- 1The Affiliated Cancer Hospital of Nanjing Medical University & Jiangsu Cancer Hospital & Jiangsu Institute of Cancer Research, Nanjing, Jiangsu, China
- 2The Fourth School of Clinical Medicine, Nanjing Medical University, Nanjing, Jiangsu, China
- 3Postgraduate College, Xuzhou Medical University, Xuzhou, China
Background: Recurrent or metastatic nasopharyngeal carcinoma (R/M NPC) that progresses following first-line treatment often ends up with a poor prognosis, and no standard regimens have been established universally. Preclinical studies have suggested that combining vascular endothelial growth factor (VEGF) inhibitors with immune checkpoint inhibitors (ICIs) may exert synergistic antitumor effects. This real-world study aimed to evaluate the efficacy and safety of programmed death-1 (PD-1) inhibitors plus either apatinib or anlotinib, with or without chemotherapy, as a subsequent-line treatment in patients with R/M NPC.
Methods: Between January 1, 2018, and December 12, 2024, a total of 154 patients with R/M NPC were included and treated with various modes of combinations (ITC, IT, IC, I). Among them, 65 received apatinib or anlotinib plus PD-1 inhibitors (ITC+ IT, combination group), and 89 did not receive the addition of apatinib or anlotinib (IC+I, non-combination group). The primary endpoint was progression-free survival (PFS); the secondary endpoints included overall survival (OS), objective response rate (ORR), disease control rate (DCR), and treatment-related adverse events (TRAEs).
Results: As of February 28, 2025, the median follow-up duration was 28.7 months (range 1.3-62.7 months). Compared with the non-combination group, the combination group showed significantly prolonged PFS (20.8 vs. 8.2 months; HR: 0.46, 95% CI: 0.32–0.69; P < 0.001) and OS (34.7 vs. 23.6 months; HR: 0.58, 95% CI: 0.35–0.96; P = 0.042). The combination group also demonstrated higher ORR (47.0% vs. 31.5%; P = 0.041) and DCR (90.8% vs. 82.0%; P = 0.126). The overall incidence of TRAEs was slightly higher in the combination group (96.9% vs. 93.3%; P = 0.599). No treatment-related deaths were reported in either group.
Conclusion: In patients with R/M NPC that progressed after first-line therapy, the combination of anti-angiogenic agents (apatinib or anlotinib) with PD-1 inhibitors based therapy demonstrated a promising antitumor efficacy and an acceptable safety profile. These findings were consistent even among patients from non-endemic regions.
Introduction
Nasopharyngeal carcinoma (NPC), a malignant tumor arising from the nasopharyngeal epithelium, is classified as undifferentiated non-keratinizing carcinoma in approximately 95% of cases. NPC is strongly associated with Epstein-Barr virus (EBV) infection (1). Globally, NPC accounts for an estimated 133,000 new cases and approximately 80,000 deaths annually, representing 0.7% of all the cancer-related mortality (2). The incidence of NPC elevates markedly in Southern China, Southeast Asia, North Africa, and the Middle East, compared to the global average (3). Due to its deep anatomical location and early non-symptoms, NPC has already progressed into a locally advanced stage in approximately 70% of patients at diagnosis. Subsequent to initial treatments, around 30% of patients still experience local recurrence or distant metastasis, a setting in which conventional salvage chemotherapy just provides limited survival benefit, with a median OS ranging from 10 to 20 months (4, 5).
In 2021, three pivotal multicenter phase III randomized controlled trials—CAPTAIN-1st, JUPITER-02, and RATIONALE-309—demonstrated that the combination of PD-1 inhibitors with chemotherapy significantly improved the OS in patients with R/M NPC receiving first-line therapy (6–8). However, no consensus has reached to propose the optimal treatment strategy for patients who experience disease progression following the first-line therapy. PD-1 inhibitors have been evaluated as a monotherapy or a combiner with chemotherapy in subsequent-line treatments, survival outcomes remain suboptimal (9). Accordingly, novel therapeutic strategies are urgently needed.
Apatinib and anlotinib are small-molecule tyrosine kinase inhibitors (TKIs) that exert antitumor effects by selectively silencing vascular endothelial growth factor (VEGF) signaling. VEGF has been shown to suppress the activation of tumor-associated endothelial cells and downregulate endothelial cell-selective adhesion molecule (ESAM), a key mediator of leukocyte adhesion and transendothelial migration. The downregulation of ESAM impairs antitumor immune responses, thereby facilitating immune evasion (10, 11). Anti-angiogenic agents not only inhibit tumor proliferation by modulating immune cell activity and remodeling the immunosuppressive tumor microenvironment, but also enhance immune surveillance by constraining regulatory T cell expansion and promoting the infiltration of immune effector cells into the tumor (12). Moreover, PD-1 inhibitors can suppress VEGF expression, thereby attenuating tumor-associated angiogenesis and further inhibiting tumor progression and metastasis (13). This synergistic interaction between anti-angiogenic therapy and immune checkpoint blockade has been validated in dealing with multiple solid tumors, including cervical, pancreatic, and gastric cancers (14–16).
Beyond these molecular mechanisms, tumors can also been conceptualized as dynamic pathological ecosystems, where cancer cells act as invasive species that interact, compete, and co-evolve with their microenvironment. In NPC, the tumor microenvironment (TME) is composed of malignant cells, stromal elements, vascular networks, and immune cell populations, together forming a complex ecological community. Through reciprocal interactions, tumor cells continuously adapt to evolve, contributing to tumor progression and therapeutic resistance. On this basis, a combination of immune checkpoint inhibitors with anti-angiogenic agents is hypothesized to achieve dual benefits: restoring immune activity while breaking tumor vasculature, thereby facilitating immune cell infiltration and ultimately improving treatment efficacy (17, 18).
Increasing evidence has underscored notable differences in the pathogenesis and tumor immune microenvironment (TIME) of NPC between patients from endemic and non-endemic regions. In endemic regions, NPC is predominantly classified as non-keratinizing undifferentiated carcinoma, and is strongly associated with EBV infection (19). Persistent EBV infection induces chronic inflammatory responses, leading to a tumor microenvironment enriched with regulatory T cells (Tregs), myeloid-derived suppressor cells (MDSCs), and elevated PD-L1 expression, collectively establishing a profoundly immunosuppressive and exhausted immune state. Consequently, patients from endemic regions tend to exhibit higher response rates and more durable clinical benefits, when treated with PD-1/PD-L1 immune checkpoint inhibitors. In contrast, NPC arising in non-endemic regions is more frequently driven by genomic alterations, such as TP53 mutations and CDKN2A inactivation, and is less commonly associated with EBV infection (20). Accordingly, the TIME in non-endemic NPC is typically characterized by a reduced immune cell infiltration and a low PD-L1 expression, reflecting a “cold tumor” phenotype (21). Under such immunologically quiescent conditions, a monotherapy with immune checkpoint inhibitors often fails to elicit a robust antitumor immune response, leading to a lower response rate and a limited clinical efficacy.
In the light of these regional disparities, we conducted a retrospective analysis of 154 patients with R/M NPC from a non-endemic region. This real-world study aimed to evaluate the efficacy and safety of incorporating apatinib or anlotinib into PD-1 inhibitors-based regimens, with or without chemotherapy.
Materials and methods
Inclusion and exclusion criteria
Inclusion criteria were as follows: (1) Histologically confirmed R/M NPC; (2) Disease progression following at least first-line of systemic therapy for recurrent or metastatic disease; (3) Eastern Cooperative Oncology Group (ECOG) performance status of 0-1; (4) Ages between 15 and 80 years; (5) At least one measurable lesion according to the Response Evaluation Criteria in Solid Tumors, version 1.1 (RECIST 1.1); (6) Receipt of at least two cycles of PD-1 inhibitor therapy, administered either as monotherapy or in combination with apatinib or anlotinib.
Exclusion criteria included the following: (1) History of other malignancies; (2) Inability to evaluate treatment efficacy; (3) Receipt of fewer than two cycles of immunotherapy; (4) Receipt of first-line therapy after disease progression; (5) Prior exposure to VEGF inhibitors other than apatinib or anlotinib.
Treatment regimens
Chemotherapy agents: paclitaxel, 260 mg/m²on day 1, intravenous infusion (IV); docetaxel, 75 mg/m²on day 1, IV; gemcitabine, 1 g/m²on days 1 and 8, IV; cisplatin, 70 mg/m²on day 1, IV; 5-fluorouracil, 600 mg/m²on day 1, IV; capecitabine, 650 mg/m², orally twice daily.
PD-1 inhibitors: camrelizumab, tislelizumab, and sintilimab, 200 mg on day 1, IV; toripalimab, 240 mg on day 1, IV.
Anti-angiogenic agents: apatinib, 250 mg orally, once daily; anlotinib, 12 mg orally, once daily on days 1–14 in each 21-day cycle.
One regimen was administered every 3 weeks and continued until disease progression, unacceptable toxicity, or necessary treatment modification or discontinuation decided by the physician.
Endpoints and assessment
Objective response rate (ORR) was defined as the proportion of patients achieving a complete response (CR) or partial response (PR). Disease control rate (DCR) was defined as the proportion of patients achieving CR, PR, or stable disease (SD). OS was calculated from the date of treatment initiation to the date of death from any cause. Progression-free survival (PFS) was calculated from treatment initiation to the first documented disease progression or death. Treatment response was evaluated according to the RECIST v1.1, after 2–3 treatment cycles. Adverse events (AEs) were assessed and graded in accordance with the Common Terminology Criteria for Adverse Events, version 5.0 (CTCAE v5.0). The primary endpoint was PFS. The secondary endpoints included OS, ORR, DCR, and the incidence of treatment-related AEs.
Statistical analysis
Baseline characteristics, tumor response rates, and AEs were compared using the chi-square (χ²) test. The Kaplan-Meier analysis was employed to estimate survival outcomes, and differences between groups were assessed using the log-rank test. Prognostic factors and subgroup analyses were evaluated using the Cox proportional hazards regression model. All statistical analyses were performed using R software and GraphPad Prism version 10.2.3. P-value <0.05 was considered statistically significant.
Results
Patient characteristics
A total of 154 patients with R/M NPC progressing after at least one line of salvage therapy were included and categorized into four treatment groups. The median follow-up duration was 28.7 months (range 1.3–62.7 months), during which 103 patients (66.9%) experienced disease progression. Among these, 31 patients (30.1%) showed locoregional recurrence, while 72 (69.9%) showed distant metastases. As of the data cutoff (February 28, 2025), 63 patients (40.9%) had died.
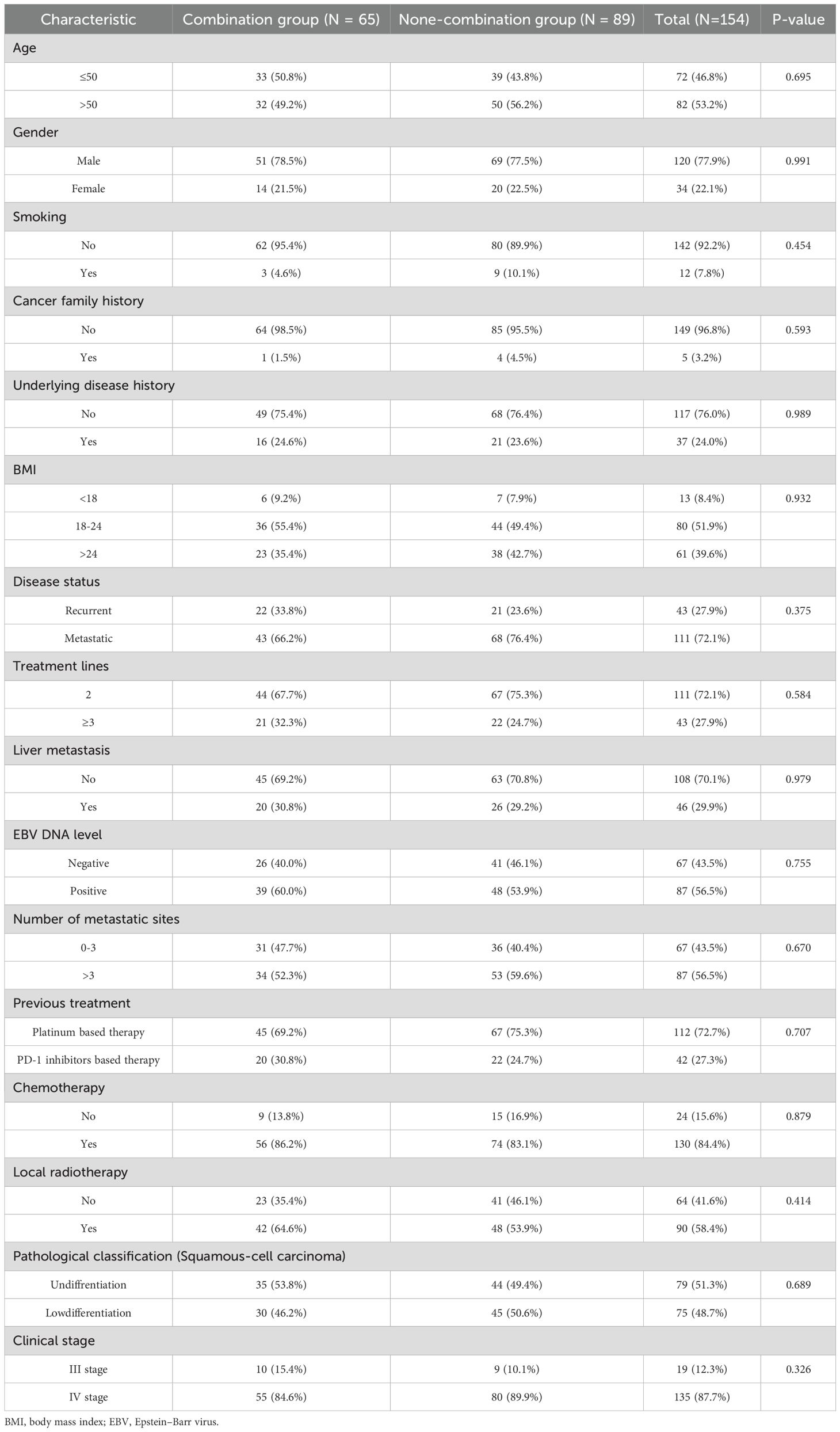
Of the enrolled patients, 56 received the ITC regimen (PD-1 inhibitor + chemotherapy + apatinib/anlotinib), 74 received the IC regimen (PD-1 inhibitor + chemotherapy), 15 received the I regimen (PD-1 inhibitor monotherapy), and 9 received the IT regimen (PD-1 inhibitor + apatinib/anlotinib). Considering the relatively small sample sizes of the IT and I subgroups, to evaluate the impact of incorporating anti-angiogenic agents on clinical outcomes, the patients receiving the ITC and IT regimens were grouped into the combination arm (n = 65), while those receiving the IC and I regimens into the non-combination arm (n = 89). The patient selection process is detailed in Figure 1. The median age of the study population was 46 years (range 18–71 years), with a male-to-female ratio of 3.5:1. Within the combination arm, 13 patients (20.0%) received apatinib and 52 (80.0%) received anlotinib. Regarding PD-1 inhibitor administration, 47 patients (30.5%) were treated with camrelizumab, 41 (26.6%) with toripalimab, 38 (24.7%) with tislelizumab, and 28 (18.2%) with sintilimab. Baseline clinical and demographic characteristics are summarized in Table 1; Supplementary Table S1, with no significant differences observed between the two arms or two major treatment subgroups (ITC subgroup and IC subgroup).
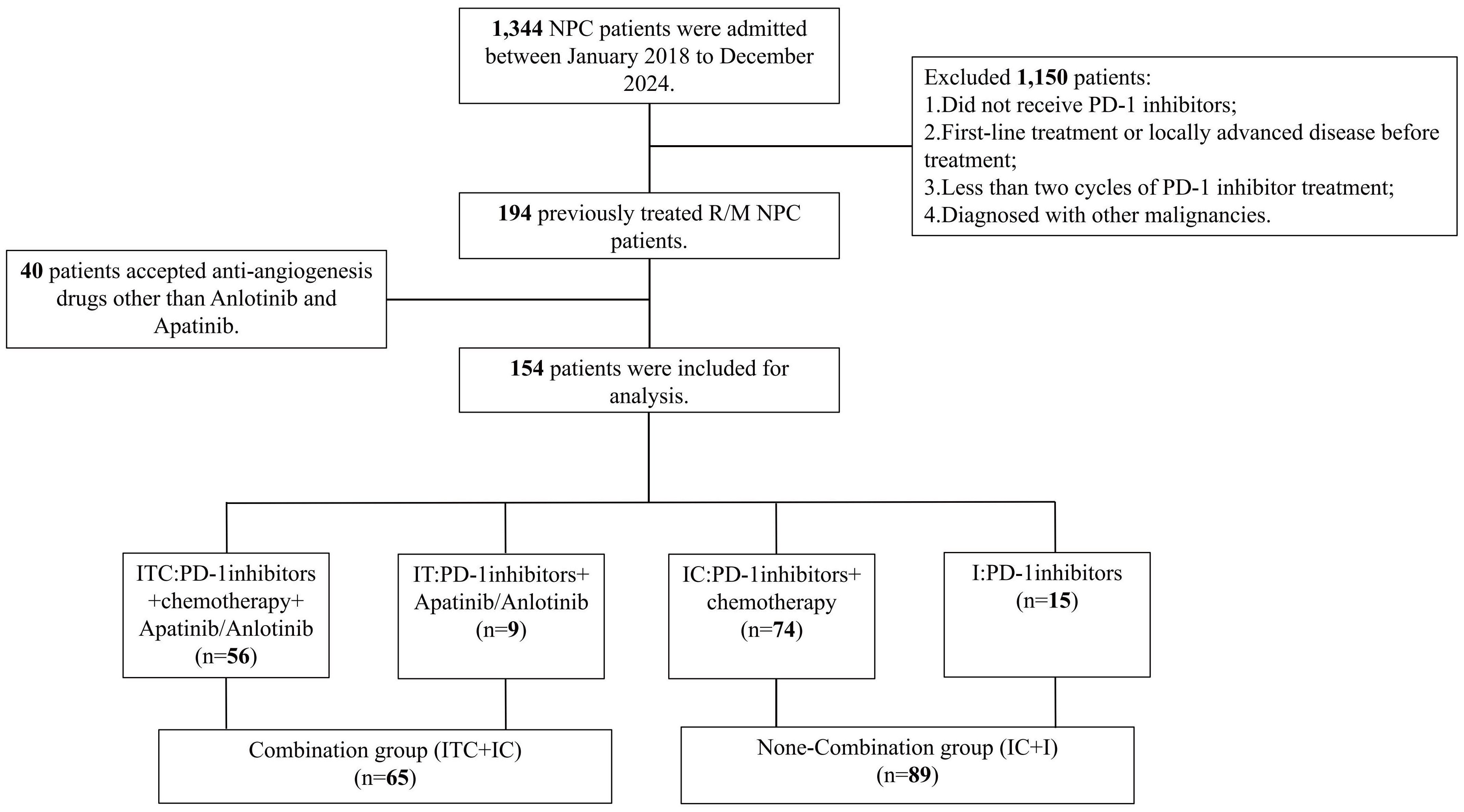
Figure 1. Detailed patient selection process. ITC, PD-1 inhibitors plus apatinib or anlotinib and chemotherapy; IT, PD-1 inhibitors plus apatinib or anlotinib; IC, PD-1 inhibitors plus chemotherapy.
Tumor response
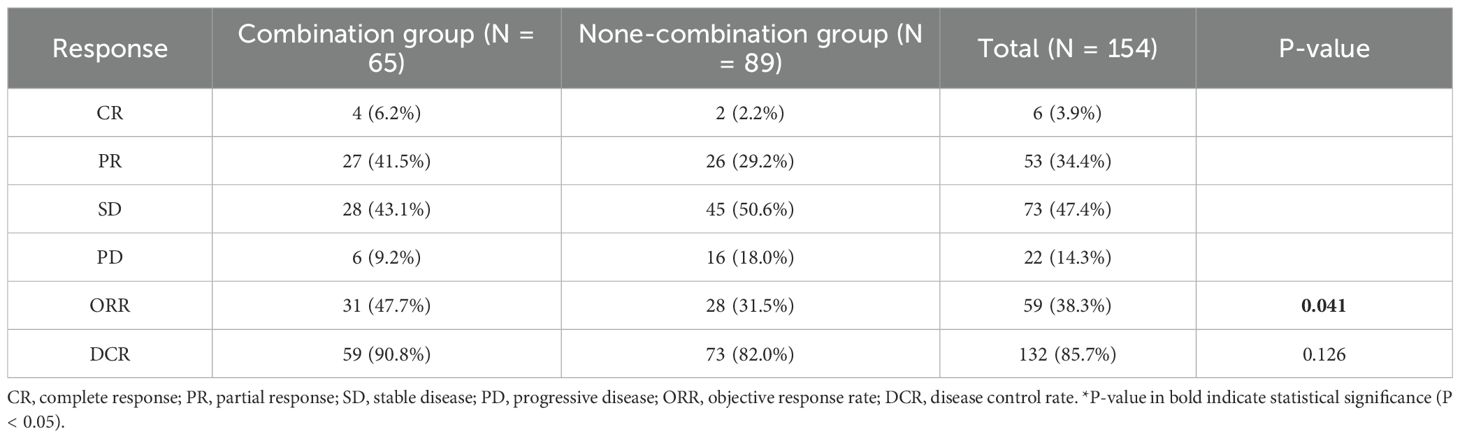
Among the 65 patients in the combination arm, 4 (6.2%) achieved CR, 27 (41.5%) achieved PR, 28 (43.1%) had SD, and 6 (9.2%) experienced PD. The ORR in the combination group was 69.1%, significantly higher than that observed in the non-combination group (49.5%) (P = 0.011). However, no significant difference in DCR was noted between the two groups (90.8% vs. 82.0%, P = 0.837). Detailed results are presented in Table 2. In addition, no statistically significant differences in ORR (48.2% vs. 31.7%, P = 0.139) and DCR (92.9% vs. 81.1%, P = 0.157) were observed between the ITC subgroup and the IC subgroup. (Supplementary Table S2).
Survival outcomes
The median follow-up duration was 28.7 months. The median PFS was significantly longer in the combination group, compared with the non-combination group (20.8 months vs. 8.2 months; P < 0.001; Figure 2A). Among the four cohorts, the median PFS was as follows: ITC, 20.8 months; IT, 11.6 months; IC, 8.4 months; and I, 6.7 months. Notably, the ITC cohort demonstrated a statistically significant PFS advantage over the IC cohorts (P < 0.001; Figure 2B). Similarly, the OS was significantly prolonged in the combination arm, compared with the non-combination arm (34.7 months vs. 23.6 months; P = 0.043; Figure 3A). When comparing individual cohorts, a statistically significant difference in OS was observed only between the ITC and IC cohorts (34.7 months vs. 23.1 months; P = 0.016), while no significant differences were identified among the remaining cohorts (Figure 3B).
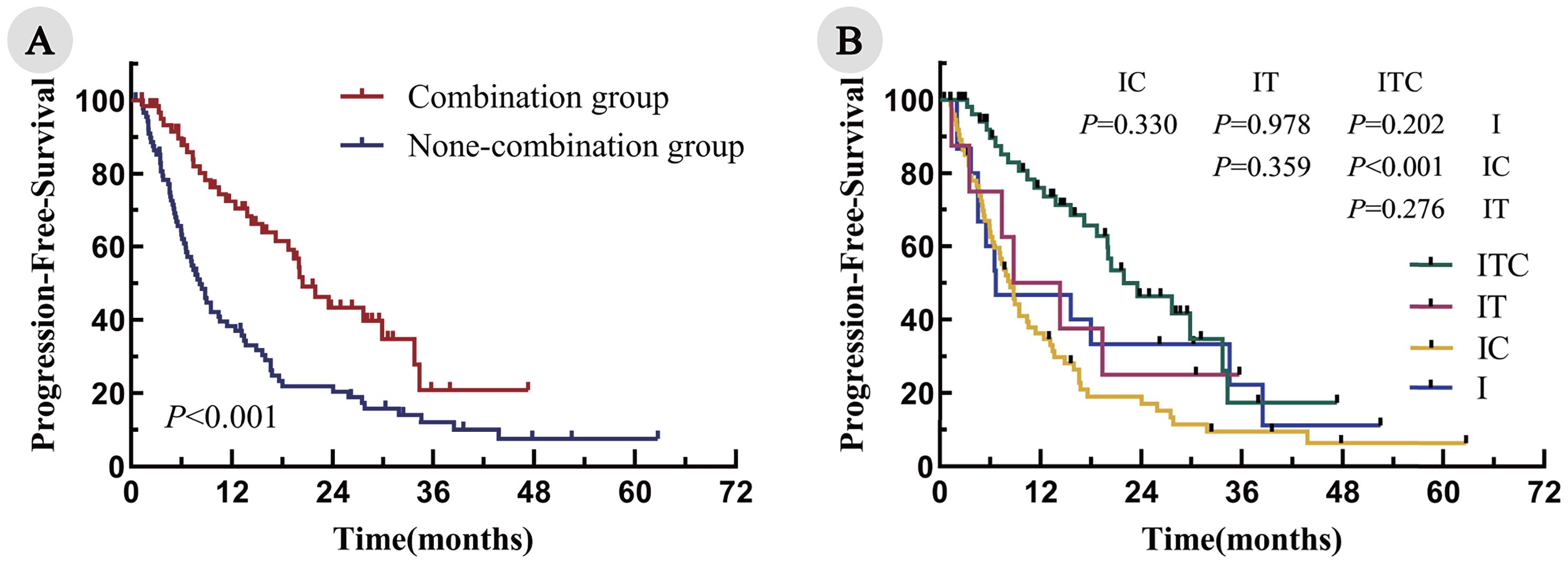
Figure 2. Kaplan–Meier curves for PFS in all enrolled patients. (A) Combination group vs. non-combination group; (B) ITC, IT, IC, and I cohorts.
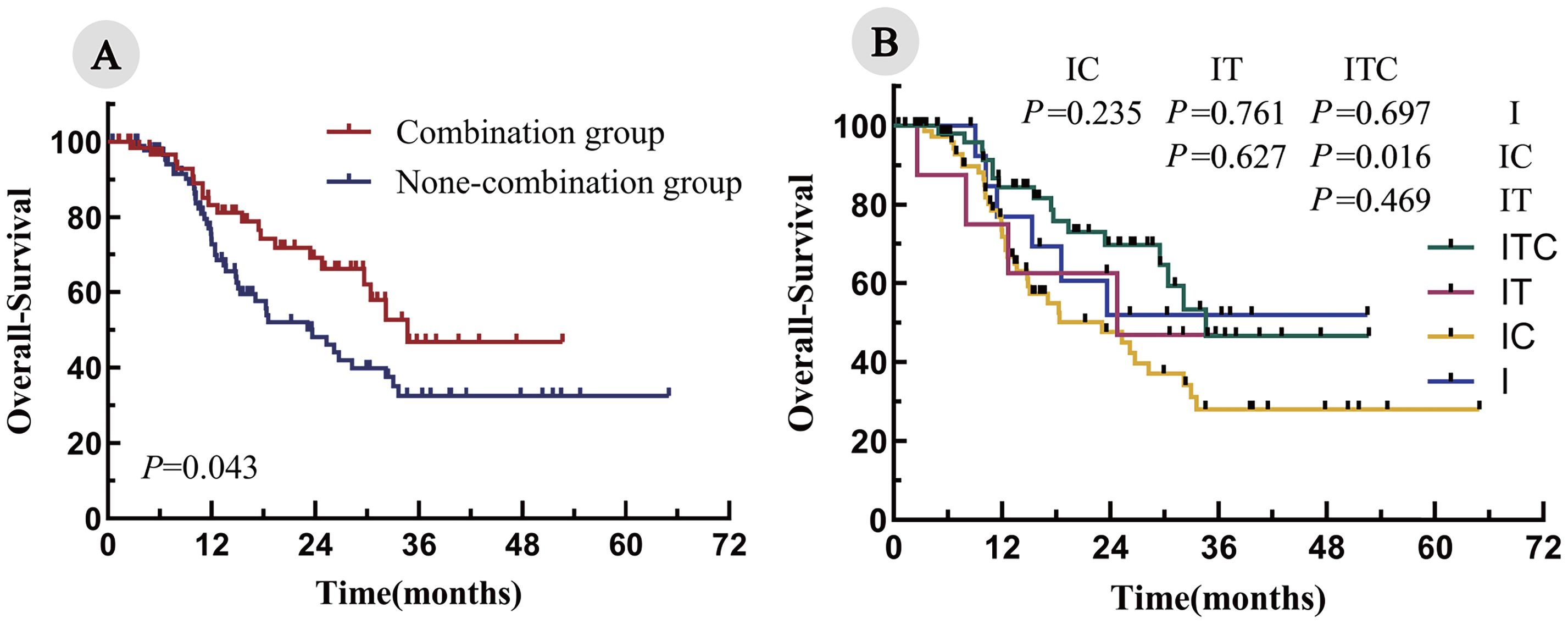
Figure 3. Kaplan–Meier curves for OS in all enrolled patients. (A) Combination group vs. none-combination group; (B) ITC, IT, IC, and I cohorts.
The multivariate Cox regression analysis, adjusted for relevant prognostic factors, identified combination therapy as an independent predictor of improved PFS (hazard ratio [HR] = 0.391; P < 0.001). Additional favorable prognostic indicators included serum lactate dehydrogenase (LDH) ≤240 U/L, prognostic nutritional index (PNI) >47.2, 0–3 metastatic sites, and receipt of more than six treatment cycles (Table 3; Supplementary Figure S1). However, OS did not demonstrate independent prognostic value (hazard ratio [HR] = 0.823; P = 0.522) (Table 4). The analysis of the ITC and IC subgroups also revealed similar results (Supplementary Tables S3, S4).
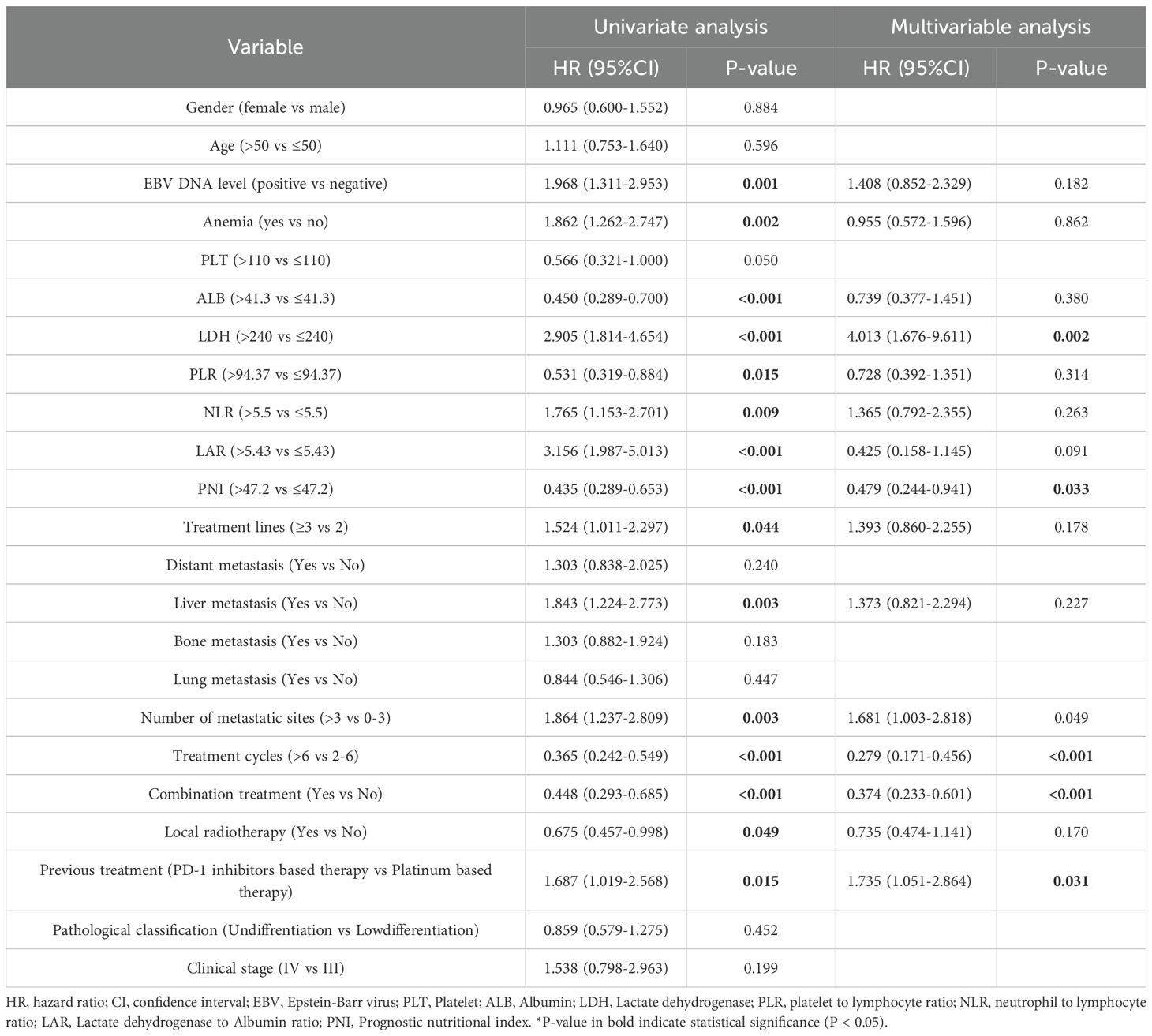
Table 3. Univariate and multivariate Cox regression analysis of prognostic factors in all enrolled patients (PFS).
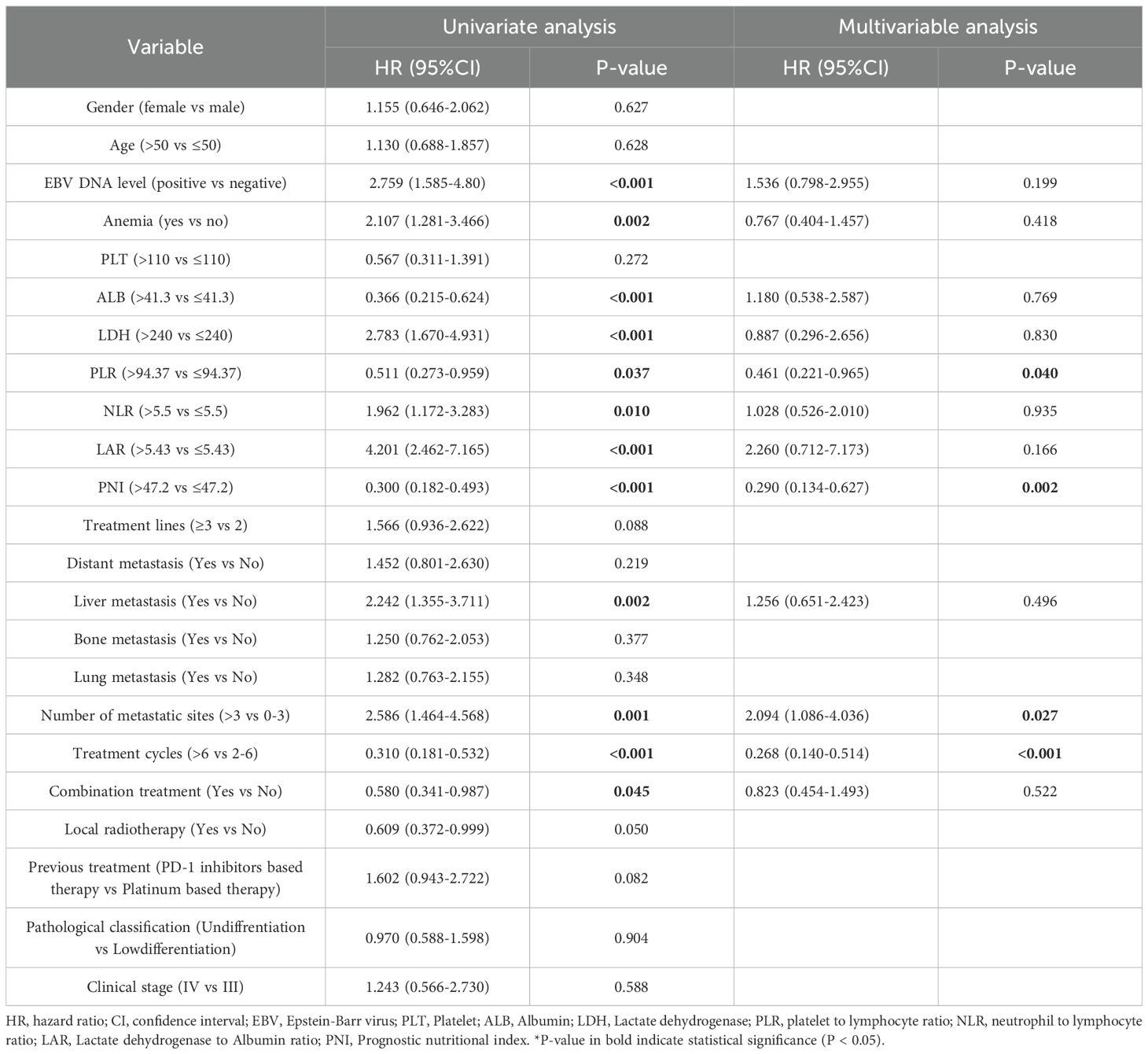
Table 4. Univariate and multivariate Cox regression analysis of prognostic factors in all enrolled patients (OS).
The subgroup analysis further demonstrated that the combination group was associated with the prolonged PFS and OS across all subgroups, with the most pronounced benefit observed in male patients or an absence of liver metastases (P < 0.05). No significant interaction effects were identified among subgroups (Figures 4A, B; Supplementary Figures S2A, B). In addition, the stratified analysis suggested that, across all treatment subgroups, patients with prior PD-1 inhibitor exposure had shorter PFS and OS compared with PD-1 naïve patients, as specifically shown in Supplementary Figure S3.
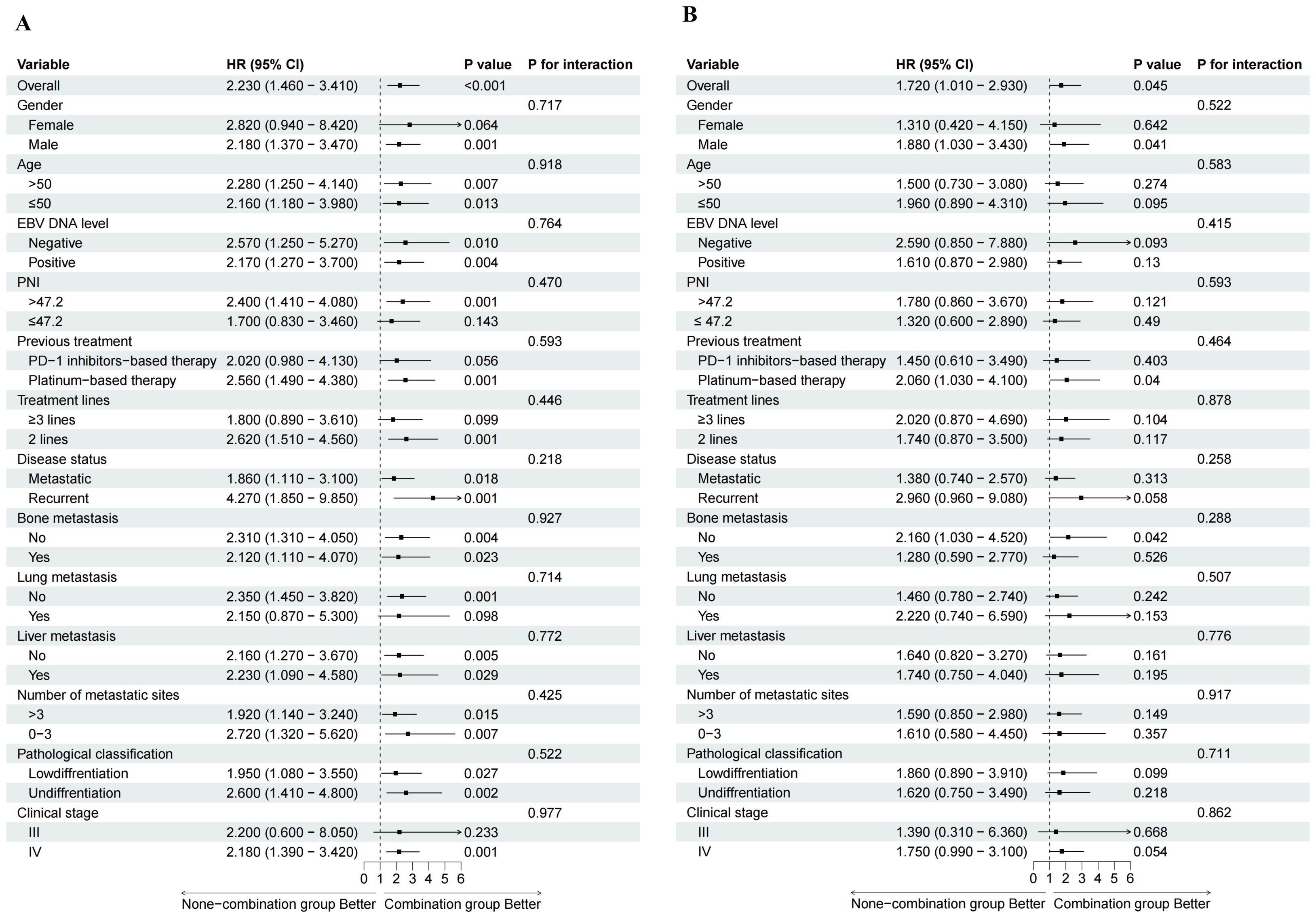
Figure 4. Forest plot of subgroup analyses. HR, hazard ratio; CI, confidence interval; EBV, Epstein-Barr virus; PNI, Prognostic nutritional index. (A) subgroup analyses for PFS; (B) subgroup analyses for OS.
AEs
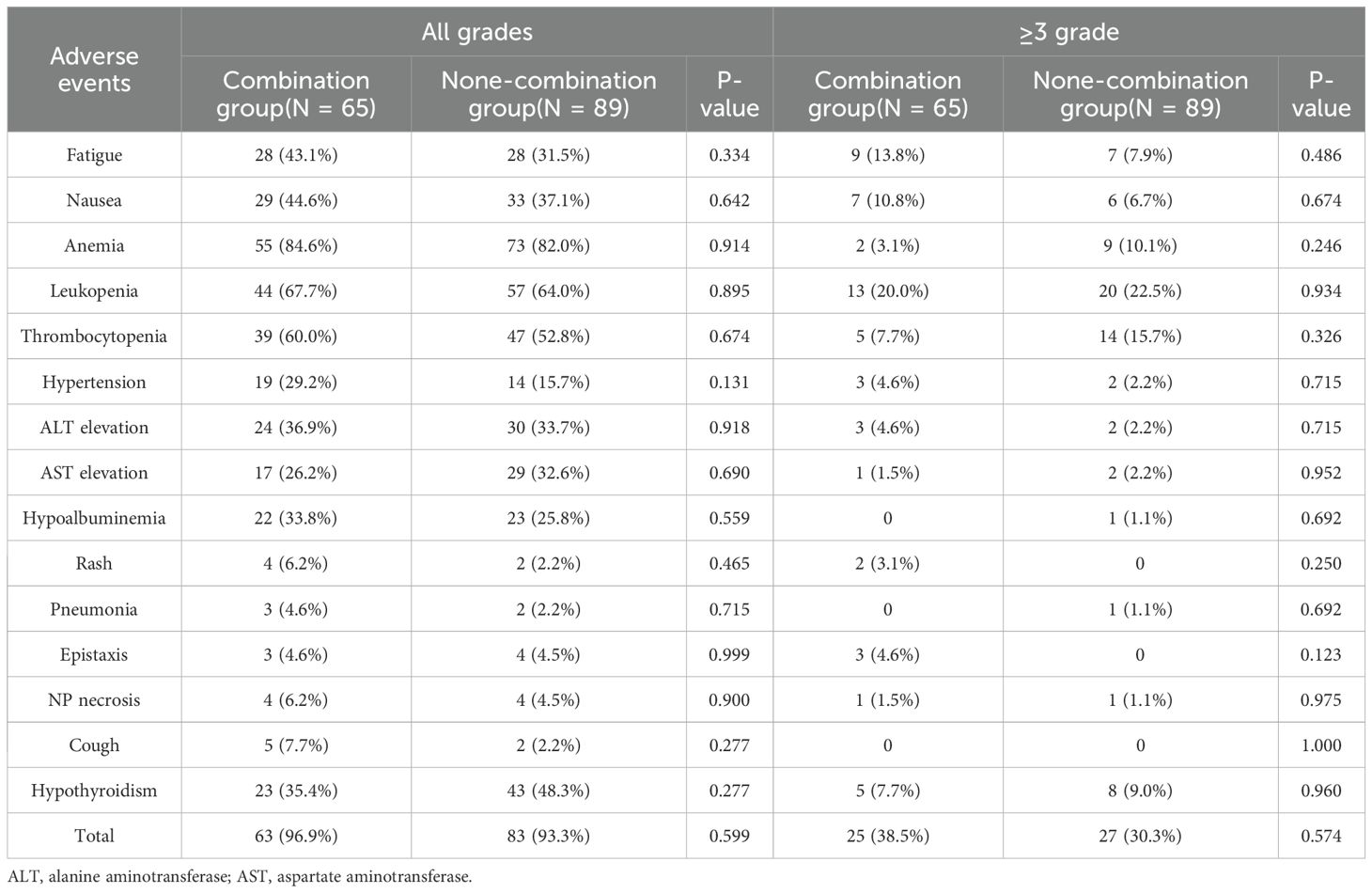
As of the last follow-up, no treatment-related deaths were reported. The most frequently observed AEs in both arms involved anemia (84.6% vs. 82.0%; P = 0.914) and leukopenia (67.7% vs. 64.0%; P = 0.895). The overall incidence of any-grade AEs was slightly higher in the combination group, compared to the non-combination group (96.9% vs. 93.3%; P = 0.599), so was the incidence of grade ≥3 AEs (38.5% vs. 30.3%; P = 0.574). The combination group exhibited higher incidences of nasopharyngeal necrosis (6.2% vs. 4.5%; P = 0.900) and hypertension (29.2% vs. 15.7%; P = 0.131), although these differences were not statistically significant. Conversely, the combination group demonstrated lower rates of grade ≥3 anemia (3.1% vs. 10.1%; P = 0.246), thrombocytopenia (7.7% vs. 15.7%; P = 0.326), and leukopenia (20.0% vs. 22.5%; P = 0.934) (Table 5; Supplementary Table S5).
Discussion
To the best of our knowledge, this is the first study to evaluate the efficacy and safety of PD-1 inhibitors combined with apatinib or anlotinib, with or without chemotherapy, in patients with R/M NPC who had experienced disease progression following the first-line therapy in non-endemic regions. Although the introduction of ICIs has improved clinical outcomes in this population, the overall prognosis remains unsatisfactory. Previous studies have reported that PD-1 inhibitor monotherapy yields a median PFS of approximately 1.9 to 6.5 months and an ORR of 21%-26% (22). In this light, new combination strategies are urgently needed to enhance treatment efficacy.
Several clinical trials, conducted in highly endemic regions, have investigated the antitumor efficacy of PD-1 inhibitors combined with anti-angiogenic agents in patients with advanced head and neck squamous cell carcinoma (HNSCC), reporting an ORR ranging from 33.3% to 65.5% and a median PFS between 6.0 and 14.3 months (23–27). In a retrospective analysis, Jiang et al. have compared the efficacy of ICIs in combination with either VEGF/VEGFR or EGFR inhibitors plus chemotherapy in the subsequent-line setting (28). The addition of targeted agents significantly prolongs the median PFS, compared with chemotherapy alone or chemotherapy plus ICIs (19.1 vs. 9.8 months; P < 0.001), consistent with our study findings. Moreover, our data demonstrated a significant improvement in the median OS in the combination group. Prognostic factor analysis further identified hematological markers, such as LDH and PNI, as independent predictors of clinical outcomes. Previous studies have shown that elevated baseline LDH levels are frequently associated with an increased tumor burden and a hypoxic tumor microenvironment (29). Additionally, LDH has been implicated in the activation of oncogenic signaling pathways, tumor metabolism, invasiveness, and immunologic modulation (30). Here, our subgroup analysis revealed that combination therapy provided a greater clinical benefit among male patients, those who were EBV-DNA positive, or those who had previously failed the platinum-based chemotherapy. These findings offer valuable insight for future patient stratification in clinical practice.
In recent years, disease progression has increasingly challenged the efficacy of immunotherapy, underscoring the urgent need to improve clinical outcomes in this setting. The mechanisms underlying the immune resistance to PD-1 inhibitors are multifactorial and remain incompletely understood. On the one hand, tumor cells can evade immune surveillance by downregulating the expression of major histocompatibility complex class I (MHC-I) molecules and other key components of the antigen presentation machinery. On the other hand, an elevated PD-L1 expression on tumor cells facilitates PD-1/PD-L1 binding, thus initiating inhibitory signaling pathways that impair T cell activation and attenuate antitumor immune responses (31, 32). Ding et al. and Yuan et al. have independently assessed the efficacy of camrelizumab in combination with either apatinib or famitinib in patients exhibiting immune-refractory disease, reporting an ORR of approximately 34% (26, 33). In addition, Xiang et al. have shown that the combination of ICIs with targeted therapies yields a significantly higher DCR and a prolonged median PFS, compared with chemotherapy alone (P < 0.001). Although our stratified and subgroup analyses indicated a favorable prognostic trend for the combination group or the ITC subgroup in immune-resistant patients, the unique benefit of this combination strategy for this population could not be confirmed. Future studies with larger sample sizes and prospective designs are warranted to further validate these findings and to provide more robust evidence for precision therapy in immune-resistant patients.
With regard to treatment-related AEs, no significant differences were observed between the two groups, and no treatment-related deaths occurred, indicating that the combination regimen maintains a manageable profile of safety. Myelosuppression, gastrointestinal symptoms, and hypothyroidism were the most commonly reported AEs in both arms. Notably, the incidence of grade ≥3 myelosuppression was lower in the combination group, potentially attributed to dose reductions in platinum-based chemotherapy when used alongside apatinib or anlotinib. However, the combination groups exhibited a higher incidence of severe nasopharyngeal necrosis or epistaxis, compared to the non-combination group. Prior studies have identified radiotherapy doses ≥72 Gy, re-irradiation, locally advanced disease, diabetes mellitus, and smoking history as risk factors for nasopharyngeal necrosis (34–36). Therefore, early identification of high-risk patients and timely clinical intervention during the combination therapy are essential to mitigate the risk of serious complications.
While this study contributes valuable data on the efficacy of combination therapy in R/M NPC patients from non-endemic regions, several limitations should be acknowledged. First, given that all patients were enrolled from a single center, the generalizability of the findings may be limited. Second, this study failed to fully demonstrate an independent advantage of the combination regimen in extending the OS, which may be attributed to the relatively small sample size and insufficient follow-up duration; therefore, larger, multicenter, randomized controlled trials are warranted to validate these results. Third, as this was a retrospective study, inherent heterogeneity could not be fully avoided, particularly in terms of the diversity of chemotherapy regimens and PD-1 inhibitors administered across groups, which also limited our ability to directly determine the impact of specific combination strategies on patient prognosis. Moreover, the relatively small sample sizes of the IT and I subgroups precluded the performance of multi-arm subgroup analyses. Lastly, the subset of patients lacked data on PD-L1 tumor expression, which prevented its inclusion in subgroup analyses and related exploratory assessments.
Conclusion
The combination of anti-angiogenic agents (apatinib or anlotinib) with PD-1 inhibitors based therapy significantly prolonged the PFS and OS in patients with R/M NPC who had undergone failures in prior therapies, without a notable increase in treatment-related AEs. This combination strategy offers a promising antitumor effect and an acceptable safety in patients with R/M NPC from non-endemic regions, supporting its potential as a subsequent-line therapeutic option.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Ethics Committee of Jiangsu Cancer Hospital (Approval No. 2020-055). The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because Given the retrospective nature of the study, the requirement for informed consent was waived.
Author contributions
JG: Writing – original draft, Writing – review & editing. JX: Writing – review & editing, Writing – original draft. YH: Writing – review & editing, Writing – original draft. DZ: Validation, Supervision, Formal analysis, Writing – review & editing. TJ: Writing – review & editing, Formal analysis, Data curation. XH: Writing – review & editing, Funding acquisition, Resources, Supervision.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was supported by grants from the National Natural Science Foundation of China (No. 82172804), the Key Project of Jiangsu Provincial Health Commission (No. K2019028), the Nanjing Science and Technology Plan Project (No. 2022SX00001663), and the Nasopharyngeal Carcinoma Cohort Program of Nanjing Medical University (No. NMUC2021011A).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2025.1624286/full#supplementary-material
Supplementary Table 1 | Baseline characteristics of the two treatment subgroups (ITC and IC). BMI, body mass index; EBV, Epstein–Barr virus.
Supplementary Table 2 | Tumor response in the two treatment groups (ITC and IC). CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; ORR, objective response rate; DCR, disease control rate.
Supplementary Table 3 | Univariate and multivariate Cox regression analysis of prognostic factors in ITC and IC subgroups (PFS). HR, hazard ratio; CI, confidence interval; EBV, Epstein-Barr virus; PLT, Platelet; ALB, Albumin; LDH, Lactate dehydrogenase; PLR, platelet to lymphocyte ratio; NLR, neutrophil to lymphocyte ratio; LAR, Lactate dehydrogenase to Albumin ratio; PNI, Prognostic nutritional index.
Supplementary Table 4 | Univariate and multivariate Cox regression analysis of prognostic factors in ITC and IC subgroups (OS). HR, hazard ratio; CI, confidence interval; EBV, Epstein-Barr virus; PLT, Platelet; ALB, Albumin; LDH, Lactate dehydrogenase; PLR, platelet to lymphocyte ratio; NLR, neutrophil to lymphocyte ratio; LAR, Lactate dehydrogenase to Albumin ratio; PNI, Prognostic nutritional index.
Supplementary Table 5 | Treatment-related adverse events in ITC and IC subgroups. ALT, alanine aminotransferase; AST, aspartate aminotransferase.
Supplementary Figure 1 | The optimal cut-off values for hematological markers were determined using the surv_cutpoint function from the survminer package in R, which identifies the most statistically significant threshold based on maximally selected rank statistics. (PLT, Platelet; ALB, Albumin; LDH, Lactate dehydrogenase; PLR, platelet to lymphpcyte ratio; NLR, neutrophil to lymphocyte ratio; LAR, Lactate dehydrogenase to Albumin ratio; PNI, Prognostic nutritional index).
Supplementary Figure 2 | Forest plot of subgroup analyses (ITC and IC). HR, hazard ratio; CI, confidence interval; EBV, Epstein-Barr virus; PNI, Prognostic nutritional index. (A) subgroup analyses for PFS; (B) subgroup analyses for OS.
Supplementary Figure 3 | Stratified analysis of PFS and OS across all treatment subgroups, categorized by prior exposure to PD-1 inhibitor–based therapy. (A) stratified analysis of PFS across all enrolled patients (154); (B) stratified analysis of OS across all enrolled patients (154); (C) stratified analysis of PFS across ITC and IC subgroups (130); (D) stratified analysis of OS across ITC and IC subgroups (130); (E) stratified analysis of PFS across combination arm (65); (F) stratified analysis of OS across combination arm (65); (G) stratified analysis of PFS across non-combination arm (89); (H) stratified analysis of OS across non-combination arm (89); (I) stratified analysis of PFS across ITC subgroup (56); (J) stratified analysis of OS across ITC subgroup (56); (K) stratified analysis of PFS across IC subgroup (74); (L) stratified analysis of OS across IC subgroup (74).
References
1. Chen YP, Chan ATC, Le QT, Blanchard P, Sun Y, Ma J, et al. Nasopharyngeal carcinoma. Lancet (London England). (2019) 394:64–80. doi: 10.1016/S0140-6736(19)30956-0
2. Prawira A, Oosting SF, Chen TW, Delos Santos KA, Saluja R, Wang L, et al. Systemic therapies for recurrent or metastatic nasopharyngeal carcinoma: a systematic review. Br J Cancer. (2017) 117:1743–52. doi: 10.1038/bjc.2017.357
3. Chang ET, Ye W, Zeng YX, and Adami HO. The evolving epidemiology of nasopharyngeal carcinoma. Cancer Epidemiol Biomarkers Prev. (2021) 30:1035–47. doi: 10.1158/1055-9965.EPI-20-1702
4. Cheng G, Dong H, Yang C, Liu Y, Wu Y, Zhu L, et al. A review on the advances and challenges of immunotherapy for head and neck cancer. Cancer Cell Int. (2021) 21:406. doi: 10.1186/s12935-021-02024-5
5. Du XJ, Wang GY, Zhu XD, Han YQ, Lei F, Shen LF, et al. Refining the 8th edition TNM classification for EBV related nasopharyngeal carcinoma. Cancer Cell. (2024) 42:464–73.e3. doi: 10.1016/j.ccell.2023.12.020
6. Mai HQ, Chen QY, Chen D, Hu C, Yang K, Wen J, et al. Toripalimab plus chemotherapy for recurrent or metastatic nasopharyngeal carcinoma: the JUPITER-02 randomized clinical trial. JAMA. (2023) 330:1961–70. doi: 10.1001/jama.2023.20181
7. Yang Y, Qu S, Li J, Hu C, Xu M, Li W, et al. Camrelizumab versus placebo in combination with gemcitabine and cisplatin as first-line treatment for recurrent or metastatic nasopharyngeal carcinoma (CAPTAIN-1st): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. (2021) 22:1162–74. doi: 10.1016/S1470-2045(21)00302-8
8. Yang Y, Pan J, Wang H, Zhao Y, Qu S, Chen N, et al. Tislelizumab plus chemotherapy as first-line treatment for recurrent or metastatic nasopharyngeal cancer: A multicenter phase 3 trial (RATIONALE-309). Cancer Cell. (2023) 41:1061–72. doi: 10.1016/j.ccell.2023.04.014
9. Yan L, Ren B, Hu R, Zhang H, and Gou H. Are PD-1 inhibitors effective for recurrent/metastatic nasopharyngeal carcinoma? Meta-analysis and systematic review. Front Pharmacol. (2022) 13:131095734. doi: 10.3389/fphar.2022.1095734
10. Yap TA, Parkes EE, Peng W, Moyers JT, Curran MA, Tawbi HA, et al. Development of immunotherapy combination strategies in cancer. Cancer Discov. (2021) 11:1368–97. doi: 10.1158/2159-8290.CD-20-1209
11. Zhang X, Zeng L, Li Y, Xu Q, Yang H, Lizaso A, et al. Anlotinib combined with PD-1 blockade for the treatment of lung cancer: a real-world retrospective study in China. Cancer Immunol Immunother. (2021) 70:2517–28. doi: 10.1007/s00262-021-02869-9
12. Ma X, Huang J, Wu X, Li X, Zhang J, Xue L, et al. Epidermal growth factor receptor could play a prognostic role to predict the outcome of nasopharyngeal carcinoma: A meta-analysis. Cancer Biomarkers: Section A Dis Markers. (2014) 14:267–77. doi: 10.3233/CBM-140401
13. Chen L, Lin G, Chen K, Liang R, Wan F, Zhang C, et al. VEGF promotes migration and invasion by regulating EMT and MMPs in nasopharyngeal carcinoma. J Cancer. (2020) 11:7291–301. doi: 10.7150/jca.46429
14. Cheng D, Hu J, Wu X, Wang B, Chen R, Zhao W, et al. PD-1 blockade combined with gemcitabine plus nab-paclitaxel is superior to chemotherapy alone in the management of unresectable stage III/IV pancreatic cancer: a retrospective real-world study. Front Oncol. (2023) 13:131281545. doi: 10.3389/fonc.2023.1281545
15. Janjigian YY, Kawazoe A, Bai Y, Xu J, Lonardi S, Metges JP, et al. Pembrolizumab plus trastuzumab and chemotherapy for HER2-positive gastric or gastro-oesophageal junction adenocarcinoma: interim analyses from the phase 3 KEYNOTE-811 randomised placebo-controlled trial. Lancet (London England). (2023) 402:2197–208. doi: 10.1016/S0140-6736(23)02033-0
16. Lorusso D, Xiang Y, Hasegawa K, Scambia G, Leiva M, Ramos-Elias P, et al. Pembrolizumab or placebo with chemoradiotherapy followed by pembrolizumab or placebo for newly diagnosed, high-risk, locally advanced cervical cancer (ENGOT-cx11/GOG-3047/KEYNOTE-A18): a randomised, double-blind, phase 3 clinical trial. Lancet (London England). (2024) 403:1341–50. doi: 10.1016/S0140-6736(24)00317-9
17. Luo W. Nasopharyngeal carcinoma ecology theory: cancer as multidimensional spatiotemporal “Unity of ecology and evolution” Pathological ecosystem. Theranostics. (2023) 13:1607–31. doi: 10.7150/thno.82690
18. Luo WR. Rethinking cancer. Zhonghua zhong liu za. (2025) 47:463–7. doi: 10.3760/cma.j.cn112152-20250401-00145
19. Young LS and Rickinson AB. Epstein-Barr virus: 40 years on. Nat Rev Cancer. (2004) 4:757–68. doi: 10.1038/nrc1452
20. Hildesheim A and Wang CP. Genetic predisposition factors and nasopharyngeal carcinoma risk: a review of epidemiological association studies, 2000-2011: Rosetta Stone for NPC: genetics, viral infection, and other environmental factors. Semin Cancer Biol. (2012) 22:107–16. doi: 10.1016/j.semcancer.2012.01.007
21. Chang ET and Adami HO. The enigmatic epidemiology of nasopharyngeal carcinoma. Cancer Epidemiol Biomarkers Prev. (2006) 15:1765–77. doi: 10.1158/1055-9965.EPI-06-0353
22. Sun H, Bu F, Li L, Zhang X, Xin X, Yan J, et al. Efficacy and safety of immune checkpoint inhibitors combined with chemotherapy as first-line treatment for recurrent or metastatic nasopharyngeal carcinoma: A network meta-analysis of randomized controlled trials. Ann Pharmacother. (2024) 58:349–59. doi: 10.1177/10600280231188171
23. Chong WQ, Low JL, Tay JK, Le TBU, Goh GS, Sooi K, et al. Pembrolizumab with or without bevacizumab in platinum-resistant recurrent or metastatic nasopharyngeal carcinoma: a randomised, open-label, phase 2 trial. Lancet Oncol. (2025) 26:175–86. doi: 10.1016/S1470-2045(24)00677-6
24. Ding X, Zhang WJ, You R, Zou X, Wang ZQ, Ouyang YF, et al. Camrelizumab plus apatinib in patients with recurrent or metastatic nasopharyngeal carcinoma: an open-label, single-arm, phase II study. J Clin Oncol. (2023) 41:2571–82. doi: 10.1200/JCO.22.01450
25. Mo Y, Pan Y, Zhang B, Zhang J, Su Y, Liu Z, et al. Apatinib combined with camrelizumab in the treatment of recurrent/metastatic nasopharyngeal carcinoma: a prospective multicenter phase II study. Front Immunol. (2023) 14:141298418. doi: 10.3389/fimmu.2023.1298418
26. Yuan L, Jia GD, Lv XF, Xie SY, Guo SS, Lin DF, et al. Camrelizumab combined with apatinib in patients with first-line platinum-resistant or PD-1 inhibitor resistant recurrent/metastatic nasopharyngeal carcinoma: a single-arm, phase 2 trial. Nat Commun. (2023) 14:4893. doi: 10.1038/s41467-023-40402-x
27. Zhang Y, Zou Q, Zhao B, Su N, Li Z, Wang X, et al. Toripalimab plus anlotinib in patients with recurrent or metastatic nasopharyngeal carcinoma: A multicenter, single-arm phase 2 trial (TORAL). CR Med. (2024) 5:101833. doi: 10.1016/j.xcrm.2024.101833
28. Jiang Y, Chen C, Liu G, Fang T, Lu N, Bei W, et al. Combination strategy exploration for prior treated recurrent or metastatic nasopharyngeal carcinoma in the era of immunotherapy. Sci Rep. (2024) 14:1768. doi: 10.1038/s41598-024-52326-7
29. Yang M, Yang Y, Cui H, Guan Z, and Yang Y. The natural compound gambogic acid radiosensitizes nasopharyngeal carcinoma cells under hypoxic conditions. Tumori. (2016) 102:135–43. doi: 10.5301/tj.5000411
30. Claps G, Faouzi S, Quidville V, Chehade F, Shen S, Vagner S, et al. The multiple roles of LDH in cancer. Nat Rev Clin Oncol. (2022) 19:749–62. doi: 10.1038/s41571-022-00686-2
31. Cheng C, Zhuge L, Xiao X, Luan S, and Yuan Y. Overcoming resistance to PD-1/PD-L1 inhibitors in esophageal cancer. Front Oncol. (2022) 12:955163. doi: 10.3389/fonc.2022.955163
32. Sun JY, Zhang D, Wu S, Xu M, Zhou X, Lu XJ, et al. Resistance to PD-1/PD-L1 blockade cancer immunotherapy: mechanisms, predictive factors, and future perspectives. Biomark Res. (2020) 8:35. doi: 10.1186/s40364-020-00212-5
33. Ding X, Hua YJ, Zou X, Chen XZ, Zhang XM, Xu B, et al. Camrelizumab plus famitinib in patients with recurrent or metastatic nasopharyngeal carcinoma treated with PD-1 blockade: data from a multicohort phase 2 study. EClinicalMedicine. (2023) 61:102043. doi: 10.1016/j.eclinm.2023.102043
34. Guo SS, Yang JH, Sun XS, Liu LZ, Yang ZC, Liu LT, et al. Reduced-dose radiotherapy for Epstein-Barr virus DNA selected staged III nasopharyngeal carcinoma: A single-arm, phase 2 trial. Eur J Cancer. (2023) 194:113336. doi: 10.1016/j.ejca.2023.113336
35. Li Y, Xu T, Qian W, Lu X, and Hu C. Radiation-induced nasopharyngeal ulcers after intensity modulated radiotherapy in primary nasopharyngeal carcinoma patients: A dose-volume-outcome analysis. Oral Oncol. (2018) 84:41–6. doi: 10.1016/j.oraloncology.2018.06.017
Keywords: nasopharyngeal carcinoma, recurrence, metastasis, immune checkpoint inhibitors, anti-angiogenic agent
Citation: Gao J, Xu J, He Y, Zong D, Jin T and He X (2025) Efficacy and safety of apatinib or anlotinib combined with PD-1 inhibitors-based therapy as subsequent-line treatment for recurrent or metastatic nasopharyngeal carcinoma: a real-world retrospective study. Front. Oncol. 15:1624286. doi: 10.3389/fonc.2025.1624286
Received: 07 May 2025; Accepted: 23 October 2025;
Published: 06 November 2025.
Edited by:
Sharon R. Pine, Division of Medical Oncology, Department of Medicine, School of Medicine, University of Colorado Anschutz Medical Campus, Aurora, United StatesReviewed by:
Weiren Luo, The Second Affiliated hospital of Southern University of Science and Technology, Shenzhen, ChinaMyrto K. Moutafi, University General Hospital Attikon, Athens, Greece
Copyright © 2025 Gao, Xu, He, Zong, Jin and He. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xia He, aGV4aWFibUAxNjMuY29t
†These authors have contributed equally to this work and share first authorship
 Jingjing Gao
Jingjing Gao Jingshu Xu3†
Jingshu Xu3† Dan Zong
Dan Zong Xia He
Xia He