Abstract
Background:
Hashimoto’s thyroiditis (HT) is often associated with papillary thyroid carcinoma (PTC) and increases the difficulty of thyroidectomy. The clinical outcomes of applying the transoral approach and the transthoracic approach—the two most widely practiced endoscopic thyroid surgery techniques—in patients with PTC complicated by HT remain unclear.
Materials and methods:
This study is a single-center retrospective design. Clinical data on 500 patients with PTC who underwent endoscopic thyroidectomy between January 2016 and December 2022 were collected. Patients voluntarily chose either the transoral endoscopic thyroidectomy via a vestibular approach (TOETVA) or endoscopic thyroidectomy via a chest-breast approach (ETCB), were grouped accordingly, and were further subdivided into HT and non-HT groups.
Results:
Of 500 patients included, 140 had HT and 360 did not. The proportion of patients with stage T1 tumors was larger in the HT group than in the non-HT group. All endoscopic thyroidectomies (202 ETCBs and 298 TOETVAs) completed successfully without conversion to open surgery. The total number of retrieved lymph nodes was larger in the HT group than in the non-HT group, but the number of positive lymph nodes was smaller. Among patients treated by ETCB, the operative time was longer and the incidence of complications (transient hypoparathyroidism and transient recurrent laryngeal nerve injury) was greater in the HT group than in the non-HT group. For patients treated by TOETVA, the operative time and incidence of complications did not differ significantly between groups.
Conclusions:
HT appears to be associated with less aggressive tumor characteristics. TOETVA could represent a preferable option compared with ETCB for managing PTC with concomitant HT, although further prospective studies are warranted to confirm these findings.
Introduction
The incidence of papillary thyroid carcinoma (PTC), the most common endocrine system malignancy, has increased rapidly in recent years (1, 2). Surgery is the primary treatment for PTC, while the first endoscopic thyroid lobectomy was reported in 1997 (3). After nearly three decades of development, numerous endoscopic approaches for thyroidectomy have been reported. Among these, endoscopic thyroidectomy via a chest-breast approach (ETCB) represents the earliest and most extensively adopted technique. The first totally transoral video-assisted thyroidectomy was documented in 2009 (4) and was classified as a type of NOTES procedure. Transoral endoscopic thyroidectomy via a vestibular approach (TOETVA) completely avoids the need to make visible skin incisions and is rapidly becoming a popular alternative (5). ETCB and TOETVA are currently used in the treatment of PTC in many medical centers, but they have shortcomings. ETCB is suboptimal for central neck dissection due to obstruction by the clavicle, while TOETVA carries increased risks of infection and mental nerve injury associated with the transoral vestibular approach (6).
Hashimoto thyroiditis (HT), the most common human autoimmune disease (7), co-occurs with PTC in approximately 23% of cases (8, 9). Since it was first reported in 1955 (10), different perspectives on this relationship have emerged. Some authors have suggested that HT is a protective factor that reduces tumor aggressiveness, possibly due to the increase in inflammatory factors or lower prevalence of BRAF mutations in co-occurring PTC and HT relative to PTC alone (11–14). However, others believe that HT negatively affects the prognosis of PTC (15).
In addition, HT increases the difficulty and complication rates of thyroidectomy (16, 17), and the same may be true for minimally invasive thyroid surgery (18, 19). Common complications associated with thyroidectomy include transient/permanent recurrent laryngeal nerve (RLN) injury, transient/permanent hypoparathyroidism, mental nerve injury, severe hematoma, and infection. This study was performed to investigate the effects of HT on PTC, ETCB, and TOETVA through the analysis of intraoperative and postoperative clinical outcomes, with the aim of identifying the most appropriate minimally invasive thyroid surgery for the treatment of PTC with concomitant HT.
Materials and methods
Sample
Data on patients with pathologically confirmed PTC who underwent endoscopic thyroidectomy (ETCB or TOETVA) at the Guangdong Provincial Hospital of Traditional Chinese Medicine between January 2016 and December 2022 were retrospectively reviewed, including age, gender, body mass index, tumor size, blood loss, hospital stay, operative time, No. of retrieved lymph nodes, No. of retrieved positive lymph nodes, tumor T stages, and complications. Patients with lateral lymph-node or distant metastasis, poorly differentiated PTC or other thyroid malignancies, and/or histories of cancer or thyroid surgery were excluded. After receiving detailed information about ETCB and TOETVA, all patients indicated their choice of operative procedure. The patients were allocated to TOETVA and ETCB groups, and further to HT and non-HT groups according to postoperative pathological examination findings. The ipsilateral CND was performed routinely in our center. Central compartment was identified by brachiocephalic trunk artery inferiorly, carotid artery laterally, and deep layer of deep cervical fascia posteriorly. In addition, all operations done by the same team, with You Qin serving as the main surgeon. The hospital’s Institutional Review Board approved this study, and all patients provided informed consent to participation.
Preoperative preparation
Thyroid function tests, neck ultrasound, and other necessary preoperative tests were performed for all included patients. For patients who underwent TOETVA, gargle was provided on the day before surgery and prophylactic antibiotics were administered 30 min before surgery.
Operative procedures
ETCB
First, a 10-mm trocar was set at nipple level next to the sternum. Two 5-mm trocars were positioned at 10–11 o’clock on the left areola and 1–2 o’clock on the right areola (Figure 1). Second, we established the initial working space, bounded superiorly by the larynx, inferiorly by the sternal notch, and laterally by the middle edges of the sternocleidomastoid. Third, the linea alba cervicalis was divided to reveal the thyroid, the isthmus was separated from the surface of the trachea and transected, the thyroid was retracted medially, and the carotid artery was exposed on the outside. Fourth, the upper pole of the gland was dissected and the superior thyroid vessels were ligated with the preservation of the superior laryngeal nerve and superior parathyroid gland. Fifth, the inferior thyroid vessel was ligated and the thyroid was cut close to the capsule to preserve the recurrent laryngeal nerve (RLN) and inferior parathyroid gland. Sixth, the thyroid lobe was removed completely. The contralateral thyroid lobe was also removed when necessary. Finally, the lymph nodes in the central region were dissected.
Figure 1
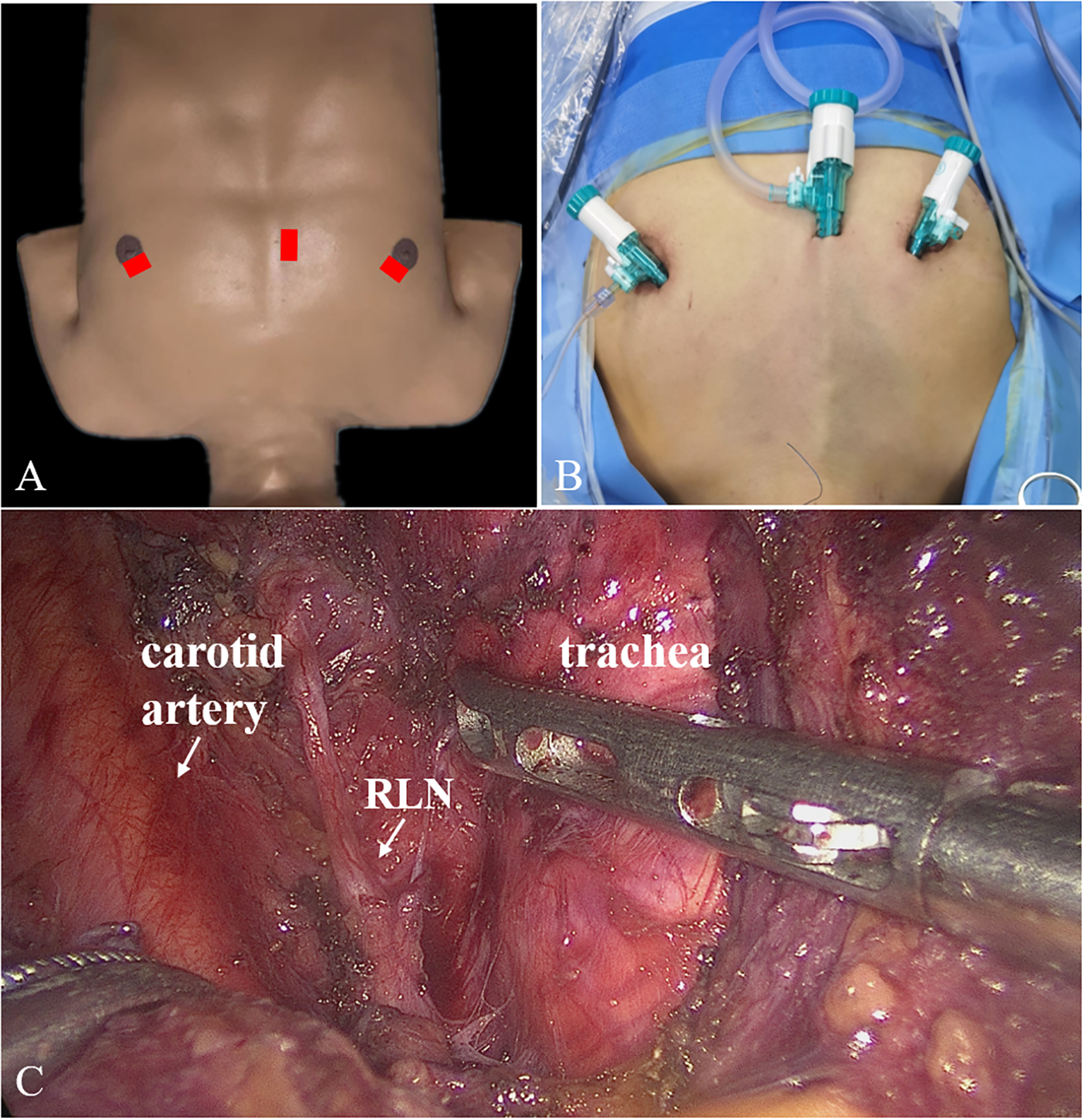
Trocars placement (A, B) and surgical scene (C) for ETCB. ETCB, endoscopic thyroidectomy via chest-breast approach.
TOETVA
First, a 10-mm trocar was set in the middle of the vestibule above the inferior labial frenulum. Two 5-mm trocars were set symmetrically on the mucosa at the level of the first premolars (Figure 2). The second and third steps were the same as for ETCB. Fourth, the sternothyroid was partially transected to expose the superior pole of the gland, and the superior thyroid vessels were ligated. Fifth, the RLN was identified near the laryngeal entry point, and thyroid was cut close to the capsule to protect the RLN and inferior parathyroid gland. Sixth, the rest of Berry’s ligament was dissected, the inferior thyroid vessel was ligated, and the thyroid lobe was removed completely. When necessary, the contralateral thyroid lobe was also removed. Finally, CND was performed.
Figure 2
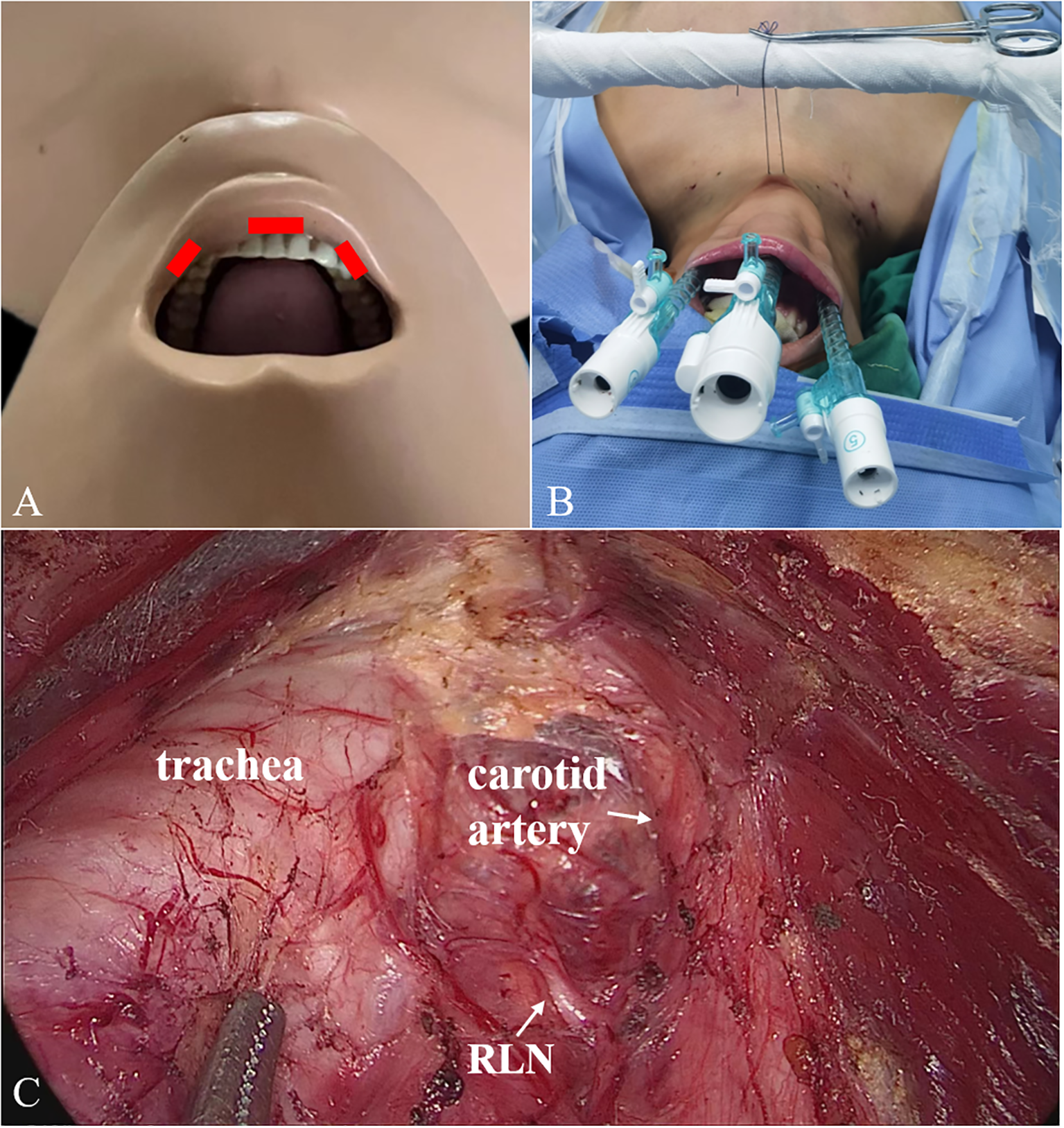
2 Trocars placement (A, B) and surgical scene (C) for TOETVA. TOETVA, transoral endoscopic thyroidectomy vestibular approach.
Postoperative follow up
After surgery, pathology results and postoperative complications were recorded. Parathyroid hormone (PTH) levels < 11 pg/mL were considered to indicate transient hypoparathyroidism. For patients with voice impairment after surgery, laryngoscopy was regularly recommended to confirm transient RLN injury. When the PTH level and RLN injury had not recovered by 6 months after surgery, the injury was considered to be permanent. Mental nerve injury is defined as the loss of chin sensation persisting for more than six months postoperatively without recovery. Severe hematoma refers to a hematoma formed by postoperative bleeding that leads to respiratory distress and necessitates reoperation for hemostasis. Infection in this study denotes a postoperative infection that requires reoperation for debridement and drainage (Table 1). The patients were asked to return for evaluation by neck ultrasound every 6 months for 5 years postoperatively. Diagnostic fine needle aspiration (FNA) was performed when suspicious lesions or lymph nodes were detected.
Table 1
| Complications | Definition |
|---|---|
| Transient hypoparathyroidism | Parathyroid hormone levels < 11 pg/mL were considered to indicate transient hypoparathyroidism. |
| Permanent hypoparathyroidism | The Parathyroid hormone levels had not recovered by 6 months after surgery. |
| Transient RLN injury | For patients with voice impairment after surgery, laryngoscopy was regularly recommended to confirm transient RLN injury. |
| Permanent RLN injury | The RLN injury had not recovered by 6 months after surgery. |
| Mental nerve injury | Loss of sensation in the chin persisting beyond six months post-surgery without recovery. |
| Severe Hematoma | Postoperative bleeding led to a severe hematoma, which caused respiratory distress and required a second surgery for hemostasis. |
| Infection | Postoperative infection requiring reoperation for debridement and drainage. |
Complications and definitions.
RLN, Recurrent laryngeal nerve.
Statistical analysis
All statistical analyses were performed with SPSS 18.0 software (SPSS Inc). Differences between groups were analyzed using the chi-squared test, chi-squared test with correction for continuity, Fisher’s exact test, and Student’s independent t test. Logistic regression was used for univariate analysis. All variables with p < 0.05 after univariate analysis were subsequently subjected to multivariate regression to determine the independence of effects. All tests were two sided, and p values < 0.05 were considered to be significant.
Results
Overall sample characteristics
In total, 500 patients with PTC (140 with and 360 without HT) were enrolled in the study. All patients underwent endoscopic thyroidectomy successfully with no conversion to open surgery. The HT group had a larger proportion of female patients, younger age, lower body mass index, and earlier tumor T stage than did the non-HT group. The total number of retrieved lymph nodes was larger in the HT group than in the non-HT group, but the number of positive lymph nodes was smaller (Table 2). Multivariate analysis, as shown in Table 2, suggested that there were several independent factors associated with the HT, including female (p = 0.002), more retrieved lymph nodes (p < 0.001), and earlier tumor T stage (p = 0.006).
Table 2
| Variable | With thyroiditis (n = 140) | Without thyroiditis (n = 360) | Univariate analysis p-value | OR | 95%CI | Multivariate analysis p-value |
|---|---|---|---|---|---|---|
| Age (year) | 40.6±10.7 | 42.9±11.5 | 0.035 | 0.987 | 0.968-1.006 | 0.182 |
| Gender (female/male) | 127/13 | 275/85 | <0.001 | 2.837 | 1.481-5.437 | 0.002 |
| BMI (kg/m2) | 20.5±7.6 | 21.9±6.4 | 0.038 | 0.980 | 0.952-1.010 | 0.196 |
| Tumor size (cm) | 0.80±0.56 | 0.78±0.57 | 0.708 | / | / | / |
| No. of retrieved lymph nodes | 8.1±4.4 | 5.8±3.9 | <0.001 | 1.150 | 1.092-1.211 | <0.001 |
| No. of retrieved positive lymph nodes | 0.92±1.4 | 1.2±2.1 | 0.074 | / | / | / |
| T1/T2+T3 | 125/15 | 287/73 | 0.012 | 2.435 | 1.294-4.584 | 0.006 |
Comparison of clinical outcomes according to coexistence of HT.
HT, Hashimoto's thyroiditis; BMI, body mass index.
ETCB group
In total, 202 patients (59 with and 143 without HT) were treated by ETCB. Operative time did not differ significantly between the HT group (135.7 ± 75.2 min) and non-HT group (117.9 ± 50.8 min) group. Besides, although the total number of retrieved lymph nodes was significantly higher in the HT group than in the non-HT group, there was no significant difference in the number of positive lymph nodes between the two groups. In the HT group, 12 patients had transient hypoparathyroidism, 7 patients had transient RLN injury, and 3 patients had permanent RLN injury. In the non-HT group, 12 patients had transient hypoparathyroidism, 2 patients had permanent hypoparathyroidism, 4 patients had transient RLN injury, 1patient had permanent RLN injury, and 1 patient had severe hematoma. The incidences of transient hypoparathyroidism (p = 0.017) and transient RLN injury (p = 0.025) were higher in the HT group than in the non-HT group (Table 3).
Table 3
| Variable | With thyroiditis (n = 59) | Without thyroiditis (n = 143) | Univariate analysis p-value | OR | 95%CI | Multivariate analysis p-value |
|---|---|---|---|---|---|---|
| Operative time (min) | 135.7±75.2 | 117.9±50.8 | 0.100 | / | / | |
| No. of retrieved positive lymph nodes | 1.0±1.5 | 1.2±2.2 | 0.632 | / | / | |
| No. of retrieved lymph nodes | 7.9±4.4 | 5.1±4.0 | <0.001 | 1.168 | 1.076-1.268 | <0.001 |
| Age (year) | 42.7±11.0 | 47.4±11.4 | 0.009 | 0.969 | 0.940-0.999 | 0.045 |
| Gender (female/male) | 52/7 | 105/38 | 0.022 | 2.512 | 1.000-6.310 | 0.050 |
| BMI (kg/m2) | 22.7±3.8 | 23.4±3.9 | 0.269 | / | / | |
| Tumor size (cm) | 0.82±0.60 | 0.84±0.55 | 0.876 | / | / | |
| Blood loss (ml) | 22.5±29.4 | 18.9±23.2 | 0.367 | / | / | |
| Hospital stay (day) | 4.4±1.6 | 4.0±1.3 | 0.077 | / | / | |
| T1/T2+T3 | 54/5 | 113/30 | 0.033 | 3.988 | 1.303-12.200 | 0.015 |
| Complications | ||||||
| Transient hypoparathyroidism | 47/12 | 131/12 | 0.017 | |||
| Permanent hypoparathyroidism | 59/0 | 141/2 | 1.000 | |||
| Transient RLN injury | 52/7 | 139/4 | 0.025 | |||
| Permanent RLN injury | 56/3 | 142/1 | 0.076 | |||
| Severe Hematoma | 59/0 | 142/1 | 1.000 | |||
| Infection | 0 | 0 | / | |||
| Reoperation | 59/0 | 142/1 | 1.000 | |||
| Recurrence | 59/0 | 142/1 | 1.000 | |||
| Other complicationsa | 0 | 0 | / | |||
Comparison of clinical outcomes according to coexistence of HT (treated by ETCB).
Including common carotid artery injury, vagus nerve injury and so on. HT, Hashimoto's thyroiditis; ETCB, endoscopic thyroidectomy via chest-breast approach; BMI, body mass index; RLN, recurrent laryngeal nerve.
TOETVA group
In total, 298 patients (81 with and 217 without HT) were treated by TOETVA. The OTs in the HT (117.7 ± 52.4 min) and non-HT (111.3 ± 41.5 min) groups were similar. There was also no significant difference between the two groups regarding positive lymph nodes; however, the total number of retrieved lymph nodes was significantly higher in the HT group. In the HT group, 17 patients had transient hypoparathyroidism and 3 patients had transient RLN injury. In the non-HT group, 28 patients had transient hypoparathyroidism, 2 patients had permanent hypoparathyroidism, 3 patients had transient RLN injury, 1 patient had permanent RLN injury, and 1 patient had severe hematoma. The incidences of postoperative complications did not differ between groups (Table 4). Notably, all patients who underwent the TOETVA were completely free from mental nerve injury and infection.
Table 4
| Variable | With thyroiditis (n = 81) | Without thyroiditis (n = 217) | Univariate analysis p-value | OR | 95%CI | Multivariate analysis p-value |
|---|---|---|---|---|---|---|
| Operative time (min) | 117.7±52.4 | 111.3±41.5 | 0.272 | / | / | |
| No. of retrieved positive lymph nodes | 0.85±1.4 | 1.3±2.1 | 0.057 | / | / | |
| No. of retrieved lymph nodes | 8.2±4.4 | 6.2±3.7 | <0.001 | 1.139 | 1.066-1.218 | <0.001 |
| Age (year) | 39.0±10.2 | 40.0±10.5 | 0.441 | / | / | |
| Gender (female/male) | 75/6 | 170/47 | 0.004 | 3.811 | 1.523-9.537 | 0.004 |
| BMI (kg/m2) | 18.9±9.1 | 20.9±7.5 | 0.269 | / | / | |
| Tumor size (cm) | 0.78±0.53 | 0.74±0.59 | 0.570 | / | / | |
| Blood loss (ml) | 16.9±14.0 | 15.7±14.1 | 0.504 | / | / | |
| Hospital stay (day) | 3.7±1.1 | 3.5±1.0 | 0.112 | / | / | |
| T1/T2+T3 | 71/10 | 174/43 | 0.134 | / | / | |
| Complications | ||||||
| Transient hypoparathyroidism | 64/17 | 189/28 | 0.083 | |||
| Permanent hypoparathyroidism | 81/0 | 215/2 | 1.000 | |||
| Transient RLN injury | 78/3 | 214/3 | 0.350 | |||
| Permanent RLN injury | 81/0 | 216/1 | 1.000 | |||
| Mental nerve injury | 0 | 0 | / | |||
| Severe Hematoma | 81/0 | 216/1 | 1.000 | |||
| Infection | 0 | 0 | / | |||
| Reoperation | 81/0 | 216/1 | 1.000 | |||
| Recurrence | 81/0 | 215/2 | 1.000 | |||
| Other complicationsa | 0 | 0 | / | |||
Comparison of clinical outcomes according to coexistence of HT (treated by TOETVA).
Including common carotid artery injury, vagus nerve injury and so on. HT, Hashimoto's thyroiditis; TOETVA, transoral endoscopic thyroidectomy vestibular approach; BMI, body mass index; RLN, recurrent laryngeal nerve.
Discussion
The relationship between PTC and HT is a matter of debate (20, 21). Different rates of the coexistence of HT and PTC have been reported. The reported coexistence rate of HT and PTC from epidemiological studies is approximately 23% on average, ranging from 5% to 85% (8, 9). In this study, 28% of patients with PTC had HT. Second, no consensus on the effect of HT on the prognosis of PTC has been reached. In this study, a larger proportion of patients in the HT group had earlier tumor T stages, suggesting that HT is a protective factor for PTC. This inference is consistent with the conclusions of most researchers (22, 23). Third, the effect of HT on lymph-node metastasis is controversial. In some studies, lymph-node metastasis was more common in the presence of concomitant HT (15, 24), which may be related to increased programmed death ligand-1 (PD-L1) levels (25). In contrast, the frequency of lymph-node metastasis was lower in patients with HT than in those without HT in other studies (26, 27), which may be due to decreased PD-L1 levels caused by major histocompatibility complex class I expression in HT (13). In this study, despite a higher total retrieved lymph nodes in the HT group, the number of positive nodes was lower, indicating a lower lymph node positivity rate (p < 0.001). This suggests that HT may help inhibit lymph node metastasis.
Little research has been performed to examine the application of endoscopic thyroidectomy for patients with PTC and concomitant HT (28, 29), and the sample for this study is the largest examined to date. In contrast to the approach taken in previous studies, we investigated the effects of HT on ETCB and TOETVA outcomes separately. For ETCB, HT may lead to OT prolongation (135.7 ± 75.2 min vs. 117.9 ± 50.8 min) and increased complication rates. Diffuse enlargement of the thyroid gland and the formation of dense fibrotic adhesions may have adverse effects on the performance of this surgery (16, 28). For TOETVA, the OT and complication rates did not differ between the HT and non-HT groups. We attribute this lack of difference to several factors. First, all patients included in this study underwent central lymph-node dissection, and TOETVA has been suggested to be more suitable than ETCB for CND (6). The obstruction of the clavicle increases the difficulty of CND in ETCB, which may lead to increased OTs and complication rates. Second, our previous study indicated that TOETVA aids the localization and protection of the RLN and superior parathyroid gland (30).
It is noteworthy that no complications of mental nerve injury or infection occurred in the TOETVA group. Given that the reported incidence of these complications is also very low in other literature on the TOETVA (5, 31), we believe that the risks of these two specific complications can likely be effectively controlled through standardized prophylactic antibiotic use and trocar placement.
In recent years, the transoral endoscopic thyroidectomy submental vestibular approach (TOETSMVA) has been proposed and demonstrated advantages compared to the TOETVA (32). The outcomes of applying this technique in patients complicated with HT will be a focus of our subsequent attention. Additionally, postoperative thyroid dysfunction in patients with HT undergoing thyroid lobectomy has attracted attention, with the study reporting a new disease entity—painless thyroiditis (33). The incidence of thyroid dysfunction in patients with HT undergoing endoscopic thyroid lobectomy is also one of the issues we intend to investigate in the future.
The main limitation of this study is its single-center retrospective design. Multicenter prospective studies, research on underlying mechanisms, and controlled trials examining the impacts of HT on PTC are urgently needed. Second, the short follow-up time of enrolled patients has resulted in recurrence data of limited meaningfulness and a lack of survival data. Third, the small number of specific complication events limits the strength of the conclusions in the complication analysis. Fourth, our study, along with others, has demonstrated that age and gender can influence the outcomes, potentially introducing confounding and bias into the analysis (34). Besides, the six-year duration of this study may have introduced learning curve effects, which could potentially influence both operative time and complication rates. In addition, the choice of surgical approach (TOETVA vs ETCB) was made by patients rather than randomized, which may have led to systematic differences between groups. The main strength of the study is that we enrolled a large number of cases who underwent endoscopic thyroidectomies performed by the same surgical team, minimizing operator-related differences.
In summary, this study suggested that PTC with concomitant HT appears to be associated with indolent tumor characteristics. TOETVA may be safer and more effective than ETCB in the treatment of co-occurring PTC and HT.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Institutional Review Board for ethics at the Guangdong Provincial Hospital of Traditional Chinese Medicine. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
Z-XC: Writing – review & editing, Supervision, Writing – original draft, Formal Analysis, Software, Funding acquisition, Data curation, Validation, Methodology. J-BC: Funding acquisition, Writing – original draft, Supervision, Formal Analysis, Software, Data curation, Methodology, Validation. F-SP: Software, Writing – original draft, Conceptualization, Visualization, Investigation. Z-HL: Investigation, Conceptualization, Software, Writing – original draft, Visualization. J-HL: Conceptualization, Writing – original draft, Software, Visualization, Investigation. W-WZ: Project administration, Methodology, Writing – original draft, Resources. Q-PL: Project administration, Methodology, Writing – original draft, Resources. BX: Writing – review & editing, Validation. YQ: Validation, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. Special Funding for Traditional Chinese Medicine Science and Technology Research at Guangdong Provincial Hospital of Chinese Medicine (No.YN2024MS057).
Acknowledgments
We thank Medjaden Inc. for scientific editing of this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Wang J Yu F Shang Y Ping Z Liu L . Thyroid cancer: incidence and mortality trends in China, 2005-2015. Endocrine. (2020) 68:163–73. doi: 10.1007/s12020-020-02207-6
2
Kim J Gosnell JE Roman SA . Geographic influences in the global rise of thyroid cancer. Nat Rev Endocrinol. (2020) 16:17–29. doi: 10.1038/s41574-019-0263-x
3
Huscher CS Chiodini S Napolitano C Recher A . Endoscopic right thyroid lobectomy. Surg Endosc. (1997) 11:877. doi: 10.1007/s004649900476
4
Benhidjeb T Harlaar J Kerver A Kleinrensink GJ Wilhelm T . Transoral endoscopic thyroidectomy: Part 2: Surgical technique. Chirurg. (2010) 81:134–8. doi: 10.1007/s00104-009-1825-6
5
Anuwong A Ketwong K Jitpratoom P Sasanakietkul T Duh QY . Safety and outcomes of the transoral endoscopic thyroidectomy vestibular approach. JAMA Surg. (2018) 153:21–7. doi: 10.1001/jamasurg.2017.3366
6
Zhang WD Dai L Wang YC Xie YY Guo JY Li JJ et al . Transoral endoscopic thyroidectomy vestibular approach versus endoscopic thyroidectomy via areola approach for patients with unilateral papillary thyroid carcinoma: A retrospective study. Surg Laparosc Endosc Percutan Tech. (2021) 31:550–3. doi: 10.1097/SLE.0000000000000932
7
Benvenga S Trimarchi F . Changed presentation of Hashimoto’s thyroiditis in North-Eastern Sicily and Calabria (Southern Italy) based on a 31-year experience. Thyroid. (2008) 18:429–41. doi: 10.1089/thy.2007.0234
8
Caturegli P De Remigis A Chuang K Dembele M Iwama A Iwama S . Hashimoto’s thyroiditis: celebrating the centennial through the lens of the Johns Hopkins hospital surgical pathology records. Thyroid. (2013) 23:142–50. doi: 10.1089/thy.2012.0554
9
Lee JH Kim Y Choi JW Kim YS . The association between papillary thyroid carcinoma and histologically proven Hashimoto’s thyroiditis: a meta-analysis. Eur J Endocrinol. (2013) 168:343–9. doi: 10.1530/EJE-12-0903
10
Dailey ME Lindsay S Skahen R . Relation of thyroid neoplasms to Hashimoto disease of the thyroid gland. AMA Arch Surg. (1955) 70:291–7. doi: 10.1001/archsurg.1955.01270080137023
11
Lu ZW Hu JQ Liu WL Wen D Wei WJ Wang YL et al . IL-10 restores MHC class I expression and interferes with immunity in papillary thyroid cancer with hashimoto thyroiditis. Endocrinology. (2020) 161. doi: 10.1210/endocr/bqaa062
12
Kim SJ Lee SE Kim YI Nam-Goong IS Jung HW Kim ES . Papillary thyroid cancer with Hashimoto’s thyroiditis attenuates the tumor aggressiveness through the up-regulation of E-cadherin and TGF-beta expression. Clin Exp Med. (2023) 23:833–40. doi: 10.1007/s10238-022-00857-6
13
Hu JQ Lei BW Wen D Ma B Zhang TT Lu ZW et al . IL-2 enhanced MHC class I expression in papillary thyroid cancer with Hashimoto’s thyroiditis overcomes immune escape in vitro. J Cancer. (2020) 11:4250–60. doi: 10.7150/jca.38330
14
Janicki L Patel A Jendrzejewski J Hellmann A . Prevalence and Impact of BRAF mutation in patients with concomitant papillary thyroid carcinoma and Hashimoto’s thyroiditis: a systematic review with meta-analysis. Front Endocrinol (Lausanne). (2023) 14:1273498. doi: 10.3389/fendo.2023.1273498
15
Demir AN Kara Z Sulu C Uysal S Sahin S Zulfaliyeva G et al . Does the association of Hashimoto’s thyroiditis with differentiated thyroid cancer really have a protective role? Horm Metab Res. (2023) 55:388–94. doi: 10.1055/a-2065-0845
16
McManus C Luo J Sippel R Chen H . Is thyroidectomy in patients with Hashimoto thyroiditis more risky? J Surg Res. (2012) 178:529–32. doi: 10.1016/j.jss.2012.09.017
17
D’Orazi V Sacconi A Trombetta S Karpathiotakis M Pichelli D Di Lorenzo E et al . May predictors of difficulty in thyroid surgery increase the incidence of complications? Prospective study with the proposal of a preoperative score. BMC Surg. (2019) 18:116. doi: 10.1186/s12893-018-0447-7
18
Pellizzo MR . Difficult thyroidectomies. G Chir. (2015) 36:49–56. doi: 10.11138/gchir/2015.36.2.049
19
Zhang D Caruso E Sun H Anuwong A Tufano R Materazzi G et al . Classifying pain in transoral endoscopic thyroidectomy. J Endocrinol Invest. (2019) 42:1345–51. doi: 10.1007/s40618-019-01071-0
20
Cunha LL Ferreira RC Marcello MA Vassallo J Ward LS . Clinical and pathological implications of concurrent autoimmune thyroid disorders and papillary thyroid cancer. J Thyroid Res. (2011) 2011:387062. doi: 10.4061/2011/387062
21
Resende de Paiva C Gronhoj C Feldt-Rasmussen U von Buchwald C . Association between Hashimoto’s thyroiditis and thyroid cancer in 64,628 patients. Front Oncol. (2017) 7:53. doi: 10.3389/fonc.2017.00053
22
Xu J Ding K Mu L Huang J Ye F Peng Y et al . Hashimoto’s thyroiditis: A “Double-edged sword” in thyroid carcinoma. Front Endocrinol (Lausanne). (2022) 13:801925. doi: 10.3389/fendo.2022.801925
23
Xu S Huang H Qian J Liu Y Huang Y Wang X et al . Prevalence of Hashimoto thyroiditis in adults with papillary thyroid cancer and its association with cancer recurrence and outcomes. JAMA Netw Open. (2021) 4:e2118526. doi: 10.1001/jamanetworkopen.2021.18526
24
Shen CT Zhang XY Qiu ZL Sun ZK Wei WJ Song HJ et al . Thyroid autoimmune antibodies in patients with papillary thyroid carcinoma: a double-edged sword? Endocrine. (2017) 58:176–83. doi: 10.1007/s12020-017-1401-7
25
Lubin D Baraban E Lisby A Jalali-Farahani S Zhang P Livolsi V . Papillary thyroid carcinoma emerging from hashimoto thyroiditis demonstrates increased PD-L1 expression, which persists with metastasis. Endocr Pathol. (2018) 29:317–23. doi: 10.1007/s12022-018-9540-9
26
Dvorkin S Robenshtok E Hirsch D Strenov Y Shimon I Benbassat CA . Differentiated thyroid cancer is associated with less aggressive disease and better outcome in patients with coexisting Hashimotos thyroiditis. J Clin Endocrinol Metab. (2013) 98:2409–14. doi: 10.1210/jc.2013-1309
27
Zhu F Shen YB Li FQ Fang Y Hu L Wu YJ . The effects of hashimoto thyroiditis on lymph node metastases in unifocal and multifocal papillary thyroid carcinoma: A retrospective chinese cohort study. Med (Baltimore). (2016) 95:e2674. doi: 10.1097/MD.0000000000002674
28
Dinc B Gunduz UR Belen NH . Transoral endoscopic thyroidectomy vestibular approach (TOETVA) in thyroiditis. Surg Laparosc Endosc Percutan Tech. (2020) 31:188–92. doi: 10.1097/SLE.0000000000000864
29
Wang MF Xia H Cai J . The impact of coexisting Hashimoto’s thyroiditis on the feasibility of endoscopic thyroidectomy in papillary thyroid carcinoma. Heliyon. (2024) 10:e26793. doi: 10.1016/j.heliyon.2024.e26793
30
Chen ZX Song YM Chen JB Zhang XB Pang FS Lin ZH et al . Safety and feasibility of the transoral endoscopic thyroidectomy vestibular approach with neuroprotection techniques for papillary thyroid carcinoma. BMC Surg. (2022) 22:270. doi: 10.1186/s12893-022-01707-8
31
Ahn JH Yi JW . Transoral endoscopic thyroidectomy for thyroid carcinoma: outcomes and surgical completeness in 150 single-surgeon cases. Surg Endosc. (2020) 34:861–7. doi: 10.1007/s00464-019-06841-8
32
Hindawi MD Ali AHG Qafesha RM Soliman W Salem H Bali E et al . Transoral endoscopic thyroidectomy submental vestibular approach for early-stage papillary thyroid carcinoma: a systematic review and meta-analysis. Langenbecks Arch Surg. (2024) 409:204. doi: 10.1007/s00423-024-03377-x
33
Sato S Nagayama Y Shindo H Katsuyama K Tatsushima D Mori Y et al . Development of painless thyroiditis after thyroid lobectomy in patients with Hashimoto’s thyroiditis. Endocr Res. (2025), 1–8. doi: 10.1080/07435800.2025.2556056
34
Ma C Xu J Zheng G Liu L Song X Zheng H . Hashimoto’s thyroiditis shows sex- and age-dependent inverse associations with papillary thyroid carcinoma progression: A propensity score-matched analysis of 6963 surgical cases. Ann Surg Oncol. (2025) 32:7498–504. doi: 10.1245/s10434-025-17842-4
Summary
Keywords
Hashimoto’s thyroiditis (HT), papillary thyroid carcinoma, transoral endoscopic thyroidectomy via a vestibular approach, endoscopic thyroidectomy via a chest-breast approach, outcomes
Citation
Chen Z-X, Chen J-B, Pang F-S, Lin Z-H, Lin J-H, Zheng W-W, Liu Q-P, Xu B and Qin Y (2025) The impact of Hashimoto’s thyroiditis on endoscopic thyroidectomy in patients with papillary thyroid carcinoma. Front. Oncol. 15:1623966. doi: 10.3389/fonc.2025.1623966
Received
06 May 2025
Accepted
09 October 2025
Published
22 October 2025
Volume
15 - 2025
Edited by
Pietro Princi, Ospedale Cristo Re, Italy
Reviewed by
Selen Soylu, Istanbul University-Cerrahpasa, Türkiye
Mahmoud Diaa Hindawi, Al-Azhar University, Egypt
Updates
Copyright
© 2025 Chen, Chen, Pang, Lin, Lin, Zheng, Liu, Xu and Qin.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: You Qin, gzqinyou@163.com; Bo Xu, aabb97@163.com; Zhen-Xin Chen, chenzhxn@126.com
†These authors have contributed equally to this work
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.