- Department of Radiation Oncology, Rutgers Cancer Institute, New Brunswick, NJ, United States
Magnetic resonance-guided radiotherapy (MRgRT) represents a significant advancement in lung cancer treatment, integrating non-ionizing high-resolution magnetic resonance imaging (MRI) with linear accelerators to enable real-time tumor visualization and adaptive treatment planning. This review highlights recent advancements in MRgRT technology and explores its clinical applications, particularly in managing lung cancer patients. MRgRT has proven particularly advantageous in stereotactic body radiotherapy (SBRT) treatment for lung cancer, where motion management is critical due to respiratory-induced tumor motion. Real-time tumor monitoring and online adaptive plan modifications ensure target accuracy, reduce margins, and mitigate radiation-induced toxicity. Additionally, MRgRT could potentially allow multileaf collimator (MLC) tracking to further improve the treatment efficiency. Recent technological innovations, including AI-powered auto-contouring algorithms, deep-learning (DL) based prediction models, and adaptive treatment strategies, further optimize MRgRT by improving workflow efficiency and reducing treatment time. Despite these benefits, the widespread adoption of MRgRT is challenged by high infrastructure costs, prolonged treatment time, and the need for specialized expertise. Ongoing research is addressing these challenges through workflow optimization, remote treatment models, and AI-driven decision support systems. As MRgRT technology continues to evolve, its integration with functional imaging, radiomics, and adaptive protocols is expected to expand its applications beyond lung cancer treatment. MRgRT represents a paradigm shift in precision oncology, delivering personalized care. Future research and prospective clinical trials should be warranted to generate high-quality clinical evidence supporting MRgRT’s clinical adoption for lung cancer patient management. As these advancements progress, MRgRT is poised to transform the future of lung cancer treatment.
Introduction
Lung cancer remains a leading cause of cancer-related mortality worldwide, accounting for over 1.8 million deaths annually (1). Non-small cell lung cancer (NSCLC) represents approximately 85% of all lung cancer cases (2). Despite advancements in systemic therapies, radiotherapy continues to play a critical role in lung cancer patient management. Advanced radiotherapy techniques, such as stereotactic body radiotherapy (SBRT) and image-guided radiotherapy (IGRT), have significantly improved local control and reduced toxicity (3–5). However, there are still challenges in lung cancer radiotherapy treatment, including intrafractional motion, anatomic changes, and dose delivery to critical organs (6–8).
Magnetic resonance-guided radiotherapy (MRgRT), such as the MRIdian Viewray system and the Elekta Unity system, has emerged as a promising solution to address these challenges. By integrating real-time magnetic resonance imaging (MRI) with a linear accelerator (linac), MRgRT enables continuous visualization of target volumes and critical structures during treatment without additional ionizing imaging dose (9, 10). The ability to perform real-time visualization and adaptive radiotherapy (ART) using MRgRT has proven beneficial in improving target dose coverage while protecting surrounding organs at risk (OARs) (11–17), especially in complex clinical scenarios such as ultracentral lung tumors (ULT) and tumors exhibiting significant or irregular motion during respiration (18–20). MRgRT allows for dose escalation strategies that were previously infeasible with conventional modalities (13, 21).The growing body of evidence from clinical trials and institutional experiences highlights the potential of MRgRT to redefine standards of care in lung cancer radiotherapy. Moreover, the incorporation of artificial intelligence (AI) (22, 23) and radiomics (24–27) into MRgRT workflows offers new opportunities to personalize and optimize treatment by assessing biological and anatomical changes throughout the treatment course.
This review explores the latest technological advancements, clinical applications, and emerging trends in MRgRT for lung cancer treatment. We discuss the advantages, limitations, and future directions of MRgRT, with a focus on its role in enhancing treatment precision, reducing toxicity, and improving clinical outcomes for lung cancer patients.
Technological advancements in MR-guided radiotherapy in lung cancer treatment
MR-linac system
The two most commonly used MR-linac platforms are the Elekta Unity (10, 28) and the ViewRay MRIdian (29, 30). ViewRay MRIdian uses a split 0.35T MRI scanner. The ViewRay system was equipped with a cobalt-60 source in earlier versions and started first clinical use at Washington University in 2014 (29). The system was later upgraded with linac technology in the new MRIdian version, which was introduced in 2017 (30, 31). Elekta Unity uses a 1.5T MRI scanner integrated with a 7 MV flattening filter-free (FFF) linac. This system started clinical use in 2018 (32), after the first clinical cases treated in 2017 at UMC Utrecht in close collaboration with Elekta and Philips with their prototype MRI-Linac (10).
Both platforms provide continuous cine-MRI during treatment, enabling clinicians to visualize and track tumor motion in real-time (33). This capability is particularly advantageous in lung cancer treatment, where respiratory-induced motion can significantly affect target positioning. With the instantaneous tumor motion information from MRI images, these systems could potentially achieve real-time multileaf collimator (MLC) adjustments following target motion (34), enhancing treatment accuracy and efficiency and reducing the need for large treatment margins. Additionally, the automatic beam gating became possible on both systems with the cine-MRI images and this function ensures that radiation is delivered only when the tumor is within the predefined target boundary, significantly reducing the risk of damage to surrounding tissues while maintaining adequate tumor dose. Both platforms offer advanced imaging sequences, including T2-weighted and diffusion-weighted imaging (DWI), enhancing soft-tissue contrast and functional imaging capabilities. These features allow clinicians to assess tumor response and identify functional changes.
Online adaptive radiotherapy
Online ART is one of the most significant advancements and cornerstones of MRgRT (11, 35). Both ViewRay MRIdian and Elekta Unity systems have built-in TPS and treatment planning workflow that enables online ART (29, 32). Traditional radiotherapy relies on a treatment plan generated prior to the first session and applied across the entire course of treatment. When anatomical changes are observed, a revised treatment plan will be generated in an offline manner. In contrast, online ART allows clinicians to modify treatment plans daily based on changes in tumor size, position, and the surrounding anatomy. This is achieved through online MR imaging, contouring based on the anatomy of the day, and re-optimization with patient on the treatment table within the same session. This capability is particularly beneficial for lung cancer patients, whose tumors can shift substantially due to respiratory motion, weight loss, or disease response or progression (13).
Clinical implementation of online ART has demonstrated the ability to reduce planning target volume (PTV) margins and spare organs at risk (OARs) more effectively (36). Modern MRgRT platforms now can potentially incorporate AI-assisted auto-contouring, auto-registration, and dose prediction tools that significantly accelerate the adaptive process (22, 23). A growing number of studies have validated the feasibility, safety, and clinical benefits of daily adaptation, including improved tumor control and reduced toxicity (17, 37, 38). Workflow enhancements and the increasing availability of trained personnel are making online ART more accessible for routine use. At the same time, integration with radiomics and biological imaging may enable biologically adaptive planning, further personalizing treatment and improving outcomes.
With existing MRgRT platforms, clinical focus on lung cancer treatment has primarily focused on stereotactic treatment (39), which has been proven to be effective in local control of early-stage lung tumors. SBRT is the recommended standard treatment option for medically inoperable early-stage non-small cell lung cancer (40) and has shown effectiveness in treating oligometastic disease in lung (41). Existing evidence supports the treatment of peripherally located tumors, where the tumor is away from critical OARs such as major airways.
The MRgRT platform, with superior soft-tissue contrast and the ability to provide online adaptive workflow, yields excellent clinical results when compared to traditional SBRT treatment without adaptive workflow (42). For example, Finazzi et al. proposed the stereotactic MR-guided adaptive radiation therapy (SMART) approach (43) and their study including 25 patients with peripheral lung lesions demonstrated great clinical results using the SMART approach (14). An example schematic visualization of the SMART workflow is shown in Figure 1 (14). In this gated breath-hold study, the PTVs generated on daily breath-hold 3D MRI were on average only 53.7% of the volume of the ITV-based PTV generated using 4DCT. In addition, the online ART process improved prescription dose coverage of the PTV from a median of 92.1% in predicted plans, to 95.0% in reoptimized ones. A single-institution retrospective study investigated the clinical outcomes of SMART in primary tumors and lung oligometastases (17). This study included 64 patients with 92 lung tumors with 80.4% of the tumors peripherally located. The follow-up study shows 1-year and 3-year local progression-free survival rates of 96.3 and 86.4% respectively without ≥grade 3 toxicity.
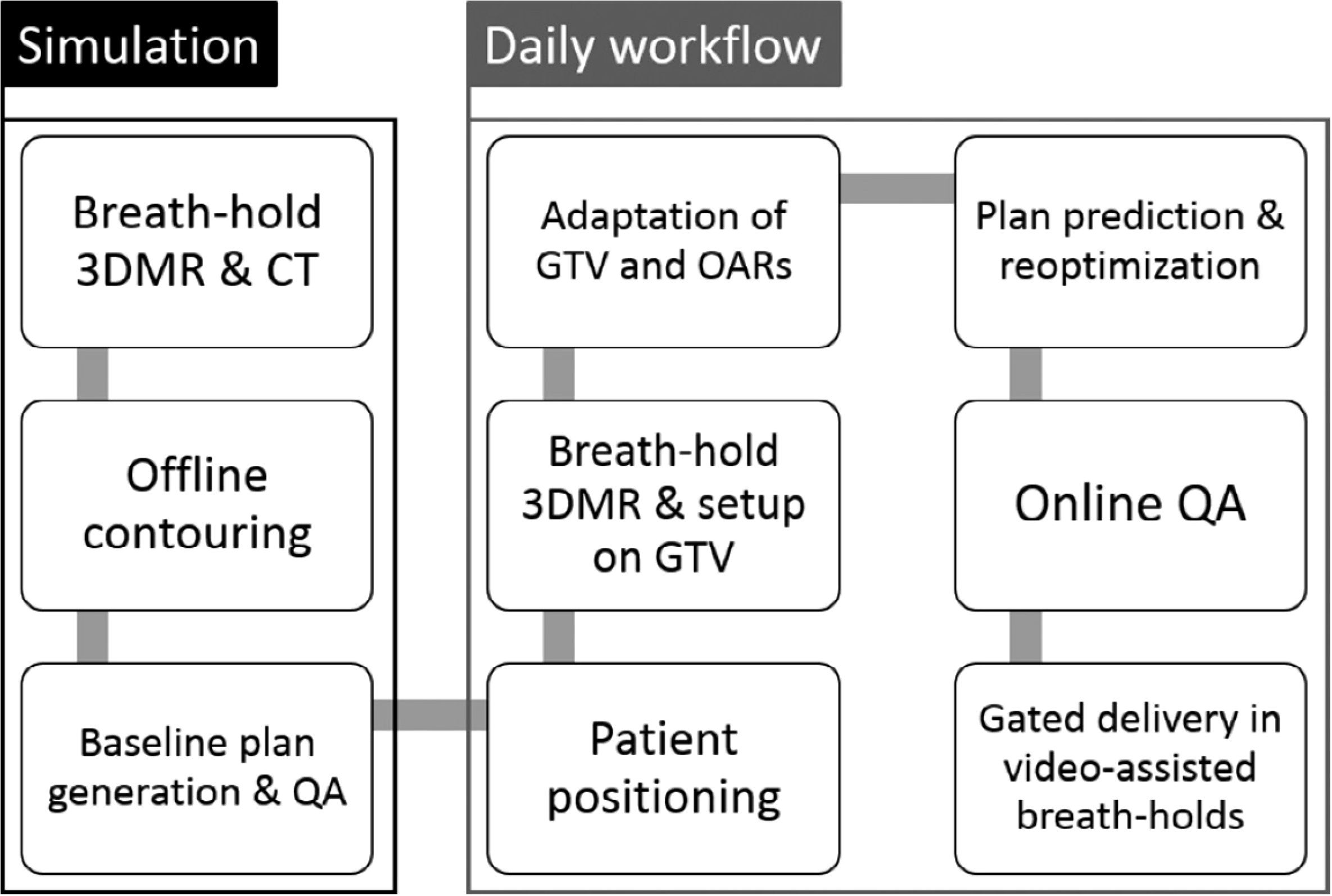
Figure 1. Schematic visualization of the SMART procedure for lung tumors. During simulation, patients undergo a breath-hold 3DMR and CT scan. A baseline treatment plan, to be used for daily plan adaptation, is generated offline. The daily workflow then consists of MR-guided patient setup, online plan adaptation including on-table QA, and gated breath-hold delivery. Reprinted with permission from ref. 14. Copyright 2020, Elsevier.
SBRT treatment of central and ultracentral tumor (ULT) has been controversial, due to the toxicity caused by the proximity of target to major airways (44). The centrality of tumor was defined according to RTOG 0813 criteria, which include tumors located within 2 cm of the proximal bronchial tree or those with a planning target volume (PTV) in contact with the mediastinal or pericardial pleura. Additionally, tumors were sub-classified as “ultracentral” if the gross tumor volume (GTV) directly abutted the proximal bronchial tree or trachea (45).
MRgRT has also garnered interest in addressing the challenges associated with SBRT for central and ultra-central lung tumors. The in silico studies conducted by Henke et al. on the treatment of central tumors within a simulated MRgRT online ART environment have demonstrated promising results, showing improved sparing of OARs compared to non-adaptive treatment approaches (46).
Since then, various studies have been proposed to investigate the characteristics of SMART approach treating ULT. A prospective study by Regnery et al. investigates the long-term outcomes and safety of SMART in treating ULT tumors (44). In this study, 16 ULT underwent SMART workflow, online ART was performed in 91% of fractions. After a median follow-up of 23.6 months, the overall survival rate was 67%, and the 2-year local progression rate was 7%. Their study supports SMART as potentially effective treatment of ULT. An adaptation example where original planning objectives were violated is demonstrated in Figure 2. The same group published another study posting results of the MAGELLAN trial (20), which aims to find the maximum tolerated dose of MR-guided SBRT of ULT. The analysis focused on the proximal bronchial tree (PBT) dose on 19 patients with ULT treated with SMART (18). They found that both intrafractional breathing motion and interfractional translations may impact doses to the PBT during SBRT of ULT. SMART protects the PBT from overdoses and maintains high PTV coverage, while non-SMART approach appears safe with advanced breathing motion management and planning organ at risk volume (PRV) but yields inferior PTV coverage.
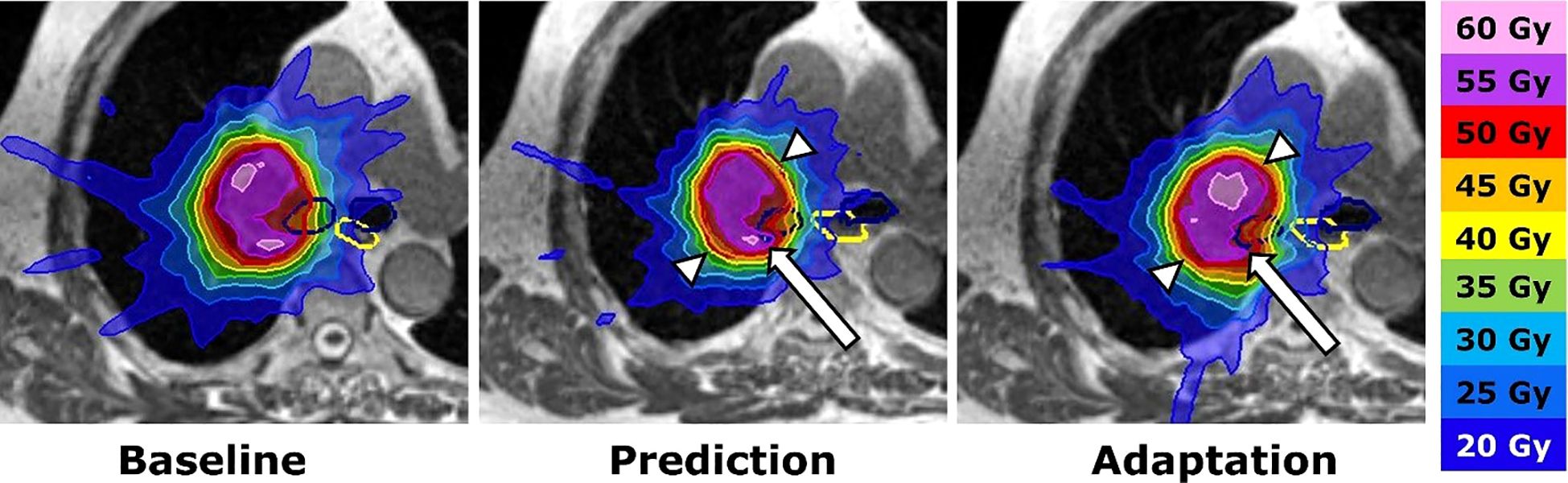
Figure 2. Adaptation procedure. SMART of an ultracentral lymph node metastasis. Left: Baseline plan with contours of the planning target volume (PTV: red), main stem bronchi (dark blue) and esophagus (yellow). Middle: The dose distribution of the baseline plan was predicted on the daily anatomy (fraction 7). The dose declines at the anteromedial and posterolateral PTV border (white arrowheads), while the right main bronchus receives an overdose (white arrow). Right: Plan adaptation optimizes the PTV coverage and avoids overdoses inside the right main bronchus. Reprinted with permission from ref. 44. Copyright 2023, Elsevier.
In addition to SBRT approach, hypofractionated approach has been investigated by La Rosa et al. in ULT (19, 47). In this institutional study, thirteen patients with 14 ULT tumors were treated with 60 Gy in 15 fractions using MRgRT, with daily online adaptation for all 195 delivered fractions. The study achieved a 92.3% crude in-field locoregional control with no reported grade 3 or higher treatment-related toxicities (CTCAE v5.0), showing promise of online ART with MRgRT in the context of non-SBRT setting.
At the moment of this writing, most of the studies described in this review have been done with 0.35 T ViewRay systems (MRIdian MR-Linac or older Co-60 system).
Due to relative late introduction to the clinic, reports with large number of patients on the 1.5 T Elekta system have been scarce. Merkel et al. reports the first clinical experiences of SBRT for ULT using Elekta Unity. The clinical data from 10 patients were collected from 2020-2022 (37). In this study, online ART was performed before each fraction using a T2-weighted 3D MRI acquired during free breathing. Adaptation improved ITV coverage in 34% of the cases, while 14% experienced reductions due to OAR dose constraints. Figure 3 demonstrated a case where decrease in ITV volume occurred in this study.
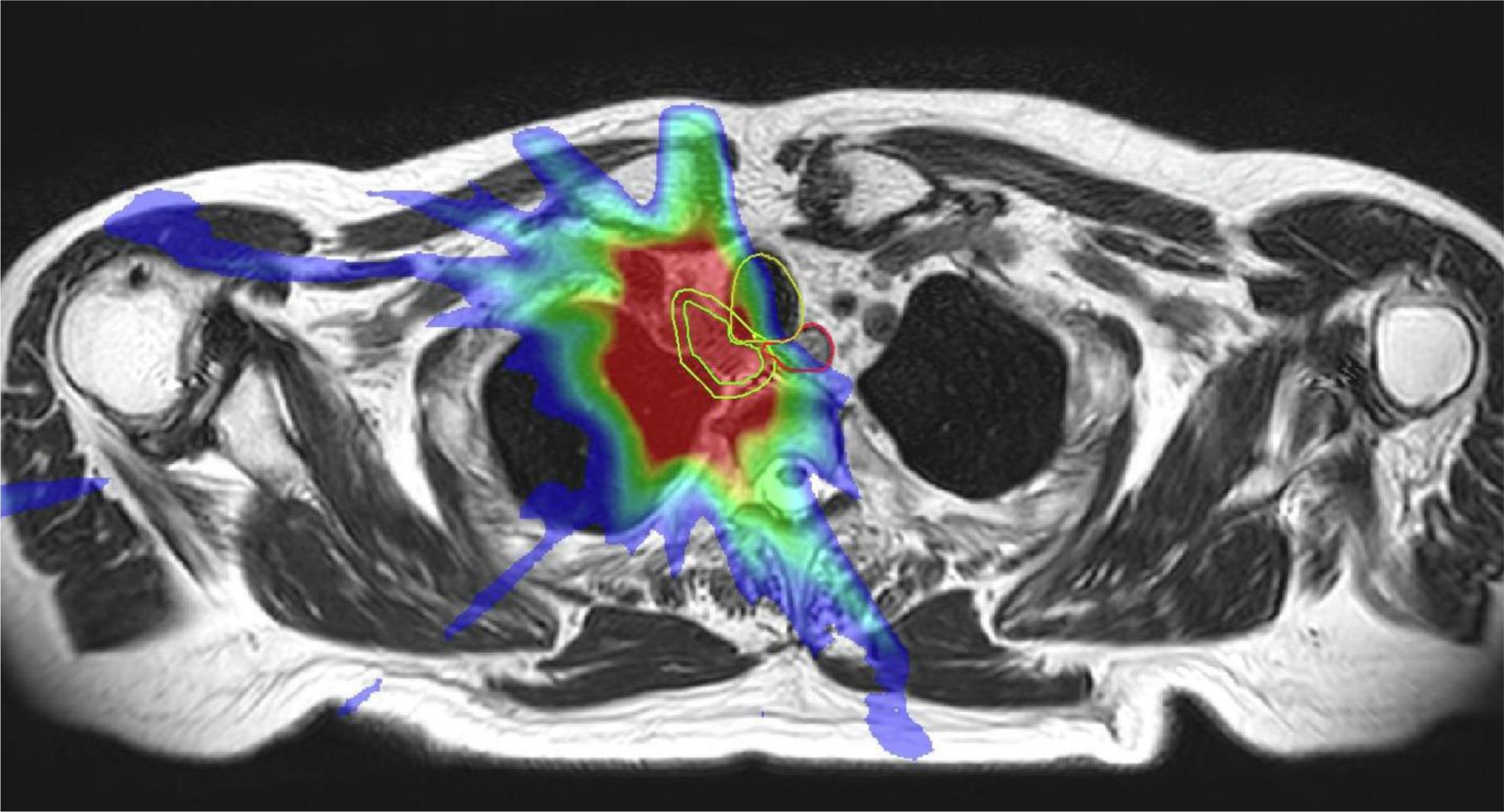
Figure 3. Online MRI and physical dose distribution of a patient with a mediastinal lymph node metastasis of a SCLC in whom a 58% decrease in ITV occurred. The dose distribution of the original plan is projected on the reduced target size at the 12th fraction The ITV (inner green line) and OARs (oesophagus, trachea, spinal cord) within a 3 cm expansion (yellow) around the ITV were modified each fraction by a radiation oncologist. Reprinted with permission from ref. 37. Copyright 2024, Elsevier.
Motion management systems
Tumor motion is a critical challenge in thoracic radiation treatment, particularly for lung cancers that are subject to continuous movement due to respiration and cardiac activity. In addition, the dose conformity of IMRT and VMAT increases the need for respiratory motion management (48).
MRgRT provides powerful tools for motion management through real-time imaging and tracking (49). Motion tracking system from ViewRay MRIdian has been enabling breath-hold gating treatment technique, by taking 2D cine MR images at a rate of 4 fps (33, 50). The system was recently upgraded with compressed sensing and additional motion tracking algorithms, which enabled respiratory gating at 8 fps, with better in-plane resolution and larger matrix size (51). However, planar images with in-plane analysis can only evaluate one-dimensional motion, for example, motion along the SI direction (51). For more complex three-dimensional geometries with motion in various directions, a single imaging slice at high frame rate may not be sufficient.
Recently, Elekta introduced the comprehensive motion management (CMM) system to Unity MR-Linac (52). With CMM, 3D anatomical motion is monitored in two planes (sagittal and coronal) during treatment using a live 2D MR cine balanced turbo field echo (bTFE) imaging sequence acquired with 170 ms temporal resolution per plane, or a temporal resolution of 340 ms two planes combined. 3D-2D registration enables contour tracking in three directions. Multiple automatic gating options are provided in this CMM system. Respiratory gating techniques within CMM include expiration gating, average gating, and breath hold. A non-respiratory gating technique is also provided that can automatically pause the beam if the target moves outside of a user defined threshold in the bTFE cine MRI images (53). This motion management system also allows for correction of tumor drift and bulk shifts. A recent commission study demonstrated CMM system has accurate target positioning prediction with a low beam delivery latency resulting in negligible impact on the overall gated dose distribution (54). The bi-plane approach of motion tracking also has limitations. One noticeable limitation is that the current CMM can only monitor/measure motion for one target belonging to both coronal and sagittal planes of cine MRI (54).
MLC tracking
MLC tracking is a technique used to compensate for intrafraction motion during radiotherapy taking an input from a real-time target position monitoring system and adjusting the radiation beam using the MLC to improve the alignment between the beam and the target (55, 56). This technique has been actively explored in various radiation therapy systems including MRgRT (55).
One research group focused on MLC tracking with MRI-linac with IMRT and VMAT plans on lung plans (34, 48). This group later refined their methods by using a moving dosimetry phantom platform with new leaf sequencer and dose optimization algorithm (57). Their most recent results showed VMAT plans maintained clinical dose constraints and achieved an average local gamma pass rate of 93% under motion with MLC tracking, significantly outperforming untracked deliveries. The system demonstrated a high delivery efficiency of 83%, a notable improvement over gated IMRT. However, current implementation of real-time tumor tracking in MRgRT is associated with approximately a 300–350 ms system latency, which is still higher than the desired latency of 150 ms to safely eliminate ITV in VMAT delivery (58).
Delta radiomics
The variation of radiomic features across different imaging time points during treatment—referred to as delta radiomics—has been proposed in the literature as a predictive tool in various oncologic settings, showing potential for forecasting both treatment response and toxicity outcomes (59, 60).
Proof-of-concept delta radiomics studies have been conducted with 0.35T MRgRT scans on ViewRay MRIdian system with different disease sites such as pancreatic cancer (24) and rectal cancer (25, 26).
A 2024 study focused on lung cancer evaluated 47 SBRT treatments in 39 lung cancer patients using ViewRay MRIdian MR-Linac and analyzed MR delta radiomic features extracted via Pyradiomics (27). These features spanned seven classes, including shape, histogram, and texture descriptors. The researchers focused on stable and non-collinear features, narrowing down from 107 to 15 core metrics. The study demonstrated the feasibility of delta radiomics with MRgRT for lung cancer and underscored its potential in adaptive radiotherapy, where treatment is tailored in real time based on biological response.
AI integration
AI has been the core of technical innovations in recent years, and the influence has been quickly adapted in the healthcare sector, especially in the technology-driven field of radiation oncology. AI-driven solutions have been increasingly incorporated into auto-segmentation (61) and advanced imaging techniques within adaptive planning framework, these advances aim to address MRgRT’s main limitations such as extended session durations and move closer to treatment timeframes typical of conventional radiotherapy.
Precise delineation of target volumes and OARs is essential for effective treatment planning and adaptation, yet the process is time-consuming and prone to inter-observer variability. Although commercial tools for automatic contouring exist for CT-based workflows, dedicated solutions tailored to MRI are still required to fully support online MRgRT. Automatic DL-based contouring has been explored for MR images from MRgRT systems in abdomen and pelvis regions, no studies are currently available that specifically focus on the thoracic region (62–65).
4D imaging is another key field of needs and advances driven by AI in MRgRT, namely time-resolving 3D MRI (4D-MRI) and synthetic 4D-CT. The need for 4D-MRI primarily stems from the requirement for MRI images with high temporal and spatial resolution for online adaptive therapy (66, 67). 4D-MRI has been incorporated into abdomen SBRT workflow (67) and liver SBRT in a prospective non-randomized patient study (66, 68) on Elekta Unity with 1.5T MRI scanner. In both studies, two pre-beam 4D-MRIs are acquired, one for the purpose of adaptive re-planning, then another one immediately before beam-on for additional verification. In the liver SBRT study, one additional 4D-MRI is acquired intra-fraction to provide information regarding if additional adaptation is required (66). This approach, independent from vendor provided motion management, has its limitation especially for intra-fraction monitoring. For each 4D-MRI, 4min acquisition time and 40s reconstruction time are needed. This does not meet the sub 500ms latency recommended to capture breathing-induced motion, which is key in lung cancer treatment. Although deep learning based methods to increase spatial resolution on sparsely sampled high temporal resolution are attempted by various research studies (69, 70), To date, MRgRT workflow incorporating 4DMRI has not been established with lung cancer patients.
The need for synthetic CT images in MRgRT stem from the desire for accurate dose calculation in the absence of CT in pure MRI environment with the paradigm of MRgRT, and ultimately, an MRI-only treatment planning framework. For lung cancer treatment on MRgRT, more accurate intrafractional dose accumulation required synthetic 4D-CT datasets, which can be generated from 4D-MRI images (72). Orthogonal cine MRI generated during beam delivery at clinical MR-linacs can also be utilized to generate synthetic 4D-CT datasets. Using a propagation method (71), continuous time-resolved estimated synthetic 4D-CTs were generated for dose reconstruction of lung tumor treatments using ViewRay MRIdian MR-linac (72).Various attempts have been conducted at using AI to generate synthetic CT. These DL networks-based methods cover clinical sites such as brain, H&N and abdomen and pelvis regions. However, no studies solely focused on synthetic 4D-CT with AI approaches (22).
Challenges and future directions
While MRgRT presents numerous promising advancements in lung cancer treatment, several challenges remain to be addressed to fully harness its potential and achieve widespread clinical adoption.
Technical and logistical challenges
One of the significant barriers to broader MRgRT implementation is the complexity and resource-intensive nature of the technology.MR-Linac systems require substantial investment in infrastructure, specialized equipment, and highly trained personnel. The integration of MRI with linacs necessitates specialized facilities designed to minimize magnetic interference and accommodate safety protocols associated with strong magnetic fields.
MRI of the lung presents unique challenges due to the inherently low proton density of the pulmonary parenchyma and the presence of numerous air–tissue interfaces, which cause rapid signal decay and susceptibility artifacts. As a result, conventional MRI sequences often yield poor SNR in lung tissue, making it difficult to visualize fine parenchymal structures. In diagnostic radiology, this limitation is addressed through the use of specialized pulse sequences such as ultra-short echo time (UTE) (73, 74), which are designed to capture signal from tissues with very short T2* relaxation times. However, in the context of MRgRT, the clinical imaging requirements differ. The goal of lung MRgRT is not primarily focused on detailed parenchymal imaging, but rather accurate localization of gross tumor volumes (GTVs), surrounding soft tissues, and organ motion tracking. MR-linac systems optimize for tumor and boundary visualization using sequences like TRUFI (56) or other balanced SSFP sequences (52), which provide sufficient contrast for target delineation and motion management even without parenchymal detail. Thus, while the limited lung parenchymal signal remains a technical constraint, it does not critically impair the clinical efficacy of MRgRT. Nevertheless, ongoing research into MR pulse sequence development, including UTE applications on MR-linacs, may further enhance imaging performance in thoracic radiotherapy.
Note that other online ART platform, such as Ethos system (Varian Medical Systems, Palo Alto, CA, USA) that uses daily cone beam computed tomography (CBCT) scans in the online ART workflow, shows potential to treat lung tumors in recent feasibility study (48, 49). The visualization of small lung tumors with large motion, especially those located close to diaphragm, could still be challenging for both CBCT-based ART and MR-based ART modalities, due to different reasons. Compared with MR-base ART, the CBCT-based ART currently does not allow real-time visualization of lung tumors during beam delivery and adds extra ionization imaging dose (49).
Recent studies have highlighted that the introduction of real-time tumor tracking and online ART significantly increases the procedural complexity and may initially decrease departmental throughput, creating practical challenges for clinical implementation. Furthermore, MR-Linac systems often require extensive quality assurance (QA) protocols to maintain the accuracy and consistency of MR component in addition to regular linac QA, adding further logistical complexity (75).
Addressing these technical barriers will require innovations such as improved system integration, streamlined imaging techniques, faster and more efficient adaptive software, and optimized patient workflow strategies. Collaboration between equipment manufacturers, clinical experts, and healthcare providers will be crucial to overcoming these challenges and fostering broader adoption of MRgRT.
Treatment time and patient compliance
Extended treatment time, inherent to online ART sessions, poses challenges related to patient comfort, compliance, and clinical throughput (76). Studies utilizing MR-Linac systems have demonstrated treatment durations significantly longer than conventional radiotherapy, with single fraction sometimes exceeding an hour due to the necessity of repeated imaging, adaptive replanning, and gated treatment processes (77). Lengthy treatment sessions can result in increased patient discomfort, potential motion during treatment, and reduced patient compliance. Future efforts should focus on reducing treatment durations through advancements in rapid imaging techniques, accelerated adaptive planning algorithms, and improved patient immobilization and comfort strategies. Enhanced patient education and engagement protocols could further improve patient experience and compliance during treatment.
Standardization and quality assurance
The variability in MR imaging protocols, adaptive treatment planning strategies, and workflow practices across different institutions highlights the need for standardization and QA guidelines. Initiatives led by professional societies are underway to develop detailed guidelines for standardized imaging and adaptive planning protocols (78, 79). Rigorous training programs for radiation oncologists, medical physicists, and radiation therapists in standardized MR-guided techniques can further enhance consistency in clinical outcomes.
Clinical evidence and prospective trials
Although retrospective studies and early clinical experiences demonstrate MRgRT’s potential benefits, robust prospective clinical trials and long-term outcome data are needed to conclusively establish its clinical efficacy, safety profile, and comparative effectiveness versus conventional radiotherapy approaches (20).
In a recent narrative review, Cheng et al. summarized ongoing MRgRT lung clinical trials, emphasizing the critical need for prospectively collected data to evaluate outcomes such as overall survival, local control, and toxicity profiles (80). These trials are particularly important in high-risk clinical scenarios—such as ultra-central tumors (ULT) or re-irradiation cases—where prospective evidence is needed to define safe and effective dose regimens and to characterize associated toxicity (18).
Future research should focus on conducting multi-center, randomized controlled trials to generate high-quality evidence supporting MRgRT’s clinical adoption and incorporation into standard clinical guidelines. Establishing multicenter collaborative networks and patient registries dedicated to MRgRT research will facilitate large-scale data collection, rigorous outcome analysis, and meaningful comparisons across clinical settings (16). These coordinated efforts will ultimately help facilitate the integration of MRgRT into standardized management of lung cancer patients.
Conclusion
MRgRT has emerged as a transformative technology in lung cancer treatment, offering daily online adaptive capabilities and unprecedented non-ionizing real-time imaging during radiation treatment. Its application in treating lung cancer patients has demonstrated superior dosimetric and clinical outcomes. As technological advancements continue, including advanced motion management techniques, AI-driven adaptive planning and workflow optimization, and delta radiomics, MRgRT is poised to be further integrated into standard oncological practice.
Author contributions
CM: Writing – original draft, Writing – review & editing. XW: Writing – review & editing. KN: Writing – review & editing. ZX: Writing – review & editing. KX: Writing – review & editing. NY: Writing – review & editing. YZ: Writing – review & editing, Writing – original draft.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2024) 74:229–63. doi: 10.3322/caac.21834
2. Li C, Lei S, Ding L, Xu Y, Wu X, Wang H, et al. Global burden and trends of lung cancer incidence and mortality. Chin Med J (Engl). (2023) 136:1583–90. doi: 10.1097/CM9.0000000000002529
3. Prezzano KM, Ma SJ, Hermann GM, Rivers CI, Gomez-Suescun JA, Singh AK, et al. Stereotactic body radiation therapy for non-small cell lung cancer: A review. World J Clin Oncol. (2019) 10:14–27. doi: 10.5306/wjco.v10.i1.14
4. Ijsseldijk MA, Shoni M, Siegert C, Wiering B, van Engelenburg AKC, Tsai TC, et al. Oncologic outcomes of surgery versus SBRT for non-small-cell lung carcinoma: A systematic review and meta-analysis. Clin Lung Cancer. (2021) 22:e235–92. doi: 10.1016/j.cllc.2020.04.017
5. Corral J, Borras JM, and Lievens Y. Utilisation of radiotherapy in lung cancer: A scoping narrative literature review with a focus on the introduction of evidence-based therapeutic approaches in Europe. Clin Transl Radiat Oncol. (2024) 45:100717. doi: 10.1016/j.ctro.2023.100717
6. Keall PJ, Mageras GS, Balter JM, Emery RS, Forster KM, Jiang SB, et al. The management of respiratory motion in radiation oncology report of AAPM Task Group 76. Med Phys. (2006) 33:3874–900. doi: 10.1118/1.2349696
7. Ladjal H, Beuve M, Giraud P, and Shariat B. Towards non-invasive lung tumor tracking based on patient specific model of respiratory system. IEEE Trans BioMed Eng. (2021) 68:2730–40. doi: 10.1109/TBME.2021.3053321
8. Li H, Dong L, Bert C, Chang J, Flampouri S, Jee KW, et al. AAPM Task Group Report 290: Respiratory motion management for particle therapy. Med Phys. (2022) 49:e50–81. doi: 10.1002/mp.15470
9. Lagendijk JJ, Raaymakers BW, and van Vulpen M. The magnetic resonance imaging-linac system. Semin Radiat Oncol. (2014) 24:207–9. doi: 10.1016/j.semradonc.2014.02.009
10. Raaymakers BW, Jurgenliemk-Schulz IM, Bol GH, Glitzner M, Kotte A, van Asselen B, et al. First patients treated with a 1.5 T MRI-Linac: clinical proof of concept of a high-precision, high-field MRI guided radiotherapy treatment. Phys Med Biol. (2017) 62:L41–50. doi: 10.1088/1361-6560/aa9517
11. Padgett KR, Simpson GN, Llorente R, Samuels MA, and Dogan N. Feasibility of adaptive MR-guided stereotactic body radiotherapy (SBRT) of lung tumors. Cureus. (2018) 10:e2423. doi: 10.7759/cureus.2423
12. Finazzi T, Haasbeek CJA, Spoelstra FOB, Palacios MA, Admiraal MA, Bruynzeel AME, et al. Clinical outcomes of stereotactic MR-guided adaptive radiation therapy for high-risk lung tumors. Int J Radiat Oncol Biol Phys. (2020) 107:270–8. doi: 10.1016/j.ijrobp.2020.02.025
13. Nierer L, Eze C, da Silva Mendes V, Braun J, Thum P, von Bestenbostel R, et al. Dosimetric benefit of MR-guided online adaptive radiotherapy in different tumor entities: liver, lung, abdominal lymph nodes, pancreas and prostate. Radiat Oncol. (2022) 17:53. doi: 10.1186/s13014-022-02021-6
14. Finazzi T, Palacios MA, Haasbeek CJA, Admiraal MA, Spoelstra FOB, Bruynzeel AME, et al. Stereotactic MR-guided adaptive radiation therapy for peripheral lung tumors. Radiother Oncol. (2020) 144:46–52. doi: 10.1016/j.radonc.2019.10.013
15. Bryant JM, Weygand J, Keit E, Cruz-Chamorro R, Sandoval ML, Oraiqat IM, et al. Stereotactic magnetic resonance-guided adaptive and non-adaptive radiotherapy on combination MR-linear accelerators: current practice and future directions. Cancers (Basel). (2023) 15(7):2081. doi: 10.3390/cancers15072081
16. Regnery S, de Colle C, Eze C, Corradini S, Thieke C, Sedlaczek O, et al. Pulmonary magnetic resonance-guided online adaptive radiotherapy of locally advanced: the PUMA trial. Radiat Oncol. (2023) 18:74. doi: 10.1186/s13014-023-02258-9
17. Ugurluer G, Dincer N, Mustafayev TZ, Gungor G, Abacioglu MU, Sengoz M, et al. MR-guided online adaptive stereotactic body radiotherapy (MRgSBRT) of primary lung tumors and lung oligometastases. Strahlenther Onkol. (2024) 201(6):637–44. doi: 10.1007/s00066-024-02328-1
18. Regnery S, Katsigiannopulos E, Lau H, Hoegen-Sassmannshausen P, Weykamp F, Renkamp CK, et al. How to protect the proximal bronchial tree during stereotactic radiotherapy of ultracentral lung tumors: Lessons from MR-guided treatment. Clin Transl Radiat Oncol. (2025) 51:100899. doi: 10.1016/j.ctro.2024.100899
19. La Rosa A, Mittauer KE, Bassiri N, Rzepczynski AE, Chuong MD, Yarlagadda S, et al. Accelerated hypofractionated magnetic resonance guided adaptive radiation therapy for ultracentral lung tumors. Tomography. (2024) 10:169–80. doi: 10.3390/tomography10010013
20. Regnery S, Ristau J, Weykamp F, Hoegen P, Sprengel SD, Paul KM, et al. Magnetic resonance guided adaptive stereotactic body radiotherapy for lung tumors in ultracentral location: the MAGELLAN trial (ARO 2021-3). Radiat Oncol. (2022) 17:102. doi: 10.1186/s13014-022-02070-x
21. Bryant JM, Sim AJ, Feygelman V, Latifi K, and Rosenberg SA. Adaptive hypofractionted and stereotactic body radiotherapy for lung tumors with real-time MRI guidance. Front Oncol. (2023) 13:1061854. doi: 10.3389/fonc.2023.1061854
22. Cusumano D, Boldrini L, Dhont J, Fiorino C, Green O, Gungor G, et al. Artificial Intelligence in magnetic Resonance guided Radiotherapy: Medical and physical considerations on state of art and future perspectives. Phys Med. (2021) 85:175–91. doi: 10.1016/j.ejmp.2021.05.010
23. Psoroulas S, Paunoiu A, Corradini S, Horner-Rieber J, and Tanadini-Lang S. MR-linac: role of artificial intelligence and automation. Strahlenther Onkol. (2025) 201:298–305. doi: 10.1007/s00066-024-02358-9
24. Tomaszewski MR, Latifi K, Boyer E, Palm RF, El Naqa I, Moros EG, et al. Delta radiomics analysis of Magnetic Resonance guided radiotherapy imaging data can enable treatment response prediction in pancreatic cancer. Radiat Oncol. (2021) 16:237. doi: 10.1186/s13014-021-01957-5
25. Boldrini L, Cusumano D, Chiloiro G, Casa C, Masciocchi C, Lenkowicz J, et al. Delta radiomics for rectal cancer response prediction with hybrid 0.35 T magnetic resonance-guided radiotherapy (MRgRT): a hypothesis-generating study for an innovative personalized medicine approach. Radiol Med. (2019) 124:145–53. doi: 10.1007/s11547-018-0951-y
26. Boldrini L, Chiloiro G, Cusumano D, Yadav P, Yu G, Romano A, et al. Radiomics-enhanced early regression index for predicting treatment response in rectal cancer: a multi-institutional 0.35 T MRI-guided radiotherapy study. Radiol Med. (2024) 129:615–22. doi: 10.1007/s11547-024-01761-7
27. Zha Y, Ye Z, Zapaishchykova A, He J, Hsu SH, Leeman JE, et al. Delta radiomics to track radiation response in lung tumors receiving stereotactic magnetic resonance-guided radiotherapy. Phys Imaging Radiat Oncol. (2024) 31:100626. doi: 10.1016/j.phro.2024.100626
28. Winkel D, Bol GH, Kroon PS, van Asselen B, Hackett SS, Werensteijn-Honingh AM, et al. Adaptive radiotherapy: The Elekta Unity MR-linac concept. Clin Transl Radiat Oncol. (2019) 18:54–9. doi: 10.1016/j.ctro.2019.04.001
29. Mutic S and Dempsey JF. The ViewRay system: magnetic resonance-guided and controlled radiotherapy. Semin Radiat Oncol. (2014) 24:196–9. doi: 10.1016/j.semradonc.2014.02.008
30. Liney GP, Whelan B, Oborn B, Barton M, and Keall P. MRI-linear accelerator radiotherapy systems. Clin Oncol (R Coll Radiol). (2018) 30:686–91. doi: 10.1016/j.clon.2018.08.003
31. Slotman BJ, Clark MA, Ozyar E, Kim M, Itami J, Tallet A, et al. Clinical adoption patterns of 0.35 Tesla MR-guided radiation therapy in Europe and Asia. Radiat Oncol. (2022) 17:146. doi: 10.1186/s13014-022-02114-2
32. Werensteijn-Honingh AM, Kroon PS, Winkel D, Aalbers EM, van Asselen B, Bol GH, et al. Feasibility of stereotactic radiotherapy using a 1.5 T MR-linac: Multi-fraction treatment of pelvic lymph node oligometastases. Radiother Oncol. (2019) 134:50–4. doi: 10.1016/j.radonc.2019.01.024
33. Green OL, Rankine LJ, Cai B, Curcuru A, Kashani R, Rodriguez V, et al. First clinical implementation of real-time, real anatomy tracking and radiation beam control. Med Phys. (2018) 45(8):3728–40. doi: 10.1002/mp.13002
34. Uijtewaal P, Borman PTS, Woodhead PL, Hackett SL, Raaymakers BW, Fast MF, et al. Dosimetric evaluation of MRI-guided multi-leaf collimator tracking and trailing for lung stereotactic body radiation therapy. Med Phys. (2021) 48:1520–32. doi: 10.1002/mp.14772
35. Henke L, Kashani R, Robinson C, Curcuru A, DeWees T, Bradley J, et al. Phase I trial of stereotactic MR-guided online adaptive radiation therapy (SMART) for the treatment of oligometastatic or unresectable primary Malignancies of the abdomen. Radiother Oncol. (2018) 126:519–26. doi: 10.1016/j.radonc.2017.11.032
36. Kishan AU, Ma TM, Lamb JM, Casado M, Wilhalme H, Low DA, et al. Magnetic resonance imaging-guided vs computed tomography-guided stereotactic body radiotherapy for prostate cancer: the MIRAGE randomized clinical trial. JAMA Oncol. (2023) 9:365–73. doi: 10.1001/jamaoncol.2022.6558
37. Merckel LG, Pomp J, Hackett SL, van Lier A, van den Dobbelsteen M, Rasing MJA, et al. Stereotactic body radiotherapy of central lung tumours using a 1.5 T MR-linac: First clinical experiences. Clin Transl Radiat Oncol. (2024) 45:100744. doi: 10.1016/j.ctro.2024.100744
38. Lee HY, Lee G, Ferguson D, Hsu SH, Hu YH, Huynh E, et al. Lung sparing in MR-guided non-adaptive SBRT treatment of peripheral lung tumors. BioMed Phys Eng Express. (2024) 10(4):045048. doi: 10.1088/2057-1976/ad567d
39. Chuong MD, Clark MA, Henke LE, Kishan AU, Portelance L, Parikh PJ, et al. Patterns of utilization and clinical adoption of 0.35 Tesla MR-guided radiation therapy in the United States - Understanding the transition to adaptive, ultra-hypofractionated treatments. Clin Transl Radiat Oncol. (2023) 38:161–8. doi: 10.1016/j.ctro.2022.11.013
40. Postmus PE, Kerr KM, Oudkerk M, Senan S, Waller DA, Vansteenkiste J, et al. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. (2017) 28:iv1–iv21. doi: 10.1093/annonc/mdx222
41. Palma DA, Olson R, Harrow S, Gaede S, Louie AV, Haasbeek C, et al. Stereotactic ablative radiotherapy versus standard of care palliative treatment in patients with oligometastatic cancers (SABR-COMET): a randomised, phase 2, open-label trial. Lancet. (2019) 393:2051–8. doi: 10.1016/S0140-6736(18)32487-5
42. Hering S, Nieto A, Marschner S, Hofmaier J, Schmidt-Hegemann NS, da Silva Mendes V, et al. The role of online MR-guided multi-fraction stereotactic ablative radiotherapy in lung tumours. Clin Transl Radiat Oncol. (2024) 45:100736. doi: 10.1016/j.ctro.2024.100736
43. Finazzi T, Palacios MA, Spoelstra FOB, Haasbeek CJA, Bruynzeel AME, Slotman BJ, et al. Role of on-table plan adaptation in MR-guided ablative radiation therapy for central lung tumors. Int J Radiat Oncol Biol Phys. (2019) 104:933–41. doi: 10.1016/j.ijrobp.2019.03.035
44. Regnery S, Katsigiannopulos E, Hoegen P, Weykamp F, Sandrini E, Held T, et al. To fly or not to fly: Stereotactic MR-guided adaptive radiotherapy effectively treats ultracentral lung tumors with favorable long-term outcomes. Lung Cancer. (2023) 179:107175. doi: 10.1016/j.lungcan.2023.03.011
45. Chaudhuri AA, Tang C, Binkley MS, Jin M, Wynne JF, von Eyben R, et al. Stereotactic ablative radiotherapy (SABR) for treatment of central and ultra-central lung tumors. Lung Cancer. (2015) 89:50–6. doi: 10.1016/j.lungcan.2015.04.014
46. Henke L, Kashani R, Yang D, Zhao T, Green O, Olsen L, et al. Simulated online adaptive magnetic resonance-guided stereotactic body radiation therapy for the treatment of oligometastatic disease of the abdomen and central thorax: characterization of potential advantages. Int J Radiat Oncol Biol Phys. (2016) 96:1078–86. doi: 10.1016/j.ijrobp.2016.08.036
47. La Rosa A, Mittauer KE, Chuong MD, Hall MD, Kutuk T, Bassiri N, et al. Accelerated hypofractionated magnetic resonance-guided adaptive radiotherapy for oligoprogressive non-small cell lung cancer. Med Dosim. (2023) 48:238–44. doi: 10.1016/j.meddos.2023.05.002
48. Uijtewaal P, Borman PTS, Woodhead PL, Kontaxis C, Hackett SL, Verhoeff J, et al. First experimental demonstration of VMAT combined with MLC tracking for single and multi fraction lung SBRT on an MR-linac. Radiother Oncol. (2022) 174:149–57. doi: 10.1016/j.radonc.2022.07.004
49. Cervino LI, Du J, and Jiang SB. MRI-guided tumor tracking in lung cancer radiotherapy. Phys Med Biol. (2011) 56:3773–85. doi: 10.1088/0031-9155/56/13/003
50. Ehrbar S, Braga Kaser S, Chamberlain M, Krayenbuhl J, Wilke L, Mayinger M, et al. MR-guided beam gating: Residual motion, gating efficiency and dose reconstruction for stereotactic treatments of the liver and lung. Radiother Oncol. (2022) 174:101–8. doi: 10.1016/j.radonc.2022.07.007
51. Charters JA, Abdulkadir Y, O'Connell D, Yang Y, and Lamb JM. Dosimetric evaluation of respiratory gating on a 0.35-T magnetic resonance-guided radiotherapy linac. J Appl Clin Med Phys. (2022) 23:e13666. doi: 10.1002/acm2.13666
52. Grimbergen G, Hackett SL, van Ommen F, van Lier A, Borman PTS, Meijers LTC, et al. Gating and intrafraction drift correction on a 1.5 T MR-Linac: Clinical dosimetric benefits for upper abdominal tumors. Radiother Oncol. (2023) 189:109932. doi: 10.1016/j.radonc.2023.109932
53. Jassar H, Tai A, Chen X, Keiper TD, Paulson E, Lathuiliere F, et al. Real-time motion monitoring using orthogonal cine MRI during MR-guided adaptive radiation therapy for abdominal tumors on 1.5T MR-Linac. Med Phys. (2023) 50:3103–16. doi: 10.1002/mp.16342
54. Smith BR, St-Aubin J, and Hyer DE. Commissioning of a motion management system for a 1.5T Elekta Unity MR-Linac: A single institution experience. J Appl Clin Med Phys. (2025):e70005. doi: 10.1002/acm2.70005
55. Keall PJ, Sawant A, Berbeco RI, Booth JT, Cho B, Cervino LI, et al. AAPM Task Group 264: The safe clinical implementation of MLC tracking in radiotherapy. Med Phys. (2021) 48:e44–64. doi: 10.1002/mp.14625
56. Keall PJ, Colvill E, O'Brien R, Ng JA, Poulsen PR, Eade T, et al. The first clinical implementation of electromagnetic transponder-guided MLC tracking. Med Phys. (2014) 41:020702. doi: 10.1118/1.4862509
57. Borman PTS, Uijtewaal P, Snyder J, Allen B, Atienza CK, Woodhead P, et al. Demonstration of motion-compensated volumetric modulated arc radiotherapy on an MR-linac. Phys Imaging Radiat Oncol. (2025) 33:100729. doi: 10.1016/j.phro.2025.100729
58. Bedford JL, Fast MF, Nill S, McDonald FM, Ahmed M, Hansen VN, et al. Effect of MLC tracking latency on conformal volumetric modulated arc therapy (VMAT) plans in 4D stereotactic lung treatment. Radiother Oncol. (2015) 117:491–5. doi: 10.1016/j.radonc.2015.07.044
59. Rao SX, Lambregts DM, Schnerr RS, Beckers RC, Maas M, Albarello F, et al. CT texture analysis in colorectal liver metastases: A better way than size and volume measurements to assess response to chemotherapy? United Eur Gastroenterol J. (2016) 4:257–63. doi: 10.1177/2050640615601603
60. Fave X, Zhang L, Yang J, Mackin D, Balter P, Gomez D, et al. Delta-radiomics features for the prediction of patient outcomes in non-small cell lung cancer. Sci Rep. (2017) 7:588. doi: 10.1038/s41598-017-00665-z
61. Vrtovec T, Mocnik D, Strojan P, Pernus F, and Ibragimov B. Auto-segmentation of organs at risk for head and neck radiotherapy planning: From atlas-based to deep learning methods. Med Phys. (2020) 47:e929–50. doi: 10.1002/mp.14320
62. Liang F, Qian P, Su KH, Baydoun A, Leisser A, Van Hedent S, et al. Abdominal, multi-organ, auto-contouring method for online adaptive magnetic resonance guided radiotherapy: An intelligent, multi-level fusion approach. Artif Intell Med. (2018) 90:34–41. doi: 10.1016/j.artmed.2018.07.001
63. Hyer DE, Caster J, Smith B, St-Aubin J, Snyder J, Shepard A, et al. A technique to enable efficient adaptive radiation therapy: automated contouring of prostate and adjacent organs. Adv Radiat Oncol. (2024) 9:101336. doi: 10.1016/j.adro.2023.101336
64. Nachbar M, Lo Russo M, Gani C, Boeke S, Wegener D, Paulsen F, et al. Automatic AI-based contouring of prostate MRI for online adaptive radiotherapy. Z Med Phys. (2024) 34:197–207. doi: 10.1016/j.zemedi.2023.05.001
65. Vagni M, Tran HE, Romano A, Chiloiro G, Boldrini L, Zormpas-Petridis K, et al. Auto-segmentation of pelvic organs at risk on 0.35T MRI using 2D and 3D Generative Adversarial Network models. Phys Med. (2024) 119:103297. doi: 10.1016/j.ejmp.2024.103297
66. van de Lindt TN, Nowee ME, Janssen T, Schneider C, Remeijer P, van Pelt VWJ, et al. Technical feasibility and clinical evaluation of 4D-MRI guided liver SBRT on the MR-linac. Radiother Oncol. (2022) 167:285–91. doi: 10.1016/j.radonc.2022.01.009
67. Paulson ES, Ahunbay E, Chen X, Mickevicius NJ, Chen GP, Schultz C, et al. 4D-MRI driven MR-guided online adaptive radiotherapy for abdominal stereotactic body radiation therapy on a high field MR-Linac: Implementation and initial clinical experience. Clin Transl Radiat Oncol. (2020) 23:72–9. doi: 10.1016/j.ctro.2020.05.002
68. van de Lindt TN, Fast MF, van den Wollenberg W, Kaas J, Betgen A, Nowee ME, et al. Validation of a 4D-MRI guided liver stereotactic body radiation therapy strategy for implementation on the MR-linac. Phys Med Biol. (2021) 66(10):105010. doi: 10.1088/1361-6560/abfada
69. Kim T, Park JC, Gach HM, Chun J, and Mutic S. Technical Note: Real-time 3D MRI in the presence of motion for MRI-guided radiotherapy: 3D Dynamic keyhole imaging with super-resolution. Med Phys. (2019) 46:4631–8. doi: 10.1002/mp.13748
70. Zhao C, Shao M, Carass A, Li H, Dewey BE, Ellingsen LM, et al. Applications of a deep learning method for anti-aliasing and super-resolution in MRI. Magn Reson Imaging. (2019) 64:132–41. doi: 10.1016/j.mri.2019.05.038
71. Paganelli C, Portoso S, Garau N, Meschini G, Via R, Buizza G, et al. Time-resolved volumetric MRI in MRI-guided radiotherapy: an in silico comparative analysis. Phys Med Biol. (2019) 64:185013. doi: 10.1088/1361-6560/ab33e5
72. Rabe M, Paganelli C, Schmitz H, Meschini G, Riboldi M, Hofmaier J, et al. Continuous time-resolved estimated synthetic 4D-CTs for dose reconstruction of lung tumor treatments at a 0.35 T MR-linac. Phys Med Biol. (2023) 68(23):235008. doi: 10.1088/1361-6560/acf6f0
73. Togao O, Tsuji R, Ohno Y, Dimitrov I, and Takahashi M. Ultrashort echo time (UTE) MRI of the lung: assessment of tissue density in the lung parenchyma. Magn Reson Med. (2010) 64:1491–8. doi: 10.1002/mrm.22521
74. Dournes G, Yazbek J, Benhassen W, Benlala I, Blanchard E, Truchetet ME, et al. 3D ultrashort echo time MRI of the lung using stack-of-spirals and spherical k-Space coverages: Evaluation in healthy volunteers and parenchymal diseases. J Magn Reson Imaging. (2018) 48:1489–97. doi: 10.1002/jmri.26212
75. Palacios MA, Verheijen S, Schneiders FL, Bohoudi O, Slotman BJ, Lagerwaard FJ, et al. Same-day consultation, simulation and lung Stereotactic Ablative Radiotherapy delivery on a Magnetic Resonance-linac. Phys Imaging Radiat Oncol. (2022) 24:76–81. doi: 10.1016/j.phro.2022.09.010
76. Gungor G, Serbez I, Temur B, Gur G, Kayalilar N, Mustafayev TZ, et al. Time analysis of online adaptive magnetic resonance-guided radiation therapy workflow according to anatomical sites. Pract Radiat Oncol. (2021) 11:e11–21. doi: 10.1016/j.prro.2020.07.003
77. Parikh NR, Lee PP, Raman SS, Cao M, Lamb J, Tyran M, et al. Time-driven activity-based costing comparison of CT-guided versus MR-guided SBRT. JCO Oncol Pract. (2020) 16:e1378–85. doi: 10.1200/JOP.19.00605
78. Corradini S, Alongi F, Andratschke N, Azria D, Bohoudi O, Boldrini L, et al. ESTRO-ACROP recommendations on the clinical implementation of hybrid MR-linac systems in radiation oncology. Radiother Oncol. (2021) 159:146–54. doi: 10.1016/j.radonc.2021.03.025
79. Tanadini-Lang S, Budgell G, Bohoudi O, Corradini S, Cusumano D, Gungor G, et al. An ESTRO-ACROP guideline on quality assurance and medical physics commissioning of online MRI guided radiotherapy systems based on a consensus expert opinion. Radiother Oncol. (2023) 181:109504. doi: 10.1016/j.radonc.2023.109504
Keywords: magnetic resonance-guided radiotherapy (MRgRT), adaptive therapy, lung cancer, artificial intelligence, radiomics, lung SBRT
Citation: Ma C, Wang X, Nie K, Xiong Z, Xu K, Yue N and Zhang Y (2025) Recent technical advancements and clinical applications of MR-guided radiotherapy in lung cancer treatment. Front. Oncol. 15:1622060. doi: 10.3389/fonc.2025.1622060
Received: 02 May 2025; Accepted: 13 June 2025;
Published: 01 July 2025.
Edited by:
Kai Ding, Johns Hopkins University, United StatesReviewed by:
William Hrinivich, Johns Hopkins University, United StatesCopyright © 2025 Ma, Wang, Nie, Xiong, Xu, Yue and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yin Zhang, eXo3MThAY2luai5ydXRnZXJzLmVkdQ==
 Chi Ma
Chi Ma Xiao Wang
Xiao Wang Ke Nie
Ke Nie Zhenyu Xiong
Zhenyu Xiong Keying Xu
Keying Xu Ning Yue
Ning Yue Yin Zhang
Yin Zhang