- 1Department of Nuclear Medicine, Tianjin Cancer Hospital Airport Hospital, National Clinical Research Center for Cancer, Tianjin, China
- 2Department of Molecular Imaging and Nuclear Medicine, Tianjin Medical University Cancer Institute and Hospital, National Clinical Research Center for Cancer, Key Laboratory of Cancer Prevention and Therapy, Tianjin’s Clinical Research Center for China, Tianjin, China
Background: Radioactive iodine (RAI) is the standard treatment for distant metastatic differentiated thyroid cancer (dmDTC). However, many patients fail to achieve satisfactory effects and prognosis. Anlotinib is a highly effective antiangiogenic tyrosine kinase inhibitor (TKI) that has shown promising efficacy in RAIR-DTC patients. This study evaluated the efficacy and safety of anlotinib in combination with 131I in dmDTC.
Methods: This single-arm, phase II study was prospectively registered on the Chinese Clinical Trial Registry (ChiCTR2500095313). The key eligible criteria included patients with dmDTC who had at least one measurable metastatic lesion capable of iodine uptake and were planned to receive RAI therapy. Previous treatment with TKI was not permitted. Patients underwent a whole-body iodine scan (Rx-WBS) following iodine administration on days 3-5. When confirmed iodine uptake in metastatic lesions, anlotinib would be given at 12 mg (QD, 2 weeks on/1 week off, Q3W) initially. One combination treatment cycle consisted of 12 weeks of anlotinib and 1 dose of iodine-131. The primary endpoints were the objective response rate (ORR) and changes in thyroglobulin (Tg) levels. The secondary endpoints included disease control rate (DCR), progression-free survival (PFS), and safety.
Results: From October 2022 to September 2024, 20 patients (4 males and 16 females) with distant metastatic DTC were enrolled. All patients who had completed at least one cycle of combined treatment were eligible for data analysis. The median follow-up was 13.7 months. 11 patients achieved partial response (PR), 8 patients achieved stable disease (SD), and 1 patient had progressive disease (PD). ORR and DCR were 55.0% [95% Confidence Interval (CI): 31.5%-76.9%] and 94.7% (95% CI: 75.1%-99.9%) respectively. Median PFS was not reached. All patients achieved a biochemical response according to protocol-defined criteria, defined as a ≥25% decrease in Tg levels. Grade 3 or higher treatment-related adverse events (TRAEs) were observed in 10 (50%; most common hypertension) patients. Dose reductions of anlotinib were required in 10 (50%) patients due to AEs, and no patient discontinued treatment because of AEs. No serious adverse events (SAEs) or deaths were reported.
Conclusions: This study demonstrates the promising efficacy and safety of combining the TKI with 131I therapy, suggesting that anlotinib may be a viable option for dmDTC.
Clinical Trial Registration: https://www.chictr.org.cn/showproj.html?proj=226033, identifier ChiCTR2500095313.
1 Introduction
Radioactive iodine (RAI) remains the cornerstone treatment for distant metastatic differentiated thyroid cancer (dmDTC) (1). However, a significant proportion of patients either do not achieve a satisfactory therapeutic effect or eventually develop RAI-refractory DTC (RAIR-DTC), leading to a poorer prognosis (2). This challenge highlights the urgent need for novel therapeutic strategies to overcome RAI resistance and improve outcomes in dmDTC.
Anlotinib is a novel, multi-targeted tyrosine kinase inhibitor (TKI) that primarily functions by inhibiting angiogenesis and suppressing tumor proliferation through its actions on various targets, including VEGFR, FGFR, PDGFR, and c-Kit (3, 4). While anlotinib has demonstrated promising efficacy as a monotherapy in advanced RAIR-DTC (5), a growing body of evidence suggests a synergistic potential when TKIs are combined with RAI therapy (6).
The rationale for combining TKIs like anlotinib with RAI stems from their complementary mechanisms of action. Preclinical and clinical studies have indicated that TKIs can potentially re-differentiate RAIR-DTC cells, thereby restoring or enhancing their iodine-uptaking capability (7). This “re-sensitization” to iodine, often achieved by inhibiting pathways involved in dedifferentiation (such as MAPK or PI3K/Akt pathways, which TKIs can modulate), allows for the re-application of effective RAI therapy in previously refractory cases. Furthermore, anlotinib’s direct anti-angiogenic and anti-tumor proliferative effects can independently contribute to tumor control and potentially reduce tumor burden, creating a more favorable microenvironment for RAI uptake and efficacy (8, 9). The combination aims to leverage these distinct yet synergistic mechanisms: anlotinib addresses tumor growth and potentially enhances iodine avidity, while ¹³¹I delivers targeted cytotoxic radiation to iodine-avid cells (10, 11). This dual approach is hypothesized to provide a more comprehensive and potent anti-tumor response compared to either monotherapy alone, particularly in patients with dmDTC.
Recent efforts have begun to explore the potential benefits of TKI and RAI combination strategies. For example, a phase II clinical trial by Song et al. investigated sequential use of anlotinib followed by RAI in patients with advanced DTC, demonstrating promising efficacy and safety (6). Additionally, the ongoing prospective trial NCT04988248 is evaluating lenvatinib in combination with RAI in iodine-avid metastatic thyroid cancer (12). These studies highlight a growing interest in integrated approaches, further supporting the rationale for our investigation.
Building upon this compelling preclinical and early clinical evidence, this study aimed to further evaluate the efficacy and safety of anlotinib in combination with ¹³¹I therapy in patients with distant metastatic differentiated thyroid cancer, with the goal of identifying a novel and effective treatment paradigm for this challenging patient population.
2 Materials and methods
2.1 Study design
This was a single-arm, exploratory phase II trial designed to evaluate the preliminary efficacy and safety of anlotinib combined with radioiodine-131 (¹³¹I) in dmDTC patients. The rationale for combining anlotinib and ¹³¹I in this study was based on both biological plausibility and unmet clinical need. Anlotinib, a novel multi-target TKI, has been approved in China for the treatment of RAIR-DTC, yet its potential to enhance or restore RAI uptake in iodine-avid metastatic lesions remains underexplored. Unlike traditional studies that focus solely on TKI monotherapy in RAIR-DTC, this trial was specifically designed to evaluate whether the sequential combination of anlotinib and RAI could offer synergistic clinical benefits in patients with measurable, iodine-avid metastatic lesions.
In this study, we strictly required evidence of iodine avidity before initiating anlotinib therapy, thereby ensuring that each enrolled lesion had the biological potential to respond to RAI. The treatment protocol involving a 12-week course of anlotinib per cycle was determined based on prior pharmacokinetic and clinical tolerability data from the ALTER01032 study. In addition, our dose-adjustment strategy for anlotinib followed the manufacturer’s guidelines and clinical best practices, allowing sequential reductions (12 → 10 → 8 mg) based on adverse event severity and patient tolerance. All efficacy assessments followed Response Evaluation Criteria In Solid Tumors (RECIST) 1.1, and biochemical response was evaluated using Tg level reductions as a surrogate for residual tumor activity.
The sample size of 20 patients was not based on formal power calculations but was determined according to feasibility considerations and in alignment with similar early-phase studies in this population. The results of this trial are intended to inform the design of future large-scale randomized studies. The study adhered to Good Clinical Practice (GCP) guidelines and the ethical principles of the 2013 Declaration of Helsinki. It was approved by the Institutional Review Board (IRB) of Tianjin Cancer Hospital Airport Hospital (approval JS-2022-0047), which provided ongoing ethical oversight. All study procedures were conducted after obtaining written approval. The study is registered on the Chinese Clinical Trial Registry (ChiCTR, Identifier: ChiCTR2500095313). It can be accessed at: https://www.chictr.org.cn/showproj.html?proj=226033. Written informed consent was obtained from all participants before study initiation, confirming their voluntary participation and understanding of the study objectives, procedures, and potential risks and benefits.
2.2 Patients
Patients aged 18–70 years who had histologically or radiologically confirmed DTC with distant metastasis following total or subtotal thyroidectomy, with at least one measurable metastatic lesion [according to RECIST version 1.1] capable of iodine uptake and were planned to RAI therapy, and Eastern Cooperative Oncology Group performance status (ECOG PS) of 0 – 1, and adequate bone marrow, renal, hepatic, and cardiac function. Previous treatments with VEGFR TKIs, such as anlotinib, vandetanib, cabozantinib, lenvatinib, sorafenib, and RET inhibitors like pralsetinib, and selpercatinib, were not permitted. Patients who have received other localized antitumor therapy within the last 3 months, or who are scheduled to undergo systemic antitumor therapy external irradiation, or other interventions during the period of dosing in this study will not be allowed to enroll, including cytotoxic therapies, immunotherapies. Patients with factors that may affect the administration of oral medications (e.g., inability to swallow), severe and/or uncontrolled diseases, participated in other anti-tumor drug clinical trials within 3 months, or are currently enrolled in other clinical trials were not included in this study.
2.3 Study treatment
RAI therapy was administered using a fixed-dose protocol: 200 mCi (7.4 GBq) for pulmonary metastases and 250 mCi (9.25 GBq) for bone metastases (It should be noted that all enrolled patients had concomitant pulmonary and/or bone metastases). Patients underwent a whole-body iodine scan (Rx-WBS) following iodine administration on day 3-5. After confirmation of iodine uptake in metastatic lesions, anlotinib therapy was initiated. Patients initially received anlotinib at 12 mg (QD, 2 weeks on/1 week off, 3 weeks for 1 cycle). Each combined treatment cycle comprised 1 dose iodine-131 and a 12-week course of anlotinib treatment until disease progression, intolerability, non-iodine-avid metastatic lesions, or withdrawal from the study. Anlotinib may be administered at reduced doses of 10 mg/day and 8 mg/day sequentially if the patient experiences an intolerable adverse event. If more than 2 dose levels need to be reduced, treatment would be terminated.
2.4 Study endpoints
The primary endpoints were the ORR [defined as the number of patients achieving an overall best response of complete response (CR) or partial response (PR) divided by the total number of patients]) and biochemical response (BRR defined as a decreased Tg level ≥ 25%). The secondary endpoints included disease control rate (DCR), median progression-free survival (mPFS), and safety. Adverse events (AEs) were evaluated according to National Cancer Institute Common Terminology Criteria for Adverse Events version 5.0 (NCI-CTCAE 5.0). The efficacy assessment is performed every 12 weeks, according to RECIST 1.1 criteria.
2.5 Statistical analyses
All patients who had completed at least one combined treatment cycle (the intent-to-treat population) were included in the Full Analysis Set (FAS). The primary efficacy analyses would be based on FAS. The Safety Analysis Set (SAS) included all patients who had completed at least one combined treatment cycle. The ORR was calculated based on the best response and is presented with 2-sided 95% Clopper and Pearson confidence intervals (CIs). Patient demographic data and safety parameters were summarized using descriptive statistics. Median PFS and overall survival are presented with 2-sided 95% CIs and were calculated using the Kaplan-Meier method and plotted over time after therapy. Clinical statistical analyses were performed using SAS version 9.4.
3 Results
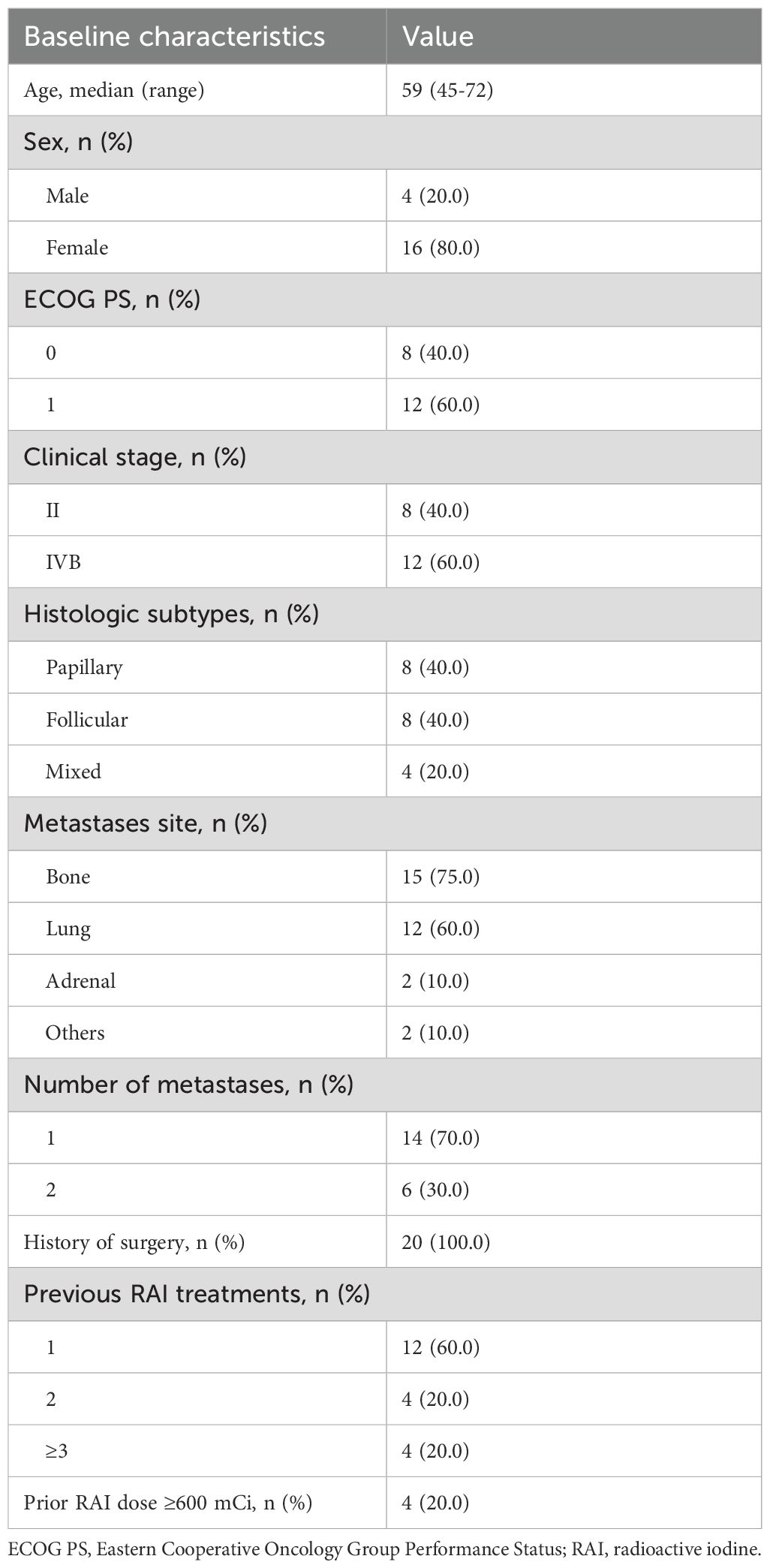
3.1 Patient characteristics
From October 2022 to September 2024, 27 patients were screened, and 7 were excluded because they did not meet the inclusion criteria. Finally, 20 patients (4 males and 16 females) with distant metastatic DTC were enrolled. All patients who had completed at least one cycle of combined treatment were eligible for data analysis. The baseline clinical characteristics of the patients are shown in Table 1. The median patient age was 59 years and had a baseline Eastern Cooperative Oncology Group performance status of 1 (60%). Forty percent of the patients have PTC,40%have FTC, and the remaining 20% have mixed cell carcinoma. The most common sites of distant metastases were bone (75%) and lung (60%). At the time of data cutoff (January 14, 2025), the median duration of follow-up was 13.7 months. Twelve patients had exited the study group. Among them, 7 patients stopped iodine treatment due to non-iodine-avid metastatic lesions, and 5 of these patients continued with anlotinib monotherapy. Four patients stopped iodine treatment due to personal preference, and 3 of these patients continued with anlotinib monotherapy. One patient received other treatments due to new lesions. Eight patients continued on treatment.
3.2 Efficacy
Within the ITT population, none of the cases achieved a complete response. Eleven patients achieved PR, 8 achieved SD and 1 had PD because of new pulmonary lesions. ORR and DCR were 55.0% (95%CI: 31.5%-76.9%) and 94.7% (95%CI: 75.1%-99.9%) respectively. A waterfall plot of each efficacy-evaluable patient’s maximum percentage tumor change in the sum of the greatest dimensions from baseline to postbaseline nadir is provided in Figure 1, and the changes in each evaluation are shown in Figure 2. Median PFS was not reached at the cut-off date (Figure 3). After completing a full treatment cycle, all patients were observed to have achieved biochemical response, defined as a decrease in Tg levels of ≥25%. Eighteen patients (90%) experienced a Tg decrease of more than 50%. ORR for subgroups is shown in Figure 4.
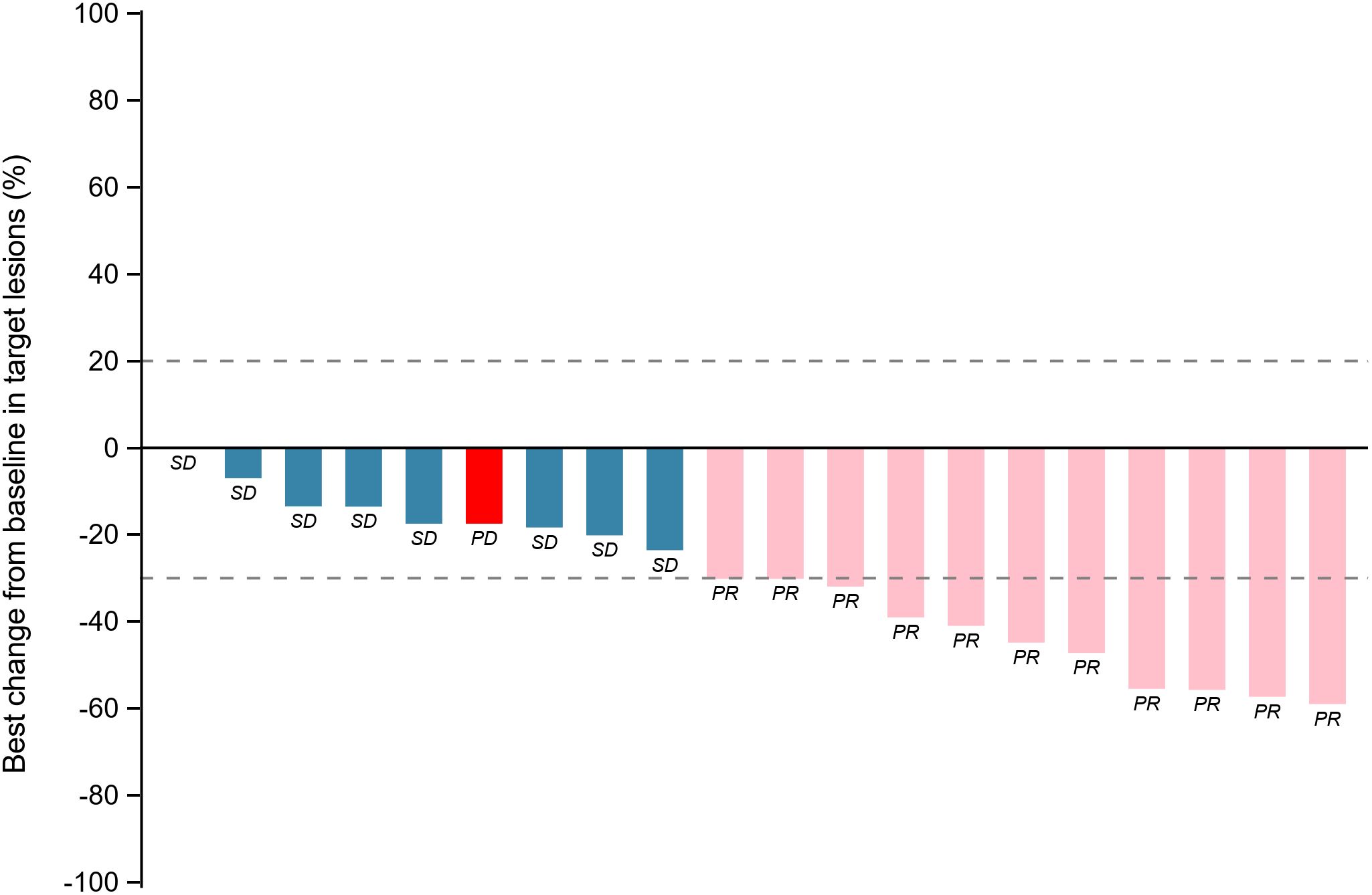
Figure 1. Tumor response assessment: maximum percentage change in target lesions from baseline by patient. Waterfall plot depicting the maximum percentage change in target lesion size from baseline for each patient. Bars are color-coded to indicate the best overall response. Blue: Stable disease (SD, change within −30% to +20%). Pink: Partial response (PR, ≥−30% decrease). Red: Progressive disease (PD, ≥+20% increase or new lesions). PR: partial response; SD: stable disease; PD: progressive disease.
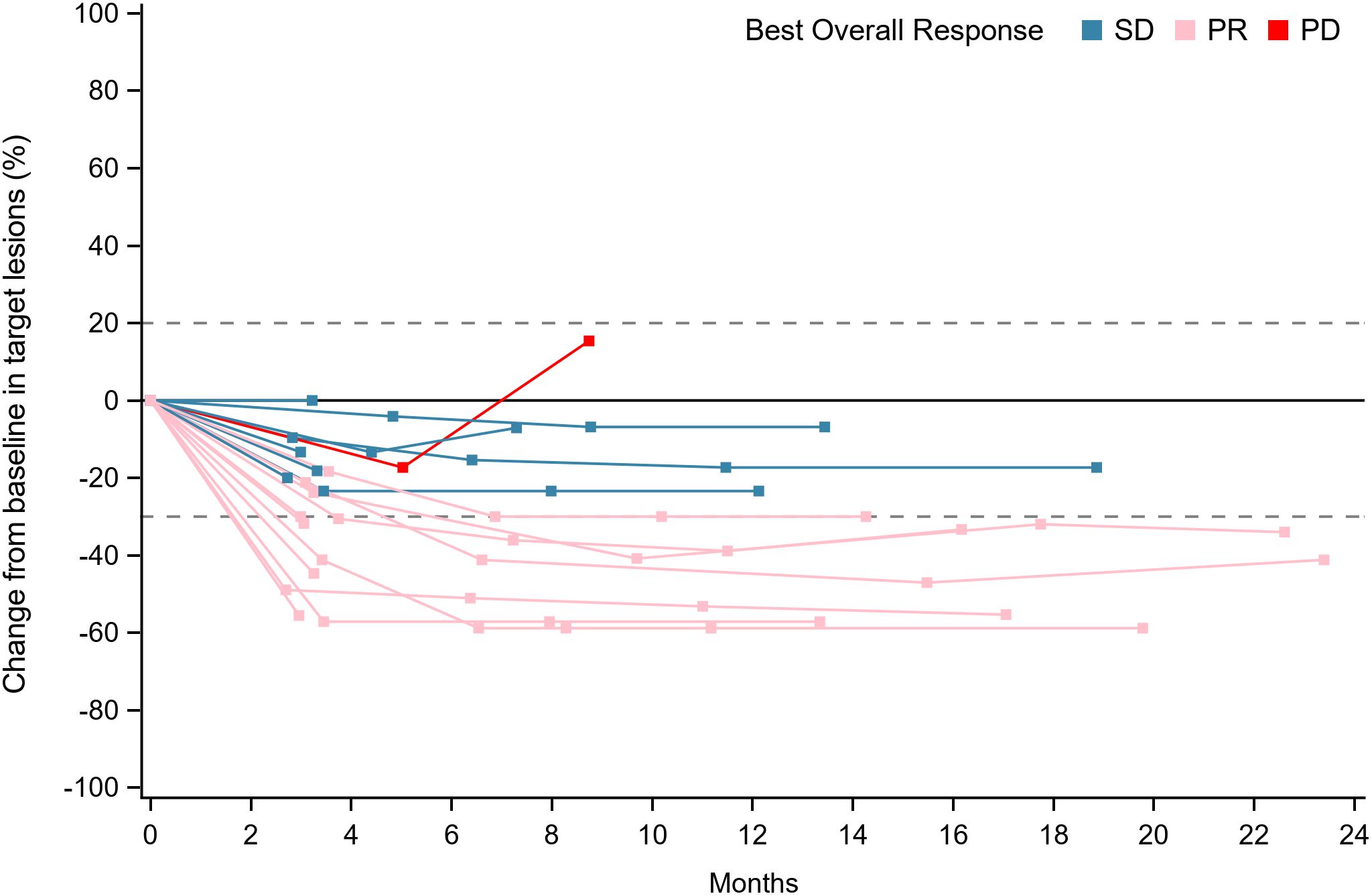
Figure 2. Longitudinal evaluation of tumor response categories over time. Swimmer’s plot showing individual patient responses (SD, PR, or PD) across the study duration (0–24 months). Each horizontal line represents a patient, with segments colored by response category. Blue: Stable disease (SD, change within −30% to +20%). Pink: Partial response (PR, ≥−30% decrease). Red: Progressive disease (PD, ≥+20% increase or new lesions). PR, partial response; SD, stable disease; PD, progressive disease.
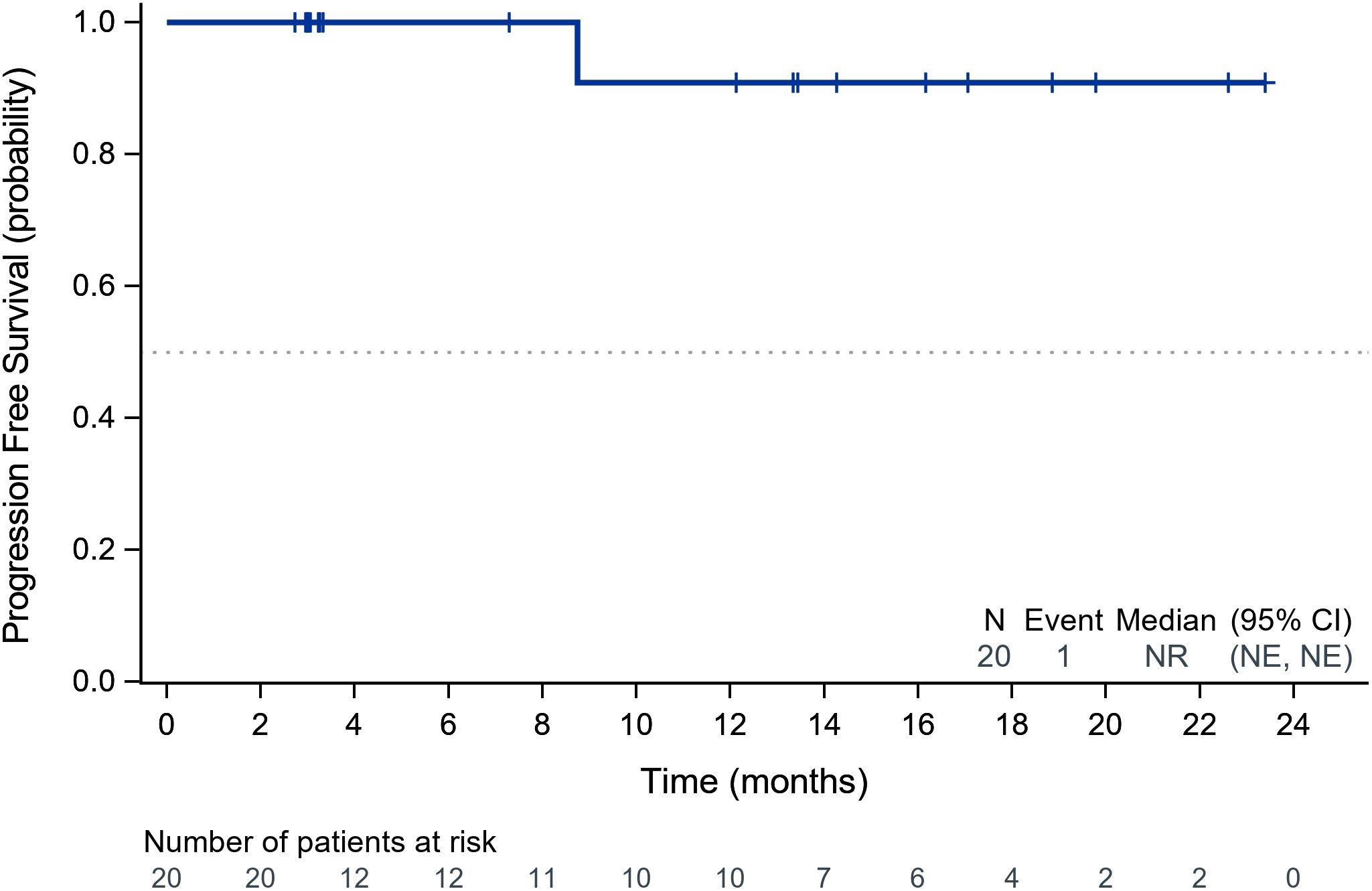
Figure 3. Kaplan-Meier analysis of progression-free survival in the study cohort. Kaplan-Meier curve illustrating PFS probability over time (months). The dashed line indicates the median PFS (95% CI). Numbers below the graph represent patients at risk at each timepoint. PFS, progression-free survival; CI, confidence interval.
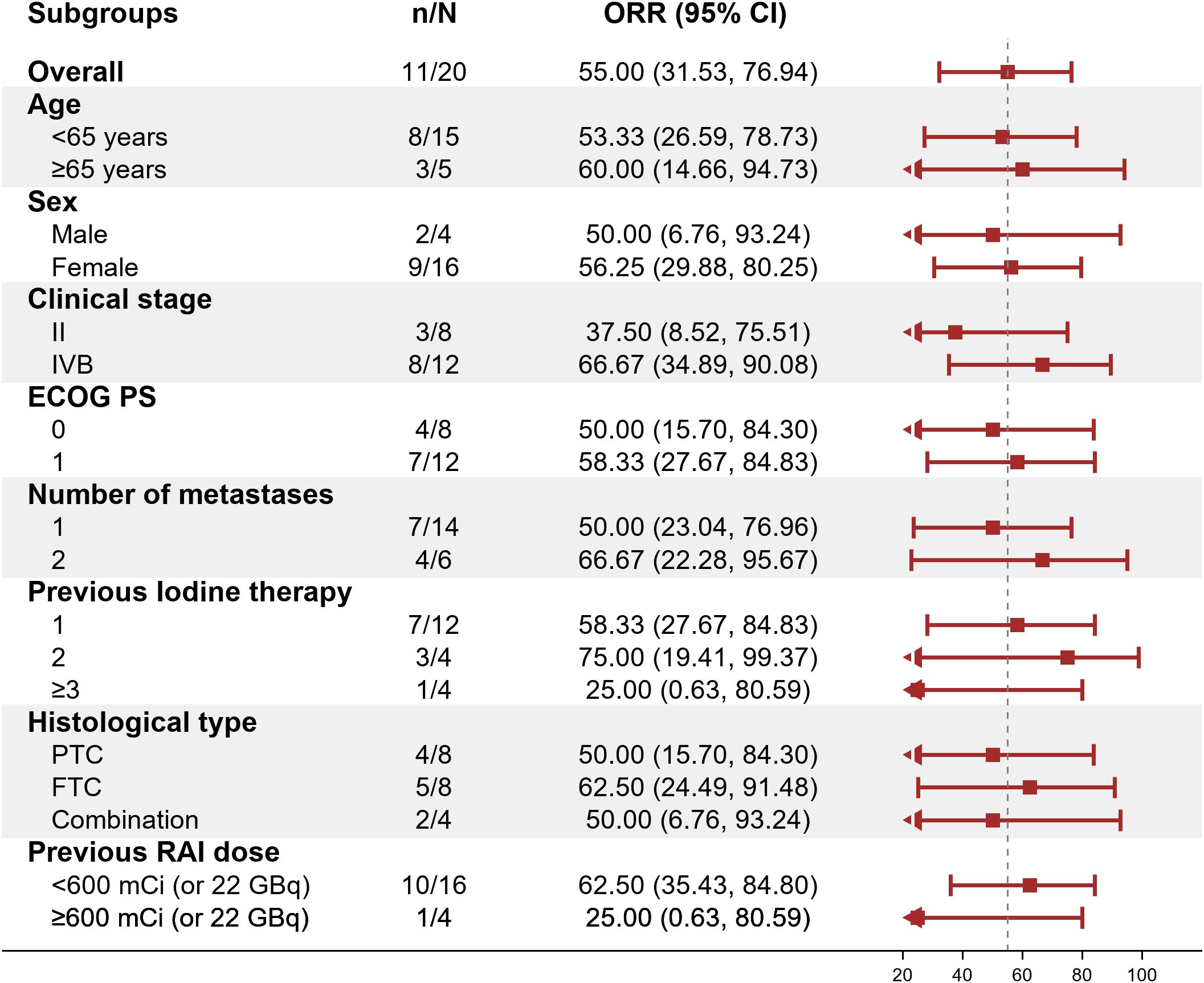
Figure 4. Objective response rate (ORR) across subgroups. Forest plot displaying ORR (95% CI) for prespecified subgroups (e.g., age, sex, clinical stage). The dashed vertical line marks the overall ORR (55%). ORR, objective response rate; CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group Performance Status; PTC, papillary thyroid carcinoma; FTC, follicular thyroid carcinoma; RAI, radioactive iodine.
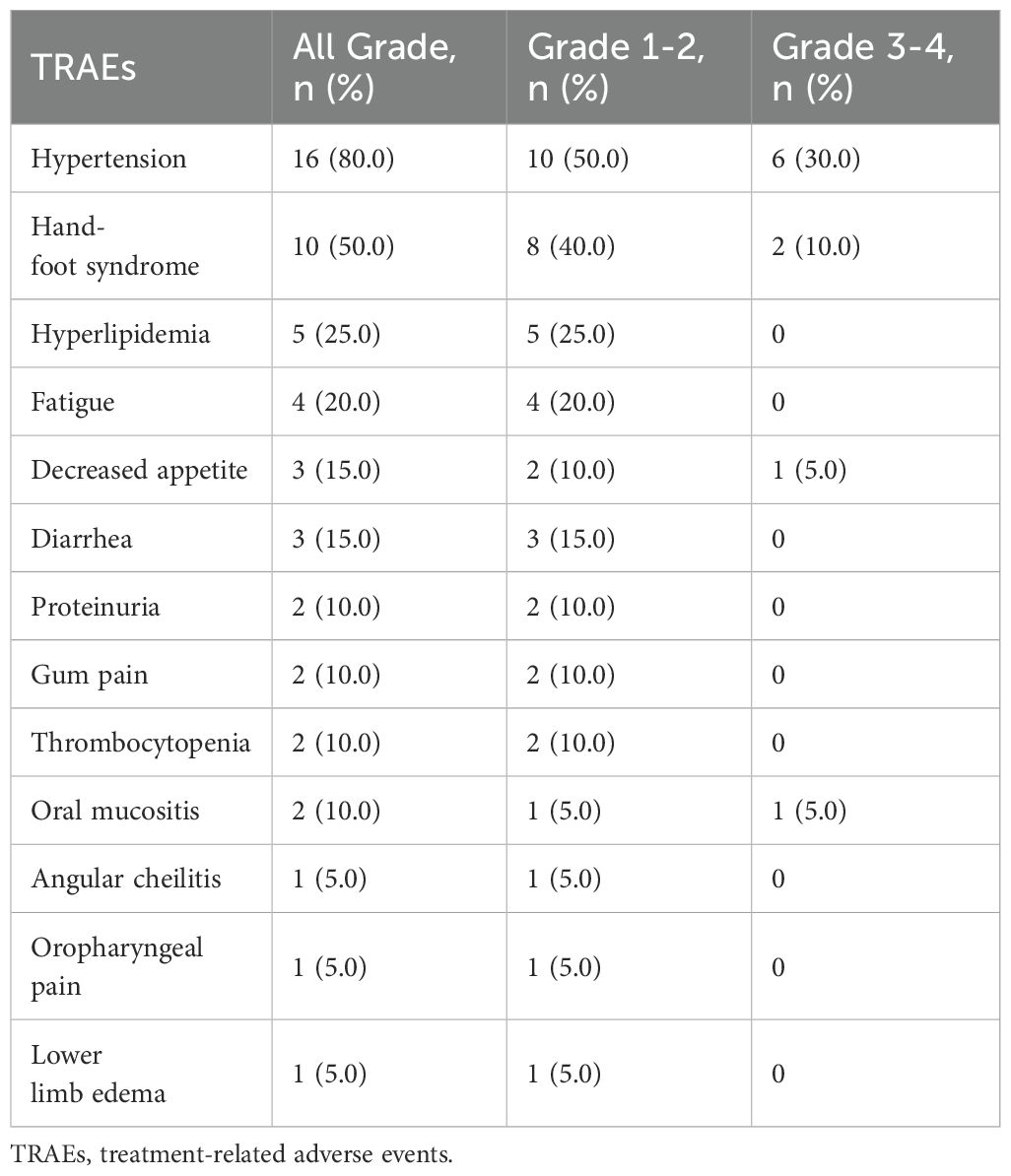
3.3 Safety
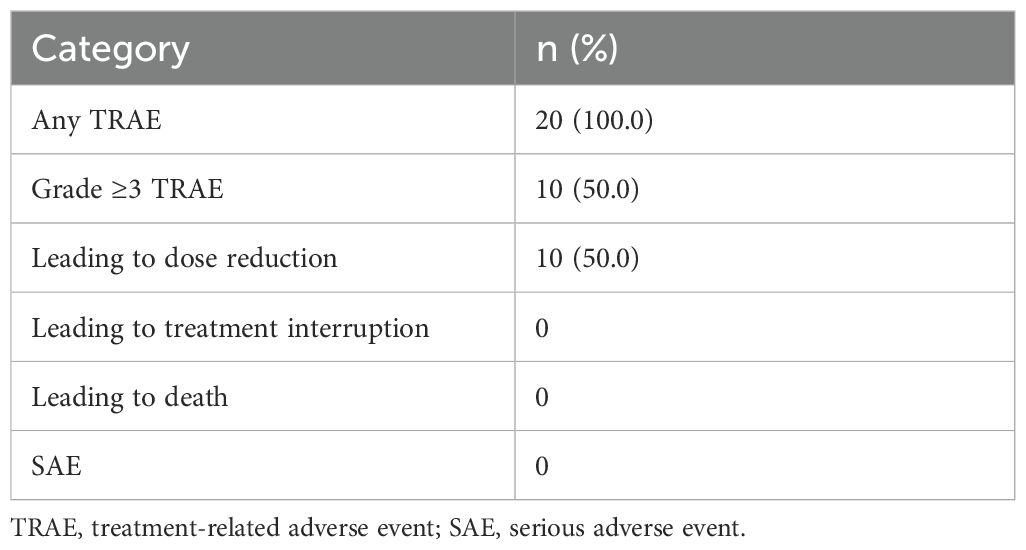
All patients experienced at least 1 treatment-related adverse events (TRAEs), and the most common TRAE was hypertension, followed by hand-foot syndrome (Tables 2, 3). Grade 3 or higher TRAEs were observed in 10 (50%) patients. Dose reductions of anlotinib were necessitated in 10 (50%) patients due to AEs, and no patient discontinued treatment because of AEs. No serious adverse events (SAEs) or deaths were reported.
4 Discussion
The prognosis of DTC markedly worsens with distant metastases, with 10-year survival rates decreasing from over 95% for localized disease to approximately 50% for metastatic cases (13). The lungs and bones are the most common metastatic sites, with bone metastases associated with poorer outcomes compared to pulmonary involvement (2, 14, 15). According to the 2015 American Thyroid Association (ATA) guidelines, RAI therapy is recommended for iodine-avid metastatic lesions and can be repeated when objective clinical benefits are demonstrated (1). However, durable complete remission remains rare—only 6.8% of metastatic DTC patients achieve disease-free status following RAI (16). Thies et al. (17) reported that patients requiring more than 22.2 GBq of ¹³¹I to achieve remission had comparable long-term outcomes to those who failed to achieve remission, highlighting the limitations of RAI monotherapy.
Approximately one-third of patients with distant metastases eventually lose their ability to concentrate RAI, progressing to RAIR-DTC (18). In this context, combining RAI with targeted therapies has gained increasing attention. Our study aimed to evaluate the efficacy and safety of combining anlotinib with ¹³¹I in patients with iodine-avid advanced DTC.
To contextualize our findings, we compared them with previous clinical trials involving anlotinib and other TKIs. The pivotal phase III ALTER0103 trial demonstrated that anlotinib monotherapy achieved an ORR of 59.21% and a DCR of 97.37% in patients with RAIR-DTC (5). Although our study design differed—our cohort included iodine-avid patients, and we utilized combination therapy rather than monotherapy—the observed ORR of 55% and DCR of 94.7% support a potential synergistic effect between anlotinib and RAI, particularly in patients who still retain iodine avidity. Importantly, our study was not designed as a direct comparator to anlotinib monotherapy, and further randomized controlled trials are needed to confirm these observations.
Lenvatinib and sorafenib, two multi-targeted TKIs, have been approved for the treatment of RAIR-DTC and represent foundational systemic options in this setting (3, 19). Although we did not directly compare our combination regimen to these agents, our findings contribute to the expanding evidence base supporting the role of TKIs in advanced thyroid cancer, while also highlighting the feasibility and potential advantages of integrating traditional RAI therapy with modern targeted agents. The broad inhibitory profile of anlotinib—targeting VEGFR, FGFR, PDGFR, and c-Kit—may play a role in enhancing the efficacy of this combination.
The rationale for combining anlotinib with RAI stems from their complementary mechanisms of action. RAI efficacy depends on adequate sodium-iodide symporter (NIS) expression for tumor uptake. However, in advanced or metastatic DTC, NIS expression is often downregulated due to activation of the mitogen-activated protein kinase (MAPK) and phosphatidylinositol-3-kinase (PI3K)/protein kinase B (AKT) pathways (20, 21). Anlotinib has been shown not only to inhibit angiogenesis via VEGFR2/3 but also to indirectly modulate the MAPK signaling cascade (4, 5), potentially restoring NIS function and enhancing RAI uptake in partially dedifferentiated tumors.
Other targeted therapies, such as selumetinib and dabrafenib, have also demonstrated the ability to restore RAI sensitivity through MAPK inhibition in both preclinical and early-phase clinical studies (22–24). Our approach differed in that we applied anlotinib after confirming iodine avidity, rather than using it as a redifferentiation agent. This strategy reflects a pragmatic and safer clinical design that aims to potentiate RAI responsiveness while minimizing the risk of overtreatment and drug-related toxicities.
The results of the ASTRA trial, a randomized phase III study, did not demonstrate significant benefit from using selumetinib prior to RAI in high-risk DTC patients after surgery (CR rate: 40% vs. 38%, P = 0.82) (25). In contrast, our patient selection focused exclusively on those with iodine-avid lesions, potentially avoiding premature systemic therapy and preserving RAI efficacy. This approach may partly explain the higher ORR (55%) and biochemical response observed in our cohort.
Our findings are also consistent with prior phase II studies that explored the combination of TKIs and RAI. In one such study, 16 patients with locally advanced or metastatic DTC received anlotinib (12 mg QD, 2 weeks on/1 week off) for four cycles. At week 13, diagnostic ¹³¹I whole-body scans (Dx-WBS) identified eight patients with iodine-avid lesions, who then underwent ¹³¹I treatment. Among them, the ORR was 33.3% and DCR was 83.3%, with biochemical responses observed in all patients (6). Similarly, Shi et al. (26) reported significant tumor reduction and SUVmax decline in five patients treated with apatinib and RAI. Song et al. also reported an ORR of 33.3% and complete biochemical responses following sequential anlotinib and RAI therapy (6). Additionally, an ongoing prospective trial (NCT04988248) is expected to provide further insights into the synergistic potential of lenvatinib and RAI in iodine-avid metastatic DTC (12).
Our study further explored the impact of cumulative RAI dosage on treatment efficacy. Patients with cumulative doses >600 mCi exhibited a lower ORR (25%) compared to those with <600 mCi (62.5%), suggesting that prolonged RAI exposure may reduce treatment sensitivity. We retrospectively reviewed 35 patients from our center who continued RAI therapy after exceeding 600 mCi. Although most lesions remained iodine-avid, none achieved radiological complete response. Only 14% (5/35) achieved >50% decline in thyroglobulin (Tg) levels, highlighting the limited efficacy of continued RAI beyond a certain threshold (unpublished data). These findings underscore the need to identify optimal timing for transitioning from RAI to systemic therapy.
Importantly, the combination of anlotinib with RAI was well-tolerated, with no additional adverse events observed compared to monotherapies. This indicates the safety and clinical feasibility of the combined regimen for appropriately selected patients with advanced, iodine-avid DTC.
This study, however, has several limitations. First, the single-arm design of this study precludes causal inference regarding the efficacy of anlotinib in combination with ¹³¹I. In the absence of a control group, it is not possible to rule out the influence of natural disease evolution or other external variables. This limitation restricts the interpretation of treatment effect size and hinders direct comparison with standard therapies. Future randomized controlled trials are necessary to confirm these preliminary findings and establish the causal relationship between the intervention and clinical outcomes. Second, the relatively small sample size (n=20) restricts the statistical power of our findings and may limit the generalizability of the results. It also means that rare adverse events might not have been captured, and some observed effects might be subject to greater variability. Finally, we also acknowledge that the relatively short follow-up period limits our ability to assess long-term outcomes, such as overall survival (OS), and the long-term safety profile of this combination therapy. We have also clearly stated that these limitations underscore the need for larger, randomized, controlled Phase III trials with longer follow-up periods to validate our preliminary findings and provide more robust evidence for the anlotinib-¹³¹I combination.
5 Conclusion
This phase II study demonstrates promising efficacy and manageable toxicity of anlotinib combined with ¹³¹I in distant metastatic DTC. Randomized studies are essential to confirm these findings and explore more therapeutic options for patients with distant metastatic DTC.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Tianjin Cancer Hospital Airport Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
YL: Writing – review & editing, Conceptualization, Writing – original draft. JL: Data curation, Writing – review & editing. QS: Writing – review & editing, Methodology. XL: Writing – review & editing, Software. ZheY: Data curation, Writing – review & editing. ZhaY: Investigation, Writing – review & editing. JF: Writing – review & editing, Resources. YZ: Writing – review & editing, Validation. LT: Visualization, Writing – review & editing. FY: Supervision, Writing – review & editing. DD: Funding acquisition, Writing – review & editing, Project administration.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by Beijing Kangmeng Charitable Foundation (KM222009).
Acknowledgments
We sincerely thank all the patients and their families for their support and cooperation. We are grateful to the radioactive iodine therapy team at Tianjin Cancer Hospital Airport Hospital for their professional guidance and assistance during the study. We also appreciate the contributions of our colleagues at Tianjin Medical University Cancer Hospital in data collection and analysis.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 american thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the american thyroid association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. (2016) 26:1–133. doi: 10.1089/thy.2015.0020
2. Durante C, Haddy N, Baudin E, Leboulleux S, Hartl D, Travagli JP, et al. Long-term outcome of 444 patients with distant metastases from papillary and follicular thyroid carcinoma: benefits and limits of radioiodine therapy. J Clin Endocrinol Metab. (2006) 91:2892–99. doi: 10.1210/jc.2005-2838
3. Silaghi H, Lozovanu V, Georgescu CE, Pop C, Nasui BA, Catoi AF, et al. State of the art in the current management and future directions of targeted therapy for differentiated thyroid cancer. Int J Mol Sci. (2022) 23(7):3470. doi: 10.3390/ijms23073470
4. Shen G, Zheng F, Ren D, Du F, Dong Q, Wang Z, et al. Anlotinib: a novel multi-targeting tyrosine kinase inhibitor in clinical development. J Hematol Oncol. (2018) 11:120. doi: 10.1186/s13045-018-0664-7
5. Chi Y, Zheng X, Zhang Y, Shi F, Cheng Y, Guo Z, et al. Anlotinib in locally advanced or metastatic radioiodine-refractory differentiated thyroid carcinoma: A randomized, double-blind, multicenter phase II trial. Clin Cancer Res. (2023) 29:4047–56. doi: 10.1158/1078-0432.CCR-22-3406
6. Song H, Qiu X, He Z, and Chen L. The efficacy and safety of sequential use of anlotinib and 131i for locally advanced or metastatic differentiated thyroid cancer: A single-arm phase ii clinical trial. J Clin Oncol. (2023). doi: 10.1200/JCO.2023.41.16_suppl.e18102
7. Hiromasa T, Wakabayashi H, Watanabe S, Yamase T, and Kinuya S. Enhancing radioactive iodine (RAI) incorporation in RAI-refractory differentiated thyroid cancer: current insights. Eur Thyroid J. (2025) 14(2):e240319. doi: 10.1530/ETJ-24-0319
8. Lu Y, Han X, Zhang H, Zheng L, and Li X. Multi-omics study on the molecular mechanism of anlotinib in regulating tumor metabolism. Eur J Pharmacol. (2024) 975:176639. doi: 10.1016/j.ejphar.2024.176639
9. Lin B, Song X, Yang D, Bai D, Yao Y, and Lu N. Anlotinib inhibits angiogenesis via suppressing the activation of VEGFR2, PDGFRbeta and FGFR1. Gene. (2018) 654:77–86. doi: 10.1016/j.gene.2018.02.026
10. Li S and Wang H. Research progress on mechanism and management of adverse drug reactions of anlotinib. Drug Des Devel Ther. (2023) 17:3429–37. doi: 10.2147/DDDT.S426898
11. Wang G, Sun M, Jiang Y, Zhang T, Sun W, Wang H, et al. Anlotinib, a novel small molecular tyrosine kinase inhibitor, suppresses growth and metastasis via dual blockade of VEGFR2 and MET in osteosarcoma. Int J Cancer. (2019) 145:979–93. doi: 10.1002/ijc.32180
12. Sharma S, Nazar AH, Mishra P, Ora M, Arya A, Barai S, et al. A new therapeutic paradigm: radioiodine combined with lenvatinib for radioiodine-avid metastatic well-differentiated thyroid cancer. Nucl Med Commun. (2025) 46:605–12. doi: 10.1097/MNM.0000000000001980
13. Zhang S, Zhu M, Zhang H, Liu H, Fan X, Zhang J, et al. The effect of radioiodine therapy on the prognosis of differentiated thyroid cancer with lung metastases. Biomedicines. (2024) 12(3):532. doi: 10.3390/biomedicines12030532
14. Lang BH, Wong KP, Cheung CY, Wan KY, and Lo C. Evaluating the prognostic factors associated with cancer-specific survival of differentiated thyroid carcinoma presenting with distant metastasis. Ann Surg Oncol. (2013) 20:1329–35. doi: 10.1245/s10434-012-2711-x
15. Sampson E, Brierley JD, Le LW, Rotstein L, and Tsang RW. Clinical management and outcome of papillary and follicular (differentiated) thyroid cancer presenting with distant metastasis at diagnosis. Cancer. (2007) 110:1451–56. doi: 10.1002/cncr.22956
16. Aziz A, Khan SA, Suchal ZA, and Islam N. Clinicopathological characteristics and treatment outcome of patients with metastatic differentiated thyroid cancer. Indian J Endocrinol Metab. (2022) 26:137–40. doi: 10.4103/ijem.ijem_455_21
17. Thies E, Tanase K, Maeder U, Luster M, Buck AK, Hanscheid H, et al. The number of 131I therapy courses needed to achieve complete remission is an indicator of prognosis in patients with differentiated thyroid carcinoma. Eur J Nucl Med Mol Imaging. (2014) 41:2281–90. doi: 10.1007/s00259-014-2851-2
18. Chai J, Zhang R, Zheng W, Zhang G, Jia Q, Tan J, et al. Predictive value of clinical and pathological characteristics for metastatic radioactive iodine-refractory differentiated thyroid carcinoma: A 16-year retrospective study. Front Endocrinol (Lausanne). (2022) 13:930180. doi: 10.3389/fendo.2022.930180
19. Capdevila J, Krajewska J, Hernando J, Robinson B, Sherman SI, Jarzab B, et al. Increased progression-free survival with cabozantinib versus placebo in patients with radioiodine-refractory differentiated thyroid cancer irrespective of prior vascular endothelial growth factor receptor-targeted therapy and tumor histology: A subgroup analysis of the COSMIC-311 study. Thyroid. (2024) 34:347–59. doi: 10.1089/thy.2023.0463
20. Aashiq M, Silverman DA, Na’Ara S, Takahashi H, and Amit M. Radioiodine-refractory thyroid cancer: molecular basis of redifferentiation therapies, management, and novel therapies. Cancers (Basel). (2019) 11(9):1382. doi: 10.3390/cancers11091382
21. Durante C, Puxeddu E, Ferretti E, Morisi R, Moretti S, Bruno R, et al. BRAF mutations in papillary thyroid carcinomas inhibit genes involved in iodine metabolism. J Clin Endocrinol Metab. (2007) 92:2840–43. doi: 10.1210/jc.2006-2707
22. Dunn LA, Sherman EJ, Baxi SS, Tchekmedyian V, Grewal RK, Larson SM, et al. Vemurafenib redifferentiation of BRAF mutant, RAI-refractory thyroid cancers. J Clin Endocrinol Metab. (2019) 104:1417–28. doi: 10.1210/jc.2018-01478
23. Rothenberg SM, McFadden DG, Palmer EL, Daniels GH, and Wirth LJ. Redifferentiation of iodine-refractory BRAF V600E-mutant metastatic papillary thyroid cancer with dabrafenib. Clin Cancer Res. (2015) 21:1028–35. doi: 10.1158/1078-0432.CCR-14-2915
24. Ho AL, Grewal RK, Leboeuf R, Sherman EJ, Pfister DG, Deandreis D, et al. Selumetinib-enhanced radioiodine uptake in advanced thyroid cancer. N Engl J Med. (2013) 368:623–32. doi: 10.1056/NEJMoa1209288
25. Ho AL, Dedecjus M, Wirth LJ, Tuttle RM, Inabnet WBR, Tennvall J, et al. Selumetinib plus adjuvant radioactive iodine in patients with high-risk differentiated thyroid cancer: A phase III, randomized, placebo-controlled trial (ASTRA). J Clin Oncol. (2022) 40:1870–78. doi: 10.1200/JCO.21.00714
Keywords: anlotinib, 131I therapy, distant metastatic differentiated thyroid cancer, efficacy, safety
Citation: Li Y, Liu J, Su Q, Liu X, Yang Z, Yang Z, Fu J, Zhang Y, Tong L, Yang F and Dai D (2025) Efficacy and safety of anlotinib in combination with ¹³¹I therapy in the treatment of distant metastatic differentiated thyroid cancer: a single-arm, phase II study. Front. Oncol. 15:1622676. doi: 10.3389/fonc.2025.1622676
Received: 04 May 2025; Accepted: 30 June 2025;
Published: 14 July 2025.
Edited by:
Daniela Vrinceanu, Carol Davila University of Medicine and Pharmacy, RomaniaReviewed by:
Beatrice Anghel, Carol Davila University of Medicine and Pharmacy, RomaniaAlina Ghazou, Jordan University of Science and Technology, Jordan
Copyright © 2025 Li, Liu, Su, Liu, Yang, Yang, Fu, Zhang, Tong, Yang and Dai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dong Dai, ZG9uZ2RhaUB0bXUuZWR1LmNu
 Yan Li
Yan Li Jianjing Liu
Jianjing Liu Qian Su2
Qian Su2 Dong Dai
Dong Dai