther. (2024) 20:2021–8. doi: 10.4103/jcrt.jcrt_500_24
44. Luan T, Shen PX, Zhang YH, Xie XH, Lin XQ, Liu M, et al. Annual therapeutic advances in advanced non-small cell lung cancer in 2024. Zhonghua Jie He He Hu Xi Za Zhi. (2025) 48:72–7. doi: 10.3760/cma.j.cn112147-20241103-00655
45. Yoshida K, Watanabe K, Nishimura T, Ikushima H, Ohara S, Takeshima H, et al. Improvement in survival in patients with advanced non-small cell lung cancer. Anticancer Res. (2025) 45:295–305. doi: 10.21873/anticanres.17417
46. Poddubskaya E, Suntsova M, Lyadova M, Luppov D, Guryanova A, Lyadov V, et al. Biomarkers of success of anti-PD-(L)1 immunotherapy for non-small cell lung cancer derived from RNA- and whole-exome sequencing: results of a prospective observational study on a cohort of 85 patients. Front Immunol. (2024) 15:1493877. doi: 10.3389/fimmu.2024.1493877
47. Gelibter A, Tuosto L, Asquino A, Siringo M, Sabatini A, Zizzari IG, et al. Anti-PD1 therapies induce an early expansion of Ki67+CD8+ T cells in metastatic non-oncogene addicted NSCLC patients. Front Immunol. (2024) 15:1483182. doi: 10.3389/fimmu.2024.1483182
48. Zeng X, Zeng D, Cheng J, Xu C, Sun C, Long H, et al. Influence of hypertension on the survival of non-small cell lung cancer patients with type 2 diabetes mellitus. Med Sci Monit. (2020) 26:e921676. doi: 10.12659/MSM.921676
49. Chen J, Lu Y, and Zheng Y. Incidence and risk of hypertension with bevacizumab in non-small-cell lung cancer patients: a meta-analysis of randomized controlled trials. Drug Des Devel Ther. (2015) 9:4751–60. doi: 10.2147/DDDT.S87258
50. Yan LZ, Dressler EV, and Adams VR. Association of hypertension and treatment outcomes in advanced stage non-small cell lung cancer patients treated with bevacizumab or non-bevacizumab containing regimens. J Oncol Pharm Pract. (2018) 24:209–17. doi: 10.1177/1078155217690921
51. Shi J, Chen G, Wang H, Wang X, Han B, Li K, et al. Occurrence of hypertension during third-line anlotinib is associated with progression-free survival in patients with squamous cell lung cancer (SCC): A post hoc analysis of the ALTER0303 trial. Thorac Cancer. (2021) 12:2345–51. doi: 10.1111/1759-7714.14076
52. Naito T, Minegishi Y, Shiraishi H, Hoshino T, Maeda J, Yokota T, et al. Influence of background cardiovascular risk factors on VEGF inhibitor-related adverse vascular events in patients with non-small cell lung cancer: a retrospective study. J Cancer Res Clin Oncol. (2023) 149:12435–42. doi: 10.1007/s00432-023-05092-4
Keywords: immune checkpoint inhibitor, hypertension, non-small cell lung cancer, PD-1/PD-L1 inhibitors, prognosis
Citation: Yuan J, Lei K, Kong Q, Chang T, Gu X, Wang J, He L-n, Gan J and Chen B (2025) Application of immunotherapy in advanced non-small cell lung cancer with hypertension: a multicenter retrospective analysis. Front. Oncol. 15:1621363. doi: 10.3389/fonc.2025.1621363
Received: 01 May 2025; Accepted: 20 August 2025;
Published: 12 September 2025.
Edited by:
M. Teresa Agulló Ortuño, Research Institute Hospital 12 de Octubre, SpainReviewed by:
Agnes S. Kim, UCONN Health, United StatesSara Diaz Saravia, Icahn School of Medicine at Mount Sinai, United States
Copyright © 2025 Yuan, Lei, Kong, Chang, Gu, Wang, He, Gan and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bojiang Chen, Y2poY2JqQG91dGxvb2suY29t; Jiadi Gan, TWVkX2dhbmpkQDE2My5jb20=
†These authors have contributed equally to this work
 Jingyi Yuan1†
Jingyi Yuan1† Tao Chang
Tao Chang Juan Wang
Juan Wang Li-na He
Li-na He Jiadi Gan
Jiadi Gan