- Hepatic Disease and Oncology Minimally Invasive Interventional Center, Beijing Youan Hospital, Capital Medical University, Beijing, China
Background & Aims: Recombinant human adenovirus type 5 (H101) was demonstrated to enhance the efficacy of transcatheter arterial embolization (TAE) for hepatocellular carcinoma (HCC). This study aims to analyze the efficacy and safety of oncolytic adenovirus H101 combined with TAE sequential thermal ablation in HCC.
Methods: This single-center retrospective study evaluated the progression-free survival (PFS) and overall survival (OS) of HCC patients who received H101 combined with TAE sequential thermal ablation therapy from July 2015 to January 2022. Adverse reactions during treatment were recorded.
Results: A total of 55 HCC patients were included, with a median follow-up of 39 months (range: 10–106 months). During the follow-up period, disease progression was observed in 43 of 55 patients, and 22 of the 55 patients died. The median OS and PFS time were 77 and 12.3 months, respectively. The one-, two-, and three-year OS rates were 94.5%, 86.3%, and 77.3%, respectively. The PFS rates at one, two and three years were 56.4%, 26.3% and 20%, respectively. Multivariate analysis revealed that the diameter was independent predictors of PFS (P = 0.023). No patient experienced a serious adverse event, or a fatal or disabling event, due to the injection of oncolytic virus.
Conclusion: This study suggests that HI01 combined with TAE sequential thermal ablation is both safe and effective for HCC, warranting further investigation through prospective randomized controlled trials.
Introduction
According to Global Cancer Statistics 2022, primary liver cancer (PLC), which remains the third most lethal cancer among all malignant tumors, accounts for 7.8% of all cancer - related deaths, with hepatocellular carcinoma (HCC) making up 75 - 85% of PLC cases (1). Although traditional treatment methods such as hepatectomy, liver transplantation, transcatheter arterial chemoembolization (TACE), radiofrequency ablation (RFA) and microwave ablation (MWA) have been widely used, due to the high heterogeneity and immune escape mechanisms of HCC, a single treatment modality often fails to achieve a durable treatment effect. Moreover, it is important to recognize that the rate of local tumor progression in HCC remains high when relying on a single treatment modality such as surgery, ablation, or TACE. This underscores the necessity for a multimodal and personalized approach in HCC management to enhance patient outcomes and reduce the risk of tumor recurrence and progression (2, 3). TACE is primarily used for HCC cases that are considered inoperable or for patients who are not suitable candidates for surgical resection. Nevertheless, TACE alone often exhibits limited tumor necrosis and is prone to recurrence in residual lesions (4). The combination of TACE and ablation has demonstrated a synergistic effect, leading to more extensive tumor necrosis and improved survival rates compared to monotherapy (5–8). A retrospective study conducted at our institution demonstrated that the 5-year recurrence rate in patients treated with a combination of TACE and ablation was still as high as 80% (9). It is therefore necessary to optimize the current routine treatment of HCC and explore new treatment strategies.
As a novel tumor immunotherapy approach, oncolytic adenovirus H101 can specifically infect and kill tumor cells and simultaneously activate the host’s anti-tumor immune response. H101 induces the immunogenic death of tumor cells through virus replication and tumor cell lysis, thereby enhancing the body’s anti-tumor immune response (10, 11). Previous studies have shown that H101 has demonstrated good preclinical and clinical effects in the treatment of various solid tumors (12–14). In addition, H101 was approved for market release by the China National Medical Products Administration (NMPA) in 2005, some studies have further confirmed that H101 combined with TACE improves the prognosis of patients with HCC (15–17). However, there are currently no relevant literature reports on the treatment of transarterial embolization (TAE) combined with H101 in sequence with thermal ablation. Therefore, in this study, a retrospective analysis was conducted to evaluate the efficacy and safety of H101 combined with TAE in sequence with thermal ablation in the treatment of HCC. The primary endpoints examined in this analysis were the tumor recurrence, progression, and overall survival (OS) status of the patients.
Materials and methods
Patient selection
A retrospective analysis was conducted on HCC patients who underwent H101 combined with TAE sequential ablation treatment at Beijing You’an Hospital, Capital Medical University, between July 2015 and January 2022. The inclusion criteria were as follows: a) HCC diagnosis confirmed by magnetic resonance imaging (MRI), computed tomography (CT), digital subtraction angiography (DSA), or tissue pathology. b) Patients who underwent TAE followed by ablation to treat HCC. It is also required that during the TAE procedure, H101 be injected into the tumor-feeding artery through the catheter. c) Patients categorized as Barcelona Clinic Liver Cancer (BCLC) Staging System stages 0, A, or B based on information available in the hospital’s Picture Archiving and Communication Systems (PACS) and Electronic Medical Record System (EMRS). d) All patients underwent follow-up for a minimum of three months after the ablation procedure. The study was in line with the Declaration of Helsinki. This retrospective study has been approved by the Ethics Committee of Beijing You’an Hospital Affiliated to Capital Medical University (LL-2022-130-k).
Treatment protocols
In the study, all patients initially received TAE as the primary treatment. H101 was administered to all enrolled patients. The specific procedure was as follows: during the TAE operation, after super-selectively inserting a microcatheter into the tumor-feeding artery, H101 was injected first (at a dose of 1.0×10¹² viral particles dissolved in 10 mL of 0.9% normal saline), followed by embolization with lipiodol (Ethiodol; Laboratoires Guerbet, Roissy, France) and the gelatin sponge particles embolization (Ailikang pharmaceutical technology co., LTD., Hangzhou, China). The sterile and purified viral batches, manufactured for clinical application in humans, were supplied by Shanghai Sunway Biotech (Shanghai, China) and underwent testing for potency, sterility, and overall safety at the National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China) (18, 19). The operation process of TAE is shown in Figure 1. After TAE, patients typically underwent ablation treatment within a month, within 1–2 weeks after TAE. The main percutaneous ablation techniques applied were RFA or MWA, both performed by skilled attending physicians. Each method operates through a unique mechanism. All ablation procedures were conducted under CT guidance. Generally, RFA was utilized for single tumors smaller than 3 cm, while MWA was preferred for tumors exceeding 5 cm in diameter. Nonetheless, the final selection of the ablation technique was based on the tumor’s size, location, and proximity to major blood vessels, as assessed by the treating physician. The operation process of ablation is shown in Figure 2.
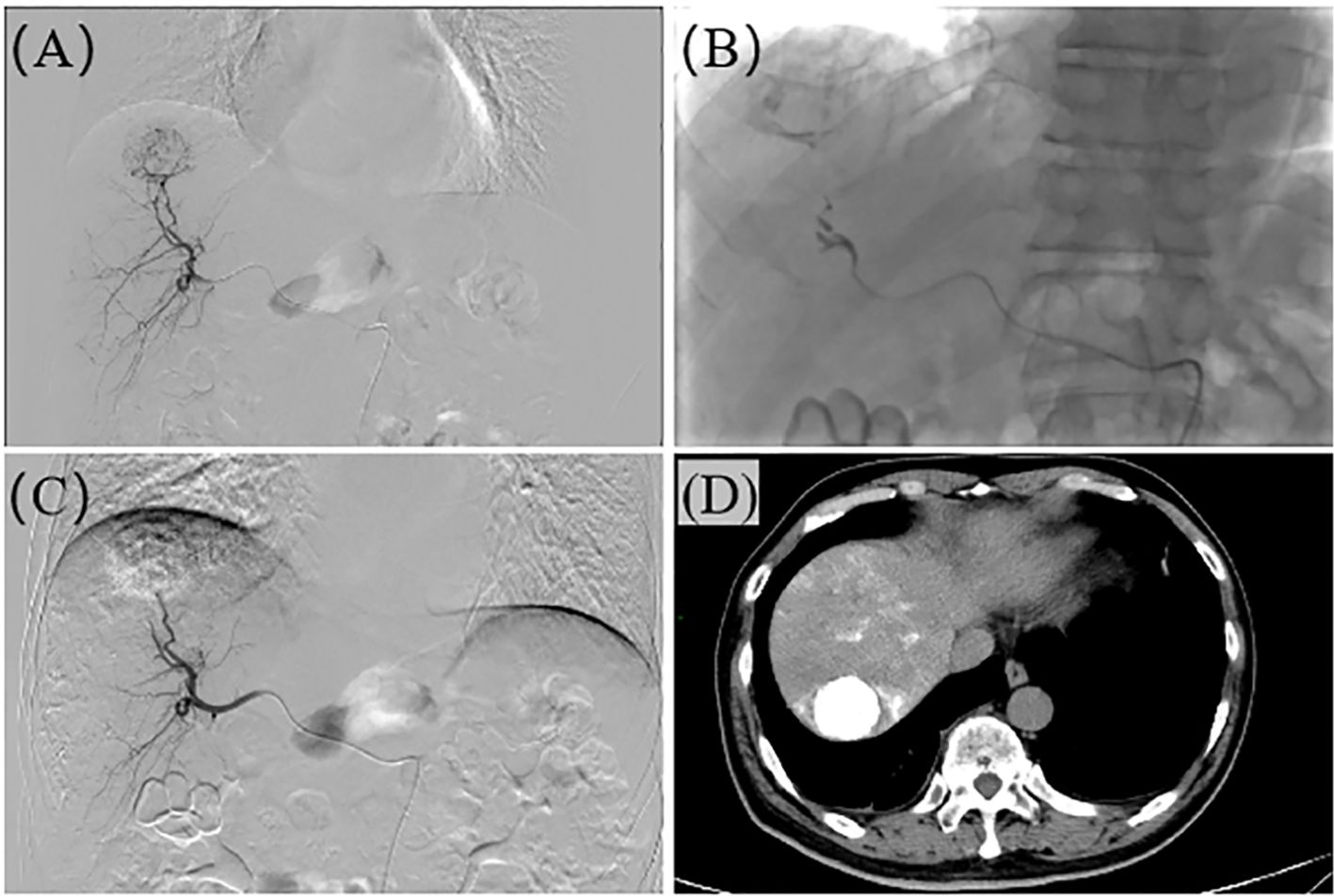
Figure 1. The operation process of TAE. (A) Hepatic arteriography reveals a roundish tumor stain in segment S7 of the liver. (B) The microcatheter is super-selectively advanced to the tumor-feeding artery, and embolization is performed with 101, lipiodol, and gelfoam respectively. (C) After the embolization is completed, re-angiography shows no tumor staining, and the embolic agents are filled within the tumor. (D) Plain CT after TAE confirms the presence of roundish lipiodol deposition foci in the liver, with dense lipiodol deposition within the tumor.
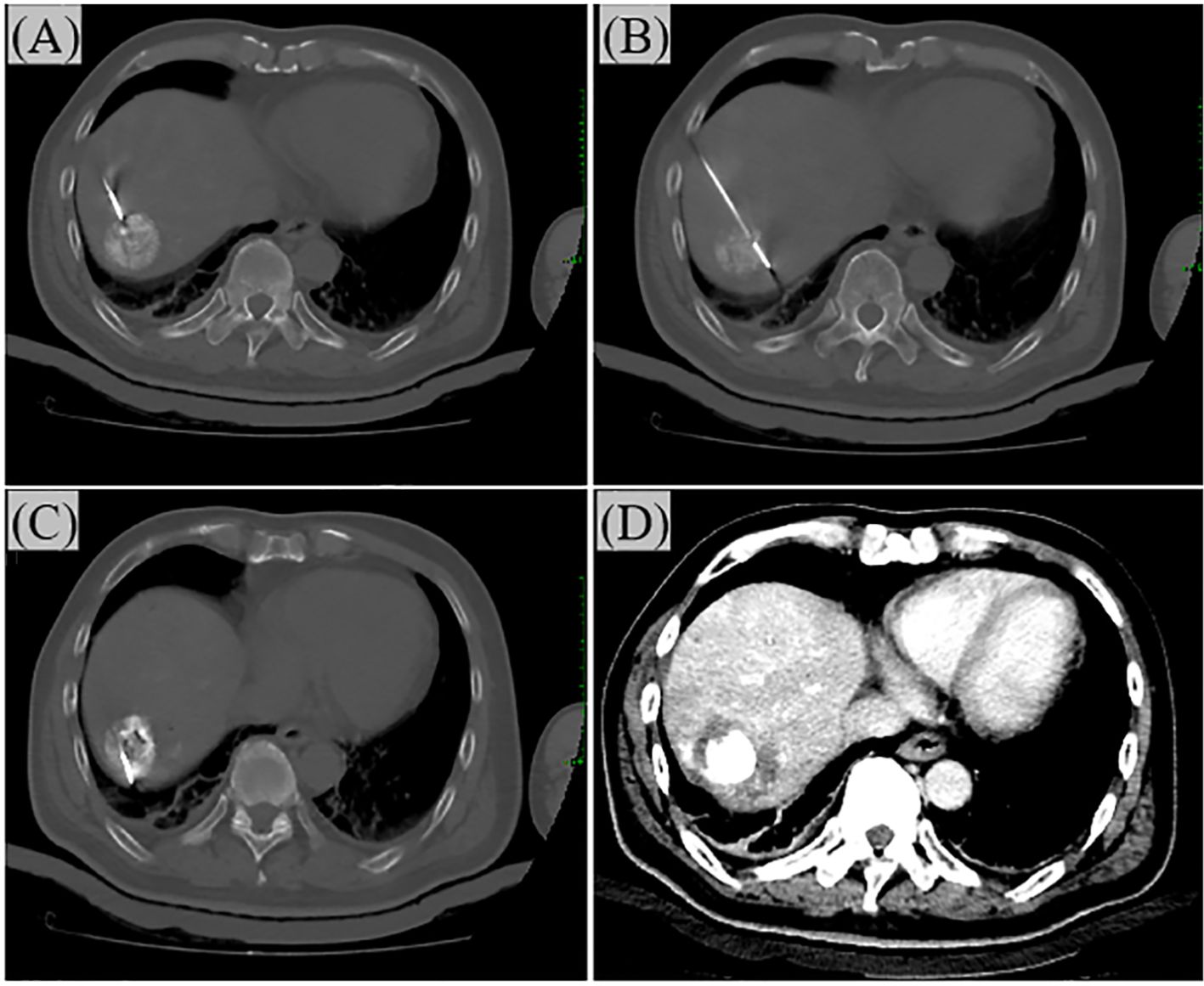
Figure 2. The operation process of Ablation. (A) Under CT guidance, the needle is inserted from the ventral side of the tumor. In the figure, the ablation needle has just entered the edge of the tumor. (B) Progressing step by step, the ablation needle enters the center of the tumor. (C) Insert the needle again to ablate the dorsal side of the tumor. (D) After the ablation is completed, perform a CT reexamination to ensure complete ablation. The ablation margin should be at least 0.5 cm.
Follow-up
Patient follow-up data were retrieved from PACS and EMRS. All assessments were reviewed independently by two radiologists to reduce subjective bias. Progression-free survival (PFS) was defined as the duration from the initiation of H101 therapy to the occurrence of tumor recurrence, the appearance of new lesions, metastasis, death, or the end of follow-up. Progression was assessed using RECIST 1.1 criteria: Imaging (CT/MRI/DSA) evidence of ≥20% increase in the longest diameter of existing lesions, appearance of new lesions, or distant metastasis. PFS was evaluated using imaging modalities such as CT, MRI, and DSA. Clinical AEs were graded according to the National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events (CTCAE) v5.0 criteria. The follow-up cutoff date was October 31, 2024.
Statistical analysis
Continuous variables were expressed as mean ± standard deviation (SD), while categorical variables were presented as percentages. The Student’s t-test was employed for comparisons of continuous data, whereas the chi-square test was utilized for categorical data. PFS and OS were estimated using the Kaplan-Meier method, and differences between groups were assessed with Log-rank tests. Univariate and multivariate analyses were performed using the Cox regression model. Variables with P-values less than 0.1 in the univariate analysis were subsequently included in the multivariate analysis. A statistically significant difference was indicated by P-values less than 0.05. Data analysis was carried out using IBM SPSS software version 25.0 (IBM Corp., Armonk, NY).
Results
Patients characteristics
We conducted a retrospective analysis of data from 55 patients, comprising 43 men and 12 women with a median follow-up of 39 months (range: 10–106 months). The average age at HCC diagnosis was 63.9 ± 10.0 years, with a range of 41 to 87 years old in our hospital. The primary cause of tumors was cirrhosis (81.8%), followed by hepatitis B virus (HBV) infection alone (70.9%). According to the BCLC staging, there were 32 patients with BCLC stage 0/A(early-stage HCC) and 23 patients with intermediate-stage HCC (BCLC stage B). Additional details of the patients’ characteristics are presented in Table 1.
Outcome and follow-up
In the patients of this cohort, disease progression was observed in 43 of 55 patients, and 22 of the 55 patients died. The median OS and PFS time were 77 and 12.3 months, respectively (Figure 3A, B). The one-, two-, and three-year OS rates were 94.5%, 86.3%, and 77.3%, respectively. The PFS rates at one, two and three years were 56.4%, 26.3% and 20% respectively. The median PFS of BCLC stage 0/A HCC patients is 13.1 months, while that of intermediate-stage HCC patients is 12.1 months. When it comes to the one-year PFS, the rates are 56.2% and 56.5% for early- and intermediate- stage HCC patients respectively. Looking at the three-year PFS, the percentages are 21.4% and 14.5% for these two groups (Figure 3C). The median OS of BCLC stage 0/A patients is 87 months, and for intermediate-stage HCC patients, it is 54 months. The one-year OS rates are 96.9% and 90.9% for early- and intermediate-stage HCC patients respectively. The three-year OS rates are 82.3% and 70.0% for these two stages (Figure 3D).
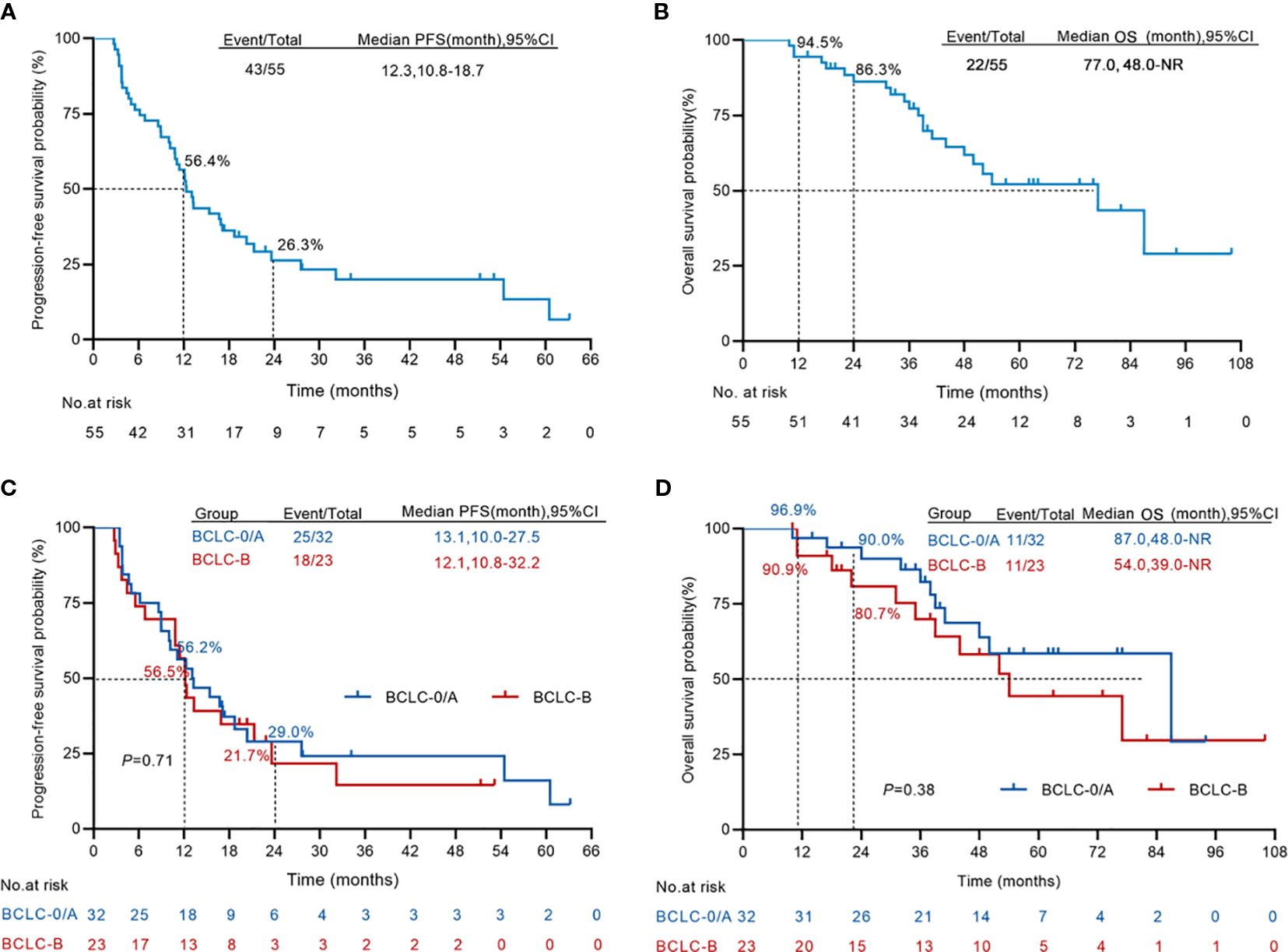
Figure 3. Kaplan–Meier curves for progression-free survival and overall survival. (A, B) The Kaplan–Meier survival analysis of overall survival (A) and progression-free survival (B) from the first H101 therapy (n = 55). (C, D) The Kaplan–Meier survival analysis comparing the overall survival (C) and progression-free survival (D) from the first H101 therapy of early-stage HCC (n = 32) and intermediate-stage HCC (n = 23). NR, not reach.
Univariate and multivariate analyses
Multivariate Cox regression analysis was conducted, including gender, age, family medical history, viral infection, liver cirrhosis, Child Pugh score, BCLC stage, alpha fetoprotein (AFP), tumor number, and maximum tumor diameter (Table 2). The univariate analysis demonstrated that the liver cirrhosis, tumor number, and maximum tumor diameter were risk factors for PFS (P < 0.05). The risk of progressive disease events among patients with liver cirrhosis was 3.020 times greater than that observed in patients without liver cirrhosis (P = 0.022). The risk of progressive disease events in patients with multiple tumors was 2.407 times higher than in patients with single tumor (P = 0.007). The maximum tumor diameter was positively correlated with progressive disease, and the risk of progressive disease in patients with a large maximum diameter was 2.771 times higher than that in patients with a smaller maximum diameter (P = 0.002). Subsequently, we incorporated liver cirrhosis, tumor number, and maximum tumor diameter into the multivariate analysis. The results showed that the maximum tumor diameter was an independent prognostic factor for progressive disease events (HR = 2.310, P = 0.023). Furthermore, we conducted a Cox regression analysis on OS (Table 3). Univariate and multivariate analyses showed that none of these indicators, including group variables, had a statistically significant effect on OS.
Safety
According to the CTCAE 5.0 criteria, 65% (36/55) of the fever events were grade 1 (body temperature 38.1-39.0°C), with no grade 2 or higher fever (>39°C) observed. All fever events resolved within 24–48 hours after symptomatic treatment. Among ablation-related complications, 100% (55/55) were grade 2 transaminase elevations (Alanine Aminotransferase/Aspartate Aminotransferase 2–5 times the upper limit of normal), which recovered within one week after hepatoprotective treatment, with no grade 3 or higher severe events. No typical immune-related adverse events (irAEs) such as rash, colitis, or thyroid dysfunction were observed caused by H101. (Table 4).
Discussion
TACE combined with ablation therapy has been reported in many studies to reduce the recurrence rate of patients and improve their prognosis (5, 7, 20). However, according to the early retrospective study reports from our center, the 5-year recurrence rate of TACE combined with ablation still remains as high as 80% (9). Although oncolytic viruses have been reported in many tumors, most of the reports are about the direct injection of H101 into the tumor body (13, 14, 21). In addition, there are very few reports on the efficacy of TACE combined with oncolytic viruses for HCC at present. Whether the chemotherapeutic drugs during hepatic artery embolization affect H101 remains to be further verified. In this study, TAE combined with H101 can avoid the influence of chemotherapeutic drugs on the efficacy of H101. In addition, research has confirmed that oncolytic viruses can enhance anti-tumor immunity and induce pyroptosis of vascular endothelial cells to inhibit tumor growth (12). Ablation therapy can also promote the release of tumor antigens and stimulate the immune response in the body (22). The sequential thermal ablation treatment of TAE combined with H101 is reported for the first time. This combined therapy offers a novel therapeutic strategy for the treatment of HCC.
Although more than 41.8% of patients in this study were non-treatment-naïve, the median 3-year OS for early and intermediate-stage HCC remained higher than those reported in the literature. According to the literature, the median survival time from recurrence to death for patients with HCC undergoing surgical resection is reported to be 21 months (23). Additionally, a study with a slightly larger sample size, involving 497 patients with recurrent HCC, developed a prognostic model to predict survival after recurrence (SAR), demonstrating a median SAR of 41.2 months (24). For patients classified as having intermediate-stage HCC according to the BCLC staging system, this study indicates that the 3-year OS rate is 70%, which similar to the results of our previous retrospective study (9). On one hand, it is believed that the combination of TAE and ablation can improve patient prognosis. On the other hand, in the study, only 4 out of 23 patients had AFP levels greater than 400 ng/ml. Elevated serum AFP levels are associated with poor prognosis in HCC patients (25). This also suggests that H101 may have a better effect on HCC patients who are AFP-negative. The favorable outcomes observed in patients with tumors ≤ 3 cm or AFP-negative status in this study are merely retrospective correlations, and a causal relationship has not been established. These outcomes may be related to confounding factors such as lower tumor burden and more indolent biological behavior in such patients, requiring further verification through prospective studies. Regrettably, the tumor progression rate in this study remains high, with 1-year PFS rates for HCC patients at 56.4%. This is consistent with the majority of literature reports and represents a challenge in the treatment of HCC (26). For example, Yun et al. reported that the cumulative 1-year recurrence rate of patients with early-stage HCC after TACE was 43.9%, while Wang et al. reported that the cumulative 1-, 3-, and 5-year recurrence rates of patients with early-stage HCC after TACE combined locoregional ablation were 26.0% (142/547), 57.8% (316/547), and 68.2% (373/547), respectively (27, 28). However, this does not mean that H101 is of no significance in the treatment of HCC. The reasons are analyzed as follows: First, in this study, nearly 41.8% of the patients had previously undergone surgical resection or multiple TACE or ablation treatments. In addition, 4 patients had undergone transjugular intrahepatic portosystemic shunt (TIPS) treatment due to gastrointestinal bleeding. Although most patients had Child-Pugh class A liver function, this was largely achieved through pharmacological intervention. These patients had undergone multiple treatments, suggesting that they might be at high risk for recurrence or progression. Second, the majority of patients exhibit good compliance; among the patients, 69.1% (38/55) have hepatitis B, which implies that these patients require long-term antiviral treatment and regular follow-ups to monitor viral load and liver function. Third, after ablation, patients are required to attend follow-ups at our hospital for three consecutive months, which allows for the early detection of any new lesions. For patients with BCLC stage B, the median PFS time in this study was 12.1 months. Zhang et al. reported that the median PFS time of patients after transarterial chemoembolization - microwave ablation (TACE-MWA) was also 10 months (29). However, all the patients included in their study were treatment-naive. In contrast, in this study, only 61% (14/23) of the patients with BCLC stage B were treatment-naive, while the remaining patients had received multiple previous local treatments, including surgery, ablation, and TACE. This indirectly indicates that for patients with BCLC stage B, the combination treatment of H101 with TAE and ablation is superior to TACE - MWA in therapeutic efficacy, but further research is needed to confirm this. Additionally, in the present study, all six patients (10.9%) with BCLC stage 0 disease presented with individual clinical factors precluding standard curative therapy: three exhibited severe cirrhosis, for whom the combined modality approach was selected to mitigate procedure-related hemorrhagic risk during ablation; and three had tumors critically abutting major vasculature or biliary structures at the hepatic hilum, where achieving an adequate ablative margin with stand-alone ablation was not feasible. In this cohort, we employed a sequential regimen of H101 combined with TAE followed by thermal ablation. This therapeutic strategy was designed to leverage the synergistic oncolytic, embolic, and ablative effects, thereby reducing procedural invasiveness while enhancing local tumor control.
The characteristics of cirrhosis are diffuse liver fibrosis and the replacement of normal liver structures with regenerated liver nodules, which have been proven to be positively correlated with liver cancer (30). The findings of this study consistently indicate that patients with cirrhosis have a 3.020 times higher risk of progression compared to those without cirrhosis. Moreover, tumor-related factors have been reported to be risk factors for recurrence after curative treatment in patients with early- stage HCC, such as tumor size, number of tumors, and pathological type (28). In this study, we found that the number of tumors and the maximum tumor diameter were risk factors for tumor progression. Furthermore, multivariate analysis showed that only the maximum tumor diameter was an independent prognostic factor for disease progression in HCC patients after H101 combined with TAE sequential thermal ablation. Our results suggest that HCC patients with a maximum tumor diameter of ≤ 3 cm are more likely to benefit from H101 combined with TAE sequential thermal ablation therapy.
In terms of safety, this study did not identify any serious adverse events (SAEs) caused by the injection of H101. The most common complication associated with H101 was fever that was consistent with the reports in the literature (14, 31). However, upon reviewing medical records, we found that such fever typically does not persist beyond 24 hours. Notably, this fever differs from that caused by TAE-induced post-embolization syndrome or infection secondary to tumor necrosis. The primary distinction lies in the timing of onset, as the latter usually occurs more than 24 hours after the procedure. However, strictly speaking, due to the lack of a control group in this study, and the fact that fever and abnormal transaminase levels can also occur after TAE, a randomized controlled study would be better able to distinguish between the two.
This study has several limitations. Firstly, it is a single-center, small-sample retrospective study, introduces multiple potential biases including selection bias, information bias, and confounding factors. Secondly, due to the lack of control group, it is difficult to distinguish whether the observed treatment effects result from H101, TAE, thermal ablation, or their synergistic combination. Therefore, the results of this study need to be further verified through prospective studies. Thirdly, this study included some non-treatment-naïve patients who had received other treatments before the start of the study, which made the interpretation of the research results more complex. Furthermore, univariate and multivariate analyses did not identify variables with a significant impact on OS, possibly due to the small sample size. Based on this, we are conducting a prospective randomized controlled trial (ChiCTR2300067319), using traditional TACE combined with ablation therapy as the control group, to investigate whether the combination of H101 and TAE sequential ablation therapy can reduce the recurrence of HCC and provide stronger treatment outcomes, thereby identifying the population that may respond effectively to H101 immunotherapy.
Conclusion
HI01 combined with TAE followed by sequential thermal ablation is safe and effective in the treatment of HCC. In observational analyses, this therapeutic approach is associated with more favorable outcomes in patients with a maximum tumor diameter of ≤ 3 cm, AFP, or in BCLC stage B. This approach provides a new local treatment option for HCC. However, due to the limited number of current studies, future research with larger sample sizes and higher levels of evidence is needed to confirm these findings.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Ethics Committee of Beijing You’an Hospital Affiliated to Capital Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
CJ: Writing – review & editing, Writing – original draft. TZ: Validation, Writing – review & editing. YZ: Resources, Writing – review & editing, Writing – original draft. JL: Funding acquisition, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, and/or publication of this article.
Acknowledgments
We would like to thank the medical staff at the Liver Disease and Tumor Interventional Treatment Center for their contributions to the patients’ pre-treatment care.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
NR, Not Reached; HR, Hazard Ratio; CI, confidence interval; HBV, hepatitis B virus; HCV, hepatitis C virus; BCLC, Barcelona Clinic Liver Cancer; AFP, alpha fetoprotein.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2024) 74:229–63. doi: 10.3322/caac.21834
2. Ng KKC, Chok KSH, Chan ACY, Cheung TT, Wong TCL, Fung JYY, et al. Randomized clinical trial of hepatic resection versus radiofrequency ablation for early-stage hepatocellular carcinoma. Br J Surg. (2017) 104:1775–84. doi: 10.1002/bjs.10677
3. Lai EC and Tang CN. Radiofrequency ablation versus hepatic resection for hepatocellular carcinoma within the Milan criteria–a comparative study. Int J Surg. (2013) 11:77–80. doi: 10.1016/j.ijsu.2012.11.019
4. Najmi Varzaneh F, Pandey A, Aliyari Ghasabeh M, Shao N, Khoshpouri P, Pandey P, et al. Prediction of post-TACE necrosis of hepatocellular carcinoma usingvolumetric enhancement on MRI and volumetric oil deposition on CT, with pathological correlation. Eur Radiol. (2018) 28:3032–40. doi: 10.1007/s00330-017-5198-9
5. Liu C, Li T, He JT, and Shao H. TACE combined with microwave ablation therapy vs. TACE alone for treatment of early- and intermediate-stage hepatocellular carcinomas larger than 5cm: a meta-analysis. Diagn Interv Radiol. (2020) 26:575–83. doi: 10.5152/dir.2020.19615
6. Zheng L, Li HL, Guo CY, and Luo SX. Comparison of the efficacy and prognostic factors of transarterial chemoembolization plus microwave ablation versus transarterial chemoembolization alone in patients with a large solitary or multinodular hepatocellular carcinomas. Korean J Radiol. (2018) 19:237–46. doi: 10.3348/kjr.2018.19.2.237
7. Zhang YJ, Chen MS, Chen Y, Lau WY, and Peng Z. Long-term outcomes of transcatheter arterial chemoembolization combined with radiofrequency ablation as an initial treatment for early-stage hepatocellular carcinoma. JAMA Netw Open. (2021) 4:e2126992. doi: 10.1001/jamanetworkopen.2021.26992
8. Shi F, Wu M, Lian SS, Mo ZQ, Gou Q, Xu RD, et al. Radiofrequency ablation following downstaging of hepatocellular carcinoma by using transarterial chemoembolization: long- term outcomes. Radiology. (2019) 293:707–15. doi: 10.1148/radiol.2019181991
9. Jing C, Li J, Yuan C, Hu C, Ma L, Zheng J, et al. Therapeutic analysis of 632 cases treated by transcatheter arterial chemoembolization combined with ablation in hepatocellular carcinoma: A retrospective study. Eur J Radiol. (2024) 178:111619. doi: 10.1016/j.ejrad.2024.111619
10. Zhang Y, Zhang H, Wei M, Mou T, Shi T, Ma Y, et al. Recombinant adenovirus expressing a soluble fusion protein PD-1/CD137L subverts the suppression of CD8+ T cells in HCC. Mol Ther. (2019) 27:1906–18. doi: 10.1016/j.ymthe.2019.07.019
11. Mandlik DS, Mandlik SK, and Choudhary HB. Immunotherapy for hepatocellular carcinoma: Current status and future perspectives. World J Gastroenterol. (2023) 29:1054–75. doi: 10.3748/wjg.v29.i6.1054
12. Wang ZM, Li MK, Yang QL, Duan SX, Lou XY, Yang XY, et al. Recombinant human adenovirus type 5 promotes anti-tumor immunity via inducing pyroptosis in tumor endothelial cells. Acta Pharmacol Sin. (2024) 45:2646–56. doi: 10.1038/s41401-024-01349-x
13. Lei J, Li QH, Yang JL, Liu F, Wang L, Xu WM, et al. The antitumor effects of oncolytic adenovirus H101 against lung cancer. Int J Oncol. (2015) 47:555–62. doi: 10.3892/ijo.2015.3045
14. Zhang J, Zhang Q, Liu Z, Wang J, Shi F, Su J, et al. Efficacy and safety of recombinant human adenovirus type 5 (H101) in persistent, recurrent, or metastatic gynecologic Malignancies: A retrospective study. Front Oncol. (2022) 12:877155. doi: 10.3389/fonc.2022.877155
15. Wu K, You N, and Zheng L. Effects of recombinant human adenovirus type 5 combined with transarterial chemoembolization on postoperative metastasis and recurrence of hepatocellular carcinoma patients. J Gastrointest Oncol. (2021) 12:2999–3007. doi: 10.21037/jgo-21-792
16. Lu S-N, He C-B, and Lin X-J. Inflammation scores predict the survival of patients with hepatocellular carcinoma who were treated with transarterial chemoembolization and recombinant human type-5 adenovirus H101. PloS One. (2017) 12:e0174769. doi: 10.1371/journal.pone.0174769
17. He C-B, Lao X-M, and Lin X-J. Transarterial chemoembolization combined with recombinant human adenovirus type 5 H101 prolongs overall survival of patients with intermediate to advanced hepatocellular carcinoma: a prognostic nomogram study. Chin J Cancer. (2017) 36:59. doi: 10.1186/s40880-017-0227-2
18. He Q, Liu Y, Zou Q, and Guan YS. Transarterial injection of H101 in combination with chemoembolization overcomes recurrent hepatocellular carcinoma. World JGastroenterol. (2011) 17:2353–5. doi: 10.3748/wjg.v17.i18.2353
19. Dong J, Li W, Dong A, Mao S, Shen L, Li S, et al. Gene therapy for unresectable hepatocellular carcinoma using recombinant human adenovirus type 5. Med Oncol. (2014) 31:95. doi: 10.1007/s12032-014-0095-4
20. Hirooka M, Hiraoka A, Ochi H, Kisaka Y, Joko K, Michitaka K, et al. Transcatheter arterial chemoembolization with or without radiofrequency ablation: outcomes in patients with barcelona clinic liver cancer stage B hepatocellular carcinoma. AJR Am J Roentgenol. (2018) 210:891–8. doi: 10.2214/AJR.17.18177
21. Huang L, Zhao H, Shan M, Chen H, Xu B, He Y, et al. Oncolytic adenovirus H101 ameliorate the efficacy of anti-PD-1 monotherapy in colorectal cancer. Cancer Med. (2022) 11:4575–87. doi: 10.1002/cam4.4845
22. Zerbini A, Pilli M, Laccabue D, Pelosi G, Molinari A, Negri E, et al. Radiofrequency thermal ablation for hepatocellular carcinoma stimulates autologous NK-cell response. Gastroenterology. (2010) 138:1931–42. doi: 10.1053/j.gastro.2009.12.051
23. Tabrizian P, Jibara G, Shrager B, Schwartz M, and Roayaie S. Recurrence of hepatocellular cancer after resection: patterns, treatments, and prognosis. Ann Surg. (2015) 261:947–55. doi: 10.1097/SLA.0000000000000710
24. Moazzam Z, Alaimo L, Endo Y, Lima HA, Woldesenbet S, Rueda BO, et al. A prognostic model to predict survival after recurrence among patients with recurrent hepatocellular carcinoma. Ann Surg. (2024) 279:471–8. doi: 10.1097/SLA.0000000000006056
25. Galle PR, Foerster F, Kudo M, Chan SL, Llovet JM, Qin S, et al. Biology and significance of alpha- fetoprotein in hepatocellular carcinoma. Liver Int. (2019) 39:2214–29. doi: 10.1111/liv.14223
26. Villanueva A. Hepatocellular carcinoma. N Engl J Med. (2019) 380:1450– 62. doi: 10.1056/NEJMra1713263
27. Yun BY, Lee HW, Min IK, Kim SU, Park JY, Kim DY, et al. Prognosis of early-stage hepatocellular carcinoma: comparison between trans-arterial chemoembolization and radiofrequency ablation. Cancers. (2020) 12:2527. doi: 10.3390/cancers12092527
28. Wang Q, Qiao W, Zhang H, Liu B, Li J, Zang C, et al. Nomogram established on account of Lasso-Cox regression for predicting recurrence in patients with early-stage hepatocellular carcinoma. Front Immunol. (2022) 13:1019638. doi: 10.3389/fimmu.2022.1019638
29. Zhang R, Shen L, Zhao L, Guan Z, Chen Q, and Li W. Combined transarterial chemoembolization and microwave ablation versus transarterial chemoembolization in BCLC stage B hepatocellular carcinoma. Diagn Interv Radiol. (2018) 24:219–24. doi: 10.5152/dir.2018.17528
30. Deng Y, Huang J, and Wong MCS. Associations of non-alcoholic fatty liver disease and cirrhosis with liver cancer in European and East Asian populations: A Mendelian randomization study. Cancer Rep (Hoboken NJ). (2024) 7:e1913. doi: 10.1002/cnr2.1913
Keywords: hepatocellular carcinoma, oncolytic adenovirus, H101, transcatheter arterial embolization, thermal ablation
Citation: Jing C, Zhu T, Zhang Y and Li J (2025) The combination treatment of oncolytic adenovirus H101 with transcatheter arterial embolization sequential thermal ablation for hepatocellular carcinoma: a retrospective study. Front. Oncol. 15:1623877. doi: 10.3389/fonc.2025.1623877
Received: 06 May 2025; Accepted: 29 August 2025;
Published: 19 September 2025.
Edited by:
Zong Sheng Guo, University at Buffalo, United StatesReviewed by:
Shuanggang Chen, Sun Yat-sen University Cancer Center (SYSUCC), ChinaZhu-Ting Fang, Fujian Provincial Hospital, China
Copyright © 2025 Jing, Zhu, Zhang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jianjun Li, bWFpbHRvOmxqamlyQGNjbXUuZWR1LmNu
 Changyou Jing
Changyou Jing Tong Zhu
Tong Zhu Yonghong Zhang
Yonghong Zhang Jianjun Li
Jianjun Li